Abstract
The central nervous system (CNS) is prone to heterogeneous insults of diverse etiologies that elicit multifaceted responses. Acute and focal injuries trigger wound repair with tissue replacement. Diffuse and chronic diseases provoke gradually escalating tissue changes. The responses to CNS insults involve complex interactions among cells of numerous lineages and functions, including CNS intrinsic neural cells, CNS intrinsic non-neural cells, and CNS extrinsic cells that enter from the circulation. The contributions of diverse non-neuronal cell types to outcome after acute injury, or to the progression of chronic disease, are of increasing interest as the push towards understanding and ameliorating CNS afflictions accelerates. In some cases considerable information is available, in others, comparatively little, as examined and reviewed here.
Introduction
A major goal of contemporary neuroscience is to understand and ameliorate a wide range of central nervous system (CNS) disorders. Towards this end, there is increasing interest in cellular and molecular mechanisms of CNS responses to damage, disease and repair. Neurons are the principal cells executing neural functions and have long dominated investigations into mechanisms underlying CNS disorders. Nevertheless, mounting evidence indicates that treating all types of CNS disorders will require a deeper understanding of how multicellular responses to injury and disease are triggered, evolve, resolve (or not) and impact on neuronal function.
The ability to repair tissue damaged by injury is fundamental to vertebrate biology and central to survival. Evolutionary pressure is likely to have forged certain fundamental cellular and molecular responses to damage that are common across different tissues. The wound or injury response in skin has long served as a model system for dissecting mechanisms of tissue repair after acute focal tissue damage and has provided insight into core cellular and molecular interactions (Greaves et al., 2013; Gurtner et al., 2008; Singer and Clark, 1999). In addition, organ-specific features exist. Organ-intrinsic cells that are specialized in inflammatory regulation and tissue repair are emerging as critical elements in organ-specific responses to insults. Organ-specific features apply particularly in the CNS, where glial cells, which maintain the cytoarchitecture and homeostatic regulation without which neurons could not function normally in healthy tissue, are also principal responders to CNS insults. Changes in glial cell function during responses to insults have the potential to impact markedly on neuronal interactions and CNS functions.
CNS insults are caused by diverse etiologies that can elicit a wide range of responses. For example, acute and focal injuries trigger wound repair with tissue replacement, whereas diffuse and chronic diseases can trigger gradually escalating tissue changes. Analysis of similarities and differences in such responses can provide valuable insights. Cellular responses to CNS insults involve complex interactions among cells of numerous lineages and functions, including CNS intrinsic neural cells, CNS intrinsic non-neural cells, and CNS extrinsic cells that enter from the circulation. The biology of cell types that participate in CNS responses to injury and disease models has generally been studied in isolation. There is increasing need to study interplay of different cells to understand mechanisms. This article examines and reviews the multiple cell types involved in, and contributing to, different types of CNS insults. In some cases extensive information is available, in others, comparatively little.
Terminology
Various terms used in discussions of CNS injury and disease can be subject to different interpretations. In this article we will define and use certain specific terms as follows. ‘Reactive gliosis’ will refer not only to microglia and astroglia, but also to glial cells that have come to be known as NG2-positive oligodendrocyte progenitor cells (NG2-OPC). Glial cells in healthy CNS tissue will not be referred to as “resting” or “quiescent”. This is an antiquated concept. Glia are highly active in healthy CNS and dynamically exert complex functions that play critical roles in normal CNS functions (Barres, 2008; Sofroniew and Vinters, 2010). For example, astrocytes exhibit physiological activation in the form of transient, ligand-evoked elevations in intracellular calcium ([Ca2+]i) that represent a type of astrocyte excitability, which is under intense investigation as a potential means of mediating dynamic astrocyte functions, including interactions with synapses and regulation of blood flow (Attwell et al., 2010; Halassa and Haydon, 2010; Tong et al., 2013; Verkhratsky et al., 1998). Microglia perform essential roles in synapse development and turnover (Stephan et al., 2012; Stevens et al., 2007). The term ‘activated’ is often used in a binary all-or-none fashion to define glial cells that have responded to insults. We feel that use of the term in this manner is inaccurate in two ways. First it does not recognize that glia are continually being ‘activated’ in physiological contexts. Second, as discussed throughout this review, glial cell responses to CNS insults are not binary and are highly diverse and specifically regulated. To differentiate physiological activation of glial cells in healthy contexts from responses associated with injury or disease we will use the term ‘reactive’, which is also meant denote a broad spectrum of potential responses of glial cells to CNS insults. Lastly, we will avoid use of the term ‘scar’ on its own, and will instead distinguish between ‘astrocyte scars’ that form compact borders around tissue lesions, and ‘fibrotic scars’ that are formed by multiple non-neural cell types and extracellular matrix within lesion cores (as discussed in detail below). We will equate ‘glial scar’ with ‘astrocyte scar’.
Multicellular response to CNS insults
Before discussing the different types of responses to CNS damage and disease, it is useful briefly to introduce different cells types involved in these responses. For ease of consideration we have grouped cells according to lineages as (i) neural and non-neural cells intrinsic to CNS, and (ii) blood-borne non-neural cells that derive primarily from bone marrow (Table 1). Some of these cell types have been studied extensively in CNS disorders, others comparatively little. Most often they have been studied in isolation. In subsequent sections we will endeavor to examine their interactions.
Table 1.
Diverse cell types in CNS responses to damage and disease
| CNS intrinsic cells: | Blood borne non-neural cells: |
|---|---|
|
CNS intrinsic neural cells: Neurons Oligodendrocytes Astrocytes NG2-OPC Neural stem/progenitor cells Ependyma |
Leukocytes: Monocyte/Macrophage Neutrophils Eosinophils NK cells T cells B cells |
|
CNS intrinsic non-neural cells: Microglia Perivascular fibroblasts Pericytes Endothelia & progenitors |
Other bone marrow-derived cells: Platelets (thrombocytes) Fibrocytes Mesenchymal (bone marrow stromal) stem cells |
CNS intrinsic neural cells
The principal neural-lineage cells in the CNS, neurons, oligodendrocytes and astrocytes, have been studied and reviewed extensively as regards their individual responses to CNS injury and disease (Barres, 2008; Franklin and Ffrench-Constant, 2008; Mattson, 2000; Sofroniew and Vinters, 2010). Less well studied are glial cells that have come to be known as NG2-positive oligodendrocyte progenitor cells (NG2-OPC). Like other glia, NG2-OPC tile the CNS and respond to CNS insults (Nishiyama et al., 2009). Features of the NG2-OPC response include migration towards injury and cell proliferation (Franklin and Ffrench-Constant, 2008; Hughes et al., 2013). Besides replacing lost oligodendrocytes (Sun et al., 2010), other roles of NG2-OPC in CNS injury and disease await future study and elucidation. In addition, the adult CNS harbors neural stem cells (NSC) of different potencies that reside in the peri-ependymal regions along the ventricles and central canal in adult brain and spinal cord (Garcia et al., 2004; Kriegstein and Alvarez-Buylla, 2009). These adult NSC can also respond to CNS injury and generate progenitors and cells of different types that migrate to sites of injury (Benner et al., 2013; Lagace, 2012; Meletis et al., 2008; Ohab and Carmichael, 2008).
CNS intrinsic non-neural cells
Various non-neural lineage cells intrinsic to CNS play critical roles in CNS damage and disease (Table 1). Microglia are well documented as highly sensitive early responders that stimulate and recruit other cells, as well phagocytose debris (Hanisch and Kettenmann, 2007; Kreutzberg, 1996; Nimmerjahn et al., 2005; Ransohoff and Perry, 2009). Fibroblast-related cells including perivascular fibroblasts, meningeal fibroblasts and pericytes, contribute to tissue replacement by forming fibrotic scar tissue after severe damage (Goritz et al., 2011; Klapka and Muller, 2006; Logan and Berry, 2002; Soderblom et al., 2013; Winkler et al., 2011). Endothelia and endothelial progenitors are prominent during tissue replacement after CNS injury (Loy et al., 2002; Oudega, 2012) and are of increasing interest in CNS repair in light of their emerging roles as trophic support cells in CNS development (Dugas et al., 2008), and their ability to produce and present laminin (Davis and Senger, 2005), a preferred growth substrate for many migrating cells and axons.
Blood borne non-neural cells
Blood-borne immune and inflammatory cells of different kinds (Table 1) play prominent roles in CNS responses to damage and disease and have been studied and reviewed (Perry, 2010; Popovich and Longbrake, 2008). In addition to well-known roles in phagocytosis and removal of debris, there is also now increasing evidence that subtypes of leukocytes play active roles in tissue repair (Derecki et al., 2012; London et al., 2013; Popovich and Longbrake, 2008). Platelets aggregate rapidly after damage for clot formation and haemostasis. Other blood-borne, bone marrow derived cell types that home to tissue injury, including in CNS, include fibrocytes (Aldrich and Kielian, 2011; Reilkoff et al., 2011) and bone marrow derived mesenchymal stem cells (Askari et al., 2003; Bianco et al., 2001; Jaerve et al., 2012), but their functions are not well understood.
Extracellular matrix (ECM)
ECM generated by different cells plays critical roles in tissue repair and replacement after acute insults, as well as in chronic tissue remodeling during chronic disease (Midwood et al., 2004). Molecules that modify ECM matrix, such as metaloproteases (MMP), are also important in this regard (Midwood et al., 2004). In the CNS, ECM generated by different cell types responding to damage or disease can include a wide variety of molecules such as laminin, collagens and glycoproteins such as chondroitin or heparan sulfate proteoglycans (CSPG or HSPG) that are implicated both in supporting tissue repair as well as in contributing to the failure of axonal regeneration (Davis and Senger, 2005; Klapka and Muller, 2006; Logan and Berry, 2002; Silver and Miller, 2004).
Response to focal insults
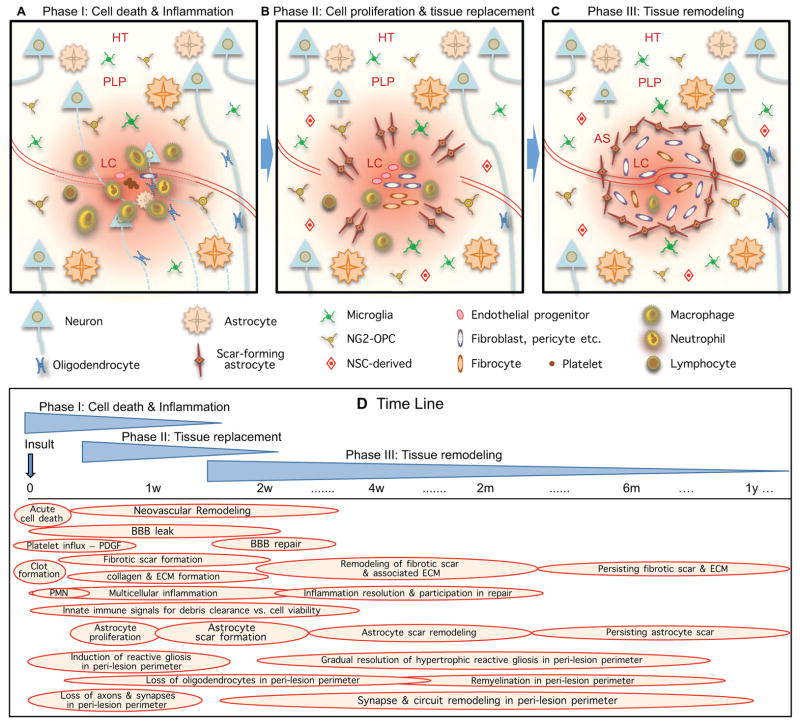
Two principal types of acute focal insults in the CNS are focal traumatic injury and ischemic stroke. In addition to being major clinical problems, they have for decades served as prototypical experimental models with which to study CNS mechanisms of response to damage and repair. In this section, we will examine basic features of CNS multicellular responses to acute focal injuries and how these change over time, using information from response to trauma or stroke as models. In this regard it is noteworthy that acute focal trauma caused by contusion or crush results in severe vascular damage and therefore exhibits many sequelae similar to stroke. We will make comparisons with the acute wound response in skin as a model system for cellular and molecular mechanisms of wound repair (Greaves et al., 2013; Gurtner et al., 2008; Shechter and Schwartz, 2013; Singer and Clark, 1999). As in skin repair, CNS responses to acute focal damage can also be divided broadly into three overlapping but distinct phases: (i) cell death and inflammation, (ii) cell proliferation for tissue replacement, and (iii) tissue remodeling (Fig. 1). We will first examine CNS responses in a context where the acute insults remain uninfected and resolve. Below, we will also briefly consider more chronic focal CNS insults, including focal infections with abscess formation, primary and secondary tumors, and chronic focal autoimmune lesions, such as multiple sclerosis plaques, which exhibit various features and cellular responses similar to traumatic and ischemic injury. In order to present succinct summaries of cellular responses and interactions, discussion of information about underling molecular signals is deferred to a later section below. It deserves mention that the time courses given for response phases and events are generalized approximations (Fig. 1), and there is much potential for overlap of events as well as variations in duration or timing in specific situations.
Figure 1. Phases and time course of multicellular responses to acute focal CNS damage.
During periods of cell death (A), cell replacement (B) and tissue remodeling (C), numerous overlapping events occur (D) that involve interactions among CNS intrinsic neural cells, CNS intrinsic non-neural cells and cells infiltrating from the circulation.
