Abstract
Inflammation is associated with development of atherosclerosis, and cholesterol crystals (CC) have long been recognized as a hallmark of atherosclerotic lesions. CC appear early in the atheroma development and trigger inflammation by NLRP3 inflammasome activation. In this study we hypothesized whether CC employ the complement system to activate the inflammasome-caspase-1 leading to release of mature IL-1β, and if complement activation regulates CC-induced cytokine production. We here describe that CC activated both the classical and alternative complement pathways and C1q was found to be crucial for the activation. CC employed C5a in the release of a number of cytokines in whole blood, including IL-1β and TNF. CC induced minimal amounts of cytokines in C5-deficient whole blood, until reconstituted with C5. Furthermore, C5a and TNF in combination acted as a potent primer for CC-induced IL-1β release by increasing IL-1β transcripts. CC-induced complement activation resulted in up-regulation of Complement receptor 3 (CD11b/CD18) leading to phagocytosis of CC. Also, CC mounted a complement-dependent production of reactive oxygen species and active caspase-1. We conclude that CC employs the complement system to induce cytokines and activate the inflammasome-caspase-1 by regulating several cellular responses in human monocytes. In light of this, complement inhibition might be an interesting therapeutic approach for treatment of atherosclerosis.
Introduction
Cholesterol crystals (CC) have long been recognized as a hallmark of atherosclerotic lesions (1, 2). Identified as “cholesterol crystal clefts”, these crystalline structures were thought to arise late in the course of the disease (3). Using hyperlipidemic ApoE−/− mice, we previously reported that CC are associated with early atheroma development (4). Oxidized LDL is endocytosed by CD36 that coordinates the intracellular conversion of this ligand to CC (5, 6). However, the phagocytosis receptor for CC has yet to be discovered. Phagocytosis of CC induces lysosomal damage that results in the activation of the NLRP3 inflammasome, with subsequent activation of caspase-1 and secretion of IL-1β (4, 7), suggesting that the interaction between CC and NLRP3 inflammasomes could be linking lipids and inflammation, the two fundamental hallmarks of atherosclerosis.
IL-1β has long been described as a potent inflammatory cytokine, and its activation is associated with the severity of atherosclerosis (8). Release of the mature form of IL-1β is controlled by two signals in macrophages. The transcription of pro-IL-1β and NLRP3 are NFκB-dependent and induced by a priming signal that either is provided by activation of pattern recognition receptors or via the presence of pro-inflammatory cytokines (9). Once activated, NLRP3, its adaptor ASC and pro-caspase-1form an inflammasome complex, which activates caspase-1that leads to cleavage of the pro-forms of IL-1β and IL-18 to their mature forms (10). While CC has been shown to activate NLRP3 inflammasomes, the endogenous primers for this activation are not well understood. One candidate is the complement system, however, this has so far not been explored in detail.
The complement system is an integral component of the innate immunity and has been shown to contribute to the pathology of several inflammatory diseases (11, 12). Complement can be activated by the classical-, the lectin- and the alternative pathways. All three pathways converge at the central C3 molecule, generating convertases that catalyse the conversion of C3 into its active fragments C3a and C3b. C3b is the amplification step that leads to all downstream complement events with the generation of C5a, a highly potent inflammatory mediator, and the terminal complement complex (TCC) (12). Activation of the classical pathway starts with C1q that binds to immunoglobulins, C-reactive protein (CRP) and distinct structures on microbial or apoptotic cells. The lectin pathway is initiated through mannose-binding lectin (MBL) and the ficolins, whereas the alternative pathway is spontaneously activated by hydrolysis of the internal C3 thioester and acts to substantially amplify activation induced by the classical and lectin pathway. The molecular mechanisms by which a damage associated molecular pattern, like CC, employ the complement system to activate inflammasome-caspase-1 is currently not known.
Here we report that CC activate both the classical- and alternative complement pathway that results in cytokine release. Our data demonstrate that the complement system controls several cellular processes involved in CC-induced inflammasome-caspase-1 activation. Moreover, we propose that C5a in combination with TNF may act as an endogenous priming signal for the CC-induced inflammasome, and identify complement receptor 3 (CR3) as a candidate receptor for phagocytosis of CC.
Materials and methods
Reagents
Cells were isolated with LymphoPrep™ (Axis-Shield, PBMC) or Polymorphprep™ (Axis-Shield, Granulocytes or PBMC). Anticoagulant in whole-blood experiments was Lepirudin/Refludan® (Celgene). C1q depleted serum and purified C1q were from Complement Technology. TNF was from Genentech. Purified C5 and recombinant C5a, ultrapure cholesterol, ATP, Cytochalasin-D and zymosan were from Sigma-Aldrich, LPS from E. coli (0111:B4, Invivogen), recombinant C3a (R&D), human serum albumin (HSA, Octapharma). The following reagents were used for qPCR analyses: RNeasy Mini kit (Qiagen), DNase and High Capacity RNA-to-cDNA Kit (Applied Biosystems), PerfeCTa® qPCR FastMix™ (Quanta Biosciences), probes and primers were from Applied Biosystems: GAPDH (Hs99999905_m1), NLRP3 (Hs00918082_m1), and IL-1β (Hs01555410_m1). The following antibodies were used: anti-CD11b PE (D12, BD Biosciences), anti-CD14 FITC (MφP9, BD Biosciences), and anti-CD14 PE (MφP9, BD Biosciences), CD45-PerCP (2D1, BD Biosciences), anti-IgG Detector, rabbit, PE (C101-359, BD Biosciences), antihuman C1q (A0136, Dako), cleaved IL-1β (2021, Cell signaling), polyclonal goat anti-mouse HRP (P0447, Dako), normal rabbit IgG (Ab105-C, R&D systems), infliximab (Janssen Biologics), eculizumab (Alexion), rituximab (Roche) and anti-C7 (Quidel). C3-inhibitor compstatin analog 22 CP40 (Ac-Ile-[Cys-Val-Trp(Me)-Gln-Asp-Trp-Sar-Ala-His-Arg-Cys]-mIle-NH2) and its control scrambled peptide (13). The specific C5a-receptor antagonist (AcF[OPdChaWR]) was synthesized as previously described (14).
Preparation of CC
100 mg ultrapure cholesterol was dissolved in 1-propanol (50 ml). The solution was mixed with distilled water, respectively 1:1.5, and rested for at least 10 minutes for monohydrate crystals to stabilize. 1-propanol was removed by evaporation and CC were re-suspended in PBS/0.05% HSA. All steps were performed at room temperature yielding CC with a size range of 1–2 µm that were stored at 4°C.
Cells and human whole blood assay
Human primary cells were isolated from buffycoats (Blood Bank, St. Olav’s Hospital, Trondheim) as previously described (15), and were maintained in RPMI with 10% heat inactivated pooled human serum (The Blood Bank, St. Olav’s Hospital, Trondheim) unless otherwise noted. Cells were primed with C5a (1µg/ml), C3a (1µg/ml) TNF (10 ng/ml), a combination of C5a and TNF or C3a and TNF, ultrapure LPS 100 pg/ml, or PBS/HSA for 2 hours. Following stimuli were applied: CC (1.5 × 107 particles/ml), ATP (3mM) and LPS (100 ng/ml). Granulocytes and PBMCs were isolated using polymorphprep according to the manufacturer's instructions. The cells were re-suspended in plasma/PBS before stimulation and analysis. The whole blood assay was performed as described before(16). Compstatin (20 µM), control peptide (20 µM), infliximab (10 µg/ml), eculizumab (100 µg/ml), rituximab (100 µg/ml), C5a receptor antagonist (10 µM) or anti-C7 (25 µg/ml) were added to whole blood incubated at 37°C for 5 minutes before stimulation. Samples were incubated with CC (1.5-6 × 107 particles/ml) unless otherwise indicated, or LPS (100 ng/ml) and incubated at 37°C under constant rotation. Aliquots of blood were collected and analyzed for complement (TCC, 30 minutes), changes in cell surface marker (CD11b, 15 minutes), and cytokine production (multiplex, 6 hours). Plasma was collected and frozen until analyzed.
