Abstract
Glutathione (GSH) protects cells against oxidative stress by playing an antioxidant role. Protecting brain endothelial cells under oxidative stress is key to treating cerebrovascular diseases and neurodegenerative diseases including Alzheimer's disease and Huntington's disease. In present study, we investigated the protective effect of GSH on brain endothelial cells against hydrogen peroxide (H2O2). We showed that GSH attenuates H2O2-induced production of nitric oxide (NO), reactive oxygen species (ROS), and 8-Oxo-2'-deoxyguanosine (8-OHdG), an oxidized form of deoxiguanosine. GSH also prevents H2O2-induced reduction of tight junction proteins. Finally, GSH increases the level of nuclear factor erythroid 2-related factor 2 (Nrf2) and activates Nrf2-mediated signaling pathways. Thus, GSH is a promising target to protect brain endothelial cells in conditions of brain injury and disease.
Keywords: glutathione (GSH), murine brain endothelial cells (bEnd.3 cells), apoptosis, hydrogen peroxide (H2O2), Reactive oxygen species (ROS), nuclear factor erythroid 2-related factor 2 (Nrf2)
INTRODUCTION
Glutathione (GSH) is a ubiquitous thiol-containing tripeptide that plays a cellular protective role under oxidative stress [1]. GSH modulates the response of a cell to redox changes by regulating antioxidant gene expression [2, 3]. Oxidative stress contributes to the progression of neurodegenerative diseases [4] and stroke [5]. Several studies demonstrated that GSH prevents the apoptotic death of endothelial cells in response to oxidative stress [6, 7]. The GSH-dependent antioxidant pathway plays a role in cell survival [8, 9], and its dysregulation contributes to the initiation and progression of the neurodegenerative diseases including dementia and Huntington's disease [10, 11, 12]. The blood brain barrier (BBB) is a barrier formed by endothelial cells [13], which protects against the entry of pathogens and neurotoxic agents into the brain [14]. Disruption of the BBB, by degradation of tight junction proteins, leads to cell death, brain edema and hemorrhage [15]. Nuclear factor erythroid 2-related factor 2 (Nrf2), a leucine zipper redox-sensitive transcription factor, is a key regulator of antioxidant and detoxification gene expression [16, 17, 18]. Under oxidative stress, Nrf2 translocates from the cytoplasm to the nucleus and subsequently activates the transcription of antioxidant genes whose promoters contain the antioxidant response element (ARE) [19, 20, 21]. Evidence indicates that Nrf2 promotes cell survival by preventing an increase in ROS [22, 23] in various conditions of oxidative stress [24, 25]. In present study, we investigated whether GSH ameliorates oxidative stress-induced damages of brain endothelial cells. We show that GSH prevents the decrease of tight junction proteins, protects BBB, and activates the Nrf2 pathway. Therefore, our results suggest that GSH is a promising therapeutic target to protect BBB in central nervous system injury and diseases.
MATERIALS AND METHODS
Cell culture
Murine brain endothelial cells (bEnd.3 cells, Manassas, VA, USA) were purchased from ATCC and cultured in Dulbecco's modified Eagle's medium (DMEM, Hyclone Laboratories, UT, USA), supplemented with 10% (v/v) fetal bovine serum (FBS, Hyclone Laboratories, UT, USA) and 100 units/ml of penicillin/streptomycin (Hyclone Laboratories, UT, USA), at 37℃ in a humidified atmosphere in the presence of 5% CO2. Culture medium was changed every 2 days [26].
Drug treatment
GSH (Sigma Aldrich, MO, USA) was melted with PBS. H2O2 (Invitrogen, CA, USA) was diluted with PBS. Cultured bEnd.3 cells were divided into six groups as follows: (1) Control group, cultured in completed media, (2) H2O2 (500 µM) group, cultured in completed media with H2O2 (500 µM) for 24 h, (3) GSH (1 mM) group, cultured in completed media with GSH (1 mM) for 24 h, (4) GSH (10 mM) group, cultured in completed media with GSH (10 mM) for 24 h, (5) H2O2 (500 µM) +GSH (1 mM) group, cultured in completed media with H2O2 (500 µM) and GSH (1 mM) for 24 h, (6) H2O2 (500 µM) +GSH (10 mM) group, cultured in completed media with H2O2 (500 µM) and GSH (10 mM) for 24 hr.
Lactate dehydrogenase (LDH) assay
H2O2-induced cytotoxicity was quantified by measuring the amounts of LDH released into the culture medium from H2O2-injured cells [27, 28]. LDH release (cytotoxicity %) was calculated by dividing the value at the experimental time point by the maximum value. The maximum LDH release was measured after freezing each culture at -70℃ overnight, followed by rapid thawing, which induced nearly complete cell damage.
Measurement of nitrite production
Nitrite production was determined using the Griess reaction [28]. Duplicate 100 µl aliquots of culture media collected from each culture were added to a 96-well plate and mixed with 100 µl modified Griess reagent (Sigma Aldrich, MO, USA). The plate was incubated in the dark for 15 min at room temperature. The absorbance of the reaction product was measured at 540 nm using a microplate reader.
Determination of intracellular ROS
The level of the intracellular ROS in all groups was measured using a fluorescent probe, 2', 7'-dichlorodihydrofluorescein diacetate (DCF-DA; Invitrogen, CA, USA) as previously described [29]. The 1×106 cells/ml were seeded in the plate and were treated with H2O2 or/and GSH for 24 h. Then, b END.3 cells were treated with 5 µM DCF-DA for 30 min at 37℃, and after washing with PBS, the fluorescence was measured in a microscope (Nikon TS100-F ECLIPSE) equipped with a CCD camera (Hamamatsu Photonics, Shizuoka, Japan) [30].
