Abstract
Amyotrophic lateral sclerosis (ALS) is a devastating progressive neurodegenerative disorder characterized by a selective loss of motor neurons in the spinal cord, brainstem, and motor cortex, leading to weakness of the limb and bulbar muscles. Although the immediate cause of death in ALS is the destruction of motor neurons, ALS is a multi-organ disease that also affects the lungs, spleen, and liver. Melittin is one of components of bee venom and has anti-neuroinflammatory effects in the spinal cord, as shown in an ALS animal model. To investigate the effects of melittin on inflammation in the lungs and spleen, we used hSOD1G93A transgenic mice that are mimic for ALS. Melittin treatment reduced the expression of inflammatory proteins, including Iba-1 and CD14 by 1.9- and 1.3-fold (p<0.05), respectively, in the lungs of symptomatic hSOD1G93A transgenic mice. In the spleen, the expression of CD14 and COX2 that are related to inflammation were decreased by 1.4 fold (p<0.05) and cell survival proteins such as pERK and Bcl2 were increased by 1.3- and 1.5-fold (p<0.05) in the melittin-treated hSOD1G93A transgenic mice. These findings suggest that melittin could be a candidate to regulate the immune system in organs affected by ALS.
Keywords: Amyotrophic lateral sclerosis (ALS), hSOD1G93A, Melittin, inflammation
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is an adult-onset, devastating, and progressive neurodegenerative disorder characterized by a selective loss of motor neurons in the spinal cord, brainstem, and motor cortex, leading to weakness of the limbs and bulbar muscles [1]. The majority of patients with ALS have sporadic ALS (sALS) caused by environmental factors; however, 5~10% inherit a familial ALS (fALS) form, of which 20% are caused by mutations in the genomic insertion of the human copper-zinc superoxide dismutase 1 (hSOD1) gene [2]. Especially, hSOD1G93A mice expressing a hSOD1 transgene with a glycine-to-alanine substitution at the 93rd codon have generally been used for an ALS animal model because of their symptom similarity to ALS in human [3, 4].
A variety of immunological dysfunctions has been demonstrated in some patients and in animal models with ALS [5,6,7,8,9]. ALS immunocytes induce the activation of monocytes and T cell numbers related to disease progression [7, 10]. In addition, Zhang R et al., have shown that an increase of abnormally immunogic activation of sALS was related to the level of monocyte/macrophage activation [7]. It suggested that the inflammation-involved markers might supply valuable support in monitoring the treatment of ALS and immune dysfunction diseases.
T cell numbers and adaptive immune molecules were changed in postmortem ALS and SOD1 Tg mice [11, 12, 13]. Postmortem examinations of ALS neural tissues reveal associations between immune and immune effector changes and exhibit reactive microglia, astrocytes, blood-borne macrophages, mast cells, increased number of dendritic cells, elevated chemoattractant factors, and histocompatibility complex (MHC) class I and II molecules as well as infiltrating CD4+ and CD8+ T lymphocytes surrounding degenerating neurons and the area affected in ALS [13, 14, 15]. The numbers of experimental and clinical observations have shown inflammatory reactions in ALS tissue indicating the involvement of both innate and adaptive immune responses [14, 16, 17]. Gendelman HE et al., have demonstrated that the spleens of SOD1Tg mice at symptomatic ALS display significant reductions in size and weight and profound lymphopenia. In addition, this study suggested that the possibility of immune dysfunction and loss of adaptive immune cells reflect mechanisms for the production of ALS symptoms [18].
Evidences of dysregulation within the peripheral adaptive immune system of patients with ALS reveal many adaptive deficits. Peripheral blood lymphocytes from these patients have been reported to exhibit abnormalities in mitochondrial and calcium metabolism [19]. In our previous study, we demonstrated that inflammation increased in the lungs of symptomatic hSOD1G93A transgenic mice [20].