Cytokine and enzyme measurements
IL-1β from PBMC or monocyte supernatants was detected using IL-1β ELISA (BD Biosciences). Plasma samples from the whole blood experiments, granulocyte or PBMC suspensions were analyzed according to the manufacturer’s instructions by multiplex cytokine assay (Bio-Plex; Bio-Rad Laboratories Inc.) for IL-1β, IL-1 receptor antagonist (IL-1ra), IL-6, IL-8, monocyte chemotactic protein (MCP-1), macrophage inflammatory protein (MIP-1α), MIP-1β (only whole blood), tumor necrosis factor (TNF).
Complement measurements
Serum from donors was incubated for 30 minutes at 37°C in the presence of CC (1.5 × 107 particles/ml), or PBS/HSA. Positive control was a mixture of zymosan (10 mg/ml) and heat aggregated human IgG (Octapharma AB, 10 mg/ml). The following complement activation products were measured by ELISA as described elsewhere (16, 17): soluble TCC, C1rs-C1-INH, C3bBbP, C3bc, and C4bc. These assays are based on monoclonal capture antibodies specific for neoepitopes expressed after a component is activated, thus, only the activated form is detected. TCC is detected by an antibody to a neoepitope expressed in C9.The C3b fragment is composed of C3c and C3dg and is rapidly split into these two fragments upon activation. For C3bc measurements, the antibody detects a neoepitope that appears on the C3c part of C3b when C3 is cleaved. This neoepitope is preserved on the C3c fragment after cleavage of C3b and is therefore designated anti-C3bc and measure the total amount of this neoepitope expressed after C3 activation. The same principle also applies for detection of C4 activation which is denoted C4bc. C1q depleted serum (diluted 1:2) with or without reconstitution with purified C1q (5 µg/ml), was incubated with CC (1.5 × 107 particles/ml), or PBS/HSA, TCC was measured as above. C1q deposition on CC was determined in plasma that had been incubated with CC (1.5 × 107 particles/ml) for 30 minutes and stained with antihuman C1q (5 µg/ml) and anti-IgG Detector, rabbit, PE as described by the manufacturer. Analyses were performed on a Beckman Coulter Epics XL-MCL (Coulter Corp, FL).
Quantitative Real Time PCR
Total RNA was extracted from PBMC or monocytes with RNeasy Mini kit, and QIAcube robotic work station (Qiagen) following the manufacturer’s instructions. Cell extracts were DNase treated, before reverse transcribed using High Capacity RNAto-cDNA Kit. mRNA were analyzed by StepOnePlusTM Realtime PCR System and its software (Applied Biosystems), TaqMan® Gene Expression Assays, and TaqMan® Universal Master Mix (ABI) with 20 µl reaction volume in triplicate wells. All data were normalized to GAPDH and expressed as fold change over controls.
Western Blot
Cleaved IL-1β was detected in serum-free supernatants from monocytes. Samples were immune-precipitated by methanol/chloroform, loaded into 10–12% Bis-Tris gel (Invitrogen), and subjected to SDS-PAGE. Gels were blotted onto nitrocellulose membranes using iBlot® 7-Minute Blotting System (Invitrogen), and stained with cleaved IL-1β antibody (2 µg/ml) overnight at 4°C. Membranes were washed three times with 0.1% Tween-20 containing PBS, incubated with HRP conjugated goat anti-mouse (1:25000) for 1 hours at RT, washed three times, and scanned using Odyssey® Infrared Imaging System (LI-COR Biosciences).
Caspase-1 detection
Whole blood was stimulated with CC for 4 hours, and incubated for 2 hours with probes for caspase-1 detection (FAM FLICATM Caspase 1 Assay Kit, Immunochemistry technologies). Blood was stained with anti-CD14 PE (50 µg/ml) before red blood cell lysis with FACS Lysing Solution (BD Bioscience). Analyses were performed on a BD FACS CantoTM II (BD Bioscience).
CD11b and ROS detection
For CD11b detection, whole blood was fixed with 1% paraformaldehyde for 4 minutes at 37°C and stained with anti-CD11b PE (50 µg/ml), anti- CD14 FITC (25 µg/ml). Identification of nucleated cells was done with LDS-751 (Sigma- Aldrich, 8µg/ml). Oxidative burst was determined using the Burstest® (Phagoburst®) kit, BD Biosciences) with some modifications. Whole blood was incubated with complement inhibitors or PBS-control and stimulated with CC or PBS/HSA. After 10 minutes of incubation, DHR 123 was added according to kit procedure, incubated for 10 minutes prior to lysis of red blood cells with FACS Lysing Solution, washed and stained with anti-CD14-PE and anti-CD45-PerCP for 15 minutes on ice. Samples were washed once and ran on a FACSCalibur flow cytometer (BD Bioscience). Data was analyzed with FlowJo (version 10, Tree Star).
Phagocytosis of CC
Whole blood was preincubated with compstatin CP40 (20 µM) and other inhibitors as described above. Samples were stimulated with CC (3 × 107 particles/ml) or PBS/HSA, for 20 minutes at 37°C. Cells were fixed with 0.5% paraformaldehyde for 4 minutes and stained with anti-CD45-PerCP, anti- CD14-FITC and anti-CD11b-PE. After staining, red blood cells were lysed using FACS Lysing Solution, washed, ran on a LSRII flow cytometer (BD Bioscience) and analyzed using FACSDiva software (BD Bioscience). Granulocytes and monocytes were selected as CD45 positive cells and gated based on CD14 expression. Phagocytosis was determined based on shift in SSC induced by CC ingestion, gated such that no more than 4% of negative cells were defined as positive for phagocytosis.
Statistics
SPSS version 21 (IBM Corp.) was used for the non-parametric Two-way ANOVA, used in Fig. 5A. GraphPad Prism version 5 (Graphpad Software) was used for the remaining analyzes, and p < 0.05 was considered statistically significant. Statistical analysis was performed on at least six independent experiments from different donors, unless otherwise indicated. Fig. 1A-1E were analyzed using Two-way ANOVA on log transformed data with Bonferroni posttests. Fig.1G, Fig. 2 and Fig. 3 were analyzed with Wilcoxon matched-pairs signed rank test, while Fig. 5C and D were analyzed with One-way ANOVA and Dunn’s multiple comparison test. The statistical significance of the decrease compared to the control peptide group in each panel on Fig. 6A-6D and Fig. 7C-7D, 7G-7H were analyzed using One-way ANOVA and Dunnett’s multiple comparisons test on log transformed data.