Western blot analyses
Equal amounts of protein (50 µg) were extracted from bEND.3 cells. They were electrophoresed on 10%~12% SDS-polyacrylamide gels. Separated proteins were electrotransferred to Immunobilon-NC membranes (Millipore). Membranes were blocked for 1 hour at room temperature with 5% skim milk in Tris-buffered saline and 0.1% Tween-20 (TBST). The primary antibodies used were Nrf2 (1:2000, Millipore, MA, USA), extracellular-regulated protein kinases (ERK) (1:1000, Millipore, MA, USA), p-ERK (1:2000, Millipore, MA, USA) and β-actin (1:1000, Santa Cruz, CA, USA). Blots were incubated with the primary antibodies overnight at 4℃. Membranes were washed three times (5 min each) with TBST. The secondary antibodies were anti-rabbit and anti-mouse (1:3000, New England Biolabs, CA, USA) and were incubated for 1 hour at room temperature. After washing with TBST (0.05% Tween 20) three times, immunoreactive signals were detected using chemiluminescence and an ECL detection system (Amersham Life Science, UK) with the LAS 4000 program.
Immunocytochemistry (ICC)
The expression of 8-Oxo-2'-deoxyguanosine (8-OHdG) and Claudin 5 in bEND.3 cells was confirmed by immunocytochemistry. All the experimental groups was washed 3 times with PBS, fixed with 4% paraformaldehyde for 3 hours, and then washed with PBS. bEND.3 cells were permeabilized with 0.025% Triton X-100 and were blocked for 1 hour at room temperature with dilution buffer (Invitrogen, CA, USA). Primary antibody anti-rabbit 8-OHdG (1:500, Santa Cruz, CA, USA), anti-rabbit Claudin 5 (1:500, Millipore, MA, USA) prepared in the dilution buffer was added to the samples and incubated for 3 hours at room temperature. Primary antibody was removed and cells were washed 3 times for 3 min each with PBS. Later samples were incubated with FITC-conjugated goat anti rabbit second antibodies (1:200, Jackson Immunoresearch, PA, USA) for 2 hours at room temperature. Cells were washed again 3 times for 3 min each with PBS and stained with 1 µg/ml 4',6-diamidino-2-phenylindole (DAPI) (1:100, Invitrogen, CA, USA) for 10 minutes at room temperature. The fixed samples were imaged using Zeiss LSM 700 confocal microscope (Carl Zeiss, NY, USA).
Statistical analyses
Statistical comparisons were performed using independent t-tests for two groups. SPSS software was used for all analyses. Data were expressed as the mean±S.E.M of 3 independent experiments. Differences were considered significant at #p<0.1, *p<0.05, and **p<0.001.
RESULTS
GSH suppresses H2O2-induced cell death
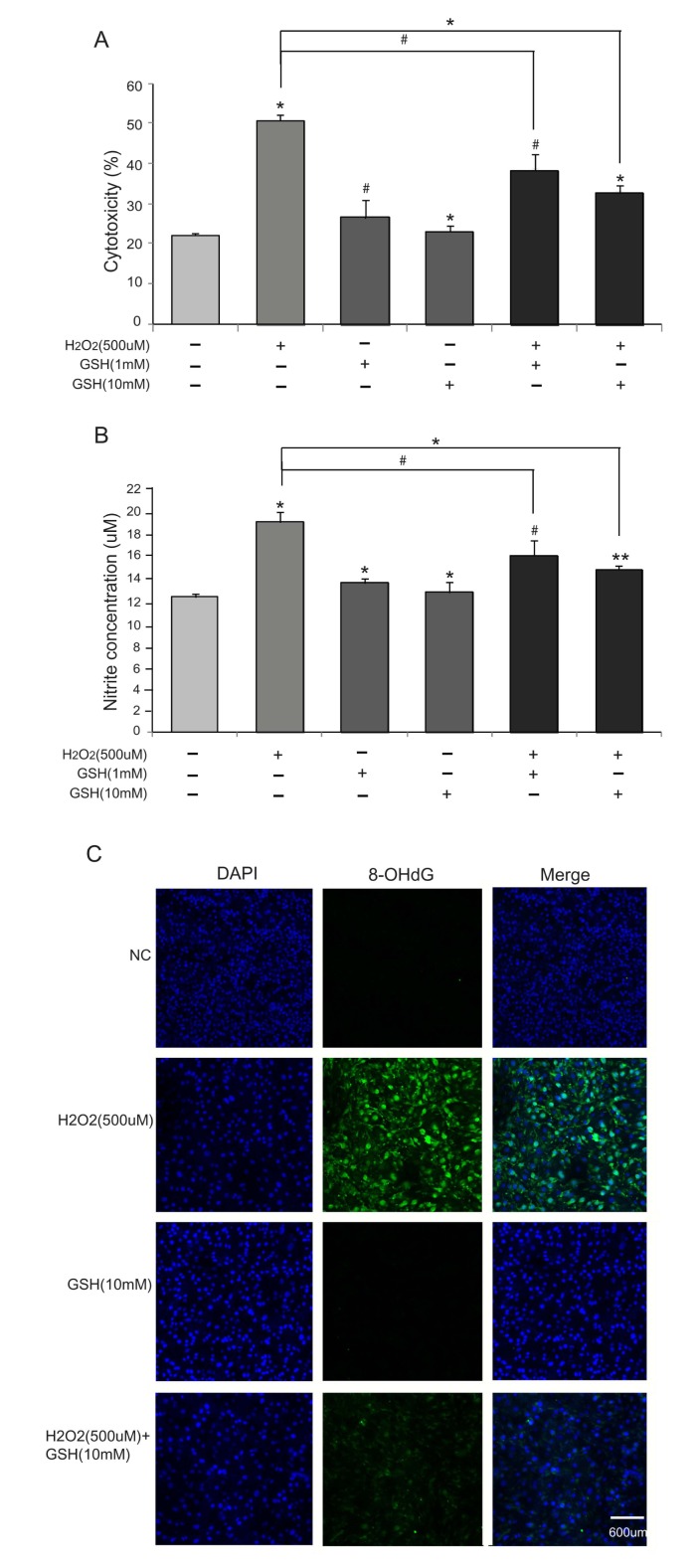
We first evaluated the cytotoxicity of H2O2 to bEND.3 cells using LDH assays. Treating cells with 500 µM H2O2 resulted in 50% cytotoxicity, which were partially rescued by co-treatment of GSH (1 mM and 10 mM) (Fig. 1A). GSH alone (1 mM and 10 mM) did not affect the rate of cell death. The protective effect of GSH was dose-dependent (32% cytotoxicity in 10 mM, 39% cytotoxicity in 1mM) confirming the specificity. We also measured nitrite levels using Griess reagent to confirm the production of nitric oxide in bEND.3 cells (Fig. 1B). The increase in nitrites in response to the treatment of 500 µM H2O2 was partially prevented by 10 mM GSH (12 µM in control, 19 µM in H2O2 only, 14 µM in H2O2+GSH 10 mM). GSH treatment alone did not change nitrite levels (Fig. 1B). The protective role of GSH was dose-dependent (Fig. 1A, B). As 10 mM GSH treatment alone promoted more cell survival and decreased more nitrite concentration (Fig. 1A, B), we decided to use 10 mM GSH in all the following experiments. Finally, we visualized 8-OHdG by immunocytochemistry to measure oxidative damages to DNA. 8-OHdG level increased in H2O2 (500 µM)-treated cells indicating DNA damages. 8-OHdG-positive cells were decreased by co-treatment of GSH (10 mM) (Fig. 1C). Taken together, these results suggest that GSH attenuates H2O2-induced damages in bEND.3 cells.