Phase I: Cell death and Inflammation
After focal CNS tissue damage from insults such as trauma or ischemia that cause acute local cell death, the first phase of response in the injury center or core includes both very rapid events that occur over time scales of seconds to hours, and more gradually progressing events that develop over days (Fig. 1A, D). Very rapid events include haemostasis with coagulation cascade, platelet aggregation and clot formation. These rapid events are followed by overlapping sequences of responses of tissue intrinsic cells, recruitment of inflammatory and immune cells, subacute death of parenchymal cells, and initiation of debris removal (Fig. 1A, D). In healthy CNS tissue, large and polar molecules in the circulation are excluded from diffusion into CNS parenchyma by the blood-brain barrier (BBB) (Abbott et al., 2006; Zlokovic, 2008). After focal insults with BBB damage, blood-borne molecules normally excluded from the CNS influx and signal to local cells as discussed in more detail below in the section on molecular signaling. Platelets influx and rapidly form aggregates for haemostasis, and also signal to local cells. Fibrin and collagen matrices begin to form over a period from hours to days, which serve as a scaffold for neutrophil and then macrophage and other leukocyte infiltration. Leukocytes then infiltrate heavily to monitor for pathogens, remove debris and provide molecular signals involved in wound repair over a variable number of days or longer depending on severity of tissue damage or presence of exacerbating factors (Fig. 1A, D) (Perry, 2010; Popovich and Longbrake, 2008). Endogenous mesenchymal stem cells may also enter the lesion core, but their roles are not well understood (Askari et al., 2003; Bianco et al., 2001; Jaerve et al., 2012). These basic cellular events reflect classic wound responses as found in other tissues (Greaves et al., 2013; Gurtner et al., 2008; Singer and Clark, 1999).
Certain CNS intrinsic cells also respond rapidly to CNS tissue damage or BBB leak. Live imaging studies in CNS in vivo show that microglia and NG2-OPC immediately migrate to sites of tissue damage and BBB leak (Hughes et al., 2013; Nimmerjahn et al., 2005). Astrocytes, in contrast, remain in situ and do not migrate either to or away from injury sites, but can swell osmotically, and depending on the severity of injury or ischemia, can die in the center of severe lesions or can become reactive and hypertrophy and in some cases proliferate (Bardehle et al., 2013; Zheng et al., 2010). Different aspects of this first phase of response occur in overlapping sequences during the first few days and then begin gradually diminishing (Fig. 1D) provided that the insult is a single acute event not complicated by bony compression, infection or other exacerbations that might prolong the damaging insult. As acute responses diminish, there is overlap with the onset of different types of cell proliferation associated with the next phase of responses (Fig. 1B, D).
Phase II: Cell proliferation and tissue replacement
The second phase of response to acute CNS tissue damage occurs from about two to ten days after the insult and is characterized by the proliferation and local migration of cells that implement tissue repair and replacement. These cells include endothelial progenitors, fibroblast lineage cells, different types of inflammatory cells, and various types of glia and neural-lineage progenitor cells, including scar-forming astrocytes and their progenitors (Fig. 1B, D). Some of these cellular proliferative phenomena, such as proliferation of endothelia for neovascularization (Casella et al., 2002) or proliferation of certain fibroblast lineage cells or inflammatory cells reflect classic wound responses (Gurtner et al., 2008; Singer and Clark, 1999), while others, such as proliferation of scar-forming astrocytes are specific and unique to CNS. This phase is also characterized throughout its duration by the absence of a BBB in the lesion core during the period where damaged blood vessels are replaced by new ones (Fig. 1D). As a consequence, endogenous (as well as exogenous) proteins and other charged molecules can freely diffuse into the surrounding neural parenchyma (Bush et al., 1999). These molecules can include serum proteins (e.g. thrombin, albumin) that signal to local cells, immunoglobulins or pathogen associated molecules as discussed below. This leaky BBB creates the potential for the lesion core to serve as source of circulating molecules that generate gradients of molecular signaling that can extend for considerable distances into neural tissue around the lesion until the BBB is repaired (Fig. 1B, D, 2A, B). This signaling may contribute to the perimeter of tapering gradient of reactive gliosis and other changes observed in tissue around mature lesions as discussed below.
Figure 2. Lesions exhibit tissue compartments and signaling gradients.
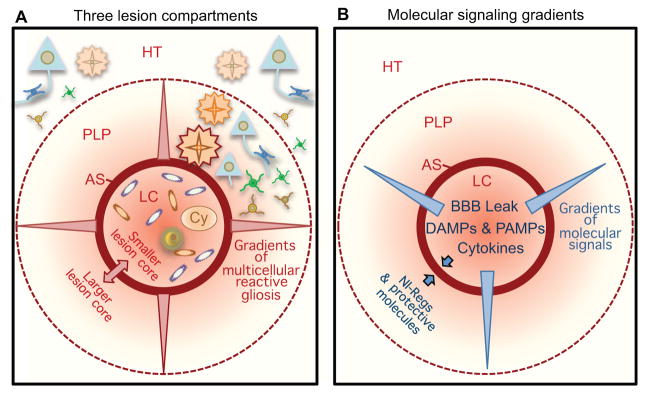
(A, B) Focal damage gives rise to lesions with distinct tissue compartments in the form of non-neural lesion cores (LC), newly-proliferated compact astrocyte scars (AS) and peri-lesion perimeters (PLP) that exhibit diminishing gradients of reactive gliosis that transition gradually to healthy tissue (HT). AS abruptly demarcate the transition between non-neural LC and PLP with viable neural cells. Size of the lesion core and the location of AS and this transition is regulated by opposing molecular signals. Gradients of molecular signals emanating from LC impact on cells in PLP.
During the proliferative phase, cellular elements are generated that will in mature lesions form two of the three major tissue compartments of the mature lesion, (i) the central lesion core of non-neural tissue and (ii) the compact astrocyte scar that surrounds the lesion core (Fig. 1B, D). Major cellular components of the non-neural lesion core derive from expansion of CNS intrinsic perivascular fibroblasts and pericytes (Goritz et al., 2011; Soderblom et al., 2013) and from proliferating endothelial progenitors. The compact astrocyte scar is formed primarily from newly proliferated elongated astrocytes generated by local astroglial progenitors that gather around the edges of severely damaged tissue containing inflammatory and fibroblast-lineage cells (Fig. 1B, D) (Faulkner et al., 2004; Sofroniew and Vinters, 2010; Wanner et al., 2013). It is noteworthy that during this proliferative period, the location of compact astrocyte scar borders is being determined. In mature lesions, these astrocyte scar borders will precisely demarcate and separate persisting areas of non-functional, non-neural lesion core tissue from immediately surrounding and potentially functional neural tissue (Fig. 1B, 2A, B). Cellular and molecular mechanisms that underlie the determination of precisely where such astrocyte scar borders are formed are incompletely understood, but are likely to involve a complex interplay and balance of molecular signals that on the one hand foster phagocytosis and debris clearance, and signals that on the other hand foster protection and preservation of healthy self (Fig. 2B), as discussed in more detail below in the section on molecular signals of cell damage and death. Several decades of experimental studies on ischemic infarcts show that final lesion size can be influenced by subacute metabolic events that continue for hours to days within penumbral zones around the lesions (Astrup et al., 1981; Dirnagl et al., 1999; Lo, 2008). In addition, astrocyte intrinsic signaling cascades have been identified that play critical roles (Herrmann et al., 2008; Okada et al., 2006; Wanner et al., 2013). A better understanding of the multi-cellular and molecular interactions that determine the location of astrocyte scar borders around areas of compromised tissue may lead to novel therapeutic strategies for reducing lesion size (Fig. 2B). These events occur over a timeframe of days after the insult (Fig. 1D), during which intervention is a realistic possibility.
Recent findings also indicate that during the proliferative phase after acute CNS damage in the forebrain, neural stem cells in periventricular germinal zones give rise to considerable numbers of neural progenitors that migrate to the injury sites in cortex or striatum (Carmichael, 2006; Kokaia et al., 2012; Lagace, 2012; Lindvall and Kokaia, 2006). These cells form part of the mix of cells in peri-lesion perimeters (Figs. 1B, C). There are various lines of evidence suggesting that these cells may contribute beneficially to tissue remodeling in peri-lesion tissue, but the nature of the contributions made by these cells remains nebulous. Under normal, unmanipulated circumstances, very few newly generated neurons mature and survive, and other potential contributions by these intriguing, migrating progenitor cells, such a contributions to immune regulation are still in the process of being defined (Kokaia et al., 2012; Lagace, 2012).
Phase III: Tissue remodeling
The third phase of response to acute tissue damage generally begins towards the end of the first week after the insult and is distinguished by tissue remodeling that includes events that are completed within weeks, such as BBB repair and scar organization, as well as chronic events that can continue over many months (Fig. 1C, D). BBB repair or restoration along newly formed blood vessels in the lesion vicinity is generally completed within several weeks after acute uncomplicated insults and requires the presence of functional astrocytes (Bush et al., 1999) and pericytes (Daneman et al., 2010; Winkler et al., 2011). The lesion core of non-neural tissue becomes surrounded by a well-organized, interdigitating compact astrocyte scar by two to three weeks after acute insults not exacerbated by ongoing tissue damage (Fig. 1C, D) (Wanner et al., 2013). These scar-forming astrocytes appear actively to corral and surround inflammatory and fibroblast-lineage cells during tissue remodeling and scar formation (Wanner et al., 2013). This compact astrocyte scar forms a structural and functional border between non-neural tissue in the central lesion core and the immediately adjacent and surrounding neural tissue that contains all viable cells of all three neural-lineage cell types, astrocytes, oligodendrocytes and neurons (Fig. 1C, 2) (Wanner et al., 2013). This astrocyte scar border serves as a protective barrier that restricts the migration of inflammatory cells form the non-neural lesion core into surrounding viable neural tissue. Disruption of astrocyte scar formation in different kinds of transgenic loss-of-function models leads to increased lesion size, increased death of local neurons, increased demyelination and decreased recovery of function after traumatic or ischemic focal insults (Bush et al., 1999; Faulkner et al., 2004; Herrmann et al., 2008; Li et al., 2008; Wanner et al., 2013). After compact astrocyte scar formation, the second period of tissue remodeling is long lasting with gradual remodeling of lesion core, astrocyte scar border and surrounding perimeter regions (Fig. 1D, 2). For example, with time there is gradual contraction of lesion cores and astrocyte scars. As transected axons continue to die back away from lesions, oligodendrocyte death can continue, leading to local tissue responses and remodeling. There is long term remodeling of ECM in the lesion core, with modulation of collagen and glycoprotein components (Klapka and Muller, 2006; Logan and Berry, 2002; Silver and Miller, 2004). In addition, there is gradual and chronically ongoing tissue remodeling in reactive perimeter regions that surround mature lesions as discussed next.
Mature lesions exhibit three tissue compartments
Astrocyte scar formation around central lesion cores is essentially complete by two to four weeks after acute insults that are not complicated by prolonged tissue damage, and the lesion can at this time be considered mature and entering its chronic stage (Figs. 1,2). In mature lesions, it is useful to recognize three very distinct lesion compartments: (i) the central lesion core of non-neural tissue comprised of locally derived fibroblast lineage cells, blood vessels, infiltrating fibrocytes and inflammatory cells, and extracellular matrix; (ii) the compact astrocyte scar that immediately surrounds the lesion core and consists of densely packed astrocytes with few if any neurons or oligodendrocytes; and (iii) the peri-lesion perimeter of viable neural tissue that is adjacent to the compact astrocyte scar and contains all three types of neural lineage cells (neurons, oligodendrocytes, astrocytes) and exhibits a tapering reactive gliosis that gradually transitions to healthy tissue (Fig. 2). All of these compartments will exhibit further tissue remodeling for weeks to months. Some of this remodeling has the potential to impact on functional outcome and may provide targets for therapeutic interventions, particularly in the perimeter of neural tissue that exhibits diffuse reactive gliosis (Fig. 1D, 2). It is useful to consider separately these compartments and their impact on functional outcome.
Lesion core
Tissue in the mature lesion core contains few or no neural-lineage cells and is non-functional in neurological terms. Initially, the main cell types in mature lesion cores include fibroblast lineage cells, endothelia, fibrocytes and inflammatory cells (Fig. 2A). With time and remodeling, and in the absence of exacerbating damage or infection, inflammatory elements will gradually withdraw although some may persist for long times. Elements of lesion core will persist permanently as fibrotic scar containing various non-neural cells and ECM. In some areas of lesion core, cells and ECM will recede to leave fluid filled cysts of quite variable size (Fig. 2A) (Tuszynski and Steward, 2012). Functionally, cells and ECM of lesion core serve as substrates for rapid wound repair and tissue replacement. The rapidity of wound closure allowed by fibrotic replacement as compared with parenchymal renewal has been suggested to afford advantages in other tissues (Greaves et al., 2013; Gurtner et al., 2008; Singer and Clark, 1999). Nevertheless, this mechanism can come with the cost of lost tissue functions. In CNS, lesion core tissue has since the 19th century been thought to have a negative long-term functional impact as an impediment to axon regeneration, confirmed by recent studies as well (Zukor et al., 2013). Molecular evaluations show that lesion core ECM contains collagens and proteoglycans that poorly support or overtly inhibit regrowth of damaged axons (Hermanns et al., 2006; Kimura-Kuroda et al., 2010; Klapka and Muller, 2006; Yoshioka et al., 2010).