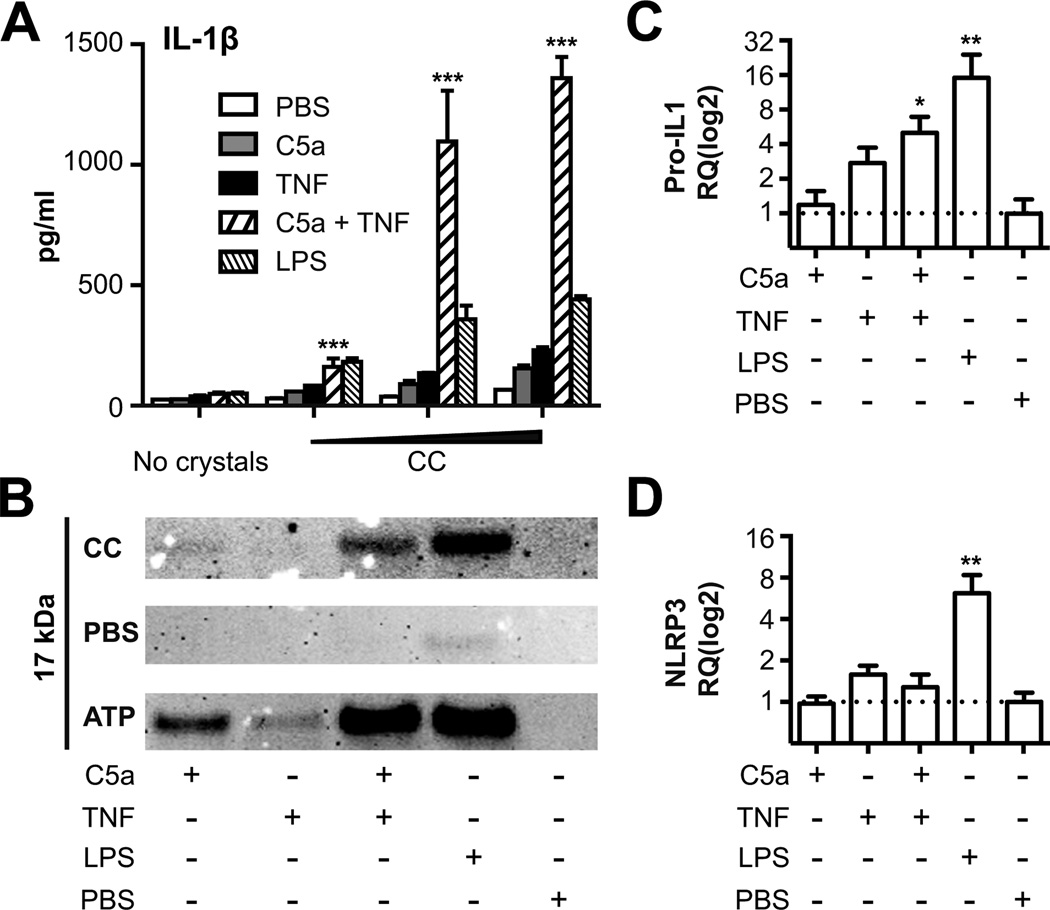
Figure 5. Combining C5a and TNF prime PBMC and monocytes for CC-induced IL-1β.
Human (A) PBMCs and (B-D) monocytes were primed for 2 hours in 10% heat inactivated human serum with PBS/HSA, C5a, TNF, a combination of the two, or a priming dose of LPS (100 pg/ml) prior to stimulation with CC. (A) IL-1β was detected by ELISA in supernatants 16 hours after stimulation with increasing concentrations of CC (0.15 × 1070.75 × 1071.5 × 107 particles/ml). Combination of C5a and TNF are compared to TNF alone for all concentrations (***, p < 0.001). (B) Cleaved IL-1β detected by western blot in monocytes, primed as indicated by matrix below blot, and stimulated for 6 hours with CC (1.5 × 107 particles/ml), ATP (3 mM) or PBS/HSA. (C) Pro-IL-1β - or (D) NLRP3 mRNA were measured by qRT-PCR and normalized against PBS control (*, p < 0.05, **, p < 0.01). One experiment of at least three performed is shown.
Figure 1. CC activate the alternative- and classical complement pathways.
Human serum was incubated at 37°C, for indicated times, in the presence of CC (3 × 107 particles/ml), zymosan and heat aggregated IgG (Zym-IgG) or PBS. (A) The end product in complement activation, TCC, showed a significant increase (***, p < 0.001) in response to CC compared to PBS at every time points, (B) the activation product C3bc, from the common complement component C3 for all three initial pathways, showed a significant increase (***, p < 0.001) at all time points in response to CC compared to PBS, (C) the alternative pathway convertase, C3bBbP, showed a significant increase (***, p < 0.001) in response to CC compared to PBS at every time points. The increase in (D) the common activation product for the classical and lectin pathways, C4bc, and (E) the activation product for the classical pathway, C1rs-C1-INH, did not reach statistical significance. Data plotted are mean ± SEM from six independent experiments with serum from healthy donors. (F) C1q binding to CC, when incubated in plasma for 30 minutes, measured by flow cytometry. (G) TCC in C1q depleted serum (C1q dep.) with or without reconstitution (C1q rec.) (*, p < 0.05) with purified C1q upon CC (1.5 × 107 particles/ml) stimulation, measured by ELISA. One out of six independently performed experiments is shown. In the lower panel, human whole blood was incubated with CC (1.5 × 107 particles/ml) for 30 minutes. (H) Binding of TCC to the crystals was detected using anti C5b-9 and anti-mouse IgG conjugated with Alexa-488, and for C3bc (I) anti-C3bc antibody directly conjugated to FITC. (J) Control IgG2a conjugated to FITC. Scale bars represent 10 µm. Data are representative of two independent experiments. AU = arbitrary units. DIC = Differential Interference Contrast.
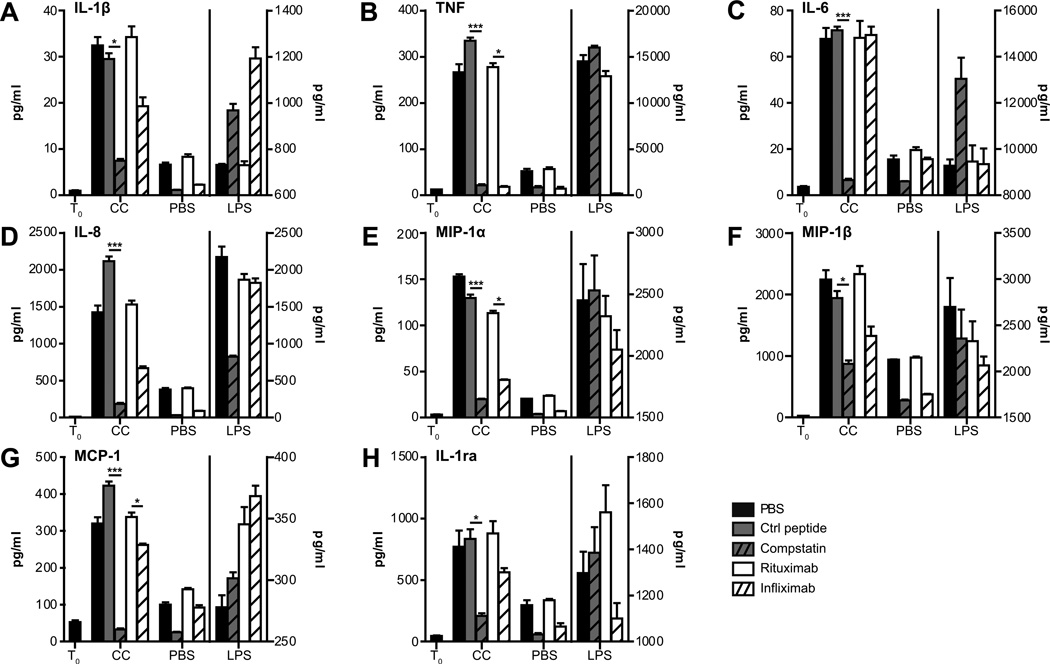
Figure 2. CC induce complement-dependent cytokine release.