Fig. 1.
The effect of GSH H2O2-induced cell death. bEDN.3 cells were treated with H2O2 (500 µM) and/or GSH (1 mM or 10 mM) for 24 hr. (A) Cytotoxicity was determined by the release of LDH into the culture media. GSH reduced H2O2's cytotoxicity. (B) H2O2-induced nitrite production was measured by using Griess reagent. GSH reduced H2O2-induced nitrite production. Data are expressed as mean±S.E.M. (#p < 0.1, *p < 0.05, **p < 0.001). (C) 8-OHdG levels were measured by immunocytochemistry. 8-OHdG-positive cells (green color) were increased in the H2O2 (500 µM) treatment group compared to the normal control (NC) group. GSH decreased 8-OHdG levels in bEND.3 cells under H2O2-induced oxidative stress. Scale bar: 600 µm, 8-Oxo-2'-deoxyguanosine (8-OHdG): green, 4', 6-diamidino-2-phenylindole (DAPI): blue.
GSH decreases H2O2-induced ROS production
We measured ROS levels using DCF-DA reagent, a fluorescent dye that visualizes ROS. DCF-DA-positive cells were increased by H2O2 treatment (500 µM), and these were partially blocked by co-treatment of GSH (10 mM) (Fig. 2). Co-treatment of GSH (10 mM) evidently were decreased DCF-DA-positive cells compared with H2O2 (500 µM) treatment group. This result indicates that GSH prevents H2O2-induced ROS production.
Fig. 2.
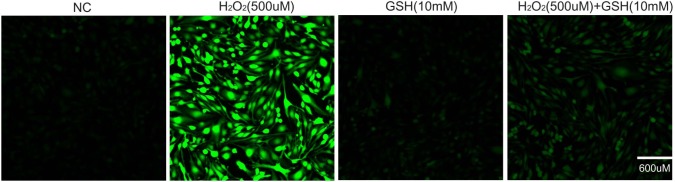
The effect of GSH on H2O2-induced ROS generation. H2O2 (500 µM) and/or GSH (10 mM) were treated in bEND.3 cells for 24 hr. ROS levels were measured using DCF-DA. ROS level in bEND.3 cells was increased in the H2O2 (500 µM) treatment group compared with normal control (NC) group. Also, ROS level was not change in the GSH (10 mM) treatment group compared with NC group. GSH decreased the H2O2-induced increase of DCF-DA-positive cells (green). Scale bar: 600 µm, 2', 7'-dichlorodihydrofluorescein diacetate (DCF-DA): green, 4', 6-diamidino-2-phenylindole (DAPI): blue.
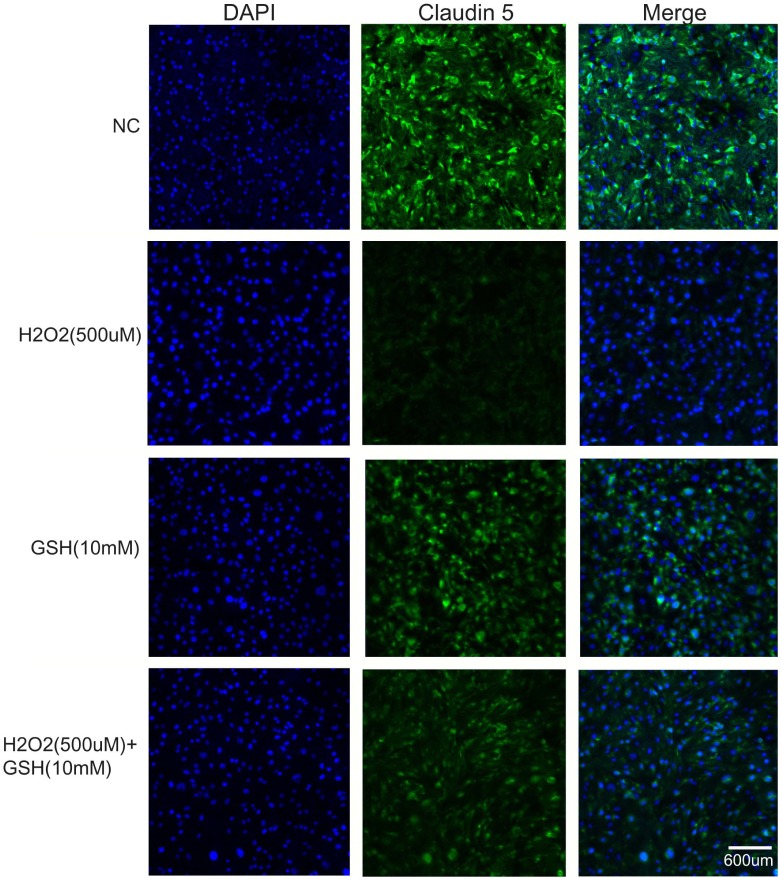
GSH prevents H2O2-induced decrease in tight junction proteins
To check the protective effect of GSH on the integrity of tight junctions during oxidative stress, we measured the level of Claudin 5, a tight junction protein, by immunocytochemisty. H2O2 (500 µM) treatment decreased the expression of Claudin 5 (Fig. 3). GSH treatment alone was not change the expression of Claudin 5 compared to normal control group. The expression of Claudin 5 was attenuated by GSH co-treatment. This suggests that GSH protects the degradation of Claudin 5 and may protect deterioration of tight junctions in response to oxidative stress.
