Abstract
An intensive study of 443 isolates of Campylobacter jejuni and Campylobacter coli from 2031 fecal samples excreted by animal sources including cattle, sheep, and pigs, a range of wild and domesticated avian species and pets is described. The prevalence found in the majority of animal sources ranged from 22% to 28% with poultry being highest at 41% and cats and dogs lowest (<5%). The average count excreted for each animal source was found not to be significantly different ranging from approximately 102 to 105 cfu/g. Multilocus sequence typing (MLST) identified phylogenies that exhibited host specificity. A number of clonal complexes (CCs) and sequence types (STs) were characteristic of particular hosts (e.g., CC-179, ST-637, and ST-1341 found only in pigeons and gulls). Analysis of genetic distance demonstrated numerous significant differences in the distribution of MLST types (CC, ST, and allele) between animal sources. Host association was quantified using structure that correctly assigned the nine animal sources with accuracies of 28%, 24%, and 55% at the CC, ST, and allele levels, respectively. This is substantially higher than would be expected by random allocation (11%) but farmyard poultry had the lowest assignment accuracy (13%, 13%, and 21%) suggesting that isolates were shared with a wide range of other animals. This study demonstrates the link between MLST type and host and provides data that can be used in risk assessment and food attribution models. Further, it demonstrates the applicability of MLST to characterize Campylobacter strains from a broad range of environmental sources.
Introduction
Human Campylobacteriosis is a zoonosis and the leading cause of recognized bacterial gastroenteritis in industrialized countries (Olson et al., 2008). The majority of human infections (>80%) are due to Campylobacter jejuni with the remainder being predominantly Campylobacter coli. Recent reported incidence rates were 95.3 per 100,000 in Scotland in 2006 (Locking et al., 2007) and 12.7 per 100,000 in the United States in 2007 (Centers for Disease Control and Prevention, 2007). These rates are likely to be the substantial underestimates of the actual disease burden because it is estimated that only 1 in 7 cases are reported in the United Kingdom (Wheeler et al., 1999) and only 1 in 38 cases in the United States (Mead et al., 1999).
Campylobacter are found in the intestines of many wild, domestic, and farm animals (Moore et al., 2005). Cattle, sheep (Stanley and Jones, 2003), poultry (Bull et al., 2006), and pigs (Boes et al., 2005) are reservoirs, and they harbor high levels of strain diversity (Colles et al., 2003). Companion animals, including cats and dogs, also carry Campylobacter (Lee et al., 2004; Workman et al., 2005). Campylobacter excreted from all reservoirs can be found throughout the environment including soil (Santamaria and Toranzos, 2003), beach sand (Bolton et al., 1999), sewage (Jones, 2001), and groundwater (Stanley et al., 1998).
Foodborne pathways of human Campylobacter infection have been intensively studied, with retail chicken being the most frequently implicated factor. As many as 90% of the birds (Gormley et al., 2008) and 87% of the breast fillets (Luber and Bartelt, 2007) are contaminated according to the retail chicken surveys in Scotland and Germany, respectively. Chicken consumption, especially when undercooked or cross contamination with other foods (Rodrigues et al., 2001), is a key risk factor for infection in several case–control studies and also the 1999 dioxin crisis in Belgium where chicken was removed from sale because the dioxin-contaminated feed coincided with a 40% reduction of human Campylobacter cases (Vellinga and Van Loock, 2002). Other vehicles of transmission include raw milk (Eberhart-Phillips et al., 1997; Michaud et al., 2004), salad vegetables (Evans et al., 2003), barbequed meat (Kapperud et al., 2003; Neimann et al., 2003), and untreated drinking water (Kapperud et al., 2003).
In contrast, environmental pathways of human Campylobacter infection remain poorly understood with comparatively few risk factors identified. Those included are the ingestion of contaminated water during swimming (Schonberg-Norio et al., 2004), visiting or living on a farm (Friedman et al., 2004), and of children living in the countryside (Ethelberg et al., 2005). The multiplicity of potential environmental sources, the rarity of environment-sourced outbreaks to help infection source identification (Cowden, 1992; Miller and Mandrell, 2005), the limited spatial and temporal scales of most reported studies, and the use of different typing methods yielding strain categories that are difficult to compare (Frost et al., 2002) all conspire to hinder further elucidation of environmental sources of human Campylobacter infections.
One approach that promises further insights into environmental sources of Campylobacter infection is to identify strains characteristic of particular hosts and estimate the fecal shedding from those hosts into the environment. This approach is most effective when strains are categorized using a universal typing method based on portable data (i.e., readily allowing results from different labs to be compared and also storage on an electronic database). For many bacterial pathogens, including Campylobacter, these requirements are satisfied by multilocus sequence typing (MLST) (Dingle et al., 2001a, 2001b). Campylobacter strains characterized using MLST are categorized as sequence types (STs), and STs and their component alleles are compared using an open-access database (http://pubmlst.org/campylobacter/). Host specificity of Campylobacter STs is evident in isolates from farm animals and wildlife (French et al., 2005) and from cattle, sheep, and poultry (McCarthy et al., 2007). However, these studies were of limited spatial and temporal scales, whereas human Campylobacter infection is usually reported at the national level.
The aim of this study was to characterize farm animals, pets, and wild birds from a national-scale collection in Scotland according to the prevalence and concentration of Campylobacter in their feces and to the extent of association of the different hosts by categorizing Campylobacter strains using MLST.