Melittin is a major component (40~60%) of whole bee venom. It is a small linear peptide composed of 26 amino acids with a hydrophobic N-terminal region and a hydrophilic C-terminal region [21]. Many studies have shown that melittin has an anti-viral, anti-arthritic, and anti-inflammatory effect [22, 23, 24]. Recent studies have shown that melittin induced apoptosis in hepatic and vascular smooth muscle cells through nuclear factor kappa B (NF-κB) inhibition and alpha serine/threonine-protein kinase 1 (AKT) activation [25]. Furthermore, we have shown that melittin treatment improved proteasome function by its anti-neuroinflammatory effects in the brain and spinal cord regions of an animal model of ALS [26]. Based on our understanding of the immune system's involvement in central nervous system maintenance [27], we examined the effects of melittin on the lung and spleen of hSOD1G93A transgenic mice. We found that melittin treatment attenuated inflammation and stimulated the signal leading to cell survival in the lung and spleen in these mice. These findings suggested that melittin treatment could help increasing immunity in an ALS animal model.
MATERIALS AND METHODS
Animals
All mice were treated in accordance with the United States National Institutes of Health guidelines (Bethesda, MD). Experimental procedures were approved by the Institutional Animal Care and Use Committees of the Korea Institute of Oriental Medicine. In this study, hemizygous transgenic B6SJL mice carrying a glycine-to-alanine mutation at the 93rd codon of the cytosolic Cu/Zn superoxide dismutase gene (hSOD1G93A) were originally obtained from the Jackson Laboratory (Bar Harbor, ME). Transgenic mice were identified by polymerase chain reaction as described previously [2]. All mice were kept in standard housing with free access to water and standard rodent chow.
Melittin treatment
Melittin was purchased from Sigma (St. Louis, MO) and diluted with saline. Melittin was subcutaneously injected at a dose of 0.1 µg/g bilaterally into 14-week-old hSOD1G93A transgenic mice (hSOD1G93A-MT, n=4~5) at the Joksamni (ST36) acupoint, which is known to mediate anti-inflammatory effects [28]. Melittin treatment was performed three times for a week at a dose of 0.1 µg/g. According to the human acupoint landmark and a mouse anatomical reference [29], the ST36 point is anatomically located 5 mm below and lateral to the anterior tubercle of the tibia. Age-matched control animals were injected bilaterally and subcutaneously with an equal volume of saline at the ST36 acupoint (hSOD1G93A, n=4~5).
Western blot analysis
The lungs and spleens of melittin- or saline-treated hSOD1G93A transgenic mice were dissected and homogenized in RIPA buffer (50 mM Tris-Cl pH 7.4, 1% NP-40, 0.1% sodium dodecyl sulfate (SDS), and 150 mM NaCl) containing a protease inhibitor cocktail (Calbiochem, CA, USA). Homogenized tissues were centrifuged at 14,000 rpm for 20 min at 4℃. Total protein was quantified using the BCA assay kit (Pierce, IL, USA). Samples denatured with SDS sampling buffer were separated through SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane for Western blot. For the detection of target proteins, the membranes were blocked with 5% non-fat milk in tris-buffered saline (TBS) and then incubated with various primary antibodies: anti-tubulin (Abcam, Cambridge, UK), anti-phospho-ERK (Cell Signaling Technologies), anti-Bcl2 (SantaCruz, CA), anti-HO1 (Abcam, Cambridge, UK), anti-Iba-1 (Wako, Osaka, Japan), anti-CD14 (BD Pharmingen), anti-COX2 (BD Biosciences), and anti-TNF-α (Abcam, Cambridge, UK). The blots were then probed with HRP-conjugated antibodies (Santa Cruz Biotechnology, CA, USA) and visualized by using enhanced chemiluminescence (ECL) reagents (Amersham Pharmacia, NJ, USA). An LAS-3000 image analyzer was used for detecting immunoblotted bands (Fujifilm, Tokyo, Japan).
