Abstract
The ability to jointly consider several structured mental representations, or relations, is fundamental to human cognition. Prior studies have consistently linked this capacity for relational integration to rostrolateral prefrontal cortex (RLPFC). Here, we sought to test two competing hypotheses: (1) RLPFC processes relations in a domain‐general manner, interacting with different brain regions as a function of the type of lower‐level relations that must be integrated; or (2) A dorsal‐ventral gradient exists within RLPFC, such that relational integration in the visuospatial domain involves relatively more dorsal RLPFC than integration in the semantic domain. To this end, we examined patterns of fMRI activation and functional connectivity during performance of visuospatial and semantic variants of a relational matching task. Across the two task variants, the regions that were most strongly engaged during relational comparison were left RLPFC and left intraparietal sulcus (IPS). Within left RLPFC, there was considerable overlap in activation for the semantic and visuospatial tasks. However, visuospatial task activation peaks were located dorsally to the semantic task peaks. In addition, RLPFC exhibited differential functional connectivity on the two tasks, interacting with different brain regions as a function of the type of relations being compared. While neurons throughout RLPFC may share the function of integrating diverse inputs, individual RLPFC neurons may have privileged access to particular representations depending on their anatomical inputs, organized along a dorsal‐ventral gradient. Thus, RLPFC is well‐positioned as a locus of abstraction from concrete, domain‐specific details to the general principles and rules that enable higher‐level cognition. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc
Keywords: fMRI, relational reasoning, functional connectivity, abstraction, domain‐sensitivity, BA 10, aPFC, semantic, visuospatial, representation
INTRODUCTION
The ability to recognize and reason with relations is a central component of many complex cognitive tasks. Of particular importance to the most complex and uniquely human mental operations is the capacity to compare or integrate distinct structured mental representations: i.e., second‐order relational processing (Gentner and Holyoak, 1997; Halford et al., 1998; Penn et al., 2008; Robin and Holyoak, 1995]. Although first‐order relational processing involves encoding and manipulating individual relationships, second‐order relational processing involves joint consideration of multiple relationships, such as occurs when distinct relationships are compared, combined, or incorporated into a more complex information structure (i.e. relations among first‐order relations). It is the capacity for relational comparison or integration that is thought to underlie the human capacity for abstract thought [Penn et al., 2008].
Although multiple regions in lateral prefrontal and parietal cortices are engaged during performance of relational reasoning tasks, fMRI studies involving adults have shown that one brain region in particular, rostrolateral prefrontal cortex (RLPFC), corresponding to the lateral aspect of anterior prefrontal cortex (BA 10/46 and 10/47), is engaged specifically by the need to compare or integrate previously distinct relations for pairs of items. fMRI studies of the Raven's Progressive Matrices (RPM) task demonstrate RLPFC activation when subjects have to integrate two relational patterns (Christoff et al., 2001; Crone et al., 2009; Kroger et al., 2002]. Studies of analogical reasoning have demonstrated activation of this region associated with the integration of semantic relations in propositional analogy tasks involving either words [Bunge et al., 2005; Green et al., 2006; Wendelken et al., 2008b] or pictures of nameable objects [Wright et al., 2008]. A recent study of transitive inference, which involves the integration of multiple relations to reach a logical conclusion, has also been shown to particularly engage RLPFC [Wendelken and Bunge, 2009]. Finally, the contrast between joint and separate consideration of two relations in a simple relational matching task reliably engages RLPFC (Bunge et al., 2009; Christoff et al., 2003; Smith et al., 2007].
In principle, the information structures required for second‐order relational processing are independent of the items and relations being processed. By several accounts, RLPFC processes information at the highest level of abstraction (see (Badre and D'Esposito 2007; Koechlin et al., 2003]); we could expect the same neural circuits that integrate semantic relationships (as in a verbal analogy task) to also integrate visuospatial relationships (as in the RPM task). Consistent with this hypothesis, the one time that we have found a functional dissociation between left and right RLPFC, it consisted of a difference in the specificity of relational processing, with left but not right RLPFC meeting a stringent test of the relational integration hypothesis [Bunge et al., 2009]. Indeed, we have seen no evidence for differences in lateralization in RLPFC that correspond to those seen for ventrolateral PFC (VLPFC) in humans, with verbal/semantic information preferentially processed in the left hemisphere and nonverbal information preferentially processed in the right (Morimoto et al., 2008; Smith and Jonides, 1997].
On the other hand, we had previously observed, in an informal meta‐analysis of fMRI studies of relational integration, different coordinates of peak activation for experiments involving visuospatial stimuli and those involving verbal/semantic stimuli [Wendelken et al., 2008b]. Specifically, RLPFC activation peaks associated with verbal/semantic relational integration on propositional analogy tasks [Bunge et al., 2005; Wendelken et al., 2008b] tended to be located more ventrally than RLPFC activation peaks associated with visuospatial relational integration on Raven's Progressive Matrices (Christoff et al., 2001; Crone et al., 2009; Kroger et al., 2002] and relational matching tasks [Christoff et al., 2003]. On the basis of this pattern, we speculated that there might be a dorsal‐ventral distinction or gradation in RLPFC as a function of stimulus domain, as has been proposed for lateral PFC [Courtney et al., 1996; Yee et al., 2010]. By this account, relatively more dorsal RLPFC would preferentially process and integrate visuospatial relations, and more ventral RLPFC would preferentially process and integrate semantic relations. However, these visuospatial and semantic reasoning studies differed along multiple dimensions and were conducted in different participants. Therefore, the observed difference in peak coordinates did not provide compelling evidence of domain‐sensitivity within RLPFC. The primary goal of this study is to test the competing hypotheses of domain‐specificity versus domain‐generality of second‐order relational processing in RLPFC.
A second goal of the current experiment was to test whether RLPFC interacts differentially with brain regions that process first‐order relations, depending on the type of relations to be integrated. Broadly consistent with this claim, a prior fMRI study examining the ability to reorder items in working memory demonstrated that right lateral RLPFC was tightly coupled with left dorsolateral prefrontal cortex (DLPFC) when participants were instructed to reorder spatial memoranda, but more with left ventrolateral prefrontal cortex (VLPFC) when participants were instructed to reorder verbal memoranda [Sakai and Passingham, 2003]. Here, we sought to test for differences in functional connectivity when participants were asked to compare pairs of visuospatial versus semantic relations.
