Abstract
Objective
To investigate the role of water-soluble extract of Salvia fruticosa (Greek sage) (S. fruticosa) leaves in reducing both intrinsic cellular and H2O2-induced DNA oxidation in cultured human embryonic kidney 293 cells. S. fruticosa, native to the Eastern-Mediterranean basin, is widely used as a medicinal herb for treatment of various diseases.
Methods
Dried leaves of S. fruticosa were extracted in phosphate buffer saline and purified using both vacuum and high pressure filtrations. Each mL of the preparation contained (7.1±1.0) mg of extract. HEK-293 cells were incubated in one set with S. fruticosa extract in the presence of 0.1 mmol/L H2O2, and in the other set with the addition of the extract alone. The DNA oxidation was measured using fluorescence upon fluorescein isothiocyanate derivatization of 8-oxoguanine moieties. The fluorescence was measured using flow cytometry technique.
Results
Cells incubated 3 h with 150 µL extract and exposed to 0.1 mmol/L H2O2 showed lower intensity of fluorescence, and thus lower DNA oxidation. Moreover, cells incubated 3 h with 100 µL of the extract showed lower intensity of fluorescence, and thus lower intrinsic cellular DNA oxidation compared to control (without S. fruticosa).
Conclusions
The results from this study suggest that the water-soluble extract of S. fruticosa leaves protects against both H2O2-induced and intrinsic cellular DNA oxidation in human embryonic kidney 293 cells.
Keywords: Salvia fruticosa, DNA oxidation, Oxidative stress, Human embryonic kidney 293 cells, Flow cytometry
1. Introduction
Antioxidants are substances capable of counteracting the oxidative damage of the free radicals in body tissues, and reducing the cellular oxidative stress[1],[2], thus, decreasing DNA, protein and lipid oxidation[3]–[5]. Enhancement of body defenses via oral antioxidant supplementation would seem to provide a reasonable and a practical approach to reduce the level of oxidative stress, and thus preventing the degenerative disorders such as cancer and diabetes[6],[7].
Recently, the development of new technologies has revolutionized the screening of natural products for potent antioxidants and anticancer materials. Plant-derived extracts are emerging as major players in this field.
Salvia fruticosa (also called a Greek sage) (S. fruticosa), is a perennial herb native to the eastern Mediterranean region. Cohorts in the Mediterranean region have used water-soluble leaf extract of S. fruticosa to treat various diseases, especially digestive system diseases[8]. It has been suggested that S. fruticosa treatment produces hypoglycemia mainly by reducing intestinal absorption of glucose[9],[10]. Pitarokili et al. (2003) showed that volatile metabolites of S. fruticosa exhibited high antifungal activities[11]. S. fruticosa oil extract as well as its alcoholic extract revealed a strong antioxidant activity[12],[13]. Moreover, Orhan et al. (2008) showed that S. fruticosa has a significant anticholinesterase activity[14]. Later reports showed that S. fruticosa stimulates DNA repair mechanism in cultured HeLa cells[15]. Recent study found that the crude ethanol extract of S. fruticosa has antiproliferative activity against breast cancer cells[16]. A newly published study by Sevindik and Rencuzogullari (2013) concluded that S. fruticosa leaf extract had no cytotoxic effect against human blood lymphocytes[17].
The vast majority of S. fruticosa research has investigated the possible health benefits of S. fruticosa oil and its constituents; little information is available about its water-soluble material. This study aimed to assess the H2O2-induced DNA oxidation protection activity of water-soluble extract of S. fruticosa leaves in human embryonic kidney 293 cells (HEK-293 cells). To do this, we measured the 8-oxoguanine moieties, sensitive biomarkers for oxidative DNA lesions, using flow cytometry. This study is the first one that utilizes flow cytometry to measure directly the anti-DNA oxidation activity of a plant extract.