Statistical analysis
All data were analyzed using GraphPad Prism 5.0 (GraphPad Software, CA, USA) and are presented as the mean±standard error of mean (SEM) where indicated. A t-test was used to compare the significance of the differences of the immunoblotting data between the melittin-treated and age-matched control hSOD1G93A mice.
RESULTS
Melittin treatment reduces the expression level of inflammatory proteins in the lungs of hSOD1G93A mice
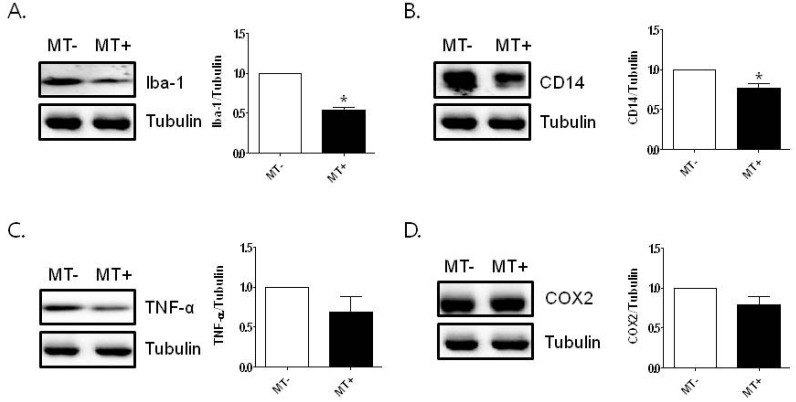
To examine the effect of melittin treatment on inflammation in the lungs of hSOD1G93A mice, we investigated the expression level of inflammatory proteins by Western blotting. As shown in Fig. 1A and B, Iba-1 and CD14 expressions, associated with inflammation, were significantly decreased 1.9- and 1.3- fold, respectively, compared to age-matched hSOD1G93A transgenic mice (*p<0.05, n=4~5/each group; Fig. 1A). To confirm the effect of melittin on inflammation in the lungs, we examined the level of pro-inflammatory proteins using anti-TNF-α and anti-COX2. Melittin treatment reduced the level of TNF-α and COX2 proteins, although the reduction was not significant (Fig. 1C, D).
Fig. 1.
The effect of melittin on inflammation in the lungs of hSOD1G93A mice. Melittin (0.1 µg/g) was injected subcutaneously bilaterally three times for a week in 14-week-old hSOD1G93A transgenic mice. Representative data from independent Western blot experiments of Iba-1 (A) and CD14 (B) in the lung of hSOD1G93A transgenic mice (n=4~5 per group). Immunoblotting of TNF-α (C) and COX2 (D) proteins from the lungs of melittin- or saline-treated hSOD1G93A transgenic mice. Tubulin is used as a loading control of total protein. Data are shown as the mean±SEM. Data were analyzed with PRISM analysis and statistical significance was calculated by t-test. *p<0.05, compared to saline-treated hSOD1G93A transgenic mice. MT-: saline-treated hSOD1G93A mice, MT+: melittin-treated hSOD1G93A mice.
