Tbet and STAT4 have distinct roles in effector T cell differentiation, but are not required for generation of T cell memory.
Keywords: cytokines, memory, effector, Tbet, STAT4, LCMV
Abstract
Effector and memory CD4 and CD8 T cell responses are critical for the control of many intracellular pathogens. The development of these populations is governed by transcription factors that molecularly control their differentiation, function, and maintenance. Two transcription factors known to be involved in these processes are Tbet and STAT4. Although Tbet has been shown to regulate CD8 T cell fate decisions and effector CD4 T cell choices, the contribution of STAT4 is less well understood. To address this, we examined the impact of STAT4 on T cell responses in the presence or absence of Tbet, following LCMV infection by using mice lacking Tbet, STAT4, or both transcription factors. STAT4 was not required for Tbet or Eomes expression; however, virus-specific effector CD8 T cells are skewed toward a memory-precursor phenotype in the absence of STAT4. This altered proportion of memory precursors did not result in an increase in memory CD8 T cells after the resolution of the infection. We also demonstrate that virus-specific effector and memory CD4 T cells formed independently of Tbet and STAT4, although a slight reduction in the number of antigen-specific CD4 T cells was apparent in mice lacking both transcription factors. Collectively, we have discovered distinct roles for Tbet and STAT4 in shaping the phenotype and function of virus-specific T cell responses.
Introduction
The eradication of intracellular pathogens is achieved by the integrated functions of CD4 and CD8 T cells. CD4 T cells differentiate into Th1 cells and augment the cytotoxicity of CD8 T cells, activate macrophages, provide help to B cells, and interfere directly with pathogen growth [1, 2]. In addition to their ability to lyse infected cells directly, CD8 T cells also produce IFN-γ, a cytokine involved in the clearance of intracellular bacteria and viruses [3]. The transcription factors Tbet and STAT4 have both been shown to influence the development of effector populations; however, whether these transcription factors have overlapping and redundant roles is unclear.
Although the transcription factors Tbet and Eomes are each sufficient for IFN-γ production by CD8 T cells [4], they do have discrete functions [5]. It has been proposed that high levels of Tbet promote the differentiation of short-lived effector cells, which are defined by the up-regulation of KLRG1 and the loss of IL-7Rα (CD127) [6]. Conversely, early expression of Eomes is hypothesized to give rise to memory precursor cells that possess low levels of KLRG1 and increased expression of CD127 and IL-2 [7]. IL-12 is thought to be a molecular switch between these two transcription factors, promoting high levels of Tbet expression while suppressing Eomes [5]. Very little is known about whether STAT4 fits into this paradigm. As IL-12 signals through STAT4, we posit that STAT4 and potentially other cytokines that signal via STAT4 play a role in the balance of Tbet versus Eomes, consequently influencing the fate of the cell.
In differentiating CD4 Th1 cells, Tbet is induced via TCR and IFN-γ signaling [2, 8, 9] and is integral for the generation of effector CD4 T cell responses to intracellular pathogens [10]. Th1 commitment is cemented by IL-12 signaling through STAT4 [11, 12], a transcription factor that is also critical for IFN-γ production [13, 14]. Nevertheless, STAT4KO CD4 T cells are capable of IFN-γ production if Tbet is overexpressed [11] or if the IL-4/STAT6 pathway is blocked [15, 16]. Whether these molecules participate in overlapping or complimentary pathways in the differentiation of effector CD4 T cell responses to intracellular pathogens in vivo is not well defined. Moreover, even less is known regarding the contribution of these transcription factors to the development and maintenance of memory CD4 T cells.
In this study, we probed the roles of Tbet and STAT4 in regulating the generation of effector and memory CD4 and CD8 T cell responses following infection with LCMV. With the use of TbetKO, STAT4KO, or both transcription factors, we demonstrate overlapping and distinct requirements for these molecules in effector CD8 T cell development and function, but notably memory generation remained intact even in the absence of both of these transcription factors. In CD4 T cells, whereas Tbet but not STAT4 is required for production of IFN-γ, effector and memory T cell responses are generated in the absence of both Tbet and STAT4. Overall, although Tbet and STAT4 work in concert for the elicitation of effector functions, they are not required for the generation and maintenance of virus-specific T cells.
MATERIALS AND METHODS
Mice and infection
BALB/c, TbetKO, and STAT4KO mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Tbet/STAT4 DKO mice were generated by crossing the TbetKO and STAT4KO strains. All mice were bred and maintained in fully accredited facilities at UAB (AL, USA). Mice were infected with 2 × 105 PFU LCMV-ARM by i.p. injection.
Cell preparation
Spleens were disrupted into single-cell suspensions, and erythrocytes were removed by lysis using 0.83% (w/v) NH4Cl. Cells were resuspended in RPMI-1640 medium, supplemented with 10% FCS, 50 μM 2-ME, 100 U/ml penicillin, 100 μg/ml streptomycin, sodium pyruvate, and nonessential amino acids (R10).