2. Materials and methods
2.1. Preparation of Salvia Fruticosa Extract
Fresh S. fruticosa leaves were collected from the Marzoog garden in the northern Jordan. The leaves were dried in the shade for one week and stored in the dark for three months before use. The dried leaves were grounded by mortar and pestle to fine particles then dissolved in PBS buffer. A volume of 5 mL buffer per gram of S. fruticosa leaves was added, and the final suspension was homogenized, transferred to a centrifuge tube, shaken overnight at room temperature and stored at 4 °C in the dark. The homogenized mixture was centrifuged at 10 000 r/min for 10 min and the supernatant was transferred to a new tube. The extract supernatant was further passed through an ultra-centrifugation membrane (<30 000 kDa; Amicon, Bedford) under high-pressure conditions (12 psi), in a filtration device (Amicon, Bedford). The extract passing the membrane was collected and stored at 4 °C in the dark for future use. Each mL of the preparation will contain (7.1±1.0) mg of extract dry weight (0.7% w/v).
2.2. Cell culture
We used human kidney cells (the HEK-293 cell line, ATCC, Manassas, VA, USA) as a cell model in our investigation. HEK-293 cells have been extensively used in cell biology research for many years. The possible genotoxicity of the extracts were examined previously in these cells[18].
HEK-293 cells were incubated in the Dulbecco's Modified Eagle Medium (DMEM) supplemented with non-essential amino acids, 2 mmol/L L-glutamine, 5% penicillin/streptomycin and Earle's BSS adjusted to contain 1.5 g/L sodium bicarbonate. Cells were kept at 37 °C in a humidified incubator containing 5% CO2 in air.
2.3. H2O2 treatment
HEK-293 cells (1×106 cells/mL) are plated and exposed to different treatments (a though c below) before flow cytometry analysis:
a. Addition of freshly prepared H2O2 and incubation for 3 h at 37 °C. The final concentration of H2O2 in the cultured cell plates was 0.1 mmol/L. Control assays were prepared in the absence of H2O2.
b. Addition of freshly prepared H2O2 followed by 150 µL S. fruticosa extract and incubation for 3 h at 37 °C. The final concentration of H2O2 in the cultured cell plates was 0.1 mmol/L. Control assays were prepared in the absence of S. fruticosa extract.
c. Incubated for 3 h at 37 °C with only 100 µL of S. fruticosa extract. Control assays were prepared in the absence of the extract.
2.4. Flow cytometry
The level of DNA oxidation was measured using a flow cytometric OxiDNA assay kit (Calbiochem, San Diego). The method used was adapted and standardized in our previous work to assess the oxidative DNA damage in human sperm[19]. This assay is based on utilizing a direct fluorescent protein binding method for detection the DNA oxidation moieties (8-oxoguanines). Briefly, HEK-293 cells were washed twice in PBS, resuspended in 1% paraformaldehyde at a concentration of (1-2)106 cell/mL and placed on ice for 15 to 30 min. These cells were again washed and resuspended in 70% ice-cold ethanol by 5 min centrifugation at 1 600 r/min. The ethanol supernatant was removed and the cell pellets were washed twice in wash buffer and resuspended in 100 µL of the staining solution for 1 h at room temperature.
The staining solution contained fluorescein isothiocyanate (FITC) labeled protein conjugate, and deionized water. All cells were further washed using rinse buffer, resuspended in 250 µL and incubated for 30 min in the dark on the ice for flow cytometry measurements.
Data acquisition was operated within 1 h on a flow cytometer equipped with a 515-nm argon laser as a light source (FACScan; Becton Dickinson, San Jose, CA). 10 000 cells were interrogated for each single assay at a flow rate of 100 cells/second. The FITC fluorescence (log green fluorescence) was measured on FL1 channel and data analysis was done using FlowJo v4.4.4 software (Tree Star Inc., Ashland, OR).
2.5. Statistical analysis
Differences in the mean values of FITC fluorescence were considered significant at P<0.05. Statistical analyses were performed using paired t-test and two-tailed distribution by the SPSS/PC computer software (SPSS 10.0.7, SPSS Inc.).
3. Results
The direct effect of H2O2 on DNA oxidation in HEK-293 cells is shown in Figure 1. Flow cytometry analysis of FITC-labeled HEK-293 showed that cells treated with 0.1 mmol/L H2O2 and incubated 3 h at 37 °C exhibit increased intensity of FITC fluorescence (P<0.05), indicating an expected positive effect of H2O2 on the intrinsic baseline of cellular DNA oxidation.
Figure 1. The effect of H2O2 in inducing DNA oxidation in HEK-293 cells as evaluated by flow cytometry.
