Abstract
Stroke is one of the common causes of death and disability. Despite extensive efforts in stroke research, therapeutic options for improving the functional recovery remain limited in clinical practice. Experimental stroke models using genetically modified mice could aid in unraveling the complex pathophysiology triggered by ischemic brain injury. Here, we optimized the procedure for generating mouse stroke model using an intraluminal suture in the middle cerebral artery and verified the blockage of blood flow using indocyanine green coupled with near infra-red radiation. The first week after the ischemic injury was critical for survivability. The survival rate of 11% in mice without any treatment but increased to 60% on administering prophylactic antibiotics. During this period, mice showed severe functional impairment but recovered spontaneously starting from the second week onward. Among the various behavioral tests, the pole tests and neurological severity score tests remained reliable up to 4 weeks after ischemia, whereas the rotarod and corner tests became less sensitive for assessing the severity of ischemic injury with time. Further, loss of body weight was also observed for up 4 weeks after ischemia induction. In conclusion, we have developed an improved approach which allows us to investigate the role of the cell death-related genes in the disease progression using genetically modified mice and to evaluate the modes of action of candidate drugs.
Keywords: blood flow, middle cerebral artery, survival, stroke, brain ischemia, behavior
INTRODUCTION
Ischemic stroke is one of the major causes of death and disability worldwide. According to American Heart & Stroke association, one in six people in the world have a chance of stroke in their lifetime, and stroke claims a life every six seconds. With the rise in aging population, the incidence of ischemic stroke is likely to increase in the future. Ischemic stroke is a heterogeneous disease caused by the reduced blood flow and energy supply to the brain, which triggers multitude of pathophysiological processes leading to cell death. The known, major fundamental mechanisms leading to cell death in ischemic stroke include early excitotoxicity, ionic imbalance and oxidative stress which later cause inflammation and apoptosis in the ischemic core and peri-infarct region.
Studies with rodent stroke models provide useful information for elucidating multiple pathways of cellular injury. Among various methods to induce ischemic brain injury in rodents, transient occlusion of the middle cerebral artery (MCA) by intraluminal suture has become most widely accepted because these animal models mimic clinical stroke and readily allow the study of reperfusion injury [1]. The majority of pre-clinical stroke studies utilize rats as experimental models due to their resemblance to humans in cerebrovascular anatomy and physiology [2]. Moreover, their moderate size permits relative ease for, performing surgery, monitoring physiologic parameters, and examining the brain specimens [3]. In contrast, mouse stroke models have been reluctantly used despite the mouse's genetic modifiability that could be advantageous in studies on the molecular pathophysiology of stroke [4, 5]. Thus, reliable methods for generating mouse stroke models are lacking.
The major reasons limiting the utilization of mouse stroke models are as follows. First, high mortality rates secondary to bacterial infections which hampers longitudinal stroke studies [6]. In stoke patients, bacterial infections are common medical complications that hinder both the neurological as well as general medical outcomes [7]. Likewise, immunodeficiency triggered by brain ischemia in mouse models causes the animals to be susceptible to bacterial infection and often leads to death from bacterial pneumonia, urinary tract infection, and sepsis [8, 9]. Thus, controlling infections in experimental stroke with prophylactic antibiotic treatment is of utmost importance to produce a stable focal ischemic stroke model with high survivability. Second, methods for reproducibly assessing the functional deficits are lacking. Compared to rat and gerbil models, models exhibit higher degrees of spontaneous recovery despite persistent ischemic damage; therefore, behavioral test batteries for mice are not well established and standardized [10]. Thus, detection of subtle functional deficits several weeks after ischemia is more difficult in mice, particularly, when comparing mice with diverse genetic backgrounds. Our aim was to develop and test novel and improved behavioral testing methods for evaluating post-stroke long-term functional outcomes, focusing on motor as well as sensory deficits. Third, appropriate methods to assess cerebral blood flow (CBF) require either expensive equipment or practically inconvenient. Unlike rat models, it is critical to verify the occlusion of targeted artery and measure hemodynamic kinetics in cerebrum, which ensures equal levels of blockage of blood flow in the wild type and genetically modified animal groups. Tracking an intravenous tracer agent, indocyanine green (ICG), coupled with near infrared radiation (NIR) allows a cost effective and minimally invasive method to measure cerebral blood flow in stroke animal models [11, 12]. This technique has also been widely used in detecting the reduction in regional CBF in acute ischemic stroke patients [13, 14].
In this study, we generated an improved and relatively stable mouse model of focal cerebral ischemia by transiently (60 min) occluding the MCA using an intraluminal suture. We analyzed the CBF by using minimally invasive optical method; i.e., tracking the kinetics of ICG in blood stream. We also used a battery of behavioral tests, namely the pole, rotarod, corner, and neurological severity score (NSS) tests to assess the behavioral deficits in ischemic mice until the recovery reached the plateau.
MATERIALS AND METHODS
Animals
All animal protocols were approved by the Institutional Animal Care and Use Committee of the Ajou University Medical School. C57Bl/6J male mice of 13 weeks old (equivalent to humans around 40 years old) (http://research.jax.org/faculty/harrison/ger1vLifespan1.html) were kept in cages on bedding (light cycle 12/12 h light/dark) with adequate access to food and water. One day before the surgery, animals were fasted overnight only with free access to water. Mice usually weighed 25~32 grams at the time of surgery. For one week after surgery, the animals were fed with wet mashed food on the floor. Animals were monitored daily to ensure their survival and condition. To prevent bacterial infection, animals were intraperitoneally administered once daily, a fluoroquinolone antibiotic, enrofloxacin (Bayer Korea, Ansan, Korea), dissolved in 0.625 mg/ml saline, was injected intraperitoneally once a day at a dose of 2.5 µg/g body weight. To reduce the pain and suffering, an opioid analgesic, Tramadol (Jeil Pharmaceutical Co, Yongin, Korea) was also given intraperitoneally in a dose of 0.2 mg/g body weight twice a day. Administration of both the drugs was done immediately after MCAo surgery for seven days. Sham animals also received similar treatment.
