Abstract
Astrocytes respond to injury and disease in the central nervous system (CNS) with a process referred to as reactive astrogliosis. Recent progress demonstrates that reactive astrogliosis is not a simple all-or-none phenomenon, but is a finely gradated continuum of changes that range from reversible alterations in gene expression and cell hypertrophy, to scar formation with permanent tissue rearrangement. There is now compelling evidence that reactive astrocytes exhibit a substantial potential for heterogeneity at multiple levels, including gene expression, cell morphology, topography (distance from lesions), CNS regions, local (among neighboring cells), cell signaling and cell function. Structural and functional changes are regulated in reactive astrocytes by many different potential signaling events that occur in a context dependent manner. It is noteworthy that different stimuli of astrocyte reactivity can lead to similar degrees of GFAP upregulation while causing substantially different changes in transcriptome profiles and cell function. Thus, it is not possible to equate simple and uniform measures such as cell hypertrophy and upregulation of GFAP expression with a single, uniform concept of astrocyte reactivity. Instead, it is necessary to recognize the considerable potential for heterogeneity and determine the functional implications of astrocyte reactivity in a context specific manner as regulated by specific signaling events.
Keywords: reactive astrocytes, reactive astrogliosis, glial scar, trauma, inflammation, autoimmune disease, heterogeneity
1. Introduction
Astrocytes respond to all forms of injury and disease in the central nervous system (CNS) through a process referred to as astrogliosis [48]. Studies over the past twenty years provide compelling evidence that reactive astrogliosis is not a simple all-or-none phenomenon, but is a finely gradated continuum of changes that range from reversible alterations in gene expression and cell hypertrophy, to scar formation with permanent tissue rearrangement. It has also become clear that the structural and functional changes associated with reactive astrogliosis occur in context dependent manners as regulated by many different potential signaling events [45, 48]. Observations such as these have gradually revealed various levels of heterogeneity among reactive astrocytes. This article summarizes the increasing evidence for diversity and heterogeneity of reactive astrogliosis and reactive astrocytes.
2. Diversity of astrocyte responses to CNS insults
Based on a large body of observations in experimental animals and human pathological specimens, we have recently proposed a definition of reactive astrogliosis that includes several grades of severity that may be commonly encountered in experimental and clinical histopathological examinations [45, 48]. This definition encompasses four key features: (i) reactive astrogliosis is a spectrum of potential molecular, cellular and functional changes in astrocytes that occur in response to all forms and severities of CNS injury and disease; (ii) the changes undergone by reactive astrocytes vary with severity of the insult along a graded continuum, (iii) the changes of reactive astrogliosis are regulated in a context-specific manner by inter- and intra-cellular signaling molecules; (iv) the changes undergone during reactive astrogliosis have the potential to alter astrocyte activities both through gain and loss of functions [45, 48]. Although the increasing severities of reactive astrogliosis transition seamlessly along a continuum, it is convenient to recognize several broad categories.
2.1 Terminology
Use of certain terms can vary considerably among authors. We will use “reactive astrogliosis” and “reactive astrocytes” as general all-inclusive descriptors of all forms of astrocyte responses associated with injury or disease. As discussed below, these terms encompass astrocyte responses of considerable diversity and heterogeneity. We will not use “activation” or “activated astrocytes” as terms that solely denote astrocyte responses to injury or disease. Astrocytes in healthy tissue continually exhibit physiological activation in the form of transient, ligand-evoked elevations in intracellular calcium ([Ca2+]i) that represent a type of astrocyte excitability, involved in mediating many critical dynamic astrocyte functions, including interactions with synapses and regulation of blood flow [3, 20, 50, 52]. Astrocyte activation can thus range from physiological contexts in healthy to pathophysiologic contexts involved in mediating or modulating responses to injury and disease. Lastly, we will use the terms ‘glial scar’, ‘astroglial scar’ and ‘scar-forming astrocytes’ only in specific contexts where astrocytes form borders between healthy and necrotic tissue.
2.2 Mild to moderate reactive astrogliosis
Mild to moderate reactive astrogliosis consists of changes (up or down) in gene expression that occur together with variable degrees of hypertrophy of cell body and stem processes, without substantive loss of individual astrocyte domains and without astrocyte proliferation [45, 48]. There is increased expression of various astrocyte structural proteins such as glial fibrillary acid protein (GFAP) that is somewhat proportional to the degree of reactivity. Mild to moderate reactive astrogliosis is generally associated with mild non-penetrating and non-contusive trauma, or with diffuse innate immune activation (viral infections, system bacterial infections), or with areas that are some distance to focal CNS lesions. In healthy grey matter, individual astrocytes occupy contiguous, essentially non-overlapping domains [11] that are more or less preserved in mild to moderate reactive astrogliosis [55].
2.3 Severe diffuse reactive astrogliosis
Severe diffuse reactive astrogliosis included changes (up or down) in gene expression with pronounced upregulation of GFAP, cellular hypertrophy, dispersed astrocyte proliferation and some loss of individual astrocyte domains with overlapping of neighboring astrocyte processes [45, 48]. These changes can extend diffusely over substantive areas, and generally occur in response to certain types of infection, or in areas responding to chronic neurodegenerative triggers or multiple areas of small local ischemia. Because there can be considerable tissue reorganization, the potential for resolution and return to normal structure is reduced [48].
