Abstract
Specialized sensory organs in the vertebrate head originate from thickenings in the embryonic ectoderm called cranial sensory placodes. These placodes, as well as the neural crest, arise from a zone of ectoderm that borders the neural plate. This zone separates into a precursor field for the neural crest that lies adjacent to the neural plate, and a precursor field for the placodes, called the pre-placodal region (PPR), that lies lateral to the neural crest. The neural crest domain and the PPR are established in response to signaling events mediated by BMPs, FGFs and Wnts, which differentially activate transcription factors in these territories. In the PPR, members of the Six and Eya families, act in part to repress neural crest specific transcription factors, thus solidifying a placode developmental program. Subsequently, in response to environmental cues the PPR is further subdivided into placodal territories with distinct characteristics, each expressing a specific repertoire of transcription factors that provides the necessary information for their progression to mature sensory organs. In this review we summarize recent advances in the characterization of the signaling molecules and transcriptional effectors that regulate PPR specification and its subdivision into placodal domains with distinct identities.
Keywords: Pre-placodal ectoderm, cranial sensory placodes, Six, Eya, Pax, Wnt, BMP, FGF, Gene regulatory network
I. Introduction
During the evolution of the vertebrate head, a number of specialized sensory organs arose that are derived from thickenings in the embryonic ectoderm called cranial sensory placodes. During gastrulation, the embryonic ectoderm is separated into neural and non-neural domains by signals from underlying tissues such as the organizer in frogs and the hypoblast in amniotes. Subsequent interactions lead to the expression of different sets of transcription factors in the neural and non-neural ectoderm whose expression overlaps in an intermediate ectodermal domain, herein referred to as the neural border (NB) zone. This region will give rise to a large number of cell types that include the precursors of the peripheral nervous system. The derivatives of this intermediate domain include the neural crest and the cranial sensory placodes. The precursor region of the placodes is first recognizable as a U-shaped domain restricted to the anterior border of the neural plate called the pre-placodal ectoderm or the pre-placodal region (PPR). The PPR subsequently breaks into individual patches of thickened ectoderm, called placodes, each of which gives rise to a specific subset of cells ranging from neurosecretory cells to sensory neurons to cranial sensory organs. In this review we will discuss what is known about how the PPR is specified, how it is molecularly distinct from the cranial neural crest with which it shares some early features, and how it is subdivided into specific cranial sensory placodes with very different developmental fates.
II. Cranial sensory placodes and their derivatives
The cranial sensory placodes give rise to several important sensory structures in the vertebrate head (reviewed in LeDouarin et al., 1986; Webb and Noden, 1993; Baker and Bronner-Fraser, 2001; Streit, 2004; Schlosser, 2005; Schlosser, 2010). The most anterior placodes include the single, midline adenohypophyseal placode, and the bilateral olfactory and lens placodes. The adenohypophyseal placode invaginates into the roof of the mouth as Rathke’s pouch, and eventually forms the anterior pituitary that contains several types of hormone secreting cells (e.g., somatotropin, prolactin, gonadotropins, thyrotropins, corticotropins). The olfactory placodes form just lateral to the adenohypophyseal placode, and invaginate as pits that eventually line parts of the nasal cavity as the olfactory sensory epithelium. This tissue produces supporting cells, basal stem cells and the primary sensory neurons that project to the olfactory bulbs of the forebrain. In some animals, a separate domain of the olfactory placode forms the vomeronasal organ, which is specialized for pheromone detection. There is some evidence that other embryonic precursors also contribute to the olfactory sensory epithelium. Di-I fate mapping in zebrafish placed olfactory and neural crest precursors in such close apposition as to suggest there may be a neural crest contribution to the olfactory epithelium (Harden et al., 2012). More recently a zebrafish Sox10-GFP reporter line indicated a large neural crest contribution to the microvillus olfactory neurons (Saxena et al., 2013). In mouse, the P0-Cre/EGFP reporter line showed that after birth neural crest contribute to the horizontal basal stem cells (Suzuki et al., 2013). However, neural crest grafts in chick and a different reporter line in mouse do not confirm these observations but instead show that the olfactory ensheathing cells are of neural crest origin (Barraud et al., 2010). These different observations raise the issue of whether the reporter constructs unquestionably distinguish between the neural crest and placode precursors (which have a common early developmental program), and whether there are species differences in the embryonic origins of some of the olfactory epithelial cells. Other derivatives of the olfactory placode include neuropeptide- and gonadotropin releasing hormone-secreting neurons that migrate into the forebrain (Murakami and Arai, 1994; Northcutt and Muske, 1994; Hillal et al., 1996; Sabado et al., 2012). The lens placode invaginates as a vesicle in response to signals from the growing optic cup. The anterior layer of the lens vesicle differentiates into lens epithelial cells that provide homeostatic support for the organ and act as stem cells to produce new lens fibers. The posterior layer of the lens vesicle differentiates into highly specialized lens fibers that are filled with transparent crystalline proteins to provide optical clarity.
Separating the anterior and posterior sets of placodes lie two trigeminal placodes (ophthalmic/profundal and maxillomandibular) on the dorsal and posterior margins of the optic vesicle at the hindbrain level of rhombomere 2. Cells delaminate from these placodes and migrate a short distance to produce the large neurons in the distal parts of the ophthalmic and maxillomandibular lobes of the trigeminal ganglion; neural crest cells migrate adjacent to the placode-derived neurons to contribute the small neurons of the proximal lobes and glia for the entire ganglion. In some species the ophthalmic/profundal ganglion develops as two separate entities (Schlosser and Northcutt, 2000; see also Streit, 2004).
The posterior set of placodes consists of the otic and epibranchial placodes. At the hindbrain level of rhombomere 5 (rhombomere 4 in some species; Ruiz i Altaba and Jessell, 1991), the bilateral otic placodes form. This thickening invaginates as the otic cup, which pinches closed as an otic vesicle. The vesicle undergoes complex morphogenetic movements to form the inner ear (auditory cochlea, vestibular semicircular canals and the otolithic organs [utricle, saccule]), and produces both the structural and neural elements of these organs, as well as the sensory ganglion cells that innervate them. In aquatic species, the lateral line system, which is specialized for detection of water currents and electrical fields, is derived from placodes that surround the otic placode (see Baker and Piotrowski, this volume). Just anterior and posterior to the otic placode is a series of epibranchial placodes that arise just dorsal to each postotic branchial/pharyngeal cleft. At the hindbrain level of rhombomere 4, the geniculate placode forms just dorsal to the first branchial/pharyngeal cleft. It gives rise to the large neurons of the geniculate (distal) ganglion of the facial cranial nerve; neural crest give rise to the small neurons of the proximal facial ganglion and the glia of both ganglia. The paratympanic organ in birds and the spiracular organ in some fishes are derived from a placode located just dorsal to the geniculate placode (O’Neill et al., 2012). At the hindbrain levels of rhombomeres 6-8, other epibranchial placodes give rise to sensory neurons that delaminate and migrate a short distance to form the large neurons of the distal sensory ganglia of the glossopharyngeal (petrosal ganglion) and vagus (nodose ganglion) cranial nerves. Neural crest cells give rise to the smaller neurons of the proximal ganglia of these nerves as well as the glia for both sets of ganglia. In certain fishes there are additional branchial arches, and thus additional placodes and cranial ganglia. In some species there also are ventrally located hypobranchial placodes of unknown function (Schlosser, 2003). Thus, like the related neural crest, cranial sensory placode cells can give rise to several cell types: neurosecretory cells; forebrain neurons; sensory ganglion neurons; sensory receptor cells; non-neural crystalline producing cells; and non-neural supportive cells.
III- The pre-placodal region (PPR): a common origin for all sensory placodes
1- The PPR forms at the border between the neural plate and the epidermis
In the latter part of the 19th century, the cranial sensory placodes were identified by histological preparations of vertebrate embryos, and described as forming lateral to the cranial portion of the neural tube. Following the movements of the placode and neural crest cells over developmental time showed that both populations contribute to cranial sensory structures. The early histological analyses were remarkable in their ability to identify placode precursors without the aid of molecular markers. von Kupffer (1895) described two precursor regions in Petromyzon (sea lamprey), one that is dorsolateral and one that is ventrolateral. Platt (1896) agreed with this arrangement in Necturus (the aquatic salamander or mudpuppy), but determined that these two zones arose from a single band of thickened ectoderm adjacent to the neural folds. von Kupffer posited that the placodes arise from unspecified epidermis after an interaction with the neural crest affiliated with each cranial nerve, whereas Platt posited that they arise from a defined zone of ectoderm that is distinct from the epidermis (see Knouff, 1935). Analyses of two species of terrestrial salamanders (Ambystoma) also observed thickenings in the epidermis prior to the emergence of definitive placodes (Landacre, 1926; Stone, 1922). Initially there was a disagreement over whether these thickenings were placode precursors or simply thickenings caused by the epidermis folding over underlying organs. Stone showed, by experimentally removing these thickenings, that some were indeed placode precursors whereas others were simply tissue folds. In an analysis of carefully staged frog embryos, Knouff (1935) provided extensive evidence that there is a distinct pan-placodal band lying between the neural ectoderm and the epidermal ectoderm to which all placodes can be histologically traced. It is thicker than the epidermal ectoderm and is cytologically more similar to neural than epidermal cells. Although fate mapping studies in chick (Couly and Le Douarin, 1987; Couly and Le Douarin, 1990; Streit, 2002) and in several amphibians (reviewed in Schlosser and Ahrens, 2004; Streit, 2004; Pieper et al., 2011) also indicate that all placodes originate from a narrow band surrounding the neural plate, Knouff’s histologically identified pre-placodal band may not accurately represent the PPR because it extends into the trunk and it only partially coincides with the domains of PPR molecular markers (Schlosser and Ahrens, 2004; Litsiou et al., 2005). Nonetheless, similar histological studies in several vertebrates, as well as the expression of common sets of molecular markers (discussed in section III-4) support the idea that there is a common pre-placodal field of progenitors surrounding the anterior neural plate (see also Streit, 2004; Schlosser, 2006).
2- Specification of the PPR
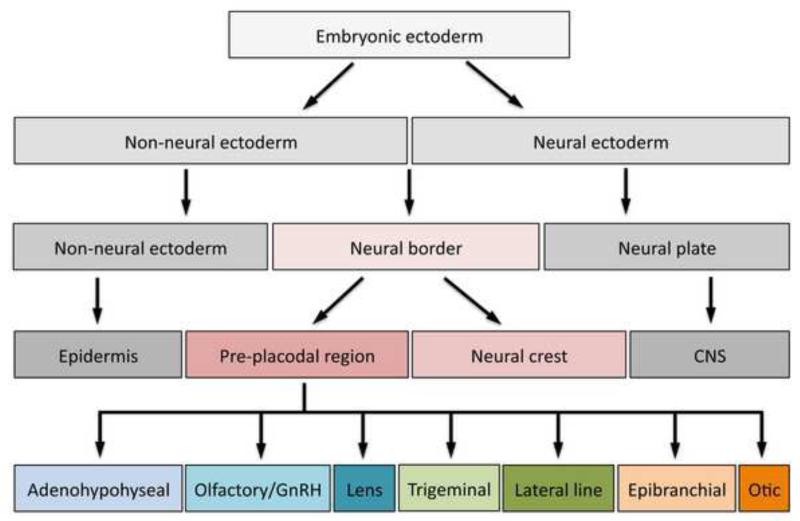
The specification of the PPR, the process that segregates the cells that will form placodes from the three other ectodermal domains (epidermis, neural crest and neural plate) occurs through a gradual progression of tissue interactions involving multiple signaling pathways that result in the dynamic expression of several transcriptional regulators. As a first step, the naïve embryonic ectoderm is separated into neural and non-neural ectodermal domains during gastrulation (Figure 1); while non-neural ectoderm is promoted by BMP and Wnt signaling, neural ectoderm is promoted by FGF signaling and by antagonizing the BMP and Wnt pathways (Streit et al., 2000; Wilson et al., 2000; De Robertis and Kuroda, 2004; Stern, 2005; Stern, 2006; Levine and Brivanlou, 2007; Rogers et al., 2009). In response, neural-specific and epidermal-specific transcription factors are expressed in different regions of the ectoderm. This leads to a second step in PPR specification: the formation of a NB zone that surrounds the neural plate and contains cells competent to become placodes and neural crest (Figure 1). One example of the formation of this new region comes from observing the dynamic expression of neural plate and epidermal genes: in Xenopus, the neural (Sox2-expressing) and the non-neural (Foxi1a/b-expressing) ectoderm domains initially abut; soon after, however, an intervening territory appears that expresses neither of these genes, indicating that a new domain has formed (Matsuo-Takasaki et al., 2005).
Figure 1. Major subdivisions of the embryonic ectoderm.
At gastrulation, the embryonic ectoderm is subdivided into two domains: the non-neuronal ectoderm and neural ectoderm. They give rise to the epidermis and central nervous system (CNS), respectively. At their boundary a third domain is subsequently generated, the neural border zone, which gives rise to two cell populations the neural crest and pre-placodal region. The pre-placodal region eventually segregates into individual cranial placodes: the adenohypophyseal, olfactory, lens, trigeminal, lateral line, otic and epibranchial placodes (from anterior to posterior).
What other molecules identify this new, intermediate ectodermal domain? Two decades of intensive molecular cloning activity involving a number of animals has identified several genes that are expressed in the NB zone, many of which have keys roles in neural crest as well as PPR specification. Comprehensive summaries can be found elsewhere (Baker and Bronner-Fraser, 2001; Bhattacharyya and Bronner-Fraser, 2004; McCabe and Bronner-Fraser 2009; Schlosser and Ahrens, 2004; Schlosser, 2006; Grocott et al., 2012; Pieper et al., 2012). However, in brief, TFAP2α, FoxI1a/b, GATA, Dlx, Msx, Zic and Pax genes are expressed in overlapping patterns in the NB zone; some are expressed in the epidermis and NB zone, some are expressed in neural plate plus NB zone and some are mostly restricted to the NB zone (for details see Yang et al., 1998; Feledy et al., 1999; Luo et al., 2001, 2002, 2003; Streit, 2002; Schlosser and Ahrens, 2004; Litsiou et al, 2005; Phillips et al., 2006; Hong and Saint-Jeannet, 2007; Khudyakov and Bronner-Fraser, 2009; Grocott et al., 2012; Pieper et al., 2012). Some of these have been called “neural border specifier” genes based on their requirement for early steps in neural crest specification (Meulemans and Bronner-Fraser, 2004), but many of them also are required for PPR specification, as discussed in more detail in Section III-3.
A third step of PPR specification is the separation of the NB zone into two different precursor populations of the peripheral nervous system: the neural crest and the placodes (Figure 1). There remain differences in opinion as to whether the neural crest cells and placode cells arise from distinct subsets of cells in the NB zone or from common progenitors (reviewed in Pieper et al., 2012). In histological preparations at neural tube stages, they are distinct entities, the neural crest migrating from the dorsal neural tube and the placode cells forming patches in the epidermis lateral to the neural tube. Although both neural crest and placode precursors arise from the NB zone, it was recognized very early (e.g., Knouff, 1935) that neural crest cells do not arise from the region of that zone that surrounds the anterior tip of the neural plate, whereas the placodes do not arise from its posterior trunk regions.
One method to determine whether neural crest and placodes share common progenitors is to fate map the NB zone at different developmental stages. Fate maps in chick at gastrula stages demonstrate that precursors for all four ectodermal domains are extensively intermingled throughout the ectoderm; fate maps made in chick, fish and frog at neural plate stages also show extensive intermixing within the NB zone (reviewed in Grocott et al., 2012). Consider two fate maps that were created by labeling small groups of cells with DiI and DiO. In chick, otic precursors are scattered over a wide region and are intermingled with cells that give rise to neural plate, neural crest and epidermis at early neural plate stages. Even at later neural fold stages, cells that give rise the neural crest, otic placode and epibranchial placodes are intermingled, indicating that there is no fate-restricted domain in the NB zone (Streit, 2002). When the types of cells that descend from a single labeled group are analyzed, it is interesting to note that at neural plate stages many groups are composed only of otic placode cells (53%). Mixed cell type groups included: otic placode cells + epidermal cells (29%); epibranchial placode cells + epidermal cells (12%); and placode cells + neural tube cells (6%). After labeling at the neural fold stage, 16% contained placode cells + neural crest cells. These results suggest that while neural crest and placode cells can arise from overlapping regions of the NB zone, they do not often arise from the same progenitor cell. Similar results were reported in Xenopus (Pieper et al., 2011). When small groups of cells in the NB zone were labeled at neural plate stages, placode-labeled groups were intermingled with those of giving rise to epidermis, neural plate and neural crest. Although the cells derived from a single labeled group often contained cells in more than one placode, only about 10% contained both neural crest and placode cells; these “mixed clone” groups were not observed when labeling was performed at neural fold or neural tube stages. In both studies, “mixed clones” may be due to placing the dye at the border between two separate progenitor domains or may be an artifact of the labeling approach, as discussed in detail by Pieper et al. (2011). Although both fate mapping studies suggest that it is unlikely that neural crest and placode cells arise very frequently from a common progenitor cell by neural plate stages, this question will only be definitively resolved by single cell lineage tracing.