Induction of ischemia
Transient middle cerebral artery occlusion (MCAo) was induced by an intraluminal suture method. Mice were anaesthetized with isoflurane (3% for induction and 2% for maintenance) in a mixture of N2:O2 (70:30) while being intubated and mechanically ventilated. Rectal temperature was maintained at 37℃ throughout the surgical procedure. After the animals were deeply anaesthetized, they were kept in supine position under an operating microscope. A midline neck incision was given between manubrium and the jaw. The underlying muscle fascia and submandibular glands were bluntly dissected to expose the right common carotid artery (rCCA). The isolated rCCA was temporarily ligated with a silk suture during whole period of occlusion. The right external carotid artery (rECA), and the right internal carotid artery (rICA) were also isolated by blunt dissection of fascia. Two temporary ligations were made in rECA: one proximal to the ECA bifurcation and the other distal to the bifurcation (Fig. 1). The rECA was cauterized between the ligations to make a rECA stump with a cauterizer (Daiwha Co, Seoul, Korea). A temporary ligation was made in rICA. A small hole was made with a 31 gauge needle (0.25 mm × 8 mm) in the rECA stump. A nylon filament suture with a silicon coated tip (Cat No. 602123PK10 or 602312PK10, Doccol co, Sharon, MA, USA) (Fig. 1) was inserted through the hole in rECA stump and further pushed along the lumen of ICA while the ligation of rICA was loosened. Usually the suture length was 1 cm from the hole to the bifurcating point of the right middle cerebral artery (rMCA). To steadily hold the suture and effectively occlude the rMCA, rICA was religated temporarily. The rECA stump over the hole was temporarily ligated with a silk suture in order to stop bleeding from the hole. Then midline neck incision was closed and animals were immediately placed in the CBF machine to measure the CBF as described below. After 60 or 90 minute of occlusion, the animals were re-anaesthetized and the nylon suture was gently removed from rMCA to reperfuse the rMCA territory. The hole in rECA stump was permanently ligated and the temporary ligation in rCCA was removed to allow normal blood flow to the brain. The midline neck incision was closed and the animals were placed in their normal habitat. As a control, the sham-operated animals were subjected to the same procedure without occlusion of MCA.
Fig. 1.
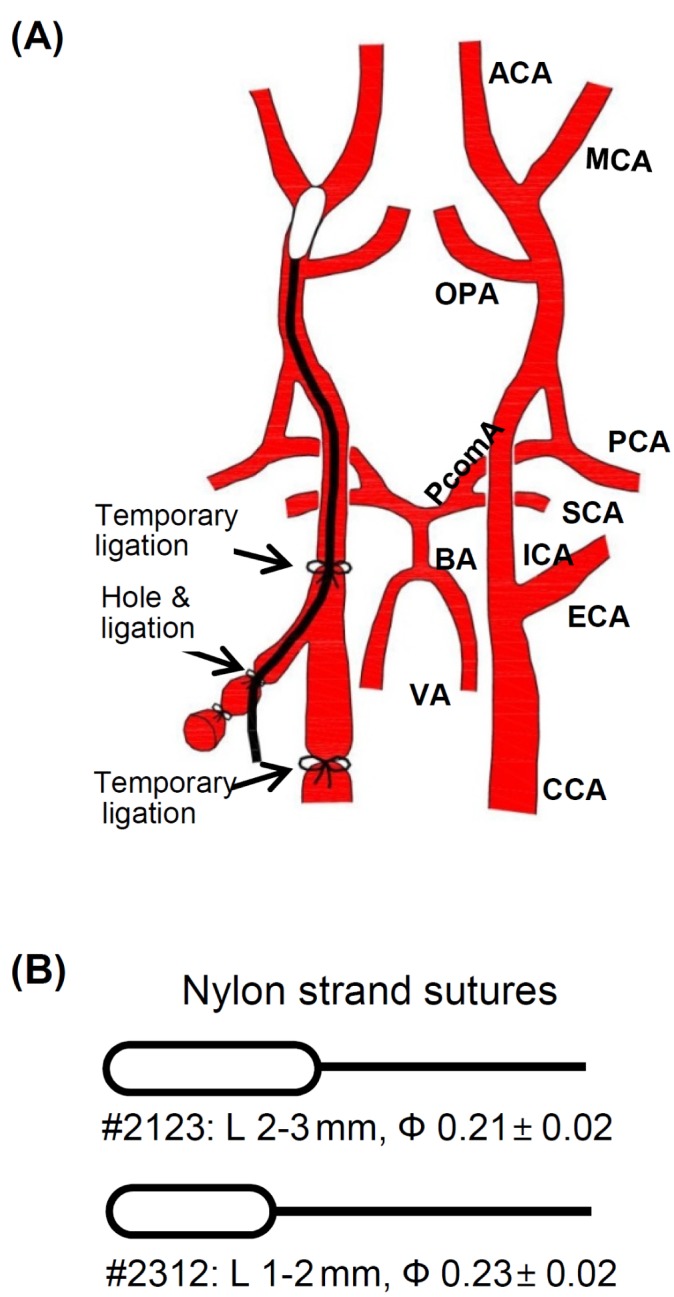
Schematic diagram showing intraluminal suture MCAo (A) The right middle cerebral artery (rMCA) was occluded with a nylon suture which was inserted via right external carotid artery (rECA) and then passed along the lumen of the internal carotid artery (ICA). (B) The nylon suture with different silicon coated tip diameter and length. #2312 suture has a silicon-coated tip which is shorter but thicker than #2123. (L: Length, Ф: diameter).