Human whole blood was incubated with CC (3 × 107 particles/ml), PBS or LPS for 6 hours after preincubation with PBS, control peptide, compstatin, anti-TNF infliximab, or control anti-CD20 rituximab at 37°C. Cytokines and chemokines were quantified in plasma by multiplex analysis. T0 represents the start of the experiment. Dataset on the left of the dividing line (T0, CC, PBS) are plotted on the left y-axis, and dataset on the right of the dividing line (LPS) are plotted on the right y-axis. Data plotted are mean ± SD from triplicate determinations in one out of at least six independently performed experiments from healthy donors (*, p < 0.05, ***, p < 0.001).
Figure 3. CC-induced release of cytokines from PBMC and granulocytes.
Human PBMC and granulocytes were isolated from whole blood and resuspended in plasma/PBS before incubation with CC (3 × 107 particles/ml) for 5.5 hours. CC-induced production of the cytokines (A) IL-1β, (B) TNF, (C) IL-6, (D) IL-8, (E) MIP-1α, and (F) MCP-1 from PBMC and granulocytes were quantified by multiplex analysis and compared (*, p <0.05). (G) Myeloperoxidase (MPO) from PBMC and granulocytes was detected by ELISA (*, p <0.05). Data plotted are mean ± SD in triplicate determinations in one out of six independent experiments.
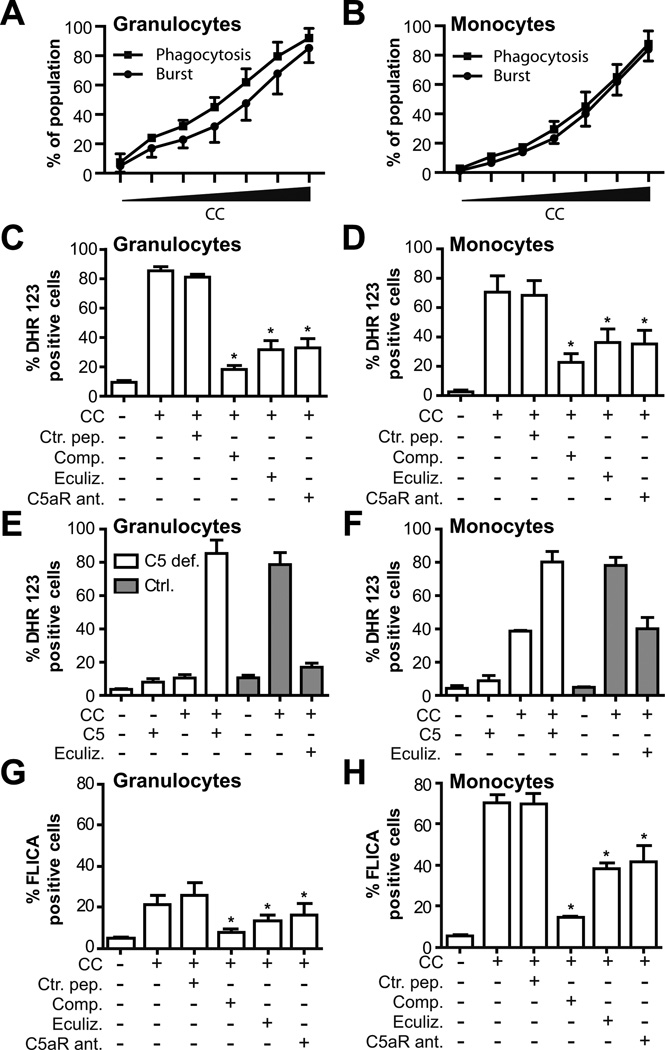
Figure 6. CC-induced up-regulation of CD11b (CR3) and phagocytosis of CC are complement-mediated.
Whole blood pre-incubated with the complement inhibitors compstatin (Comp.), eculizumab (Eculiz.), C5a receptor antagonist (C5aR ant.), control peptide (Ctrl. Pep.) or anti-C7 (C7 antibody) was incubated with CC (3 × 107 particles/ml), or PBS/HSA. (A, B) Median fluorescent intensity (MFI) of CD11b on granulocytes and monocytes measured after 15 minutes. (C, D) Phagocytosis was determined based on shift in SSC induced by CC ingestion, as described in Materials and Methods. The decrease compared to the control peptide group in each panel (A-D) was significant (*, p < 0.05) for all comparisons, except for C7 antibody. Results are mean ± SEM, n=6. (E, F) Phagocytosis of CC in granulocytes and monocytes in whole blood from a C5 deficient donor (C5 def.), or C5 deficient donor reconstituted with purified C5 (50 µg/ml). Blood from three controls (Ctrl.) were analyzed in parallel. Results from the C5 deficient donor represent mean ± SD of two experiments performed at two time points.
Figure 7. CC-induced ROS production and caspase-1 activation are complement-mediated.
Whole blood was incubated with increasing concentrations of CC (0 – 6 × 107 particles/ml). A dose-dependent relationship between CC and phagocytosis/oxidative burst in granulocytes (A) and monocytes (B) was measured. Whole blood pre-incubated with the complement inhibitors compstatin (Comp.), eculizumab (Eculiz.), C5a receptor antagonist (C5aR ant.), control peptide (Ctrl. Pep.) and incubated with CC (3 × 107 particles/ml) or PBS/HSA. ROS production is shown as % dihydrorhodamine (DHR) 123 positive (C) granulocytes or (D) monocytes. (E, F) CC-induced ROS production in granulocytes and monocytes from a C5 deficient donor, or C5 deficient donor reconstituted with purified C5. Results from the C5 deficient donor represent mean ± SD of two experiments performed at two time points and blood from three controls (Ctrl.) were analyzed in parallel. Activation of caspase-1 was detected as % FLICA positive (G) granulocytes or (H) monocytes in whole blood (mean ± SEM, n=3, *, p < 0.05). The decrease compared to the control peptide group in (C, D) and (G, H) was significant (mean ± SEM, n=6,*, p < 0.05) for all comparisons.
Study approval
Approval No S-04114 from the Regional Ethical Committee, South-East Regional Health Authority and approval No 32-2004 from the Regional Ethical Committee, Northern Norway Regional Health Authority for the use of whole blood. Approval No2009/224 from the Regional Ethical Committee, NTNU, for the use of human buffy coats and leukocytes. Blood from the C5-deficient individual was obtained and used in accordance with a protocol approved by the regional ethics committee (REK Nord 2010/1141). The study confirms with the principles outlined in the Declaration of Helsinki for use of human tissue or subjects. Signed informed consent was obtained from all participants.
Results
CC activate the classical- and alternative complement pathway
In the first set of experiments CC were added to serum for 30, 60 and 120 minutes and the complement activation products were measured. This led to a strong, time-dependent and significant (p < 0.001) increase in both TCC and C3bc (Figs. 1A, 1B). Moreover, marked deposition of C3bc and TCC was observed on the crystal surface, demonstrating the strong opsonisation and complement activation potential of CC (Figs.1H, 1I). The alternative pathway convertase C3b-Bb-properdin complex (C3BbP) was also markedly and significantly (p < 0.001) increased by CC at all time points (Fig. 1C). The modest increase in C4bc, a marker of both classical- and lectin pathway activation, did not reach statistical significance(Fig. 1D). No increase in C1rs-C1-INH in the soluble phase was observed (Fig. 1E), consistent with attachment of C1rs-C1-INH complexes to the CC surface, which were not liberated into the liquid phase. Supporting this, C1q bound strongly to the surface of the CC incubated in plasma for 30 minutes (Fig. 1F). To further investigate the involvement of the classical pathway, we compared the formation of TCC in C1q depleted serum in the absence and presence of purified C1q. CC were unable to activate complement in C1q depleted serum, while C1q reconstitution significantly (p < 0.05) restored the complement activation potential (Fig. 1G). Collectively, these data demonstrate the robust complement activation potential of CC, where the classical pathway plays an instrumental role in the initial phase of the activation, propagated by the alternative amplification loop.