Semantic processing has been linked to the lateral temporal lobes and to left VLPFC (Binder et al., 2009; Cabeza and Nyberg, 2000; Gainotti et al., 1995; Tranel et al., 1997]. Thus, we hypothesized that RLPFC would be more tightly coupled with these regions when participants were required to retrieve and integrate knowledge about common objects and animals from semantic memory than when they reasoned about novel, abstract shapes.
By contrast, visuospatial processing has been linked most closely to the superior parietal lobule (SPL) (Amorapanth et al., 2010; Cabeza and Nyberg, 2000; Kesner, 2009; Sack, 2009] and also with the superior frontal sulcus (SFS) [Courtney et al., 1996; Goldman‐Rakic et al., 1991; Sala et al., 2003]. Both of our relational matching tasks, described below, require visuospatial processing of relations between four components of a spatial array. However, we designed the visuospatial task with the goal of taxing this form of processing more heavily than the semantic task. Thus, we hypothesized that RLPFC would be more tightly coupled with the SPL and SFS on second‐order visuospatial than semantic trials.
MATERIALS AND METHODS
Experimental Task
To test for the effects of stimulus domain on RLPFC activation and functional connectivity, we collected functional magnetic resonance imaging (fMRI) data in healthy adults for a relational matching task that included both semantic and visuospatial conditions. Our experimental task was adapted from a relational matching task that reliably engages RLPFC (Bunge et al., 2009; Christoff et al., 2003; Smith et al., 2007]. On each trial, participants viewed an array of four visual stimuli (see Fig. 1], and were asked to make similarity judgments about each of two pairs of items (two first‐order relational judgments) or pairs of relations between items (one second‐order relational judgment). On first‐order trials, participants were presented with two pairs of items and their task was to indicate, separately for each pair, whether they matched along a specified feature dimension. On second‐order trials, participants were presented with two pairs of items and were asked to indicate whether the items within each pair were related in the same way—i.e., whether the dimension of feature similarity for the second pair was the same as for the first pair.
Figure 1.
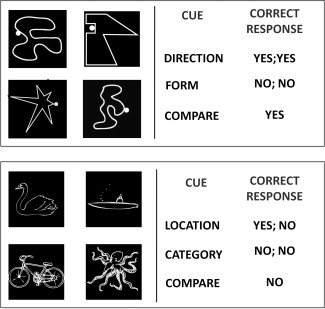
Elements of the visuospatial (top) and semantic (bottom) relational matching tasks. On the left are sample stimulus pictures. Each set of four pictures was presented with an instructional cue positioned between the top and bottom pairs. The first text column lists each of the possible cues, and the second column indicates the appropriate response for this stimulus array, given a particular cue.
The visuospatial (VIS) version of the task was designed to emphasize analysis of visuospatial relations, and to de‐emphasize conceptual knowledge. The items in this version of the task were abstract line drawings. On first‐order relational trials (VIS1), participants were asked to make one of two feature judgments for both the top and bottom pairs of stimuli: (1) Form: whether both drawings consisted of straight or curvy lines, and (2) Direction: whether both dots were on the left or right side of the drawings. On second‐order relational trials (VIS2), participants were asked whether the top and bottom pair of stimuli shared the same dimension of feature similarity (Form or Direction).
The semantic (SEM) version of the task was designed to emphasize conceptual knowledge, and to deemphasize analysis of visuospatial features. The items in this version of the task were pictures of animals and vehicles. On first‐order relational trials (SEM1), participants were asked to make one of two feature judgments for both the top and bottom pairs of stimuli: (1) Category—whether or not the picture was of an animal or vehicle, and 2) Location—whether or not the picture was of something that resides/operates on land or on water. On second‐order relational trials (SEM2), participants were asked whether the top and bottom pair of stimuli shared the same dimension of feature similarity (Category or Location).
That the stimuli differed between the VIS and SEM tasks is a crucial element of the task design, insofar as it helped to ensure that participants were not inadvertently processing semantic relations during the visuospatial task, or vice versa. The presence of second‐order trials, alongside second‐order trials, allowed us to control for stimulus differences between tasks, such that we would be able to examine domain differences at the level of the relational processing, as well as at the level of stimulus processing.
For a second‐order trial to be counted as correct, the single second‐order relational judgment (which hinged on the correct processing of two first‐order relations) needed to be correct. Thus, chance performance was 50% for second‐order trials. Response times (RTs) were measured from the onset of stimulus presentation until the last button press (i.e., the second button press for first‐order trials, and the only button press for second‐order trials). For a first‐order trial to be counted as correct, both independent first‐order relational judgments (i.e., for the top and the bottom pairs of stimuli) had to be correct. Thus, chance performance was 25% for first‐order trials. RTs were measured from the onset of stimulus presentation until the button press for the second relational judgment.
In previous versions of the relational matching task (Bunge et al., 2009; Christoff et al., 2003; Smith et al., 2007], subjects were only asked to provide a single response for first‐order trials, answering, in effect, “Is there a match on the top or bottom pair?” As such, it was possible for participants to respond correctly without considering both pairs, in which case observed second‐order > first‐order differences could be explained by an increase in the number of single relations processed (since consideration of both relations was always necessary for second‐order trials) rather than by the added demand for relational integration. To avoid this potential confound in this study, we required subjects to provide two responses on first‐order trials. Thus, the current experiment, which compared trials requiring one second‐order relational judgment to trials requiring two first‐order relational judgments, provides the clearest test yet that it is the need to jointly consider two relations, and not simply the need to consider multiple relations, that drives activation in RLPFC.
The VIS and SEM tasks were presented in separate scans so as to minimize task‐switching and rule maintenance demands. First‐ and second‐order trials were randomly intermixed within a scan. An experimental trial proceeded as follows: first, subjects were presented with a cue stimulus for 500 ms—Form/Direction/Compare for the VIS scan, or Category/Location/Compare for the SEM scan. This cue remained present onscreen for the duration of the trial. Subsequently, an array of four items was presented on the screen. Participants responded twice via button press for first‐order trials (making one yes/no judgment of similarity for the top pair of items and another for the bottom pair), and once for second‐order trials (making a yes/no judgment of similarity in the first‐order relationships between pairs of items). They were given up to 5.5 s to make this/these response(s), and the trial terminated as soon as they had done so. The intertrial interval was randomly jittered between 1 and 5 s.