Measurement of regional CBF
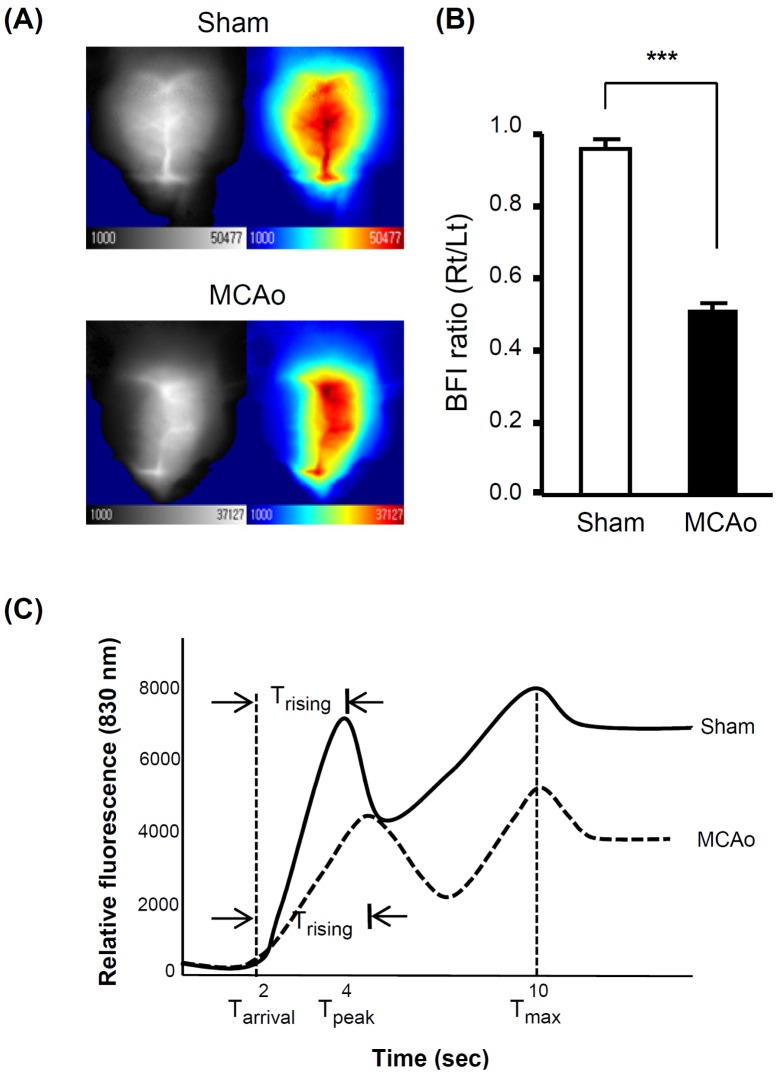
CBF was measured directly over the scalp after shaving the fur, without exposing or opening skull using a custom-made machine and a software (Vieworks, Seongnam, Korea). While the MCA was occluded with a suture as described above, the animals were anesthetized with ketamine (0.19 mg/g body weight) and placed in the dark chamber of the CBF machine. The hair covering the head skin was removed. ICG (0.001 mg/g body weight) was injected to tail vein and 760 nm lights were illuminated overhead. The 12-bit gray scale ICG fluorescent signals through a 830 nm filter were captured every 580 msec for 3 min. The initial 40 frames were used to generate blood flow maps using a software provided by the manufacturer and calculated according to the previous report by Ku et al. [11]. Trising value was calculated as the duration between Tarrival (time of first appearance of the ICG fluorescence) and Tpeak (first peak time). The slope of the first peak in the time-intensity curve was used to calculate the blood flow index (BFI) by dividing the relative fluorescence intensity at Tpeak with Trising. The BFI represented overall blood volume information with respect to time and correlated with CBF.
Histological evaluation
To measure the infarct volume, the animals were deeply anesthetized with high dose of isoflurane gas 24 h after the surgery and were decapitated. The brains were extracted rapidly and coronally sliced into 1 or 2 mm sections on an ice-cold mouse mold (Leica Biosystems, Buffalo Grove, IL, USA). Each section was incubated in 2% of 2,3,5 triphenyltetrazolium chloride (TTC) in phosphate buffered saline, pH 7.4 (PBS) for 10~12 min at 37℃. After TTC solution was drained, the brain slices were fixed with 4% paraformaldehyde in PBS. The images of both sides of each slice were acquired with a stereoscopic microscope (SZX2-ILLB, Oympus Co. Toyko, Japan) and the average was used to obtain the infarct volume using Image J software (http://rsbweb.nih.gov/ij/). The unstained area in the section was regarded as an infarct area whereas the stained area as non-infarct area.
Behavior tests
All behavioral tests were carried out on 2nd, 4th, 7th, 14th, 21st and 28th day after MCA occlusion. Rotarod test evaluates the balance and coordination function [15]. Prior to surgery, the mice were trained for balancing on the rotating drum (Acceler Rota-Rod 7650, UGO BASILE, Varese, Italy) for 5 days (3 trials per day) [16]. The rotarod was accelerated from 4 to 40 r.p.m. for 250 sec as preoperative baseline. Animals not achieving the baseline criteria were excluded from the subsequent study. The latency before falling off the accelerating drum was recorded with the maximum of 250 second. The longest latency from three consecutive trials for each testing day was extracted for data analysis using Sigma plot software (Systat Software Inc, San Jose, CA, USA).
Corner test is used to detect unilateral abnormalities of sensory and motor functions in the stroke model [17]. Corner was made by placing two wooden cardboards (30 cm × 20 cm × 1 cm) at an angle of 30 degree. Mouse was made to enter the corner upon its placement at midway to the corner. As the mouse reached deep into the corner, both sides of the vibrissae were stimulated together. Upon the stimulation of vibrissae mouse reared forward and upward and finally turned backward towards the open end. The direction towards which the mouse turned was recorded. A total of 10 trials were recorded per each animal pre-operatively and on indicated days. Sham animals did not show any preference in the direction while the ischemic mice showed marked preference in turning towards the non-impaired side (right turn). The percentage of right turn was analyzed as the indicator of deficit.