Melittin treatment reduces the expression level of inflammatory proteins and increases the level of cell survival-related proteins in the spleen of hSOD1G93A mice
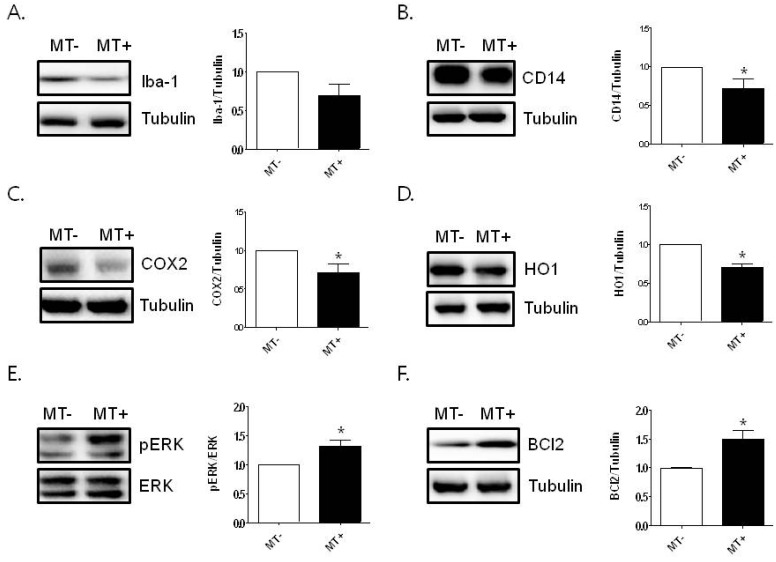
The spleen plays a role in the immune system by attacking foreign toxins with macrophages. Therefore, we investigated the anti-inflammatory effects of melittin in the spleen of hSOD1G93A transgenic mice. We found that the expression of Iba-1 protein was decreased by melittin treatment in the spleen, compared to that of saline-treated hSOD1G93A transgenic mice; however, the decrease was not significant (Fig. 2A). To confirm whether melittin reduces inflammation in the spleen, we analyzed the expression level of inflammatory proteins. As shown in Fig. 2B and C, inflammation-related proteins, CD14 and COX-2, were significantly decreased by each 1.4-fold by 0.1 µg/g melittin treatment in hSOD1G93A mice compared to age-matched control (*p<0.05, n=4-5/each group). These findings suggest that melittin attenuates inflammation in the lungs and spleen of hSOD1G93A mice. Next, we examined the anti-inflammatory effects of melittin on cell-survival signaling in the organs of hSOD1G93A mice. As shown in Fig. 2D, melittin attenuated the expression level of HO1 induced by oxidative stress in the spleen of hSOD1G93A transgenic mice. Furthermore, we found that the expression level of pERK and Bcl2 proteins related to cell survival significantly increased, by 1.3- and 1.5-fold, respectively, compared to those in age-matched control mice (Fig. 2E, F). These findings suggest that melittin could be one of candidates increasing immunity in ALS.
Fig. 2.
The effect of melittin on cell survival of the spleen of hSOD1G93A mice. Representative data from independent experiments of Western blotting of Iba-1 (A) and CD14 (B) in the spleen of hSOD1G93A transgenic mice (n=4~5 per group). COX2 (C) and HO1 (D) proteins levels are determined by immunoblotting in the spleen of melittin- or saline-treated hSOD1G93A transgenic mice. The expression level of pERK (E) and Bcl2 (F) leading to cell survival is increased by melittin treatment in the spleen of hSOD1G93A mice compared to saline-treated hSOD1G93A mice. Tubulin is used as a loading control for protein. Data are shown as the mean±SEM. *p<0.05 compared to saline-treated hSOD1G93A transgenic mice.
DISCUSSION
ALS is characterized by the death of motor neurons in the brainstem and spinal cord, leading to respiratory failure. ALS is caused by both environmental and genetic factors, with 90-95% sporadic ALS (sALS) showing no clear genetic linkage. Familial ALS (fALS) causing genetic factors includes superoxide dismutase 1 (SOD1), TAR DNA-Binding Protein 43 (TDP-43), C9ORF72, and ubiquilin-2 gene (UBQLN-2). Inflammation plays an important role in recovering against infection and restoring health and normal functioning of the body. Although ALS is not an inflammatory or immune-mediated disease, and anti-inflammatory agents are not effective for patients with ALS in clinical trials, both patients with ALS and animal models of ALS have shown inflammatory responses [14].