Cell-surface staining and flow cytometry
Splenocytes were stained as described previously [17]. Cells were first treated with anti-CD16/CD32 (2.4G2; UAB Epitope Recognition and Immunoreagent Core Facility). Cells were then stained with anti-CD127 (A7R34; eBioscience, San Diego, CA, USA), anti-KLRG1 (2F1; eBioscience), and anti-CD8α (53-6.7; eBioscience) mAb in conjunction with the LCMV epitope-specific Ld(NP118) tetramer (prepared in-house at UAB). All samples were acquired on an LSRII flow cytometer (BD Biosciences, San Jose, CA, USA). Data were analyzed using FlowJo software (TreeStar, Ashland, OR, USA).
In vitro stimulation and intracellular staining
For peptide stimulation, splenocytes were incubated for 5 h at 37°C in 5–6% CO2 with the indicated peptide(s) and Brefeldin A (Golgi Plug; BD Biosciences). CD4 T cell responses were stimulated by the H-2d MHC class II-restricted peptides: NP6-20 and Z31-45 or both [18]. CD8 T cell responses were stimulated by the H-2d MHC class I-restricted NP118-126 peptide [19]. For cytokine stimulation, splenocytes were cultured for 5.5 h in the presence or absence of murine rIL-12 (Biosource/Invitrogen, Camarillo, CA, USA, or PeproTech, Rocky Hill, NJ, USA), IL-18 (Biosource/Invitrogen), IL-21 (R&D Systems, Minneapolis, MN, USA, or PeproTech), or combinations of these cytokines. Cytokines were used at a final concentration of 20 ng/ml. Brefeldin A was added for the last 1.5 h of culture to facilitate the intracellular accumulation of IFN-γ.
Intracellular cytokine staining was performed using the Cytofix/Cytoperm Plus Fixation/Permeablization kit, according to the manufacturer's directions (BD Biosciences). To detect Tbet and Eomes expression, cells were stained using the Fixation and Permeabilization kit, according to the manufacturer's directions, as described previously (eBioscience) [20]. Cells were stained with combinations of anti-CD8α (53-6.7), anti-CD4 (RM4-5), anti-CD11a (M17/4), anti-IFN-γ (XMG.12), anti-IL-2 (JES6-5H4), anti-TNF-α (MP6-XT22), anti-Tbet (eBio4B10), and anti-Eomes (Dan11mag; all eBioscience) mAb. For certain experiments, cells were costained with Ld(NP118) tetramers prior to intracellular staining.
In vitro stimulation and phosflow staining
Splenocytes were left unstimulated or stimulated with IL-12 (10 ng/mL; Biosource/Invitrogen) or IFN-α A/D (103U/mL; Biosource/Invitrogen) for 30 min at 37°C in 5–6% CO2. Cells were treated with Lyse/Fix buffer, permeablized with Perm Buffer III, and stained with anti-phosphorylated STAT4 mAb (all BD Biosciences) in conjunction with anti-CD8α (53-6.7), anti-CD4 (RM4-5), and anti-CD11a (M17/4; all eBioscience) mAb.
Statistical analysis
Statistical significance was determined using a one-way ANOVA with a Tukey multicomparison post-test or unpaired Student's t-test on Prism software (Graph Pad, La Jolla, CA, USA). Statistical significance is denoted as *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001.
RESULTS
Combined absence of Tbet and STAT4 leads to reduced effector CD8 T cell response
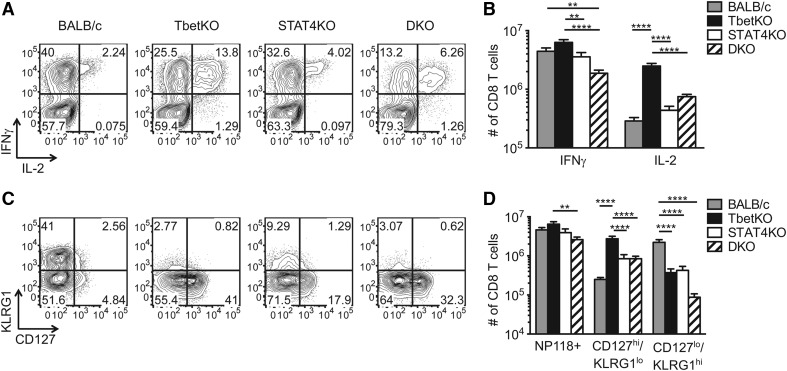
Multiple reports have demonstrated that Tbet is dispensable for production of IFN-γ in CD8 T cells but plays a critical role in memory differentiation, yet the importance of STAT4 in effector and memory CD8 T cell development has not been examined extensively. We infected BALB/c, TbetKO, STAT4KO, and Tbet/STAT4-DKO mice with LCMV-ARM and analyzed the LCMV NP118-specific CD8 T cell response 8 days later by tetramer and intracellular cytokine staining (Fig. 1 and Supplemental Fig. 1). No differences in the numbers and frequencies of NP118-specific IFN-γ-producing or tetramer-binding CD8 T cells were detected among BALB/c, TbetKO, and STAT4KO mice, but the IFN-γ-producing population was reduced in DKO mice (Fig. 1A, B, and D and Supplemental Fig. 1A, B, and D). This reduction reflects the diminished ability of the CD8 T cells from DKO mice to produce IFN-γ, as ∼72% of their tetramer-positive CD8 T cells were capable of secreting IFN-γ compared with >90% in the other cohorts (Fig. 1B and D and Supplemental Fig. 1D).
Figure 1. Tbet and STAT4 differentially regulate effector CD8 T cell phenotypes.