CC induce complement-dependent cytokine release
The ability of CC to induce cytokine responses in the whole blood system was then examined. Addition of CC for 6 hours resulted in a marked induction of proinflammatory cytokines (TNF, IL-1β, IL-6), chemokines (IL-8, MIP-1α, MIP-1β, MCP-1) and the inhibitor IL-1ra (Figs. 2A-2H). LPS contamination of the CC was eliminated as a cause for these responses as the amount of LPS present in the CC was below the detection limit in the limulus amebocyte lysate assay. Furthermore, anti-CD14 antibodies or lipid IVa did not reduce the CC-induced cytokines, while the LPS response was completely inhibited with the anti-CD14 antibody (data not shown). By separation of blood leukocytes into peripheral mononuclear cells (PBMC) and granulocytes, we found that the PBMC fraction were responsible for the CC-induced IL-1β, TNF, IL-6, MCP-1 and MIP-1α as significantly less (p < 0.05) of these cytokines were detected from CC stimulated granulocytes (Figs. 3A-3G). The granulocytes responded to CC with a minor IL-8 release compared to PBMC, whereas a vigorous secretion of myeloperoxidase occurred in granulocytes, but not in PBMC (p < 0.05).
The cytokine responses induced with the CC in whole blood were complement dependent as the C3 convertase inhibitor compstatin significantly reduced (p < 0.05) release of all cytokines measured (Fig. 2). For TNF, IL-6, IL-8, MIP-1α and MCP-1, the inhibition with compstatin was more than 90%.In general, cytokine responses to LPS were greater than to CC. In contrast to CC, compstatin did not inhibit LPS induced cytokine responses, except for IL-8, which was reduced by 50%,but overall did not reach statistical significance (Fig. 2D). LPS has in earlier studies been shown to have a weak complement activating potential that can contribute to the IL-8 response in whole blood (18). Since compstatin inhibits generation of C3a, we also included a C5 blocking antibody (eculizumab), which blocks the cleavage of C5 to C5a and C5b. In general, the results obtained with eculizumab showed the same inhibition profile as for compstatin (Supplemental Figs.1A-1H).
Addition of CC to whole blood resulted in a 5–10 fold increase in the TNF release compared to non-stimulated controls. Since TNF represents an early cytokine response, we addressed whether TNF induced by CC could act in a feedback loop and affect the production of other cytokines in the whole blood model. Thus, we added the TNF blocking antibody infliximab together with CC and measured cytokine release and observed significant inhibition (p < 0.05) of MIP-1α, and MCP-1,as compared to the negative control antibody rituximab, but no effect was found on IL-6 release(Fig. 2). Infliximab also reduced CC-stimulated release of IL-1β, IL-8, MIP-1β, and IL-1ra, however, these effects did not reach statistical significance. These results suggest that TNF is involved in the release of cytokines that are induced by CC in whole blood.
CC-induced CD11b/CD18 (CR3) expression and cytokine release is reconstituted by C5 in whole blood from a C5 deficient person
In the next set of experiments the role of C5 in the inflammatory response induced by CC was investigated in whole blood from a C5 deficient person. C5 deficiency is a rare condition and is previously described in around 40 cases worldwide. The case report for the C5 deficient person used in this study has previously been published (19). No formation of TCC occurred in whole blood from the C5 deficient person incubated with CC, whereas reconstitution with purified C5 completely restored the TCC formation to that of healthy individuals (Fig.4A). Furthermore, CC did not result in granulocyte- or monocyte CR3 expression in the C5 deficient person, whereas addition of C5 completely restored the CR3 expression to levels seen in healthy individuals (Figs. 4B, 4C). Some CR3 expression was induced by LPS in the C5 deficient person, confirming earlier reports of a complement-dependent and -independent CR3 expression by LPS (18).
Figure 4. CC-induced CD11b expression and cytokine production in whole blood from a C5-deficient person.
Whole blood from a C5 deficient donor, or C5 deficient donor reconstituted with purified C5 (50 µg/ml), or blood from three healthy donors were incubated with CC (3 × 107 particles/ml), PBS/HSA or LPS at 37°C. (A) TCC detected by ELISA in plasma after 30 minutes. (B, C) Median fluorescent intensity (MFI) of CD11b on granulocytes and monocytes measured after 15 minutes. Plasma collected after 6 hours incubation was analyzed by multiplex for (D) IL-1β, (E) TNF, (F) IL-6, and (G) IL-8. Mean of triplicate determinations are shown for the C5 defect donor samples, and mean of three control donors are shown. Error bars: ±SD.
The CC induced cytokine response was then investigated in whole blood from the C5 deficient person. In C5 deficient blood, CC induced low secretion of IL-1β, TNF, IL-6 and IL-8 that was comparable with levels in non-stimulated blood from healthy individuals (Figs.4D-4G). Reconstitution with purified C5 resulted in a 2.5 to 5-fold increase in the CC induced cytokine secretion. Addition of C5 to C5 deficient whole blood led to a slight increase in TCC and cytokines in the non-stimulated samples, consistent with purified C5 being slightly activated spontaneously as seen previously (20). Since we only have one available case of C5 deficiency, proper statistical analysis of this data is not feasible. However, this case supports that C5 contributes to the CC-induced cytokine response in whole blood.
C5a activates the C5a receptor (CD88) on immune cells resulting in several types of inflammatory responses (21). Of interest, we observed that addition of a C5a receptor antagonist to whole blood reduced the CC-inducedIL-1β by 81 ± 8%(mean ± SEM, n=3, p < 0.01) and CC-induced TNF by 60 ±3% (mean ± SEM, n=3, p < 0.01) compared to untreated control. Together these data support a role of C5 in the CC-induced cytokine responses in whole blood and point to C5a as a mediator of these responses.
Combining C5a and TNF prime PBMC and monocytes for CC-induced IL-1β
We have previously reported that CC activate the NLRP3 inflammasome in phagocytes in vitro (4). Before CC can activate NLRP3, the phagocytes must be primed in an NFκB dependent manner so that the pro-IL-1β and NLRP3 are induced and present in sufficient amounts (9). Low doses of exogenously added LPS can act as a potent primer for CC induced IL-1β processing (4), however, endogenous primers for NLRP3 by CC have not yet been fully addressed. Since we observed involvement of C5 on the CC-induced cytokine responses as well as markedly enhanced TNF release, C5a and TNF were examined alone or in combination for their priming properties. C5a and TNF were found to synergize in providing a potent priming signal in CC-activated human PBMC (p < 0.001) (Fig. 5A) while a minimal priming effect was observed with the C3a and TNF combination (Supplemental Fig. 2).