Fig. 3.
The effect of GSH H2O2-induced decrease in tight junction proteins. H2O2 (500 µM) and/or GSH (10 mM) were treated in bEND.3 cells for 24 hr. The level of Claudin 5, a tight junction protein, was evaluated by immunocytochemistry. GSH attenuated H2O2-induced decrease in the number of Claudin 5-positive cells (green). Scale bar: 600 µm, Claudin 5: green, 4', 6-diamidino-2-phenylindole (DAPI): blue.
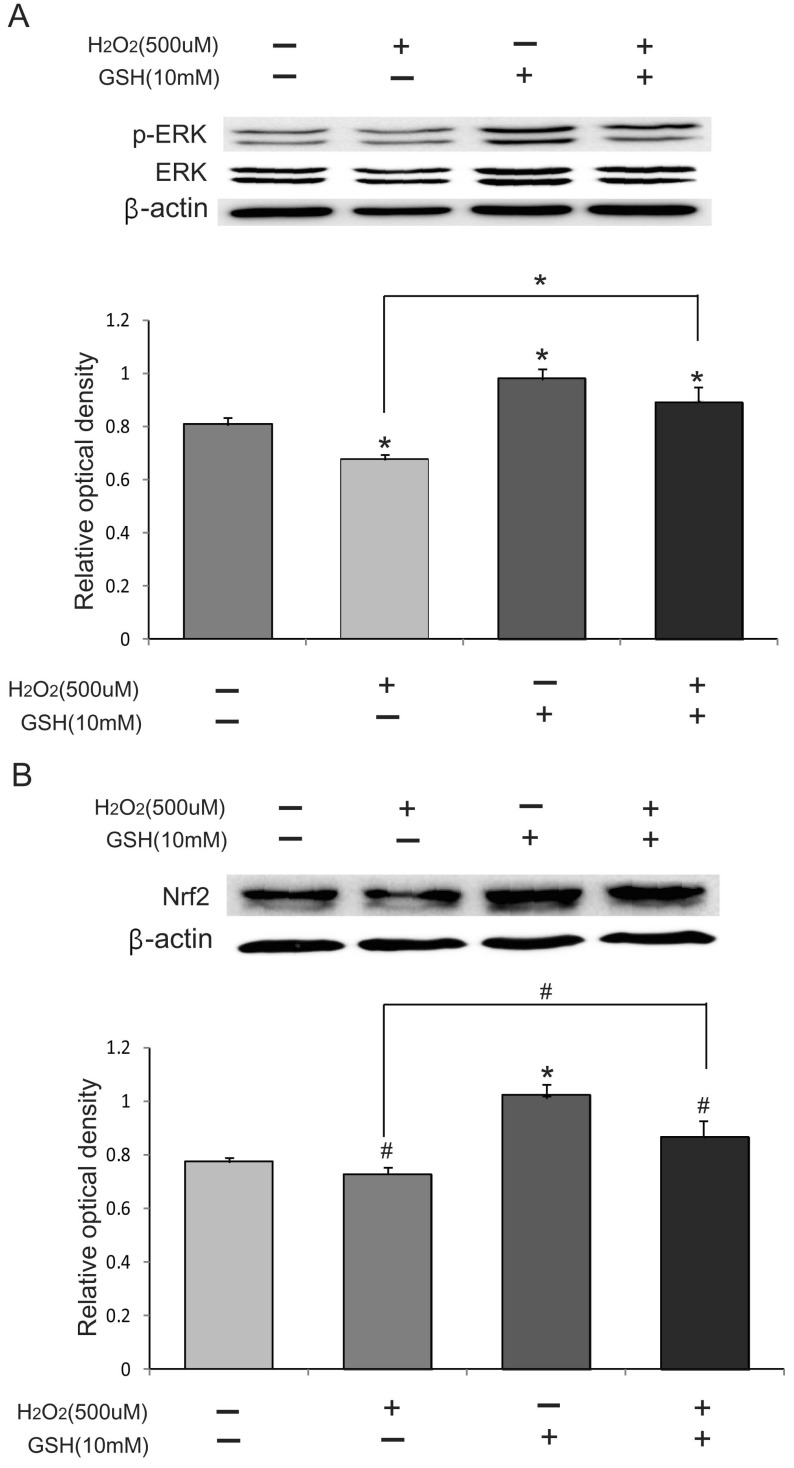
GSH promotes the Nrf2-mediated signaling
Nrf2 is a key regulator of anti-oxidative responses. To investigate whether Nrf2 signaling is activated in H2O2-induced oxidative stress, we first measured the phosphorylation status of ERK by Western blot analysis because the phosphorylation of ERK means the activation of ERK pathway. These results suggested that the protein level of phosphor-ERK/ERK in the H2O2 (500 µM) group attenuated compared to those of in the normal control (NC) group (Fig. 4A). The protein level of phosphor-ERK in the H2O2 (500 µM) +GSH (10 mM) group was higher than those of the H2O2 (500 µM) group (Fig. 4A). Also, we checked the expression of Nrf2 and found that H2O2 treatment decreases Nrf2 levels. GSH co-treatment attenuated this decrease. These results suggest that GSH increases Nrf2 levels and activates its downstream signaling pathway.
Fig. 4.
The effect of GSH on Nrf2 signaling. H2O2 (500 µM) and/or GSH (10 mM) were treated in bEND.3 cells for 24 hr. The protein level of Nrf2, ERK, phospho-ERK was evaluated by using Western blot analysis. (A) The phospho-ERK protein levels decreased by H2O2 treatment. GSH treatment increased the level of phospho-ERK. Bar graph showed the quantification of phosphor-ERK/ERK protein in all groups. (B) Nrf2 protein levels decreased by H2O2 treatment. GSH increased the basal level of Nrf2 proteins. GSH co-treatment increased Nrf2 protein levels compared to H2O2 treatment. β-actin was used as an internal control (mean±S.E.M., n=3) (#p<0.1, *p<0.05, **p<0.001).