Splenocytes from BALB/c, TbetKO, STAT4KO, and DKO mice were analyzed for effector CD8 T cell responses at Day 8 postinfection. (A and B) Splenocytes were stimulated for 5 h with the NP118 peptide, followed by intracellular cytokine staining for IFN-γ and IL-2. (A) Plots are gated on CD8+ cells and show the percentages of IFN-γ+ or IL-2+ cells. (B) The numbers of IFN-γ+ or IL-2+ NP118-specific CD8 T cells/spleen. (C and D) The expression of CD127 and KLRG1 on splenic Ld(NP118) tetramer-positive CD8 T cells at Day 8 postinfection. (C) Representative plots are gated on Ld(NP118)+ CD8+ cells. (D) The numbers of Ld(NP118)+ CD8 T cells, CD127hiKLRG1lo Ld(NP118)+ CD8 T cells, and CD127loKLRG1hi Ld(NP118)+ CD8 T cells. Data shown are from three separate experiments with 11–15 mice/group.
Our data demonstrate that Tbet and STAT4 together are crucial for the production of IFN-γ by virus-specific effector CD8 T cells in response to antigen-dependent TCR stimulation. As effector CD8 T cells also respond to inflammatory cytokines [21–23], we tested the requirement of Tbet and STAT4 for TCR-independent IFN-γ production driven by such cytokines. A combination of IL-12, IL-18, and IL-21 induced maximal IFN-γ release by LCMV-specific CD8 T cells; ∼70% of the tetramer-positive CD8 T cells from BALB/c mice produced IFN-γ (Fig. 2). In contrast, the cytokine-induced IFN-γ response by LCMV-specific CD8 T cells from TbetKO and STAT4KO mice was markedly impaired, with only ∼30% and ∼19% of the tetramer-positive CD8 T cells producing IFN-γ, respectively (Fig. 2). Unexpectedly, cytokine-induced IFN-γ production was almost extinguished in LCMV-specific CD8 T cells from DKO mice (Fig. 2), demonstrating important, nonredundant roles for Tbet and STAT4 in mediating IFN-γ production by effector CD8 T cells.
Figure 2. Optimal cytokine-dependent, antigen-independent stimulation of virus-specific CD8 T cells requires Tbet and STAT4.
Splenic CD8 T cells from BALB/c, TbetKO, STAT4KO, and DKO mice infected 8 days prior with LCMV-ARM were analyzed for the production of IFN-γ following NP118 peptide and cytokine stimulation. (A) Representative plots show IFN-γ staining from activated CD11ahi CD8 T cells after stimulation with NP118 peptide (top row) or a combination of IL-12, IL-18, and IL-21 (middle row), as well as IFN-γ staining in conjunction with Ld(NP118) tetramer staining after stimulation with IL-12, IL-18, and IL-21 (bottom row). Plots are gated on CD8+ cells, and the values represent the percent of cells in each quadrant. (B) The frequencies of Ld(NP118)+ CD8+ cells that produced IFN-γ after 5.5 h stimulation with indicated cytokines. Data shown are from three to five experiments with nine to 15 mice/group. Statistics shown are WT compared with the other cohorts within each stimulation group.
STAT4 does not repress IL-2 production in CD8 T cells
In addition to the antiviral cytokine IFN-γ, we examined whether Tbet and STAT4 influence the ability of CD8 T cells to produce IL-2 following peptide stimulation. As published previously, the frequency and number of virus-specific IL-2-producing CD8 T cells were increased in TbetKO mice (Fig. 1A and B and Supplemental Fig. 1C) [24]. By contrast, STAT4KO had little impact on IL-2 production, as there was no significant increase in the number of NP118-specific, IL-2-producing CD8 T cells compared with WT mice. Interestingly, DKO mice resembled their TbetKO counterparts, with a similar proportion of the NP118-specific IFN-γ+ CD8 T cells coproducing IL-2, highlighting the dominant role of Tbet in suppressing IL-2 production by effector CD8 T cells (Fig. 1A and B and Supplemental Fig. 1E). Still, the total number of IL-2-producing, virus-specific CD8 T cells was reduced compared with the single TbetKO mice (Fig. 1B), which is consistent with the decrease in the numbers of Ld(NP118) tetramer-positive CD8 T cells between the TbetKO and DKO groups (Fig. 1D).
STAT4 impacts the formation of CD127loKLRG1hi-expressing CD8 T cells
Tbet has been proposed to be important for the balance between effector and memory cell development, as mice lacking Tbet have a higher frequency of CD127hiKLRG1lo memory precursors and a lower frequency of CD127loKLRG1hi short-lived effector CD8 T cells [6]. As IL-12 promotes Tbet expression through STAT4 [5, 6], we examined these subsets in all four cohorts of mice. Our data confirmed that in the absence of Tbet, there is a higher frequency of CD127hiKLRG1lo tetramer-binding CD8 T cells (Fig. 1C and ref. [6]), and in this study, we also note an increased number of this cell population (Fig. 1D). An increased percentage and number of CD127hiKLRG1lo and a reduced frequency and number of CD127loKLRG1hi tetramer-positive CD8 T cells were detected in STAT4KO mice compared with WT mice (Fig. 1C and D) but not to the extent observed in TbetKO mice. CD8 T cells in mice lacking both transcription factors display a surface phenotype similar to TbetKO mice, with a majority of the virus-specific CD8 T cells displaying the CD127hiKLRG1lo memory-precursor phenotype and a dramatically reduced frequency and number of CD127loKLRG1hi Ld(NP118)-specific CD8 T cells (Fig. 1C and D). Of note, Tbet expression in STAT4KO effector CD8 T cells resembled that of their WT counterparts (Fig. 3A). This indicates that Tbet determines the differentiation state of the effector CD8 T cells; however, STAT4 can also influence this process.