Compact astrocyte scars (glial scars)
Mature astrocyte scars consist of relatively narrow zones of newly generated astrocytes with elongated processes that intermingle and intertwine extensively, and that immediately abut and surround on all sides regions of lesion core tissue (Fig. 2A) (Sofroniew and Vinters, 2010; Wanner et al., 2013). These narrow zones of densely intertwined elongated scar-forming astrocytes are generally not more than a mm across and are generally devoid of other neural-lineage cells (neurons or oligodendrocytes) (Wanner et al., 2013). These scar-forming astrocytes are newly proliferated in response to the insult and the packing density of astrocyte cell bodies in astrocyte scars is at least double that in healthy tissue (Wanner et al., 2013). The densely packed elongated scar-forming astrocytes transition seamlessly and rapidly to less densely packed and less-densely intertwined hypertrophic reactive astrocytes that are intermingled with viable neurons and other elements of neural tissue in the surrounding perimeter of reactive neural tissue discussed below (Fig. 2A) (Wanner et al., 2013). Transgenic loss-of-function studies show that newly-proliferated compact astrocyte scars exert essential neuroprotective functions by restricting the spread of inflammatory cells away from tissue lesions caused by trauma, stroke and autoimmune inflammation. When astrocyte scar formation is experimentally prevented or attenuated in vivo, inflammation spreads, lesion size increases, neuronal loss and demyelination are exacerbated and functional recovery is diminished (Bush et al., 1999; Drogemuller et al., 2008; Faulkner et al., 2004; Wanner et al., 2013). Scar-forming astrocytes are also widely regarded as a, if not the, major impediment to axon regeneration after CNS injury, based in part on in vitro studies showing astrocyte production of certain proteoglycans that can inhibit elongation (Silver et al., 1993). Nevertheless, other studies show that axon regeneration in vivo occurs along astrocyte bridges, and that in the absence of such bridges, axon regeneration does not occur and seems more to be impeded be confrontation with non-neural lesion core tissue (Kawaja and Gage, 1991; Williams et al., 2013; Zukor et al., 2013). Transgenic loss-of-function studies conducted in vivo, may be help to clarify the precise nature and scale of effects exerted by scar-forming astrocytes on axon regrowth after injury.
Peri-lesion perimeters
All mature lesion cores and astrocyte scars are surrounded by perimeters of viable neural tissue that exhibits a gradient of tapering reactive gliosis that gradually transitions to healthy tissue (Fig. 2A) (Wanner et al., 2013). The tissue in these perimeter zones contains all neural lineage cell types including neurons and oligodendrocytes, and these cells are intermingled with reactive gliosis that can extend for considerable distances (Fig. 2A). Reactive perimeter tissue begins immediately adjacent to compact astrocyte scars where the elongated cell-processes of scar forming astrocytes overlap with hypertrophic reactive astrocytes and reactive microglia that are intermingled with surviving viable neurons and other elements of neural tissue (Fig. 2A). It is somewhat remarkable that viable neurons can be present within a few hundred μm of compact astrocyte scars and the lesion core tissue filled with potentially cytotoxic inflammatory elements just beyond (Bush et al., 1999; Wanner et al., 2013). The reactive gliosis in perimeters includes hypertrophic reactive astrocytes and microglia (Fig. 2A). Both the cause and functions of perimeters of tapering reactive gliosis are not clear. Regarding cause, it is possible that gradients of signaling molecules diffusing out from the lesion core, either entering during the period of BBB leak, or produced there by infiltrating and proliferating cells, may trigger such a gradient of gradually tapering responses among local glia (Fig. 2A, B). From a functional perspective, the degree to which specific aspects of this reactive gliosis are beneficial or harmful (or some mixture of both) remains to be determined.
Reactive perimeter areas around lesion cores in brain and spinal cord are likely to exhibit long term, and potentially intense, tissue remodeling, with new synapse formation and the potential for formation of new relay circuits (Bareyre et al., 2004; Carmichael, 2006; Clarkson et al., 2010; Courtine et al., 2008; Li et al., 2010; Overman et al., 2012). Numerous factors have the potential to impact on this synapse and circuit remodeling, including extracellular matrix molecules such as proteoglycans in the peri-neuronal (Garcia-Alias et al., 2009). Reactive glia may play important roles in this remodeling, but this topic is understudied. In the healthy CNS, astrocytes interact with neurons on multiple spatial and temporal scales that can influence or modulate neural function in various ways (Halassa and Haydon, 2010). Both astrocytes and microglia have the potential to play fundamental roles in the synapse remodeling in the peri-lesion perimeter (Stephan et al., 2012; Stevens et al., 2007) and the impact of reactive gliosis on such remodeling is not yet understood. Such peri-lesion perimeter areas are likely to provide fertile ground for investing new strategies for interventions that may influence neural plasticity and relay circuit formation, which in combination with appropriate rehabilitative training (van den Brand et al., 2012) have the potential to beneficially influence functional outcome.
Chronic focal insults
In addition to acute focal trauma and stroke, which often serve as models to study mechanisms CNS damage as just discussed, other more chronic forms of damage warrant consideration. Important chronic insults include focal infections with abscess formation, tumors and autoimmune lesions. All of these conditions invoke reactive gliosis and multicellular responses that have strong similarities with those that occur after acute focal damage. This multicellular response plays critical roles in progression, severity and resolution (or not) of the insults.
Infection
Focal infections or abscesses can form when traumatic wounds become infected or can be seeded spontaneously, particularly in immune compromised individuals. Such infections elicit reactive gliosis with astrocyte scar formation similar to that after acute focal trauma (Sofroniew and Vinters, 2010). The time course of lesion persistence and tissue remodeling is protracted and dependent on resolution of the infection. Transgenic loss-of-function studies show that astrocyte scar formation is critical to focal containment of the infection. When astrocyte scars are disrupted, infection and inflammation spread rapidly through adjacent neural tissue with devastating effects (Drogemuller et al., 2008).
Cancer
Both primary and metastatic tumors elicit reactive gliosis and multicellular responses that have similarities to other forms of focal tissue damage. Interactions of tumor cells with CNS glia and infiltrating inflammatory cells is complex and heavily dependent on tumor cell type. Non-invasive tumors are surrounded by reactive gliosis and encapsulating scar similar to that seen around traumatic tissue damage. Aggressively invasive tumors are not encapsulated or surrounded by well-defined astrocytes scars, but evoke other forms of reactive gliosis and multicellular responses. Successful infiltration and spread of certain tumor cell types is thought to be associated with their ability to create an environment for growth, vascularization and spread, which may include successfully neutralizing barrier-forming functions of local CNS cells and co-opting programs for creating proliferative niches (Louis, 2006; Silver et al., 2013; Watkins and Sontheimer, 2012).
Autoimmune disease
Focal autoimmune lesions are interesting to consider from the perspective of being a response to CNS damage. Although CNS autoimmune disease is often viewed as diffuse, chronic and caused primarily by dysfunctions of the peripheral immune system, individual autoimmune lesions bear many similarities to acute focal traumatic wounds. For example, active multiple sclerosis plaques are filled with inflammatory cells and chronic plaques exhibit central lesion cores that are devoid of all neural lineage cell types (no neurons, oligodendrocytes or astrocytes) surrounded by astrocyte scars that separate non-neural tissue from perimeters of reactive gliosis in tissue with all three neural cell types (Frohman et al., 2006; Lucchinetti et al., 1996) in manners similar to tissue damage caused by trauma or ischemia (Figs. 1,2). Neuromyelitis optic (NMO) is an autoimmune inflammatory disease where causal auto-antibodies are directed against aquaporin-4 on the astrocyte cell membrane, and NMO lesions are associated with complement-mediated, lytic destruction of astrocytes (Lennon et al., 2005; Popescu and Lucchinetti, 2012). There is remarkable congruence between clinical evidence from NMO and experimental loss-of-function studies demonstrating that transgenically mediated ablation or attenuation of scar-forming astrocytes results in severe exacerbation of autoimmune CNS inflammation (Haroon et al., 2011; Voskuhl et al., 2009). Thus, both clinical and experimental evidence implicate critical roles for scar-forming astrocytes in limiting the spread of autoimmune CNS inflammation. These observations strongly suggest that dysfunction of CNS intrinsic cells such as astrocytes may contribute causally to the onset or progression of CNS autoimmune conditions in ways not generally considered by research focused only on trying to identify causal mechanisms the peripheral immune system. This notion may be further supported by suggestions that a subgroup of patients with multiple sclerosis have disease related autoantibodies against the potassium channel Kir4.1, which in the CNS is located on astrocyte membranes (Srivastava et al., 2012). It will be interesting to determine the extent to which gain or loss of astrocyte functions contributes to the pathophysiology of CNS autoimmune conditions.
Response to diffuse insults
Diffuse insults to CNS tissue can be associated with neurodegenerative diseases, certain seizure disorders or mild traumatic brain injury. Diffuse insults tend initially to be less intense than acute focal damage caused by trauma or stroke, and may not initially cause overt tissue damage, but instead accumulate gradually over chronic periods of time. As the tissue damage becomes more severe, small individual lesions form, each of which invokes reactive gliosis and multi-cellular responses similar to those caused by acute tissue damage with breakdown of the BBB, inflammation and recruitment of leukocytes (Fig. 1,2) but on a smaller scale. Over time the diffuse damage comes to resemble a diffuse collections of many small focal lesions that are intermingled and can be dispersed over large areas (Fig. 3A). Each of these small lesions gives rise to its own peri-lesion perimeter zones of tapering reactive gliosis, with the consequence that overlapping zones of severe hypertrophic reactive gliosis and multicellular responses can be distributed over large areas of tissue (Fig. 3A). It is important to realize that these many small lesions are interspersed among viable neurons and neural circuitry, which become enveloped by these over-lapping peri-lesion perimeters of reactive gliosis (Fig. 3A, B). As discussed above, there is now substantive evidence for the participation of astrocytes and microglia in normal neural circuit function, but the impact of reactive gliosis on such functions is not known. In the context of diffuse CNS insults, large areas of functioning neural tissue may be exposed to intense reactive gliosis that is likely to impact on synaptic interactions and neural circuit functions (Fig. 3B). A better understanding of such effects may open inroads to new therapeutic strategies.
Figure 3. Diffuse tissue damage leads to extended regions of reactive gliosis.
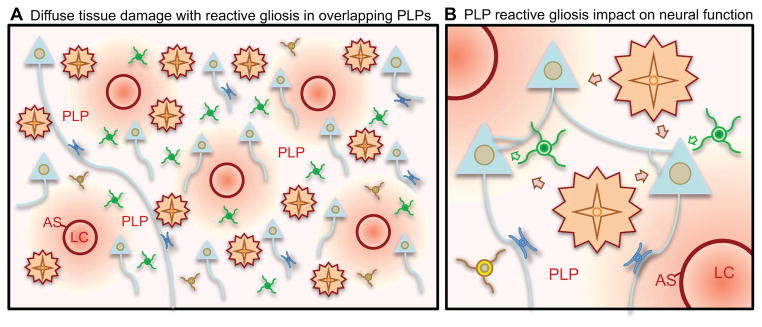
(A) Small chronic insults can lead to diffuse tissue damage with formation of dispersed small lesion cores (LC), astrocyte scars (AS) and peri-lesion perimeters (PLP) that can overlap and coalesce into regions of contiguous reactive gliosis that can extend over large areas.
(B) Reactive astrocytes and reactive microglia in PLP can impact on synapses and neuronal functions in various ways.
Neurodegenerative disease
Reactive gliosis and multicellular responses are triggered in different and selective ways in different neurodegenerative disease. In some cases, the trigger can be accumulation of extracellular toxins, such as β-amyloid in Alzheimer’s disease (AD) (Prokop et al., 2013; Zlokovic, 2011), which can accumulate and cause many small areas of focal tissue damage that trigger gliosis with many similarities to other forms of diffuse insults (Fig. 3A). In other cases, the trigger can be neuronal or synaptic damage or death secondary to neuronal intrinsic changes. Some conditions, such as amyotrophic lateral sclerosis (ALS) or Huntington’s disease, cause intrinsic cellular changes in both neurons and glia can perturb cellular functions and interactions, which can serve as triggers (Boillee et al., 2006; Maragakis and Rothstein, 2006). Neurodegenerative insults can also lead to disturbance of the neurovascular unit, resulting in BBB leak which can serve as a further trigger for reactive gliosis and also signal to recruit blood borne inflammatory cells (Zlokovic, 2008, 2011). In many cases, reactive gliosis and multicellular responses may not be apparent at onset or in early stages of the neurodegenerative conditions, but appear later and may then become involved in disease progression. Reactive gliosis triggered in various ways, either by accumulation of abnormal proteins, ischemic or traumatic cellular damage, or microbes, can lead to also lead to BBB leak via specific molecular signaling cascades as discussed below, with resultant inflammation that may contribute to disease progression.
The contributions of glial cells and reactive gliosis to neurodegenerative conditions are complex and only beginning to be understood. Glia not only become reactive in response degenerative cues as just mentioned, but can also participate in trying to combat them. For example, both microglia and astrocytes are thought to contribute to clearing β-amyloid (Prokop et al., 2013; Wyss-Coray et al., 2003), and attenuation of reactive astrogliosis can increase β-amyloid load in a transgenic mouse model of AD (Kraft et al., 2013). In addition, glia may be affected by the disease-causing molecular defects and become dysfunctional, further complicating efforts to understand the roles of reactive gliosis in the disease process. For example, in ALS, glial cell dysfunction is causally implicated in mediating neuronal degeneration (Yamanaka et al., 2008), and ongoing reactive gliosis and inflammation are also thought to contribute to disease progression (Maragakis and Rothstein, 2006). Distinctions between disease-induced dysfunctions of glial cells and the reactive responses that are triggered in glial cells by cell and tissue damage are sometimes blurred or equated, but should not be. Understanding these different phenomena and the ways in which they interact is likely to have important consequences for understanding the mechanisms that underlie cellular pathophysiology and drive disease progression. It is important to remember that although some aspects of glial reactivity may contribute to disease progression, others are likely to be protective. Defining the molecular basis of such differences may help to identify novel therapeutic strategies.