2.4. Compact astroglial scar formation
Compact astroglial scars derive almost entirely from newly proliferated astrocytes with elongated shapes [54], whose cell processes overlap and intertwine extensively to form compact borders that surround and demarcate areas of severe tissue damage, necrosis, infection or autoimmune-triggered inflammatory infiltration [10, 14, 17, 53, 54]. Astrocyte scar borders directly interface with and surround a variety of non-neural cell types in the central core of tissue lesions, including perivascular derived fibroblasts, fibrocytes, pericytes and other glial cells [1, 9, 24, 40, 45, 48]. The large composite of multicellular lesion core also contains a rich deposit of collagenous extracellular matrix that contains many molecular cues that inhibit axonal and cellular migration [43]. It is noteworthy that glial scar formation is associated with substantive tissue reorganization and structural changes that are essentially permanent and persist even when triggering insults may have resolved.
3. Multiple levels of reactive astrocyte heterogeneity
There is a growing awareness of heterogeneity among astrocytes in healthy CNS [51, 57]. This awareness, plus observations of different forms of astrogliosis as just discussed, are leading to an appreciation of heterogeneity among reactive astrocytes at multiple levels (Fig. 1).
Fig. 1.
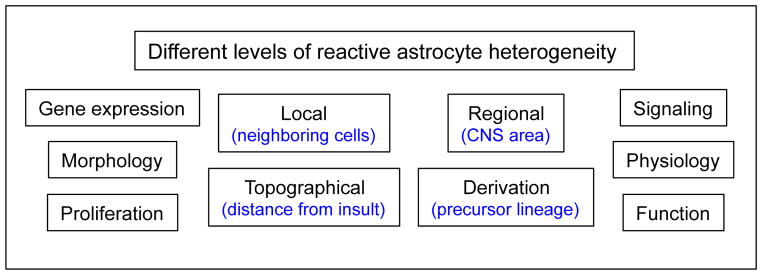
Schema depicts multiple levels at which reactive astrocytes can exhibit heterogeneity.
3.1. Gene expression
Astrocytes exhibit a wide range of changes in gene expression (both in the up and down directions) when they become reactive in response to different CNS insults in vivo, or when they are exposed to triggers of reactive astrogliosis in vitro. [15, 45]. GFAP appears to be upregulated with most forms of reactive astrogliosis [15, 45], and is widely used as a reliable marker in experimental and clinical settings [48]. Additional molecules appear to be upregulated in many forms of reactive astrogliosis, for example Lcn2 [12, 28], and may become useful as broad markers of astrogliosis. In contrast, many other molecules are regulated selectively only in response to different specific triggers of reactive astrogliosis. Microarray profiling to generate transcriptome databases has revealed considerable heterogeneity of reactive astrocytes after different types of insults in vivo or after stimulation with different mediators in vitro. For example, there are major differences in the transcriptome profiles of reactive astrocytes isolated from in vivo tissue after stroke or peripheral LPS injections [56] or hyperammonemia [30] or in normal aging versus young adult [36]. In addition, related studies demonstrate major differences in the transcriptome profiles in astrocytes in cell cultures after stimulation with different mediators of reactive astrogliosis such as LPS, IL1β, TNFα, INFγ or TGFβ individually or in combinations [21, 27, 31, 37]. Stimulation of astrocytes with specific molecular mediators of reactivity can significantly alter from several hundreds to several thousands of astrocyte transcripts in ways that are selective and exhibit some overlap, but also considerable differences [21, 27, 31, 37, 56]. Such findings are consistent with the model that the changes in reactive astrocyte function induced by reactive astrogliosis are context dependent and driven by specific signaling mechanisms [45]. For example, common functional themes of changes induced in reactive astrocytes by cytokines and inflammatory mediators include modulation of molecular and cellular networks associated with regulation of cell morphology, cell maintenance, cell growth and cell proliferation, consistent with the changes undergone by reactive astrocytes in vivo such as cell hypertrophy and proliferation in response to CNS injury and tissue repair [21, 56]. Other common themes include antigen presentation and regulation of astrocyte production of cytokines and molecules involved in immune and inflammatory regulation [21, 56]. Such changes are likely to drive astrocyte functions towards interactions with immune and inflammatory cells. In contrast, after stroke, gene expression changes induced in reactive astrocytes appeared more directed towards repair and protective functions [56]. These studies show that astrocytes exhibit broad, diverse and selective changes in transcriptome profiles in response to stimulation with different triggers of reactive astrogliosis. It is particularly noteworthy that different stimuli of astrocyte reactivity can lead to similar degrees of GFAP upregulation, while causing substantially different changes in transcriptome profiles, indicating that GFAP upregulation on its own provides little information about reactive astrocyte function.
3.2. Morphology: Hypertrophy with domain preservation or process interdigitation
Hypertrophy of cell soma and processes is a canonical morphological feature of reactive astrocytes [48]. Nevertheless, reactive astrocyte hypertrophy is not a stereotypic, uniform response. Instead, the degree of cellular hypertrophy is heterogeneous and highly variable among reactive astrocytes and is more or less proportional to the severity of the insult and the proximity of the astrocyte to the insult [48]. The functional implications of this heterogeneity of cellular hypertrophy among reactive astrocytes are not yet understood. An additional level of morphological heterogeneity among reactive astrocytes involves the degree to which cellular processes do or do not interdigitate. In healthy tissue, processes of individual astrocytes occupy essentially non-overlapping domains that are preserved during cellular hypertrophy in mild to moderate reactive astrogliosis [11, 55]. However, during severe astrogliosis that involves cell proliferation, there is clearly evident interdigitation of reactive astrocyte processes [48], in particular in scar borders around tissue lesions, where the cell processes of newly proliferated, elongated reactive astrocytes intertwine and intermingle extensively (Fig. 2) [54]. Thus, there is clear evidence for different forms of morphological heterogeneity of reactive astroglia as regards the degree of hypertrophy and the degree of interaction and interdigitation of cell processes.