Data Collection
Twenty‐two right‐handed young adults were scanned on a Siemens 3T Trio at the UC‐Berkeley Brain Imaging Center. High‐resolution anatomical images (MPRAGE) were acquired first from each subject, followed by acquisition of echoplanar functional images during performance of the task. Two 8‐min functional scans were collected for each participant, one for SEM trials and one for VIS trials. The order of these two scans was counterbalanced across participants. For the functional images, thirty‐two 3.45 mm axial slices (3 mm thick with a 0.45‐mm gap between slices) were collected (with TR = 1.5 s, TE = 25 ms, FOV = 230 mm, and 128 × 128 voxels). Visual stimuli were projected to a screen that participants were able to view by means of a mirror. Subjects responded by pressing one of two buttons on a button box that was held in the right hand. Stimulus presentation and response acquisition were controlled using Presentation® software (www.neurobs.com)
Data Analysis
Data were preprocessed and analyzed using SPM5 (Wellcome Department of Cognitive Neurology, London). Functional images were corrected for differences in slice acquisition timing and were realigned to the first volume by means of rigid body motion correction with sinc interpolation. Structural images were co‐registered to the functional images and then spatially normalized to SPM5′s T1 template. These normalization parameters were then applied to the functional images. Functional images were spatially smoothed with an 8‐mm full‐width half‐maximum isotropic Gaussian kernel. The data were then high‐pass filtered with a limit of 120‐s and submitted to statistical analyses.
Whole‐brain exploratory analysis was performed using a general linear model that incorporated task effects, session effects, and a general linear trend. Task effects were modeled via epoch regressors, aligned to the onset of each trial and with durations equal to response times. Incorporating response time into the model in this manner means that differences in parameter estimates cannot be driven by increased time‐on‐task, as it relates to response time. Separate regressors were specified for first‐order and second‐order trials separately for the SEM and VIS tasks, and there was a separate regressor of no interest modeling incorrect trials. These regressors were convolved with SPM's canonical hemodynamic response function (HRF) to produce a general linear model (GLM) of the BOLD response associated with each condition.
This GLM was used to compute the least‐squares parameter estimate of the height of the best‐fitting synthetic response function for each condition at each voxel. Parameter estimates associated with each experimental condition were combined to produce contrast images for target contrasts. Group‐level t‐tests were performed on these contrast images to produce group activation maps. All activation clusters that survived a voxel‐level threshold of P < 0.001 (uncorrected) with a 10‐voxel extent threshold are reported; in addition, we indicate which activations clusters survive a whole‐brain FWE correction for multiple comparisons at P < 0.05.
Region‐of‐interest (ROI) analyses were performed using Marsbar (http://marsbar.sourceforge.net). Functionally defined ROIs were obtained from activation clusters identified in the whole‐brain contrasts. Anatomical template regions were obtained from the AAL repository [Tzourio‐Mazoyer et al., 2002], included with the Marsbar distribution. The mean signal across all voxels in a defined region was submitted to the GLM analysis as described earlier to produce an ROI parameter estimate for each experimental condition for each subject. These ROI parameter estimates were then submitted to repeated measures ANOVA in SPSS. Event‐related timecourses were extracted from selected regions by averaging across trial‐specific timeseries (i.e. the 15 s of detrended raw signal following each trial onset) for each condition.
We sought to test for positional differences in peak activations associated with the SEM and VIS tasks. Inter‐individual differences in the location of functional activation within an ROI could potentially mask systematic domain‐related differences in the group analysis. Thus, we conducted an analysis at the single‐subject level focused on peak activations in anterior middle frontal gyrus (aMFG), defined as MFG anterior to y = 40 mm, as well as in pMFG (MFG posterior to y = 40 mm). For each ROI and each contrast of interest, we first obtained the MNI coordinates of the peak activation for each subject (i.e., the voxel with the highest T‐statistic value within the ROI). Next, because MFG is angled relative to MNI coordinate system, we transformed each set of coordinates from MNI‐space into “gyrus‐space,” separately for aMFG and pMFG. Transformation of the coordinates into gyrus space would enable us test for dorsal/ventral differences with respect to the local orientation of the gyrus. This process entailed selecting points along the gyrus to define an axis, calculating an affine transformation matrix based on these coordinates, and then applying that transformation matrix to each of the original coordinates that we had obtained. We took care to ensure that the transformation preserved all distance relationships between points. Transformed coordinate values, for selected pairs of contrasts—in particular, VIS2 > VIS1 and SEM2 > SEM1—were submitted to repeated measures ANOVAs within SPSS.
To assess correlated activity between an ROI and other brain regions, we used the β‐correlation method [Rissman et al., 2004], implemented via SPM5 and custom MATLAB scripts. For each subject, SPM's canonical HRF was fit to each occurrence of each condition, and the resulting parameter estimates (betas) were sorted according to condition to produce a condition‐specific β‐series for each voxel. The β‐series associated with a functional ROI seed were correlated with voxels across the brain to produce β‐correlation images. Contrasts between β‐correlation images were subjected to an arc‐hyperbolic tangent transform [Fisher, 1921] to allow for statistical inference based on the correlation magnitudes [Rissman et al., 2004]. Group‐level t‐tests were performed on the resulting subject contrast images to produce group correlation contrast maps.
RESULTS
Behavioral Performance
Accuracy and response times (RTs) for the relational matching task are presented in Table I. There were no significant differences in performance (all F's < 1) between the first‐order SEM and VIS trials. Thus, we collapsed across these trial type pairs for all subsequent analyses. There was no difference in accuracy between first‐ and second‐order trials (F 1,22 < 1), but participants were slower on second‐order than first‐order trials (F 1,22 = 12.2, P = 0.002). The SEM task was more difficult than the VIS task, both in terms of accuracy (F 1,22 = 47.0, P < 0.001) and RTs (F 1,22 = 47.1, P < 0.001). This finding is not surprising, given that the SEM task required retrieval of relevant knowledge about each stimulus from long‐term memory. There was also a significant interaction between stimulus domain and relational complexity (F 1,22 = 8.1, P = 0.009), such that integration demands had a greater effect on RTs for VIS than SEM trials.
Table I.