In the context of neurodegenerative disease it is also interesting briefly to consider the potential for non-cell autonomous neuronal degeneration precipitated by dysfunction of glia or other non-neural cells. Loss- or gain-of-function genetic mutations in microglia (Derecki et al., 2012) or astrocytes (Brenner et al., 2001; Rothstein, 2009; Tao et al., 2011) have the potential to cause non-cell autonomous degeneration of different types of neurons in different human diseases and animal experimental models. Such observations indicate that glial cell dysfunction may be the primary causal event in certain neurological conditions. Such observations also raise the possibility that dysfunction of specific aspects of reactive gliosis during responses to CNS insults may exacerbate damage or lead to worse tissue repair and worse outcome. The potential for genetic polymorphisms to impact on glial cell functions and responses to CNS injury is an as yet untapped area that may reveal causal factors underlying differences in the responses of different individuals to seemingly similar types and severities of CNS insults.
Epilepsy
Astrocytes and microglia can be involved in epilepsy in multiple ways. Astrocytes play multiple critical roles in regulating synapse activity and neuronal excitability and astrocytes may play critical roles in the generation of seizure activity (Devinsky et al., 2013; Jabs et al., 2008; Wetherington et al., 2008). In addition, reactive gliosis of both microglia and astrocytes is a prominent feature of chronically epileptic neural tissue, which can also exhibit BBB breakdown and multicellular inflammation (Devinsky et al., 2013; Jabs et al., 2008; Wetherington et al., 2008). With increasing loss of neurons, tissue sclerosis sets in, which has similarities with severe diffuse astrogliosis due to other causes as discussed above, with many smaller tissue lesions surrounded by a continuum of peri-lesion tissue with a mix of surviving neurons and increasingly severe hypertrophic gliosis (Fig. 3A). The impact of reactive gliosis and the multicellular response to tissue damage on the surviving neurons in the peri-lesion perimeter regions is not well understood (Fig. 3B), but may contribute to reduced seizure thresholds. Selective experimental induction of astrocyte reactivity is associated with a reduction of inhibition in local hippocampal neurons (Ortinski et al., 2010). More work in this area is warranted.
Diffuse traumatic brain injury (TBI)
Large focal lesions caused by severe focal TBI (contusion or crush) have been discussed above. Nevertheless, clinically relevant TBI is often diffuse and widely distributed. The cellular and molecular mechanisms of diffuse TBI that cause functional disturbances without large focal lesions are incompletely understood. Diffuse TBI is generally characterized by small foci of vascular breakdown and tissue damage that can be diffusely distributed over large areas of CNS (DeKosky et al., 2013; McIntosh et al., 1989). It is interesting to consider that each of these small areas of tissue or cellular damage may represent a small focal injury with compartments equivalent to core, astrocyte scar and perimeter, which may be spread over large areas without an obvious single large focal region (Fig. 3A). As discussed above, the interspersed viable neural tissue in the many overlapping peri-lesion perimeter regions will be exposed to intense hypertrophic reactive gliosis and inflammation, which can impact on neural functions (Fig. 3B). Specific cellular and molecular mechanisms of reactive gliosis and multicellular responses triggered by mild or moderate diffuse TBI are only beginning to be characterized. Newly proliferated reactive astrocytes exert protective functions essential for neuronal survival (Myer et al., 2006). Microglia can exert a broad range of effects depending on specific molecular stimuli, which and either protect or exacerbate tissue loss (Hanisch and Kettenmann, 2007). Understanding and manipulating the reactive gliosis and multicellular sequelae triggered by mild to moderate TBI has enormous potential to identify new therapeutic targets in a field of large epidemiological importance. Emerging molecular mechanisms are discussed in the next section.
Molecular signaling and multicellular interactions
Molecular signaling among the different cell types that respond to CNS insults is complex, combinatorial and densely interwoven. Different cells have the capacity to produce and respond to similar sets of molecules, and there are hints that some molecules may coordinate multicellular responses. Many intercellular signaling molecules have been identified, but we are in the early stages of determining how these multiple signals regulate multi-cellular interactions and the temporal progression from one phase to another during responses to specific CNS insults. Here we provide a general overview of molecular functional classes (Fig. 4A) and a few representative examples of the many specific molecules that regulate or influence CNS cellular responses to insults (Table 2).
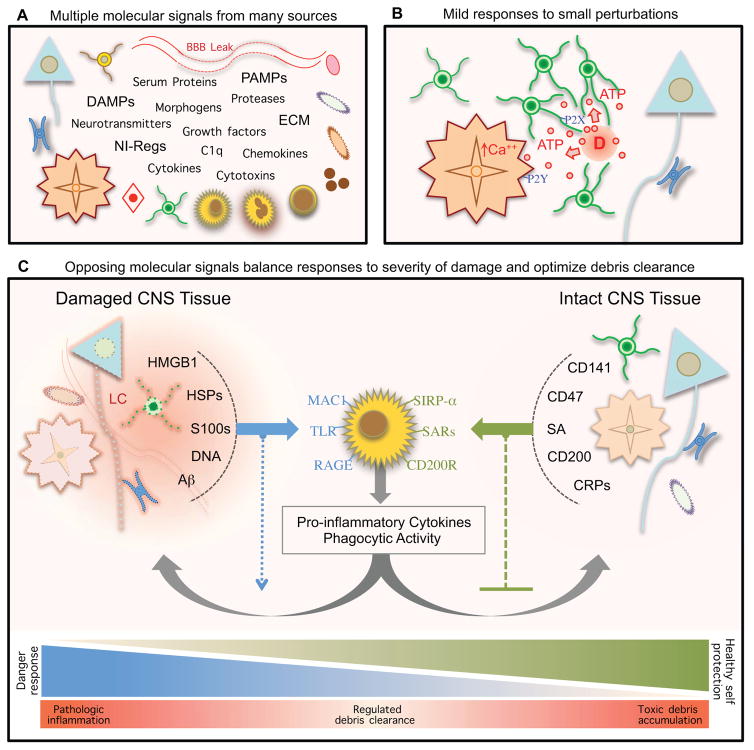
Figure 4. Multi-molecular regulation and graded responses to damage.
(A) Responses to CNS damage are regulated and influenced by diverse categories of molecular signals derived from many types of CNS intrinsic and extrinsic cells.
(B) Low levels of ATP released by cells during mild CNS insults can act through (i) P2X receptors to induce rapid chemotaxis of microglial processes, and (ii) P2Y receptors on local astrocytes to evoke calcium signaling and connexin-dependent ATP release, which can increase extracellular ATP levels and thereby amplify this “danger” signal.
(C) Following severe injury, signaling molecules from “altered self” and “healthy self” vie to balance debris clearance and preservation of viable tissue. Severely damaged or dead cells release a number of hydrophobic intracellular molecules (left), or “alarmins”, which express damage associated molecular patterns (DAMPs). These “danger” signals alert CNS innate immune cells to tissue injury and promote clearance of debris via activation of pattern recognition receptors on phagocytic immune cells (center). Viable CNS intrinsic cells at lesion borders (right) express membrane-bound and soluble neuroimmune regulatory molecules to prevent attack and phagocytosis by immune cells.
Table 2.
Major classes and a few specific examples of intercellular signaling molecules that regulate cellular responses to CNS damage
| Class | Molecule | Source | Lesion compartment | Phase | Targets and effects |
|---|---|---|---|---|---|
| Protease & Related | Thrombin | S | LC, AS | I | Clot formation; astrocyte proliferation |
| MMP-9 | M, O, S | LC, AS, PLP | I–III | OPC proliferation, remyelination; BBB leak; neovascular remodeling | |
| Kallikreins | A, M, N, S | LC, AS, PLP | I–III | Pro-inflammatory; demyelination | |
| Serpins | A, M, O | LC, PLP | I–III | Inhibit deleterious protease | |
| NTs | ATP Glutamate | N, O, A | LC, AS, PLP | I | Microglia chemotaxis; reactive astrogliosis |
| DAMPs | Alarmins: HMGB1, S100s, DNA | Damaged Cells | LC, PLP | I. II | “Altered self” signals; pro-inflammatory; increase phagocytosis |
| PAMPs: LPS | Microbes | LC, PLP | I. II | “Non-self invader” signals; pro-inflammatory; increase phagocytosis | |
| Growth Factors Morphogens | FGF | A, N, E, | LC, AS, PLP | I – III | Fibrotic scar; ECM; neovascular remodeling; reactive astrogliosis |
| VEGF | E, P, F, A | LC, AS | I–III | BBB permeability; neovascular remodeling | |
| PDGF-B PDGF-A |
E, A | LS, PLP | I–III | Neovascular remodeling Remyelination; OPC Proliferation |
|
| Endothelin, EGF, BMP | N, A, O | LC, AS | II, III | Astrocyte proliferation, glial scar formation | |
| Cytokines Chemokines | TGFβ | A, M, L | AS, PLP | I–III | Fibrotic scar formation; ECM production; astrocyte proliferation |
| IL-1β, TNFα, INFγ | A, M, L | LC, AS, | I, II | Pro-inflammatory regulation | |
| IL-6 | A, M, L | I, II | Leukocyte instruction, astrocyte scar formation | ||
| NIRegs | CD141 | A, O, E | LC, AS | I–III | Protection of healthy self; resolution of inflammation |
| CD200, CD47 | N | LC, AS, PLP | I, II | Protection of healthy self | |
| Neural Remodeling | Neurotrophins, BDNF etc. | N, A | PLP | III | Synapse remodeling |
| Peri-neuronal net | A, OPC | PLP | III | Restrict terminal sprouting | |
| Thmbs, C1q | A, M | PLP | III | Synapse formation and pruning |
A (astrocyte), E (endothelia), F (fibroblast), L (leukocyte), M (microglia), N (neuron), NTs (neurotransmitters), O (oligodendrocyte), OPC (O progenitor cell), S (serum), Thmbs (thrombospondin)
Cell damage and death
Cells that are dead, dying, or temporarily damaged but recoverable, release or express molecules that signal damage. These molecules fall into many categories including neurotransmitters, cytokines, chemokines, neuroimmune-regulators (NI-Regs) and danger associated molecular patterns (DAMPs) that stimulate reactive gliosis and other cellular responses including debris clearance by immune cells (Fig. 4B, C) (Table 2). Insults of different types and severities release different combinations of these molecules, which in turn trigger different responses. For example, mild cellular insults can subtly elevate extracellular concentrations of glutamate or ATP that attract microglial cell processes without initially unleashing full-blown inflammation (Fig. 4B) (Davalos et al., 2005; Liu et al., 2009). ATP can also elicit calcium responses in astrocytes and cause connexin-dependent ATP release that increases extracellular ATP levels and amplify this “danger” signal (Fig 4B). More severe cell damage or death will release additional DAMPs and alarmins in the form of cytosolic and nuclear contents such as K+, heat shock proteins such as Aβ-crystallin, S100 family calcium-binding proteins, DNA-binding HMGB1 (high mobility group box 1), as well of DNA and RNA. All of these molecules can serve as “danger” signals to alert innate immune cells to tissue injury and promote clearance of “altered self” cellular debris via activation of pattern recognition receptors (PRRs) on phagocytic innate immune cells (Fig. 4C) (Chan et al., 2012; Griffiths et al., 2010; Kono and Rock, 2008). Molecules such as beta-amyloid (Aβ) produced during neurodegenerative disorders can also act as alarmins (Fig. 4C). A single type of alarmin may bind multiple classes of PPRs. For example, HMBG1 elicits pro-inflammatory signaling through activation of multiple TLRs, RAGE (receptor for advanced glycation end products) and MAC1 (macrophage antigen complex-1) (Chan et al., 2012; Gao et al., 2011), and S100 activates multiple TLRs and RAGE (Chan et al., 2012).
Healthy neurons and glia near CNS insults express neuroimmune regulatory molecules (NI-Regs) and self defense proteins that signal “healthy self” and serve to confine potentially noxious pro-inflammatory signaling and prevent phagocytosis of viable cells (Fig. 4C) (Table 2) (Griffiths et al., 2010). These “healthy self” molecular signals include both membrane-bound and soluble NI-Reg molecules. For example, sialic acids (SA) in membranes of healthy cells interact with SA receptors (SARs), including Siglecs, on innate immune cells and serve as “don’t eat me” signals (Fig. 4C) (Varki and Gagneux, 2012). CD200 and CD47 similarly act through receptors CD200R and SIRP-α on inflammatory cells to prevent phagocytic attack (Fig. 4C) (Griffiths et al., 2010). Complement regulatory proteins (CRPs) can reduce complement activation (Fig. 4C) (Griffiths et al., 2010). Thrombomodulin (CD141) binds and sequesters HMGB1, thereby reducing alarmin bioavailability (Fig. 4C) (Abeyama et al., 2005).
Thus, specific receptor-mediated signals that indicate “altered self” or “healthy self” can tailor the nature of the reactive gliosis and multi-cellular responses to the type and severity of cellular damage or death (Fig. 4B, C). It is important to note that the final level of cellular damage inflicted by insults of all kinds, is determined in part by prolonged periods of different types of secondary events (Fig. 1D), including a balancing act among innate immune mechanisms that regulate the clearance of “altered-self” debris and “non-self” pathogens, while preserving “healthy self” (Fig. 4C) (Griffiths et al., 2010). These events will impact on final size, location of glial scar and signaling to per-lesion perimeters (Fig. 2).