Materials and Methods
Fecal samples from potential Campylobacter hosts were collected in rural and urban areas during August 2005 to September 2006. The rural areas included 116 randomly selected farms (sampled once each) in NE and SW Scotland. The farms were chosen as follows. The postcode districts (indicated by the first four digits of the postcode) in the rural areas were tabulated, the central town or village was identified, farmers listed in the local telephone directory (www.yell.com) under that location were contacted by phone, and one willing farmer in each postcode district randomly chosen for each collection visit. NE and SW areas were visited concurrently. The species sampled in rural areas were cattle, sheep, pigs, dogs, farmyard poultry (hens), pigeons, corvids, and gulls. The urban areas were sites in the cities of Edinburgh (10 sites), Glasgow (8), and Aberdeen (17) with high risks of Campylobacter transmission to people, for example, parks, waterfronts, open-air eating areas, public squares, and small petting farms. The species sampled in the urban areas were dogs, cats, feral pigeons, gulls, ducks, geese, and swans. Avian samples from unknown sources were categorized as birds. In all cases, fresh fecal mammalian (25 g) and fresh avian (<5 g) grab samples were collected (n = 2023). The grab samples involved placing a sterile plastic bag inside-out over the hand and then “grabbing” the sample. Some smaller avian samples were collected using a sterile swab moistened with saline. Samples were transported chilled (4°C) to the laboratory for immediate analysis.
Identification and enumeration of Campylobacter
Fecal aliquots (10 g) were homogenized (10:90 w:v) in Campylobacter enrichment broth (see below) and 0.1 mL decimal dilutions plated directly onto the modified Campylobacter Blood-Free Selective Agar Base (CCDA base, CM0739; Oxoid, Basingstoke, UK) with CCDA selective supplement (SR 155; Oxoid) for target enumeration (minimum count was therefore 100 cfu/g). The swabs were individually tested by adding a portion of a measured 10 mL volume enrichment broth into the swab tube. The sample was gently vortexed, and the contents of the swab tube and the swab were placed into the remaining enrichment broth and gently mixed. Two further dilutions were prepared from this sample and then 0.1 mL plated for enumeration. The remainder of the sample was incubated for presence or absence. The presence or absence of Campylobacter was ascertained by incubating the remaining enrichment broth microaerobically in an atmosphere of 10% CO2, 5% O2, balance N2, 100 mL volumes of nutrient broth base (Mast, Bootle, UK) with 5% horse blood, growth supplement (Mast Selectavial SV61), amphotericin (2 μg/mL), cefoperazone (15 μg/mL), and trimethoprim (10 μg/mL); polymixin B (2500 IU/L) and rifampicin (5 μg/mL) were added, and the broths incubated for 2 days at 37°C (Bull et al., 2006). All antimicrobials were purchased from Sigma-Aldrich (Gillingham, UK). Enrichment broths (0.1 mL) were plated onto mCCDA agar incubated microaerobically for 2 days at 37°C. Five colonies were taken from each plate and presumptively identified as Campylobacter microscopically (Gram stain) and by agglutination with Microscreen latex (Microgen, Camberley, UK). Individual colonies were stored (−80°C, nutrient broth, glycerol added to 15% [v/v]) for MLST. Campylobacter presence was confirmed using isolate ST as determined by MLST.
Genotyping
Archived isolates were plated from frozen onto CCD agar and incubated microaerobically for 48 h at 37°C. Bacterial DNA was prepared and MLST performed as previously described (Gormley et al., 2008). Sequences were assembled using STARS software available at http://pubmlst.org and novel alleles, and STs were submitted to the Campylobacter MLST database at this website.
Analysis of prevalence
Campylobacter prevalence values with 95% binomial confidence intervals (CI) were calculated using Microsoft Excel. Differences in prevalence values between sources were tested for statistical significance using Fisher’s exact test (www.quantitativeskills.com/sisa/statistics/twoby2.htm), and odds ratios (OR), CI, and p-values obtained. OR (Bland and Altman, 2000) were defined as the odds of a Campylobacter being found in a particular source divided by the odds of being found in a different source. An OR value >1 indicates a higher prevalence in the first source.
Analysis of microbiological enumeration
Counts were estimated for each source and the average calculated. The 95% CI were determined using a bootstrap method that involved sampling the original data with replacement. This process was repeated 10,000 times using Poptools (www.cse.csiro.au/poptools/). Enumerations were compared between all pairs of sources. Briefly, the difference of the average counts for each pair of hosts was calculated and tested for significance in Visual Basic Application under Excel (VBAE) by comparing this difference with a distribution of 10,000 differences obtained by randomizing the data without replacement (Manly, 2007).
Clonal frame
The clonal genealogy of STs was estimated using the model-based approach in determining bacterial microevolution: clonalframe (Didelot and Falush, 2007). This model calculates clonal relationships with improved accuracy distinguishing point mutations from imported chromosomal recombination events, the source of the majority of allelic polymorphisms. Analysis was carried out on concatenated sequences representing 150 STs, from 16 animal species. The program was run with 50,000 burnin followed by 50,000 subsequent iterations. The consensus trees represent combined data from three independent runs with 75% consensus required for inference of relatedness.
Analysis of diversity
Rarefaction analysis (Heck et al., 1975) was used to estimate the diversity of each of the nine animal sources (rural and urban combined) that had 10 or more MLST-typed isolates. The method was implemented in VBAE and incorporated MLST data at the levels of clonal complex (CC—a group of STs whose members are linked to at least one other member by being identical for six of the seven MLST genes) and ST. Diversity of strains between any two sources was carried out as follows. Rarefaction estimated the number of new types found in a particular number of isolates for each of the individual sources and the difference in diversity was determined by subtraction. Testing for significance was performed using a randomization test (Manly, 2007) with correction for differences in sample size. Briefly, the data from the two sources were randomized without replacement, the rarefaction was then rerun and the difference in diversity calculated. This was repeated 10,000 times. The posterior distribution of diversity differences obtained was then compared with the diversity difference calculated between the two original animal sources to obtain the level of significance (p-value).