DISCUSSIONS
Oxidative stress aggravates neurodegenerative diseases [4] and brain injury [5], and excessive ROS and/or reactive nitrogen species (RNS) levels are strongly associated with such states [31, 32, 33, 34]. GSH, the most abundant non-protein thiols, decreases ROS levels and activates cellular oxidative stress responses by several mechanisms [35, 36]. In present study, we investigated the protective effect of GSH on H2O2- induced oxidative stress in the brain capillary endothelial cells. Our results that GSH inhibits H2O2-induced increases in ROS and nitric oxide suggest that GSH may protect brain capillary endothelial cells from oxidative stress. Several studies demonstrated that GSH inhibits the cell death in oxidative stress [37, 38]. In addition, recent studies demonstrated that GSH inhibits DNA damage of cells against oxidative stress [39] and GSH attenuates the generation of ROS under oxidative stress [40]. Mitochondrial DNA (mt DNA) is one of the cellular components most severely affected by oxidative stress [41]. The oxidative modification of deoxyguanosine to 8-OHdG in mtDNA is the major DNA lesion induced by oxidative stress [42, 43] and is considered as an index of DNA damage [42, 44]. Considering that the elevated level of 8-OHdG is related with increased mtDNA deletions, mutation, and mtDNA loss [45, 46, 47], our result suggests that GSH protects the mtDNA in brain capillary endothelial cell from H2O2-induced damages. We also showed that Claudin 5, a tight junction protein [48], is stabilized by GSH. Because the degradation of tight junction proteins is associated to the disruption of BBB and the progression of central nervous system diseases [49, 50, 51, 52], our result suggests that GSH may protect BBB by inhibiting the degradation of tight junction proteins under oxidative stress. ROS regulates directly or indirectly a number of transcription factors [53]. Especially, ROS can promote the activation of Nrf2, which regulates antioxidant gene expression [53, 54]. Several studies demonstrated that Nrf2 activation inhibits ROS generation [55] and NO generation [56] to protect cell damage against oxidative stress [22]. In addition, Nrf2 modulates the apoptosis and autophagy related signaling [23]. Nrf2 signaling is activated by the PI3K/Akt pathway and the ERK pathway to promote the expression of antioxidant genes during oxidative stress [57, 58, 59, 60, 61]. Specifically, Nrf2 is activated by phosphorylation of ERK [61,62,63,64]. Gunjima et al. demonstrated that SH-SY5Y cells were protected against oxidative stress through Nrf2-glutathione pathway [65]. However, the research on the protective effect of GSH through Nrf2/ERK pathway in brain capillary endothelial cells has not studied until now. In present study, we investigated the protective effect of GSH against oxidative stress in brain capillary endothelial cells. In addition, we showed that GSH may promote phosphorylation of ERK in brain capillary endothelial cells under oxidative stress (Fig. 4). Based on our results, we suggested that the protective mechanism of GSH may be related to the Nrf2/ERK pathway in brain capillary endothelial cells against oxidative stress. Several studies suggested that Nrf2 activates to protect the cells in early oxidative stress. However, in present study, we analyzed the expression of ERK and Nrf2 at late oxidative stress state (at 24 hrs after H2O2-induced oxidative injury). Even though the expression of ERK and Nrf2 were decreased in only H2O2 treatment group, GSH co-treatment group were protected brain capillary endothelial cells through activation of ERK and Nrf2 against oxidative stress. In addition, considering recent studies that excessive ROS inhibits the phosphorylation of ERK [66, 67], decreased ROS levels by GSH treatment in present study may promote the phosphorylation of ERK and Nrf2 activation. In conclusion, we show that GSH protects brain capillary endothelial cells from H2O2-induced damages. GSH does this possibly by stabilizing tight junction proteins and activating Nrf2 signaling. Hence, this study suggests that GSH is a promising target to treat various central nervous system disorders and injuries characterized by oxidative stress.
ACKNOWLEDGEMENTS
This research was supported by the Brain Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2012-0005827). This work was supported by the Brain Korea 21 Plus Project for Medical Science, Yonsei University. We would like to thank professor Hosung Jung for his critical reading of this manuscript and editorial assistance.
References
- 1.Reliene R, Schiestl RH. Glutathione depletion by buthionine sulfoximine induces DNA deletions in mice. Carcinogenesis. 2006;27:240–244. doi: 10.1093/carcin/bgi222. [DOI] [PubMed] [Google Scholar]
- 2.Anderson ME. Glutathione: an overview of biosynthesis and modulation. Chem Biol Interact. 1998;111-112:1–14. doi: 10.1016/s0009-2797(97)00146-4. [DOI] [PubMed] [Google Scholar]
- 3.Wu WC, Bhavsar JH, Aziz GF, Sadaniantz A. An overview of stress echocardiography in the study of patients with dilated or hypertrophic cardiomyopathy. Echocardiography. 2004;21:467–475. doi: 10.1111/j.0742-2822.2004.03083.x. [DOI] [PubMed] [Google Scholar]
- 4.Patten DA, Germain M, Kelly MA, Slack RS. Reactive oxygen species: stuck in the middle of neurodegeneration. J Alzheimers Dis. 2010;20(Suppl 2):S357–S367. doi: 10.3233/JAD-2010-100498. [DOI] [PubMed] [Google Scholar]
- 5.Nanetti L, Raffaelli F, Vignini A, Perozzi C, Silvestrini M, Bartolini M, Provinciali L, Mazzanti L. Oxidative stress in ischaemic stroke. Eur J Clin Invest. 2011;41:1318–1322. doi: 10.1111/j.1365-2362.2011.02546.x. [DOI] [PubMed] [Google Scholar]