Reactive gliosis
Reactive gliosis is regulated by a large array of different extracellular signals generated after CNS insults. This array includes not only molecules released by damaged or dead cells as just described, but also molecules entering via leaky BBB, molecules released by infiltrating leukocytes and molecules released by local cells including reactive glia themselves (Fig. 5A) (Sofroniew, 2009). In response to these many different and often combinatorial molecular signals, reactive glia alter their gene expression, structure and function in selective and specific ways depending on the type and severity of the insults and depending on the specific combinations of molecular signals that are impinging on them. It is important to emphasize that glia do not exist in in simple all-or-none “quiescent” or “activated” states, and that there is no single program of “reactive gliosis” that is triggered in an all-or-none fashion and is similar in all situations. Instead, reactive glia can exhibit a vast range of responses as determined by combinations of specific signalling events. For example, exposure to PAMPs such as lipopolysaccharide (LPS) can markedly skew the transcriptome of reactive astrocytes towards chemokines, cytotoxicity and inflammation (Hamby et al., 2012; Zamanian et al., 2012). It is also noteworthy that different combinations of stimulatory factors can lead to synergistic changes in molecular expression that could not be predicted from individual effects (Hamby et al., 2012).
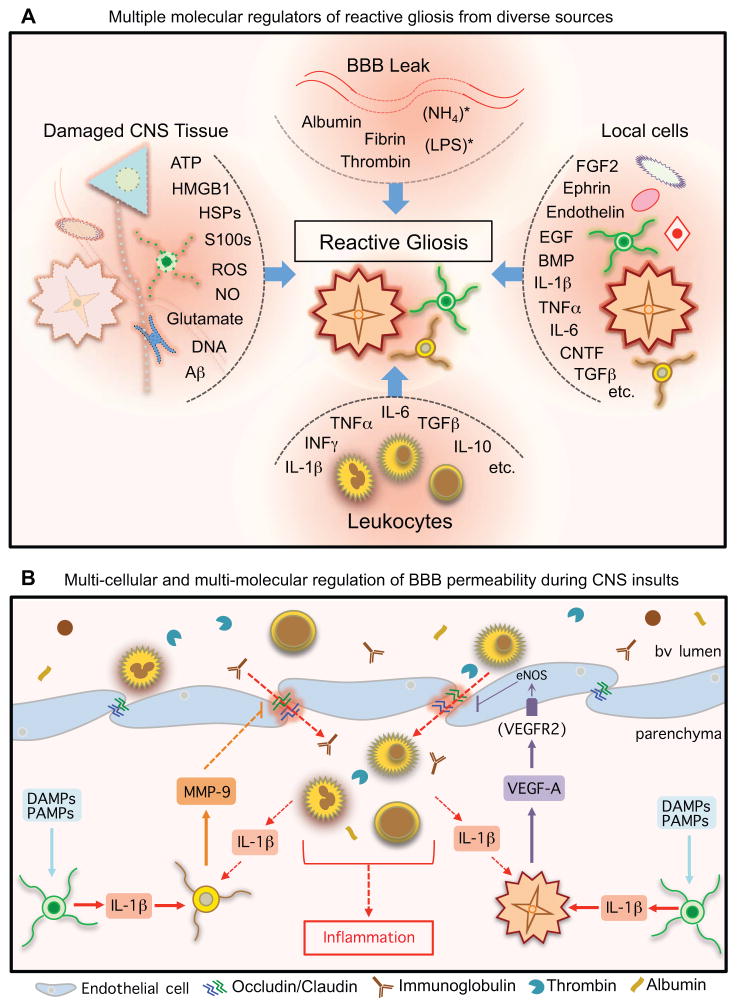
Figure 5. Multi-cellular and multi-molecular regulation of reactive gliosis and BBB.
(A) Reactive gliosis can be induced, regulated and influenced by a wide variety of molecular signals that can derive from many types of CNS intrinsic and extrinsic sources.
(B) BBB permeability can be regulated by specific molecular signaling cascades involving interactions among different cell types that converge on endothelial tight junctions (Argaw et al., 2012; Argaw et al., 2009; Seo et al., 2013).
Information about the effects mediated by reactive glia in response to specific signals is also steadily increasing and it is clear that many aspects of reactive gliosis are part of coordinated multicellular innate and adaptive immune responses to CNS insults. It is interesting to consider that certain molecules are emerging as potential coordinators of multicellular responses. One interesting set of converging data implicates IL-1β as a key trigger that stimulates different types of glial cells to modulate BBB permeability to serum proteins and leukocytes during reactive gliosis in response to CNS insults (Fig. 5B). Numerous DAMPs and PAMPs generated by CNS insults will stimulate IL-1β release from microglia, and this IL-1β will stimulate (i) local reactive astrocytes to release VEGF, and (ii) local NG2-OPCs to release MMP-9, which in turn influence tight junctions and barrier properties of local endothelial cells and allow entry of serum proteins (including IgGs and signaling proteins such as thrombin, albumin, proteases etc.) and leukocytes into local CNS parenchyma (Fig. 5B) (Argaw et al., 2012; Argaw et al., 2009; Seo et al., 2013). Entering leukocytes have the potential to generate more IL-1β and sustain local BBB permeability, creating the potential for a feed-forward loop under adverse circumstances. Understanding the molecular mechanisms whereby such signaling is downregulated and the BBB reseals may help to reveal rational therapeutic interventions for certain conditions. Repair of the BBB after an uncomplicated acute injury generally occurs within several weeks (Fig. 1D).
Astrocyte scar formation
Formation of compact astrocyte scars is a specialized aspect of reactive astrogliosis that occurs in response to severe tissue damage and leukocyte infiltration and involves phases of cell proliferation and cell organization (Fig. 1) (Sofroniew and Vinters, 2010; Wanner et al., 2013). Molecular signals that regulate astrocyte proliferation signals after CNS damage can derive from serum proteins or local cells and include thrombin, endothelin, FGF2, ATP, BMPs (bone morphogenic proteins), SHH (sonic hedge hog) and others (Table 2) (Gadea et al., 2008; Neary et al., 2003; Sahni et al., 2010; Shirakawa et al., 2010; Sirko et al., 2013). The location of astrocyte scar borders around lesions can be influenced by interactions among proliferating or newly proliferated scar-forming astroglia, fibroblast-linage cells and inflammatory cells (Wanner et al., 2013), and can be modulated by transgenic manipulation of astrocyte intrinsic signaling cascades involving STAT3, SOCS3 or NFκB, which can increase or decrease lesion sizes (Brambilla et al., 2005; Herrmann et al., 2008; Okada et al., 2006; Wanner et al., 2013), providing targets for potential intervention. Signals for organization of newly proliferated astrocytes into compact scars include the IL-6 receptor-STAT3 signaling system, which critically regulates astrocyte scar formation after trauma, infection and autoimmune inflammation (Drogemuller et al., 2008; Haroon et al., 2011; Herrmann et al., 2008; Wanner et al., 2013).
Neural remodeling in peri-lesion perimeters
Reactive gliosis is not only important in responses to very local CNS damage, but also is likely to play important roles in the neural remodeling that continues for prolonged times in peri-lesion perimeters after acute focal insults such as stroke and trauma (Figs. 1,2), as well as in chronic diffuse CNS insults (Figs. 3) including neurodegenerative conditions such as Alzheimer’s disease. Such peri-lesion perimeter regions can extend for considerable distances away from large focal lesions (Figs. 1,2), or can cover large areas of diffuse tissue damage (Figs. 3). In such cases, the associated reactive gliosis has the potential to impact on substantial territories of functioning neural tissue. There is mounting evidence that the diverse changes in molecular signaling associated with this diffuse reactive gliosis (Table 2) will impact on the ongoing neural remodeling and changes in neural function. Astrocytes interact with neurons on multiple spatial and temporal scales that can influence or modulate neural function in various direct and indirect ways, including by modulation of the extracellular balance of ions, transmitters and water critical for neural function, as well as direct influences on synaptic activity directly via release of ‘gliotransmitters’; (Halassa and Haydon, 2010; Hamilton and Attwell, 2010; Henneberger et al., 2010; Volterra and Meldolesi, 2005). Microglia are also implicated in participating in the regulation of synaptic turnover (Stephan et al., 2012). Peri-neuronal net molecules such as chondroitin sulfate proteoglycans generated by astrocytes and NG2-OPC influence synaptic plasticity (Table 2) (Wang and Fawcett, 2012). The effects of reactive gliosis on such functions is only beginning to be understood, but reactive astrogliosis has been reported to impact on neural functions in various ways, including (i) by down-regulation of astrocyte glutamine synthase which is associated with reduced inhibitory synaptic currents in local neurons (Ortinski et al., 2010), (ii) increased expression of xCT (Slc7a11), a cysteine-glutamate transporter associated with increased glutamate signaling, seizures and excitotoxicity (Buckingham et al., 2011; Jackman et al., 2010), and (iii) changes in the expression of multiple GPCRs and G proteins and calcium signaling evoked by their ligands that have the potential to alter astrocyte-neuron interactions (Hamby et al., 2012). There is also a growing body of evidence that in response to inflammatory mediators and cytokines, reactive astrocytes can modulate their expression of multiple molecules such as transmitter and ion transporters, neuromodulators such as nitric oxide and prostaglandins, growth factors and synapse regulatory proteins, which can impact on synaptic and neuronal functions including complex behaviors such as sickness behavior, pain, appetite, sleep, and mood, as reviewed in more detail elsewhere (Sofroniew, 2013). Taken together, such findings provide evidence that different molecular mediators and signaling mechanisms associated with reactive gliosis can modulate functions of reactive astrocytes and microglia in different ways that have the potential to impact on synaptic and other neuronal functions in peri-lesion perimeters. Such signaling mechanisms are likely to have effects on the efficacy of training and functional rehabilitation after acute damage such as trauma or stroke, as well as effectors to mitigate functional deterioration during degenerative conditions. Understanding and manipulating the signaling mechanisms and effects of reactive gliosis in peri-lesion perimeter tissue has the potential to reveal novel therapeutic strategies (Gleichman and Carmichael, 2013).
Blood brain barrier (BBB)
The BBB is generated and regulated by a complex multicellular neurovascular unit that involves critical interactions among endothelia, pericytes, astrocytes and other cells (Abbott et al., 2006; Zlokovic, 2008). Compromise of the BBB can occur in various manners and to various degrees, ranging from specific molecular signaling events that open BBB permeability during certain types of reactive gliosis as discussed above (Fig. 5B), to severe breakdown of the BBB by caused traumatic or ischemic endothelial destruction resulting in the need for neovascularization (Fig. 1). In addition to overt trauma and ischemia, considerable evidence implicates a central role for BBB dysfunction in aging and various neurodegenerative disorders. For example, both transgenically-induced and aging-related pericyte deficiencies result in BBB breakdown that precede neuronal degeneration (Bell et al., 2010). Pericyte deficiency is also apparent in postmortem amyotrophic lateral sclerosis tissue (Winkler et al., 2013) and BBB dysfunction precedes neurodegeneration in a murine model (Zhong et al., 2008). Polymorphisms in APOE isoforms are implicated in increased vulnerability to BBB breakdown in neurodegenerative disorders and traumatic injury (Bell et al., 2012).
Regardless of triggering events, BBB leaks lead immediately to CNS parenchymal entry of serum proteins such as thrombin, albumin, immunoglobulins and proteases (Fig. 5B) that can have a wide range of direct and indirect effects. Thrombin can signal directly via protease-activated receptors on various CNS cells including microglia and astrocytes (Shigetomi et al., 2008; Suo et al., 2002). Immunoglobulins contribute to clearing of pathogens but may contribute to autoimmune dysfunctions. Platelets can enter and form aggregates for clotting and release PDGFs, important for neovascular remodeling as well as for signaling to pericytes and NG2 cells. Metaloproteases act on multiple proteins and contribute to extracellular matrix and tissue remodeling (Table 2). Serum kallikrein proteases activate inflammation. Tissue kallikreins, which can interact with serum proteases as part of larger proteolytic cascades, are implicated in oligodendrocyte pathology, demyelination, axonopathy and neuronal degeneration (Burda et al., 2013; Radulovic et al., 2013). Serpins inhibit and balance protease effects (Table 2).
In response to signals associated with BBB leak, local glial cells produce cytokines and chemokines that attract leukocytes, which on arrival produce additional cytokines and chemokines. The result is a rich extracellular mixture of molecular signals that can instruct or influence multiple cell types in lesion cores and can also diffuse away into surrounding CNS parenchyma and influence neural cells that may not have been involved in the initial insult (Fig. 2). These molecular gradients may influence or take part in shaping cellular events in the peri-lesion perimeters such as the long lasting gradient of reactive gliosis and the long lasting neural remodeling that occurs in perimeter tissue. The mix of molecular signals generated in lesion cores changes over time as the injury response progresses through different phases and the BBB leak is repaired and different cell types occupy this area and produce different effector molecules (Fig. 1D, Table 2). Depending on the severity of the insult, lesion cores exhibit a BBB leak for at least several days up to two weeks throughout the initial period (Phase I) of damage response (Fig. 1D). BBB repair requires the presence of functional astrocytes (Bush et al., 1999). Release of the protein, HMGB1 (high mobility group box 1), by reactive astrocytes promotes endothelial cell neovascular remodeling, whereas blockade of HMGB1 release by reactive astrocytes prevents neovascular remodeling and worsens neurological deficits (Hayakawa et al., 2012). In complex, chronic or diffuse insults, BBB leaks may persist and have long lasting effects on extended areas of neural tissue in peri-lesion perimeters (Fig. 3), and late BBB disruption may lead to secondary tissue injury (Zlokovic, 2008). Even when BBB leaks are repaired, lesion cores continue to contain collections of non-neural cells within the BBB that have the potential to continue producing high levels of many different signaling molecules for prolonged periods. Thus, lesion core tissue has the potential to generate diffusion gradients of many potent molecules that spread into neighboring tissue parenchyma and form gradients of molecular signals that influence host cells (Fig. 2). It is also noteworthy that while the BBB is open, there is also the potential for entry of various pathogen associated molecular patterns (PAMPs) (Table 2) that are generated by microbial infections in the periphery and which can substantively alter the response of local cells to CNS damage, even when there is no local infection, as discussed below.