Analysis of genetic distance between populations
Pairwise comparisons of the genetic distance between each of the nine animal sources (rural and urban combined) were calculated using the genetic distance, d1, described by Manly (1985).
where pi and qi are the proportions of a particular genotype found in each of the animal sources under comparison. When d1 = 1, there are no genotypes in common and when d1 = 0 the two animal sources have the same distribution of genotypes. Genetic distance was determined at the level of ST, CC, and allele level (for the allele level the right-hand side of the equation was modified to sum the contribution across all seven MLST alleles, which was then divided by seven). The statistical significance of the genetic distance was found using the randomization test method as described above. Analysis of molecular variance was also performed and is supplied in the Supplemental Material.
Analysis of host association
Structure software (Pritchard et al., 2000) was used to assign MLST isolates to animal source populations (rural and urban combined) based on the difference in frequency of genotypes between populations. The methodology used was a modification of that described previously (McCarthy et al., 2007). Briefly, a nonadmixture model (i.e., isolates come from a single-source population) with uncorrelated gene frequencies between populations was used. A jacknife method (15,000 iterations of the program, implemented in C++) was used in assigning unknown MLST isolates to animal sources, and the probabilities of assignment were determined. Then, 10,000 Monte Carlo steps were used to calculate the average proportion, and corresponding 95% CI, of the correct assignment to the host animal sources.
All p-values obtained by statistical techniques were corrected by sequential Bonferroni correction where multiple comparisons were carried out. This involved multiplying the p-value obtained by the number of comparisons with the resulting p-value being considered statistically significant when p < 0.05. The VBAE programs are available by request.
Results
Prevalence and microbiological enumeration
Campylobacter prevalence was lowest in dogs and highest in poultry (Table 1). The main farm animal sources (cattle, sheep, and pigs) had similar prevalence values (Table 1) and had OR not significantly different from 1.0 ( p > 0.05), whereas dogs had a lower prevalence than most other hosts (p < 0.05, Tables 1 and 2). Campylobacter concentrations were lowest in poultry and highest in ducks, sheep, pigeons, and geese. Sheep had concentrations approximately 10-fold higher than cattle (Table 1). None of these differences was significant in pairwise comparisons ( p > 0.05, data not presented).
Table 1.
Source, Location, Prevalence, and Concentrations (cfu/g) of Campylobacter in Feces from Different Hosts
| Prevalenc |
Campylobacter concentrations (cfu/g) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Host | Rural +ve/total | Urban +ve/total | Total prevalence % (95% CI)a | 0 | 100–102 | 102–103 | 103–104 | 104–105 | 105–106 | 106–107 | 107–108 | Mode | Average (95% CI)a |
| Cattle | 104/474 | – | 21.9 (18.2–25.7) | 369 | 80 | 2 | 10 | 5 | 1 | 0 | 1 | 4×102 | 3×104 (2×102–8×104) |
| Sheep | 97/292 | – | 24.9 (20.6–29.2) | 291 | 52 | 5 | 13 | 12 | 8 | 1 | 6 | 2×105 | 2×105 (7×104–4×105) |
| Pig | 27/101 | – | 26.7 (18.1–35.3) | 72 | 9 | 4 | 5 | 5 | 4 | 1 | 0 | 3×104 | 3×104 (3×103–7×104) |
| Birdb | 11/34 | 16/80 | 23.7 (15.9–31.5) | 86 | 17 | 2 | 3 | 2 | 3 | 0 | 0 | 8×103 | 7×103 (1×103–2×104) |
| Duck | 1/3 | 11/43 | 26.1 (13.4–38.8) | 31 | 10 | 0 | 2 | 0 | 0 | 2 | 0 | 2×105 | 4×105 (5×101–1×106) |
| Goose | 4/12 | 13/56 | 25.0 (14.7–35.3) | 49 | 13 | 1 | 2 | 0 | 0 | 2 | 0 | 2×105 | 2×105 (1×102–4×105) |
| Gull | 1/1 | 47/215 | 22.2 (16.7–27.8) | 165 | 38 | 2 | 3 | 2 | 2 | 2 | 1 | 2×104 | 6×104 (4×103–2×105) |
| Pigeon | 2/13 | 69/242 | 27.8 (22.3–33.3) | 181 | 58 | 1 | 5 | 3 | 4 | 1 | 1 | 2×105 | 2×105 (3×103–4×105) |
| Poultry | 21/51 | 3/7 | 41.4 (28.7–54.1) | 33 | 20 | 0 | 4 | 0 | 0 | 0 | 0 | 1×102 | 2×102 (5×101–4×102) |
| Cat | 0/6 | 2/38 | 4.5 (0–10.7) | ||||||||||
| Dog | 0/11 | 1/66 | 1.3 (0–2.8) | ||||||||||
| Swan | – | 4/39 | 10.3 (1–19.8) | ||||||||||
| Otherc | 8/36 | 1/14 | 18.0 (0.07–28.6) | ||||||||||
Confidence interval calculated by the bootstrap method.
Bird denotes samples of avian origin but unknown species.
Other includes crow (1), deer (2), donkey (1), ferret (1), fly (3), fox (1), goat (4), hedgehog (1), horse (12), jackdaw (1), llama (1), pheasant (8), rabbit (5), rook (4), sparrow (1), starling (2), swallow (1), and toad (1). The Campylobacter-positive isolates were crow (1), horse (1), jackdaw (1), pheasant (5), and starling (1).
CI, confidence intervals.
Table 2.