In order to verify that the released IL-1β represented the mature form and a result of inflammasome activation, human monocytes were primed and activated. Supernatants were precipitated and subjected to Western blot analysis (Fig. 5B), showing the cleaved and mature 17 kDa form of IL-1β. Priming of monocytes with a combination of C5a and TNF followed by addition of CC resulted in a robust release of the mature form of IL-1β. Also the NLRP3 activator ATP gave a strong release of mature IL-1β from monocytes treated with the C5a/TNF combination (Fig. 5B). We observed that the C5a/TNF combination increased the IL-1β transcripts in monocytes(p < 0.05) (Fig. 5C). TNF alone also increased the IL-1β mRNA, whereas C5a alone had no or minimal effect. The NLRP3 mRNA was hardly increased by the C5a/TNF combination (Fig. 5D), suggesting that the priming effect of C5a/TNF is predominantly to induce increased amounts of pro-IL-1β.
CC-induced up-regulation of CR3 and phagocytosis of CC are complement-dependent
Phagocytosis of CC is required for NLRP3 activation (4). Thus, the role of complement activation in the phagocytosis of the crystals was investigated. Compstatin inhibited phagocytosis of CC in monocytes by 50–60% after 20 minutes of CC incubation, with minimal inhibition after 60 minutes (Supplemental Fig.2B). This result indicates that complement activation contributes to the phagocytosis of CC in isolated human monocytes at early time points, which may be crucial for the initial inflammasome activation and subsequent IL-1β release.
To further address the involvement of complement activation in the phagocytosis of CC by leukocytes in whole blood, the effect of complement inhibition was examined. Compstatin, eculizumab and the C5a receptor antagonist reduced the CC-induced CR3 up-regulation down to baseline levels (p < 0.05) (Figs. 6A,6B). No inhibitory effect was seen with the anti-C7 antibody that inhibits TCC generation without affecting C3 and C5. Granulocytes were more effective than monocytes to phagocytose CC (Figs. 6C, 6D). Compstatin, eculizumab and the C5a receptor antagonist reduced granulocyte phagocytosis by more than 80% (p < 0.05) and monocyte phagocytosis by more than 60% (p < 0.05). In line with these findings, granulocytes, and to lesser extent monocytes, from the C5 deficient person had markedly reduced phagocytosis of CC, and reconstitution with C5 restored the phagocytosis to control levels (Figs. 6E, 6F). Taken together these data suggest that CR3 might be an important phagocytosis receptor for CC in monocytes and, in particular, in granulocytes. Furthermore, the clear inhibitory effect by the C5a receptor antagonist demonstrates that C5a is a mediator of CC phagocytosis in leukocytes, consistent with its potential to up-regulate expression of CR3.
CC induce production of reactive oxygen species and activation of inflammasome-caspase-1 in a complement-dependent manner
A consequence of complement dependent phagocytosis is the induction of reactive oxygen species (ROS) which has been implicated in activation of inflammasomes (10). Addition of CC to human whole blood resulted in a dose-dependent relationship between CC and phagocytosis as well as CC and ROS production in both granulocytes and monocytes (Figs. 7A, 7B). The CC-induced ROS production was reduced by compstatin, eculizumab and C5aR antagonist (p < 0.05)(Figs. 7C,7D). Of interest, eculizumab and the C5aR antagonist were equally effective in reducing the CC-induced oxidative burst suggesting that C5a, and not the sublytic TCC, is an important mediator in this response. Moreover, granulocytes and monocytes from the C5 deficient person had attenuated CC-induced ROS responses that were restored to control levels by C5 reconstitution (Figs.7E, 7F). Importantly, the CC-induced ROS production was accompanied by a marked caspase-1 activation in the monocytes, whereas a lower caspase-1 activity was detected in granulocytes(Figs. 7G, 7H). Compstatin was particularly effective in reducing caspase-1 activity, however, also eculizumab and the C5aR antagonist significantly (p < 0.05) inhibited caspase-1 in monocytes suggesting that C5a is a mediator also for CC-induced caspase-1 activation.
Discussion
Despite early identifications of their physical properties, CC have, until recently, been considered as relatively inert (1). However, we and others have shown that CC- induced inflammation is a critical contributor to atheroma development and progression in experimental atherosclerosis (4, 7). In the present study we have identified new and important principles of CC-induced inflammatory responses and for the first time documented that the complement system is crucial for CC-induced inflammasome-caspase-1 activation.
Our data demonstrate that CC induced a robust complement activation that resulted in high amounts of C3bc and the soluble TCC. Previous studies have suggested that CC activate the alternative pathway (22–24). Our data confirm these findings, as the amount of C3BbP was strongly increased by the CC. Notably, we also found a clear activation of the classical pathway by the CC suggesting an initial activation of the classical pathway by C1q binding to the CC surface. Further studies are needed to clarify whether C1q binds directly to the CC, or indirectly via other molecules like immunoglobulins or C-reactive protein. It cannot be excluded that also the lectin pathway may contribute to CC-induced complement activation. However, since the CC lack carbohydrates, the contribution of the lectin pathway is likely to be minimal. Moreover, methods to detect specific complement activation products for the lectin pathway are, to our knowledge, not available. The alternative pathway may play an important role in the downstream effect of initial classical pathway activation by the CC, as the alternative pathway has been reported to amplify the outcome of total complement activation initially triggered by the classical or lectin pathway by 80–90%(25, 26).
The human whole blood model is a unique tool to explore the role of the complement system in CC-induced inflammation. It allows inflammatory cross-talk by using an anticoagulant that does not affect complement activation (16). In this model we demonstrate a marked release of pro-inflammatory cytokines, in particular TNF, when CC is added. Minimal cytokine response and strong myeloperoxidase release were observed from granulocytes upon addition of CC suggesting that monocytes are the primary cell type that respond to CC by releasing cytokines. By adding infliximab, some of the CC-induced cytokines were reduced, suggesting that TNF may act as an amplifier of CC-induced cytokine release. The inflammatory cytokine response to CC was strongly dependent on complement activation as both C3 and C5 inhibitors attenuated the response. Furthermore, the CC-induced cytokines were lower in whole blood from a C5-deficient person, which were restored by reconstitution with purified C5.
Among the complement activation products, C5a is described as the most potent inflammatory peptide, with a broad spectrum of functions in immune activation and inflammation(11, 27, 28). Early studies have shown that C5a can potentiate secretion of TNF by human PBMC (29) while others have shown that C5a stimulates transcription of TNF (30). Among other effector functions of complement is the ability of the terminal pathway to form sublytic TCC that can trigger cell activation, including the release of inflammatory cytokines like TNF, without inducing cell death (31–34). The sublytic TCC may also trigger intracellular Ca2+ fluxes leading to NLRP3 activation(35). However, addition of an anti-C7 antibody that inhibited the TCC formation by 50–60% did not reduce either CC-induced IL-1β - or TNF release. Notably, the CC-induced TNF- and IL-1β responses were reduced with the C5a receptor antagonist, suggesting that C5a is a key player in the induction of pro-inflammatory cytokines induced by CC.
Adding TNF and C5a together to monocytes in vitro enhanced the pro-IL-1β mRNA, however, the NLRP3 mRNA was not increased by this combined treatment. Fully differentiated macrophages have modest levels of basal NLRP3 expression whereas monocytes show high endogenous NLRP3 levels (36). Thus, induction of pro-IL-1β may be sufficient as a priming signal in monocytes for CC induced NLRP3 activation. Furthermore, C5a has the ability to increase the expression of its own signaling receptor CD88 (37). The priming effect was strongest for the C5a/TNF combination as only minimal priming was observed for the C3a/TNF combination. These findings suggest that TNF, which is released early during an inflammatory response, may act together with C5a as an endogenous primer for CC induced NLRP3 activation in monocytes by enhancing the transcripts of pro-IL-1β.