Figure 3. STAT4 is not essential for Tbet or Eomes expression in virus-specific effector CD8 T cells.
BALB/c, TbetKO, STAT4KO, and DKO mice were analyzed for expression of Tbet and Eomes by effector CD8 T cells on Day 8 post-LCMV infection. (A) Representative plots show ex vivo Tbet staining in CD11ahi CD8 T cells. (B) Eomes and IFN-γ expression in CD8 T cells after a 5-h stimulation with media or the LCMV NP118 peptide. Plots are gated on CD8+ cells, and values indicate the frequency of cells in each quadrant. Data shown are from two separate experiments with eight to 10 mice/group.
STAT4 signaling is not required for Eomes expression
The expression of Eomes in CD8 T cells is associated with memory formation and has been shown to compensate for the loss of Tbet for IFN-γ production. Notably, IL-12, which signals through STAT4, has been documented to repress Eomes in effector CD8 T cells. It is unclear, however, if loss of STAT4 leads to increased Eomes expression in effector CD8 T cells. Therefore, we investigated the levels of Eomes in the four cohorts of LCMV-infected mice and found peptide stimulation had very little effect on the frequencies of Eomes-expressing CD8 T cells. The highest levels of Eomes were detected in TbetKO CD8 T cells, and interestingly, STAT4KO CD8 T cells did not display an elevation in the frequency of Eomes-positive cells compared with WT CD8 T cells (Fig. 3B). Surprisingly, effector CD8 T cells in DKO mice did not show an increase in the percentage of cells that expressed Eomes compared with WT mice; however, this may be a reflection of the overall dampened CD8 T cell response in these mice.
Memory CD8 T cell generation is intact in the absence of Tbet and STAT4
The absence of Tbet and STAT4 results in an increased proportion of memory precursor CD8 T cells at the peak of the LCMV response; however, it is important to examine whether this culminates in a higher number of memory cells. We analyzed the memory response >50 days post-LCMV infection by tetramer staining and cytokine production (Fig. 4). No differences were observed in the numbers of LCMV-specific, tetramer-binding and IFN-γ-producing CD8 T cells from the various experimental cohorts, and the only exception is a modest reduction in the number of IFN-γ+ CD8 T cells in the DKO mice compared with the TbetKO mice. These data highlight that whereas the effector phenotype of the virus-specific CD8 T cells is regulated by Tbet and STAT4 (Fig. 1), the absence these transcription factors does not facilitate the generation of memory CD8 T cells. Interestingly, the number of LCMV-specific memory CD8 T cells producing IL-2 was different among the four genotypes of mice (Fig. 4), indicating that Tbet impedes the expression of IL-2 in effector and memory T cells.
Figure 4. CD8 T cell memory is unaltered in the absence of Tbet and STAT4.

Splenocytes from BALB/c, TbetKO, STAT4KO, and DKO mice were analyzed on Days 51–175 for LCMV-specific memory CD8 T cells responses. Cells were stained ex vivo with the Ld(NP118) tetramer or intracellularly for IFN-γ+ and IL-2+ after a 5-h NP118 peptide stimulation. Numbers of tetramer+, IFN-γ+, or IL-2+ NP118-specific CD8 T cells are shown; cumulative data from three separate experiments with seven to 22 mice/group.
STAT4 is not necessary for Tbet and IFN-γ expression in CD4 T cells
Tbet and STAT4 are also critical for the development of Th1 effector CD4 T cells. To interrogate how these molecules impact the generation of antiviral CD4 T cell responses in vivo, we analyzed effector CD4 T cell responses 8 days post-LCMV infection of BALB/c, TbetKO, STAT4KO, and DKO mice (Fig. 5). Of note, the H-2d-restricted NP6- and Z31-specific CD4 T responses in BALB/c mice are much lower in frequency than the H-2b-restricted GP61- and NP309-specific CD4 T cell responses detected in C57BL/6 mice [18]; however, these responses are highly specific, as cytokine production is not detected when these peptides are used to stimulate splenocytes from noninfected mice, and the irrelevant OVA323–338 peptide does not induce cytokine production (data not shown). In the absence of Tbet, there was a marked defect in the ability of the CD4 T cells to produce IFN-γ, as shown by the reduced frequency and number of IFN-γ-producing, virus-specific CD4 T cells in TbetKO and DKO mice (Fig. 5A and B and Supplemental Fig. 2A). STAT4 was still readily phosphorylated in TbetKO CD4 T cells by stimulation with type I IFN (Supplemental Fig. 3), demonstrating that the absence of Tbet does not corrupt the STAT4 signaling pathway. Moreover, we found that STAT4 was dispensable for IFN-γ production, as there was no difference in the frequency and number of IFN-γ-producing, virus-specific CD4 T cells in the STAT4KO mice compared with WT mice (Fig. 5A and B and Supplemental Fig. 2A). Importantly, the levels of Tbet were similar in WT and STAT4KO effector CD4 T cells (Fig. 6), establishing that during an acute LCMV infection, the induction of this transcription factor and the production of IFN-γ do not require STAT4.