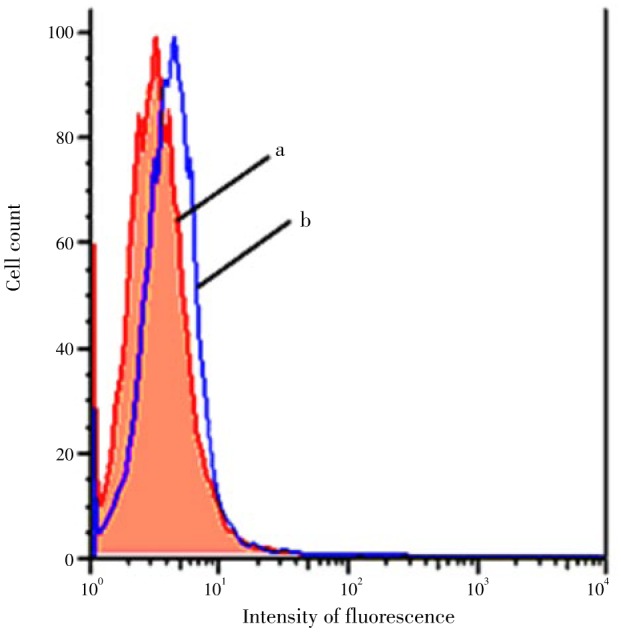
a: Represents the flow cytometry histogram for cells without H2O2 treatment; b: represents the flow cytomety histogram for cells incubated 3 h with 0.1 mmol/L H2O2. Data are representative of 6 independent experiments; the mean values of the histograms (a, and b) are statistically different (P<0.05).
Figure 2 shows the effect of the S. fruticosa extract in decreasing DNA oxidation induced by 0.1 mm H2O2 in HEK-293 cells. Cells incubated 3 h with 150 µL of the extract and exposed to 0.1 mmol/L H2O2 showed lower intensity of fluorescence (P<0.05), and thus lower DNA damage.
Figure 2. The DNA-oxidation protection activity of S. fruticosa extract.
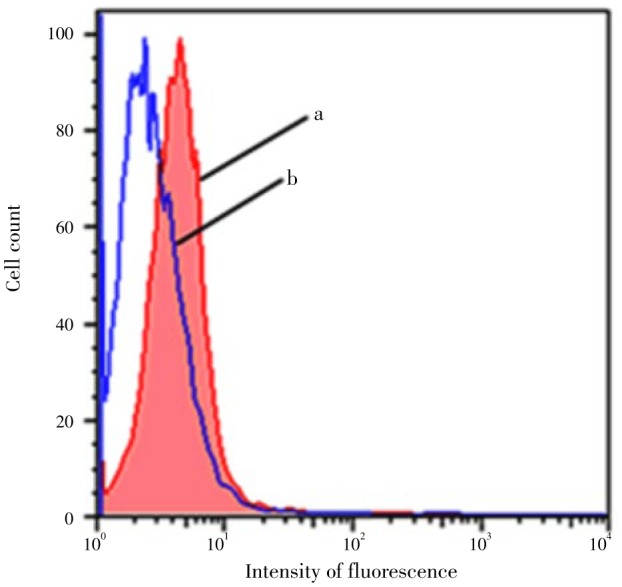
a: Represents the flow cytometry histogram for cells incubated 3 h with 0.1 mmol/L H2O2; b: represents the population incubated 3 h with 0.1mmol/L H2O2 in the presence of 150 µL S. fruticosa extract. Data are representative of 6 independent experiments; the mean values of the histograms (a, and b) are statistically different (P<0.05).
Figure 3 shows the effect of the S. fruticosa extract in reducing the intrinsic cellular DNA oxidation in HEK-293 cells. As shown in the figure, cells incubated 3 h with 100 µL of the extract showed a lower intensity of fluorescence (P<0.05), and thus lower intrinsic cellular DNA damage compared to the control (in absence of S. fruticosa).
Figure 3. The DNA-oxidation protection activity of S. fruticosa extract as evaluated by flow cytometry.