Activation of the NLRP3 inflammasome is also dependent on ROS generation. Many of the NLRP3 activators, including ATP and crystalline structures such as asbestos and monosodium urate, are triggers of short-lived ROS (38). Our data from the whole blood assay show that monocytes and granulocytes produce ROS in response to CC in a complement dependent manner, which may contribute to inflammasome activation. Indeed, we show in the same model that CC also activated caspase-1 in a complement-dependent manner, however, little caspase-1 activation occurred in granulocytes compared to monocytes, which corresponds well with the low CC-induced IL-1β release from granulocytes. Moreover, our data suggest that monocytes and granulocytes employ CR3 for the phagocytosis of CC. CR3is expressed on monocytes, macrophages and granulocytes and is activated during the innate immune response to microbes. C3b, a cleavage product of C3, coats invading microbes and is further cleaved to form C3bi, which is the main ligand for CR3, resulting in a phagocytic response. CR3 is a central receptor that mediates phagocytosis of E. coli by monocytes and granulocytes (18). Several lines of evidence suggest that CR3 is an essential phagocytosis receptor also for CC. Addition of CC to whole blood resulted in C3 opsonisation and a substantial up-regulation of CR3 (detected by CD11b) in monocytes and granulocytes. Complement inhibitors virtually abolished both the CR3 expression and phagocytosis of CC and a C5 deficient donor had blunted CR3 expression and phagocytosis in response to CC. Moreover, C5a was found to be a mediator of both the CC phagocytosis and CR3 expression, which is in line with previous data showing that C5a directly induces CR3 expression in monocytes and granulocytes (18).
The results presented in this paper demonstrate that the complement system participates in CC-induced inflammasome activation in monocytes by regulating several cellular responses. The combination of C5a and TNF acts as a primer for inflammasome-caspase-1 activation and complement activation leads to C5a generation with subsequent up-regulation of CR3, CC phagocytosis, ROS production and formation of functional caspase-1. We suggest that the connection observed between CC-induced complement activation and inflammasome activation is important for development of atherosclerosis. Complement components are detected in atherosclerotic plaques and circulating levels of C5a is increased in persons with advanced atherosclerosis (39). Moreover, a C5a inhibitor has been reported to reduce atherosclerosis in ApoE−/− mice (40). Interestingly, treatment with this inhibitor did not reduce the number of immune cells in lesion sites, but instead significantly inhibited the lipid content within the plaques. The lowering effect of C5a inhibition on cholesterol content in local atherosclerotic lesions may be attributed to the effect of C5a on phagocytosis of CC, suggesting a role for complement not only in inflammation, but also in lipid accumulation. The bidirectional interaction between lipids and inflammation is a hallmark of atherosclerosis and our findings suggest that CC and complement are important mediators in these processes.
IL-1β is proposed to be involved in the development of atherosclerosis. A clinical trial is currently testing the long-term effect of an IL-1β antibody on recurrent cardiovascular events (41). In summary, our data demonstrate that CC induce cytokine responses that are dependent on complement activation, and that C5a and TNF together play a role in controlling inflammasome activation by the crystals. Further we describe data implying that CR3 is a candidate receptor for mediating phagocytosis of CC. Altogether, results presented in this paper point to the complement system as a key trigger involved in CC-induced inflammation and suggest that complement inhibition, in particular at the level of C5, is an interesting target for treatment of cardiovascular disease.
Supplementary Material
Acknowledgements
We thank Grethe Bergseth for performing excellent complement activation analyses, and Dr. Hongchang Qu for synthesizing the complement inhibiting peptides used in this study. The confocal imaging was performed at the Cellular & Molecular Imaging Core Facility, Norwegian University of Science and Technology (NTNU).
Source of support:
This work was partly supported by the Research Council of Norway through its Centres of Excellence funding scheme, project number 223255/F50(TE), Central Norway Regional Health Authority (TE), The Norwegian Council on Cardiovascular Disease (TEM), The Odd Fellow Foundation (TEM) and National Institutes of Health Grants AI-068730 (JDL).
Nonstandard Abbreviations and Acronyms
- CC
cholesterol crystals
- ApoE−/−
apolipoprotein E-deficient
- TCC
terminal complement complex
- CR3
complement receptor 3
- ROS
reactive oxygen species
Footnotes
Disclosures:
Conflict of interest: The co-author, Prof. J.D.L., is the holder of several patent applications on complement inhibitors and the founder of Amyndas Pharmaceuticals, which is developing complement inhibitors for clinical applications. The other authors report no conflict.
References
- 1.Small DM. George Lyman Duff memorial lecture. Progression and regression of atherosclerotic lesions. Insights from lipid physical biochemistry. Arteriosclerosis. 1988;8:103–129. doi: 10.1161/01.atv.8.2.103. [DOI] [PubMed] [Google Scholar]
- 2.Abela GS. Cholesterol crystals piercing the arterial plaque and intima trigger local and systemic inflammation. J. Clin. Lipidol. 2010;4:156–164. doi: 10.1016/j.jacl.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler. Thromb. Vasc. Biol. 1995;15:1512–1531. doi: 10.1161/01.atv.15.9.1512. [DOI] [PubMed] [Google Scholar]
- 4.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, Becker CE, Ediriweera HN, Mullick AE, Golenbock DT, Stuart LM, Latz E, Fitzgerald KA, Moore KJ. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat. Immunol. 2013;14:812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajamaki K, Lappalainen J, Oorni K, Valimaki E, Matikainen S, Kovanen PT, Eklund KK. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One. 2010;5:e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galea J, Armstrong J, Gadsdon P, Holden H, Francis SE, Holt CM. Interleukin-1 beta in coronary arteries of patients with ischemic heart disease. Arterioscler. Thromb. Vasc. Biol. 1996;16:1000–1006. doi: 10.1161/01.atv.16.8.1000. [DOI] [PubMed] [Google Scholar]
- 9.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu. Rev. Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 11.Klos A, Tenner AJ, Johswich KO, Ager RR, Reis ES, Kohl J. The role of the anaphylatoxins in health and disease. Mol. Immunol. 2009;46:2753–2766. doi: 10.1016/j.molimm.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qu H, Ricklin D, Bai H, Chen H, Reis ES, Maciejewski M, Tzekou A, DeAngelis RA, Resuello RR, Lupu F, Barlow PN, Lambris JD. New analogs of the clinical complement inhibitor compstatin with subnanomolar affinity and enhanced pharmacokinetic properties. Immunobiology. 2013;218:496–505. doi: 10.1016/j.imbio.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finch AM, Wong AK, Paczkowski NJ, Wadi SK, Craik DJ, Fairlie DP, Taylor SM. Low-molecular-weight peptidic and cyclic antagonists of the receptor for the complement factor C5a. J. Med. Chem. 1999;42:1965–1974. doi: 10.1021/jm9806594. [DOI] [PubMed] [Google Scholar]