The various components of immune reactions such as the innate and adaptive immune systems act in concert to maintain the functional integrity of the body and brain under non-pathological conditions [30]. In patients with ALS, there is persuasive evidence that immunity is involved in promoting disease progression even though the pathogenesis of ALS is still unclear. In addition, complementary factors C1q and C3 mRNA were upregulated with the progression of the pathology in hSOD1G93A mice [31]. Recent study has demonstrated that the activation of classical (C1qB and C4) and alternate (factor B) factors in the complementary system expressed on motor neurons and microglia were increased in hSOD1G93A mice [32]. It suggests that complementary activation detected in the motor neurons may cause a decrease in immunity as disease progresses in hSOD1G93A mice.
Melittin is a major component composed of 26 amino acids and is a biologically active substance in bee venom. In addition, it has anti-apoptotic, anti-inflammatory, anti-viral, and anti-bacterial effects [22, 33]. Furthermore, we have shown that melittin reduced neuroinflammation in the spinal cord of symptomatic hSOD1G93A transgenic mice [26]. However, there have been no reports on the effects of melittin in the organs of in vivo ALS animal models, or on the associated molecular mechanisms of melittin. Based on these findings, we hypothesized that melittin affects inflammation in the lungs and spleen of symptomatic hSOD1G93A transgenic mice. We found that melittin treatment reduced inflammatory proteins such as TNF-α, CD14, and COX2 in the lungs and spleen and increased the expression level of proteins related to cell survival signaling such as Bcl2 and pERK in the spleen of symptomatic hSOD1G93A transgenic mice.
The spleen, thymus, and liver, all shrink in size during ALS disease progression. However, the change in lymphocyte proportions in these organs does not necessarily reflect a change in their absolute number. CD4+CD25+ T regulatory cells, CD8+ regulatory cells, and natural killer T (NKT) cells are all involved in the immune system [34, 35, 36]. NKT cells are elevated in the lymphoid organs and central nervous system of mSOD1 mice. In addition, NKT cells are dramatically increased in the liver and spinal cord of hSOD1G93A transgenic mice [37].
Compared to age-matched wild-type mice, in ALS mice, we previously observed defects in the lungs and increased inflammatory proteins such as TNF-α, Iba-1, and IL-6 via immunoblotting and immunostaining [20]. In this study, melittin treatment reduced the expression level of TNF-α and COX2 in the lung of hSOD1G93A transgenic mice, even though it was not significant (Fig. 1A, B). Furthermore, we found that the expression level of Iba-1 and CD14 proteins was decreased by 1.9- and 1.3-fold respectively in the lungs of symptomatic hSOD1G93A animal (Fig. 1A, B). This suggests that melittin could be helpful for the reduction of inflammation in the lungs of hSOD1G93A transgenic mice.
Respiratory insufficiency is a common feature of ALS and is present in almost all cases at some stage of the illness. It is the commonest cause of death in ALS. There are multiple causes of respiratory muscle failure, all of which act to produce a progressive decline in pulmonary function. Respiratory complications in patients with ALS often require invasive ventilation because of increased difficulty in clearing secretions by cough. Melittin could be helpful for avoiding invasive ventilation in some patients with ALS.
Immunity in ALS involves the function of the spleen in innate immune reactions. Previous papers have shown that the spleens of SOD1Tg mice at the end stage of ALS display significant reductions in size and weight and profound lymphopenia. In addition, this study suggested that the possibility of immune dysfunction and loss of adaptive immune cells reflect mechanisms for the production of ALS symptoms [18]. In this study, we showed that melittin treatment reduced the expression level of inflammatory proteins CD14 and COX2 by 1.4 fold in the spleen compared to age-matched saline-treated mice (Fig. 2C, D). Furthermore, we found that pERK and Bcl2 proteins, which are related to cell survival, were significantly increased (1.3- and 1.5- fold, respectively) in the melittin-treated hSOD1G93A mice (Fig. 2E, F). In addition, we observed an increase of the expression level of phospho-Akt in the spleen of melittin-treated mice compared to saline-treated hSOD1G93A transgenic mice but it was not significant. These findings suggest that melittin may be a biologically effective substance that can reduce inflammation and increase cell survival in the organs of an ALS animal model.