Fig. 2. Photomicrographs depict heterogeneity of reactive astrocytes that form scar borders around tissue lesions or are distant to focal tissue lesions.
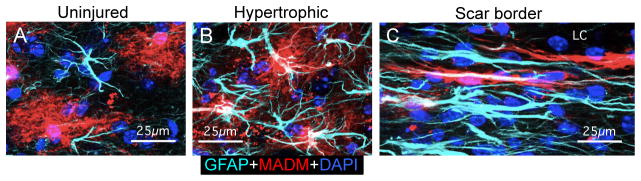
Images show mouse spinal cord stained for GFAP (blue) and a reporter protein (red) derived from the MADM system of sparsely labeling astrocytes. (A) In uninjured tissue, astrocyte processes labeled with GFAP or reporter protein respect individual cellular domains. (B) In tissue somewhat removed from an SCI lesion, the processes of hypertrophic astrocytes exhibit some, but minimal overlap. (C) In the astrocyte scar border that immediately abuts and surrounds the SCI lesion core (LC), the cell processes of elongated astrocytes overlap, make contacts and intertwine extensively. Adapted from [54].
3.3. Proliferation
Astrocyte proliferation in response to CNS insults has been recognized for some time [23, 33], but has been a less well-characterized aspect of reactive astrogliosis. Recent studies demonstrate that proliferation by reactive astrocytes is heterogeneous, and is in particular an essential feature of the formation of the compact astrocyte scar borders that surround tissue lesions [17, 54]. These studies show that scar forming astrocytes that immediately interface with and surround damaged and inflamed tissue after SCI are comprised almost entirely of newly proliferated astroglia that have elongated and highly interdigitating cell processes [54]. If proliferation of reactive astrocytes is prevented, then the formation of these scar borders is prevented [17]. These studies also show that (i) there is a gradient of astroglial proliferation that diminishes rapidly with distance from the SCI lesion, (ii) the density of astroglial cell bodies in the compact scar border is nearly double that in uninjured tissue, (iii) astrocyte scar borders are compact structures of limited (several hundred micrometer) thickness, and (iv) accompanying the gradient of diminishing astroglial proliferation there is also a gradient of rapidly diminishing density of astroglial cells with distance from the SCI lesion, which transitions in a graded fashion to a density similar to that seen in healthy tissue [54]. The newly proliferated elongated astroglia in mature scar borders appear to derive from actively dividing elongated GFAP-positive astroglia with shapes and molecular profiles that are similar to GFAP-positive radial glial progenitors that express markers associated with gliogenic, but not neuronogenic potential [26, 54]. In addition, proliferation can occur among mature scattered astrocytes not directly involved in scar border formation. For example, in tissue not immediately adjacent to the SCI lesion, actively proliferating GFAP-positive astrocytes have stellate appearances associated with mature astrocytes in healthy tissue [54] and look similar to the proliferating perivascular astrocytes recently identified by in vivo imaging of live tissue after ischemia [5]. Together, these findings point towards the potential for different forms of reactive astrocyte proliferation after different types and different severities of insults. Such findings also begin to define different roles for proliferation during reactive astrogliosis and underscore the heterogeneity of reactive astrocytes that have or have not proliferated.
3.4. Topography (with respect to distance from insults)
The previous three sections summarize evidence that reactive astrogliosis is not a simple all or none response, but instead is highly and specifically variable as regards changes in molecular expression, morphology, and proliferation, which are tuned in a context specific manner to different CNS insults. Such findings support a concept of phenotypic heterogeneity of reactive astrocytes. This concept can be extended further by evidence that reactive astrocytes exhibit phenotypic heterogeneity in all three of the parameters molecular expression, morphology, and proliferation, in a manner that is graded with respect to distance from acute focal tissue lesions resulting from traumatic, ischemic or autoimmune injury [48, 53, 54]. A recent analysis of phenotypic heterogeneity of reactive astrocytes after SCI found two broad and fundamentally different categories of reactive astrocytes comprising: (i) newly proliferated, elongated astroglia with extensively overlapping and interacting cell processes that form the compact scar borders, and (ii) hypertrophic stellate reactive astroglia that for the most part are not proliferative and derive directly from mature local astroglia, and whose processes overlap far less extensively or remain within their original territories [54]. Compared with astrocyte appearance in healthy tissue, phenotypic changes are maximal immediately adjacent to severely damaged tissue, where the majority of reactive astrocytes are newly proliferated and exhibit elongated shapes with overlapping processes. With increasing distance away from areas of tissue damage, the degree of proliferation and overlap of astrocyte processes diminishes, transition first into areas where processes remain within their individual domains, but the astrocytes are still hypertrophic and reactive, and then transitioning gradually to areas where astrocytes are indistinguishable in phenotype from those in healthy tissue. Such findings provide clear evidence of phenotypic heterogeneity of reactive astrocytes with respect to distance from tissue lesions.