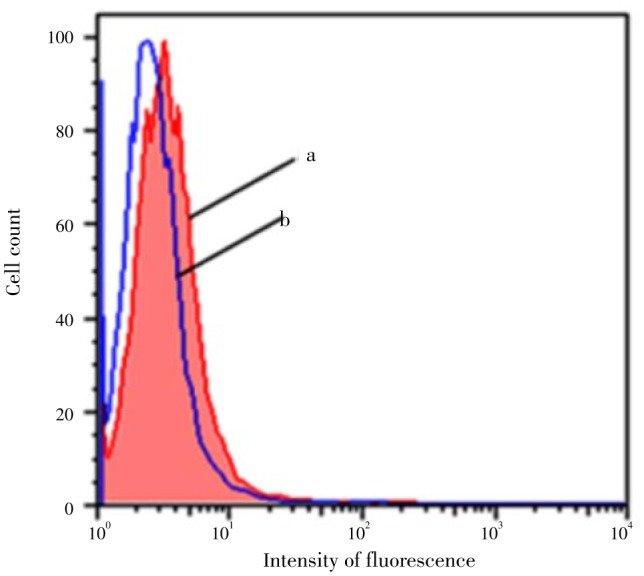
a: Represents the flow cytometry histogram for cells without S. fruticosa extract treatment; b: Represents the flow cytomety histogram for cells incubated 3 h with 100 µL S. fruticosa extract. Data are representative of 6 independent experiments; the mean values of the histograms (a, and b) are statistically different (P<0.05).
4. Discussion
In cellular systems, in the presence of free ferrous and cuprous ions, and superoxide anion (•O2-), H2O2 can produce hydroxyl radicals (•OH), a very short-lived reactive oxygen species, which recognized as Fenton's reaction[20]–[22]. •OH causes DNA lesion; when created adjacent to the DNA it strikes its main building blocks such as deoxyribose sugar and nitrogen bases (purines and pyrimidines), which may result in chemical alteration, and subsequently leads to injury, death, or enhancement of abnormal growth of cells (cancer development) [23].
The first flow cytometry experiment aimed to standardize the level of H2O2-induced DNA oxidation in HEK-293 cells as determined using 8-oxoguanine as oxidative damage marker. As expected for H2O2, our flow cytometry experiments showed an increase in the DNA oxidation after the addition of H2O2. However, in the presence of S. fruticosa extract, we show a lower FITC-fluorescence intensity compared to H2O2 alone, and hence, a lower level of DNA oxidation. Similar changes in DNA oxidation, have been reported in the study the protective effect of L-carnitine to reduce the in vitro oxidative stress on human spermatozoa using this same 8-oxoguanine marker in flow cytometry[19]. Although, the constituents of our extract in this study are unknown, the antioxidant effect may due to the presence of polyphenols such as rosmarinic acid and luteolin-7-glucoside[15].
In the last flow cytometry experiment, we intended to investigate the effect of water-soluble extract of S. fruticosa on the basal-intrinsic cellular DNA oxidation in HEK-293 cells. Cells incubated 3 h with the extract showed lower levels of 8-oxoguanine moieties compared to controls in the absence of extract. This decrease in the cellular DNA oxidation may due to an increase in the activity of DNA repair machinery in the presence of S. fruticosa extract. These results are in line with similar reports by Ramos et al.(2010)[15], who used the comet assay to investigate the antioxidant activity of the water-soluble extract of S. fruticosa. The exact mechanism by which S. fruticosa boosts the repair machinery of HEK-293 cells is not known at this point. However, like the recent reports about extracting polyphenols[24],[25], a possible mechanism by which our water extract of S. fruticosa reduces the level of DNA oxidation is probably via alleviating the load of cellular oxidative stress by directly scavenging the reactive oxygen species.
In conclusion, our results suggest that water-soluble extract of S. fruticosa leaves mediates protection against both intrinsic cellular and H2O2-induced DNA oxidation in HEK-293 cells. The reduction in the intrinsic cellular DNA oxidation may reflect an enhanced activity of the DNA repair machinery.
Acknowledgments
This work was supported by Cleveland State University and Jordan University of Science and Technology with grant number 20130097.
Comments
Background
The oxidation of lipid, DNA, protein, carbohydrate, and other biological molecules by toxic ROS may cause DNA mutation or/and serve to damage target cells or tissues, and this often results in cell senescence and death. Cancer chemoprevention by using antioxidant approaches has been suggested to offer a good potential in providing important fundamental benefits to public health. Antioxidants of plant extracts have formed the basis of many applications, including processed food preservation, pharmaceuticals, alternative medicine and natural therapies.