In future, it should be investigated whether melittin affects T cells, lymphoid cells, and complementary factors in hSOD1G93A transgenic mice in order to demonstrate its anti-inflammatory effects. In addition, it needs to be investigated whether the effects of melittin on other organs such as the liver and thymus are able to demonstrate anti-inflammatory effects of melittin since hSOD1G93A overexpression in the mice causes organ disability. Furthermore, the molecular mechanism of melittin action should be investigated to analyze its anti-inflammatory effects in the organs of an ALS animal model.
Neurodegenerative diseases, including Alzheimer's disease (AD) and Parkinson's disease (PD), are caused by neuroinflammation associated with dysregulation of immune homeostasis by the combined action of a series of defects arising in the body. Therefore, it will be worth investigating the effects of melittin on neurodegenerative diseases.
ACKNOWLEDGEMENTS
We would like to thank Dr. Kang-Woo Lee for kind discussion, and Mu-Dan Chai for animal maintenance. This research was supported by grants (K12010) from the Korea Institute of Oriental Medicine (KIOM).
References
- 1.Cleveland DW, Rothstein JD. From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci. 2001;2:806–819. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- 2.Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng HX, Rahmani Z, Krizus A, McKenna-Yasek D, Cayabyab A, Gaston SM, Berger R, Tanzi RE, Halperin JJ, Herzfeldt B, van den Bergh R, Hung WY, Bird T, Deng G, Mulder DW, Smyth C, Laing NG, Soriano E, Pericak-Vance MA, Haines J, Rouleau GA, Gusella JS, Horvitz HR, Brown RH., Jr Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 3.Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, Chen W, Zhai P, Sufit RL, Siddique T. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 4.Bendotti C, Atzori C, Piva R, Tortarolo M, Strong MJ, DeBiasi S, Migheli A. Activated p38MAPK is a novel component of the intracellular inclusions found in human amyotrophic lateral sclerosis and mutant SOD1 transgenic mice. J Neuropathol Exp Neurol. 2004;63:113–119. doi: 10.1093/jnen/63.2.113. [DOI] [PubMed] [Google Scholar]
- 5.Provinciali L, Laurenzi MA, Vesprini L, Giovagnoli AR, Bartocci C, Montroni M, Bagnarelli P, Clementi M, Varaldo PE. Immunity assessment in the early stages of amyotrophic lateral sclerosis: a study of virus antibodies and lymphocyte subsets. Acta Neurol Scand. 1988;78:449–454. doi: 10.1111/j.1600-0404.1988.tb03686.x. [DOI] [PubMed] [Google Scholar]
- 6.Bartfeld H, Dham C, Donnenfeld H, Jashnani L, Carp R, Kascsak R, Vilcek J, Rapport M, Wallenstein S. Immunological profile of amyotrophic lateral sclerosis patients and their cell-mediated immune responses to viral and CNS antigens. Clin Exp Immunol. 1982;48:137–146. [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang R, Gascon R, Miller RG, Gelinas DF, Mass J, Hadlock K, Jin X, Reis J, Narvaez A, McGrath MS. Evidence for systemic immune system alterations in sporadic amyotrophic lateral sclerosis (sALS) J Neuroimmunol. 2005;159:215–224. doi: 10.1016/j.jneuroim.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Boillée S, Vande Velde C, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 9.Lobsiger CS, Cleveland DW. Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nat Neurosci. 