However, the neural crest and placodes certainly share a common early developmental history. In fact, both cell types are induced around the border of the neural plate when small pieces of neural plate are grafted into an ectopic location in the non-neural ectoderm (Selleck and Bronner-Fraser, 1995, 2000; Mancilla and Mayor, 1996; Woda et al., 2003; Glavic et al., 2004a; Litsiou et al., 2005; Ahrens and Schlosser, 2005). However, there are conflicting observations of whether the induced neural crest and placode cells arise from the grafted neural plate or host epidermis (reviewed in Pieper et al., 2012). A recent study addressed this issue in Xenopus by evaluating the expression of a number of neural plate, NB zone, neural crest and placode markers (Pieper et al., 2012). Transplanting lineage labeled anterior neural plate into the epidermis resulted in neural plate, NB zone and neural crest markers only in the grafted tissue and never in the host epidermis; PPR markers were induced exclusively in the adjacent host epidermis. Transplanting early neural plate into the NB zone induced neural crest but not placode markers, and transplanting epidermis into the NB zone induced placode but not neural crest markers. Thus, these authors propose that the neural and non-neural ectoderm domains have differing competence to produce neural crest and placodes. By performing these grafting experiments at different developmental stages, they show, however, that this bipotential competence is not achieved until the end of gastrulation; earlier epidermis or animal cap ectoderm grafts can produce both cell types. These results support the idea that the neural crest and placodes share an early developmental history that becomes restricted via competence factors to differing programs by neural plate stages.
3- The tissue interactions, signaling pathways and transcription factors that specify the PPR
What tissue interactions account for the formation of the PPR? Several studies from chick and Xenopus suggest that an interaction between the neural and non-neural ectoderm is involved. As mentioned above, grafting pieces of neural plate into non-neural ectoderm produces cells expressing placode markers around its border. However, there is evidence that other tissues also contribute to PPR induction. For example, in Xenopus the Organizer mesoderm and the dorsolateral endomesoderm, which later gives rise to the pharyngeal arches/pouches and heart, can induce placode markers, whereas the axial mesoderm can not (Ahrens and Schlosser, 2005). Ablation studies showed that the anterior neural plate and the dorsolateral mesoderm are required for PPR induction, whereas the midline mesoderm and the midline neural plate are not. In chick, when neural plate was transplanted into non-neural ectoderm neural crest markers were induced in a high percentage of cases, whereas PPR markers were induced infrequently, indicating that an important signaling source was missing from the ectopic site (Litsiou et al., 2005). Ablation and transplantation experiments further showed that the head mesoderm, similar to that identified in frog, provides an important additional PPR-inducing signal. Further experiments in the chick demonstrated that the mesoderm signal most likely is one or more member of the FGF family (Litsiou et al., 2005). Ahrens and Schlosser (2005) also showed the importance of FGF8 signaling. In frog, FGF8 has multiple expression domains within the neural plate, including the midbrain-hindbrain boundary (Ahrens and Schlosser; 2005), however the source of FGF8 is likely both the endomesoderm and the anterior neural ridge. There also is evidence that FGF signaling favors PPR formation in fish (Esterberg and Fritz, 2009; Kwon et al., 2010). It has been proposed that FGFs promote the expression of “pre-neural” genes, prevent the expansion of PPR-repressing factors, and/or regulate PPR-specific transcription factors (reviewed in Grocott et al., 2012). However, FGF signaling is not sufficient to induce ectopic PPR markers in the epidermis (Ahrens and Schlosser, 2005; Litsiou et al., 2005).
As mentioned above, if embryonic ectoderm is subjected to high levels of BMP it forms epidermis, whereas if it is protected from BMP signaling by secreted antagonists it forms neural ectoderm. In the patterning of the mesoderm, blood islands form in regions of high BMP signaling, and notochord forms at the lowest levels of BMP signaling; so-called intermediate mesoderm (kidney, gonads) forms in regions of intermediate BMP signaling. Does the same occur in the ectoderm? Indeed, several studies showed that the BMP gradient patterns the ectoderm (Neave et al., 1997; Nguyen et al., 1998) and that intermediate levels of BMP signaling induce neural crest formation (Morgan and Sargent, 1997; Wilson et al., 1997; Marchant et al., 1998; Mayor et al., 1997; Mayor and Aybar, 2001; Aybar and Mayor, 2002). The interpretations of some experiments argue that the same is true for placode formation. In both fish and frog, reduction of BMP signaling, either by expressing dominant-negative receptors or BMP antagonists, induces placode genes (Glavic et al., 2004a; Brugmann et al., 2004; Kwon et al., 2010). In fact, Xenopus animal cap ectodermal explants, which endogenously express high levels of BMP, optimally express placode genes in the presence of low levels of the BMP antagonist Noggin, whereas neural crest genes are optimally expressed in the presence of intermediate Noggin levels, and neural plate genes in the presence of high Noggin levels (Brugmann et al., 2004; Hong and Saint-Jeannet, 2007; Park and Saint-Jeannet, 2008). In fish, BMP levels that induce a neural crest fate activate TFAP2 genes, whereas the BMP levels that induce a PPR fate activate TFAP2 plus Foxi1 and Gata3 genes (Bhat et al., 2013). These results indicate that the different ectodermal domains require different, graded levels of BMP signaling. However, there also is evidence that does not support the BMP gradient model of PPR induction. First, in both chick and frog, BMP is highly expressed in the NB zone/neural folds, a position that is incongruous with a simple gradient. In addition, local reduction of BMP in the epidermis of the embryos alone does not induce ectopic PPR markers, although it can expand endogenous PPR domains (Brugmann et al., 2004; Ahrens and Schlosser, 2005; Litsiou et al., 2005). However, the combination of reducing BMP signaling in the presence of active FGF signaling does induce ectopic PPR marker at high frequency (Streit and Stern, 1999; Ahrens and Schlosser, 2005; Litsiou et al., 2005). Zebrafish embryos carrying mutations in various components of the BMP signaling pathway show an expanded neural crest domain associated with a lateral shift of the PPR (Neave et al., 1997; Nguyen et al., 1998). Recent time-course experiments demonstrate that there are two phases of PPR specification by BMP signaling; during gastrula stages high levels of BMP activate NB zone genes that are required for PPR formation, and during neural plate stages lower levels are required to initiate PPR genes (Kwon et al., 2010). Differential timing of Wnt and BMP signaling also may discriminate between rostral and caudal NB zone derivatives (Patthey et al., 2008; 2009). Thus, combinations of signaling factors available at different developmental stages are crucial in PPR specification. In an interesting parallel, neural crest induction requires Wnt signaling plus low BMP signaling at gastrula stages, and high BMP at neurula stages (Steventon et al., 2009).
It is well established that other factors are required for neural crest induction, including Wnt, FGFs and retinoic acid (RA) (Saint-Jeannet et al., 1997; Mayor et al., 1997; LaBonne and Bronner-Fraser, 1998; Chang and Hemmati-Brivanlou, 1998; Mayor and Aybar, 2001; Villanueva et al., 2002; Monsoro-Burq et al., 2003; Glavic et al., 2004b). These factors also are known to play important roles in establishing the posterior axis of all germ layers, and therefore are called posteriorizing factors. Xenopus animal cap explants and anterior neural plate explants will express neural crest genes when cultured in the presence of both BMP and posteriorizing factors (Sasai et al., 2001; Villaneueva et al., 2002; Hong and Saint-Jeannet, 2007). In contrast, posteriorizing factors are not required for animal cap explants to express placode genes in the presence of BMP (Brugmann et al., 2004). In fact, in the intact Xenopus embryo canonical Wnt signaling needs to be reduced for ectopic placode gene expression to occur, and ectopic activation of Wnt signaling represses endogenous placode gene expression (Brugmann et al., 2004; Hong and Saint-Jeannet, 2007). Concordant with these findings, the anterior neural plate and underlying chordomesoderm are sources of anti-Wnt factors (Dkk1) that inhibit neural crest formation (Pera and DeRobertis, 2000; Carmona-Fontaine et al., 2007; Takai et al., 2010). Similar results were obtained in chick (Litsiou et al., 2005): reduction of Wnt signaling adjacent to the endogenous PPR expands placode genes and activation of Wnt signaling represses them. They elegantly showed that simultaneous reduction of BMP and Wnt signaling additively expanded the PPR, but did not ectopically induce PPR genes unless an early pulse of FGF signaling was provided. These authors propose that FGF signaling confers a “neural border” state, and that if cells within that state receive a Wnt signal they become neural crest whereas if they are protected from Wnt signaling they express a PPR fate.
An apparent discrepancy is the role of FGFs in PPR induction. As mentioned above, FGF8 is required for PPR formation, but FGFs also are posteriorizing (Fletcher et al., 2006) and thereby can be inhibitory to PPR formation (Brugmann et al., 2004). Alternatively, this could be due to the neuralizing activity of FGF. This also suggests that there is either specificity in PPR responsiveness to different FGF ligands, to the timing of exposure or to the level of ligand present. Although this issue is yet to be fully resolved, there is evidence that low levels of FGF8 promote neural crest and PPR fates whereas high levels inhibit them (Hong and Saint-Jeannet, 2007).
Also unresolved is the influence of RA signaling in PPR specification. It has been proposed the RA is required for anterior placode induction based on its synthesis in adjacent tissues, e.g., the forebrain and facial mesenchyme (reviewed in Paschaki et al., 2013). However, recent studies in chick and mouse suggest that RA signaling is required for neurogenesis rather than placode induction (Paschaki et al., 2013). It also has been suggested that RA would inhibit PPR formation because of its role in posterior axis formation and induction of the Hox genes. However, RA signaling may have a role in PPR specification independent of its posteriorizing activity, in a similar manner as the posteriorizing activity of FGF and canonical Wnt can be uncoupled from their role in neural crest induction (Monsoro-Burq et al., 2005; Wu et al., 2005). Consistent with this possibility, in Xenopus the RA-synthesizing enzyme (Raldh2) is expressed in the trunk mesoderm, as well as in a discrete U-shaped ectodermal domain around the anterior neural plate (Chen et al., 2001). During PPR formation, decreasing RA signaling expands the domain of FGF8 expression, suggesting a role for RA signaling in limiting the posterior boundary of the PPR (Shiotsugu et al., 2004). But, RA signaling also induces two genes: Tbx1, a T-box transcription factor, and Ripply3/Dscr6, a Groucho-associated co-repressor (Arima et al., 2005; Janesick et al., 2012). Ripply3 and Tbx1 expression domains in the PPR partially overlap; in regions where only Tbx1 is expressed, PPR genes are induced, whereas in regions where Tbx1 and Ripply3 overlap, PPR genes are repressed in a Groucho-dependent manner (Janesick et al., 2012). Thus, while RA signaling is required for formation of the posterior, Tbx1-positive part of the PPR, it also restricts its posterior boundary.
What transcription factors are downstream of these signals to specify the PPR? Cells in the NB zone may differentially become neural crest and PPR based on their different levels of expression of NB specifier genes. These genes were first identified as being expressed in the area surrounding the nascent neural ectoderm (i.e., the NB zone), and to be required for the activation of a set of genes that are expressed in the early-forming neural crest (e.g., FoxD3, Snail2, Sox10), which are called “neural crest specifier” genes (Meulemans and Bronner-Fraser, 2004). Msx1 is one of the earliest expressed NB specifiers and is a direct target of BMP. In frog, it is expressed throughout the non-neural ectoderm and is required for epidermis specification as well as neural crest specification (Tribulo et al., 2003; Monsoro-Burq et al., 2005); in fish, several members of the Msx family have different, overlapping domains in the NB zone and are involved in neural crest formation (Phillips et al., 2006). Interestingly, however, regardless of species, the domains of Msx genes overlap with those of Dlx genes in the NB zone. As mentioned above, formation of the NB zone requires Dlx activity in the non-neural ectoderm. Based on the observations that Msx and Dlx proteins can inhibit each other through the formation of heterodimers (Zhang et al., 1997), an exhaustive series of experiments that knocked down multiple members of the Msx and Dlx families demonstrated that different levels of these proteins, regulated by their mutual antagonism, biases NB cells towards neural crest (Msx-high, Dlx-low) versus PPR (Msx-low, Dlx-high) fates (Phillips et al., 2006). Msx1 also is known to activate both Pax3 and Zic1, two other NB specifier genes, and interactions between Pax3 and Zic1 are required to initiate neural crest specific genes (Tribulo et al., 2003; Monsoro-Burq et al., 2005; Sato et al., 2005). By temporally controlling the timing of Pax3 expression with a hormone-inducible construct, Hong and Saint-Jeannet (2007) demonstrated that the Pax3 influence on neural crest genes was later than its role in forming the NB zone. They also showed that while Zic1 synergizes with Pax3 to produce neural crest, in the absence of Pax3, Zic1 promotes PPR gene expression. It was recently shown that Msx1 directly binds to the anterior PPR enhancer of the Six1 gene (see section III-4) to repress it, whereas Dlx binding leads to activation (Sato et al., 2010).
In frog, fish and chick, Dlx genes have been shown to be required for PPR formation (Solomon and Fritz 2002; Woda et al., 2003; McLarren et al., 2003; Phillips et al., 2006,). There is evidence that the Dlx factors may act by regulating a BMP antagonist at the NB zone (Esterberg and Fritz, 2009; Reichert et al., 2013). Recent work has linked the expression of Dlx3 and GATA2 to the non-neural ectoderm acquiring the competence to form PPR (Pieper et al., 2012). Gain-of-function of either gene reduces neural plate, NB zone and neural crest genes whereas loss-of-function expands them. Epidermal, PPR and placode gene expression is significantly reduced when either Dlx3 or GATA2 is depleted. However, the genes are not equivalent: although increased Dlx3 expands PPR markers, increased GATA2 only rarely does so. These authors propose that Dlx and GATA2 act as competence factors because they are required in the non-neural ectoderm for PPR markers to be induced. Recent studies in zebrafish concur with these observations; in this organism GATA3, TFAP2a, TFAP2c and Foxi1 have been proposed to play a similar role (Kwon et al., 2010; Bhat et al., 2013). It is important to note a dependence on the signaling environment for the effects on the PPR genes; the upregulation of Six1 by Dlx3 is significantly increased in the presence of BMP and/or Wnt inhibitors (Pieper et al., 2012).
The initial expression of two other non-neural ectoderm genes also appear to play critical roles in specifying the PPR in Xenopus: TFAP2α and Foxi1a/b (Matsuo-Takasaki et al., 2005; Luo et al., 2002; Zhang et al., 2006). Knock-down of Foxi1a/b causes expansion of neural plate genes and reduction of NB zone and epidermal genes, whereas gain-of-function reduces both neural plate and NB zone genes and expands epidermal genes (Matsuo-Takasaki et al., 2005). Experiments using a hormone-inducible construct demonstrated that Foxi1a/b initially acts during mid-gastrulation downstream of BMP signaling to reinforce the separation of the neural and non-neural ectoderm domains. Next, it is required for “neural border specifier” gene expression (e.g., Msx1), and still later it must be down-regulated to allow for neural crest and placode gene expression (Matsuo-Takasaki et al., 2005). TFAP2α also is involved in the formation of the PPR in frogs and fish (Nguyen et al., 1998; Luo et al., 2002). It is initially expressed throughout the embryonic ectoderm where it is a key regulator of epidermis-specific keratins. Next it is down-regulated in the neural ectoderm and later still is down-regulated in the PPR but not in the premigratory neural crest cells. Loss-of-function of TFAP2α causes a down-regulation of epidermis-specific keratin genes and NB specifier genes and up-regulation of neural plate genes (Luo et al., 2002; de Croze et al., 2011). In contrast, TFAP2α gain-of-function expands NB specifier genes as well as neural crest genes and reduces neural plate genes (Luo et al., 2003; Hoffman et al., 2007; de Croze et al., 2011). Epistasis analyses show that TFAP2α acts upstream of NB specifier genes, and both Pax3 and epidermis-specific keratin genes are direct transcriptional targets (Luo et al., 2002; de Croze et al., 2011).
In summary, both the neural crest and the PPR are induced by the combined activity of several signals emanating from different tissues at different times. In addition to differentially affected cell fates, these signals also differentially influence the positioning of neural crest versus placodes in the embryonic axis. Posteriorizing factors positively influence neural crest to form from midbrain to tail, whereas they repress PPR genes and confine them to the head. Likewise, Wnt antagonists expressed in cranial domains likely allow PPR to form around the anterior tip of the neural plate but prevent the neural crest to form in this region. In addition, differing levels of members of the Msx, Dlx, GATA, Pax and Zic protein families contribute to producing neural crest versus PPR cells. The specifics of the contributions of each gene will not be fully understood until ChIP sequencing studies are completed, but a proposed gene regulatory network based on the available epistasis data from several species has recently been constructed that should serve the field well as a starting point for these analyses (Grocott et al., 2012).