Behavioral performance for each experimental condition
| Condition | Instruction | Mean accuracy (± std. error) | Mean RT (± std. error) |
|---|---|---|---|
| SEM1 | Location | 85% (±2%) | 3.78 s (±0.18 s) |
| SEM1 | Category | 84% (±3%) | 3.67 s (±0.16 s) |
| SEM2 | Compare | 86% (±2%) | 3.77 s (±0.14 s) |
| VIS1 | Direction | 95% (±1%) | 2.88 s (±0.10 s) |
| VIS1 | Form | 92% (±1%) | 2.96 s (±0.13 s) |
| VIS2 | Compare | 93% (±2%) | 3.25 s (±0.10 s) |
Whole‐Brain fMRI Results
We first sought to determine which brain regions were engaged on second‐ relative to first‐order trials, collapsing across VIS and SEM conditions. The only clusters that survived FWE correction were in left RLPFC and in the vicinity of the intraparietal sulcus (IPS; BA 40/7). However, a broader network that also includes right RLPFC, bilateral DLPFC, dorsomedial PFC, and bilateral SPL is evident in the map of subthreshold activation (Supp. Info. Fig. 1). Deactivations for this contrast were observed in anterior medial PFC and posterior cingulate cortex, key nodes of the so‐called default network that is frequently deactivated by demanding tasks. Relative deactivation was also observed in bilateral, but especially left, motor and somatosensory cortices, consistent with the fact that two right‐hand responses were required on first‐order trials, and only one on second‐order trials.
To compare brain activation related to relational integration across the VIS and SEM tasks, we computed the contrast of second‐order >first‐order trials separately for each task (Fig. 2A). For SEM trials (Table IIB), integration‐related activation was observed in left RLPFC and left inferior pariatel lobule (IPL), bordering the intrapariatal sulcus (IPS), as well as a small cluster in left DLPFC (BA 9). Only the RLPFC cluster survived whole‐brain FWE correction for multiple comparisons for SEM2 > SEM1. For VIS trials (Table IIC), integration‐related activation was more widespread, including bilateral RLPFC, bilateral DLPFC, bilateral VLPFC, medial frontal gyrus, bilateral posterior parietal cortex, and bilateral middle occipital gyrus. Only left RLPFC and left IPS clusters survived correction for multiple comparisons for VIS2 > VIS1. Figure 2B, which shows BOLD activation timecourses for each condition from the region of overlap, reveals that VIS and SEM trials elicited a similar temporal activation profile.
Figure 2.
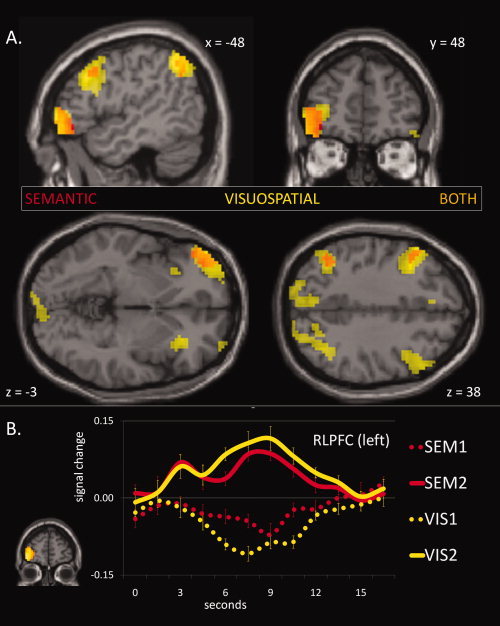
(A) Activation clusters associated with relational integration (second‐order >first‐order). Clusters associated with visuospatial integration (VIS2 > VIS1) are shown in yellow, while clusters associated with semantic integration (SEM2 > SEM1) are shown in red; overlap is shown in orange. Images are thresholded at P < 0.001 (uncorrected) with a 10‐voxel extent threshold. (B) BOLD activation timecourse associated with the RLPFC activation cluster. Error bars show the standard error of the mean at each timepoint.
Table II.
Peak coordinates for the whole‐brain comparison of second > first‐order relation trials, thresholded at P < 0.001 (uncorrected) with an extent threshold of 10 voxels
| Region | x, y, z | T‐statistic (peak voxel) | Cluster size (no. of voxels) |
|---|---|---|---|
| A. (SEM2 + VIS2) > (SEM1 + VIS1) | |||
| Left RLPFC (BA 10,11,47) | −42, 54, −3 | 8.09 | 373 |
| Right RLPFC (BA 10,11) | 45, 51, −12 | 4.45 | 15 |
| Left DLPFC (BA 9) | −54, 24, 33 | 6.49 | 232 |
| Right DLPFC (BA 9) | 51, 30, 36 | 4.59 | 43 |
| Dorsomedial PFC (BA 8) | −6, 27, 45 | 6.51 | 144 |
| Left IFG (BA 47, 13) | −30, 27, 0 | 4.85 | 16 |
| Right IFG (BA 47) | 36, 24, −3 | 5.03 | 21 |
| Left PPC (BA 40, 7) | −45, −60, 48 | 6.99 | 260 |
| Left PPC (BA 7, 19) | −9, −75, 39 | 5.52 | 73 |
| Right PPC (BA 40, 7) | 36, −57, 48 | 5.12 | 251 |
| B. SEM2 > SEM1 | |||
| Left RLPFC (BA 10,47,11) | −45, 48, −15 | 5.42 | 177 |
| Left MFG (BA 9) | −33, 15, 30 | 4.37 | 14 |
| Left PPC (BA 40, 39) | −51, −60, 48 | 5.1 | 48 |
| C. VIS2 > VIS1− | |||
| Left RLPFC (BA 10,11) | −48, 48, −3 | 6.65 | 357 |
| Left DLPFC (BA 9) | −39, 12, 33 | 5.83 | 365 |
| Right DLPFC (BA 9) | 54, 27, 36 | 4.81 | 134 |
| Dorsomedial PFC (BA 8) | −6, 30, 45 | 6.85 | 194 |
| Left IFG (BA 47, 13) | −30, 27, 3 | 5.84 | 25 |
| Right IFG (BA 47) | 30, 30, −3 | 4.52 | 25 |
| Left PPC (BA 40, 7, 39) | −33, −75, 51 | 7.51 | 1352 |
| Right PPC (BA 40, 7, 39) | 36, −60, 51 | 7.36 | (1352) |
| Left MOG (BA 19) | −51, −81, −9 | 5.45 | 80 |
| Right MOG (BA 19) | 48, −57, −15 | 4.58 | 77 |
| Medial MOG (BA 18) | 12, −99, 3 | 5.01 | 184 |
| D. (VIS2 ‐VIS1) > (SEM2 ‐SEM1), masked inclusively with VIS2 > VIS1 | |||
| Right DLPFC (BA 9) | 48, 24,30 | 4.42 | 56 |
| Right MOG (BA 19) | 27, −96, 15 | 4.60 | 26 |
| Right SPL (BA 7) | 36, −63, 54 | 3.87 | 12 |
The contrast (SEM2‐SEM1) > (VIS2‐VIS1) is not listed, as it yielded no significant clusters of activation.