Pole test is a simple behavior test used to assess motor dysfunction after stroke [18]. Mice were placed in the top of a 60 cm vertical pole with a diameter of 1 cm. The pole was placed in the home cage so that mice might prefer to descend to the floor of cage. Recording was started when the animal began the turning movement. The time to turn completely downward (Tturn) and total time to descend to the floor (Ttotal) were recorded. When the animal paused while descending, the trial was repeated. When the animal could not turn but instead descended with a lateral body position, then Ttotal was attributed to Tturn. When the animal fell off the pole immediately, the maximum scores for Tturn (10 sec) and Ttotal (15 sec) were assigned. The test was repeated for 3 trials per animal in each setting and the average Tturn and Ttotal were used for data analysis.
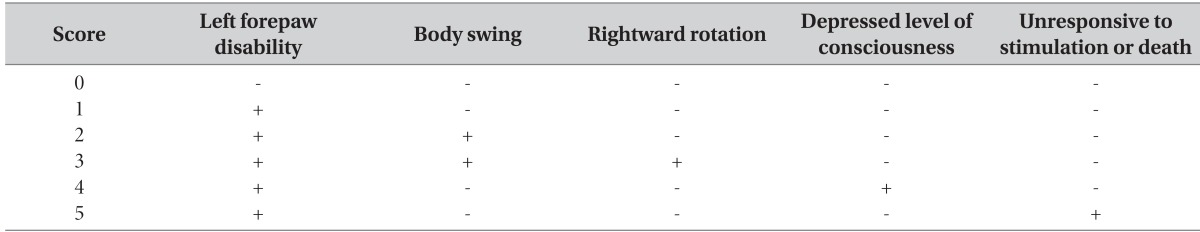
For the NSS test, a 0-5 grading scale was used, with the slight modification performed by Bederson et al. [19] (Table 1). Normal mice were scored 0, while dead mice or mice unresponsive to stimulation were scored 5.
Table 1.
Modified neurological severity score (NSS)
RESULTS
Optimization of survivability of ischemic animals
The use of nylon suture with a tip length of 2~3 mm, without any treatment post-surgery, resulted in the death of most mice between day 2 and day 4 of ischemic injury (Fig. 2A). We observed the signs of pyuria and hypothermia in the dead mice before their death, suggestive of bacterial infection and the sepsis following the immunosuppressive states as the primary cause of such a low survival rate (11%) was bacterial infection and sepsis following the immunosuppression. Mice that survived for at least a week post ischemia did not die during the time window of 28 days. When we treated the animals with prophylactic antibiotic drug, the incidence of pyuria was reduced with the increment in the survival rate at day 28, up to 40%. Reducing the suture tip length to 1~2 mm (Cat No. 2312) and occlusion time to 1 h further improved the survival rates to a significant level (60%). Interestingly, the infarct volumes assessed with TTC staining were not significantly different among three groups (Fig. 2B and 2C), suggesting that improved viability was not due to differences in the brain damage. Thus, we occluded MCA for 1 h with the suture (Cat No. 2312) and administered the animals with antibiotic and analgesic drugs in the subsequent studies.
Fig. 2.
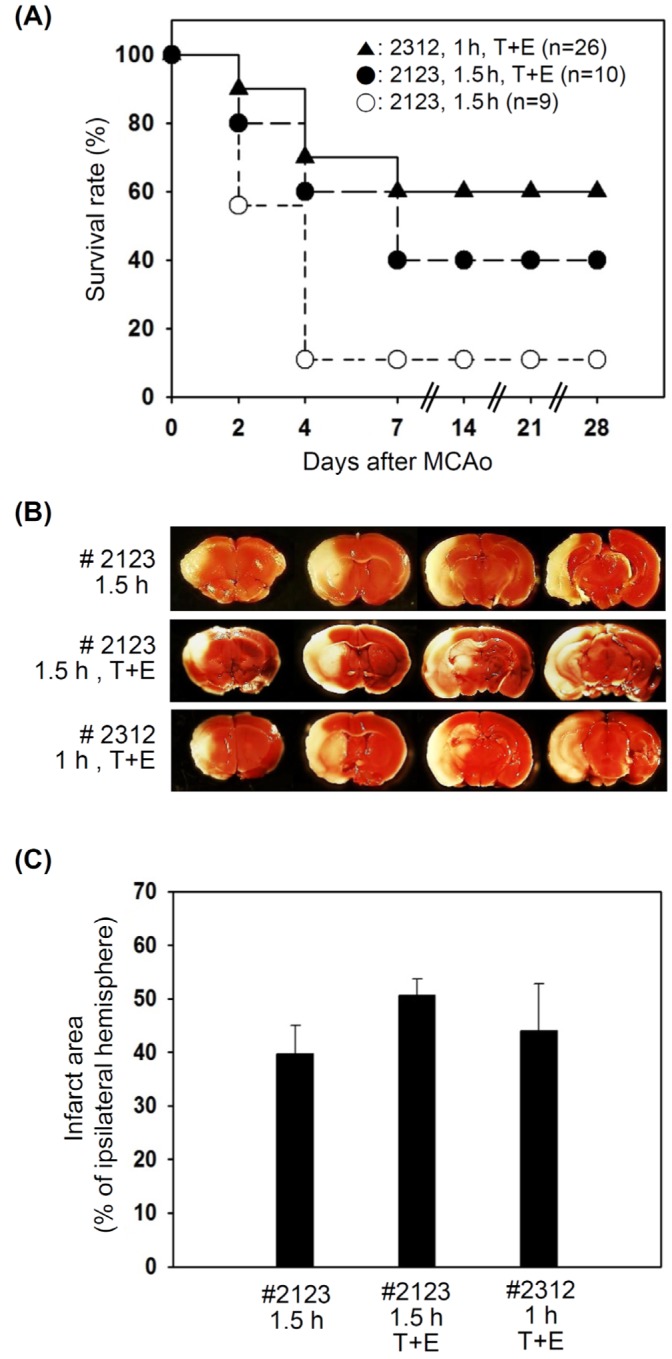
Comparison the effect of parameters on the survivability. (A) Following MCAo for 1.5 h, the mortality was too high in the animals without any treatment (○). The mortality was slightly reduced when the animals were treated with Enrofloxacin (antibiotic) and Tramadol (opioid analgesic) (●). Occlusion for 1 h with a nylon suture with #2312 and treatment with Enrofloxacin and Tramadol enhanced the survival rates up to 60% (▲). It should be noted that 7 days after the ischemic injury, animals were stabilized and no more death occurred. (B) In the brains from the MCAo animals, the infarcted area in the right hemisphere was not stainable by TTC. (C) The infarcted area was calculated and shown as the means±S.E. at least three animals per group. The difference among the groups was insignificant.