4- Molecular identity of the PPR
The PPR was first identified by morphological criteria, and then its contributions to different structures were highly refined with the application of fate mapping techniques, most importantly the quail-chick chimera approach (LeDouarin et al., 1986; Webb and Noden, 1993). However, significant progress in understanding PPR specification at a molecular level was made only when PPR-specific genes were identified. Of particular value in marking the early PPR have been members of the Six transcription factor family and the Eya co-factor family, which are considered to be pan-placodal markers. Three of the 6 members of the vertebrate Six family (Six1, Six2, Six4) and all four members of the Eya family (Eya1, Eya2, Eya3, Eya4) are expressed in U-shaped domains surrounding the anterior neural plate (Figure 2), albeit with some species variations (Ohto et al., 1998; Esteve and Bovolenta, 1999; Sahly et al., 1999; Pandur and Moody, 2000; Kobayashi et al., 2000; David et al., 2001; Ghanbari et al., 2001; Bessarab et al., 2004; Schlosser and Ahrens, 2004; Brugmann and Moody, 2005). At later developmental stages they also are strongly expressed in other developing organs, including somitic muscle, kidney and limb buds. The founding member of the Six gene family is Drosophila Sine oculis. All Six proteins contain a homeodomain-type DNA-binding region and an N-terminal Six Domain (SD) that binds co-factors such as Eya and Groucho proteins. In fly, binding of Eya to Sine oculis increases DNA binding specificity (Pignoni et al., 1997). Eya proteins do not bind to DNA directly, and they can access the nuclear compartment only by binding to other transcription factors via the C-terminally located Eya Domain (ED), which binds to the SD of Six proteins (Pignoni et al., 1997; Ohto et al., 1999). Eya proteins can interact with multiple Six proteins and also with many other transcription factors. Recent structural analyses show that the binding of EYA2 to the SIX1 SD modifies the way in which the SIX1 homeodomain interacts with DNA (Patrick et al., 2013); thus Eya proteins can be critical modifiers of Six protein functions.
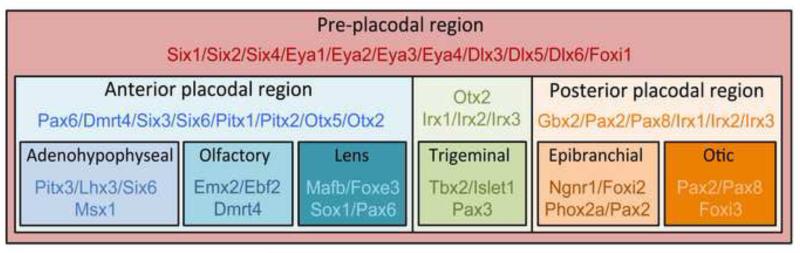
Figure 2. Combinatorial expression of transcription factors during specification of the PPR and segregation of the sensory placodes.
Once specified, the PPR becomes sequentially subdivided into incrementally smaller domains each expressing a specific repertoire of genes. Only a subset of these genes is shown here. Anterior is to the left and posterior to the right.
Six and Eya genes have proven to be more than simple markers of the PPR domain; their expression is critical for the specification of the PPR. In frog, knock-down of Six1 in the NB zone reduces the expression of other early PPR genes (Eya1, Sox11) and expands neural crest and epidermal genes (Brugmann et al., 2004). Conversely, Six1 gain-of-function in the NB zone expands the other PPR genes and reduces the domains of neural crest and epidermal genes. In chick, these gain-of-function effects also occur, but only when both Six1 and Eya2 are co-expressed (Christophorou et al., 2009). In fish, Six1 is necessary for the formation of inner ear hair cells (Bricaud and Collazo, 2006, 2010). Expressing activating Six1 constructs or co-expressing Eya1 with wild type Six1 results in up-regulation of placode genes, whereas expressing repressing Six1 constructs or co-expressing Groucho with wild type Six1 results in down-regulation of neural crest and epidermal genes (Brugmann et al., 2004; Christophorou et al., 2009). Thus, Six1 appears to have dual roles in promoting downstream placode genes by transcriptional activation, and inhibiting alternate ectoderm fates by transcriptional repression. It is not yet clear in fish, frog or chick whether Six2 and Six4 have similar or redundant functions to those of Six1 in PPR specification.
The roles of Six and Eya genes in mammals are much harder to study at the early stages of PPR specification so until recently analyses have relied on late developmental processes. In mouse, knockout of Six1 results in severe defects in the olfactory and inner ear sensory structures, as well as deficits in the other structures in which it is later expressed, such as muscle and kidney (Oliver et al., 1995; Laclef et al., 2003; Li et al., 2003; Zheng et al., 2003; Ozaki et al., 2004; Zou et al., 2004; Konishi et al., 2006). Although Six1 is expressed in the mouse PPR (Sato et al., 2010), Six1-null embryos have defects in placode morphogenesis and neurogenesis rather than PPR formation (Ikeda et al., 2007, 2010; Chen et al., 2009). The PPR may form in these embryos due to the remaining activity of Six2 and Six4. In support of this idea, although craniofacial phenotypes have not been reported in Six2-null or Six4-null mice (Self et al., 2006; Ozaki et al., 2001), Six1/Six4 double-knockouts have more severe craniofacial phenotypes than Six1-null mice (Grifone et al., 2005; Zou et al., 2006; Chen et al., 2009).
In humans, mutations in SIX1 are causative in some individuals afflicted with Branchio-otic Syndrome (specifically BOS3). These patients have minor craniofacial defects and profound hearing loss, presumably due to defects in otic placode/inner ear development (Abdelhak et al., 1997; Ruf et al., 2004). In the majority of these patients there are single amino acid changes in the SD, suggesting that interactions with co-factors cause the defects (Ruf et al., 2004; Ito et al., 2006; Sanggaard et al., 2007; Kochkar et al., 2008; Patrick et al., 2009; Noguchi et al., 2011). Bricaud and Collazo (2011) showed that when a BOS patient mutation was inserted into zebrafish Six1 and then expressed during development, hair cell formation was disrupted. Similarly, a mouse that harbors a mutation in Six1 that also is found in some BOS patients had several inner ear defects (Bosman et al., 2009).
Eya1-null mice have severely deformed inner ears and are deaf, and have deficits in cranial ganglia, other cranial structures, kidney and skeletal muscles (Johnson et al, 1999; Xu et al., 1999, 2002; Zou et al., 2004, 2006a, b). Eya4-null mice also have inner ear defects (Depreux et al., 2008). Phenotypes in Eya2-null mice have not been reported yet, and while Eya3-null mice show behavioral abnormalities suggesting nervous system defects, craniofacial deficits were not reported (Söker et al., 2008). In humans, mutations in EYA1 also are causative in some individuals afflicted with two types of Branchio-otic Syndrome (BOS1, BOR1); BOR patients have additional renal defects (Abdelhak et al., 1997; Kumar et al., 1997; Spruijt et al., 2006). In these patients the majority of the mutations cause amino acid substitutions in the ED that prevent the protein from binding to Six1 (Buller et al., 2001; Ozaki et al., 2002). Partial deletions of EYA1 that include nearby genes cause oto-facio-cervical syndrome (Rickard et al., 2001; Estefania et al., 2006). When human patient Eya1 mutations were introduced into either Xenopus or zebrafish, anterior pituitary, ear and lateral line development was perturbed (Kozlowski et al., 2005; Nica et al., 2006; Pogoda and Hammerschmidt, 2007; Li et al. 2010). There are no human mutations reported yet for EYA2 or EYA3, but two syndromes also characterized by hearing loss are associated with EYA4 mutations: autosomal dominant nonsyndromic sensorineural deafness 10 (OMIM #601316; Wayne et al., 2001; Makishima et al., 2007) and dilated cardiomyopathy with sensorineural hearing loss (OMIM #605362; Schönberger et al., 2005). Similarly, knockdown of Eya4 in zebrafish causes defects in the ear, lateral line and heart (Schönberger et al., 2005; Wang et al., 2008).
Six and Eya genes are not the only transcription factors expressed in the PPR, but they are amongst the earliest to be expressed throughout the PPR (Figure 2; Schlosser and Ahrens, 2004). Interestingly, several of the NB specifier genes continue to be expressed in subdomains of the PPR after the onset of Six and Eya gene expression (Schlosser and Ahrens, 2004; Grocott et al., 2012). In Xenopus, for example, after Foxi1a’s initial broad non-neural ectoderm expression it becomes confined to U-shaped band in the head located just lateral to the Six1 domain, with a small region of overlap (Matsuo-Takasaki et al., 2005); a similar lateral, partially overlapping position characterizes the related Foxi1c gene. Dlx3 and Dlx5 genes similarly are initially broadly expressed in the non-neural ectoderm and then are confined to stripes that partially overlap with Six1 (Schlosser and Ahrens, 2004). Prior to the formation of individual placodes, several other transcription factors (e.g., Sox2, Sox3, Sox11, Irx1) are expressed in broad, overlapping regions of the PPR. Various Pax genes, which are characteristic of specific placodes, and bHLH genes, which are characteristic of neurogenic placodes, also are expressed in subdomains of the PPR before the placodes separate, but none of these are pan-placodal. It is likely that these regional, partially overlapping domains of expression downstream of Six and Eya genes contribute to the subdivision of the PPR into distinct placodes.
IV- Subdivision of the PPR into domains with distinct identities
1- Anterior-posterior regionalization of the PPR
Classical transplantation experiments in amphibians showed that within the ectoderm adjacent to the anterior neural plate, cells were competent to give rise to any placode, but this ability was progressively lost overtime (Jacobson, 1963a). When the PPR was rotated along its anterior-posterior axis at the early neurula stage, cells within the transplanted region formed placodes largely according to their novel position. If the same experiment was performed a few hours later, placodes now developed primarily according to their original position in the PPR, suggesting that their identity was already determined at the time of transplantation. Therefore, cells within the PPR are initially competent to form any placode derivative, and over time progressively acquire their specific identity, likely in response to cues from the local environment (Jacobson, 1963a).
All placodal precursors within the PPR are generated by a common inductive mechanism, and they initially share a similar developmental program, since all placodal precursors start with the same initial ground state, a lens fate (Bailey et al., 2006). The PPR is subsequently divided along the anterior-posterior axis into individual domains in which cells will adopt fate characteristic for each sensory placode. Expression analyses of genes restricted to the PPR in frog and chicken embryos fully support the idea of a progressive restriction of fate over time. While the PPR is characterized by the broad expression of transcription factors such Six1 and Eya1, anterior and posterior differences can be visualized through differential gene expression at the early neurula stage. These two placodal domains give rise to the adenohypophyseal, olfactory and lens placodes anteriorly, and otic, lateral line and epibranchial placodes posteriorly, with the trigeminal placode forming in between (Figure 2). For example in chicken embryos, Foxi3 is initially expressed broadly in the PPR surrounding the anterior neural plate. It then becomes restricted to the posterior placodal region that will give rise to the otic and epibranchial placodes (Khatri and Groves, 2013). These placodal regions are more restricted than the PPR but retain a relatively broad potential. These subdomains of the PPR from which more than one placode can emerge are sometimes referred as “equivalence domains” or “equivalence groups” (Dutta et al., 2005; Schlosser, 2006; Toro and Varga, 2007; Ladher et al., 2010). The transcription factors Otx2 and Gbx2 are an excellent example of a cell intrinsic mechanism to establish these equivalence domains from PPR progenitor cells. At the neurula stage, Otx2 and Gbx2 have anterior and posterior expression domains, respectively. While Gbx2 is required for otic placode specification, Otx2 is necessary for olfactory, lens and trigeminal placodes formation (Steventon et al., 2012). Gbx2 and Otx2 are believed to mutually repress each other to establish boundaries between these prospective placodal domains. Other families of transcription factors show similar complementary expression patterns along the anterior-posterior axis of the PPR. For example, Pax6, Six3 and Six6 are restricted anteriorly at the neurula stage, whereas three members of the Iroquois family, Irx1, Irx2 and Irx3 are confined to the posterior PPR (Figure 2; Schlosser and Ahrens, 2004; Schlosser 2006). Whether these factors are also involved in setting up these boundaries has not been analyzed experimentally. It is also important to point out that transcription factors expressed within these two regions do not necessarily share the same boundaries of expression. At the late neurula stage, Otx2 expression domain extends more posteriorly than Pax6 and Six3/6, whereas Irx genes are more broadly expressed than Gbx2 to include the prospective trigeminal placode anteriorly - a region where Irx genes overlap with Otx2 (Figure 2). Ultimately, as development proceeds these factors become progressively restricted to smaller areas within these domains, thereby establishing the molecular identity of individual placodes.
2- Cell movements associated with sensory placodes separation
Lineage and fate map analyses in fish, frog and chicken embryos, at gastrula and neurula stages, have shown that the region of the embryonic ectoderm immediately adjacent to the prospective brain contains precursors for most cranial placodes (Kozlowski et al., 1997; Bhattacharyya et al., 2004; Streit, 2002; Pieper et al, 2011). The precursors for the different placodes appear initially intermingled in this region and are subsequently recruited into distinct areas along the anterior-posterior axis. Using focal dye labeling, recent fate maps of the lens and olfactory placodes (Bhattacharyya et al., 2004), adenohypophyseal and lens placodes (Dutta et al., 2005; reviewed in Toro and Varga, 2007), and trigeminal, epibranchial, and otic placodes (Streit, 2002; Xu et al., 2008; reviewed in Ladher et al., 2010) have established that adjacent ectodermal cell populations can contribute to distinct placodes. In the process cells of the PPR undergo extensive cells movements as documented in chicken embryos (Bhattacharyya et al., 2004; Streit, 2002). However, most labeling techniques used in these studies do not permit one to follow the behavior of single cells, therefore, it remains unclear whether the movements of these dye-labeled groups of cells reflect actual cell sorting based on cell identity, or random cell movements due to differential growth within the PPR, and changes occurring in the ectoderm layer during neurulation (Schlosser, 2010). A more recent study suggests that cell rearrangements are minimal in the PPR (Pieper et al., 2011); using time-lapse imaging to follow the movement of single cells in Xenopus head ectoderm, they described very little directed, large-scale cell rearrangements within the PPR at neurula stages, and during the initial segregation of placodal domains. Rather the cells appeared to move without changing their relative positions within the PPR and with adjacent ectodermal territories, suggesting that individualization of placodes from the pre-placodal ectoderm does not involve large-scale cell sorting in Xenopus (Pieper et al., 2011). Another study performed at a later developmental stage recently showed that cells of the epibranchial placode move actively to segregate into distinct subpopulations. Here the interaction between placode and neural crest cells is the driving force in directing the coordinated migration of epibranchial placode cells to their final position (Theveneau et al., 2013). In zebrafish, time-lapse recordings of transgenic embryos expressing Pax2a:GFP in the otic/epibranchial placode show that cells are continuously recruited from adjacent regions into the Pax2a expression domain through directed migration (Bhat and Riley, 2011). The cell recruitment in the posterior placode is severely impaired in integrin-5 depleted embryos indicating that this process is guided by integrin-ECM (extracellular matrix) interactions. Interestingly, the most anterior placodes (pituitary, olfactory and lens) were unaffected in these embryos suggesting that this phenomenon may not regulate cell movements in other regions of the PPR (Bhat and Riley, 2011). Altogether these studies point to possible species-specific differences in the relative importance and nature of the cell arrangements associated with sensory placode separation. Alternatively, these differences could be strictly experimental, due to differences in the exact developmental time point at which these analyses are carried out.
3- Regional induction of placodal domains
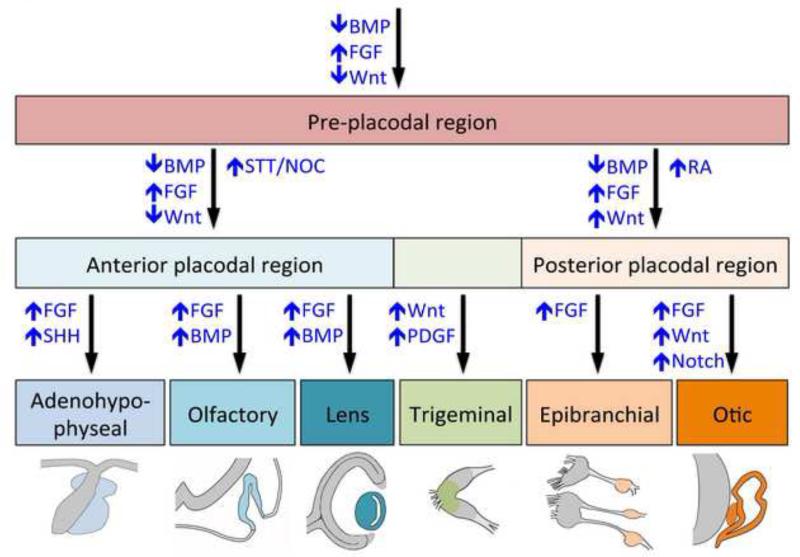
In the early 1960’s, Antone Jacobson performed experiments in amphibian embryos to evaluate the abilities of different tissues to induce lens, otic or olfactory placodes during development (Jacobson 1963b; 1963c; 1966). His work demonstrated that the lens and olfactory placodes could be induced by early signals derived from the endoderm and mesoderm, whereas the otic placode was generated in response to mesoderm- and neuroectoderm-derived signals. These observations clearly indicate that the tissues adjacent to the PPR differentially influence cell fate within the head ectoderm. However, the initial overlap between the different placode precursors within the PPR has made it very challenging to identify the specific inductive signals directing the formation of the different placode domains. Interestingly, the same classes of signaling molecules implicated in the induction of the PPR are also involved in its subsequent subdivision into domains with distinct identities. Here we summarize the activity of these molecules focusing on the BMP, FGF and Wnt signaling pathways (Figure 3).
Figure 3. Signaling pathways regulating placode formation.