To compare visuospatial and semantic integration, we examined interactions between task domain (VIS, SEM) and relational complexity (first‐order, second‐order). To probe for regions that were more engaged by semantic than visuospatial integration, we examined the interaction contrast (SEM2‐SEM1) > (VIS2‐VIS1). However, no regions were activated for this contrast. In terms of experimental power, this interaction contrast is equivalent to the main effect contrast (SEM2 + VIS2) > (SEM1 + VIS1), which produced robust activation; thus, it is unlikely that the null finding here is due simply to a lack of power. To probe for regions that were more engaged by visuospatial than semantic integration, we examined the interaction contrast (VIS2 ‐ VIS1) > (SEM2 ‐ SEM1). Three regions, right DLPFC (BA 9), right SPL (BA 7), and right MOG (BA 19), demonstrated a significantly greater effect of visuospatial than semantic integration (Table IID). A similar interaction was observed for response times; however, because response times were incorporated into the analysis model, it is unlikely that differing response times could have driven this result.
Test of Dorsal‐Ventral Gradient in Anterior MFG
To address our primary question, concerning the possibility of dorsal‐ventral differences within RLPFC in the locus of visuospatial and semantic integration, we compared RLPFC activation peaks, for each participant, from the VIS and SEM tasks. Specifically, we tested whether or not VIS activation peaks were dorsal to SEM activation peaks, with respect to the orientation of anterior MFG (Fig. 3A). Comparing the peaks associated with semantic integration to those associated with visuospatial integration (Fig. 3B), we did observe a highly significant difference in position transverse to the gyrus (F 1,21 = 9.1; P = 0.007), such that the visuospatial integration peak was located dorsally to the semantic integration peak in almost every subject (Fig. 3C). There was no difference in position longitudinal to the gyrus. We also examined activation peaks associated with the baseline contrasts (e.g. SEM1 > baseline). For the second‐order conditions (SEM2 > baseline and VIS2 > baseline), there was again a significant effect of domain on the position of the activation peak, transverse to the MFG (F 1,21 = 4.5; P < 0.05). However, there was no such effect for the first‐order conditions (SEM1 > baseline and VIS1 > baseline; F < 1).
Figure 3.
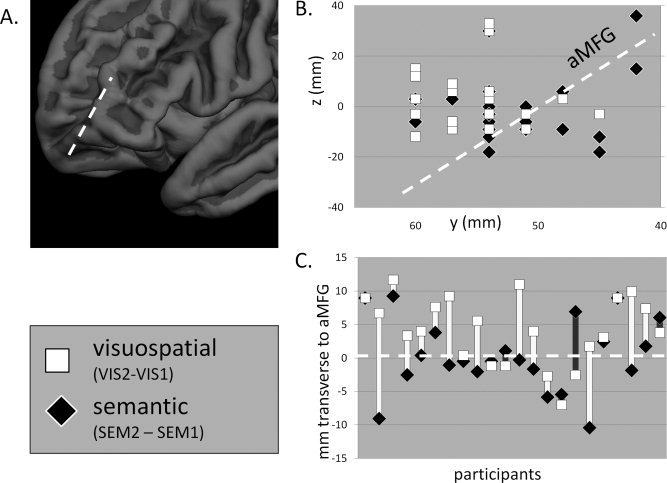
Results from the topographical analysis of left RLPFC. (A) Dotted line depicts the orientation of aMFG. (B) MNI y and z coordinates of activation peaks, from each subject, for the semantic integration (SEM2 > SEM1) and visuospatial integration (VIS2 > VIS1) contrasts. The dotted line corresponds to the orientation of aMFG. (C) Within‐subject differences, in the direction transverse to aMFG, between the peaks coordinates associated with semantic and visuospatial integration.
In addition to examining left anterior MFG, where the effects of integration demand were strongest, we also examined right anterior MFG, as well as left and right posterior MFG, in a similar manner. In each of these three regions, we observed no effect of domain on the position of peak activation, for the integration contrasts or for the baseline contrasts. In summary, among the lateral prefrontal regions that we examined, only left RLPFC—the region that had demonstrated the strongest activation for both semantic and visuospatial integration—also demonstrated a difference in the loci of semantic and visuospatial activation peaks, with peak activation for second‐order visuospatial processing observed dorsally to peak activation for second‐order semantic processing.
FMRI Results: ROI Analyses
To examine the contributions to visuospatial and semantic relational integration of regions in lateral PFC and parietal cortex that are commonly engaged on reasoning tasks, and to probe for hemispheric differences in the processing of the two domains, we next extracted parameter estimates from the following anatomically defined ROIs: left and right RLPFC (MFG anterior to y = 45 mm), VLPFC (BAs 44 & 45), DLPFC (BAs 9 & 46), IPL (BA 40), and SPL (BA 7). We submitted parameter estimates from each region to a 2 × 2 × 2 ANOVA (hemisphere × integration × stimulus domain); statistical results are presented in Table III. Among these ROIs, only RLPFC showed a significant main effect of integration demand, with stronger activation for second‐ than first‐order trials. Both IPL and VLPFC were engaged more strongly for first‐ than second‐order trials, perhaps related to the fact that first‐order trials required two separate judgments rather than just one. Only the parietal ROIs demonstrated a significant main effect of stimulus domain: indeed, both SPL and IPL were engaged more strongly by visuospatial than semantic stimuli. RLPFC and DLPFC demonstrated a significant interaction between integration demand and stimulus domain; in both cases, there was a bigger effect of relational demands for the VIS task than the SEM task. VLPFC and, to a lesser extent, DLPFC demonstrated an interaction between hemisphere and domain, with both regions showing a relative preference for semantic stimuli on the left and visuospatial stimuli on the right.
Table III.
Statistical results for separate 2 × 2 × 2 ANOVAs (hemisphere × integration demand × stimulus domain) applied to left and right‐side ROI pairs from five separate brain regions
| Region | Integration second > first‐order | Domain VIS > SEM | Domain × integration (VIS2 ‐VIS1) > (SEM2 ‐ SEM1) | Domain × hemisphere L: SEM > VIS, R: VIS > SEM |
|---|---|---|---|---|
| RLPFC | P < .001 | P = 0.02 | ||
| VLPFC | first> second (P = 0.02) | P = 0.006 | ||
| DLPFC | P = 0.008 | P = 0.03 | ||
| SPL | P = 0.07 | P = 0.002 | P = 0.07 | |
| IPL | first> second (P = 0.001) | P = 0.001 | . |
P‐values > 0.1 are not shown.