3.5. Local (among neighboring cells)
A particularly interesting type of reactive astrocyte heterogeneity to consider is the potential for heterogeneity of phenotype and function among locally intermingled neighboring astrocytes. Although little is as yet known about this topic, there is intriguing evidence for local heterogeneity among neighboring reactive astrocytes. For example, immunohistochemical analysis at the single cell in vivo shows that closely neighboring reactive astrocytes can exhibit markedly different levels of expression of different chemokine or cytokines in response to local injection of a mixture of inflammatory mediators (Fig. 3) [21]. Single cell immunohistochemical analyses in vivo also shows that closely neighboring reactive astrocytes can exhibit markedly different levels of expression of activated signaling molecules such as pSTAT3 at different times after SCI [24]. In addition, neighboring astrocytes in healthy tissue exhibit differences in their expression of the Gli family of transcription factors essential for sonic hedgehog signaling (SHH), and genetic disruption of the SHH signaling pathway from astrocytes causes GFAP upregulation and cell hypertrophy in those particular astrocytes, probably through a mechanism of disturbing astrocyte-neuronal interactions [19]. Such findings point towards the growing evidence for heterogeneity among neighboring astrocytes and begs the question of whether or not there are functional specializations among astrocytes in healthy tissue or among reactive astrocytes responding to CNS insults.
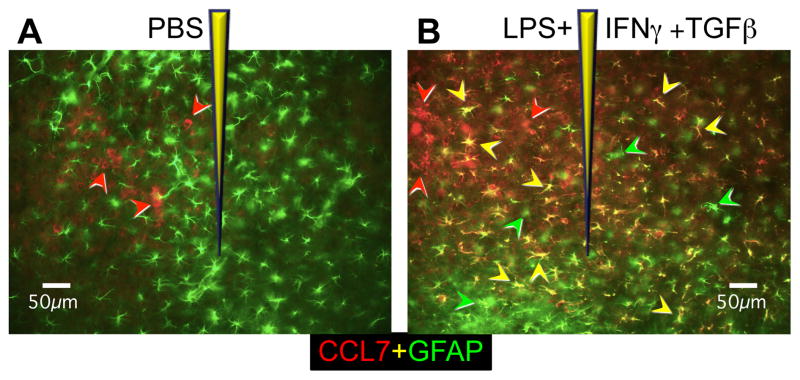
Fig. 3. Photomicrographs depict heterogeneity of reactive astrocytes in response to different timuli (A, B), as well as local heterogeneity of reactive astrocytes in response to the same stimulus (B).
Images show mouse cerebral cortex stained for GFAP (green) or the chemokine CCL7 (red) after injection of either PBS (A) or the inflammatory mediators, LPS+IFNγ+TGFβ in PBS, (B). Green arrowheads indicate reactive astrocytes that express only GFAP, red arrowheads indicate microglia or macrophages that express CCL7, and yellow arrowheads indicate reactive astrocytes that express both GFAP and CCL7. After injection of PBS, reactive astrocytes stain only for GFAP (green) and no reactive astrocytes have upregulated expression of CCL7 (A). After injection of LPS+IFNγ+TGFβ in PBS, many reactive astrocytes have upregulated both GFAP and CCL7 (yellow arrows) and these are intermingled with some reactive astrocytes that have not upregulated CCL7 and are positive only for GFAP (green arrowheads) (B). Adapted from [21].
3.6. CNS region and precursor lineage
Astrocytes from different CNS regions have long been known to exhibit subtle differences in cell morphology. Regional differences in astrocyte transcriptome profiles have also been noted [4]. Recent findings using genetic tools for cell-lineage fate mapping show that astrocytes are allocated to spatial domains in mouse spinal cord in accordance with their embryonic sites of origin [51]. Interestingly, newly proliferated reactive astrocytes generated in response to a penetrating injury were derived according their spatial site of original [51]. In addition, it has been reported that some reactive astrocytes found near ischemic injuries of cerebral cortex may derive from precursor cells in the periventricular region [6]. Such findings raise the interesting question of whether reactive astrogliosis is the same in different CNS regions, such as cerebral cortex or spinal cord, and whether reactive astrocytes derived from different precursor lineages might exhibit different responses and exert different functions or effects. At present there is little understanding about the potential for reactive astrocytes in different CNS regions or from different sites of derivation or from different lineages to exhibit different responses to similar insults. Moreover, surprisingly little information is available even about potential differences in the responses of grey and white matter astrocytes to similar insults. These are important topics that warrant investigation.
3.7. Signaling and regulation
The different types of phenotypic heterogeneity of reactive astrocytes summarized in the previous sections raise important questions about the signaling mechanisms that might underlie such heterogeneity. The high degree of heterogeneity among reactive astrocytes with respect to changes in molecular expression, morphology, and proliferation argues strongly that reactive astrogliosis (i) is not a stereotypic all-or-none phenomenon, and (ii) is not controlled by a simple on-off signaling mechanism. Instead, there is a growing body of evidence that reactive astrogliosis can be triggered and subtly regulated by a myriad of extracellular and intracellular signaling mechanisms that finely tune astrocyte reactivity to different CNS insults in a context specific manner. A vast number of extracellular molecular mediators that can trigger or modulate astrocyte reactivity can be released in response to essentially all forms of CNS insults and by all types of cells. The broad range of molecular categories that can trigger reactive astrogliosis ranges from small molecules such as purines and transmitters, to large polypeptide growth factors and cytokines as reviewed in detail elsewhere [45, 48]. Some of these molecular mediators are released via specific signaling mechanisms, while others are released by simply cell damage or cell death. Most of the extracellular molecular triggers of reactive astrogliosis have well defined receptor targets than have the potential to initiate a wide variety of different intracellular signaling cascades that involve many different second messenger systems.