Diagram illustrating the signaling pathways involved in the specification of the PPR, and the subsequent step-wise induction of sensory placodes with distinct identities. The arrows indicate whether a signaling pathway is activated (↑) or inhibited (↓). BMP, bone morphogenetic protein; FGF, fibroblast growth factor; NOC, nociceptin; PDGF, platelet derived growth factor; RA, retinoic acid; SHH, sonic hedgehog; STT, somatostatin. Anterior is to the left and posterior to the right.
Bone Morphogenetic Protein signaling
In Xenopus a balance of BMPs and their antagonists is in part responsible for positioning the PPR (Brugmann et al., 2004; Glavic et al., 2004a). Zebrafish embryos with mutations in components of the BMP signaling pathway show expanded neural crest domain associated with a lateral displacement of the placodal domain (Neave, et al., 1997; Nguyen, et al., 1998). BMP signals are also implicated in the specification of olfactory and lens placodes in the chick embryo. At gastrula stages BMP2 and BMP4 are expressed in the PPR. In an in vitro assay, using ectodermal explants of anterior border region isolated from late gastrula stage embryos (stage 4), short-term exposure to BMP signaling promotes specification of olfactory fate, whereas prolonged exposure to BMP signals promotes formation of lens cells. BMP signaling is also sufficient to promote olfactory and lens progenitors in prospective forebrain explants (Sjodal et al., 2007). BMP4-/- mouse embryos lack lens placodes, but expression of Six3 and Pax6 is detected in the prospective lens ectoderm, and the olfactory placode appears normal (Furuta and Hogan, 1998). These results suggest that placodal progenitor cells are specified in the absence of BMP4, which may reflect functional redundancy with other BMP family members such as BMP7. These results also indicate that BMP4 activity is required for differentiation of lens but not olfactory placodal cells; however, BMP4 alone is not sufficient to promote lens development suggesting that it is acting in combination with other inducers (Furuta and Hogan, 1998). BMP7 protein is present in the head ectoderm at the time of lens placode induction. Inhibition of BMP7 signaling at the time of lens placode induction significantly decreases the frequency of lens formation in an organ culture system. The expression of the lens placode marker Sox2 was also severely affected in BMP7-/- mutant embryos (Wawersik, 1999).
Fibroblast Growth Factor signaling
In frogs, FGF8 is expressed in the anterior neural plate region at the early neurula stage (Christen and Slack, 1997), and morpholino-mediated knockdown of FGF8a results in a broad loss of pre-placodal genes (Hong and Saint-Jeannet, 2007). In chick embryos, FGF8 has been proposed as the factor repressing the lens ground state of the PPR to promote olfactory fate (Bailey et al., 2006). Exposure of the presumptive lens ectoderm to FGF8 blocks expression of the lens marker Pax6 and promotes olfactory placode formation. Interestingly, under these conditions genes specific for other placodal territories were not activated (Bailey et al., 2006), consistent with the idea that following repression of lens fate, each placodal domains is specified in response to a distinct set of inductive cues. Several members of the FGF family have been implicated in specification of the posterior placodal region (prospective otic/epibranchial domain), and different species appear to use different combinations of these factors (reviewed in Schneider-Maunoury and Pujades, 2007; Schimmang 2007; Ladher et al., 2010; Chen and Streit, 2013). FGF19 expressed in the paraxial mesoderm and FGF8 derived from the endoderm, are implicated in otic/epibranchial domain induction in chicken (Ladher et al., 2000; Ladher et al., 2005). In zebrafish this process depends primarily on FGF3 and FGF8 (Philipps et al., 2001; Maroon et al., 2002; Leger and Brand, 2002; Liu et al., 2003), while in the mouse, paraxial mesoderm-derived FGF8 and FGF10 controls otic/epibranchial domain formation (Wright and Mansour, 2003; Ladher et al., 2005; Zelarayan et al., 2007). The subsequent segregation of the posterior placodal region into otic and epibranchial domains also involves FGF signaling, however, otic and epibranchial fates have different requirements. FGF signaling must be attenuated to elicit otic fate from the otic/epibranchial progenitor domain (Freter, 2008), while sustained FGF activity is required to promote epibranchial fate (Nikaido et al., 2007; Sun et al., 2007; Nechiporuk et al., 2007). In the FGF8 zebrafish mutant, acerebellar, epibranchial expression of Sox3 is disrupted. This phenotype can be rescued by implantation of an FGF8 bead near the prospective hindbrain. This requirement for FGF signaling was further demonstrated using a soluble FGF receptor antagonist, which reduced expression of the epibranchial markers Sox3 and Phox2a (Nikaido et al., 2007). In contrast, other studies using morpholino antisense oligonucleotides against both FGF3 and FGF8 pointed to a dual requirement of FGF3 and FGF8, rather than FGF8 alone (Sun et al., 2007; Nechiporuk et al., 2007). FGF misexpression studies have produced more variable and often contradictory results. A recent study, using heat shock-inducible transgenes to misexpress FGF3 or FGF8 in zebrafish, suggests that the response of prospective otic cells to FGF signaling varies greatly with developmental stage and is influenced by the distribution and levels of FGF available anteriorly (Padanad et al., 2012). Finally, FGF3 in the ventral diencephalon is required for the expression of early adenohypophyseal markers in zebrafish (Herzog et al., 2004). In the mouse, loss of FGF receptor-2b or deletion of its ligand, FGF10, leads to early pituitary defects due to excessive apoptosis in Rathke’s pouch epithelium (Ohuchi et al., 2000), suggesting an important role of FGF signaling in development of the adenohypohyseal placode, possibly as a survival factor.
Wnt signaling
At the neural border, inhibition of canonical Wnt signaling pathway favors expression of PPR-specific genes at the expense of neural crest fate (Brugmann et al., 2004; Litsiou et al., 2005). Recent studies using Xenopus animal caps suggest that cranial placodes along the antero-posterior axis may have different requirements with regard to Wnt signaling (Park and Saint-Jeannet, 2008). Expression of FGF8a promotes expression of the olfactory placode-specific gene Dmrt4 in animal caps injected with Noggin. However, simultaneous activation of canonical Wnt signaling in these explants elicited expression of the otic-specific gene Pax8 while reducing Dmrt4 expression (Park and Saint-Jeannet, 2008). Zebrafish masterblind mutants carry a point mutation in the GSK3-binding domain of axin1 leading to increased Wnt activity (Heisenberg et al., 2001). In these mutant embryos anterior forebrain structures are missing, and the most anterior placodes (olfactory and lens) are also lost, while the posterior trigeminal placode appeared expanded (Heisenberg et al., 1996). Altogether, these results in fish and frogs support the view that the most anterior cranial placodes require Wnt inhibition, while more posterior placodal territories depend on active canonical Wnt signaling. In chicken, presumptive otic ectoderm showed a stronger induction of the otic gene Pax2 when cultured in the presence of FGF19 and Wnt8C as compared to explants cultured with FGF19 alone (Ladher et al., 2000). Mouse work indicates that the presumptive otic epithelium is exposed to Wnt signals early, as the activity of a TCF/Lef-LacZ reporter is detected in the pre-otic ectoderm (Ohyama et al., 2006). Conditional knockout of β-catenin results in a smaller than normal otic vesicle, and conversely conditional stabilization of β-catenin expands the otic placode domain (Ohyama et al., 2006; 2007). The situation is however more complex, since otic fate is regulated through an interplay between canonical Wnt and Notch signaling pathways. In the mouse, activation of Notch signaling favors the expression of otic genes at the expense of epibranchial fate, similar to Wnt activation (Jayasena et al., 2008). Wnt signaling activates the expression of components of the Notch signaling cascade (Notch1, Jagged1 and Hes1) in the otic progenitor domain, which in turn enhances Wnt signaling to activate otic gene expression and solidify otic identity (Jayasena et al., 2008). By contrast, in birds Notch signaling is primarily involved in regionalization of the otic placode, by establishing the proneural domain within the otic epithelium (Abello et al., 2007). Wnt molecules are also implicated in trigeminal placode formation in chicken embryos. Blocking canonical Wnt signaling prevented the targeted cells from adopting or maintaining a ophthalmic/profundal trigeminal placodal fate, based on the expression of Pax3 and Eya4. In contrast, activation of the Wnt pathway was not sufficient to elicit Pax3 expression, suggesting that other signaling cues are also required to promote ophthalmic/profundal trigeminal placodal fate (Lassiter et al., 2007; Dude et al., 2009).
In addition to the signaling pathways discussed above other signaling molecules are also associated with various aspects of placode development. Recently, RA signaling through retinoic acid receptor alpha has been proposed as an important signal to pattern the posterior PPR (Janesick et al., 2012). Later in development, RA has been primarily implicated in the morphogenesis and patterning of the otocyst (Romand et al., 2006). This activity is largely indirect through RA’s ability to regulate rhombomere identity in the hindbrain through differential Hox genes expression. In zebrafish, application of a dose of RA that does not perturb patterning of the anterior neural plate leads to enlarged otic placodes, a process dependent on FGF signaling (Hans et al., 2007). In vitamin A-deficient quail embryos, Rathke’s pouch fails to develop, suggesting an important role of RA in adenohypophyseal placode development in birds. However, this phenotype might be secondary to the loss of other signaling molecules since BMP2, sonic hedgehog (SHH) and FGF8 also are downregulated in these animals (Maden et al., 2007).
In chicken, platelet derived growth factor (PDGF) signaling is implicated in the induction of the ophthalmic lobe of the trigeminal placode (McCabe and Bronner-Fraser, 2008). PDGF receptor is detected in the cranial ectoderm at the time of trigeminal placode formation, while the ligand PDGF-D is confined to the midbrain. In recombinant explants of quail ectoderm with chicken neural tube, which normally promote trigeminal placode fate, blocking PDGF signaling results in loss of the trigeminal placode specific gene, Pax3. Conversely, expression of PDGF-D increases the number of Pax3-expressing cells in the trigeminal placode (McCabe and Bronner-Fraser, 2008).
SHH is one of the main factors involved in adenophypophyseal placode induction. During development SHH is expressed throughout the oral ectoderm, but it is excluded from Rathke’s pouch as soon as this structure forms. In SHH-deficient mouse embryos, formation of the diencephalon is severely disrupted, which has made it difficult to assess adenohypophysis development. However, Rathke’s pouch formation was completely arrested in transgenic animals expressing a specific hedgehog inhibitor (Hip) throughout the oral ectoderm (Treier et al., 2001). In the talpid chicken mutants, SHH signaling is reduced, and formation of the pituitary is severely disrupted (Lewis et al., 1999), and ectopic lenses form from the roof of the mouth, where the adenohypophysis is normally positioned (Ede and Kelley, 1964). The zebrafish double mutants for SHH and TWHH, which encode two partially redundant hedgehog ligands, have a complete loss of anterior pituitary fates (Herzog et al., 2003). In contrast overexpression of SHH causes induction of an excessive number of pituitary cells at the expense of lens precursors (Dutta et al., 2005; Herzog et al., 2003; Sbrogna et al., 2003). These studies clearly demonstrate that SHH has the ability not only to repress lens fate in the anterior placodal region, but also to promote the alternate adenohypophyseal fate.
A recent study has identified two neuropetides, Nociceptin and Somatostatin, controlling the specification of the anterior PPR in fish and chick (Llera-Forero et al., 2013). This study reports that mesendoderm-derived Somatostatin promotes ectodermal Nociceptin expression, and both peptides regulate Pax6 expression in lens and olfactory progenitors. Moreover, loss of Somatostatin and Nociceptin signaling leads to severe reduction of the lens (Llera-Forero et al., 2013). These neuropetides represent the first class of signaling molecules directly involved in the specification of placodes of anterior character.
Finally, it is important to mention that recent in vitro studies have successfully differentiated human embryonic stem cells (hESCs) into cranial placode derivatives using various induction protocols (Chen et al., 2012; Leung et al., 2013; Dincer et al., 2013). This is primarily achieved through the temporal manipulation of the same signaling molecules (BMP, FGF, Wnt and SHH) involved in the initial specification and regionalization of the different placode domains (Fig 3). For example, hair-cell-like cells and auditory neurons with the expected electrophysiological properties have been generated in vitro, and these otic progenitors have the ability to produce spiral ganglion neurons when transplanted in vivo (Chen et al., 2012). Another study has reported the generation of trigeminal sensory neuron progenitors capable of in vivo engraftment in chick and mouse embryos, as well as the production of anterior pituitary cells capable of synthesizing human growth hormone (GH) and adrenocorticotropic hormone (ACTH) in vivo (Dincer et al., 2013).
4- Transcriptional code of sensory placodes
In response to these signaling events, transcription factors are sequentially and differentially activated in placodal precursors. The combinatorial expression of these genes provides a molecular signature for each sensory placode, and presumably drives their development into mature sensory organs. A subset of these transcription factors is presented in Figure 2. A comprehensive list of the genes surrounding the anterior neural plate, and their progressive restriction to individual placodes can be found in several recent reviews (Streit, 2004; 2007; Battacharrya and Bronner-Fraser, 2004; Schlosser and Ahrens, 2004; Brugmann and Moody, 2005; Schlosser, 2006; Bailey and Streit, 2006; Moody, 2007; McCabe and Bronner-Fraser, 2009; Schlosser, 2010; Park and Saint-Jeannet, 2010; Grocott et al., 2012).
Among the transcription factors that show restricted expression patterns as the PPR is progressively subdivided, the Pax gene family plays an especially critical role in setting up the identity of individual placodal regions (Baker and Bronner-Fraser, 2000; reviewed in Baker and Bronner-Fraser, 2001; Schlosser 2006). All placodes express one or more Pax gene at a relatively early stage in their development. At neurula stages Pax2 and Pax8 are broadly expressed in the posterior placodal region, with Pax6 more restricted to the anterior placodal region, and Pax3 located in between (Figure 2). Later in development, Pax2 is upregulated in epibranchial placode precursors at the onset of neurogenesis in chick, frogs and zebrafish (Baker and Bronner-Fraser, 2000; Schlosser and Ahrens, 2004, Nechiporuk et al, 2007). In zebrafish, Pax2 and Pax8 function synergistically to specify the otic placode (Hans et al., 2004), but act redundantly to maintain otic fate (Mackereth et al., 2005). By contrast mice lacking Pax8 function have no obvious inner ear phenotype (Mansouri et al., 1998), while Pax2 mutant mouse embryos display agenesis of the cochlear duct and associated ganglion (Torres et al., 1996; Burton et al., 2004). In humans, PAX2 mutations are linked to sensorineural hearing loss (Sanyanusin et al., 1995). In birds, Pax2 is the sole Pax gene expressed in otic/epibranchial progenitors. In the absence of Pax2 function, otic progenitors lose expression of otic markers (Eya1 and Gata3), resulting in smaller otocysts, and the Phox2a/Phox2b expression domain was dramatically reduced in epibranchial progenitors (Christophorou et al., 2010; Freter et al., 2012). More than the mere expression of Pax2 and Pax8, a recent study suggests that expression levels of these proteins are also critical for sensory placode formation (McCarroll et al., 2012). By manipulating Pax2a and Pax8 expression levels in zebrafish embryos it is possible to shift fate among placode progenitors, where cells expressing high Pax2a/Pax8 levels become otic, while low Pax2a/Pax8-expressing cells acquire epibranchial fate (McCarroll et al., 2012).
In most vertebrates Pax3 is expressed in the prospective ophthalmic/profundal trigeminal placode (Stark et al., 1997; Baker et al., 1999; Schlosser and Ahrens, 2004) where it regulates cutaneous sensory neuron identity (Baker et al., 1999; 2002; Baker and Bronner-Fraser, 2000). In the Pax3 mouse mutant, Splotch (Pax3Splotch), several cranial ganglia are hypoplastic including the trigeminal ganglion (Epstein et al., 1991; Tremblay et al., 1995). This phenotype is also associated with severe defects in the cochlear duct and vestibulocochlear ganglion formation (Buckiova and Syka, 2004). Interestingly, mis-expression of Pax3 in the otic/epibranchial placodal domain represses Pax2 expression and upregulates the ophthalmic trigeminal placode markers FGFR4 and Ngn2 (Dude et al., 2009). These results suggest that Pax3 is sufficient to alter the identity of placodal progenitors, and point to a mutual repressive activity between Pax3 and Pax2 in patterning the PPR.
Pax6 is implicated in the formation of the most anterior placodes, it is expressed in the anterior-most domain of the PPR, the region that gives rise to the adenohypophyseal, olfactory and lens placodes (Gehring and Ikeo, 1999; Bhattacharyya and Bronner-Fraser, 2004). Pax6 overexpression leads to ectopic formation of eyes and lenses (Altmann et al., 1997; Chow et al., 1999), while the Pax6 mouse mutant, Small eyes, shows reduced eyes with missing lens and olfactory placodes (Hogan et al., 1986). The adenohypophyseal placode is also defective in these mutant embryos (Bentley et al., 1999). In humans, PAX6 mutations cause aniridia, a sight-threatening disorder that affects the iris, retina, optic nerve, lens and cornea (review in Hingorani et al., 2012). A recent study in birds indicates that Pax6 functions with Pax3 to set up placodal territories. The Pax6 posterior boundary of expression initially includes the prospective ophthalmic trigeminal placode. Pax6 is progressively downregulated in this region as Pax3 starts to be expressed (Wakamatsu, 2011). Mis-expression studies indicate that Pax6 and Pax3 can repress each other’s expression in the placodal ectoderm to properly position the ophthalmic trigeminal placode (Wakamatsu, 2011). This mutual repressive activity among Pax family members may represent a common mechanism to establish boundaries between placodal territories, very reminiscent of the cross regulation between Pax6 and Pax2 in the mammalian visual system, which is critical to establish the spatial regionalization of the optic cup and optic stalk (Schwarz et al., 2000).