FMRI Results: Functional Connectivity
To better characterize the functional network within which RLPFC operated on this task, we performed a β‐series correlation analysis with left RLPFC as our seed region. The seed ROI comprised 177 voxels in the intersection between the RLPFC activation clusters obtained from the SEM2 > SEM1 and VIS2 > VIS1 contrasts. Across all trials, RLPFC activity was highly correlated with activity in a broad network that included the IPS area, lateral PFC, medial frontal gyrus, and middle temporal gyrus (Fig. 4A).
Figure 4.
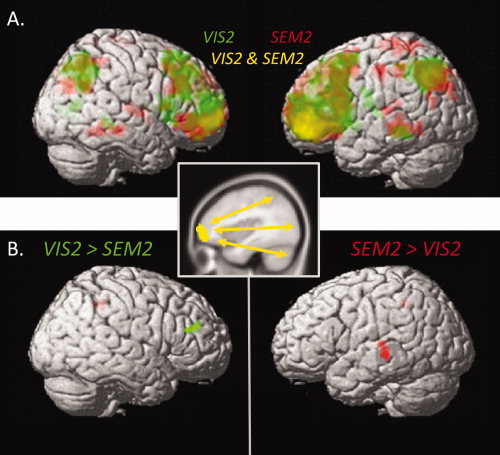
Functional connectivity maps, obtained via β‐correlations analysis with a left RLPFC seed (inset). (A) Overall connectivity with left RLPFC, separately for semantic (red) and visuospatial (green) trials, at P < 0.001 uncorrected. Areas of overlap are shown in yellow. (B) Clusters demonstrating differential connectivity with left RLPFC, for VIS2 > SEM2 (right DLPFC, green, P < 0.005 uncorrected) and SEM2 > VIS2 (left MTG, red, P < 0.001 uncorrected).
To test the hypothesis that RLPFC should be more correlated with semantic representational areas on SEM2 than VIS2 trials, and with visuospatial representational areas on VIS2 than SEM2, we contrasted the RLPFC seed β‐correlation patterns for the contrasts of SEM2 > baseline and VIS2 > baseline. Two regions, left MTG (−60, −30, −3; 17 voxels) and midline superior frontal gyrus (6, 57, 30; 27 voxels), proved to be significantly more correlated with RLPFC on SEM2 than VIS2 trials (Fig. 4B). At P < 0.001 uncorrected, no clusters exhibited stronger correlations with RLPFC on VIS2 than SEM2 trials. However, at a relaxed threshold of P < 0.005 uncorrected, right DLPFC (54, 36, 21; 10 voxels) was observed for this comparison (Fig. 4C).
DISCUSSION
The present findings demonstrate that there is a great deal of overlap in RLPFC for second‐order semantic and visuospatial relational processing. That there was so much overlap between the contrasts suggests that neurons involved in each process, whether the same cells or different ones, are distributed throughout RLPFC. Engagement of left RLPFC for semantic integration extended well into more dorsal aspects of the region, and engagement for visuospatial integration similarly extended well into the more ventral aspects of the region. At the same time, our results demonstrate that the RLPFC clusters involved in second‐order semantic and visuospatial relational processing are not equivalent. The fact that there was a systematic difference in the locus of activation peaks suggests that the distribution of cells involved in semantic and visuospatial integration is not uniform. This in turn suggests that, while many neurons may be involved in both visuospatial and semantic integration, there must be some neurons within RLPFC whose function is effectively domain‐specific by virtue of the provenance of its inputs. We propose that relational integration is a fundamental process that can be carried out, by neurons in RLPFC, on diverse kinds of inputs, depending on the information that a particular integrating circuit or assembly receives. The type of information received, in turn, depends on anatomical position: neurons located more ventrally in left RLPFC may have privileged access to first‐order semantic information represented in left VLPFC, whereas neurons located more dorsally may have privileged access to first‐order visuospatial info encoded in the superior frontal sulcus and nearby regions in DLPFC. Thus, we hypothesize that RLPFC neurons are domain‐general in principle, but that they exhibit domain‐sensitivity by virtue of a dorsal‐ventral gradient in their anatomical projections from the dorsal and ventral streams.
Understanding the extent to which processing in RLPFC is domain‐general or domain‐specific is important to understanding the role of RLPFC in abstraction [Badre et al., 2010]. The idea that RLPFC (and anterior PFC, more generally) operates at a high level of abstraction receives considerable support from many recent investigations. For example, for a set of decision tasks that ranges from concrete stimulus‐response mapping to abstract context‐based selection, only the most abstract task engages this region [Badre and D'Esposito, 2007]. Similarly, in a comparison of decision tasks that varied in their level of temporal abstraction, it was the most temporally abstract task that engaged anterior PFC [Koechlin et al., 2003]. Maintenance of short‐term bindings between verbal and spatial information has been shown to engage RLPFC [Prabhakaran et al., 2000], suggesting that temporary abstract representations might be instantiated here. Finally, in a study that compared unscrambling concrete words versus unscrambling abstract words, RLPFC was relatively more engaged when participants unscrambled abstract words [Christoff et al., 2009].
This study examined domain sensitivity within RLPFC during second‐order relational processing, which is known to engage RLPFC reliably (Bunge et al., 2005; Christoff et al., 2001; Smith et al., 2007; Wendelken and Bunge, 2009]. However, RLPFC is also engaged in other higher‐order processes, such as when participants are asked to switch between external and internal representations [Burgess et al., 2007], and when they must engage in cognitive branching [Koechlin et al., 1999; Koechlin and Hyafil, 2007]. Indeed, it is possible that RLPFC was more active on our second‐order than first‐order trials because the second‐order trials required more switching between feature dimensions [consistent with Pollmann et al., 2000], and/or between external and internal representations (consistent with Burgess et al., 2007]. Further, although we have emphasized the role of RLPFC in relational comparison, comparison of simpler mental structures can also engage this region [cf. Boorman et al., 2009; Bunge and Wendelken, 2009; Dobbins and Han, 2006].