- 6.Hermann C, Zeiher AM, Dimmeler S. Shear stress inhibits H2O2-induced apoptosis of human endothelial cells by modulation of the glutathione redox cycle and nitric oxide synthase. Arterioscler Thromb Vasc Biol. 1997;17:3588–3592. doi: 10.1161/01.atv.17.12.3588. [DOI] [PubMed] [Google Scholar]
- 7.Zhou HG, Liu L, Zhang Y, Huang YY, Tao YH, Zhang S, Su JJ, Tang YP, Guo ZL, Hu RM, Dong Q. Glutathione prevents free fatty acids-induced oxidative stress and apoptosis in human brain vascular endothelial cells through Akt pathway. CNS Neurosci Ther. 2013;19:252–261. doi: 10.1111/cns.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vali S, Chinta SJ, Peng J, Sultana Z, Singh N, Sharma P, Sharada S, Andersen JK, Bharath MM. Insights into the effects of alpha-synuclein expression and proteasome inhibition on glutathione metabolism through a dynamic in silico model of Parkinson's disease: validation by cell culture data. Free Radic Biol Med. 2008;45:1290–1301. doi: 10.1016/j.freeradbiomed.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen EM, Mieyal JJ. Protein-thiol oxidation and cell death: regulatory role of glutaredoxins. Antioxid Redox Signal. 2012;17:1748–1763. doi: 10.1089/ars.2012.4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saharan S, Mandal PK. The emerging role of glutathione in Alzheimer's disease. J Alzheimers Dis. 2014 doi: 10.3233/JAD-132483. (in press) [DOI] [PubMed] [Google Scholar]
- 11.Mason RP, Casu M, Butler N, Breda C, Campesan S, Clapp J, Green EW, Dhulkhed D, Kyriacou CP, Giorgini F. Glutathione peroxidase activity is neuroprotective in models of Huntington's disease. Nat Genet. 2013;45:1249–1254. doi: 10.1038/ng.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson WM, Wilson-Delfosse AL, Mieyal JJ. Dysregulation of glutathione homeostasis in neurodegenerative diseases. Nutrients. 2012;4:1399–1440. doi: 10.3390/nu4101399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cecchelli R, Berezowski V, Lundquist S, Culot M, Renftel M, Dehouck MP, Fenart L. Modelling of the blood-brain barrier in drug discovery and development. Nat Rev Drug Discov. 2007;6:650–661. doi: 10.1038/nrd2368. [DOI] [PubMed] [Google Scholar]
- 14.Huang Q, Deng J, Wang F, Chen S, Liu Y, Wang Z, Wang Z, Cheng Y. Targeted gene delivery to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Exp Neurol. 2012;233:350–356. doi: 10.1016/j.expneurol.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 15.Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32:200–219. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Owuor ED, Kong AN. Antioxidants and oxidants regulated signal transduction pathways. Biochem Pharmacol. 2002;64:765–770. doi: 10.1016/s0006-2952(02)01137-1. [DOI] [PubMed] [Google Scholar]
- 17.Surh YJ, Na HK. NF-kappaB and Nrf2 as prime molecular targets for chemoprevention and cytoprotection with anti-inflammatory and antioxidant phytochemicals. Genes Nutr. 2008;2:313–317. doi: 10.1007/s12263-007-0063-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW, Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest. 2006;116:984–995. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin W, Zhu L, Guan Q, Chen G, Wang QF, Yin HX, Hang CH, Shi JX, Wang HD. Influence of Nrf2 genotype on pulmonary NF-kappaB activity and inflammatory response after traumatic brain injury. Ann Clin Lab Sci. 2008;38:221–227. [PubMed] [Google Scholar]
- 20.Jain AK, Bloom DA, Jaiswal AK. Nuclear import and export signals in control of Nrf2. J Biol Chem. 2005;280:29158–29168. doi: 10.1074/jbc.M502083200. [DOI] [PubMed] [Google Scholar]
- 21.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 22.Kolamunne RT, Dias IH, Vernallis AB, Grant MM, Griffiths HR. Nrf2 activation supports cell survival during hypoxia and hypoxia/reoxygenation in cardiomyoblasts; the roles of reactive oxygen and nitrogen species. Redox Biol. 2013;1:418–426. doi: 10.1016/j.redox.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattart L, Calay D, Simon D, Roebroek L, Caesens-Koenig L, Van Steenbrugge M, Tevel V, Michiels C, Arnould T, Boudjeltia KZ, Raes M. The peroxynitrite donor 3-morpholinosydnonimine activates Nrf2 and the UPR leading to a cytoprotective response in endothelial cells. Cell Signal. 2012;24:199–213. doi: 10.1016/j.cellsig.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Ryu MJ, Kang KA, Piao MJ, Kim KC, Zheng J, Yao CW, Cha JW, Chung HS, Kim SC, Jung E, Park D, Chae S, Hyun JW. 7,8-Dihydroxyflavone protects human keratinocytes against oxidative stress-induced cell damage via the ERK and PI3K/Akt-mediated Nrf2/HO-1 signaling pathways. Int J Mol Med. 2014;33:964–970. doi: 10.3892/ijmm.2014.1643. [DOI] [PubMed] [Google Scholar]
- 25.Lee HS, Lee GS, Kim SH, Kim HK, Suk DH, Lee DS. Anti-oxidizing effect of the dichloromethane and hexane fractions from Orostachys japonicus in LPS-stimulated RAW 264.7 cells via upregulation of Nrf2 expression and activation of MAPK signaling pathway. BMB Rep. 2014;47:98–103. doi: 10.5483/BMBRep.2014.47.2.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung HJ, Jeon YH, Bokara KK, Koo BN, Lee WT, Park KA, Lee JE. Agmatine promotes the migration of murine brain endothelial cells via multiple signaling pathways. Life Sci. 2013;92:42–50. doi: 10.1016/j.lfs.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 27.Hong S, Kim CY, Lee JE, Seong GJ. Agmatine protects cultured retinal ganglion cells from tumor necrosis factor-alpha-induced apoptosis. Life Sci. 2009;84:28–32. doi: 10.1016/j.lfs.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Ahn SK, Hong S, Park YM, Lee WT, Park KA, Lee JE. Effects of agmatine on hypoxic microglia and activity of nitric oxide synthase. Brain Res. 2011;1373:48–54. doi: 10.1016/j.brainres.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Royall JA, Ischiropoulos H. Evaluation of 2',7'-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch Biochem Biophys. 1993;302:348–355. doi: 10.1006/abbi.1993.1222. [DOI] [PubMed] [Google Scholar]