The Pax gene family exemplifies the importance of having the right combination of transcriptional regulators to establish the identity of individual placodes, and to direct their differentiation. The mechanisms by which Pax genes regulate placode identity are not fully understood, however, Pax proteins are not the only factors regulating this process, and they are very likely to act in concert with others families of transcription factors with which they are co-expressed in various placodal domains (Figure 2).
V- Conclusions and perspectives
In this review we have summarized the major signaling pathways and molecular effectors involved in PPR specification and its subsequent subdivision into sensory placodes with distinct identities. Both processes are guided by the careful orchestration of complex regulatory mechanisms that are both temporally and spatially regulated, and that distinguish this precursor population from the related neural crest. It is remarkable that the activation of the same combination of signaling molecules at different time points during the ontogeny of the cranial placodes result in such drastically different outcomes. Understanding how these signaling pathways are integrated to generate the PPR and the appropriate fate within each distinct placode and its derivatives is the next challenge. Another important task is to uncover the full repertoire of transcription factors activated in each placodal domain, and to define how these factors interact with one another in order to fully appreciate the mechanisms by which this gene network drive sensory placode development. This information will be important for understanding the normal development of the cranial sensory organs, and for detecting abnormalities that occur when the underlying gene networks are altered by mutations or environmental factors.
Highlights.
The pre-placodal region (PPR) contains progenitors for all cranial sensory placodes.
Specification of the PPR and its separation into individual placodes are regulated by several classes of signaling molecules.
Six/Eya gene families are broadly expressed in the PPR and drive its development.
The combinatorial expression of transcription factors underlies the identity of cranial sensory placodes.
Acknowledgements
We would like to thank Marianne Bronner and the Fondation des Treilles for organizing the meeting that is at the origin of this review. Work in the authors’ laboratories is supported by the NIH (R01 DE014212 to J-P. S-J. and R01 DE022065 to S. A. M.).
References
- Abdelhak S, Kalatzis V, Heilig R, Compain S, Samson D, Vincent C, Weil D, Cruaud C, Sahly I, Leibovici M, Bitner-Glindzicz M, Francis M, Lacombe D, Vigneron J, Charachon R, Boven K, Bedbeder P, Van Regemorter N, Weissenbach J, Petit C. A human homologue of the Drosophila eyes absent gene underlies Branchio-Oto-Renal (BOR) syndrome and identifies a novel gene family. Nature Genet. 1997;15:157–164. doi: 10.1038/ng0297-157. [DOI] [PubMed] [Google Scholar]
- Abello G, Khatri S, Giraldez F, Alsina B. Early regionalization of the otic placode and its regulation by the Notch signaling pathway. Mech. Dev. 2007;124:631–645. doi: 10.1016/j.mod.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Ahrens K, Schlosser G. Tissues and signals involved in the induction of placodal Six1 expression in Xenopus laevis. Dev. Biol. 2005;288:40–59. doi: 10.1016/j.ydbio.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Altmann CR, Chow RL, Lang RA, Hemmati-Brivanlou A. Lens induction by Pax-6 in Xenopus laevis. Dev. Biol. 1997;185:119–123. doi: 10.1006/dbio.1997.8573. [DOI] [PubMed] [Google Scholar]
- Arima K, Shiotsugu J, Niu R, Khandpur R, Martinez M, Shin Y, Koide T, Cho KW, Kitayama A, Ueno N, et al. Global analysis of RAR-responsive genes in the Xenopus neurula using cDNA microarrays. Dev. Dyn. 2005;232:414–431. doi: 10.1002/dvdy.20231. [DOI] [PubMed] [Google Scholar]
- Aybar MJ, Mayor R. Early induction of neural crest cells: lessons learned from frog, fish and chick. Curr. Opin. Genet. Dev. 2002;12:452–458. doi: 10.1016/s0959-437x(02)00325-8. [DOI] [PubMed] [Google Scholar]
- Bailey AP, Streit A. Sensory organs: making and breaking the pre-placodal region. Curr. Top. Dev. Biol. 2006;72:167–204. doi: 10.1016/S0070-2153(05)72003-2. [DOI] [PubMed] [Google Scholar]
- Bailey AP, Bhattacharyya S, Bronner-Fraser M, Streit A. Lens specification is the ground state of all sensory placodes, from which FGF promotes olfactory identity. Dev. Cell. 2006;11:505–517. doi: 10.1016/j.devcel.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Baker CVH, Bronner-Fraser M. Establishing neuronal identity in vertebrate neurogenic placodes. Development. 2000;127:3045–3056. doi: 10.1242/dev.127.14.3045. [DOI] [PubMed] [Google Scholar]
- Baker CV, Bronner-Fraser M. Vertebrate cranial placodes I. Embryonic induction. Dev. Biol. 2001;232:1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- Baker CV, Stark MR, Bronner-Fraser M. Pax3-expressing trigeminal placode cells can localize to trunk neural crest sites but are committed to a cutaneous sensory neuron fate. Dev. Biol. 2002;249:219–236. doi: 10.1006/dbio.2002.0767. [DOI] [PubMed] [Google Scholar]
- Baker CV, Stark MR, Marcelle C, Bronner-Fraser M. Competence, specification and induction of Pax-3 in the trigeminal placode. Development. 1999;126:147–156. doi: 10.1242/dev.126.1.147. [DOI] [PubMed] [Google Scholar]
- Barraud P, Seferiadis AA, Tyson LD, Zwart MF, Szabo-Rogers HL, Ruhuberg C, Liu KJ, Baker CV. Neural crest origin of olfactory ensheathing glia. Proc. Natl. Acad. Sci. USA. 2010;107:21040–21045. doi: 10.1073/pnas.1012248107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley CA, Zidehsarai MP, Grindley JC, Parlow AF, Barth-Hall S, Roberts VJ. Pax6 is implicated in murine pituitary endocrine function. Endocrine. 1999;10:171–177. doi: 10.1385/ENDO:10:2:171. [DOI] [PubMed] [Google Scholar]
- Bessarab DA, Chong SW, Korzh V. Expression of zebrafish six1 during sensory organ development and myogenesis. Dev. Dyn. 2004;230:781–786. doi: 10.1002/dvdy.20093. [DOI] [PubMed] [Google Scholar]
- Bhat N, Riley B. Integrin-a5 coordinates assembly of posterior cranial placodes in zebrafish and enhances fgf-dependent regulation of otic/epibranchial cells. PLoS One. 2011;6:e27778. doi: 10.1371/journal.pone.0027778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat N, Kwon HJ, Riley BB. A gene network that coordinates preplacodal competence and neural crest specification in zebrafish. Dev. Biol. 2013;373:107–117. doi: 10.1016/j.ydbio.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Bronner-Fraser M. Hierarchy of regulatory events in sensory placode development. Curr. Opin. Genet. Dev. 2004;14:520–526. doi: 10.1016/j.gde.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Bailey AP, Bronner-Fraser M, Streit A. Segregation of lens and olfactory precursors from a common territory: cell sorting and reciprocity of Dlx5 and Pax6 expression. Dev. Biol. 2004;271:403–414. doi: 10.1016/j.ydbio.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Bosman EA, Quint E, Fuchs E, Hrabe de Angelis M, Steel KP. Catweasel mice: a novel role for Six1 in sensory patch development and a model for branchio-otic-renal syndrome. Dev. Biol. 2009;328:285–296. doi: 10.1016/j.ydbio.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricaud O, Collazo A. The transcription factor six1 inhibits neuronal and promotes hair cell fates in the developing zebrafish (Danio rerio) inner ear. J. Neurosci. 2006;26:10438–10451. doi: 10.1523/JNEUROSCI.1025-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricaud O, Collazo A. Balancing cell numbers during organogenesis: Six1a differentially affects neurons and sensory hair cells in the inner ear. Dev. Biol. 2011;357:191–201. doi: 10.1016/j.ydbio.2011.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugmann SA, Moody SA. Induction and specification of the vertebrate ectodermal placodes: precursors of the cranial sensory organs. Biol. Cell. 2005;97:303–319. doi: 10.1042/BC20040515. [DOI] [PubMed] [Google Scholar]
- Brugmann SA, Pandur PD, Kenyon KL, Pignoni F, Moody SA. Six1 promotes a placodal fate within the lateral neurogenic ectoderm by functioning as both a transcriptional activator and repressor. Development. 2004;131:5871–5881. doi: 10.1242/dev.01516. [DOI] [PubMed] [Google Scholar]
- Buckiova D, Syka J. Development of the inner ear in Splotch mutant mice. Neuroreport. 2004;125:2001–2005. doi: 10.1097/00001756-200409150-00002. [DOI] [PubMed] [Google Scholar]
- Buller C, Xu X, Marquis V, Schwanke R, Xu PX. Molecular effects of Eya1 domain mutations causing organ defects in BOR syndrome. Hum. Mol. Genet. 2001;10:2775–278. doi: 10.1093/hmg/10.24.2775. [DOI] [PubMed] [Google Scholar]
- Burton Q, Cole LK, Mulheisen M, Chang W, Wu DK. The role of Pax2 in mouse inner ear development. Dev. Biol. 2004;272:161–175. doi: 10.1016/j.ydbio.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Carmona-Fontaine C, Acuna G, Ellwanger K, Niehrs C, Mayor R. Neural crests are actively precluded from the anterior neural fold by a novel inhibitory mechanism dependent on Dickkopf1 secreted by the prechordal mesoderm. Dev. Biol. 2007;309:208–221. doi: 10.1016/j.ydbio.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Chang C, Hemmati-Brivanlou A. Neural crest induction by Xwnt7B in Xenopus. Dev. Biol. 1998;194:129–134. doi: 10.1006/dbio.1997.8820. [DOI] [PubMed] [Google Scholar]
- Chen B, Kim EH, Xu PX. Initiation of olfactory placode development and neurogenesis is blocked in mice lacking both Six1 and Six4. Dev. Biol. 2009;326:75–85. doi: 10.1016/j.ydbio.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Streit A. Induction of the inner ear: stepwise specification of otic fate from multipotent progenitors. Hear. Res. 2013;297:3–12. doi: 10.1016/j.heares.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Chen Y, Pollet N, Niehrs C, Pieler T. Increased XRALDH2 activity has a posteriorizing effect on the central nervous system of Xenopus embryos. Mech. Dev. 2001;101:91–103. doi: 10.1016/s0925-4773(00)00558-x. [DOI] [PubMed] [Google Scholar]
- Chen W, Jongkamonwiwat N, Abbas L, Eshtan SJ, Johnson SL, Kuhn S, Milo M, Thurlow JK, Andrews PW, Marcotti W, Moore HD, Rivolta MN. Restoration of auditory evoked response by human ES-derived otic progenitors. Nature. 2012;490:278–282. doi: 10.1038/nature11415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RL, Altmann CR, Lang RA, Hemmati-Brivanlou A. Pax6 induces ectopic eyes in a vertebrate. Development. 1999;126:4213–4222. doi: 10.1242/dev.126.19.4213. [DOI] [PubMed] [Google Scholar]
- Christen B, Slack JMW. FGF-8 is associated with anteroposterior patterning and limb regeneration in Xenopus. Dev. Biol. 1997;192:455–46. doi: 10.1006/dbio.1997.8732. [DOI] [PubMed] [Google Scholar]
- Christophorou NA, Bailey AP, Hanson S, Streit A. Activation of Six1 target genes is required for sensory placode formation. Dev. Bio. 2009;336:327–336. doi: 10.1016/j.ydbio.2009.09.025. [DOI] [PubMed] [Google Scholar]
- Christophorou NA, Mende M, Lleras-Forero L, Grocott T, Streit A. Pax2 coordinates epithelial morphogenesis and cell fate in the inner ear. Dev. Biol. 2010;345:180–190. doi: 10.1016/j.ydbio.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couly GF, LeDouarin NM. Mapping of the early neural primordium in quail-chick chimeras. II. The prosencephalic neural plate and neural folds: implications for the genesis of cephalic human congenital abnormalities. Dev. Biol. 1987;120:198–214. doi: 10.1016/0012-1606(87)90118-7. [DOI] [PubMed] [Google Scholar]
- Couly GF, LeDouarin NM. Head morphogenesis in embryonic avian chimeras: evidence for a segmental pattern in the ectoderm corresponding to the neuromeres. Development. 1990;108:543–558. doi: 10.1242/dev.108.4.543. [DOI] [PubMed] [Google Scholar]
- de Croze N, Maczkowiak F, Monsoro-Burq AH. Reiterative AP2a activity controls sequential steps in the neural crest gene regulatory network. Proc. Natl. Acad. Sci. USA. 2011;108:155–160. doi: 10.1073/pnas.1010740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David R, Ahrens K, Wedlich D, Schlosser G. Xenopus Eya1 demarcates all neurogenic placodes as well as migrating hypaxial muscle precursors. Mech. Dev. 2001;103:189–192. doi: 10.1016/s0925-4773(01)00355-0. [DOI] [PubMed] [Google Scholar]
- Depreux FF, Darrow K, Conner DA, Eavey RD, Liberman MC, Seidman CE, Seidman JG. Eya4-deficient mice are a model for heritable otitis media. J. Clin. Invest. 2008;118:651–658. doi: 10.1172/JCI32899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRobertis EM, Kuroda H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Ann. Rev. Cell Dev. Biol. 2004;20:285–308. doi: 10.1146/annurev.cellbio.20.011403.154124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dincer Z, Piao J, Niu L, Ganat Y, Kriks S, Zimmer B, Shi S-H, Tabar V, Studer L. Specification of functional cranial placode derivatives from human pluripotent stem cells. Cell Reports. 2013;5:1–16. doi: 10.1016/j.celrep.2013.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dude CM, Kuan CY, Bradshaw JR, Greene ND, Relaix F, Stark MR, Baker CV. Activation of Pax3 target genes is necessary but not sufficient for neurogenesis in the ophthalmic trigeminal placode. Dev. Biol. 2009;326:314–326. doi: 10.1016/j.ydbio.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S, Dietrich JE, Aspock G, Burdine RD, Schier A, Westerfield M, Varga ZM. Pitx3 defines an equivalence domain for lens and anterior pituitary placode. Development. 2005;132:1579–1590. doi: 10.1242/dev.01723. [DOI] [PubMed] [Google Scholar]
- Ede DA, Kelly WA. Developmental abnormalities in the head region of the talpid3 mutant of the fowl. J. Embryol. Exp. Morph. 1964;12:161–182. [PubMed] [Google Scholar]
- Epstein DJ, Vekemans M, Gros P. Splotch (Sp2H), a mutation affecting development of the mouse neural tube, shows a deletion within the paired homeodomain of Pax-3. Cell. 1991;67:767–774. doi: 10.1016/0092-8674(91)90071-6. [DOI] [PubMed] [Google Scholar]
- Estefania E, Ramirez-Camacho R, Gomar M, Trinidad A, Arellano B, Garcia-Berrocal JR, Verdaguer JM, Vilches C. Point mutation of an EYA1-gene splice site in a patient with oto-facial-cervical syndrome. Ann. Hum. Genet. 2006;70:140–144. doi: 10.1111/j.1529-8817.2005.00204.x. [DOI] [PubMed] [Google Scholar]
- Esterberg R, Fritz A. Dlx3b/4b are required for the formation of the preplacodal region and otic placode through local modulation of BMP activity. Dev. Biol. 2009;325:189–199. doi: 10.1016/j.ydbio.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteve P, Bovolenta P. cSix4, a member of the six gene family of transcription factors, is expressed during placode and somite development. Mech, Dev. 1999;85:161–165. doi: 10.1016/s0925-4773(99)00079-9. [DOI] [PubMed] [Google Scholar]
- Feledy JA, Beanan MJ, Sandoval JJ, Goodrich JS, Lim JH, Matsuo-Takasaki M, Sato SM, Sargent TD. Inhibitory patterning of the anterior neural plate in Xenopus by homeodomain factors Dlx3 and Msx1. Dev. Biol. 1999;212:455–464. doi: 10.1006/dbio.1999.9374. [DOI] [PubMed] [Google Scholar]
- Fletcher RB, Baker JC, Harland RM. FGF8 spliceforms mediate early mesoderm and posterior neural tissue formation in Xenopus. Development. 2006;133:1703–1714. doi: 10.1242/dev.02342. [DOI] [PubMed] [Google Scholar]