For many studies that demonstrate activation of RLPFC, the contrast of interest can be characterized as difficult > easy [Gilbert et al., 2006]. However, there are many studies in which a difficult > easy contrast does not engage RLPFC, and we have previously demonstrated that an easier relational integration task engages RLPFC relative to a more difficult non‐integration task [Wendelken et al., 2008b]. We have proposed that a parsimonious account of RLPFC activation across various kinds of cognitive tasks is the comparison and/or integration of previously separate mental representations [Bunge and Wendelken, 2009]. However, this study was not designed to discriminate between these related accounts of RLPFC function.
We predicted that RLPFC would be most tightly coupled with the left middle temporal gyrus and/or left VLPFC during the joint consideration of semantic relations. In fact, left middle temporal gyrus demonstrated enhanced functional connectivity to left RLPFC on SEM2 relative to VIS2 trials. By contrast, right DLPFC—a region often engaged during spatial working memory [Funahashi et al., 1989; Scherf et al., 2006]—exhibited the opposite pattern, albeit not as strongly. The asymmetry in these functional connectivity differences between the SEM and VIS tasks likely stems from the fact that both the VIS and SEM tasks required visuospatial processing to some extent, whereas only the SEM task required retrieval from semantic memory. Overall, these findings indicate that while RLPFC can process both semantic and visuospatial information, it interacts more closely with different brain regions as a function of the type of relations being processed. This finding echoes that from an earlier study demonstrating differential connectivity of RLPFC during manipulation of visuospatial versus semantic information in working memory [Sakai and Passingham, 2006].
In addition to demonstrating task‐related differences in the patterns of communication between regions, functional connectivity analysis also reveals the broader network that includes RLPFC. Indeed, RLPFC activation across all task conditions was highly correlated with activation in the IPS area, lateral PFC, and dorsomedial prefrontal cortex. The broader network observed in the current study is highly similar to the frontoparietal control network identified in a recent study of intrinsic connectivity [Vincent et al., 2008] that is typically activated in tasks that require controlled processing of information. We hypothesize that RLPFC is a key node in this network, activated in particular by the most complex information processing tasks.
Outside lateral PFC, the region that demonstrated the strongest functional connectivity to left RLPFC was the left IPS area. This is also the region that was most strongly activated alongside left RLPFC during second‐order relational processing. Strong functional connectivity between RLPFC and IPS has been demonstrated on other tasks [Boorman et al., 2009], and even in the absence of task demands [Vincent et al., 2008]. We have shown previously that the IPS area is sensitive to increases in relational processing demands, rather than being selectively engaged during relational integration (Crone et al., 2009; Wendelken and Bunge, 2009]. Thus, the current results lend additional support to the idea that the IPS area actively represents or processes the structured mental representations—visuospatial and semantic—that RLPFC integrates in the service of higher cognition.
More generally, posterior parietal cortex, including both SPL and IPL, was engaged more strongly by visuospatial than semantic processing. This finding is consistent with a large body of prior work that points to this region as a key locus of spatial processing (Amorapanth et al., 2010; Cabeza and Nyberg, 2000; Kesner, 2009; Sack, 2009]. However, it remains the case that part of this region ‐ the IPS area ‐ was also involved in the processing of semantic relations. The present findings are in keeping with the hypothesis that spatial representations in parietal cortex serve as the foundation for relational representations of all types—spatial and nonspatial [Wendelken et al., 2008a].
In conclusion, the present fMRI data support the hypothesis that the ability to jointly consider previously disparate mental representations—a hallmark of human cognition (Penn et al.)—is supported by interactions between RLPFC and the regions that actively maintain these distinct mental representations. This capacity for higher‐order relational processing across stimulus domains enables generalization from one set of representations to another, which in turn supports learning and abstract thought.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figure 1
REFERENCES
- Amorapanth PX, Widick P, Chatterjee A ( 2010): The neural basis for spatial relations. J Cogn Neurosci 22: 1739–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, D'Esposito M ( 2007): Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. J Cogn Neurosci 19: 2082–2099. [DOI] [PubMed] [Google Scholar]
- Badre D, Kayser AS, D'Esposito M ( 2010): Frontal cortex and the discovery of abstract action rules. Neuron 66: 315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J, Desai R, Graves W, Conant L ( 2009): Where is the semantic system? A critical review and meta‐analysis of 120 functional neuroimaging studies. Cereb Cortex 19: 2767–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman ED, Behrens TEJ, Woolrich MW, Rushworth MFS ( 2009): How green is the grass on the other side? Frontopolar cortex and the evidence in favor of alternative courses of action. Neuron 62: 733–743. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Wendelken C ( 2009): Comparing the bird in the hand with the ones in the bush. Neuron 62: 609–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Wendelken C, Badre D, Wagner AD ( 2005): Analogical reasoning and prefrontal cortex: Evidence for separable retrieval and integration mechanisms. Cereb Cortex 15: 239–249. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Helskog EH, Wendelken C ( 2009): Left, but not right, rostrolateral prefrontal cortex meets a stringent test of the relational integration hypothesis. Neuroimage 46: 338–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PW, Dumontheil I, Gilbert SJ ( 2007): The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends Cogn Sci 11: 290–298. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L ( 2000): Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12: 1–47. [DOI] [PubMed] [Google Scholar]
- Christoff K, Prabhakaran V, Dorfman J, Zhao Z, Kroger JK, Holyoak KJ, Gabrieli JD ( 2001): Rostrolateral prefrontal cortex involvement in relational integration during reasoning. Neuroimage 14: 1136–1149. [DOI] [PubMed] [Google Scholar]
- Christoff K, Ream JM, Geddes LP, Gabrieli JD ( 2003): Evaluating self‐generated information: Anterior prefrontal contributions to human cognition. Behav Neurosci 117: 1161–1168. [DOI] [PubMed] [Google Scholar]