Research frontiers
This study is carried out to evaluate the water extract of S. fruticosa leaves for its antioxidants activity in HEK293 cells by measuring 8-oxoguanine moieties as a sensitive biomarkers oxidation, using flow cytometry.
Related reports
L-carnitine has similar changes in DNA oxidation; to reduce the in vitro oxidative stress on human spermatozoa using this same 8-oxoguanine marker in flow cytometry.
Innovations and breakthroughs
In this study authors have demonstrated the antioxidant activity of the aqueous extract of S. fruticosa leaves in HEK-293 cells by using flow cytometry.
Applications
Enhancement of body defenses via oral supplementation with S. fruticosa leaves protects against both H2O2-induced and intrinsic cellular DNA oxidation and so reduce the level of oxidative stress, and thus preventing the degenerative disorders such as cancer.
Peer review
This is a good research in which authors cleared assess H2O2-induced DNA oxidation protection activity of the aqueous extract of S. fruticosa leaves based on measuring 8-oxoguanine moieties as a sensitive biomarkers oxidation for oxidative DNA lesions in HEK-293 cells, using flow cytometry.
Footnotes
Foundation Project: Supported by Cleveland State University and Jordan University of Science and Technology; grant number 20130097.
Conflict of interest statement: The authors declare that there are no conflicts of interest.
References
- 1.Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82:291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 2.Alok S, Jain SK, Verma A, Kumar M, Mahor A, Sabharwal M. Herbal antioxidant in clinical practice: a review. Asian Asian Pac J Trop Biomed. 2014;4:78–84. doi: 10.1016/S2221-1691(14)60213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sivonová M, Tatarková Z, Duracková Z, Dobrota D, Lehotský J, Matáková T, et al. Relationship between antioxidant potential and oxidative damage to lipids, proteins and DNA in aged rats. Physiol Res. 2007;56:757–764. doi: 10.33549/physiolres.931094. [DOI] [PubMed] [Google Scholar]
- 4.Therond P. [Oxidative stress and damages to biomolecules (lipids, proteins, DNA)] Ann Pharm Fr. 2006;64:383–389. doi: 10.1016/s0003-4509(06)75333-0. French. [DOI] [PubMed] [Google Scholar]
- 5.Banihani S, Agarwal A, Sharma R, Bayachou M. Cryoprotective effect of l-carnitine on motility, vitality and DNA oxidation of human spermatozoa. Andrologia. 2013 doi: 10.1111/and.12130. [DOI] [PubMed] [Google Scholar]
- 6.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Banihani S, Swedan S, Alguraan Z. Pomegranate and type 2 diabetes. Nutr Res. 2013;33:341–348. doi: 10.1016/j.nutres.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Gurdal B, Kultur S. An ethnobotanical study of medicinal plants in Marmaris (Mugla, Turkey) J Ethnopharmacol. 2013;146:113–126. doi: 10.1016/j.jep.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Azevedo MF, Lima CF, Fernandes-Ferreira M, Almeida MJ, Wilson JM, Pereira-Wilson C. Rosmarinic acid, major phenolic constituent of Greek sage herbal tea, modulates rat intestinal SGLT1 levels with effects on blood glucose. Mol Nutr Food Res. 2011;55(Suppl 1):S15–25. doi: 10.1002/mnfr.201000472. [DOI] [PubMed] [Google Scholar]
- 10.Perfumi M, Arnold N, Tacconi R. Hypoglycemic activity of Salvia fruticosa Mill. from Cyprus. J Ethnopharmacol. 1991;34:135–140. doi: 10.1016/0378-8741(91)90030-h. [DOI] [PubMed] [Google Scholar]