CBF measurement
We acquired time-series fluorescence images over the head of mice after a bolus injection of ICG. The contralateral (control) hemisphere or sham-operated animals showed increased 830nm fluorescent signals emitted from ICG (Fig. 3A). The map of BFI clearly separated the cerebral blood from adjacent tissues or skull (Fig. 2A). While the MCA was occluded, the BFI value was consistently reduced up to 50% blood flow in the right hemisphere compared to the left one (Fig. 3B). Trising values in the ischemic hemisphere were also increased by 24%, indicating reduced velocity of blood flow in the ipsilateral hemisphere (Fig. 3C, Table 2). In the sham-operated animals, all the values were similar in the right and left hemispheres. The results indicated that blockage of CBF in the right hemisphere was successfully induced in a consistent manner.
Fig. 3.
Verification of MCAo by CBF measurement during occlusion. (A) Representative CBF maps in the sham and a MCAo animals. Local CBF was decreased in the right hemisphere of the MCAo animals while there was no change in the sham animals. (B) The relative CBF are presented as the BFI ratio of the ipsilateral (right) to contralateral (left) hemisphere. BFI ratio was significantly decreased in the MCAo animals but not in the sham-operated animals. All data are presented as means±S.D. from 7 and 20 animals from the sham-operated and MCAo animals, respectively. (C) The graphs show the representative fluorescent signals of sham-operated (solid line) and MCAo (dotted line) animals. It should be noted that Trising, the duration between the arrival time (Tarrival) and the first peak time (Tpeak), was increased in the MCAo animal (t-test, ***p<0.001).
Table 2.
Comparison of parameters between the right and left hemispheres
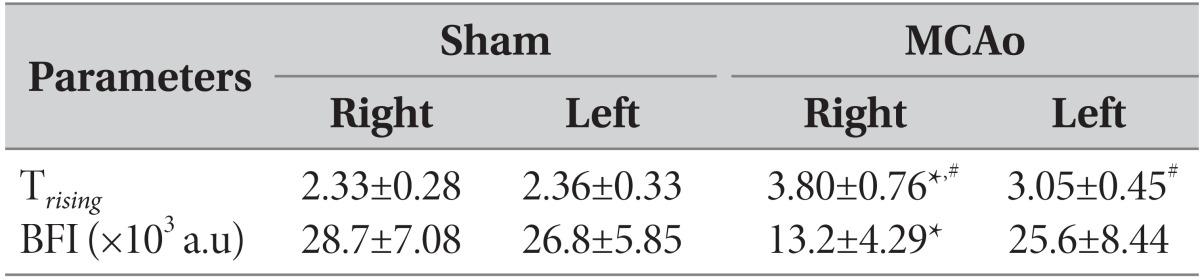
a.u: arbitrary units.
#p<0.01, compared to the sham-operated animals. *p<0.01, compared to the left (contralateral) side.
Assessment of behavior functions
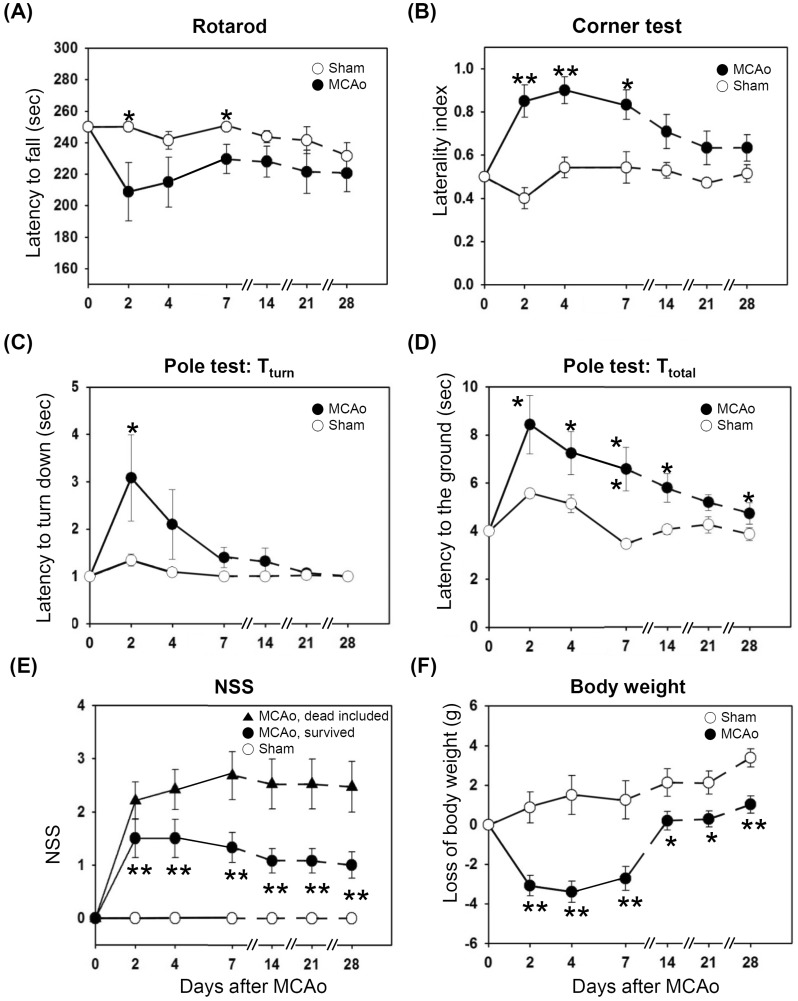
Overall, the MCAo mice exhibited poor performance in behavioral tests during the first week after ischemia, but their performance improved and was close to the performance level of the normal and sham-operated mice.