- Freter S, Muta Y, Mak SS, Rinkwitz S, Ladher RK. Progressive restriction of otic fate: the role of FGF and Wnt in resolving inner ear potential. Development. 2008;135:3415–3424. doi: 10.1242/dev.026674. [DOI] [PubMed] [Google Scholar]
- Freter S, Muta Y, O’Neill P, Vassilev VS, Kuraku S, Ladher RK. Pax2 modulates proliferation during specification of otic and epibranchial placodes. Dev. Dyn. 2012;241:1716–1728. doi: 10.1002/dvdy.23856. [DOI] [PubMed] [Google Scholar]
- Furuta Y, Hogan BLM. BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 1998;12:3764–3775. doi: 10.1101/gad.12.23.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Ikeo K. Pax 6-mastering eye morphogenesis and eye evolution. Trends Genet. 1999;15:371–377. doi: 10.1016/s0168-9525(99)01776-x. [DOI] [PubMed] [Google Scholar]
- Ghanbari H, Seo HC, Fjose A, Brändli AW. Molecular cloning and embryonic expression of Xenopus Six homeobox genes. Mech. Dev. 2001;101:271–277. doi: 10.1016/s0925-4773(00)00572-4. [DOI] [PubMed] [Google Scholar]
- Glavic A, Maris Honoré S, Gloria Feijóo C, Bastidas F, Allende ML, Mayor R. Role of BMP signaling and the homeoprotein Iroquois in the specification of the cranial placodal field. Dev. Biol. 2004a;272:89–103. doi: 10.1016/j.ydbio.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Glavic A, Silva F, Aybar MJ, Bastidas F, Mayor R. Interplay between Notch signaling and the homeoprotein Xiro1 is required for neural crest induction in Xenopus embryos. Development. 2004b;131:347–359. doi: 10.1242/dev.00945. [DOI] [PubMed] [Google Scholar]
- Grifone R, Demignon J, Houbron C, Souil E, Niro C, Seller MJ, Hamard G, Maire P. Six1 and Six4 homeoproteins are required for Pax3 and Mrf expression during myogenesis in the mouse embryo. Development. 2005;132:2235–2249. doi: 10.1242/dev.01773. [DOI] [PubMed] [Google Scholar]
- Grocott T, Tambalo M, Streit A. The peripheral sensory nervous system in the vertebrate head: A gene regulatory perspective. Dev. Biol. 2012;370:3–23. doi: 10.1016/j.ydbio.2012.06.028. [DOI] [PubMed] [Google Scholar]
- Hans S, Liu D, Westerfield M. Pax8 and Pax2a function synergistically in otic specification, downstream of the Foxi1 and Dlx3b transcription factors. Development. 2004;131:5091–5102. doi: 10.1242/dev.01346. [DOI] [PubMed] [Google Scholar]
- Hans S, Christison J, Liu D, Westerfield M. Fgf-dependent otic induction requires competence provided by Foxi1 and Dlx3b. BMC Dev. Biol. 2007;7:5. doi: 10.1186/1471-213X-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden MV, Pereiro L, Ramialison M, Wittbrodt J, Prasad MK, McCallion AS, Whitlock KE. Close association of olfactory placode precursors and cranial neural crest cells does not predestine cell mixing. Dev. Dyn. 2012;241:1143–54. doi: 10.1002/dvdy.23797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg CP, Brand M, Jiang YJ, Warga RM, Beuchle D, van Eeden FJ, Furutani-Seiki M, Granato M, Haffter P, Hammerschmidt M, Kane DA, Kelsh RN, Mullins MC, Odenthal J, Nusslein-Volhard C. Genes involved in forebrain development in the zebrafish, Danio rerio. Development. 1996;123:191–203. doi: 10.1242/dev.123.1.191. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Houart C, Take-Uchi M, Rauch GJ, Young N, Coutinho P, Masai I, Caneparo L, Concha ML, Geisler R, Dale TC, Wilson SW, Stemple DL. A mutation in the Gsk3-binding domain of zebrafish Masterblind/Axin1 leads to a fate transformation of telencephalon and eyes to diencephalon. Genes Dev. 2001;15:1427–1434. doi: 10.1101/gad.194301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog W, Zeng X, Lele Z, Sonntag C, Ting JW, Chang CY, Hammerschmidt M. Adenohypophysis formation in the zebrafish and its dependence on sonic hedgehog. Dev. Biol. 2003;25:36–49. doi: 10.1016/s0012-1606(02)00124-0. [DOI] [PubMed] [Google Scholar]
- Herzog W, Sonntag C, von der Hardt S, Roel HH, Varga ZM, Hammerschmidt M. Fgf3 signaling from the ventral diencephalon is required for early specification and subsequent survival of the zebrafish adenohypophysis. Development. 2004;131:3681–3692. doi: 10.1242/dev.01235. [DOI] [PubMed] [Google Scholar]
- Hillal EM, Chen JH, Silverman AJ. Joint migration of gonadotropin-releasing hormone (GnRH) and neuropeptide Y (NPY) neurons from olfactory placode to central nervous system. J. Neurobiol. 1996;31:487–502. doi: 10.1002/(SICI)1097-4695(199612)31:4<487::AID-NEU8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Hingorani M, Hanson I, van Heyningen V. Aniridia. Eur. J. Huma. Genet. 2012;20:1011–1017. doi: 10.1038/ejhg.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman TL, Javier AL, Campeau SA, Knight RD, Schilling TF. Tfap2 transcription factors in zebrafish neural crest development and ectodermal evolution. J. Exp. Zool. B Mol. Dev. Evol. 2007;308:679–691. doi: 10.1002/jez.b.21189. [DOI] [PubMed] [Google Scholar]
- Hogan BL, Horsburgh G, Cohen J, Hetherington CM, Fisher G, Lyon MF. Small eyes (Sey): a homozygous lethal mutation on chromosome 2 which affects the differentiation of both lens and nasal placodes in the mouse. J. Embryol. Exp. Morphol. 1986;97:95–110. [PubMed] [Google Scholar]
- Hong C-S, Saint-Jeannet J-P. The activity of Pax3 and Zic1 regulates three distinct cell fates at the neural plate border. Mol. Biol. Cell. 2007;18:2192–2202. doi: 10.1091/mbc.E06-11-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Ookawara S, Sato S, Ando Z, Kageyama R, Kawakami K. Six1 is essential for early neurogenesis in the development of olfactory epithelium. Dev. Biol. 2007;311:53–68. doi: 10.1016/j.ydbio.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Kageyama R, Suzuki Y, Kawakami K. Six1 is indispensable for production of functional progenitor cells during olfactory epithelial development. Int. J. Dev. Biol. 2010;54:1453–1464. doi: 10.1387/ijdb.093041ki. [DOI] [PubMed] [Google Scholar]
- Ito T, Noguchi Y, Yashima T, Kitamura K. SIX1 mutation associated with enlargement of the vestibular aqueduct in a patient with branchio-oto syndrome. Laryngoscope. 2006;116:797–799. doi: 10.1097/01.mlg.0000209096.40400.96. [DOI] [PubMed] [Google Scholar]
- Jacobson AG. The determination and positioning of the nose, lens and ear. III. Effects of reversing the antero-posterior axis of epidermis, neural plate and neural fold. J. Exp. Zool. 1963a;154:293–303. doi: 10.1002/jez.1401540305. [DOI] [PubMed] [Google Scholar]
- Jacobson AG. Determination and positioning of the nose, lens and ear. II. The role of the endoderm. J. Exp. Zool. 1963b;154:285–291. doi: 10.1002/jez.1401540304. [DOI] [PubMed] [Google Scholar]
- Jacobson AG. The determination and positioning of the nose, lens and ear. I. Interactions within the ectoderm, and between the ectoderm and underlying tissues. J. Exp. Zool. 1963c;154:273–283. doi: 10.1002/jez.1401540303. [DOI] [PubMed] [Google Scholar]
- Jacobson AG. Inductive processes in embryonic development. Science. 1966;152:25–34. doi: 10.1126/science.152.3718.25. [DOI] [PubMed] [Google Scholar]
- Janesick A, Shiotsugu J, Taketani M, Blumberg B. RIPPLY3 is a retinoic acid-inducible repressor required for setting the borders of the pre-placodal ectoderm. Development. 2012;139:1213–1224. doi: 10.1242/dev.071456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasena CS, Ohyama T, Segil N, Groves A. Notch signaling augments the canonical Wnt pathway to specify the size of the otic placode. Development. 2008;135:2251–2261. doi: 10.1242/dev.017905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KR, Cook SA, Erway LC, Matthews AN, Sanford LP, Paradies NE, Friedman RA. Inner ear and kidney anomalies caused by IAP insertion in an intron of the Eya1 gene in a mouse model of BOR syndrome. Hum. Mol. Genet. 1999;8:645–653. doi: 10.1093/hmg/8.4.645. [DOI] [PubMed] [Google Scholar]
- Khatri SB, Groves AK. Expression of the Foxi2 and Foxi3 transcription factors during development of chicken sensory placodes and pharyngeal arches. Gene Expr. Patterns. 2013;13:38–42. doi: 10.1016/j.gep.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khudyakov, Bronner-Fraser M. Comprehensive spatiotemporal analysis of early chick neural crest network genes. Dev. Dyn. 2009;238:716–723. doi: 10.1002/dvdy.21881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knouff RA. The developmental pattern of ectodermal placodes in Rana pipiens. J. Comp. Neurol. 1935;62:17–7. [Google Scholar]
- Kobayashi M, Osanai H, Kawakami K, Yamamoto M. Expression of three zebrafish Six4 genes in the cranial sensory placodes and the developing somites. Mech. Dev. 2000;98:151–155. doi: 10.1016/s0925-4773(00)00451-2. [DOI] [PubMed] [Google Scholar]
- Kochhar A, Orten DJ, Sorensen JL, Fischer SM, Cremers CW, Kimberling WJ, Smith RJ. SIX1 mutation screening in 247 branchio-otic-renal syndrome families: a recurrent missense mutation associated with BOR. Hum. Mutat. 2008;29:565. doi: 10.1002/humu.20714. [DOI] [PubMed] [Google Scholar]
- Konishi Y, Ikeda K, Iwakura Y, Kawakami K. Six1 and Six4 promote survival of sensory neurons during early trigeminal gangliogenesis. Brain Res. 2006;1116:93–102. doi: 10.1016/j.brainres.2006.07.103. [DOI] [PubMed] [Google Scholar]
- Kozlowski DJ, Murakami T, Ho RK, Weinberg ES. Regional cell movement and tissue patterning in the zebrafish embryo revealed by fate mapping with caged fluorescein. Bioch. Cell Biol. 1997;75:551–562. [PubMed] [Google Scholar]
- Kozlowski DJ, Whitfield TT, Hukriede NA, Lam WK, Weinberg ES. The zebrafish dog-eared mutation disrupts eya1, a gene required for cell survival and differentiation in the inner ear and lateral line. Dev. Biol. 2005;277:27–41. doi: 10.1016/j.ydbio.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Kwon HJ, Bhat N, Sweet EM, Cornell RA, Riley BB. Identification of early requirements for preplacodal etoderm and sensory organ development. PLoS Genet. 2010;6:e1001133. doi: 10.1371/journal.pgen.1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Deffenbacher K, Cremers CW, Van Camp G, Kimberling WJ. Brachio-oto-renal syndrome: identification of novel mutations, molecular characterization, mutation distribution and prospects for genetic testing. Genet. Test. 1997;1:243–251. doi: 10.1089/gte.1997.1.243. [DOI] [PubMed] [Google Scholar]
- LaBonne C, Bronner-Fraser M. Neural crest induction in Xenopus: evidence for a two-signal model. Development. 1998;125:2403–2414. doi: 10.1242/dev.125.13.2403. [DOI] [PubMed] [Google Scholar]
- Laclef C, Souil E, Demignon J, Maire P. Thymus, kidney and craniofacial abnormalities in Six 1 deficient mice. Mech. Dev. 2003;120:669–679. doi: 10.1016/s0925-4773(03)00065-0. [DOI] [PubMed] [Google Scholar]
- Ladher RK, Anakwe KU, Gurney AL, Schoenwolf GC, Francis-West PH. Identification of synergistic signals initiating inner ear development. Science. 2000;290:1965–1967. doi: 10.1126/science.290.5498.1965. [DOI] [PubMed] [Google Scholar]
- Ladher RK, Wright TJ, Moon AM, Mansour SL, Schoenwolf GC. FGF8 initiates inner ear induction in chick and mouse. Genes & Dev. 2005;19:603–613. doi: 10.1101/gad.1273605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladher RK, O’Neill P, Begbie J. From shared lineage to distinct functions: development of the inner ear and epibranchial placodes. Development. 2010;137:1777–1785. doi: 10.1242/dev.040055. [DOI] [PubMed] [Google Scholar]
- Landacre FJ. The primitive lines of Amblystoma jeffersonianum. J. Comp. Neurol. 1926;40:471–496. [Google Scholar]
- Lassiter RN, Dude CM, Reynolds SB, Winters NI, Baker CV, Stark MR. Canonical Wnt signaling is required for ophthalmic trigeminal placode cell fate determination and maintenance. Dev. Biol. 2007;308:392–406. doi: 10.1016/j.ydbio.2007.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDouarin NM, Fontaine-Perus J, Couly G. Cephalic ectodermal placodes and neurogenesis. Trends Neurosci. 1986;9:175–180. [Google Scholar]
- Leger S, Brand M. Fgf8 and Fgf3 are required for zebrafish ear placode induction, maintenance and inner ear patterning. Mech. Dev. 2002;119:91–108. doi: 10.1016/s0925-4773(02)00343-x. [DOI] [PubMed] [Google Scholar]
- Leung AW, Morest KD, Li JY. Differential BMP signaling controls formation and differentiation of multipotent preplacodal progenitors from human embryonic stem cells. Dev. Biol. 2013;379:208–220. doi: 10.1016/j.ydbio.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AJ, Brivanlou AH. Proposal of a model of mammalian neural induction. Dev. Biol. 2007;308:247–256. doi: 10.1016/j.ydbio.2007.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KE, Drossopoulou G, Paton IR, Morrice DR, Robertson KE, Burt DW, Ingham PW, Tickle C. Expression of ptc and gli genes in talpid3 suggests bifurcation in Shh pathway. Development. 1999;126:2397–2407. doi: 10.1242/dev.126.11.2397. [DOI] [PubMed] [Google Scholar]
- Li Y, Manaligod JM, Weeks DL. EYA1 mutations associated with the branchio-otic renal syndrome result in defective otic development in Xenopus laevis. Biol. Cell. 2010;102:277–292. doi: 10.1042/BC20090098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Oghi KA, Zhang J, Krones A, Bush KT, Glass CK, Nigam SK, Aggarwal AK, Maas R, Rose DW, Rosenfeld MG. Eya protein phosphatase activity regulates Six1-Dach-Eya transcriptional effects in mammalian organogenesis. Nature. 2003;426:247–25. doi: 10.1038/nature02083. [DOI] [PubMed] [Google Scholar]
- Litsiou A, Hanson S, Streit A. A balance of FGF, BMP and WNT signalling positions the future placode territory in the head. Development. 2005;132:4051–4062. doi: 10.1242/dev.01964. [DOI] [PubMed] [Google Scholar]
- Liu D, Chu H, Maves L, Yan YL, Morcos PA, Postlethwait JH, Westerfield M. Fgf3 and Fgf8 dependent and independent transcription factors are required for otic placode specification. Development. 2003;130:2213–2224. doi: 10.1242/dev.00445. [DOI] [PubMed] [Google Scholar]
- Llera-Forero L, Tambalo M, Christophorou M, Chambers D, Houart C, Streit A. Neuropeptides: developmental signals in placode progenitor formation. Dev. Cell. 2013;26:195–203. doi: 10.1016/j.devcel.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo T, Matsuo-Takasaki M, Lim JH, Sargent TD. Differential regulation of Dlx gene expression by a BMP morphogenetic gradient. Int. J., Dev. Biol. 2001b;45:681–684. [PubMed] [Google Scholar]
- Luo T, Matsuo-Takasaki M, Thomas ML, Weeks DL, Sargent TD. Transcription factor AP-2 is an essential and direct regulator of epidermal development in Xenopus. Dev. Biol. 2002;245:136–144. doi: 10.1006/dbio.2002.0621. [DOI] [PubMed] [Google Scholar]
- Luo T, Lee Y-H, Saint-Jeannet J-P, Sargent TD. Induction of neural crest in Xenopus by transcription factor AP2a. Proc. Natl. Acad. Sci. USA. 2003;100:532–537. doi: 10.1073/pnas.0237226100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackereth MD, Kwak SJ, Fritz A, Riley BB. Zebrafish pax8 is required for otic placode induction and plays a redundant role with Pax2 genes in the maintenance of the otic placode. Development. 2005;132:371–382. doi: 10.1242/dev.01587. [DOI] [PubMed] [Google Scholar]
- Maden M, Blentic A, Reijntjes S, Sequin S, Gale E, Graham A. Retinoic acid is required for specification of the ventral eye field and for Rathke’s pouch in the avian embryo. Int. J. Dev. Biol. 2007;51:191–200. doi: 10.1387/ijdb.062175mm. [DOI] [PubMed] [Google Scholar]
- Makishima T, Madeo AC, Brewer CC, Zalewski CK, Butman JA, Sachdev V, Arai AE, Holbrook BM, Rosing DR, Griffith AJ. Nonsyndromic hearing loss DFNA10 and a novel mutation of EYA4: evidence for correlation of normal cardiac phenotype with truncating mutations of the Eya domain. Am. J. Med. Genet. 2007;143A:1592–1598. doi: 10.1002/ajmg.a.31793. [DOI] [PubMed] [Google Scholar]