2007;10:1355–1360. doi: 10.1038/nn1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang R, Gascon R, Miller RG, Gelinas DF, Mass J, Lancero M, Narvaez A, McGrath MS. MCP-1 chemokine receptor CCR2 is decreased on circulating monocytes in sporadic amyotrophic lateral sclerosis (sALS) J Neuroimmunol. 2006;179:87–93. doi: 10.1016/j.jneuroim.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Alexianu ME, Kozovska M, Appel SH. Immune reactivity in a mouse model of familial ALS correlates with disease progression. Neurology. 2001;57:1282–1289. doi: 10.1212/wnl.57.7.1282. [DOI] [PubMed] [Google Scholar]
- 12.Kawamata T, Akiyama H, Yamada T, McGeer PL. Immunologic reactions in amyotrophic lateral sclerosis brain and spinal cord tissue. Am J Pathol. 1992;140:691–707. [PMC free article] [PubMed] [Google Scholar]
- 13.Troost D, Van den Oord JJ, Vianney de Jong JM. Immunohistochemical characterization of the inflammatory infiltrate in amyotrophic lateral sclerosis. Neuropathol Appl Neurobiol. 1990;16:401–410. doi: 10.1111/j.1365-2990.1990.tb01276.x. [DOI] [PubMed] [Google Scholar]
- 14.McGeer PL, McGeer EG. Inflammatory processes in amyotrophic lateral sclerosis. Muscle Nerve. 2002;26:459–470. doi: 10.1002/mus.10191. [DOI] [PubMed] [Google Scholar]
- 15.Graves MC, Fiala M, Dinglasan LA, Liu NQ, Sayre J, Chiappelli F, van Kooten C, Vinters HV. Inflammation in amyotrophic lateral sclerosis spinal cord and brain is mediated by activated macrophages, mast cells and T cells. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004;5:213–219. doi: 10.1080/14660820410020286. [DOI] [PubMed] [Google Scholar]
- 16.Moisse K, Strong MJ. Innate immunity in amyotrophic lateral sclerosis. Biochim Biophys Acta. 2006;1762:1083–1093. doi: 10.1016/j.bbadis.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Weydt P, Weiss MD, Möller T, Carter GT. Neuro-inflammation as a therapeutic target in amyotrophic lateral sclerosis. Curr Opin Investig Drugs. 2002;3:1720–1724. [PubMed] [Google Scholar]
- 18.Banerjee R, Mosley RL, Reynolds AD, Dhar A, Jackson-Lewis V, Gordon PH, Przedborski S, Gendelman HE. Adaptive immune neuroprotection in G93A-SOD1 amyotrophic lateral sclerosis mice. PLoS One. 2008;3:e2740. doi: 10.1371/journal.pone.0002740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curti D, Malaspina A, Facchetti G, Camana C, Mazzini L, Tosca P, Zerbi F, Ceroni M. Amyotrophic lateral sclerosis: oxidative energy metabolism and calcium homeostasis in peripheral blood lymphocytes. Neurology. 1996;47:1060–1064. doi: 10.1212/wnl.47.4.1060. [DOI] [PubMed] [Google Scholar]
- 20.Jiang JH, Yang EJ, Baek MG, Kim SH, Lee SM, Choi SM. Anti-inflammatory effects of electroacupuncture in the respiratory system of a symptomatic amyotrophic lateral sclerosis animal model. Neurodegener Dis. 2011;8:504–514. doi: 10.1159/000327911. [DOI] [PubMed] [Google Scholar]
- 21.Habermann E. Bee and wasp venoms. Science. 1972;177:314–322. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- 22.Raghuraman H, Chattopadhyay A. Melittin: a membrane-active peptide with diverse functions. Biosci Rep. 2007;27:189–223. doi: 10.1007/s10540-006-9030-z. [DOI] [PubMed] [Google Scholar]
- 23.Park HJ, Lee SH, Son DJ, Oh KW, Kim KH, Song HS, Kim GJ, Oh GT, Yoon DY, Hong JT. Antiarthritic effect of bee venom: inhibition of inflammation mediator generation by suppression of NF-kappaB through interaction with the p50 subunit. Arthritis Rheum. 2004;50:3504–3515. doi: 10.1002/art.20626. [DOI] [PubMed] [Google Scholar]