Although it is beyond our scope here to review the voluminous amount of information available regarding reactive astrocyte signaling, it is useful to consider a few specific examples. As discussed above, not all reactive astrogliosis induces cell proliferation, and reactive astrocyte proliferation is particularly associated with the formation of compact scar borders around necrotic or inflamed tissue lesions. In this regard, several different molecular triggers of reactive astrocyte proliferation in vivo have been identified, including EGF, FGF, endothelin 1 and ATP [18, 29, 32]. Gene array studies of astrocyte transcriptome profiles show that astrocytes can have very different responses to different molecular mediators or modulators of reactivity [21, 27, 31, 37, 56]. Other studies have examined the roles of specific signaling cascades. Transgenic loss of function studies from different laboratories have identified important roles for signaling pathways involving STAT3, SOCS3 or NFκB in regulating reactive astrogliosis and its functions in different models of CNS disorders and have highlighted the potential for different signaling mechanisms to regulate different functions. For example, deletion of STAT3 or its associated membrane receptor GP130, markedly attenuates certain aspects of reactive astrogliosis such as cell hypertrophy, upregulation of GFAP and scar formation, and was associated with increased spread of inflammation and infection, increased lesion size and demyelination and impaired functional recovery after spinal cord injury, autoimmune disease or infection, implying an overall anti-inflammatory role for this signaling mechanism in reactive astrocytes [14, 22, 24, 35]. In contrast, deletion of SOCS3 or NFkB also attenuated certain aspects of reactive astrogliosis such as cell hypertrophy and upregulation of GFAP, but was associated with reduced inflammation and lesion size after spinal cord injury or autoimmune disease, implying a pro-inflammatory role for this signaling mechanism in reactive astrocytes [7, 8, 35]. Thus, different signaling pathways that trigger comparable levels of morphological astrocyte reactivity in the form of cellular hypertrophy and increased GFAP expression, can be involved in mediating what appear to be very different pro- or anti-inflammatory effects. These results clearly demonstrate that it is not valid to assume that reactive astrocytes that exhibit similar degrees of cellular hypertrophy and induction of GFAP expression are exerting similar functional effects. Findings such as these highlight the heterogeneity of the signaling mechanisms that underlie astrocyte reactivity and point towards the need for substantial more work in identifying the roles of specific signaling pathways in specific contexts and functions.
3.8. Physiology
In comparison with the voluminous information available about morphological and genetic changes associated with reactive astrogliosis, far less is known about the impact of reactive astrogliosis on astrocyte physiology. Although astrocytes do not generate action potentials, astrocytes do exhibit ligand-evoked changes in intracellular calcium concentration ([Ca2+]i), which are thought to represent a form of astrocyte excitability that can enable astrocytes to exert functions in a dynamic fashion, including interactions with synapses and regulation of blood flow [3, 20, 25, 50, 52]. The effects of reactive astrogliosis on astrocyte physiology and calcium signaling are not well defined and are only beginning to be studied. For example, exposure to inflammatory mediators (i) markedly alters astrocyte expression of a large number of G protein-coupled receptors (GPCRs) and G proteins, and (ii) markedly alters calcium signaling in the form of [Ca2+]i transients evoked by specific ligands of various receptors whose gene expression was changed [21]. Interestingly, different inflammatory mediators caused markedly different changes in GPCR and G protein expression, providing evidence that exposure to different stimuli during reactive astrogliosis has the potential to yield reactive astrocytes with different physiological characteristics. These findings also show that molecular triggers or modulators of reactive astrogliosis such as cytokines and inflammatory mediators can have pronounced and heterogeneous effects on astrocyte physiology, which have the potential to impact on a variety of astrocyte functions.
4. Functional implications
In healthy CNS, astrocytes exert many essential and complex functions including (i) homeostatic maintenance of extracellular ions, pH and water [34, 38, 39, 44]; (ii) uptake and clearance of neurotransmitters such as glutamate and GABA [41, 42]; (iii) provision of neurons and axons with energy metabolites [2]; (iv) modulation of local blood flow [3, 49]; and (v) developmental and ongoing influences on synapses and synaptic functions [13, 20]. The degree to which execution of these functions might involve astrocyte specialization and heterogeneity is not known. The degree to which reactive astrocytes maintain or modulate their participation in these functions, and/or adopt new functions, is also not well understood. Nevertheless, in keeping with the different types of phenotypic heterogeneity of reactive astrocytes summarized in the previous sections, there is mounting evidence that reactive astrocytes exhibit functional heterogeneity in different contexts.
Perhaps the most prominent example of functional specialization among reactive astrocytes is the formation of compact scar borders. As discussed in more detail above, scar borders are comprised primarily of newly proliferated reactive astrocytes with elongated shapes and intertwining cell processes [54]. Functionally, these scar borders surround damaged and inflamed tissue lesions caused by focal trauma, ischemic stroke, infection and autoimmune disease; and loss of function studies show that astrocyte scars restrict the spread of inflammatory cells into neighboring healthy tissue that contains viable neurons [14, 17, 48, 53, 54]. These scar-forming astrocytes are clearly different in phenotype and function from stellate reactive astrocytes that have not proliferated and have hypertrophied within their individual domain structure.