In the rotarod test, the MCAo mice remained on the rotarod for lesser time than did the sham-operated mice for the first week, but the difference became non-significant between after a week (Fig. 4A).
Fig. 4.
All the behavior tests were performed on day 2, 4, 7, 14, 21, and 28 after the surgery. All data are presented as means±S.E. from 7 and 12 survived animals from the sham-operated and MCAo animals, respectively. (A) The MCAo animals remained on the rotarod for shorter time compared to the sham-operated animals. (B) The MCAo animals showed the biased turning to the right side when reaching the corner, whereas the sham operated animals did not show any directional biasness. (C, D) The latency to turn downward and reach the floor from the top of the pole was increased in the MCAo group compared to the sham group. (E) Data are presented from the survived animals (●) or from total animals (▲) including the 8 dead animals. The maximum score of 5 was given to the dead animals. Either way, MCAo animals showed severe deficits than the sham animals (○). (F) The loss of body weight was higher in the MCAo group compared to the sham (t-test, *p<0.05, **p<0.01).
In corner tests, normal or sham-operated mice displayed similar tendency in right and left turning behavior when the animals reached the corner. However, the MCAo animals showed the right-sided bias in turning back since the animals had the paresis in the left side (Fig. 4B). The laterality index, the indicator of right turning preference, was significantly increased in MCAo animals (p<0.05). Within the span of 28 days the right turning bias in the ischemic group was partially reduced owing to spontaneous improvement in their sensory-motor function on the left side of the body. The sham-operated animals turned both the directions with almost equal probability, over the entire period of behavioral testing.
In the pole test before the surgery, when the mice were placed on the top of the pole facing their head upward, mice in both the sham and MCAo groups could turn their head completely vertically downward within baseline value of 1 second and reached the floor within 4 sec, when they were placed on the top of the pole facing their head upward. However, Tturn, the time to turn the head completely downward was significantly increased after MCAo (Fig. 4C), indicating bradykinesia following ischemic brain injury in the striatum. Similarly, Ttotal, the time taken to reach the floor, was significantly increased after MCAo (Fig. 4D). Although the MCAo mice showed profound improvement in the motor function (bradykinesia) over the time, the sham-operated animals always took lesser time to reach the floor.
The NSS graphs included two data sets from the MCAo mice (Fig. 4E): the first includes data for the dead mice that were assigned the highest score of 5, as shown in Table 1 (closed triangles) and the other only the survived animals (closed circles) after the MCAo. In both cases, the MCAo animals consistently showed higher NSS score compared to the sham group through the testing period. On surviving the first week, the MCAo mice spontaneously recovered their motor functions and thus showed less severity in NSS tests. None of the sham operated animals showed any NSS value during the behavior testing window period (open circle).
After the induction of ischemia, the mice showed significant weight loss, which occurred mostly during the first week after the surgery (Fig. 4F). The sham-operated animals did not loss but continuously gained weight. MCAo animals showed a tendency to catch up the body weight closed to the sham group, probably due to the fact that the animals could freely access to water and mashed food on the floor. The sham-operated animals did not lose their body weight and continuously gained it. The body weights remained significantly lower in the MCAo group compared to the sham-operated group.
DISCUSSION
Intraluminal suture method is a commonly utilized method for inducing experimental stroke models as it mimics the clinical stroke and yields relatively reproducible infarct lesion with consistent behavioral deficits [20, 21, 22]. In this study, we standardized a method for generating a transient intraluminal MCAo model in mice by solving the primary concerns of low survival rates, validation of ischemic injury in practically achievable ways with affordable costs, and lack of reliable behavior assessment. In our mouse MCAo model, initially the post ischemic survivability was as low as 11% at day 7. Most of the dying animals showed the symptoms of pyuria and hypothermia, which are the indication of bacterial infection and sepsis. Post-ischemic treatment with a prophylactic antibiotic reduced the incidence of pyuria and the mortality of the models, yielding 40% survivability (Fig. 2A). The use of a nylon suture with short tip (Cat No. 2312) for 1 h further increased the survivability of animals, allowing 60% survival in the MCAo group at 28 days post-surgery. Because we changed the tip size and occlusion duration concurrently, which of those two parameters is more effective at increasing survivability is unclear. Since the high mortality after MCAo in C57BL/6J mice is known to be associated with the blockage of posterior communicating artery (PcomA) [23], it is likely that a shorter tip that does not interfere with the blood flow through PcomA might have contributed to higher survivability. Despite the variation in MCAo induction parameters, we could not observe the significant differences in the brain infarct area between the groups. Contrary to our findings, earlier studies have shown the variation in infarct volume with the MCAo induction parameters. Compared to vehicle-treated animals the infarct volumes were smaller in the group of animals treated with moxifloxacin, a flouroquinolone antibiotics [6] and by using a nylon suture with a shorter tip length [23] had smaller infarct volumes. This discrepancy in results may be attributed to the small sample size in our study.
Relatively high survivability of 60% in the present study allowed us to detect transient and long-lasting (up to 28 days) sensorimotor impairments following transient focal ischemic injury. The mortality and weight loss are the first indication of extent and severity of ischemic injury. In this study, most of MCAo mice having higher neurological severity score at day 1 were dead before 7 days. MCAo animals significantly lost body weights right after the induction of stroke but tended to gain body weights 7 days after ischemia. This result is in accordance to the previous report [24]. Sham-operated animals did not lose their body weight after surgery.