- Christoff K, Keramatian K, Gordon AM, Smith R, Mädler B ( 2009): Prefrontal organization of cognitive control according to levels of abstraction. Brain Res 1286: 94–105. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, Haxby JV ( 1996): Object and spatial visual working memory activate separate neural systems in human cortex. Cereb Cortex 6: 39–49. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, van Leijenhorst L, Honomichl RD, Christoff K, Bunge SA ( 2009): Neurocognitive development of relational reasoning. Dev Sci 12: 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins IG, Han S ( 2006): Isolating rule‐ versus evidence‐based prefrontal activity during episodic and lexical discrimination: A functional magnetic resonance imaging investigation of detection theory distinctions. Cereb Cortex 16: 1614–1622. [DOI] [PubMed] [Google Scholar]
- Fisher R ( 1921): On the “probable error” of a coefficient of correlation deduced from a small sample. Metron 1: 3–32. [Google Scholar]
- Funahashi S, Bruce CJ, Goldman‐Rakic PS ( 1989): Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol 61: 331–349. [DOI] [PubMed] [Google Scholar]
- Gainotti G, Silveri MC, Daniele A, Giustolisi L ( 1995): Neuroanatomical correlates of category‐specific semantic disorders: A critical survey. Memory 3: 247–264. [DOI] [PubMed] [Google Scholar]
- Gentner D, Holyoak KJ ( 1997): Reasoning and learning by analogy. Am Psychol 52: 32–34. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Simons JS, Frith CD, Burgess PW ( 2006): Performance‐related activity in medial rostral prefrontal cortex (area 10) during low‐demand tasks. J Exp Psychol Hum Percept Perform 32: 45–58. [DOI] [PubMed] [Google Scholar]
- Goldman‐Rakic P, Funahashi S, Bruce C ( 1991): Neocortical memory circuits. Q J Quant Biol 55: 1512–1515. [DOI] [PubMed] [Google Scholar]
- Green AE, Fugelsang JA, Kraemer DJ, Shamosh NA, Dunbar KN ( 2006): Frontopolar cortex mediates abstract integration in analogy. Brain Res 1096: 125–137. [DOI] [PubMed] [Google Scholar]
- Halford GS, Wilson WH, Phillips S ( 1998): Processing capacity defined by relational complexity: Implications for comparative, developmental, and cognitive psychology. Behav Brain Sci 21: 803–831; discussion 831. [DOI] [PubMed] [Google Scholar]
- Kesner R ( 2009): The posterior parietal cortex and long‐term memory representation of spatial information. Neurobiol Learn Mem 91: 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Hyafil A ( 2007): Anterior prefrontal function and the limits of human decision‐making. Science 318: 594–598. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J ( 1999): The role of the anterior prefrontal cortex in human cognition. Nature 399: 148–151. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F ( 2003): The architecture of cognitive control in the human prefrontal cortex. Science 302: 1181–1185. [DOI] [PubMed] [Google Scholar]
- Kroger JK, Sabb FW, Fales CL, Bookheimer SY, Cohen MS, Holyoak KJ ( 2002): Recruitment of anterior dorsolateral prefrontal cortex in human reasoning: A parametric study of relational complexity. Cereb Cortex 12: 477–485. [DOI] [PubMed] [Google Scholar]
- Morimoto HM, Hirose S, Chikazoe J, Jimura K, Asari T, Yamashita KI, Miyashita Y, Konishi S ( 2008): On verbal/nonverbal modality dependence of left and right inferior prefrontal activation during performance of flanker interference task. J Cogn Neurosci 20: 2006–2014. [DOI] [PubMed] [Google Scholar]
- Penn DC, Holyoak KJ, Povinelli DJ ( 2008): Darwin's mistake: Explaining the discontinuity between human and nonhuman minds. Behav Brain Sci 31: 109–130; discussion 130. [DOI] [PubMed] [Google Scholar]
- Pollmann S, Weidner R, Muller HJ, von Cramon DY ( 2000): A fronto‐posterior network involved in visual dimension changes. J Cogn Neurosci 12: 480–494. [DOI] [PubMed] [Google Scholar]
- Prabhakaran V, Narayanan K, Zhao Z, Gabrieli JD ( 2000): Integration of diverse information in working memory within the frontal lobe. Nat Neurosci 3: 85–90. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D'Esposito M ( 2004): Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage 23: 752–763. [DOI] [PubMed] [Google Scholar]
- Robin N, Holyoak KJ ( 1995): Relational complexity and the functions of prefrontal cortex In: Gazzaniga MS, editor. The Cognitive Neurosciences. Cambridge, MA: MIT Press; pp 987–999. [Google Scholar]
- Sack AT ( 2009): Parietal cortex and spatial cognition. Behav Brain Res 202: 153–161. [DOI] [PubMed] [Google Scholar]
- Sakai K, Passingham RE ( 2003): Prefrontal interactions reflect future task operations. Nat Neurosci 6: 75–81. [DOI] [PubMed] [Google Scholar]
- Sakai K, Passingham RE ( 2006): Prefrontal set activity predicts rule‐specific neural processing during subsequent cognitive performance. J Neurosci 26: 1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala JB, Rämä P, Courtney SM ( 2003): Functional topography of a distributed neural system for spatial and nonspatial information maintenance in working memory. Neuropsychologia 41: 341–356. [DOI] [PubMed] [Google Scholar]
- Scherf KS, Sweeney JA, Luna B ( 2006): Brain basis of developmental change in visuospatial working memory. J Cogn Neurosci 18: 1045–1058. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J ( 1997): Working memory: A view from neuroimaging. Cognit Psychol 33: 5–42. [DOI] [PubMed] [Google Scholar]
- Smith R, Keramatian K, Christoff K ( 2007): Localizing the rostrolateral prefrontal cortex at the individual level. Neuroimage 36: 1387–1396. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Damasio AR ( 1997): A neural basis for the retrieval of conceptual knowledge. Neuropsychologia 35: 1319–1327. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M ( 2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL ( 2008): Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol 100: 3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendelken C, Bunge SA ( 2010): Transitive inference: Distinct contributions of rostrolateral prefrontal cortex and the hippocampus. J Cogn Neurosci 22: 837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendelken C, Bunge SA, Carter CS ( 2008a) Maintaining structured information: an investigation into functions of parietal and lateral prefrontal cortices. Neuropsychologia 46: 665–678. [DOI] [PubMed] [Google Scholar]
- Wendelken C, Nakhabenko D, Donohue SE, Carter CS, Bunge SA ( 2008b) “Brain is to thought as stomach is to ??”: Investigating the role of rostrolateral prefrontal cortex in relational reasoning. J Cogn Neurosci 20: 682–693. [DOI] [PubMed] [Google Scholar]
- Wright S, Matlen B, Baym C, Ferrer E, Bunge S ( 2008): Neural correlates of fluid reasoning in children and adults. Frontiers Hum Neurosci 1: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee LTS, Roe K, Courtney SM ( 2010): Selective involvement of superior frontal cortex during working memory for shapes. J Neurophysiol 103: 557–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figure 1