Comparisons of Campylobacter Prevalence Among Hosts
| Comparison | OR | 95% CI | p |
|---|---|---|---|
| Cattle–poultry | 0.40 | 0.23–0.70 | 0.047 |
| Cattle–dog | 21.36 | 2.94–155.50 | <0.001 |
| Sheep–dog | 25.25 | 3.46–183.99 | <0.001 |
| Pigs–dog | 27.73 | 3.67–209.34 | <0.001 |
| Birds–dog | 23.59 | 3.13–177.72 | <0.001 |
| Duck–dog | 26.28 | 3.35–214.65 | 0.002 |
| Goose–dog | 25.33 | 3.27–196.35 | <0.001 |
| Gull–dog | 21.71 | 2.94–166.25 | <0.001 |
| Pigeon–cat | 8.10 | 1.91–34.36 | 0.018 |
| Pigeon–dog | 29.33 | 4.00–214.92 | <0.001 |
| Poultry–cat | 14.28 | 3.27–67.22 | <0.001 |
| Poultry–dog | 53.65 | 6.97–412.94 | <0.001 |
| Poultry–swan | 6.18 | 1.94–19.68 | 0.005 |
OR is defined as the ratio of the odds of a Campylobacter-positive isolate occurring in a given host divided by the odds of a positive isolate occurring in a second host. 95% CI, the table only shows comparisons that are statistically significant using Fisher’s exact test after sequential Bonferroni correction for multiple comparisons (n=78).
Clonal frame
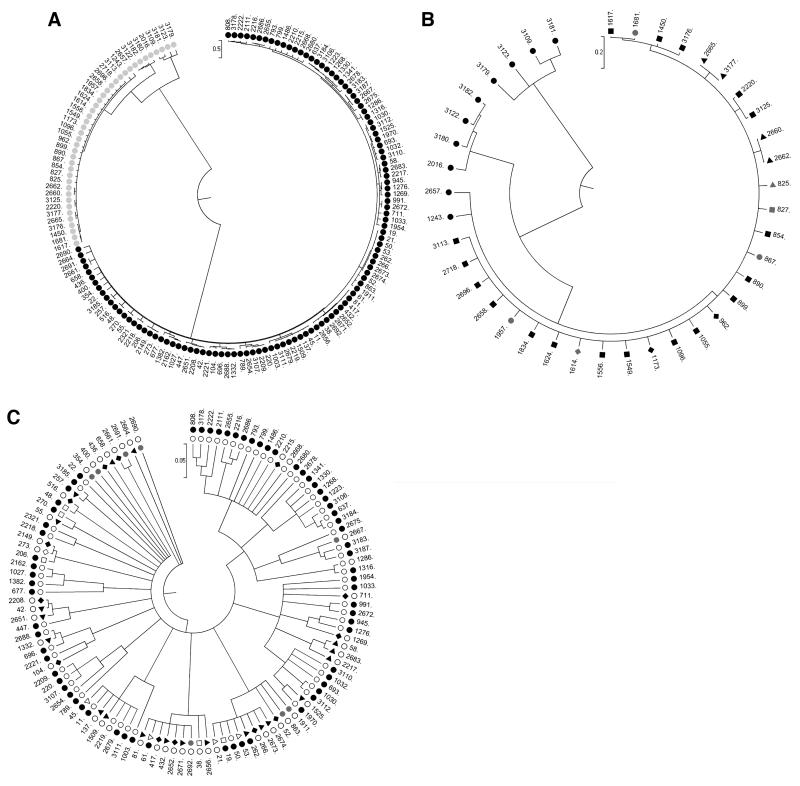
Clonal frame readily distinguished C. jejuni from C. coli STs (Fig. 1A). The C. coli tree consisted of one major and three minor clades (Fig. 1B, for ease of viewing the STs from all avian species except poultry are represented by black circles). All minor clades comprised exclusively avian species excluding poultry. The main clade had pig and poultry STs widely distributed and also contained cattle, avian, and sheep STs. Hence, the C. coli tree provides evidence of genetic segregation/host specificity between animal sources. The C. jejuni tree (Fig. 1C) comprised one major clade and one minor (with cattle, sheep, and poultry STs) clade. The major clade had a broad distribution of avian species excluding poultry STs. The sheep and poultry STs were widely distributed but where avian (excluding poultry) STs are common other animal sources are rare, and vice-versa. This further demonstrates genetic differences between animal sources.
FIG. 1.
(A) Trees generated by clonalframe of the 442 Campylobacter jejuni (black) and Campylobacter coli (gray) haplotypes (concatenated multilocus sequence typing alleles). (B) Magnified C. coli tree showing distribution of host STs (avian excluding poultry, black circle; cattle, black triangle; pigs, black square; sheep, black diamond; poultry, gray circle; poultry + gull, gray triangle; poultry + sheep + cattle + gull, gray square; poultry + sheep, gray diamond). (C) Magnified C. jejuni tree outer ring showing all avian isolates but not poultry, inner ring showing remainder of isolates (colors as in (B) but cattle + sheep + poultry combined, white triangle; cattle + sheep combined, white square; sheep + poultry, white diamond; and pets, black inverted triangle). ST, sequence type.
Strain diversity
None of the ST rarefaction curves reach a plateau indicating that the ST diversity detected in each host within this study is a proportion of the total diversity that that particular host excretes into the environment (Fig. 2B). In contrast, the CC rarefaction curves (Fig. 2B) for cattle and sheep show more evidence of a plateau indicating that a high proportion of the total CC diversity from these hosts was sampled. The rare-faction suggests that pigeons have the lowest MLST diversity, while a number of hosts (e.g., other birds) appear to have high diversity. Pairwise comparisons of diversity between hosts (Table 3) show that sheep, pigs, and pigeons have a lower diversity than unidentified birds ( p < 0.05). At the ST level, only pigeons have a lower diversity than birds and geese but here poultry shows a greater diversity than pigeons ( p < 0.05). The relatively high diversity in birds at both CC and ST levels is not surprising since birds will comprise a number of avian species that are likely to have a number of host-associated MLST types.
FIG. 2.
Rarefaction curves showing diversity by clonal complex (A) and ST (B) for animal sources with 10 or more multilocus sequence typing–typed Campylobacter isolates.
Table 3.