- Mancilla A, Mayor R. Neural crest formation in Xenopus laevis: mechanism of Xslug induction. Dev. Biol. 1996;177:580–589. doi: 10.1006/dbio.1996.0187. [DOI] [PubMed] [Google Scholar]
- Mansouri A, Chowdhury K, Gruss P. Follicular cells of the thyroid gland require Pax8 gene function. Nat. Genet. 1998;19:87–90. doi: 10.1038/ng0598-87. [DOI] [PubMed] [Google Scholar]
- Marchant L, Linker C, Ruiz P, Guerrero N, Mayor R. The inductive properties of mesoderm suggest that the neural crest cells are specified by a BMP gradient. Dev. Biol. 1998;198:319–329. [PubMed] [Google Scholar]
- Maroon H, Walshe J, Mahmood R, Kiefer P, Dickson C, Mason I. Fgf3 and Fgf8 are required together for formation of the otic placode and vesicle. Development. 2002;129:2099–2108. doi: 10.1242/dev.129.9.2099. [DOI] [PubMed] [Google Scholar]
- Matsuo-Takasaki M, Matsumura M, Sasai Y. As essential role of Foxi1a for ventral specification of the cephalic ectoderm during gastrulation. Development. 2005;132:3885–3894. doi: 10.1242/dev.01959. [DOI] [PubMed] [Google Scholar]
- Mayor R, Aybar M. Induction and development of neural crest in Xenopus laevis. Cell Tissue Res. 2001;305:203–209. doi: 10.1007/s004410100369. [DOI] [PubMed] [Google Scholar]
- Mayor R, Guerrero N, Martinez C. Role of FGF and noggin in neural crest induction. Dev. Biol. 1997;189:1–12. doi: 10.1006/dbio.1997.8634. [DOI] [PubMed] [Google Scholar]
- McCabe KL, Bronner-Fraser M. Essential role for PDGF signaling in ophthalmic trigeminal placode induction. Development. 2008;135:1863–1874. doi: 10.1242/dev.017954. [DOI] [PubMed] [Google Scholar]
- McCabe KL, Bronner-Fraser M. Molecular and tissue interactions governing induction of cranial ectodermal placodes. Dev. Biol. 2009;332:189–195. doi: 10.1016/j.ydbio.2009.05.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarroll MN, Lewis ZR, Culbertson MD, Martin BL, Kimelman D, Nechiporuk AV. Graded levels of Pax2a and Pax8 regulate cell differentiation during sensory placode formation. Development. 2012;139:2740–2750. doi: 10.1242/dev.076075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLarren KW, Litsiou A, Streit A. DLX5 positions the neural crest and preplacode region at the border of the neural plate. Dev. Biol. 2003;259:34–4. doi: 10.1016/s0012-1606(03)00177-5. [DOI] [PubMed] [Google Scholar]
- Meulemans D, Bronner-Fraser M. Gene-regulatory interactions in neural crest evolution and development. Dev. Cell. 2004;7:291–299. doi: 10.1016/j.devcel.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq AH, Fletcher RB, Harland RM. Neural crest induction by paraxial mesoderm in Xenopus embryos requires FGF signals. Development. 2003;130:3111–3124. doi: 10.1242/dev.00531. [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq AH, Wang E, Harland R. Msx1 and Pax3 cooperate to mediate FGF8 and Wnt signals during Xenopus neural crest induction. Dev. Cell. 2005;8:167–178. doi: 10.1016/j.devcel.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Moody SA. Determination of preplacodal ectoderm and sensory placodes. In: Moody SA, editor. Principles of Developmental Genetics. Elsevier; 2007. pp. 590–614. [Google Scholar]
- Morgan R, Sargent MG. The role in neural patterning of translation initiation factor eIF4AII; induction of neural fold genes. Development. 1997;124:2751–2760. doi: 10.1242/dev.124.14.2751. [DOI] [PubMed] [Google Scholar]
- Murakami S, Arai Y. Transient expression of somatostatin immunoreactivity in the olfactory-forebrain region in the chick embryo. Dev. Brain Res. 1994;82:277–285. doi: 10.1016/0165-3806(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Neave B, Holder N, Patient R. A graded response to Bmp-4 spatially coordinates patterning of the mesoderm and ectoderm in the zebrafish. Mech. Dev. 1997;62:183–185. doi: 10.1016/s0925-4773(97)00659-x. [DOI] [PubMed] [Google Scholar]
- Nechiporuk A, Linbo T, Poss KD, Raible DW. Specification of epibranchial placodes in zebrafish. Development. 2007;134:611–623. doi: 10.1242/dev.02749. [DOI] [PubMed] [Google Scholar]
- Nguyen VH, Schmid B, Trout J, Connors SA, Ekker M, Mullins MC. Ventral and lateral regions of the zebrafish gastrula, including the neural crest progenitors, are established by a bmp2b/swirl pathway of genes. Dev. Biol. 1998;199:93–110. doi: 10.1006/dbio.1998.8927. [DOI] [PubMed] [Google Scholar]
- Nica G, Herzog W, Sonntag C, Nowak M, Schwarz H, Zapata AG, Hammerschmidt M. Eya1 is required for lineage-specific differentiation, but not for cell survival in the zebrafish adenohypophysis. Dev. Biol. 2006;292:189–204. doi: 10.1016/j.ydbio.2005.12.036. [DOI] [PubMed] [Google Scholar]
- Nikaido M, Doi K, Shimizu T, Hibi M, Kikuchi Y, Yamasu K. Initial specification of the epibranchial placode in zebrafish embryos depends on the fibroblast growth factor signal. Dev. Dyn. 2007;236:564–571. doi: 10.1002/dvdy.21050. [DOI] [PubMed] [Google Scholar]
- Noguchi Y, Ito T, Nishio A, Honda K, Kitamura K. Audiovestibular findings in a branchio-oto syndrome patient with a SIX1 mutation. Acta Otolaryngol. 2011;131:413–418. doi: 10.3109/00016489.2010.543146. [DOI] [PubMed] [Google Scholar]
- Northcutt RG, Muske LE. Multiple embryonic origins of gonadotropin-releasing hormone (GnRH) immunoreactive neurons. Dev. Brain Res. 1994;78:279–290. doi: 10.1016/0165-3806(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Ohto H, Takizawa T, Saito T, Kobayashi M, Ikeda K, Kawakami K. Tissue and developmental distribution of Six family gene products. Int. J. Dev. Biol. 1998;42:141–148. [PubMed] [Google Scholar]
- Ohto H, Kamada S, Tago K, Tominaga S, Ozaki H, Sato S, Kawakami K. Cooperation of Six and Eya in activation of their target genes through nuclear translocation of Eya. Mol. Cell. Biol. 1999;19:6815–6824. doi: 10.1128/mcb.19.10.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohuchi H, Hori Y, Yamasaki M, Harada H, Sekine K, Kato S, Itoh N. FGF10 acts as a major ligand for FGF receptor 2 IIIB in mouse multi-organ development. Biochem. Biophys. Res. Commun. 2000;277:643–649. doi: 10.1006/bbrc.2000.3721. [DOI] [PubMed] [Google Scholar]
- Oliver G, Mailhos A, Wehr R, Copeland NG, Jenkins NA, Gruss P. Six3, a murine homologue of the sine oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development. 1995;121:4045–4055. doi: 10.1242/dev.121.12.4045. [DOI] [PubMed] [Google Scholar]
- O’Neill P, Mak SS, Fritzsch B, Ladher RK, Baker CV. The amniote paratympanic organ develops from a previously undiscovered sensory placode. Nat. Commun. 2012;3:1041. doi: 10.1038/ncomms2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Mohamed OA, Taketo MM, Dufort D, Groves AK. Wnt signals mediate a fate decision between otic placode and epidermis. Development. 2006;133:865–875. doi: 10.1242/dev.02271. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Groves AK, Martin K. The first steps towards hearing: mechanisms of otic placode induction. Int. J. Dev. Biol. 2007;51:463–472. doi: 10.1387/ijdb.072320to. [DOI] [PubMed] [Google Scholar]
- Ozaki H, Watanabe Y, Ikeda K, Kawakami K. Impaired interactions between mouse Eya1 harboring mutations found in patients with brachio-oto-renal syndrome and Six, Dach, and G proteins. J. Hum. Genet. 2002;47:107–11. doi: 10.1007/s100380200011. [DOI] [PubMed] [Google Scholar]
- Ozaki H, Nakamura K, Funahashi J, Ikeda K, Yamada G, Tokano H, Okamura HO, Kitamura K, Muto S, Kotaki H, Sudo K, Horai R, Iwakura Y, Kawakami K. Six1 controls patterning of the mouse otic vesicle. Development. 2004;131:551–562. doi: 10.1242/dev.00943. [DOI] [PubMed] [Google Scholar]
- Ozaki H, Watanabe Y, Takahashi K, Kitamura K, Tanaka A, Urase K, Momoi T, Sudo K, Sakagami J, Asano M, Iwakura Y, Kawakami K. Six4, a putative myogenin gene regulator, is not essential for mouse embryonic development. Mol., Cell Biol. 2001;21:3343–3350. doi: 10.1128/MCB.21.10.3343-3350.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padanad MS, Bhat N, Guo B, Riley BB. Conditions that influence the response to Fgf during otic placode induction. Dev. Biol. 2012;364:1–10. doi: 10.1016/j.ydbio.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandur PD, Moody SA. Xenopus Six1 gene is expressed in neurogenic cranial placodes and maintained in the differentiating lateral lines. Mech. Dev. 2000;96:253–257. doi: 10.1016/s0925-4773(00)00396-8. [DOI] [PubMed] [Google Scholar]
- Park BY, Saint-Jeannet J-P. Hindbrain-derived Wnt and Fgf signals cooperate to specify the otic placode in Xenopus. Dev. Biol. 2008;324:108–121. doi: 10.1016/j.ydbio.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BY, Saint-Jeannet J-P. Colloquium Series on Developmental Biology. 3. Vol. 1. Morgan & Claypool Publishers; 2010. Induction and segregation of the vertebrate cranial placodes. [PubMed] [Google Scholar]
- Paschaki M, Cammas L, Muta Y, Matsuoka Y, Mak S-S, Rataj-Baniowska M, Fraulob V, Dolle P, Ladher RK. Retinoic acid regulates olfactory progenitor cell fate and differentiation. Neural Dev. 2013;8:13. doi: 10.1186/1749-8104-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patthey C, Gunhaga L, Edlund T. Early development of the central and peripheral nervous systems is coordinated by Wnt and BMP signals. PLoS One. 2008;3:e1625. doi: 10.1371/journal.pone.0001625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patthey C, Edlund T, Gunhaga L. Wnt-regulated temporal control of BMP exposure directs the choice between neural plate border and epidermal fate. Development. 2009;136:73–83. doi: 10.1242/dev.025890. [DOI] [PubMed] [Google Scholar]
- Patrick AN, Schiemann BJ, Yang K, Zhao R, Ford HL. Biochemical and functional characterization for six SIX1 Branchio-otic-renal syndrome mutations. J. Biol. Chem. 2009;284:20781–20790. doi: 10.1074/jbc.M109.016832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick AN, Cabrera JH, Smaith AL, Chen XS, Ford HL, Zhao R. Structure function analyses of the human SIX1-EYA2 complex reveal insights into metastasis and BOR Syndrome. Nature Struct. Mol. Biol. 2013;20:447–453. doi: 10.1038/nsmb.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera EM, De Robertis EM. A direct screen for secreted proteins in Xenopus embryos identifies distinct activities for the Wnt antagonists Crescent and Frzb-1. Mech. Dev. 2000;96:183–195. doi: 10.1016/s0925-4773(00)00394-4. [DOI] [PubMed] [Google Scholar]
- Phillips BT, Bolding K, Riley BB. Zebrafish fgf3 and fgf8 encode redundant functions required for otic placode induction. Dev. Biol. 2001;235:351–365. doi: 10.1006/dbio.2001.0297. [DOI] [PubMed] [Google Scholar]
- Phillips BT, Kwon HJ, Melton C, Houghtaling P, Fritz A, Riley BB. Zebrafish msxB, msxC and msxE function together to refine the neural-nonneural border and regulate cranial placodes and neural crest development. Dev. Biol. 2006;294:376–390. doi: 10.1016/j.ydbio.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Pieper M, Eagleson GW, Wosniok W, Schlosser G. Origin and segregation of cranial placodes in Xenopus laevis. Dev. Biol. 2011;360:257–275. doi: 10.1016/j.ydbio.2011.09.024. [DOI] [PubMed] [Google Scholar]
- Pieper M, Ahrens K, Rink E, Peter A, Schlosser G. Differential distribution of competence for panplacodal and neural crest induction to non-neural and neural ectoderm. Development. 2012;139:1175–1187. doi: 10.1242/dev.074468. [DOI] [PubMed] [Google Scholar]
- Pignoni F, Hu B, Zavitz KH, Xiao J, Garrity PA, Zipursky SL. The eye-specification proteins So and Eya form a complex and regulate multiple steps in Drosophila eye development. Cell. 1997;91:881–891. doi: 10.1016/s0092-8674(00)80480-8. [DOI] [PubMed] [Google Scholar]
- Platt JB. Ontogenetic differentiation of the ectoderm in necturus. II. On the development of the peripheral nervous system. Quart. J. Mic. Sci. 1896;38:485–547. [Google Scholar]
- Pogoda HM, Hammerschmidt M. Molecular genetics of pituitary development in zebrafish. Semin. Cell Dev. Biol. 2007;18:543–558. doi: 10.1016/j.semcdb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Rawson NE, LaMantia AS. Once and again: retinoic acid signaling in the developing and regenerating olfactory pathway. J. Neurobiol. 2006;66:653–676. doi: 10.1002/neu.20236. [DOI] [PubMed] [Google Scholar]
- Reichert S, Randall RA, Hill CS. A BMP regulatory network controls ectodermal cell fate decisions at the neural plate border. Development. 2013;140:4435–444. doi: 10.1242/dev.098707. [DOI] [PubMed] [Google Scholar]
- Rickard S, Parker M, van’t Hoff W, Barnicoat A, Russell-Eggitt I, Winter RM, Bitner-Glindzicz M. Oto-facial-cervical (OFC) syndrome is a contiguous gene deletion syndrome involving EYA1: molecular analysis confirms allelism with BOR syndrome and further narrows the Duane syndrome critical regions to 1cM. Hum. Genet. 2001;108:398–403. doi: 10.1007/s004390100495. [DOI] [PubMed] [Google Scholar]
- Rogers CD, Moody SA, Casey ES. Neural induction and factors that stabilize a neural fate. Birth Defects Res. C. Embryo Today. 2009;87:249–262. doi: 10.1002/bdrc.20157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romand R, Dolle P, Hashino E. Retinoid signaling in inner ear development. J. Neurobiol. 2006;66:687–704. doi: 10.1002/neu.20244. [DOI] [PubMed] [Google Scholar]
- Ruf RG, Xu PX, Silvius D, Otto EA, Beekmann F, Muerb UT, Kumar S, Neuhaus TJ, Kemper MJ, Raymond RMJ, et al. SIX1 mutations cause branchio-oto-renal syndrome by disruption of EYA1-SIX1-DNA complexes. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8090–809. doi: 10.1073/pnas.0308475101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz i Altaba A, Jessell TM. Retinoic acid modifies the pattern of cell differentiation in the central nervous system of neurula stage Xenopus embryos. Development. 1991;112:945–958. doi: 10.1242/dev.112.4.945. [DOI] [PubMed] [Google Scholar]
- Sabado V, Barraud P, Baker CV, Streit A. Specification of GnRH-1 neurons by antagonistic FGF and retinoic acid signaling. Dev. Biol. 2012;362:254–262. doi: 10.1016/j.ydbio.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahly I, Andermann P, Petit C. The zebrafish eya1 gene and its expression pattern during embryogenesis. Dev. Genes Evol. 1999;209:399–410. doi: 10.1007/s004270050270. [DOI] [PubMed] [Google Scholar]
- Saint-Jeannet J-P, He X, Varmus HE, Dawid IB. Regulation of dorsal fate in the neuraxis by Wnt-1 and Wnt-3a. Proc. Natl. Acad. Sci. USA. 1997;94:13713–13718. doi: 10.1073/pnas.94.25.13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanggaard KM, Rendtorff ND, Kjaer KW, Eiberg H, Johnsen T, Gimsing S, Dyrmose J, Nielsen KO, Lage K, Tranebjaerg L. Branchio-otic-renal syndrome: detection of EYA1 and SIX1 mutations in five out of six Danish families by combining linkage, MLPA and sequencing analyses. Eur. J. Hum. Genet. 2007;15:1121–1131. doi: 10.1038/sj.ejhg.5201900. [DOI] [PubMed] [Google Scholar]
- Sanyanusin P, Schimmenti LA, McNoe LA, Ward TA, Pierpont MEM, Sullivan MJ, Dobyns WB, Eccles MR. Mutation of the PAX2 gene in a family with optic nerve colobomas, renal anomalies and vesicoureteral reflux. Nature Genet. 1995;9:358–364. doi: 10.1038/ng0495-358. [DOI] [PubMed] [Google Scholar]
- Sasai N, Mizuseki K, Sasai Y. Requirement of FoxD3-class signaling for neural crest determination in Xenopus. Development. 2001;128:2525–2536. doi: 10.1242/dev.128.13.2525. [DOI] [PubMed] [Google Scholar]