- 11.Pitarokili D, Tzakou O, Loukis A, Harvala C. Volatile metabolites from Salvia fruticosa as antifungal agents in soilborne pathogens. J Agric Food Chem. 2003;51:3294–3301. doi: 10.1021/jf0211534. [DOI] [PubMed] [Google Scholar]
- 12.Papageorgiou V, Gardeli C, Mallouchos A, Papaioannou M, Komaitis M. Variation of the chemical profile and antioxidant behavior of Rosmarinus officinalis L. and Salvia fruticosa Miller grown in Greece. J Agric Food Chem. 2008;56:7254–7264. doi: 10.1021/jf800802t. [DOI] [PubMed] [Google Scholar]
- 13.Senol FS, Orhan IE, Erdem SA, Kartal M, Sener B, Kan Y, et al. Evaluation of cholinesterase inhibitory and antioxidant activities of wild and cultivated samples of sage (Salvia fruticosa) by activity-guided fractionation. J Med Food. 2011;14:1476–1483. doi: 10.1089/jmf.2010.0158. [DOI] [PubMed] [Google Scholar]
- 14.Orhan I, Kartal M, Kan Y, Sener B. Activity of essential oils and individual components against acetyl- and butyrylcholinesterase. Z Naturforsch C. 2008;63:547–553. doi: 10.1515/znc-2008-7-813. [DOI] [PubMed] [Google Scholar]
- 15.Ramos AA, Azqueta A, Pereira-Wilson C, Collins AR. Polyphenolic compounds from Salvia species protect cellular DNA from oxidation and stimulate DNA repair in cultured human cells. J Agric Food Chem. 2010;58:7465–7471. doi: 10.1021/jf100082p. [DOI] [PubMed] [Google Scholar]
- 16.Abu-Dahab R, Afifi F, Kasabri V, Majdalawi L, Naffa R. Comparison of the antiproliferative activity of crude ethanol extracts of nine Salvia species grown in Jordan against breast cancer cell line models. Pharmacog Mag. 2012;8:319–324. doi: 10.4103/0973-1296.103664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sevindik N, Rencuzogullari E. The genotoxic and antigenotoxic effects of Salvia fruticosa leaf extract in human blood lymphocytes. Drug Chem Toxicol. 2013 doi: 10.3109/01480545.2013.851689. [DOI] [PubMed] [Google Scholar]
- 18.Hudecová A1, Hašplová K, Miadoková E, Magdolenová Z, Rinna A, Collins AR, et al. Gentiana asclepiadea protects human cells against oxidation DNA lesions. Cell Biochem Funct. 2012;30:101–107. doi: 10.1002/cbf.1822. [DOI] [PubMed] [Google Scholar]
- 19.Banihani S, Sharma R, Bayachou M, Sabanegh E, Agarwal A. Human sperm DNA oxidation, motility and viability in the presence of L-carnitine during in vitro incubation and centrifugation. Andrologia. 2012;44(Suppl 1):505–512. doi: 10.1111/j.1439-0272.2011.01216.x. [DOI] [PubMed] [Google Scholar]
- 20.Rodopulo AK. [Oxidation of tartaric acid in wine in the presence of heavy metal salts (activation of oxygen by iron)] Izv Akad Nauk SSSR Biol. 1951;3:115–128. [Article in Undetermined Language] [PubMed] [Google Scholar]
- 21.Goldstein S, Meyerstein D, Czapski G. The Fenton reagents. Free Radic Biol Med. 1993;15:435–435. doi: 10.1016/0891-5849(93)90043-t. [DOI] [PubMed] [Google Scholar]
- 22.Monroe EB, Heien ML. Electrochemical generation of hydroxyl radicals for examining protein structure. Anal Chem. 2013;85:6185–6189. doi: 10.1021/ac400107c. [DOI] [PubMed] [Google Scholar]
- 23.Miroslav F. Electrochemical sensors for DNA interactions and damage. Electroanalysis. 2002;14:1449–1463. [Google Scholar]
- 24.Tan X, Zhao C, Pan J, Shi Y, Liu G, Zhou B, et al. In vivo non-enzymatic repair of DNA oxidative damage by polyphenols. Cell Biol Int. 2009;33:690–696. doi: 10.1016/j.cellbi.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Keuser B1, Khobta A, Gallé K, Anderhub S, Schulz I, Pauly K, et al. Influences of histone deacetylase inhibitors and resveratrol on DNA repair and chromatin compaction. Mutagenesis. 2013;28:569–576. doi: 10.1093/mutage/get034. [DOI] [PubMed] [Google Scholar]