There is also evidence suggesting other forms of functional heterogeneity among reactive astrocytes. Various studies show that reactive astrocytes can respond to stimulation with different inflammatory mediators by selectively upregulating their production of different chemokines, cytokines and growth factors [21, 27, 31, 37, 46, 56]. These and other observations strongly suggest that reactive astrocytes take part in two-way dialogues with immune and inflammatory cells and thereby play central roles in regulating CNS immune and inflammatory responses. Whether or not there is specialization among reactive astrocytes in this context remains to be determined. However, the observation of heterogeneity in the production of chemokines by neighboring astrocytes exposed to inflammatory mediators in vivo (Fig. 3) [21], suggests that this may be the case.
The ability of reactive astrocytes to respond in different ways to different molecular signals as described in sections 3.1–3.8, strongly implies that reactive astrocytes exhibit functional heterogeneity, particularly in different contexts when exposed to different stimuli. Given that reactive astrocytes have long been implicated in having detrimental effects as well as beneficial functions [48], it will be important to determine in a context specific manner which signals lead to beneficial, and which to detrimental, effects. For example, certain reactive astrocyte responses that may be beneficial in one context, such as a local bacterial infection, may be detrimental in another context, such as a sterile traumatic or ischemic injury. Reactive astrocytes that are responding to a primary insult, such as a traumatic injury that may be sterile (uninfected) may nonetheless be influenced by peripheral infections, which generate circulating cytokines and other inflammatory mediators that can drive reactive astrocyte transcriptome profiles towards antimicrobial cytotoxic phenotypes that would not normally be implemented in sterile insults. Shifts towards cytotoxic antimicrobial functions induced in this manner in reactive astrocytes have the potential to have detrimental effects on local neural cell types. In this regard it is interesting to note that there is clinical epidemiological evidence that peripheral infections have a negative impact on neurological outcome after spinal cord injury [16].
Reactive astrocyte heterogeneity also has the potential to impact on astrocyte-neuron interactions. In the healthy CNS, astrocytes interact with neurons on multiple spatial and temporal scales that can influence or modulate neural function in various ways [20]. Stimulation by cytokines and inflammatory mediators can modulate reactive astrocyte physiology and functions in ways that have the potential to impact on neural functions including complex behaviors such as sleep and mood [47].
5. Conclusions
There is now compelling evidence that reactive astrogliosis is not a simple all-or-none phenomenon, but is a finely graded continuum of changes that range from reversible alterations in gene expression and cell hypertrophy, to scar formation with permanent tissue rearrangement. The structural and functional changes associated with astrocyte reactivity occur in context dependent manners as regulated by many different potential signaling events. Accordingly, reactive astrocytes exhibit a substantial potential for heterogeneity at multiple levels, including gene expression, cell morphology, topography (distance from lesions), CNS regions, local (among neighboring cells), cell signaling and cell function. It is noteworthy that different stimuli of astrocyte reactivity can lead to similar GFAP upregulation while causing substantially different changes in transcriptome profiles and cell function. Thus, it is not possible to equate simple and uniform measures such as cell hypertrophy and upregulation of GFAP expression with a single, uniform concept of astrocyte reactivity. Instead, it is necessary to recognize the considerable potential for heterogeneity and determine the functional implications of astrocyte reactivity in a context specific manner as regulated by specific signaling events.
Highlights.
Astrocyte reactivity is not a single stereotypic program
Reactive astrocytes exhibit substantial heterogeneity at multiple levels
Heterogeneity includes gene expression, cell morphology and cell function
Astrocyte reactivity varies in a context specific manner
Astrocyte reactivity occurs in response to many different specific signaling events
Acknowledgments
Work in the authors’ laboratory is supported by grants from the National Institutes of Health (NS057624), The Dr. Miriam and Sheldon G. Adelson Medical Foundation and Wings for Life.