In this study, we showed that corner test could effectively reveal deficits in sensorimotor functions effectively during first week after stroke induction. However, in the later phase of stroke recovery, the MCAo mice underwent spontaneous recovery and did not show significant differences compared to the sham-operated animals in most behavior tests including the corner test. Ttotal pole test remained most effective for detecting sensorimotor deficits until 21 days after ischemic injury to detect the sensorimotor deficits. Previous studies have shown that the pole test has been effective to detect motor dysfunction after proximal MCAo leading to striatal damage [25, 26]. Pole test was not effective in distal MCAo producing only cortical damage [27]. The accelerating rotarod test effectively assessed motor impairment only for the first week following ischemic injury but became unreliable thereafter; this is in contrast to results from our previous studies on rat ischemic models [28, 29]. Taken together, these results suggest that due to spontaneous recovery as well as motor learning with repetitive trials, as previously reported that rotarod test was not reliable to test motor impairment in a mouse stroke model [10, 27].
NSS test with 0~5 scales is a widely used test to measure neurological deficits in rodent model of stroke [30]. The NSS tests can assess the functional deficits immediately after the induction of ischemia and aid in confirming the induction of ischemic injury. Consistent with previous reports, we also found that the animals undergo spontaneous recovery from sensorimotor deficits as early as day 7. However, NSS tests are reliable tests, which can immediately indicate the severity of ischemic injury and long-lasting effects of MCAo-injury throughout the testing period. Thus, altered behavioral scores and loss of body weights were most prominent up to 7 days after the surgery [31, 32].
One of the advantages of using mouse MCAo model is to study in vivo functions with genetically modified mice. It should be noted that all the behavior test scores may vary depending on the ischemic damages which also can be affected by genetic modification. If a gene which regulates cell death or blood vasculature of the brain is disrupted, the extent of ischemic damages may be also affected. Thus, it is crucial that both the wild type and genetically modified animals receive similar level of injury, which can be verified with reduction of BFI values. ICG with NIR allows minimally invasive analysis of CBF in experimental stroke models as well as in clinical practice [11]. As NIR can penetrate into deeper layer of the cortex, signals emitted from ICG in brain tissues can be captured without opening scalp or skull [11]. Tarrival, Tpeak, Tmax, Trising and BFI values extracted from time-series fluorescence imaging of ICG indicated the similar level of CBF in the right and left hemispheres of the sham-operated animals. The BFI values were significantly decreased in the injured area of the right cerebral hemisphere in MCAo animals.
In summary, we have described a reliable method to induce focal cerebral ischemic injury in mice, with improved survival rate. We have also identified the behavioral tests best suited to assess subsequent functional impairments. To ensure consistency in inducing injury, we have proposed an inexpensive method for measuring CBF during MCAO. We envisage that our model will be amenable to studies in mutant mice, thus enabling investigation of the molecular mechanisms underlying the complex pathophysiology following focal cerebral ischemia and reperfusion injury.
ACKNOWLEDGEMENTS
This study was supported in part by the Bio & Medical Technology Development Program of the Korean National Research Foundation (NRF-2010-0020406 to H.S.-K).
References
- 1.Durukan A, Tatlisumak T. Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol Biochem Behav. 2007;87:179–197. doi: 10.1016/j.pbb.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 2.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 3.Mhairi Macrae I. New models of focal cerebral ischaemia. Br J Clin Pharmacol. 1992;34:302–308. doi: 10.1111/j.1365-2125.1992.tb05634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takizawa S, Hogan M, Hakim AM. The effects of a competitive NMDA receptor antagonist (CGS-19755) on cerebral blood flow and pH in focal ischemia. J Cereb Blood Flow Metab. 1991;11:786–793. doi: 10.1038/jcbfm.1991.136. [DOI] [PubMed] [Google Scholar]
- 5.Huang CY, Fujimura M, Chang YY, Chan PH. Overexpression of copper-zinc superoxide dismutase attenuates acute activation of activator protein-1 after transient focal cerebral ischemia in mice. Stroke. 2001;32:741–747. doi: 10.1161/01.str.32.3.741. [DOI] [PubMed] [Google Scholar]
- 6.Kinouchi H, Epstein CJ, Mizui T, Carlson E, Chen SF, Chan PH. Attenuation of focal cerebral ischemic injury in transgenic mice overexpressing CuZn superoxide dismutase. Proc Natl Acad Sci U S A. 1991;88:11158–11162. doi: 10.1073/pnas.88.24.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meisel C, Prass K, Braun J, Victorov I, Wolf T, Megow D, Halle E, Volk HD, Dirnagl U, Meisel A. Preventive antibacterial treatment improves the general medical and neurological outcome in a mouse model of stroke. Stroke. 2004;35:2–6. doi: 10.1161/01.STR.0000109041.89959.4C. [DOI] [PubMed] [Google Scholar]
- 8.Salat D, Campos M, Montaner J. Advances in the pathophysiology and management of infections in the acute phase of stroke. Med Clin (Barc) 2012;139:681–687. doi: 10.1016/j.medcli.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Davenport RJ, Dennis MS, Wellwood I, Warlow CP. Complications after acute stroke. Stroke. 1996;27:415–420. doi: 10.1161/01.str.27.3.415. [DOI] [PubMed] [Google Scholar]