- 24.Park HJ, Lee HJ, Choi MS, Son DJ, Song HS, Song MJ, Lee JM, Han SB, Kim Y, Hong JT. JNK pathway is involved in the inhibition of inflammatory target gene expression and NF-κB activation by melittin. J Inflamm (Lond) 2008;5:7. doi: 10.1186/1476-9255-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Son DJ, Ha SJ, Song HS, Lim Y, Yun YP, Lee JW, Moon DC, Park YH, Park BS, Song MJ, Hong JT. Melittin inhibits vascular smooth muscle cell proliferation through induction of apoptosis via suppression of nuclear factor-kappaB and Akt activation and enhancement of apoptotic protein expression. J Pharmacol Exp Ther. 2006;317:627–634. doi: 10.1124/jpet.105.095901. [DOI] [PubMed] [Google Scholar]
- 26.Yang EJ, Kim SH, Yang SC, Lee SM, Choi SM. Melittin restores proteasome function in an animal model of ALS. J Neuroinflammation. 2011;8:69. doi: 10.1186/1742-2094-8-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz M, Ziv Y. Immunity to self and self-maintenance: a unified theory of brain pathologies. Trends Immunol. 2008;29:211–219. doi: 10.1016/j.it.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Wilms H, Zecca L, Rosenstiel P, Sievers J, Deuschl G, Lucius R. Inflammation in Parkinson's diseases and other neurodegenerative diseases: cause and therapeutic implications. Curr Pharm Des. 2007;13:1925–1928. doi: 10.2174/138161207780858429. [DOI] [PubMed] [Google Scholar]
- 29.Yin CS, Jeong HS, Park HJ, Baik Y, Yoon MH, Choi CB, Koh HG. A proposed transpositional acupoint system in a mouse and rat model. Res Vet Sci. 2008;84:159–165. doi: 10.1016/j.rvsc.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- 31.Lee JD, Lee JY, Taylor SM, Noakes PG, Woodruff TM. Innate immunity in ALS. In: Maurer MH, editor. Amyotrophic lateral sclerosis. Rijeka: InTech; 2012. pp. 393–412. [Google Scholar]
- 32.Lee JD, Kamaruzaman NA, Fung JN, Taylor SM, Turner BJ, Atkin JD, Woodruff TM, Noakes PG. Dysregulation of the complement cascade in the hSOD1G93A transgenic mouse model of amyotrophic lateral sclerosis. J Neuroinflammation. 2013;10:119. doi: 10.1186/1742-2094-10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang C, Chen T, Zhang N, Yang M, Li B, Lü X, Cao X, Ling C. Melittin, a major component of bee venom, sensitizes human hepatocellular carcinoma cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by activating CaMKII-TAK1-JNK/p38 and inhibiting IkappaBalpha kinase-NFkappaB. J Biol Chem. 2009;284:3804–3813. doi: 10.1074/jbc.M807191200. [DOI] [PubMed] [Google Scholar]
- 34.La Cava A, Van Kaer L, Fu-Dong-Shi CD4+CD25+ Tregs and NKT cells: regulators regulating regulators. Trends Immunol. 2006;27:322–327. doi: 10.1016/j.it.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Pomié C, Ménager-Marcq I, van Meerwijk JP. Murine CD8+ regulatory T lymphocytes: the new era. Hum Immunol. 2008;69:708–714. doi: 10.1016/j.humimm.2008.08.288. [DOI] [PubMed] [Google Scholar]
- 36.Hammond KJ, Kronenberg M. Natural killer T cells: natural or unnatural regulators of autoimmunity? Curr Opin Immunol. 2003;15:683–689. doi: 10.1016/j.coi.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 37.Finkelstein A, Kunis G, Seksenyan A, Ronen A, Berkutzki T, Azoulay D, Koronyo-Hamaoui M, Schwartz M. Abnormal changes in NKT cells, the IGF-1 axis, and liver pathology in an animal model of ALS. PLoS One. 2011;6:e22374. doi: 10.1371/journal.pone.0022374. [DOI] [PMC free article] [PubMed] [Google Scholar]