Footnotes
The authors have no financial conflicts or interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aldrich A, Kielian T. Central nervous system fibrosis is associated with fibrocyte-like infiltrates. Am J Pathol. 2011;179:2952–2962. doi: 10.1016/j.ajpath.2011.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allaman I, Belanger M, Magistretti PJ. Astrocyte-neuron metabolic relationships: for better and for worse. Trends Neurosci. 2011;34:76–87. doi: 10.1016/j.tins.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachoo RM, Kim RS, Ligon KL, Maher EA, Brennan C, Billings N, Chan S, Li C, Rowitch DH, Wong WH, DePinho RA. Molecular diversity of astrocytes with implications for neurological disorders. Proc Natl Acad Sci USA. 2004;101:8384–8389. doi: 10.1073/pnas.0402140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bardehle S, Kruger M, Buggenthin F, Schwausch J, Ninkovic J, Clevers H, Snippert HJ, Theis FJ, Meyer-Luehmann M, Bechmann I, Dimou L, Gotz M. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat Neurosci. 2013;16:580–586. doi: 10.1038/nn.3371. [DOI] [PubMed] [Google Scholar]
- 6.Benner EJ, Luciano D, Jo R, Abdi K, Paez-Gonzalez P, Sheng H, Warner DS, Liu C, Eroglu C, Kuo CT. Protective astrogenesis from the SVZ niche after injury is controlled by Notch modulator Thbs4. Nature. 2013;497:369–373. doi: 10.1038/nature12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brambilla R, Bracchi-Ricard V, Hu WH, Frydel B, Bramwell A, Karmally S, Green EJ, Bethea JR. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med. 2005;202:145–156. doi: 10.1084/jem.20041918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brambilla R, Persaud T, Hu X, Karmally S, Shestopalov VI, Dvoriantchikova G, Ivanov D, Nathanson L, Barnum SR, Bethea JR. Transgenic inhibition of astroglial NF-kappaB improves functional outcome in experimental autoimmune encephalomyelitis by suppressing chronic central nervous system inflammation. J Immunol. 2009;182:2628–2640. doi: 10.4049/jimmunol.0802954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bundesen LQ, Scheel TA, Bregman BS, Kromer LF. Ephrin-B2 and EphB2 regulation of astrocyte-meningeal fibroblast interactions in response to spinal cord lesions in adult rats. J Neurosci. 2003;23:7789–7800. doi: 10.1523/JNEUROSCI.23-21-07789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bush TG, PN, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH, Sofroniew MV. Leukocyte infiltration, neuronal degeneration and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- 11.Bushong EA, Martone MA, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 atratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarke LE, Barres BA. Emerging roles of astrocytes in neural circuit development. Nat Rev Neurosci. 2013;14:311–321. doi: 10.1038/nrn3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drogemuller K, Helmuth U, Brunn A, Sakowicz-Burkiewicz M, Gutmann DH, Mueller W, Deckert M, Schluter D. Astrocyte gp130 expression is critical for the control of Toxoplasma encephalitis. J Immunol. 2008;181:2683–2693. doi: 10.4049/jimmunol.181.4.2683. [DOI] [PubMed] [Google Scholar]
- 15.Eddleston M, Mucke L. Molecular profile of reactive astrocytes - implications for their role in neurological disease. Neuroscience. 1993;54:15–36. doi: 10.1016/0306-4522(93)90380-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Failli V, Kopp MA, Gericke C, Martus P, Klingbeil S, Brommer B, Laginha I, Chen Y, DeVivo MJ, Dirnagl U, Schwab JM. Functional neurological recovery after spinal cord injury is impaired in patients with infections. Brain. 2012;135:3238–3250. doi: 10.1093/brain/aws267. [DOI] [PubMed] [Google Scholar]
- 17.Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gadea A, Schinelli S, Gallo V. Endothelin-1 regulates astrocyte proliferation and reactive gliosis via a JNK/c-Jun signaling pathway. J Neurosci. 2008;28:2394–2408. doi: 10.1523/JNEUROSCI.5652-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia AD, Petrova R, Eng L, Joyner AL. Sonic hedgehog regulates discrete populations of astrocytes in the adult mouse forebrain. J Neurosci. 2010;30:13597–13608. doi: 10.1523/JNEUROSCI.0830-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halassa MM, Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol. 2010;72:335–355. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamby ME, Coppola G, Ao Y, Geschwind DH, Khakh BS, Sofroniew MV. Inflammatory mediators alter the astrocyte transcriptome and calcium signaling elicited by multiple g-protein-coupled receptors. J Neurosci. 2012;32:14489–14510. doi: 10.1523/JNEUROSCI.1256-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haroon F, Drogemuller K, Handel U, Brunn A, Reinhold D, Nishanth G, Mueller W, Trautwein C, Ernst M, Deckert M, Schluter D. Gp130-dependent astrocytic survival is critical for the control of autoimmune central nervous system inflammation. J Immunol. 2011;186:6521–6531. doi: 10.4049/jimmunol.1001135. [DOI] [PubMed] [Google Scholar]
- 23.Hatten ME, Liem RK, Shelanski ML, Mason CA. Astroglia in CNS injury. Glia. 1991;4:233–243. doi: 10.1002/glia.440040215. [DOI] [PubMed] [Google Scholar]
- 24.Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, Korsak RA, Takeda K, Akira S, Sofroniew MV. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. 2008;28:7231–7243. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 26.Imura T, Nakano I, Kornblum HI, Sofroniew MV. Phenotypic and functional heterogeneity of GFAP-expressing cells in vitro: Differential expression of LeX/CD15 by GFAP-expressing multipotent neural stem cells and non-neurogenic astrocytes. Glia. 2006;53:277–293. doi: 10.1002/glia.20281. [DOI] [PubMed] [Google Scholar]
- 27.John GR, Lee SC, Song X, Rivieccio M, Brosnan CF. IL-1-regulated responses in astrocytes: relevance to injury and recovery. Glia. 2005;49:161–176. doi: 10.1002/glia.20109. [DOI] [PubMed] [Google Scholar]