Statistically Significant Pairwise Comparisons of Campylobacter Diversity Between Animal Sources Determined by Rarefaction at the Level of Clonal Complex and Sequence Type
| CC |
ST |
||
|---|---|---|---|
| Comparison | p-Value | Comparison | p-Value |
| Sheep<birds | 0.0144 | ||
| Sheep<geese | 0.0360 | ||
| Pigs<birds | 0.0216 | ||
| Pigs<geese | 0.0396 | ||
| Pigs>pigeons | 0.0036 | ||
| Birds>pigeons | 0.0036 | Birds>pigeons | 0.0036 |
| Geese>pigeons | 0.0108 | ||
| Pigeons<poultry | 0.0036 | ||
The table denotes which animal source has greater diversity of Campylobacter and associated p-value after Bonferroni correction (n=36). The p-values are determined by a randomization test.
CC, clonal complex; ST, sequence type.
Genetic distance
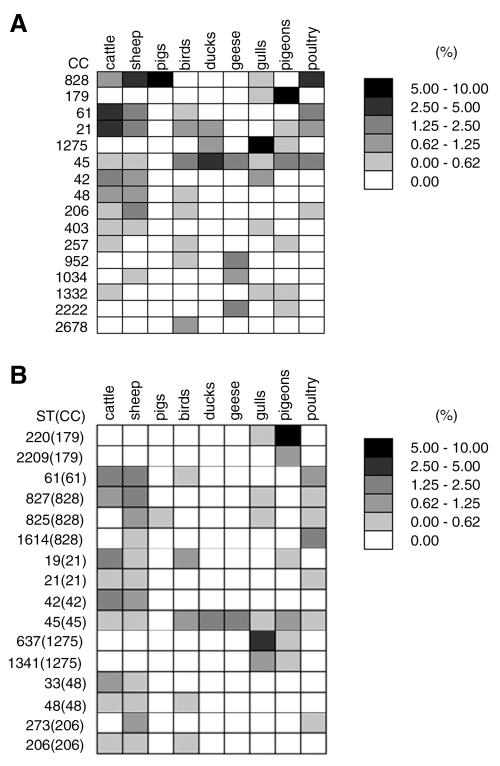
Table 4 presents standardized pairwise genetic distances between animal sources at the levels of CC, ST, and allele. The greatest number of statistically significant pairwise differences was found at the allele level (28/36) compared with 24 at the level of CC and 19 by ST. Ducks and poultry generate the lowest number of significant pairwise differences (8 and 10) when comparing all three levels of the analysis. Pigeons were found to be significantly different from the other animal sources at all three levels with CC-179 dominating (Fig. 3) and being only found occasionally elsewhere (in gulls). Results from analysis of molecular variance show similar pairwise differences (see Supplemental Material as was obtained using the standardized genetic distance.
Table 4.
Genetic Distances (Manly, 1985) Within Campylobacter Reservoirs Calculated at the Clonal Complex, Sequence Type, and Allele Level
| Level | Group | Sheep | Pigs | Birds | Ducks | Geese | Gulls | Pigeon | Poultry |
|---|---|---|---|---|---|---|---|---|---|
| CC | Cattle | 0.389 | 0.913 | 0.754 | 0.890 | 0.971 | 0.821 | 0.940 | 0.660 |
| Sheep | 0.679 | 0.771 | 0.907 | 0.961 | 0.861 | 0.965 | 0.469 | ||
| Pig | 1.000 | 1.000 | 1.000 | 0.959 | 1.000 | 0.708 | |||
| Bird | 0.756 | 0.848 | 0.959 | 0.846 | 0.719 | ||||
| Duck | 0.882 | 0.855 | 0.851 | 0.812 | |||||
| Goose | 0.959 | 0.897 | 0.895 | ||||||
| Gull | 0.895 | 0.918 | |||||||
| Pigeon | 0.888 | ||||||||
| ST | Cattle | 0.527 | 1.000 | 0.814 | 0.981 | 0.981 | 0.945 | 0.967 | 0.825 |
| Sheep | 0.965 | 0.848 | 0.990 | 0.989 | 0.938 | 0.976 | 0.729 | ||
| Pig | 1.000 | 1.000 | 1.000 | 0.980 | 1.000 | 0.963 | |||
| Bird | 0.859 | 0.902 | 0.958 | 0.904 | 0.884 | ||||
| Duck | 0.882 | 0.958 | 0.933 | 0.958 | |||||
| Goose | 0.958 | 0.921 | 0.958 | ||||||
| Gull | 0.910 | 0.927 | |||||||
| Pigeon | 0.963 | ||||||||
| Allele | Cattle | 0.323 | 0.886 | 0.693 | 0.869 | 0.854 | 0.817 | 0.784 | 0.568 |
| Sheep | 0.688 | 0.722 | 0.886 | 0.859 | 0.853 | 0.846 | 0.441 | ||
| Pig | 0.995 | 1.000 | 0.966 | 0.961 | 1.000 | 0.699 | |||
| Bird | 0.713 | 0.701 | 0.823 | 0.801 | 0.662 | ||||
| Duck | 0.749 | 0.846 | 0.863 | 0.821 | |||||
| Goose | 0.856 | 0.855 | 0.819 | ||||||
| Gull | 0.823 | 0.856 | |||||||
| Pigeon | 0.823 |
The figures in bold represent that the differences between the groups are significant at the p<0.05 level following sequential Bonferroni correction (n=36). p-Values are determined by a randomization test.
FIG. 3.
Common Campylobacter clonal complex (A) and ST (B) types (% of total dataset), each animal source is normalized by its representative number of isolates and the sum of all the grid elements in each figure totals 100% (birds are avian samples of unknown host species).
Host association
Figure 3 illustrates the distribution of types by host at the levels of CC and ST. Table 5 shows the host assignment accuracy from structure. Overall, each of the three levels (CC, ST, and allele) of analysis demonstrated better classification accuracy than would be expected at random (11.1%) with allele level analysis performing best (54.7%). Poultry exhibited the lowest classification accuracy (12.5–20.9%), while pigeons showed the greatest host association (60.1–77.5%). Farm animal sources (cattle and pigs) gave average (18.4–30.0%) classification by CC and ST but exhibited substantially higher host association at the allele level. Sheep gave similar CC and ST values as cattle and pigs, but the allele level showed only a moderate increase (40.2%).