Figure 5. IFN-γ production by CD4 T cells is Tbet-dependent but STAT4-independent.

Splenocytes from BALB/c, TbetKO, STAT4KO, or DKO mice 8 days post-LCMV-ARM infection were stimulated with a mixture of NP6 and Z31 peptides for 5 h and then stained for the production of IFN-γ, IL-2, and TNF-α. (A) Representative plots show IFN-γ and IL-2 staining (gated on CD4+ cells). The numbers of (B) IFN-γ+, (C) IL-2+, and (D) TNF-α+ CD4 T cells following NP6 or Z31 stimulation is shown. Data are compiled from three to five experiments with eight to 17 mice/group.
Figure 6. STAT4 is not required for Tbet expression in CD4 T cells following LCMV infection.
(A) Representative plots show ex vivo Tbet staining in activated, CD11ahi CD4+ splenocytes 8 days post-LCMV infection in the four cohorts of mice. The (B) frequencies and (C) numbers of Tbet+ CD4 T cells were determined. Data shown are from three separate experiments with 11–12 mice/group.
LCMV-specific CD4 T cells are elicited in the absence of Tbet and STAT4
To examine the differentiation of the effector population in the absence of Tbet, irrespective of the production of IFN-γ, we analyzed the virus-specific CD4 T cell response by assessing production of the Th1 cytokines, IL-2, and TNF-α. By 8 days following LCMV infection, a similar frequency and number of IL-2- and TNF-α-responding CD4 T cells were detected in the absence of Tbet or STAT4 (Fig. 5A, C, and D and Supplemental Fig. 2B and C). Whereas there was a trend toward a decrease in the frequency of IL-2-producing CD4 T cells in DKO mice (Fig. 5C and Supplemental Fig. 2B), enumeration of these responses showed no difference compared with WT controls (Fig. 5C); however, there was a decrease in the frequency and number of TNF-α+ CD4 T cells in DKO mice (Fig. 5D and Supplemental Fig. 2C). These data suggest that expansion of antigen-specific CD4 T cells following LCMV infection is intact in the absence of Tbet or STAT4; however, both of these transcription factors are required for an optimal CD4 T cell effector response.
LCMV-specific memory CD4 T cells are formed in the absence of Tbet and STAT4
We observed the induction of LCMV-specific effector CD4 T cells in mice lacking Tbet and STAT4; however, it was unknown if these cells would persist into the memory pool. Therefore, we analyzed the CD4 T cell memory responses in these mice >50 days following priming. As expected, almost no IFN-γ production was detected in the absence of Tbet, whereas the STAT4KO mice had similar frequencies and numbers of IFN-γ-producing memory CD4 T cells when compared with their WT counterparts (Fig. 7A and B and Supplemental Fig. 2D). The numbers of IL-2- and TNF-α-responding CD4 T cells were comparable in the absence of Tbet or STAT4, demonstrating that antigen-specific memory CD4 T cells form, and these cells are maintained until at least 175 days postinfection, even if IFN-γ production is compromised (Fig. 7B–D and Supplemental Fig. 2D–F). There was a slight reduction in the number of IL-2- and TNF-α-producing memory CD4 T cells when Tbet and STAT4 were absent, suggesting that Tbet and STAT4 can compensate for one another in the generation or maintenance of memory CD4 T cells (Fig. 7C and D). Consistent with previous reports, a small subset of IL-17A-producing CD4 T cells was uncovered in TbetKO and DKO mice [4], but this population of cells was not present in WT or STAT4KO mice (data not shown), further emphasizing the importance of Tbet in shaping CD4 T cell responses.
Figure 7. Formation of CD4 T cell memory in the absence of Tbet and STAT4.
Splenocytes from BALB/c, TbetKO, STAT4KO, or DKO mice 51–175 days post-LCMV-ARM infection were stimulated with NP6 and Z31 peptide for 5 h. (A) Representative plots show IFN-γ and IL-2 staining (gated on CD4+ cells). The numbers of (B) IFN-γ+, (C) IL-2+, and (D) TNF-α+ CD4 T cells following NP6 or Z31 stimulation are shown. Data are compiled from three to five experiments with eight to 19 mice/group.
DISCUSSION
In recent years, the Th1-associated transcription factors Tbet and STAT4 have been shown to shape the phenotype and outcome of CD4 and CD8 T cell responses. In this study, we show that virus-specific CD4 and CD8 T cell responses are elicited, and T cell memory develops in the absence of Tbet and STAT4. However, we note distinct roles for each of these molecules for effector cytokine production, cell-surface marker expression, and the responsiveness to proinflammatory cytokines.