- 15.Husebye H, Aune MH, Stenvik J, Samstad E, Skjeldal F, Halaas O, Nilsen NJ, Stenmark H, Latz E, Lien E, Mollnes TE, Bakke O, Espevik T. The Rab11a GTPase controls Toll-like receptor 4-induced activation of interferon regulatory factor-3 on phagosomes. Immunity. 2010;33:583–596. doi: 10.1016/j.immuni.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mollnes TE, Brekke OL, Fung M, Fure H, Christiansen D, Bergseth G, Videm V, Lappegard KT, Kohl J, Lambris JD. Essential role of the C5a receptor in E coli-induced oxidative burst and phagocytosis revealed by a novel lepirudin-based human whole blood model of inflammation. Blood. 2002;100:1869–1877. [PubMed] [Google Scholar]
- 17.Fure H, Nielsen EW, Hack CE, Mollnes TE. A neoepitope-based enzyme immunoassay for quantification of C1-inhibitor in complex with C1r and C1s. Scand. J. Immunol. 1997;46:553–557. doi: 10.1046/j.1365-3083.1997.d01-168.x. [DOI] [PubMed] [Google Scholar]
- 18.Brekke OL, Christiansen D, Fure H, Fung M, Mollnes TE. The role of complement C3 opsonization, C5a receptor, and CD14 in E. coli-induced upregulation of granulocyte and monocyte CD11b/CD18 (CR3), phagocytosis, and oxidative burst in human whole blood. J. Leukoc. Biol. 2007;81:1404–1413. doi: 10.1189/jlb.0806538. [DOI] [PubMed] [Google Scholar]
- 19.Grimnes G, Beckman H, Lappegard KT, Mollnes TE, Skogen V. Recurrent meningococcal sepsis in a presumptive immunocompetent host shown to be complement C5 deficient-a case report. APMIS. 2011;119:479–484. doi: 10.1111/j.1600-0463.2011.02740.x. [DOI] [PubMed] [Google Scholar]
- 20.Lappegard KT, Christiansen D, Pharo A, Thorgersen EB, Hellerud BC, Lindstad J, Nielsen EW, Bergseth G, Fadnes D, Abrahamsen TG, Hoiby EA, Schejbel L, Garred P, Lambris JD, Harboe M, Mollnes TE. Human genetic deficiencies reveal the roles of complement in the inflammatory network: lessons from nature. Proc. Natl. Acad. Sci. U. S. A. 2009;106:15861–15866. doi: 10.1073/pnas.0903613106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manthey HD, Woodruff TM, Taylor SM, Monk PN. Complement component 5a (C5a) Int. J. Biochem. Cell Biol. 2009;41:2114–2117. doi: 10.1016/j.biocel.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Hammerschmidt DE, Greenberg CS, Yamada O, Craddock PR, Jacob HS. Cholesterol and atheroma lipids activate complement and stimulate granulocytes. A possible mechanism for amplification of ischemic injury in atherosclerotic states. J. Lab. Clin. Med. 1981;98:68–77. [PubMed] [Google Scholar]
- 23.Seifert PS, Kazatchkine MD. Generation of complement anaphylatoxins and C5b-9 by crystalline cholesterol oxidation derivatives depends on hydroxyl group number and position. Mol. Immunol. 1987;24:1303–1308. doi: 10.1016/0161-5890(87)90125-8. [DOI] [PubMed] [Google Scholar]
- 24.Vogt W, von Zabern I, Damerau B, Hesse D, Luhmann B, Nolte R. Mechanisms of complement activation by crystalline cholesterol. Mol. Immunol. 1985;22:101–106. doi: 10.1016/s0161-5890(85)80003-1. [DOI] [PubMed] [Google Scholar]
- 25.Harboe M, Mollnes TE. The alternative complement pathway revisited. J. Cell. Mol. Med. 2008;12:1074–1084. doi: 10.1111/j.1582-4934.2008.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harboe M, Ulvund G, Vien L, Fung M, Mollnes TE. The quantitative role of alternative pathway amplification in classical pathway induced terminal complement activation. Clin. Exp. Immunol. 2004;138:439–446. doi: 10.1111/j.1365-2249.2004.02627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward PA. The harmful role of c5a on innate immunity in sepsis. J. Innate Immun. 2010;2:439–445. doi: 10.1159/000317194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu. Rev. Immunol. 2005;23:821–852. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- 29.Okusawa S, Yancey KB, van der Meer JW, Endres S, Lonnemann G, Hefter K, Frank MM, Burke JF, Dinarello CA, Gelfand JA. C5a stimulates secretion of tumor necrosis factor from human mononuclear cells in vitro. Comparison with secretion of interleukin 1 beta and interleukin 1 alpha. J. Exp. Med. 1988;168:443–448. doi: 10.1084/jem.168.1.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schindler R, Gelfand JA, Dinarello CA. Recombinant C5a stimulates transcription rather than translation of interleukin-1 (IL-1) and tumor necrosis factor: translational signal provided by lipopolysaccharide or IL-1 itself. Blood. 1990;76:1631–1638. [PubMed] [Google Scholar]
- 31.Cole DS, Morgan BP. Beyond lysis: how complement influences cell fate. Clin. Sci. (Lond.) 2003;104:455–466. doi: 10.1042/CS20020362. [DOI] [PubMed] [Google Scholar]
- 32.Morgan BP. Complement membrane attack on nucleated cells: resistance, recovery and non-lethal effects. Biochem. J. 1989;264:1–14. doi: 10.1042/bj2640001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, Yang C, Jin N, Xie Z, Tang Y, Fei L, Jia Z, Wu Y. Terminal complement complex C5b-9-treated human monocyte-derived dendritic cells undergo maturation and induce Th1 polarization. Eur. J. Immunol. 2007;37:167–176. doi: 10.1002/eji.200636285. [DOI] [PubMed] [Google Scholar]
- 34.David S, Biancone L, Caserta C, Bussolati B, Cambi V, Camussi G. Alternative pathway complement activation induces proinflammatory activity in human proximal tubular epithelial cells. Nephrol. Dial. Transplant. 1997;12:51–56. doi: 10.1093/ndt/12.1.51. [DOI] [PubMed] [Google Scholar]
- 35.Triantafilou K, Hughes TR, Triantafilou M, Morgan BP. The complement membrane attack complex triggers intracellular Ca2+ fluxes leading to NLRP3 inflammasome activation. J. Cell Sci. 2013 doi: 10.1242/jcs.124388. [DOI] [PubMed] [Google Scholar]
- 36.Guarda G, Zenger M, Yazdi AS, Schroder K, Ferrero I, Menu P, Tardivel A, Mattmann C, Tschopp J. Differential expression of NLRP3 among hematopoietic cells. J. Immunol. 2011;186:2529–2534. doi: 10.4049/jimmunol.1002720. [DOI] [PubMed] [Google Scholar]
- 37.Riedemann NC, Guo RF, Sarma VJ, Laudes IJ, Huber-Lang M, Warner RL, Albrecht EA, Speyer CL, Ward PA. Expression and function of the C5a receptor in rat alveolar epithelial cells. J. Immunol. 2002;168:1919–1925. doi: 10.4049/jimmunol.168.4.1919. [DOI] [PubMed] [Google Scholar]
- 38.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Speidl WS, Exner M, Amighi J, Kastl SP, Zorn G, Maurer G, Wagner O, Huber K, Minar E, Wojta J, Schillinger M. Complement component C5a predicts future cardiovascular events in patients with advanced atherosclerosis. Eur. Heart J. 2005;26:2294–2299. doi: 10.1093/eurheartj/ehi339. [DOI] [PubMed] [Google Scholar]
- 40.Manthey HD, Thomas AC, Shiels IA, Zernecke A, Woodruff TM, Rolfe B, Taylor SM. Complement C5a inhibition reduces atherosclerosis in ApoE −/− mice. FASEB J. 2011;25:2447–2455. doi: 10.1096/fj.10-174284. [DOI] [PubMed] [Google Scholar]
- 41.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) Am. Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.