Infiltrating leukocytes
Leukocytes that infiltrate from the circulation provide a major source of molecular signaling during responses to CNS damage. These molecular signals include a diverse mix of cytokines, chemokines and growth factors (Table 2) that can instruct and modify the functions of local reactive glia (Fig. 5A) and other intrinsic CNS cells as well as other local leukocytes. It is likely that the molecular signals produced by leukocytes, and their functional effects, will vary during different phases of response to acute or chronic insults (Fig. 1) and range from those mediating classical inflammatory responses with cytotoxic and phagocytic activities, to those influencing tissue repair and remodeling. Although temporal changes during wound responses are not yet well studied, different functional roles for different leukocyte subtypes are gradually coming into focus. For example, in response to stimulation by different cytokines, monocytes generate macrophages with distinct functional phenotypes that produce different effector molecules (Mosser and Edwards, 2008). In response to INFγ, classically activated macrophages generate cytotoxic antimicrobial molecules and phagocytose dead cells and debris (Fig. 4C), whereas in response to stimulation with Il-4 or Il10, macrophages take part in would healing and anti-inflammatory activities (Mosser and Edwards, 2008). Similarly, T cells stimulated by different cytokines become polarized towards different activities, such as TH1, TReg and TH17 cells, which can exert very different effects ranging from cell killing to participation in tissue repair (MacIver et al., 2013; Mills, 2011; Walsh and Kipnis, 2011).
Impact of peripheral infections on responses to CNS insults
Many of the cell types responding to CNS damage are exquisitely sensitive to molecular cues associated with microbial infection. This is not surprising because limiting infection spread is likely to have shaped the evolution of injury responses in all tissues including CNS. Various features of the response to infection such as production of cytotoxins and certain types of inflammation can damage host cells as well as microbes. Triggering such responses in the absence of overt infection has the potential to cause or exacerbate tissue damage. Thus, certain responses that may be beneficial in microbial infection may be detrimental if triggered during sterile (uninfected) tissue damage after trauma, stroke, degenerative disease or autoimmune attack. In this regard it is important to recognize that reactive glia, both microglia and astrocytes, that are responding to a sterile primary CNS insult, can be influenced by peripheral infections that generate high levels circulating cytokines and other inflammatory mediators such as LPS and other PAMPs. This signaling can occur through blood born cytokines or LPS released at the site of peripheral infection and entering through the leaky BBB in the injury core during phase I of the damage response after focal insults or through BBB leaks triggered by neurodegenerative or autoimmune processes (Figs. 5A). LPS and cytokines have powerful effects both on reactive microglia (Perry, 2010; Perry et al., 2007) and on reactive astrocytes (Hamby et al., 2012; Zamanian et al., 2012) that can drive transcriptome profiles and wound response towards pro-inflammatory and potentially cytotoxic phenotypes over prolonged times in ways that would not normally be implemented in sterile CNS insults and that can exacerbate tissue damage. Congruent with such experimental observations is clinical epidemiological evidence indicating that peripheral infections have a negative impact on neurological outcome after spinal cord injury (Failli et al., 2012).
Common features, differences and building models
Given the large number of potential CNS insults and the considerable amount of variation among the multicellular responses they elicit, looking for common features and clear differences may provide valuable insights regarding shared fundamental mechanisms. Understanding basic factors that drive responses to one type of insult may inform ideas regarding responses to another. In this regard it is interesting to consider that evolutionary pressures shaping responses to CNS damage are likely to have favored rapid responses to small CNS injuries that were not functionally incapacitating, and that kept such injuries small and uninfected, or that kept small infections from spreading. One efficient means of doing so would be to isolate small focal injuries or small infections with cellular barriers that effectively wall off these lesions allowing a robust inflammatory (and anti-microbial) responses in lesion cores while preserving as much adjacent neural tissue as possible (Sofroniew, 2005). In this article, based on available information, we present a basic model of multicellular responses to different types of focal CNS tissue damage that is compatible with this notion (Figs. 1–3). Certain common features in this model of cellular responses to CNS damage could be interpreted as serving the basic primitive function of preventing or limiting the spread of focal infection. Viewing certain cellular responses to other CNS insults in this light may provide insights regarding basic mechanisms and how to understand and eventually manipulate responses safely to improve outcomes. We of course recognize that not all responses will be determined or influenced in this manner. Nevertheless, the powerful and fundamental role of response to infection is likely to be ancestral to all responses to CNS damage and therefore has the potential to influence all types of responses that evolved subsequently. This point is underscored by the growing recognition of the role of inflammatory mechanisms in different neurodegenerative diseases, and the potential for molecules released by peripheral infections to influence far distant reactive gliosis as discussed above. It is important to realize that the multicellular responses to diffuse CNS insults are likely to have been shaped by adapting already existing responses to focal traumatic damage or focal infection. This notion is likely to apply not only to degenerative diseases, but also to autoimmune mediated damage, where the CNS response to such damage bears striking similarities to responses to other forms of CNS tissue damage. This discussion is meant to highlight the usefulness of constructing models of how multiple cells interact and how multiple molecules drive these interactions during the responses to different types of CNS damage. The models we have presented here are compatible with basic evidence currently available but no doubt will require modification as information accrues. We hope that they are flexible enough to accommodate modifications and can serve as useful frameworks.
Manipulating CNS wound responses to promote repair
As the cell biology and molecular signaling that underlie responses to CNS damage become defined, there is increasing potential for identifying ways to manipulate these responses to improve outcome. Manipulations under intense investigation include not only traditional approaches such as development of drug candidates for parenteral pharmacological delivery, but also lifestyle modifications such as reduced energy consumption and implementation of exercise strategies intended to reduce inflammatory responses, cellular degeneration and functional decline after stroke and traumatic injury (Arumugam et al., 2010; Mattson, 2012; Piao et al., 2013). In addition, novel interventional strategies in the form of biomaterial implants as molecular depots for local molecular delivery, as well as tissue regenerative approaches based on transplantation of progenitor or stem cells are showing considerable promise for future application in neural repair and regeneration. The non-neural lesion cores of focal lesions may represent particularly useful targets for such approaches (Fig. 6). For example, biomaterial implants, particularly in the form of injectable hydrogels have potential to serve both as scaffolds and depots that can influence tissue remodeling and cellular functions (Pakulska et al., 2012). Large lesion cores of non-neural tissue after stroke or severe focal trauma may represent useful sites for placement of biomaterial depots that release gradients of molecules to influence locally surrounding neural tissue without inflicting additional damage (Fig. 6A). For example, injectable hydrogel depots can provide sustained delivery of different kinds of bioactive molecules, including growth factors or small hydrophobic molecules to influence gene expression and function of local cells over prolonged subacute times after which they are degraded without substantive tissue damage (Pakulska et al., 2012; Song et al., 2012; Yang et al., 2009; Zhang et al., 2014). Such molecular delivery has the potential to influence not only the ongoing repair in the subacute period after injury such as scar formation, but also can influence cells in the large perimeter zone around CNS lesions that undergoes continual tissue remodeling for prolonged periods after tissue damage (Figs. 6A). Delivery of molecules via depots placed into lesion cores have the potential to influence the neural plasticity and tissue remodeling in this perimeter region (Clarkson et al., 2010).
Figure 6. Lesions as targets for therapeutic strategies.
Non-neural lesion cores (LC) represent targets for biomaterial depots (A) or grafts of exogenous neural stem/progenitor cells (B), or mesenchymal stem cells (C) as potential therapeutic strategies. Molecules delivered by biomaterial depots (A) or certain cell grafts (C) have potential to support and attract axon growth (arrow a), modulate cells in peri-lesion perimeter (PLP) (arrow b), modulate cells in LC (arrow c) modulate or astrocyte scar (AS) (arrow c). New neurons generated by stem/progenitor cell grafts can receive and send neural connects in PLP and beyond, and may be able to form new, functional relay circuits (B).
Various cellular regenerative approaches may have potential for CNS repair (Bliss et al., 2007; Martino and Pluchino, 2006; Snyder and Teng, 2012). Delivery of neural lineage progenitor or stem cells into lesion cores (Figs. 6B) can lead to the generation of local neurons that form afferent and efferent connections with host and have the potential to form functionally integrated circuits (Abematsu et al., 2010; Lu et al., 2012). Stromal or mesenchymal stem cells derived from bone marrow or umbilical cord, delivered by peripheral (intravascular) or CNS injection, can home to injury sites (Fig. 6C) where they may can release growth factors, cytokines and other factors that may influence the response to damage and repair process (Bliss et al., 2007; Kokaia et al., 2012; Lemmens and Steinberg, 2013). Such grafting studies are being intensely pursued but are still in the early stages of development. Beneficial effects are reported on cellular events such as immunomodulation, survival of CNS intrinsic cells including neurons and growth of axons, however, limited information is as yet available on overall functional efficacy.
Concluding remarks
The study of cellular responses to CNS injury has progressed enormously in the last two decades, as have ideas about their functions and mechanisms. It is now clear that reactive gliosis and multicellular responses to CNS damage are a complex mixture of events that balance clearance of dead cells, pathogens and debris with maximal preservation of adjacent local tissue. These events are regulated by a vast number of molecular signals that control specific cellular functions as dictated by specific situations. Reactive gliosis is not all-or-none or stereotypic, instead it is highly variable and context specific. Loss of function studies show that reactive gliosis and multicellular responses to CNS damage exert essential beneficial functions without which tissue damage (and function) would increase and tissue repair would not occur. Nevertheless, over activity of certain functions may in some cases exacerbate tissue damage. Interventions to block such activities will need to target specific molecular events rather than attempt to block the process of gliosis per se.
Understanding the ways in which dysfunction of reactive gliosis and multicellular responses to CNS damage, for example through genetic mutations or polymorphisms, has the potential to identify new mechanisms that may underlie specific CNS disorders or that may underlie population variability in responses to similar insults such as stroke or trauma. There is a compelling need to deepen our understanding of specific cellular interactions during responses to CNS insults, and how these change over time and are governed by complex molecular signaling mechanisms. Such information is fundamental to understanding most CNS disorders and for developing rationally targeted therapeutic strategies that can safely manipulate or modify cellular responses to CNS insults in ways that improve outcome.