Table 5.
Correct Assignment to Host by Structure at the Level of Clonal Complex, Sequence Type, and Allele
| Source | CC | ST | Allele |
|---|---|---|---|
| Cattle | 30.0 (28.7–31.3) | 27.3 (25.8–28.7) | 65.2 (64.2–66.2) |
| Sheep | 26.8 (25.5–28.1) | 27.6 (26.1–29.1) | 40.2 (39.1–41.2) |
| Pig | 26.8 (24.4–29.4) | 18.4 (15.9–21.1) | 94.6 (93.6–95.7) |
| Bird | 13.8 (11.9–15.7) | 12.8 (10.8–15.1) | 30.4 (28.7–32.2) |
| Duck | 13.6 (10.9–16.4) | 14.4 (11.1–18.2) | 40.9 (38.4–43.2) |
| Goose | 16.5 (13.9–19.1) | 14.9 (12.0–17.8) | 58.4 (56.1–60.5) |
| Gull | 44.3 (42.7–46.0) | 31.2 (29.2–33.2) | 64.3 (63.5–65.1) |
| Pigeon | 66.1 (64.8–67.3) | 60.1 (58.5–61.7) | 77.5 (77.1–77.8) |
| Poultry | 12.5 (10.5–14.5) | 12.7 (10.4–15.1) | 20.9 (19.7–22.1) |
| Average | 27.8 | 24.4 | 54.7 |
Discussion
Structure showed that host association was strongest at the allele level, in agreement with previous work (McCarthy et al., 2007). Here, however, an accuracy of 55% is obtained for identifying nine animal sources, whereas in the McCarthy study a value of 58% was reported for three sources (cattle, sheep, and pigs). The isolates in the current study were obtained over a discrete time interval (15 months) and within a restricted geographical area (specific areas within Scotland) that may partly explain the relatively high accuracy for the large number of animal sources. In addition, the wider range of animal sources studied here appear to have a number of genotypes that show host restriction or appear far more frequently in one source than in others (e.g., in gulls and pigeons; Fig. 3). The reasons for this may be attributed to mechanisms of isolation, host adaptation, or genetic drift. Further, an additional analysis was carried out (data not presented) applying structure solely to cattle and sheep isolates and a host association of 65% was obtained. This is closer to that obtained in previous studies (McCarthy et al., 2007). This suggests that the Campylobacter types found within cattle and sheep are more similar to each other than, for example, between bovids and wild birds.
At all three levels of analysis (CC, ST, and allele) poultry isolates exhibit the poorest degree of host association of all animal sources tested. However, the number of poultry isolates is small (n = 24), but the results do indicate that poultry may have the ability to acquire Campylobacter from a number of different animal sources. Pigs exhibited a very high degree of host association at the allele level (95%) compared with the ST (18%) and CC (27%) levels. Pig isolates were predominantly C. coli (CC-828) but exhibited a number of alleles on the tkt locus (data not presented) that are either absent or rare in C. coli isolates from other animal sources (e.g., sheep and cattle). These pig-associated alleles include tkt-35 and tkt-44 that have previously been found to be associated with pigs (Miller et al., 2006).
The lineages identified here for C. jejuni and C. coli are in agreement with previous work (Sheppard et al., 2008) that also demonstrated that C. coli has one major and a number of minor clades. Within both species, host association is apparent through the clustering of STs and this is particularly apparent for the avian (excluding poultry) hosts, originally reported by French (2007).
The rarefaction analysis indicates that only a relatively small proportion of full genetic diversity has been tested. This is particularly the case at the ST level. This gives an indication of the sampling intensity required to instigate a surveillance program to monitor the Campylobacter population in animal hosts which is essential to understand whether Campylobacter types remain relatively stable within a population or are subject to change over time as can readily be observed with both Salmonella (Cogan and Humphrey, 2003) and Campylobacter (Gormley et al., 2008) in poultry. Further, a comprehensive survey has the potential to elucidate how strains may be circulating and evolving in animal populations and which subsequently cause disease in humans by either foodborne or environmental routes.
The CC most commonly found in gulls (CC-1275) has also been reported in gulls from Sweden and Australia (PubMLST database). A number of the STs from these international gull isolates were found in the current study but the most common, ST-637 in the CC-1275 complex here, has only been reported previously from a chicken in Denmark. It is also worth noting that in the current study this ST was found in all three cities demonstrating its widespread nature in Scotland. The pigeon-associated CC (CC-179) has previously been reported (PubMLST database) in sand from UK bathing beaches and from wild birds of UK origin. The two most common STs (ST-220 and ST-2209) have been found in bathing beach sand and wild birds (including a pigeon), respectively. In the current study this CC dominated the pigeon isolates from all three cities as well as the rural isolates indicating its ubiquity across Scotland. A previous study (French et al., 2005) of Campylobacter in a rural farming environment suggested that ST-61 was overrepresented in cattle compared to birds, rabbit, and water isolates. This agrees with the current study that shows that ST-61 is more common in cattle than pigeons and gulls (see Supplemental Material. The PubMLST database also shows that this ST is well represented in both cattle and sheep but is not present in wild birds. The C. coli CC-828 has previously been reported to dominate Campylobacter in pigs (Rosef et al., 1983) which is in agreement with results here. It has also been reported (Oporto et al., 2007) that C. coli is more common in sheep than cattle, again in agreement with results here. C. coli is rare in all the avian species (2/175 isolates) reported here except poultry (7/24 isolates).