- 30.Jung HJ, Moon JS, Park AR, Choi H, Lee JE, Choi SH, Lim CJ. Anti-inflammatory, antinociceptive and anti-angiogenic activities of a phospholipid mixture purified from porcine lung tissues. Immunopharmacol Immunotoxicol. 2012;34:398–407. doi: 10.3109/08923973.2011.611137. [DOI] [PubMed] [Google Scholar]
- 31.Abramov AY, Scorziello A, Duchen MR. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neurosci. 2007;27:1129–1138. doi: 10.1523/JNEUROSCI.4468-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffman DL, Salter JD, Brookes PS. Response of mitochondrial reactive oxygen species generation to steady-state oxygen tension: implications for hypoxic cell signaling. Am J Physiol Heart Circ Physiol. 2007;292:H101–H108. doi: 10.1152/ajpheart.00699.2006. [DOI] [PubMed] [Google Scholar]
- 33.Lacza Z, Puskar M, Figueroa JP, Zhang J, Rajapakse N, Busija DW. Mitochondrial nitric oxide synthase is constitutively active and is functionally upregulated in hypoxia. Free Radic Biol Med. 2001;31:1609–1615. doi: 10.1016/s0891-5849(01)00754-7. [DOI] [PubMed] [Google Scholar]
- 34.Levrand S, Vannay-Bouchiche C, Pesse B, Pacher P, Feihl F, Waeber B, Liaudet L. Peroxynitrite is a major trigger of cardiomyocyte apoptosis in vitro and in vivo. Free Radic Biol Med. 2006;41:886–895. doi: 10.1016/j.freeradbiomed.2006.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Presnell CE, Bhatti G, Numan LS, Lerche M, Alkhateeb SK, Ghalib M, Shammaa M, Kavdia M. Computational insights into the role of glutathione in oxidative stress. Curr Neurovasc Res. 2013;10:185–194. doi: 10.2174/1567202611310020011. [DOI] [PubMed] [Google Scholar]
- 36.Yeligar SM, Harris FL, Hart CM, Brown LA. Glutathione attenuates ethanol-induced alveolar macrophage oxidative stress and dysfunction by downregulating NADPH oxidases. Am J Physiol Lung Cell Mol Physiol. 2014;306:L429–L441. doi: 10.1152/ajplung.00159.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abramov AY, Scorziello A, Duchen MR. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neurosci. 2007;27:1129–1138. doi: 10.1523/JNEUROSCI.4468-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffman DL, Salter JD, Brookes PS. Response of mitochondrial reactive oxygen species generation to steady-state oxygen tension: implications for hypoxic cell signaling. Am J Physiol Heart Circ Physiol. 2007;292:H101–H108. doi: 10.1152/ajpheart.00699.2006. [DOI] [PubMed] [Google Scholar]
- 39.Lacza Z, Puskar M, Figueroa JP, Zhang J, Rajapakse N, Busija DW. Mitochondrial nitric oxide synthase is constitutively active and is functionally upregulated in hypoxia. Free Radic Biol Med. 2001;31:1609–1615. doi: 10.1016/s0891-5849(01)00754-7. [DOI] [PubMed] [Google Scholar]
- 40.Levrand S, Vannay-Bouchiche C, Pesse B, Pacher P, Feihl F, Waeber B, Liaudet L. Peroxynitrite is a major trigger of cardiomyocyte apoptosis in vitro and in vivo. Free Radic Biol Med. 2006;41:886–895. doi: 10.1016/j.freeradbiomed.2006.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karamanlidis G, Nascimben L, Couper GS, Shekar PS, del Monte F, Tian R. Defective DNA replication impairs mitochondrial biogenesis in human failing hearts. Circ Res. 2010;106:1541–1548. doi: 10.1161/CIRCRESAHA.109.212753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klungland A, Bjelland S. Oxidative damage to purines in DNA: role of mammalian Ogg1. DNA Repair (Amst) 2007;6:481–488. doi: 10.1016/j.dnarep.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 43.Lu AL, Li X, Gu Y, Wright PM, Chang DY. Repair of oxidative DNA damage: mechanisms and functions. Cell Biochem Biophys. 2001;35:141–170. doi: 10.1385/CBB:35:2:141. [DOI] [PubMed] [Google Scholar]
- 44.Gredilla R, Bohr VA, Stevnsner T. Mitochondrial DNA repair and association with aging--an update. Exp Gerontol. 2010;45:478–488. doi: 10.1016/j.exger.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cassano P, Sciancalepore AG, Lezza AM, Leeuwenburgh C, Cantatore P, Gadaleta MN. Tissue-specific effect of age and caloric restriction diet on mitochondrial DNA content. Rejuvenation Res. 2006;9:211–214. doi: 10.1089/rej.2006.9.211. [DOI] [PubMed] [Google Scholar]
- 46.Chou YF, Yu CC, Huang RF. Changes in mitochondrial DNA deletion, content, and biogenesis in folate-deficient tissues of young rats depend on mitochondrial folate and oxidative DNA injuries. J Nutr. 2007;137:2036–2042. doi: 10.1093/jn/137.9.2036. [DOI] [PubMed] [Google Scholar]
- 47.Hiona A, Sanz A, Kujoth GC, Pamplona R, Seo AY, Hofer T, Someya S, Miyakawa T, Nakayama C, Samhan-Arias AK, Servais S, Barger JL, Portero-Otín M, Tanokura M, Prolla TA, Leeuwenburgh C. Mitochondrial DNA mutations induce mitochondrial dysfunction, apoptosis and sarcopenia in skeletal muscle of mitochondrial DNA mutator mice. PLoS One. 2010;5:e11468. doi: 10.1371/journal.pone.0011468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chai Q, He WQ, Zhou M, Lu H, Fu ZF. Enhancement of blood-brain barrier permeability and reduction of tight junction protein expression are modulated by chemokines/cytokines induced by rabies virus infection. J Virol. 2014 doi: 10.1128/JVI.03149-13. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krueger M, Härtig W, Reichenbach A, Bechmann I, Michalski D. Blood-brain barrier breakdown after embolic stroke in rats occurs without ultrastructural evidence for disrupting tight junctions. PLoS One. 2013;8:e56419. doi: 10.1371/journal.pone.0056419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Engelhardt S, Al-Ahmad AJ, Gassmann M, Ogunshola OO. Hypoxia selectively disrupts brain microvascular endothelial tight junction complexes through a hypoxia-inducible factor-1 (HIF-1) dependent mechanism. J Cell Physiol. 2013 doi: 10.1002/jcp.24544. (in press) [DOI] [PubMed] [Google Scholar]