- 28.Lee S, Park JY, Lee WH, Kim H, Park HC, Mori K, Suk K. Lipocalin-2 is an autocrine mediator of reactive astrocytosis. J Neurosci. 2009;29:234–249. doi: 10.1523/JNEUROSCI.5273-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levison SW, Jiang FJ, Stoltzfus OK, Ducceschi MH. IL-6-type cytokines enhance epidermal growth factor-stimulated astrocyte proliferation. Glia. 2000;32:328–337. doi: 10.1002/1098-1136(200012)32:3<328::aid-glia110>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 30.Lichter-Konecki U, Mangin JM, Gordish-Dressman H, Hoffman EP, Gallo V. Gene expression profiling of astrocytes from hyperammonemic mice reveals altered pathways for water and potassium homeostasis in vivo. Glia. 2008;56:365–377. doi: 10.1002/glia.20624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meeuwsen S, Persoon-Deen C, Bsibsi M, Ravid R, van Noort JM. Cytokine, chemokine and growth factor gene profiling of cultured human astrocytes after exposure to proinflammatory stimuli. Glia. 2003;43:243–253. doi: 10.1002/glia.10259. [DOI] [PubMed] [Google Scholar]
- 32.Neary JT, Zimmermann H. Trophic functions of nucleotides in the central nervous system. Trends Neurosci. 2009;32:189–198. doi: 10.1016/j.tins.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Norton WT, Aquino DA, Hozumi I, Chiu FC, Brosnan CF. Quantitative aspects of reactive gliosis: a review. Neurochem Res. 1992;17:877–885. doi: 10.1007/BF00993263. [DOI] [PubMed] [Google Scholar]
- 34.Obara M, Szeliga M, Albrecht J. Regulation of pH in the mammalian central nervous system under normal and pathological conditions: facts and hypotheses. Neurochem Int. 2008;52:905–919. doi: 10.1016/j.neuint.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 35.Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y, Okano H. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nature Med. 2006;12:829–834. doi: 10.1038/nm1425. [DOI] [PubMed] [Google Scholar]
- 36.Orre M, Kamphuis W, Osborn LM, Melief J, Kooijman L, Huitinga I, Klooster J, Bossers K, Hol EM. Acute isolation and transcriptome characterization of cortical astrocytes and microglia from young and aged mice. Neurobiol Aging. 2013 doi: 10.1016/j.neurobiolaging.2013.07.008. in press. [DOI] [PubMed] [Google Scholar]
- 37.Pang Y, Cai Z, Rhodes PG. Analysis of genes differentially expressed in astrocytes stimulated with lipopolysaccharide using cDNA arrays. Brain Res. 2001;914:15–22. doi: 10.1016/s0006-8993(01)02766-4. [DOI] [PubMed] [Google Scholar]
- 38.Papadopoulos MC, Verkman AS. Aquaporin water channels in the nervous system. Nat Rev Neurosci. 2013 doi: 10.1038/nrn3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rash JE. Molecular disruptions of the panglial syncytium block potassium siphoning and axonal saltatory conduction: pertinence to neuromyelitis optica and other demyelinating diseases of the central nervous system. Neuroscience. 2010;168:982–1008. doi: 10.1016/j.neuroscience.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reilkoff RA, Bucala R, Herzog EL. Fibrocytes: emerging effector cells in chronic inflammation. Nat Rev Immunol. 2011;11:427–435. doi: 10.1038/nri2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sattler R, Rothstein JD. Regulation and dysregulation of glutamate transporters. Handb Exp Pharmacol. 2006:277–303. doi: 10.1007/3-540-29784-7_14. [DOI] [PubMed] [Google Scholar]
- 42.Schousboe A, Sarup A, Bak LK, Waagepetersen HS, Larsson OM. Role of astrocytic transport processes in glutamatergic and GABAergic neurotransmission. Neurochem Int. 2004;45:521–527. doi: 10.1016/j.neuint.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Silver J, Miller JH. Regeneration beyond the glial scar. Nature Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 44.Simard M, Nedergaard M. The neurobiology of glia in the context of water and ion homeostasis. Neuroscience. 2004;129:877–896. doi: 10.1016/j.neuroscience.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 45.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sofroniew MV. Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators. Neuroscientist. 2013 doi: 10.1177/1073858413504466. in press. [DOI] [PubMed] [Google Scholar]
- 47.Sofroniew MV. Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators. Neuroscientist: advance publication online. 2013 doi: 10.1177/1073858413504466. [DOI] [PubMed] [Google Scholar]
- 48.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- 50.Tong X, Shigetomi E, Looger LL, Khakh BS. Genetically encoded calcium indicators and astrocyte calcium microdomains. Neuroscientist. 2013;19:274–291. doi: 10.1177/1073858412468794. [DOI] [PubMed] [Google Scholar]
- 51.Tsai HH, Li H, Fuentealba LC, Molofsky AV, Taveira-Marques R, Zhuang H, Tenney A, Murnen AT, Fancy SP, Merkle F, Kessaris N, Alvarez-Buylla A, Richardson WD, Rowitch DH. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science. 2012;337:358–362. doi: 10.1126/science.1222381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verkhratsky A, Orkand RK, Kettenmann H. Glial calcium: homeostasis and signaling function. Physiol Rev. 1998;78:99–141. doi: 10.1152/physrev.1998.78.1.99. [DOI] [PubMed] [Google Scholar]
- 53.Voskuhl RR, Peterson RS, Song B, Ao Y, Morales LB, Tiwari-Woodruff S, Sofroniew MV. Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J Neurosci. 2009;29:11511–11522. doi: 10.1523/JNEUROSCI.1514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wanner IB, Anderson MA, Song B, Levine J, Fernandez A, Gray-Thompson Z, Ao Y, Sofroniew MV. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J Neurosci. 2013;33:12870–12886. doi: 10.1523/JNEUROSCI.2121-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilhelmsson U, Bushong EA, Price DL, Smarr BL, Phung V, Terada M, Ellisman MH, Pekny M. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc Natl Acad Sci USA. 2006;103:17513–17518. doi: 10.1073/pnas.0602841103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32:6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y, Barres BA. Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr Opin Neurobiol. 2010;20:588–594. doi: 10.1016/j.conb.2010.06.005. [DOI] [PubMed] [Google Scholar]