Campylobacter MLST types in poultry here are not consistent with those from retail UK chicken from NE Scotland (Gormley et al., 2008). For example, retail chicken data showed two dominant STs (ST-257 and ST-2030) that are absent from the poultry isolates obtained in the current study. This may indicate that strains found in farmyard poultry may not be representative of those in retail poultry where birds are typically farmed in large units, often exceeding 10,000 birds. Further, the source of retail chicken may originate from another part of the United Kingdom. Alternately, the samples tested here may not be representative of the overall population of STs found in poultry, supported by the rarefaction curve (Fig. 3) that does not plateau suggesting that additional sampling would yield further STs.
Both the prevalence and counts of Campylobacter in sheep were similar to those reported in previous studies in England (Jones et al., 1999) and Northern Ireland (Madden et al., 2007). However, these values are substantially lower than found in New Zealand (Devane et al., 2005) where prevalences of 76% (sheep), 84–98% (cattle), and 65% (ducks) were reported. These higher prevalence figures may be a factor in the high incidence rates of human campylobacteriosis in New Zealand. Further, we found that Scottish sheep had a number of high shedding (>104/g) animals and it has been reported (Matthews et al., 2006) that for organisms such as E. coli O157 these “high shedding animals” are considered to be pivotal in maintaining and propagating infection within an animal group. Target enumeration therefore provides useful information on the dynamics of Campylobacter in the farm environment. The very low prevalence reported here for C. coli and C. jejuni in healthy cats (4.5%) and dogs (1.3%) is in agreement with data reported in cats (2%) (Bender et al., 2005) and dogs (0.8%) (Hackett and Lappin, 2003) in the United States and in cats (4%) and dogs (9%) in Australia (Baker et al., 1999).
The number of samples of farmyard poultry is low because free range hens are uncommon in the areas sampled in this study. Currently, the majority of poultry in Scotland (and the United Kingdom) are broilers housed in large sheds. Hence, the amount of fresh fecal material from poultry is not widely distributed (>95% is incinerated at the end of each growout in Scotland) and as such may play a lesser direct role in transmitting Campylobacter in the environment. The prevalence of Campylobacter in farmyard poultry (41%) was considerably less than observed in retail poultry (90%) (Gormley et al., 2008) suggesting that poultry in broiler houses have a higher prevalence or that there is substantial cross contamination during processing as previously reported (Allen et al., 2007). The farmyard poultry fecal counts are low compared with broilers (Nauta et al., 2007) and this may be a function of stocking density or perhaps age of the birds. Further, the farmyard poultry fecal counts are of a similar magnitude to that reported on retail poultry meat (majority approximately 102 cfu/g; Gormley et al., 2008). However, human infection is more likely through retail poultry as the prevalence is higher and the likelihood of exposure is greater (e.g., through undercooking, cross contamination of other foods in the kitchen as well as direct human contact with chicken meat being more frequent than contact with chicken feces).
The levels of host association reported here together with comprehensive data on prevalence and counts offer the potential of source tracking campylobacter strains through the food chain. This would enable the attribution of human cases to animal hosts and food sources at the population level, currently established for Salmonella (Hald et al., 2004). Indeed, a number of papers have recently been published on Campylobacter applying source attribution methods (Wilson et al., 2008; Mullner et al., 2009; Sheppard et al., 2009; Strachan et al., 2009). These workers demonstrate that chicken is the main source of infection but that strains from other sources, in particular ruminants but also to a lesser extent wild birds, play a role in infection. Further it has been reported that in rural children there is an excess of cases and that this is due predominantly to nonpoultry sources (French et al., 2009; Strachan et al., 2009). It should be noted that the etiology of Campylobacter is complex and human disease is a consequence of multiple infection pathways including environmental contact (e.g., contaminated drinking water or from farm animals), infection acquired from abroad in addition to food. Consideration should also be given to the point that the infectivity of Campylobacter types from different hosts or environments may vary.
There is an ongoing requirement to monitor Campylobacter in the environment because the abundance of the major MLST types can change with time (Gormley et al., 2008). It is also likely that the sizes of the reservoirs excreting the organism into the environment will change with time (e.g., UK pig numbers are currently declining) and there is a need for the assessment of agricultural interventions to reduce the burden of Campylobacter (e.g., providing advice on dealing with manure), food animals (e.g., improving biosecurity in broiler houses), and food products. We advise that the characterization of Campylobacter in the environment as described here should be seen as a preliminary step in understanding the ecology of this organism. The information on the strain types found in different animal hosts together with the prevalence and enumeration data can be used as a basis to develop models that examine the maintenance and cycling of Campylobacter in the environment (Skelly and Weinstein, 2003).
Supplementary Material
Acknowledgments
The Food Standards Agency, Scotland, wholly funded this work. The Scottish Agricultural College collected rural samples from SW Scotland. This publication made use of the C. jejuni MLST website (http://pubmlst.org/campylobacter/) developed by Keith Jolley and Man-Suen Chan and sited at the University of Oxford.
Footnotes
Disclosure Statement: No competing financial interests exist.