Acknowledgments
The authors are supported by the NIH (NINDS), The Dr. Miriam and Sheldon G. Adelson Medical Foundation and Wings for Life.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Abematsu M, Tsujimura K, Yamano M, Saito M, Kohno K, Kohyama J, Namihira M, Komiya S, Nakashima K. Neurons derived from transplanted neural stem cells restore disrupted neuronal circuitry in a mouse model of spinal cord injury. J Clin Invest. 2010;120:3255–3266. doi: 10.1172/JCI42957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeyama K, Stern DM, Ito Y, Kawahara K, Yoshimoto Y, Tanaka M, Uchimura T, Ida N, Yamazaki Y, Yamada S, et al. The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J Clin Invest. 2005;115:1267–1274. doi: 10.1172/JCI22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrich A, Kielian T. Central nervous system fibrosis is associated with fibrocyte-like infiltrates. Am J Pathol. 2011;179:2952–2962. doi: 10.1016/j.ajpath.2011.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argaw AT, Asp L, Zhang J, Navrazhina K, Pham T, Mariani JN, Mahase S, Dutta DJ, Seto J, Kramer EG, et al. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J Clin Invest. 2012;122:2454–2468. doi: 10.1172/JCI60842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc Natl Acad Sci USA. 2009;106:1977–1982. doi: 10.1073/pnas.0808698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam TV, Phillips TM, Cheng A, Morrell CH, Mattson MP, Wan R. Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann Neurol. 2010;67:41–52. doi: 10.1002/ana.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke. 1981;12:723–725. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardehle S, Kruger M, Buggenthin F, Schwausch J, Ninkovic J, Clevers H, Snippert HJ, Theis FJ, Meyer-Luehmann M, Bechmann I, et al. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat Neurosci. 2013;16:580–586. doi: 10.1038/nn.3371. [DOI] [PubMed] [Google Scholar]
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, Holtzman DM, Betsholtz C, Armulik A, Sallstrom J, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner EJ, Luciano D, Jo R, Abdi K, Paez-Gonzalez P, Sheng H, Warner DS, Liu C, Eroglu C, Kuo CT. Protective astrogenesis from the SVZ niche after injury is controlled by Notch modulator Thbs4. Nature. 2013;497:369–373. doi: 10.1038/nature12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- Bliss T, Guzman R, Daadi M, Steinberg GK. Cell transplantation therapy for stroke. Stroke. 2007;38:817–826. doi: 10.1161/01.STR.0000247888.25985.62. [DOI] [PubMed] [Google Scholar]
- Boillee S, Vande Velde C, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Brambilla R, Bracchi-Ricard V, Hu WH, Frydel B, Bramwell A, Karmally S, Green EJ, Bethea JR. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med. 2005;202:145–156. doi: 10.1084/jem.20041918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner M, Johnson AB, Boespflug-Tanguy O, Rodriguez D, Goldman JE, Messing A. Mutations in GFAP, encoding glial fibrillary acidic protein, are associated with Alexander disease. Nat Genet. 2001;27:117–120. doi: 10.1038/83679. [DOI] [PubMed] [Google Scholar]
- Buckingham SC, Campbell SL, Haas BR, Montana V, Robel S, Ogunrinu T, Sontheimer H. Glutamate release by primary brain tumors induces epileptic activity. Nat Med. 2011;17:1269–1274. doi: 10.1038/nm.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda JE, Radulovic M, Yoon H, Scarisbrick IA. Critical role for PAR1 in kallikrein 6-mediated oligodendrogliopathy. Glia. 2013;61:1456–1470. doi: 10.1002/glia.22534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush TG, NP, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH, Sofroniew MV. Leukocyte infiltration, neuronal degeneration and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol. 2006;59:735–742. doi: 10.1002/ana.20845. [DOI] [PubMed] [Google Scholar]
- Casella GT, Marcillo A, Bunge MB, Wood PM. New vascular tissue rapidly replaces neural parenchyma and vessels destroyed by a contusion injury to the rat spinal cord. Exp Neurol. 2002;173:63–76. doi: 10.1006/exnr.2001.7827. [DOI] [PubMed] [Google Scholar]
- Chan JK, Roth J, Oppenheim JJ, Tracey KJ, Vogl T, Feldmann M, Horwood N, Nanchahal J. Alarmins: awaiting a clinical response. J Clin Invest. 2012;122:2711–2719. doi: 10.1172/JCI62423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468:305–309. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, Qi J, Edgerton VR, Sofroniew MV. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nature Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circulation research. 2005;97:1093–1107. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Blennow K, Ikonomovic MD, Gandy S. Acute and chronic traumatic encephalopathies: pathogenesis and biomarkers. Nature reviews Neurology. 2013;9:192–200. doi: 10.1038/nrneurol.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki NC, Cronk JC, Lu Z, Xu E, Abbott SB, Guyenet PG, Kipnis J. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature. 2012;484:105–109. doi: 10.1038/nature10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Vezzani A, Najjar S, De Lanerolle NC, Rogawski MA. Glia and epilepsy: excitability and inflammation. Trends Neurosci. 2013;36:174–184. doi: 10.1016/j.tins.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Drogemuller K, Helmuth U, Brunn A, Sakowicz-Burkiewicz M, Gutmann DH, Mueller W, Deckert M, Schluter D. Astrocyte gp130 expression is critical for the control of Toxoplasma encephalitis. J Immunol. 2008;181:2683–2693. doi: 10.4049/jimmunol.181.4.2683. [DOI] [PubMed] [Google Scholar]
- Dugas JC, Mandemakers W, Rogers M, Ibrahim A, Daneman R, Barres BA. A novel purification method for CNS projection neurons leads to the identification of brain vascular cells as a source of trophic support for corticospinal motor neurons. J Neurosci. 2008;28:8294–8305. doi: 10.1523/JNEUROSCI.2010-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Failli V, Kopp MA, Gericke C, Martus P, Klingbeil S, Brommer B, Laginha I, Chen Y, DeVivo MJ, Dirnagl U, Schwab JM. Functional neurological recovery after spinal cord injury is impaired in patients with infections. Brain. 2012;135:3238–3250. doi: 10.1093/brain/aws267. [DOI] [PubMed] [Google Scholar]
- Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- Frohman EM, Racke MK, Raine CS. Multiple sclerosis--the plaque and its pathogenesis. The New England journal of medicine. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- Gadea A, Schinelli S, Gallo V. Endothelin-1 regulates astrocyte proliferation and reactive gliosis via a JNK/c-Jun signaling pathway. J Neurosci. 2008;28:2394–2408. doi: 10.1523/JNEUROSCI.5652-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Zhou H, Zhang F, Wilson BC, Kam W, Hong JS. HMGB1 acts on microglia Mac1 to mediate chronic neuroinflammation that drives progressive neurodegeneration. J Neurosci. 2011;31:1081–1092. doi: 10.1523/JNEUROSCI.3732-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia ADR, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principle source of constitutive neurogenesis in adult mouse forebrain. Nature Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- Garcia-Alias G, Barkhuysen S, Buckle M, Fawcett JW. Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nat Neurosci. 2009;12:1145–1151. doi: 10.1038/nn.2377. [DOI] [PubMed] [Google Scholar]
- Gleichman AJ, Carmichael ST. Astrocytic therapies for neuronal repair in stroke. Neurosci Lett. 2013 doi: 10.1016/j.neulet.2013.10.055. [DOI] [PubMed] [Google Scholar]
- Goritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisen J. A pericyte origin of spinal cord scar tissue. Science. 2011;333:238–242. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- Greaves NS, Ashcroft KJ, Baguneid M, Bayat A. Current understanding of molecular and cellular mechanisms in fibroplasia and angiogenesis during acute wound healing. Journal of dermatological science. 2013 doi: 10.1016/j.jdermsci.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Griffiths MR, Gasque P, Neal JW. The regulation of the CNS innate immune response is vital for the restoration of tissue homeostasis (repair) after acute brain injury: a brief review. International journal of inflammation. 2010;2010:151097. doi: 10.4061/2010/151097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol. 2010;72:335–355. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamby ME, Coppola G, Ao Y, Geschwind DH, Khakh BS, Sofroniew MV. Inflammatory mediators alter the astrocyte transcriptome and calcium signaling elicited by multiple g-protein-coupled receptors. J Neurosci. 2012;32:14489–14510. doi: 10.1523/JNEUROSCI.1256-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton NB, Attwell D. Do astrocytes really exocytose neurotransmitters? Nat Rev Neurosci. 2010;11:227–238. doi: 10.1038/nrn2803. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Haroon F, Drogemuller K, Handel U, Brunn A, Reinhold D, Nishanth G, Mueller W, Trautwein C, Ernst M, Deckert M, Schluter D. Gp130-dependent astrocytic survival is critical for the control of autoimmune central nervous system inflammation. J Immunol. 2011;186:6521–6531. doi: 10.4049/jimmunol.1001135. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Pham LD, Katusic ZS, Arai K, Lo EH. Astrocytic high-mobility group box 1 promotes endothelial progenitor cell-mediated neurovascular remodeling during stroke recovery. Proc Natl Acad Sci U S A. 2012;109:7505–7510. doi: 10.1073/pnas.1121146109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463:232–236. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermanns S, Klapka N, Gasis M, Muller HW. The collagenous wound healing scar in the injured central nervous system inhibits axonal regeneration. Advances in experimental medicine and biology. 2006;557:177–190. doi: 10.1007/0-387-30128-3_11. [DOI] [PubMed] [Google Scholar]
- Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, Korsak RA, Takeda K, Akira S, Sofroniew MV. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. 2008;28:7231–7243. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes EG, Kang SH, Fukaya M, Bergles DE. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci. 2013;16:668–676. doi: 10.1038/nn.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabs R, Seifert G, Steinhauser C. Astrocytic function and its alteration in the epileptic brain. Epilepsia. 2008;49(Suppl 2):3–12. doi: 10.1111/j.1528-1167.2008.01488.x. [DOI] [PubMed] [Google Scholar]
- Jackman NA, Uliasz TF, Hewett JA, Hewett SJ. Regulation of system x(c)(−)activity and expression in astrocytes by interleukin-1beta: implications for hypoxic neuronal injury. Glia. 2010;58:1806–1815. doi: 10.1002/glia.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaerve A, Bosse F, Muller HW. SDF-1/CXCL12: its role in spinal cord injury. The international journal of biochemistry & cell biology. 2012;44:452–456. doi: 10.1016/j.biocel.2011.11.023. [DOI] [PubMed] [Google Scholar]
- Kawaja MD, Gage FH. Reactive astrocytes are substrates for the growth of adult CNS axons in the presence of elevated levels of nerve growth factor. Neuron. 1991;7:1019–1030. doi: 10.1016/0896-6273(91)90346-2. [DOI] [PubMed] [Google Scholar]
- Kimura-Kuroda J, Teng X, Komuta Y, Yoshioka N, Sango K, Kawamura K, Raisman G, Kawano H. An in vitro model of the inhibition of axon growth in the lesion scar formed after central nervous system injury. Mol Cell Neurosci. 2010;43:177–187. doi: 10.1016/j.mcn.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Klapka N, Muller HW. Collagen matrix in spinal cord injury. J Neurotrauma. 2006;23:422–435. doi: 10.1089/neu.2006.23.422. [DOI] [PubMed] [Google Scholar]
- Kokaia Z, Martino G, Schwartz M, Lindvall O. Cross-talk between neural stem cells and immune cells: the key to better brain repair? Nat Neurosci. 2012;15:1078–1087. doi: 10.1038/nn.3163. [DOI] [PubMed] [Google Scholar]
- Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft AW, Hu X, Yoon H, Yan P, Xiao Q, Wang Y, Gil SC, Brown J, Wilhelmsson U, Restivo JL, et al. Attenuating astrocyte activation accelerates plaque pathogenesis in APP/PS1 mice. FASEB J. 2013;27:187–198. doi: 10.1096/fj.12-208660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC. Does the endogenous neurogenic response alter behavioral recovery following stroke? Behavioural brain research. 2012;227:426–432. doi: 10.1016/j.bbr.2011.08.045. [DOI] [PubMed] [Google Scholar]
- Lemmens R, Steinberg GK. Stem cell therapy for acute cerebral injury: What do we know and what will the future bring? Curr Op Neurol. 2013 doi: 10.1097/WCO.0000000000000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202:473–477. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Lundkvist A, Andersson D, Wilhelmsson U, Nagai N, Pardo AC, Nodin C, Stahlberg A, Aprico K, Larsson K, et al. Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab. 2008;28:468–481. doi: 10.1038/sj.jcbfm.9600546. [DOI] [PubMed] [Google Scholar]
- Li S, Overman JJ, Katsman D, Kozlov SV, Donnelly CJ, Twiss JL, Giger RJ, Coppola G, Geschwind DH, Carmichael ST. An age-related sprouting transcriptome provides molecular control of axonal sprouting after stroke. Nat Neurosci. 2010;13:1496–1504. doi: 10.1038/nn.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. 2006;441:1094–1096. doi: 10.1038/nature04960. [DOI] [PubMed] [Google Scholar]
- Liu GJ, Nagarajah R, Banati RB, Bennett MR. Glutamate induces directed chemotaxis of microglia. Eur J Neurosci. 2009;29:1108–1118. doi: 10.1111/j.1460-9568.2009.06659.x. [DOI] [PubMed] [Google Scholar]
- Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med. 2008;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- Logan A, Berry M. Cellular and molecular determinants of glial scar formation. Adv Exp Med Biol. 2002;513:115–158. doi: 10.1007/978-1-4615-0123-7_4. [DOI] [PubMed] [Google Scholar]
- London A, Cohen M, Schwartz M. Microglia and monocyte-derived macrophages: functionally distinct populations that act in concert in CNS plasticity and repair. Frontiers in cellular neuroscience. 2013;7:34. doi: 10.3389/fncel.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis DN. Molecular pathology of malignant gliomas. Annual review of pathology. 2006;1:97–117. doi: 10.1146/annurev.pathol.1.110304.100043. [DOI] [PubMed] [Google Scholar]
- Loy DN, Crawford CH, Darnall JB, Burke DA, Onifer SM, Whittemore SR. Temporal progression of angiogenesis and basal lamina deposition after contusive spinal cord injury in the adult rat. J Comp Neurol. 2002;445:308–324. doi: 10.1002/cne.10168. [DOI] [PubMed] [Google Scholar]
- Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, Brock J, Blesch A, Rosenzweig ES, Havton LA, et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150:1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchinetti CF, Bruck W, Rodriguez M, Lassmann H. Distinct patterns of multiple sclerosis pathology indicates heterogeneity on pathogenesis. Brain Pathol. 1996;6:259–274. doi: 10.1111/j.1750-3639.1996.tb00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol. 2013;31:259–283. doi: 10.1146/annurev-immunol-032712-095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragakis NJ, Rothstein JD. Mechanisms of Disease: astrocytes in neurodegenerative disease. Nat Clin Pract Neurol. 2006;2:679–689. doi: 10.1038/ncpneuro0355. [DOI] [PubMed] [Google Scholar]
- Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci. 2006;7:395–406. doi: 10.1038/nrn1908. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Apoptosis in neurodegenerative disorders. Nature reviews Molecular cell biology. 2000;1:120–129. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell metabolism. 2012;16:706–722. doi: 10.1016/j.cmet.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh TK, Vink R, Noble L, Yamakami I, Fernyak S, Soares H, Faden AL. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience. 1989;28:233–244. doi: 10.1016/0306-4522(89)90247-9. [DOI] [PubMed] [Google Scholar]
- Meletis K, Barnabe-Heider F, Carlen M, Evergren E, Tomilin N, Shupliakov O, Frisen J. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 2008;6:e182. doi: 10.1371/journal.pbio.0060182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midwood KS, Williams LV, Schwarzbauer JE. Tissue repair and the dynamics of the extracellular matrix. The international journal of biochemistry & cell biology. 2004;36:1031–1037. doi: 10.1016/j.biocel.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Mills KH. TLR-dependent T cell activation in autoimmunity. Nat Rev Immunol. 2011;11:807–822. doi: 10.1038/nri3095. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer DJ, Gurkoff GG, Lee SM, Hovda DA, Sofroniew MV. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain. 2006;129:2761–2772. doi: 10.1093/brain/awl165. [DOI] [PubMed] [Google Scholar]
- Neary JT, Kang Y, Willoughby KA, Ellis EF. Activation of extracellular signal-regulated kinase by stretch-induced injury in astrocytes involves extracellular ATP and P2 purinergic receptors. J Neurosci. 2003;23:2348–2356. doi: 10.1523/JNEUROSCI.23-06-02348.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- Ohab JJ, Carmichael ST. Poststroke neurogenesis: emerging principles of migration and localization of immature neurons. Neuroscientist. 2008;14:369–380. doi: 10.1177/1073858407309545. [DOI] [PubMed] [Google Scholar]
- Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y, Okano H. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nature Med. 2006;12:829–834. doi: 10.1038/nm1425. [DOI] [PubMed] [Google Scholar]
- Ortinski PI, Dong J, Mungenast A, Yue C, Takano H, Watson DJ, Haydon PG, Coulter DA. Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci. 2010;13:584–591. doi: 10.1038/nn.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudega M. Molecular and cellular mechanisms underlying the role of blood vessels in spinal cord injury and repair. Cell and tissue research. 2012;349:269–288. doi: 10.1007/s00441-012-1440-6. [DOI] [PubMed] [Google Scholar]
- Overman JJ, Clarkson AN, Wanner IB, Overman WT, Eckstein I, Maguire JL, Dinov ID, Toga AW, Carmichael ST. A role for ephrin-A5 in axonal sprouting, recovery, and activity-dependent plasticity after stroke. Proc Natl Acad Sci U S A. 2012;109:E2230–2239. doi: 10.1073/pnas.1204386109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakulska MM, Ballios BG, Shoichet MS. Injectable hydrogels for central nervous system therapy. Biomed Mater. 2012;7:024101. doi: 10.1088/1748-6041/7/2/024101. [DOI] [PubMed] [Google Scholar]
- Perry VH. Contribution of systemic inflammation to chronic neurodegeneration. Acta Neuropathol. 2010;120:277–286. doi: 10.1007/s00401-010-0722-x. [DOI] [PubMed] [Google Scholar]
- Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7:161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- Piao CS, Stoica BA, Wu J, Sabirzhanov B, Zhao Z, Cabatbat R, Loane DJ, Faden AI. Late exercise reduces neuroinflammation and cognitive dysfunction after traumatic brain injury. Neurobiol Dis. 2013;54:252–263. doi: 10.1016/j.nbd.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu BF, Lucchinetti CF. Pathology of demyelinating diseases. Annual review of pathology. 2012;7:185–217. doi: 10.1146/annurev-pathol-011811-132443. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Longbrake EE. Can the immune system be harnessed to repair the CNS? Nat Rev Neurosci. 2008;9:481–493. doi: 10.1038/nrn2398. [DOI] [PubMed] [Google Scholar]
- Prokop S, Miller KR, Heppner FL. Microglia actions in Alzheimer’s disease. Acta Neuropathol. 2013 doi: 10.1007/s00401-013-1182-x. [DOI] [PubMed] [Google Scholar]
- Radulovic M, Yoon H, Larson N, Wu J, Linbo R, Burda JE, Diamandis EP, Blaber SI, Blaber M, Fehlings MG, Scarisbrick IA. Kallikrein cascades in traumatic spinal cord injury: in vitro evidence for roles in axonopathy and neuron degeneration. J Neuropathol Exp Neurol. 2013;72:1072–1089. doi: 10.1097/NEN.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Reilkoff RA, Bucala R, Herzog EL. Fibrocytes: emerging effector cells in chronic inflammation. Nat Rev Immunol. 2011;11:427–435. doi: 10.1038/nri2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD. Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Ann Neurol. 2009;65(Suppl 1):S3–9. doi: 10.1002/ana.21543. [DOI] [PubMed] [Google Scholar]
- Sahni V, Mukhopadhyay A, Tysseling V, Hebert A, Birch D, McGuire TL, Stupp SI, Kessler JA. BMPR1a and BMPR1b signaling exert opposing effects on gliosis after spinal cord injury. J Neurosci. 2010;30:1839–1855. doi: 10.1523/JNEUROSCI.4459-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo JH, Miyamoto N, Hayakawa K, Pham LD, Maki T, Ayata C, Kim KW, Lo EH, Arai K. Oligodendrocyte precursors induce early blood-brain barrier opening after white matter injury. J Clin Invest. 2013;123:782–786. doi: 10.1172/JCI65863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter R, Schwartz M. CNS sterile injury: just another wound healing? Trends in molecular medicine. 2013;19:135–143. doi: 10.1016/j.molmed.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Shigetomi E, Bowser DN, Sofroniew MV, Khakh BS. Two forms of astrocyte calcium excitability have distinct effects on NMDA receptor-mediated slow inward currents in pyramidal neurons. J Neurosci. 2008;28:6659–6663. doi: 10.1523/JNEUROSCI.1717-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa H, Sakimoto S, Nakao K, Sugishita A, Konno M, Iida S, Kusano A, Hashimoto E, Nakagawa T, Kaneko S. Transient receptor potential canonical 3 (TRPC3) mediates thrombin-induced astrocyte activation and upregulates its own expression in cortical astrocytes. J Neurosci. 2010;30:13116–13129. doi: 10.1523/JNEUROSCI.1890-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver DJ, Siebzehnrubl FA, Schildts MJ, Yachnis AT, Smith GM, Smith AA, Scheffler B, Reynolds BA, Silver J, Steindler DA. Chondroitin sulfate proteoglycans potently inhibit invasion and serve as a central organizer of the brain tumor microenvironment. J Neurosci. 2013;33:15603–15617. doi: 10.1523/JNEUROSCI.3004-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J, Edwards MA, Levitt P. Immunocytochemical demonstration of early appearing astroglial structures that form boundaries and pathways along axon tracts in the fetal brain. J Comp Neurol. 1993;328:415–436. doi: 10.1002/cne.903280308. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nature Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Singer AJ, Clark RA. Cutaneous wound healing. The New England journal of medicine. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Sirko S, Behrendt G, Johansson PA, Tripathi P, Costa M, Bek S, Heinrich C, Tiedt S, Colak D, Dichgans M, et al. Reactive glia in the injured brain acquire stem cell properties in response to sonic hedgehog. [corrected] Cell Stem Cell. 2013;12:426–439. doi: 10.1016/j.stem.2013.01.019. [DOI] [PubMed] [Google Scholar]
- Snyder EY, Teng YD. Stem cells and spinal cord repair. The New England journal of medicine. 2012;366:1940–1942. doi: 10.1056/NEJMcibr1200138. [DOI] [PubMed] [Google Scholar]
- Soderblom C, Luo X, Blumenthal E, Bray E, Lyapichev K, Ramos J, Krishnan V, Lai-Hsu C, Park KK, Tsoulfas P, Lee JK. Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury. J Neurosci. 2013;33:13882–13887. doi: 10.1523/JNEUROSCI.2524-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005;5:400–407. doi: 10.1177/1073858405278321. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV. Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators. Neuroscientist. 2013 doi: 10.1177/1073858413504466. advance publication online. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Song J, Zhang S, Anderson MA, Ao Y, Yang CY, Deming TJ, Sofroniew MV. Sustained local delivery of bioactive nerve growth factor in the central nervous system via tunable diblock copolypeptide hydrogel depots. Biomaterials. 2012;33:9105–9116. doi: 10.1016/j.biomaterials.2012.08.060. [DOI] [PubMed] [Google Scholar]
- Srivastava R, Aslam M, Kalluri SR, Schirmer L, Buck D, Tackenberg B, Rothhammer V, Chan A, Gold R, Berthele A, et al. Potassium channel KIR4.1 as an immune target in multiple sclerosis. The New England journal of medicine. 2012;367:115–123. doi: 10.1056/NEJMoa1110740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan AH, Barres BA, Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci. 2012;35:369–389. doi: 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Sun F, Lin CL, McTigue D, Shan X, Tovar CA, Bresnahan JC, Beattie MS. Effects of axon degeneration on oligodendrocyte lineage cells: dorsal rhizotomy evokes a repair response while axon degeneration rostral to spinal contusion induces both repair and apoptosis. Glia. 2010;58:1304–1319. doi: 10.1002/glia.21009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo Z, Wu M, Ameenuddin S, Anderson HE, Zoloty JE, Citron BA, Andrade-Gordon P, Festoff BW. Participation of protease-activated receptor-1 in thrombin-induced microglial activation. J Neurochem. 2002;80:655–666. doi: 10.1046/j.0022-3042.2001.00745.x. [DOI] [PubMed] [Google Scholar]
- Tao J, Wu H, Lin Q, Wei W, Lu XH, Cantle JP, Ao Y, Olsen RW, Yang XW, Mody I, et al. Deletion of astroglial dicer causes non-cell-autonomous neuronal dysfunction and degeneration. J Neurosci. 2011;31:8306–8319. doi: 10.1523/JNEUROSCI.0567-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X, Shigetomi E, Looger LL, Khakh BS. Genetically encoded calcium indicators and astrocyte calcium microdomains. Neuroscientist. 2013;19:274–291. doi: 10.1177/1073858412468794. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Steward O. Concepts and methods for the study of axonal regeneration in the CNS. Neuron. 2012;74:777–791. doi: 10.1016/j.neuron.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brand R, Heutschi J, Barraud Q, DiGiovanna J, Bartholdi K, Huerlimann M, Friedli L, Vollenweider I, Moraud EM, Duis S, et al. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science. 2012;336:1182–1185. doi: 10.1126/science.1217416. [DOI] [PubMed] [Google Scholar]
- Varki A, Gagneux P. Multifarious roles of sialic acids in immunity. Ann N Y Acad Sci. 2012;1253:16–36. doi: 10.1111/j.1749-6632.2012.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Orkand RK, Kettenmann H. Glial calcium: homeostasis and signaling function. Physiol Rev. 1998;78:99–141. doi: 10.1152/physrev.1998.78.1.99. [DOI] [PubMed] [Google Scholar]
- Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- Voskuhl RR, Peterson RS, Song B, Ao Y, Morales LB, Tiwari-Woodruff S, Sofroniew MV. Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J Neurosci. 2009;29:11511–11522. doi: 10.1523/JNEUROSCI.1514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JT, Kipnis J. Regulatory T cells in CNS injury: the simple, the complex and the confused. Trends in molecular medicine. 2011;17:541–547. doi: 10.1016/j.molmed.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Fawcett J. The perineuronal net and the control of CNS plasticity. Cell and tissue research. 2012;349:147–160. doi: 10.1007/s00441-012-1375-y. [DOI] [PubMed] [Google Scholar]
- Wanner IB, Anderson MA, Song B, Levine J, Fernandez A, Gray-Thompson Z, Ao Y, Sofroniew MV. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J Neurosci. 2013;33:12870–12886. doi: 10.1523/JNEUROSCI.2121-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins S, Sontheimer H. Unique biology of gliomas: challenges and opportunities. Trends Neurosci. 2012;35:546–556. doi: 10.1016/j.tins.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherington J, Serrano G, Dingledine R. Astrocytes in the epileptic brain. Neuron. 2008;58:168–178. doi: 10.1016/j.neuron.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RR, Henao M, Pearse DD, Bunge MB. Permissive Schwann Cell Graft/Spinal Cord Interfaces for Axon Regeneration. Cell Transplant. 2013 doi: 10.3727/096368913X674657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14:1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler EA, Sengillo JD, Sullivan JS, Henkel JS, Appel SH, Zlokovic BV. Blood-spinal cord barrier breakdown and pericyte reductions in amyotrophic lateral sclerosis. Acta Neuropathol. 2013;125:111–120. doi: 10.1007/s00401-012-1039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, Silverstein SC, Husemann J. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat Med. 2003;9:453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11:251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CY, Song B, Ao Y, Nowak AP, Abelowitz RB, Korsak RA, Havton LA, Deming TJ, Sofroniew MV. Biocompatibility of amphiphilic diblock copolypeptide hydrogels in the central nervous system. Biomaterials. 2009;30:2881–2898. doi: 10.1016/j.biomaterials.2009.01.056. [DOI] [PubMed] [Google Scholar]
- Yoshioka N, Hisanaga S, Kawano H. Suppression of fibrotic scar formation promotes axonal regeneration without disturbing blood-brain barrier repair and withdrawal of leukocytes after traumatic brain injury. J Comp Neurol. 2010;518:3867–3881. doi: 10.1002/cne.22431. [DOI] [PubMed] [Google Scholar]
- Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32:6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Anderson MA, Ao Y, Khakh BS, Fan J, Deming TJ, Sofroniew MV. Tunable diblock copolypeptide hydrogel depots for local delivery of hydrophobic molecules in healthy and injured central nervous system. Biomaterials. 2014;35:1989–2000. doi: 10.1016/j.biomaterials.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Watts LT, Holstein DM, Prajapati SI, Keller C, Grass EH, Walter CA, Lechleiter JD. Purinergic receptor stimulation reduces cytotoxic edema and brain infarcts in mouse induced by photothrombosis by energizing glial mitochondria. PLoS One. 2010;5:e14401. doi: 10.1371/journal.pone.0014401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Deane R, Ali Z, Parisi M, Shapovalov Y, O’Banion MK, Stojanovic K, Sagare A, Boillee S, Cleveland DW, Zlokovic BV. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat Neurosci. 2008;11:420–422. doi: 10.1038/nn2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukor K, Belin S, Wang C, Keelan N, Wang X, He Z. Short Hairpin RNA against PTEN Enhances Regenerative Growth of Corticospinal Tract Axons after Spinal Cord Injury. J Neurosci. 2013;33:15350–15361. doi: 10.1523/JNEUROSCI.2510-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]