It has been postulated that IL-12 signaling plays an integral role in the CD8 T cell effector versus memory bifurcation by influencing the expression of Tbet and Eomes [25]. IL-12 signaling via STAT4 promotes Tbet while potentially repressing Eomes [5]. Hence, STAT4 activation is proposed to favor terminal differentiation of effector CD8 T cells and limit memory-precursor formation [26, 27]. Following LCMV infection, we did not observe marked alterations in the expression of Tbet or Eomes in virus-specific CD8 T cells in STAT4KO mice, indicating little involvement of STAT4 in the regulation of these molecules. We did, however, note a pronounced reduction in the frequency and number of terminally differentiated, CD127loKLRG1hi effector CD8 T cells and a trend toward increased memory-precursor CD127hiKLRG1lo CD8 T cells in the absence of STAT4. As we detected Tbet expression in the STAT4KO mice, one possibility is that STAT4 controls the expression of CD127 and KLRG1 independently of Tbet. Alternatively, Tbet may require STAT4 for the optimal induction of these surface markers. Nevertheless, no differences were detected in the numbers of LCMV-specific memory CD8 T cells in mice lacking Tbet, STAT4, or both transcription factors up to 6 months following infection, indicating that progression to memory occurs independently of these molecules.
Eomes expression by CD8 T cells can substitute for Tbet to mediate transcription of Ifng, as well as other known effector genes [28]. However, we have revealed a critical role for Tbet to induce maximal IFN-γ production following either TCR-dependent or cytokine-dependent, TCR-independent stimulation. In mice lacking Tbet and STAT4, not all virus-specific CD8 T cells were capable of producing IFN-γ, and there was a reduction in the mean fluorescence intensity of IFN-γ staining. This signifies that on a per-cell basis, Tbet and STAT4 function cooperatively for maximal IFN-γ production after antigen-specific TCR stimulation. Moreover, we observed that Tbet was necessary for maximum induction of IFN-γ by effector CD8 T cells in response to IL-12/IL-18/IL-21 stimulation, even in the presence of Eomes. Preliminary data indicate that there were no differences in the levels of IL-12Rβ2 and IL-18Rα on the Ld(NP118) tetramer-positive effector CD8 T cells (data not shown). This suggests that Tbet and Eomes are not redundant in all aspects of effector CD8 T cell function and further highlights a role for Tbet in CD8 T cell IFN-γ production.
The observation that antigen-specific CD4 T cells were able to produce IFN-γ upon peptide stimulation in the absence of STAT4 is intriguing. It has been reported that STAT4 is required for maximal IFN-γ production by T cells and that IL-12-mediated proliferation and IFN-γ induction are dependent on STAT4 [29, 30]. However, previous publications have demonstrated that IL-12 is not necessary for IFN-γ production following acute LCMV infection [31–33]. Nevertheless, it has been shown that if IL-4 or STAT6 signaling is blunted then STAT4 is no longer essential for IFN-γ secretion [15]. We speculate that the ability of CD4 T cells to produce IFN-γ in the absence of STAT4 following LCMV infection is the result of a combination of factors. First, strong, antigenic stimulation has been shown to overcome the requirement of STAT4 for IFN-γ secretion, and it has been demonstrated that LCMV infection elicits responses with high functional avidity [34–36]. Additionally, LCMV induces large amounts of type I IFN, which signals via STAT1/2 heterodimers early following infection [37–39]. This may then induce a transcriptional program in the virus-specific effector T cells that is able to induce IFN-γ production independently of STAT4. Future experiments will need to be performed to assess the accessibility of the Ifng gene in the absence of STAT4 and to determine whether STAT1 is able to facilitate reorganization of the loci under these circumstances.
Many studies have demonstrated the importance of the transcription factors Tbet and STAT4 for the development of effector CD4 T cells of the Th1 lineage in vitro and in vivo [13, 14, 39, 40]; however, the requirement of these molecules for the formation of CD4 T cell memory has not been examined extensively. We found that LCMV-specific effector and memory CD4 T cells capable of producing IL-2 and TNF-α are generated in the absence of Tbet or STAT4, but these responses are reduced in mice lacking both of the transcription factors. This reveals redundant roles for Tbet and STAT4 in the formation and/or maintenance of the functional, virus-specific CD4 T cell response. Intriguingly, TbetKO does not result in an elevated number of IL-2-producing effector and memory CD4 T cells, which is in contrast to the LCMV-specific CD8 T cell response. The underlying cause of this differential role for Tbet in the repression of IL-2 production in CD8 but not CD4 T cells is not known. This dichotomy could be a result of differences in accessibility of the Il2 gene loci, the precursor frequency of the LCMV-specific CD4 and CD8 T cell populations, or the strength of the interaction between the TCR and the peptide:MHC complex.
This study advances our understanding of the roles of Tbet and STAT4 during antiviral T cell responses by showing that these transcription factors regulate distinct phenotypic and functional aspects of LCMV-specific effector CD8 T cells. Nevertheless, robust CD8 T cell responses are elicited, and immunological memory is established in the absence of these transcription factors. Additionally, we have highlighted the separation between the production of the hallmark Th1 cytokine IFN-γ by CD4 T cells and the generation and maintenance of virus-specific CD4 T cells.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the U.S. National Institutes of Health Grants R01 DK084082 (to L.E.H.), T32 AI07051 (to S.B.M.), and R01 AI04360 and U01 AI082966 (to A.J.Z.).
We thank the other members of the L.E.H. and A.J.Z. laboratories for helpful discussions and critical reading of this manuscript.