- 10.Prass K, Meisel C, Höflich C, Braun J, Halle E, Wolf T, Ruscher K, Victorov IV, Priller J, Dirnagl U, Volk HD, Meisel A. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198:725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balkaya M, Kröber JM, Rex A, Endres M. Assessing post-stroke behavior in mouse models of focal ischemia. J Cereb Blood Flow Metab. 2013;33:330–338. doi: 10.1038/jcbfm.2012.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ku T, Choi C. Noninvasive optical measurement of cerebral blood flow in mice using molecular dynamics analysis of indocyanine green. PLoS One. 2012;7:e48383. doi: 10.1371/journal.pone.0048383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin ZJ, Ren M, Li L, Liu Y, Su J, Yang SH, Liu H. Interleaved imaging of cerebral hemodynamics and blood flow index to monitor ischemic stroke and treatment in rat by volumetric diffuse optical tomography. Neuroimage 85. Neuroimage. 2014;85(Pt 1):566–582. doi: 10.1016/j.neuroimage.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner BP, Gertsch S, Ammann RA, Pfenninger J. Reproducibility of the blood flow index as noninvasive, bedside estimation of cerebral blood flow. Intensive Care Med. 2003;29:196–200. doi: 10.1007/s00134-002-1592-z. [DOI] [PubMed] [Google Scholar]
- 15.Terborg C, Bramer S, Harscher S, Simon M, Witte OW. Bedside assessment of cerebral perfusion reductions in patients with acute ischaemic stroke by near-infrared spectroscopy and indocyanine green. J Neurol Neurosurg Psychiatry. 2004;75:38–42. [PMC free article] [PubMed] [Google Scholar]
- 16.Rogers DC, Campbell CA, Stretton JL, Mackay KB. Correlation between motor impairment and infarct volume after permanent and transient middle cerebral artery occlusion in the rat. Stroke. 1997;28:2060–2065. doi: 10.1161/01.str.28.10.2060. [DOI] [PubMed] [Google Scholar]
- 17.Choi CI, Lee YD, Kim H, Kim SH, Suh-Kim H, Kim SS. Neural induction with neurogenin 1 enhances the therapeutic potential of mesenchymal stem cells in an amyotrophic lateral sclerosis mouse model. Cell Transplant. 2013;22:855–870. doi: 10.3727/096368912X637019. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Schallert T, Zhang ZG, Jiang Q, Arniego P, Li Q, Lu M, Chopp M. A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia. J Neurosci Methods. 2002;117:207–214. doi: 10.1016/s0165-0270(02)00114-0. [DOI] [PubMed] [Google Scholar]
- 19.Bouët V, Freret T, Toutain J, Divoux D, Boulouard M, Schumann-Bard P. Sensorimotor and cognitive deficits after transient middle cerebral artery occlusion in the mouse. Exp Neurol. 2007;203:555–567. doi: 10.1016/j.expneurol.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 21.McAuley MA. Rodent models of focal ischemia. Cerebrovasc Brain Metab Rev. 1995;7:153–180. [PubMed] [Google Scholar]
- 22.Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke. 1996;27:1616–1622. doi: 10.1161/01.str.27.9.1616. [DOI] [PubMed] [Google Scholar]
- 23.Belayev L, Busto R, Zhao W, Fernandez G, Ginsberg MD. Middle cerebral artery occlusion in the mouse by intraluminal suture coated with poly-L-lysine: neurological and histological validation. Brain Res. 1999;833:181–190. doi: 10.1016/s0006-8993(99)01528-0. [DOI] [PubMed] [Google Scholar]
- 24.Yuan F, Tang Y, Lin X, Xi Y, Guan Y, Xiao T, Chen J, Zhang Z, Yang GY, Wang Y. Optimizing suture middle cerebral artery occlusion model in C57BL/6 mice circumvents posterior communicating artery dysplasia. J Neurotrauma. 2012;29:1499–1505. doi: 10.1089/neu.2011.2105. [DOI] [PubMed] [Google Scholar]
- 25.Virtanen T, Jolkkonen J, Sivenius J. Re: external carotid artery territory ischemia impairs outcome in the endovascular filament model of middle cerebral artery occlusion in rats. Stroke. 2004;35:e9–e10. doi: 10.1161/01.STR.0000107295.05923.F4. [DOI] [PubMed] [Google Scholar]
- 26.Ji S, Kronenberg G, Balkaya M, Färber K, Gertz K, Kettenmann H, Endres M. Acute neuroprotection by pioglitazone after mild brain ischemia without effect on long-term outcome. Exp Neurol. 2009;216:321–328. doi: 10.1016/j.expneurol.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Royl G, Balkaya M, Lehmann S, Lehnardt S, Stohlmann K, Lindauer U, Endres M, Dirnagl U, Meisel A. Effects of the PDE5-inhibitor vardenafil in a mouse stroke model. Brain Res. 2009;1265:148–157. doi: 10.1016/j.brainres.2009.01.061. [DOI] [PubMed] [Google Scholar]
- 28.Freret T, Bouet V, Leconte C, Roussel S, Chazalviel L, Divoux D, Schumann-Bard P, Boulouard M. Behavioral deficits after distal focal cerebral ischemia in mice: Usefulness of adhesive removal test. Behav Neurosci. 2009;123:224–230. doi: 10.1037/a0014157. [DOI] [PubMed] [Google Scholar]
- 29.Kim SS, Yoo SW, Park TS, Ahn SC, Jeong HS, Kim JW, Chang DY, Cho KG, Kim SU, Huh Y, Lee JE, Lee SY, Lee YD, Suh-Kim H. Neural induction with neurogenin1 increases the therapeutic effects of mesenchymal stem cells in the ischemic brain. Stem Cells. 2008;26:2217–2228. doi: 10.1634/stemcells.2008-0108. [DOI] [PubMed] [Google Scholar]
- 30.Yoo SW, Chang DY, Lee HS, Kim GH, Park JS, Ryu BY, Joe EH, Lee YD, Kim SS, Suh-Kim H. Immune following suppression mesenchymal stem cell transplantation in the ischemic brain is mediated by TGF-beta. Neurobiol Dis. 2013;58:249–257. doi: 10.1016/j.nbd.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Huang Z, Huang PL, Panahian N, Dalkara T, Fishman MC, Moskowitz MA. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science. 1994;265:1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- 32.Witte OW, Bidmon HJ, Schiene K, Redecker C, Hagemann G. Functional differentiation of multiple perilesional zones after focal cerebral ischemia. J Cereb Blood Flow Metab. 2000;20:1149–1165. doi: 10.1097/00004647-200008000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Okada M, Tamura A, Urae A, Nakagomi T, Kirino T, Mine K, Fujiwara M. Long-term spatial cognitive impairment following middle cerebral artery occlusion in rats. A behavioral study. J Cereb Blood Flow Metab. 1995;15:505–512. doi: 10.1038/jcbfm.1995.62. [DOI] [PubMed] [Google Scholar]