- 51.Zehendner CM, Librizzi L, Hedrich J, Bauer NM, Angamo EA, de Curtis M, Luhmann HJ. Moderate hypoxia followed by reoxygenation results in blood-brain barrier breakdown via oxidative stress-dependent tight-junction protein disruption. PLoS One. 2013;8:e82823. doi: 10.1371/journal.pone.0082823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wan W, Chen H, Li Y. The potential mechanisms of Abeta-receptor for advanced glycation end-products interaction disrupting tight junctions of the blood-brain barrier in Alzheimer's disease. Int J Neurosci. 2014;124:75–81. doi: 10.3109/00207454.2013.825258. [DOI] [PubMed] [Google Scholar]
- 53.Leonard MO, Kieran NE, Howell K, Burne MJ, Varadarajan R, Dhakshinamoorthy S, Porter AG, O'Farrelly C, Rabb H, Taylor CT. Reoxygenation-specific activation of the antioxidant transcription factor Nrf2 mediates cytoprotective gene expression in ischemia-reperfusion injury. FASEB J. 2006;20:2624–2626. doi: 10.1096/fj.06-5097fje. [DOI] [PubMed] [Google Scholar]
- 54.McMahon M, Itoh K, Yamamoto M, Hayes JD. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J Biol Chem. 2003;278:21592–21600. doi: 10.1074/jbc.M300931200. [DOI] [PubMed] [Google Scholar]
- 55.Lisk C, McCord J, Bose S, Sullivan T, Loomis Z, Nozik-Grayck E, Schroeder T, Hamilton K, Irwin DC. Nrf2 activation: a potential strategy for the prevention of acute mountain sickness. Free Radic Biol Med. 2013;63:264–273. doi: 10.1016/j.freeradbiomed.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dilshara MG, Lee KT, Jayasooriya RG, Kang CH, Park SR, Choi YH, Choi IW, Hyun JW, Chang WY, Kim YS, Lee HJ, Kim GY. Downregulation of NO and PGE2 in LPS-stimulated BV2 microglial cells by trans-isoferulic acid via suppression of PI3K/Akt-dependent NF-κB and activation of Nrf2-mediated HO-1. Int Immunopharmacol. 2014;18:203–211. doi: 10.1016/j.intimp.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 57.Huang HC, Nguyen T, Pickett CB. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J Biol Chem. 2002;277:42769–42774. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- 58.Kim JW, Li MH, Jang JH, Na HK, Song NY, Lee C, Johnson JA, Surh YJ. 15-Deoxy-Delta(12,14)-prostaglandin J(2) rescues PC12 cells from H2O2-induced apoptosis through Nrf2-mediated upregulation of heme oxygenase-1: potential roles of Akt and ERK1/2. Biochem Pharmacol. 2008;76:1577–1589. doi: 10.1016/j.bcp.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 59.Hwang YP, Jeong HG. The coffee diterpene kahweol induces heme oxygenase-1 via the PI3K and p38/Nrf2 pathway to protect human dopaminergic neurons from 6-hydroxydopamine-derived oxidative stress. FEBS Lett. 2008;582:2655–2662. doi: 10.1016/j.febslet.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 60.Xu C, Yuan X, Pan Z, Shen G, Kim JH, Yu S, Khor TO, Li W, Ma J, Kong AN. Mechanism of action of isothiocyanates: the induction of ARE-regulated genes is associated with activation of ERK and JNK and the phosphorylation and nuclear translocation of Nrf2. Mol Cancer Ther. 2006;5:1918–1926. doi: 10.1158/1535-7163.MCT-05-0497. [DOI] [PubMed] [Google Scholar]
- 61.Chen CY, Jang JH, Li MH, Surh YJ. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochem Biophys Res Commun. 2005;331:993–1000. doi: 10.1016/j.bbrc.2005.03.237. [DOI] [PubMed] [Google Scholar]
- 62.Meng X, Sun G, Ye J, Xu H, Wang H, Sun X. Notoginsenoside R1-mediated neuroprotection involves estrogen receptor-dependent crosstalk between Akt and ERK1/2 pathways: A novel mechanism of Nrf2/ARE signaling activation. Free Radic Res. 2014;48:445–460. doi: 10.3109/10715762.2014.885117. [DOI] [PubMed] [Google Scholar]
- 63.Papaiahgari S, Zhang Q, Kleeberger SR, Cho HY, Reddy SP. Hyperoxia stimulates an Nrf2-ARE transcriptional response via ROS-EGFR-PI3K-Akt/ERK MAP kinase signaling in pulmonary epithelial cells. Antioxid Redox Signal. 2006;8:43–52. doi: 10.1089/ars.2006.8.43. [DOI] [PubMed] [Google Scholar]
- 64.Papaiahgari S, Kleeberger SR, Cho HY, Kalvakolanu DV, Reddy SP. NADPH oxidase and ERK signaling regulates hyperoxia-induced Nrf2-ARE transcriptional response in pulmonary epithelial cells. J Biol Chem. 2004;279:42302–42312. doi: 10.1074/jbc.M408275200. [DOI] [PubMed] [Google Scholar]
- 65.Xu C, Yuan X, Pan Z, Shen G, Kim JH, Yu S, Khor TO, Li W, Ma J, Kong AN. Mechanism of action of isothiocyanates: the induction of ARE-regulated genes is associated with activation of ERK and JNK and the phosphorylation and nuclear translocation of Nrf2. Mol Cancer Ther. 2006;5:1918–1926. doi: 10.1158/1535-7163.MCT-05-0497. [DOI] [PubMed] [Google Scholar]
- 66.Liu WH, Chang LS. Caffeine induces matrix metalloproteinase-2 (MMP-2) and MMP-9 down-regulation in human leukemia U937 cells via Ca2+/ROS-mediated suppression of ERK/c-fos pathway and activation of p38 MAPK/c-jun pathway. J Cell Physiol. 2010;224:775–785. doi: 10.1002/jcp.22180. [DOI] [PubMed] [Google Scholar]
- 67.Kim TH, Kim YK, Woo JS. The adenosine A3 receptor agonist Cl-IB-MECA induces cell death through Ca(2)(+)/ROS-dependent down regulation of ERK and Akt in A172 human glioma cells. Neurochem Res. 2012;37:2667–2677. doi: 10.1007/s11064-012-0855-5. [DOI] [PubMed] [Google Scholar]