SEE CORRESPONDING EDITORIAL ON PAGE 699
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- ARM
- Armstrong
- DKO
- double-knockout (deficient)
- Eomes
- eomesodermin
- KLRG1
- killer cell lectin-like receptor subfamily G member 1
- LCMV
- lymphocytic choriomeningitis virus
- STAT4KO
- C.129S6-STAT4tm1Gru (STAT4 knockout)
- TbetKO
- C.129S6-Tbx21tm1Glm/J (Tbet knockout)
- UAB
- University of Alabama at Birmingham
AUTHORSHIP
S.B.M., J.T.I., A.J.Z., and L.E.H. designed research. S.B.M., J.T.I., and R.L.K. performed research. S.B.M. and J.T.I. analyzed data. S.B.M. and L.E.H. wrote the paper.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- 1. Zhu J., Paul W. E. (2008) CD4 T cells: fates, functions, and faults. Blood 112, 1557–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu J., Yamane H., Paul W. E. (2010) Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 28, 445–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harty J. T., Tvinnereim A. R., White D. W. (2000) CD8+ T cell effector mechanisms in resistance to infection. Annu. Rev. Immunol. 18, 275–308 [DOI] [PubMed] [Google Scholar]
- 4. Intlekofer A. M., Banerjee A., Takemoto N., Gordon S. M., Dejong C. S., Shin H., Hunter C. A., Wherry E. J., Lindsten T., Reiner S. L. (2008) Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science 321, 408–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Takemoto N., Intlekofer A. M., Northrup J. T., Wherry E. J., Reiner S. L. (2006) Cutting edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J. Immunol. 177, 7515–7519 [DOI] [PubMed] [Google Scholar]
- 6. Joshi N. S., Cui W., Chandele A., Lee H. K., Urso D. R., Hagman J., Gapin L., Kaech S. M. (2007) Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity 27, 281–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Banerjee A., Gordon S. M., Intlekofer A. M., Paley M. A., Mooney E. C., Lindsten T., Wherry E. J., Reiner S. L. (2010) Cutting edge: the transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J. Immunol. 185, 4988–4992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lighvani A. A., Frucht D. M., Jankovic D., Yamane H., Aliberti J., Hissong B. D., Nguyen B. V., Gadina M., Sher A., Paul W. E., O'Shea J. J. (2001) T-bet is rapidly induced by interferon-γ in lymphoid and myeloid cells. Proc. Natl. Acad. Sci. USA 98, 15137–15142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hermann-Kleiter N., Baier G. (2010) NFAT pulls the strings during CD4+ T helper cell effector functions. Blood 115, 2989–2997 [DOI] [PubMed] [Google Scholar]
- 10. Lazarevic V., Glimcher L. H. (2011) T-bet in disease. Nat. Immunol. 12, 597–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mullen A. C., High F. A., Hutchins A. S., Lee H. W., Villarino A. V., Livingston D. M., Kung A. L., Cereb N., Yao T. P., Yang S. Y., Reiner S. L. (2001) Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science 292, 1907–1910 [DOI] [PubMed] [Google Scholar]
- 12. Schulz E. G., Mariani L., Radbruch A., Hofer T. (2009) Sequential polarization and imprinting of type 1 T helper lymphocytes by interferon-γ and interleukin-12. Immunity 30, 673–683 [DOI] [PubMed] [Google Scholar]
- 13. Lawless V. A., Zhang S., Ozes O. N., Bruns H. A., Oldham I., Hoey T., Grusby M. J., Kaplan M. H. (2000) Stat4 regulates multiple components of IFN-γ-inducing signaling pathways. J. Immunol. 165, 6803–6808 [DOI] [PubMed] [Google Scholar]
- 14. Kaplan M. H. (2005) STAT4: a critical regulator of inflammation in vivo. Immunol. Res. 31, 231–242 [DOI] [PubMed] [Google Scholar]
- 15. Kaplan M. H., Wurster A. L., Grusby M. J. (1998) A signal transducer and activator of transcription (Stat)4-independent pathway for the development of T helper type 1 cells. J. Exp. Med. 188, 1191–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Usui T., Nishikomori R., Kitani A., Strober W. (2003) GATA-3 suppresses Th1 development by downregulation of Stat4 and not through effects on IL-12Rβ2 chain or T-bet. Immunity 18, 415–428 [DOI] [PubMed] [Google Scholar]
- 17. Fuller M. J., Khanolkar A., Tebo A. E., Zajac A. J. (2004) Maintenance, loss, and resurgence of T cell responses during acute, protracted, and chronic viral infections. J. Immunol. 172, 4204–4214 [DOI] [PubMed] [Google Scholar]
- 18. Mothe B. R., Stewart B. S., Oseroff C., Bui H. H., Stogiera S., Garcia Z., Dow C., Rodriguez-Carreno M. P., Kotturi M., Pasquetto V., Botten J., Crotty S., Janssen E., Buchmeier M. J., Sette A. (2007) Chronic lymphocytic choriomeningitis virus infection actively down-regulates CD4+ T cell responses directed against a broad range of epitopes. J. Immunol. 179, 1058–1067 [DOI] [PubMed] [Google Scholar]