- Sato T, Sasai N, Sasai Y. Neural crest determination by co-activation of Pax3 and Zic1 genes in Xenopus ectoderm. Development. 2005;132:2355–2363. doi: 10.1242/dev.01823. [DOI] [PubMed] [Google Scholar]
- Sato S, Ikeda K, Ochi H, Ogino H, Yajima H, Kawakami K. Conserved expression of mouse Six1 in the pre-placodal region (PPR) and identification of an enhancer for the rostral PPR. Dev. Biol. 2010;344:158–171. doi: 10.1016/j.ydbio.2010.04.029. [DOI] [PubMed] [Google Scholar]
- Sato S, Ikeda K, Shioi G, Nakao K, Yajima H, Kawakami K. Regulation of Six1 expression by evolutionarily conserved enhancers in tetrapods. Dev. Biol. 2012;368:95–108. doi: 10.1016/j.ydbio.2012.05.023. [DOI] [PubMed] [Google Scholar]
- Saxena A, Peng BN, Bronner ME. Sox10-dependent neural crest origin of olfactory microvillous neurons in zebrafish. eLife. 2013;2:e00336. doi: 10.7554/eLife.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbrogna JL, Barresi MJ, Karlstrom RO. Multiple roles for Hedgehog signaling in zebrafish pituitary development. Dev. Biol. 2003;254:19–35. doi: 10.1016/s0012-1606(02)00027-1. [DOI] [PubMed] [Google Scholar]
- Schimmang T. Expression and functions of FGF ligands during early otic development. Int. J. Dev. Biol. 2007;51:473–481. doi: 10.1387/ijdb.072334ts. [DOI] [PubMed] [Google Scholar]
- Schlosser G. Hypobranchial placodes in Xenopus laevis give rise to hypobranchial ganglia, a novel type of cranial ganglia. Cell Tiss. Res. 2003;312:21–29. doi: 10.1007/s00441-003-0710-8. [DOI] [PubMed] [Google Scholar]
- Schlosser G. Evolutionary origins of vertebrate placodes: insights from developmental studies and from comparisons with other deuterostomes. J. Exp. Zool. B. Dev. Evol. 2005;304:347–399. doi: 10.1002/jez.b.21055. [DOI] [PubMed] [Google Scholar]
- Schlosser G. Induction and specification of cranial placodes. Dev. Biol. 2006;294:303–351. doi: 10.1016/j.ydbio.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Schlosser G. Making senses development of vertebrate cranial placodes. Int. Rev. Cell Mol. Biol. 2010;283:129–234. doi: 10.1016/S1937-6448(10)83004-7. [DOI] [PubMed] [Google Scholar]
- Schlosser G, Ahrens K. Molecular anatomy of placode development in Xenopus laevis. Dev. Biol. 2004;271:439–466. doi: 10.1016/j.ydbio.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Schlosser G, Northcutt RG. Development of neurogenic placodes in Xenopus laevis. J. Comp. Neurol. 2000;418:121–146. [PubMed] [Google Scholar]
- Schneider-Maunoury S, Pujades C. Hindbrain signals in otic regionalization: walk on the wild side. Int. J. Dev. Biol. 2007;51:495–506. doi: 10.1387/ijdb.072345ss. [DOI] [PubMed] [Google Scholar]
- Schönberger J, Wang L, Shin JT, Kim SD, Depreux FFS, Zhu H, Zon L, Pizard A, Kim JB, MacRae CA, Mungall AJ, Seidman JG, Seidman CE. Mutation in the transcriptional coactivator EYA4 causes dilated cardiomyopathy and sensorineural hearing loss. Nature Genetics. 2005;37:418–422. doi: 10.1038/ng1527. [DOI] [PubMed] [Google Scholar]
- Schwarz M, Cecconi F, Bernier G, Andrejewski N, Kammandel B, Wagner M, Gruss P. Spatial specification of mammalian eye territories by reciprocal transcriptional repression of Pax2 and Pax6. Development. 2000;127:4325–4334. doi: 10.1242/dev.127.20.4325. [DOI] [PubMed] [Google Scholar]
- Self M, Lsagutin OV, Bowling B, Hendrix J, Cal Y, Dressler GR, Oliver G. Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBO J. 2006;25:5214–5228. doi: 10.1038/sj.emboj.7601381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selleck MA, Bronner-Fraser M. Origins of the avian neural crest: The role of neural plate-epidermal interactions. Development. 1995;121:525–538. doi: 10.1242/dev.121.2.525. [DOI] [PubMed] [Google Scholar]
- Selleck MA, Bronner-Fraser M. Avian neural crest cell fate decisions: a diffusible signal mediates induction of neural crest by the ectoderm. Int. J. Dev. Neurosci. 2000;18:621–627. doi: 10.1016/s0736-5748(00)00037-x. [DOI] [PubMed] [Google Scholar]
- Shiotsugu J, Katsuyama Y, Arima K, Baxter A, Koide T, Song J, Chandraratna RA, Blumberg B. Multiple points of interaction between retinoic acid and FGF signaling during embryonic axis formation. Development. 2004;131:2653–2667. doi: 10.1242/dev.01129. [DOI] [PubMed] [Google Scholar]
- Sjodal M, Edlund T, Gunhaga L. Time of exposure to BMP signals plays a key role in the specification of the olfactory and lens placodes ex vivo. Dev. Cell. 2007;13:141–149. doi: 10.1016/j.devcel.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Solomon KS, Fritz A. Concerted action of two dlx paralogs in sensory placode formation. Development. 2002;129:3127–3136. doi: 10.1242/dev.129.13.3127. [DOI] [PubMed] [Google Scholar]
- Söker T, Dalke C, Puk O, Floss T, Becker L, Bolle I, Favor J, Hans W, Holter SM, Horsch M, Kallnik M, Kling E, Moerth C, Schrewe A, Stigloher C, Topp S, Gailus-Durner V, Naton B, Beckers J, Fuchs H, Ivandic B, Klopstock T, Schulz H, Wolf E, Wurst W, Bally-Cuif L, de Angelis MH, Graw J. Pleio-tropic effects in Eya3 knockout mice. BMC Dev. Biol. 2008;8:118. doi: 10.1186/1471-213X-8-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruijt L, Hoefsloot LH, van Schaijk GH, van Waardenburg D, Kremer B, Brackel HJ, de Die-Smulders CE. Identification of a novel EYA1 mutation presenting in a newborn with laryngomalacia, glossoptosis, retrognathia, and pectus excavatum. Am. J. Med. Genet. A. 2006;140:1343–1345. doi: 10.1002/ajmg.a.31285. [DOI] [PubMed] [Google Scholar]
- Stark MR, Sechrist J, Bronner-Fraser M, Marcelle C. Neural tube-ectoderm interactions are required for trigeminal placode formation. Development. 1997;124:4287–4295. doi: 10.1242/dev.124.21.4287. [DOI] [PubMed] [Google Scholar]
- Stern CD. Neural induction: old problem, new findings, yet more questions. Development. 2005;132:2007–2021. doi: 10.1242/dev.01794. [DOI] [PubMed] [Google Scholar]
- Stern CD. Neural induction: 10 years on since the “default model”. Curr. Opin. Cell Biol. 2006;18:692–697. doi: 10.1016/j.ceb.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Steventon B, Araya C, Linker C, Kuriyama S, Mayor R. Differential requirements of BMP and Wnt signaling during gastrulation and neurulation define two steps in neural crest induction. Development. 2009;136:771–779. doi: 10.1242/dev.029017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steventon B, Mayor R, Streit A. Mutual repression between Gbx2 and Otx2 in sensory placodes reveals a general mechanism for ectodermal patterning. Dev. Biol. 2012;367:55–65. doi: 10.1016/j.ydbio.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone LS. Experiments on the development of the cranial ganglia and the lateral line sense organs in Amblystoma punctatum. J. Exp. Zool. 1922;35:421–496. [Google Scholar]
- Streit A. Extensive cell movements accompany formation of the otic placode. Dev. Biol. 2002;249:237–254. doi: 10.1006/dbio.2002.0739. [DOI] [PubMed] [Google Scholar]
- Streit A. Early development of the cranial sensory nervous system: from a common field to individual placodes. Dev. Biol. 2004;276:1–15. doi: 10.1016/j.ydbio.2004.08.037. [DOI] [PubMed] [Google Scholar]
- Streit A. The pre-placodal region: an ectodermal domain with multipotential progenitors that contribute to sense organs and cranial sensory ganglia. Int. J. Dev. Biol. 2007;51:447–461. doi: 10.1387/ijdb.072327as. [DOI] [PubMed] [Google Scholar]
- Streit A, Berliner AJ, Papanayotou C, Sirulnik A, Stern CD. Initiation of neural induction by FGF signaling before gastrulation. Nature. 2000;406:74–78. doi: 10.1038/35017617. [DOI] [PubMed] [Google Scholar]
- Streit A, Stern C. Establishment and maintenance of the border of the neural plate in the chick: involvement of FGF and BMP activity. Mech. Dev. 1999;82:51–66. doi: 10.1016/s0925-4773(99)00013-1. [DOI] [PubMed] [Google Scholar]
- Sun SK, Dee CT, Tripathi VB, Rengifo A, Hirst CS, Scotting PJ. Epibranchial and otic placodes are induced by a common Fgf signal, but their subsequent development is independent. Dev. Biol. 2007;303:675–686. doi: 10.1016/j.ydbio.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Yoshizaki K, Kobayashi T, Osumi N. Neural crest-derived horizontal basal cells as tissue stem cells in the adult olfactory epithelium. Neurosci. Res. 2013;75:112–120. doi: 10.1016/j.neures.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Takai A, Inomata H, Arakawa A, Yakura R, Matsuo-Takasaki M, Sasai Y. Anterior neural development requires Del1, a matrix-associated protein that attenuates canonical Wnt signaling via the Ror2 pathway. Development. 2010;137:3293–3302. doi: 10.1242/dev.051136. [DOI] [PubMed] [Google Scholar]
- Theveneau E, Steventon B, Scarpa E, Garcia S, Trepat X, Streit A, Mayor R. Chase-and-run between adjacent cell populations promotes directional collective migration. Nature Cell Biol. 2013;15:763–772. doi: 10.1038/ncb2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro S, Varga ZM. Equivalent progenitor cells in the zebrafish anterior preplacodal field give rise to adenohypophysis, lens, and olfactory placodes. Semin. Cell Dev. Biol. 2007;18:534–542. doi: 10.1016/j.semcdb.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Torres M, Gómez-Pardo E, Gruss P. Pax2 contributes to inner ear patterning and optic nerve trajectory. Development. 1996;122:3381–3391. doi: 10.1242/dev.122.11.3381. [DOI] [PubMed] [Google Scholar]
- Treier M, Gleiberman AS, O’Connell SM, Szeto DP, McMahon JA, McMahon AP, Rosenfeld MG. Multistep signaling requirements for pituitary organogenesis in vivo. Genes Dev. 1998;12:1691–1704. doi: 10.1101/gad.12.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay P, Kessel M, Gruss P. A transgenic neuroanatomical marker identifies cranial neural crest deficiencies associated with the Pax3 mutant Splotch. Dev. Biol. 1995;171:317–329. doi: 10.1006/dbio.1995.1284. [DOI] [PubMed] [Google Scholar]
- Tribulo C, Ayba MJ, Nguyen VH, Mullins MC, Mayor R. Regulation of Msx genes by Bmp gradient is essential for neural crest specification. Development. 2003;130:6441–6452. doi: 10.1242/dev.00878. [DOI] [PubMed] [Google Scholar]
- Villanueva S, Glavic A, Ruiz P, Mayor R. Posteriorization by FGF, Wnt, and retinoic acid is required for neural crest induction. Dev. Biol. 2002;241:289–301. doi: 10.1006/dbio.2001.0485. [DOI] [PubMed] [Google Scholar]
- Von Kupffer C. Studien zur vergleichenden Entwicklungsgeschichte des Kpofes des Cranioten. Munchen: 1895. 3. Heft: Die Entwicklung der Kopfnerven von Ammocoetes planeri. [Google Scholar]
- Wang L, Sewell WF, Kim SD, Shin JT, MacRae CA, Zon LI, Seidman JG, Seidman CE. Eya4 regulation of Na+/K+-ATPase is required for sensory system development in zebrafish. Development. 2008;135:3425–3434. doi: 10.1242/dev.012237. [DOI] [PubMed] [Google Scholar]
- Wakamatsu Y. Mutual repression between Pax3 and Pax6 is involved in the positioning of ophthalmic trigeminal placode in avian embryo. Develop. Growth Differ. 2011;53:994–1003. doi: 10.1111/j.1440-169X.2011.01311.x. [DOI] [PubMed] [Google Scholar]
- Wayne S, Robertson NG, DeClau F, Chen N, Verhoeven K, Prasad S, Tranebjarg L, Morton CC, Ryan AF, Van Camp G, Smith RJ. Mutations in the transcriptional activator Eya4 cause late-onset deafness at the DFN10 locus. Hum. Mol. Genet. 2001;10:195–200. doi: 10.1093/hmg/10.3.195. [DOI] [PubMed] [Google Scholar]
- Wawersik S, Purcell P, Rauchman M, Dudley AT, Robertson EJ, Maas R. BMP7 acts in murine lens placode development. Dev. Biol. 1999;207:176–188. doi: 10.1006/dbio.1998.9153. [DOI] [PubMed] [Google Scholar]
- Webb JF, Noden DM. Ectodermal placodes: Contributions to the development of the vertebrate head. Am. Zool. 1993;33:434–447. [Google Scholar]
- Wilson PA, Lagna G, Suzuki A, Hemmati-Brivanlou A. Concentration-dependent patterning of the Xenopus ectoderm by BMP4 and its signal transducer Smad1. Development. 1997;124:3177–3184. doi: 10.1242/dev.124.16.3177. [DOI] [PubMed] [Google Scholar]
- Wilson SI, Graziano E, Harland R, Jessell TM, Edlund T. An early requirement for FGF signaling in the acquisition of neural cell fate in the chick embryo. Curr. Biol. 2000;10:421–429. doi: 10.1016/s0960-9822(00)00431-0. [DOI] [PubMed] [Google Scholar]
- Woda JM, Pastagia J, Mercola M, Artinger KB. Dlx proteins position the neural plate border and determine adjacent cell fates. Development. 2003;130:331–342. doi: 10.1242/dev.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright TJ, Mansour SL. Fgf3 and Fgf10 are required for mouse otic placode induction. Development. 2003;130:3379–3390. doi: 10.1242/dev.00555. [DOI] [PubMed] [Google Scholar]
- Wu J, Yang J, Klein PS. Neural crest induction by the canonical Wnt pathway can be dissociated from anterior-posterior neural patterning in Xenopus. Dev. Biol. 2005;279:220–232. doi: 10.1016/j.ydbio.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Xu H, Dude CM, Baker CV. Fine-grained fate maps for the ophthalmic and maxillomandibular trigeminal placodes in the chick embryo. Dev. Biol. 2008;317:174–186. doi: 10.1016/j.ydbio.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Xu PX, Adams J, Peters H, Brown MC, Heaney S, Maas R. Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat. Genet. 1999;23:113–117. doi: 10.1038/12722. [DOI] [PubMed] [Google Scholar]
- Xu PX, Zheng W, Laclef C, Maire P, Maas RL, Peters H, Xu X. Eya1 is required for the morphogenesis of mammalian thymus, parathyroid and thyroid. Development. 2002;129:3033–3044. doi: 10.1242/dev.129.13.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Zhang H, Hu G, Wang H, Abate-Shen C, Shen MM. An early phase of embryonic Dlx5 expression defines the rostral boundary of the neural plate. J Neurosci. 1998;18:8322–8330. doi: 10.1523/JNEUROSCI.18-20-08322.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelarayan LC, Vendrell V, Alvarez Y, Dominguez-Frutos E, Theil T, Alonso MT, Maconochie M, Schimmang T. Differential requirements for FGF3, FGF8 and FGF10 during inner ear development. Dev. Biol. 2007;308:379–391. doi: 10.1016/j.ydbio.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Zhang H, Hu G, Wang H, Sciavolino P, Iler N, Shen MM, Abate-Shen C. Heterodimerization of Msx and Dlx homeoproteins results in functional antagonism. Mol. Cell Biol. 1997;17:2920–2932. doi: 10.1128/mcb.17.5.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Luo T, Sargent TD. Expression of TFAP2beta and TFAP2gamma genes in Xenopus laevis. Gene Expr. Patterns. 2006;6:589–595. doi: 10.1016/j.modgep.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Zheng W, Huang L, Wei ZB, Silvius D, Tang B, Xu PX. The role of Six1 in mammalian auditory system development. Development. 2003;130:3989–4000. doi: 10.1242/dev.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou D, Silvius D, Fritzsch B, Xu PX. Eya1 and Six1 are essential for early steps of sensory neurogenesis in mammalian cranial placodes. Development. 2004;131:5561–5572. doi: 10.1242/dev.01437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou D, Silvius D, Davenport J, Grifone R, Maire P, Xu PX. Patterning of the third pharyngeal pouch into thymus/parathyroid by Six and Eya1. Dev. Biol. 2006a;293:499–512. doi: 10.1016/j.ydbio.2005.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou D, Silvius D, Rodrigo-Blomqvist S, Enerback S, Xu PX. Eya1 regulates the growth of otic epithelium and interacts with Pax2 during the development of all sensory areas of the inner ear. Dev. Biol. 2006b;298:430–441. doi: 10.1016/j.ydbio.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]