- 19. Whitton J. L., Tishon A., Lewicki H., Gebhard J., Cook T., Salvato M., Joly E., Oldstone M. B. (1989) Molecular analyses of a five-amino-acid cytotoxic T-lymphocyte (CTL) epitope: an immunodominant region which induces nonreciprocal CTL cross-reactivity. J. Virol. 63, 4303–4310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yeh W. I., McWilliams I. L., Harrington L. E. (2011) Autoreactive Tbet-positive CD4 T cells develop independent of classic Th1 cytokine signaling during experimental autoimmune encephalomyelitis. J. Immunol. 187, 4998–5006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lertmemongkolchai G., Cai G., Hunter C. A., Bancroft G. J. (2001) Bystander activation of CD8+ T cells contributes to the rapid production of IFN-γ in response to bacterial pathogens. J. Immunol. 166, 1097–1105 [DOI] [PubMed] [Google Scholar]
- 22. Berg R. E., Forman J. (2006) The role of CD8 T cells in innate immunity and in antigen non-specific protection. Curr. Opin. Immunol. 18, 338–343 [DOI] [PubMed] [Google Scholar]
- 23. Ingram J. T., Yi J. S., Zajac A. J. (2011) Exhausted CD8 T cells downregulate the IL-18 receptor and become unresponsive to inflammatory cytokines and bacterial co-infections. PLoS Pathog. 7, e1002273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sullivan B. M., Juedes A., Szabo S. J., von Herrath M., Glimcher L. H. (2003) Antigen-driven effector CD8 T cell function regulated by T-bet. Proc. Natl. Acad. Sci. USA 100, 15818–15823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhu Y., Ju S., Chen E., Dai S., Li C., Morel P., Liu L., Zhang X., Lu B. (2010) T-bet and eomesodermin are required for T cell-mediated antitumor immune responses. J. Immunol. 185, 3174–3183 [DOI] [PubMed] [Google Scholar]
- 26. Kaech S. M., Cui W. (2012) Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 12, 749–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. D'Cruz L. M., Rubinstein M. P., Goldrath A. W. (2009) Surviving the crash: transitioning from effector to memory CD8+ T cell. Semin. Immunol. 21, 92–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pearce E. L., Mullen A. C., Martins G. A., Krawczyk C. M., Hutchins A. S., Zediak V. P., Banica M., DiCioccio C. B., Gross D. A., Mao C. A., Shen H., Cereb N., Yang S. Y., Lindsten T., Rossant J., Hunter C. A., Reiner S. L. (2003) Control of effector CD8+ T cell function by the transcription factor eomesodermin. Science 302, 1041–1043 [DOI] [PubMed] [Google Scholar]
- 29. Kaplan M. H., Sun Y. L., Hoey T., Grusby M. J. (1996) Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature 382, 174–177 [DOI] [PubMed] [Google Scholar]
- 30. Afkarian M., Sedy J. R., Yang J., Jacobson N. G., Cereb N., Yang S. Y., Murphy T. L., Murphy K. M. (2002) T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat. Immunol. 3, 549–557 [DOI] [PubMed] [Google Scholar]
- 31. Oxenius A., Karrer U., Zinkernagel R. M., Hengartner H. (1999) IL-12 is not required for induction of type 1 cytokine responses in viral infections. J. Immunol. 162, 965–973 [PubMed] [Google Scholar]
- 32. Orange J. S., Biron C. A. (1996) An absolute and restricted requirement for IL-12 in natural killer cell IFN-γ production and antiviral defense. Studies of natural killer and T cell responses in contrasting viral infections. J. Immunol. 156, 1138–1142 [PubMed] [Google Scholar]
- 33. Cousens L. P., Peterson R., Hsu S., Dorner A., Altman J. D., Ahmed R., Biron C. A. (1999) Two roads diverged: interferon α/β- and interleukin 12-mediated pathways in promoting T cell interferon γ responses during viral infection. J. Exp. Med. 189, 1315–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bot A., Rodrigo E., Wolfe T., Bot S., Von Herrath M. G. (2003) Infection-triggered regulatory mechanisms override the role of STAT 4 in control of the immune response to influenza virus antigens. J. Virol. 77, 5794–5800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Williams M. A., Ravkov E. V., Bevan M. J. (2008) Rapid culling of the CD4+ T cell repertoire in the transition from effector to memory. Immunity 28, 533–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. O'Malley J. T., Eri R. D., Stritesky G. L., Mathur A. N., Chang H. C., Hogenesch H., Srinivasan M., Kaplan M. H. (2008) STAT4 isoforms differentially regulate Th1 cytokine production and the severity of inflammatory bowel disease. J. Immunol. 181, 5062–5070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kolumam G. A., Thomas S., Thompson L. J., Sprent J., Murali-Krishna K. (2005) Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 202, 637–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Uddin S., Platanias L. C. (2004) Mechanisms of type-I interferon signal transduction. J. Biochem. Mol. Biol. 37, 635–641 [DOI] [PubMed] [Google Scholar]
- 39. Nguyen K. B., Watford W. T., Salomon R., Hofmann S. R., Pien G. C., Morinobu A., Gadina M., O'Shea J. J., Biron C. A. (2002) Critical role for STAT4 activation by type 1 interferons in the interferon-γ response to viral infection. Science 297, 2063–2066 [DOI] [PubMed] [Google Scholar]
- 40. Carter L. L., Murphy K. M. (1999) Lineage-specific requirement for signal transducer and activator of transcription (Stat)4 in interferon γ production from CD4(+) versus CD8(+) T cells. J. Exp. Med. 189, 1355–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.