References
- Allen VM, Bull SA, Corry JE, Domingue G, Jorgensen F, Frost JA, Whyte R, Gonzalez A, Elviss N, Humphrey TJ. Campylobacter spp. contamination of chicken carcasses during processing in relation to flock colonisation. Int J Food Microbiol. 2007;113:54–61. doi: 10.1016/j.ijfoodmicro.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Baker J, Barton MD, Lanser J. Campylobacter species in cats and dogs in South Australia. Aust Vet J. 1999;77:662–666. doi: 10.1111/j.1751-0813.1999.tb13159.x. [DOI] [PubMed] [Google Scholar]
- Bender JB, Shulman SA, Averbeck GA, Pantlin GC, Stromberg BE. Epidemiologic features of Campylobacter infection among cats in the upper midwestern United States. J Am Vet Med Assoc. 2005;226:544–547. doi: 10.2460/javma.2005.226.544. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistics notes. The odds ratio. BMJ. 2000;320:1468. doi: 10.1136/bmj.320.7247.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boes J, Nersting L, Nielsen EM, Kranker S, Enoe C, Wachmann HC, Baggesen DL. Prevalence and diversity of Campylobacter jejuni in pig herds on farms with and without cattle or poultry. J Food Prot. 2005;68:722–727. doi: 10.4315/0362-028x-68.4.722. [DOI] [PubMed] [Google Scholar]
- Bolton FJ, Surman SB, Martin K, Wareing DR, Humphrey TJ. Presence of Campylobacter and Salmonella in sand from bathing beaches. Epidemiol Infect. 1999;122:7–13. doi: 10.1017/s0950268898001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull SA, Allen VM, Domingue G, Jorgensen F, Frost JA, Ure R, Whyte R, Tinker D, Corry JE, Gillard-King J, Humphrey TJ. Sources of Campylobacter spp. colonizing housed broiler flocks during rearing. Appl Environ Microbiol. 2006;72:645–652. doi: 10.1128/AEM.72.1.645-652.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food—10 States, 2006. MMWR Morb Mortal Wkly Rep. 2007;56:336–339. [PubMed] [Google Scholar]
- Cogan TA, Humphrey TJ. The rise and fall of Salmonella Enteritidis in the UK. J Appl Microbiol. 2003;94(Suppl):114S–119S. doi: 10.1046/j.1365-2672.94.s1.13.x. [DOI] [PubMed] [Google Scholar]
- Colles FM, Jones K, Harding RM, Maiden MC. Genetic diversity of Campylobacter jejuni isolates from farm animals and the farm environment. Appl Environ Microbiol. 2003;69:7409–7413. doi: 10.1128/AEM.69.12.7409-7413.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowden J. Campylobacter: epidemiological paradoxes. BMJ. 1992;305:132–133. doi: 10.1136/bmj.305.6846.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane ML, Nicol C, Ball A, Klena JD, Scholes P, Hudson JA, Baker MG, Gilpin BJ, Garrett N, Savill MG. The occurrence of Campylobacter subtypes in environmental reservoirs and potential transmission routes. J Appl Microbiol. 2005;98:980–990. doi: 10.1111/j.1365-2672.2005.02541.x. [DOI] [PubMed] [Google Scholar]
- Didelot X, Falush D. Inference of bacterial microevolution using multilocus sequence data. Genetics. 2007;175:1251–1266. doi: 10.1534/genetics.106.063305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle KE, Colles FM, Wareing DR, Ure R, Fox AJ, Bolton FE, Bootsma HJ, Willems RJ, Urwin R, Maiden MC. Multi-locus sequence typing system for Campylobacter jejuni. J Clin Microbiol. 2001a;39:14–23. doi: 10.1128/JCM.39.1.14-23.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle KE, Van Den Braak N, Colles FM, Price LJ, Woodward DL, Rodgers FG, Endtz HP, Van Belkum A, Maiden MC. Sequence typing confirms that Campylobacter jejuni strains associated with Guillain-Barre and Miller-Fisher syndromes are of diverse genetic lineage, serotype, and flagella type. J Clin Microbiol. 2001b;39:3346–3349. doi: 10.1128/JCM.39.9.3346-3349.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart-Phillips J, Walker N, Garrett N, Bell D, Sinclair D, Rainger W, Bates M. Campylobacteriosis in New Zealand: results of a case-control study. J Epidemiol Community Health. 1997;51:686–691. doi: 10.1136/jech.51.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethelberg S, Simonsen J, Gerner-Smidt P, Olsen KE, Molbak K. Spatial distribution and registry-based case-control analysis of Campylobacter infections in Denmark, 1991–2001. Am J Epidemiol. 2005;162:1008–1015. doi: 10.1093/aje/kwi316. [DOI] [PubMed] [Google Scholar]
- Evans MR, Ribeiro CD, Salmon RL. Hazards of healthy living: bottled water and salad vegetables as risk factors for Campylobacter infection. Emerg Infect Dis. 2003;9:1219–1225. doi: 10.3201/eid0910.020823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French N. Campylobacter: Diversity, host specificity and population biology. Zoonoses Public Health. 2007;54(Suppl 1):9–10. [Google Scholar]
- French N, Barrigas M, Brown P, Ribiero P, Williams N, Leatherbarrow H, Birtles R, Bolton E, Fearnhead P, Fox A. Spatial epidemiology and natural population structure of Campylobacter jejuni colonizing a farmland ecosystem. Environ Microbiol. 2005;7:1116–1126. doi: 10.1111/j.1462-2920.2005.00782.x. [DOI] [PubMed] [Google Scholar]
- French NP, Midwinter A, Holland B, Collins-Emerson J, Pattison R, Colles F, Carter P. Molecular epidemiology of Campylobacter jejuni isolates from wild-bird fecal material in children’s playgrounds. Appl Environ Microbiol. 2009;75:779–783. doi: 10.1128/AEM.01979-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman CR, Hoekstra RM, Samuel M, Marcus R, Bender J, Shiferaw B, Reddy S, Ahuja SD, Helfrick DL, Hardnett F, Carter M, Anderson B, Tauxe RV. Emerging Infections Program FoodNet Working Group. Risk factors for sporadic Campylobacter infection in the United States: a case-control study in FoodNet sites. Clin Infect Dis. 2004;38(Suppl 3):S285–S296. doi: 10.1086/381598. [DOI] [PubMed] [Google Scholar]
- Frost JA, Gillespie IA, O’Brien SJ. Public health implications of Campylobacter outbreaks in England and Wales, 1995–9: epidemiological and microbiological investigations. Epidemiol Infect. 2002;128:111–118. doi: 10.1017/s0950268802006799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormley FJ, Macrae M, Forbes KJ, Ogden ID, Dallas JF, Strachan NJ. Has retail chicken played a role in the decline of human campylobacteriosis? Appl Environ Microbiol. 2008;74:383–390. doi: 10.1128/AEM.01455-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett T, Lappin MR. Prevalence of enteric pathogens in dogs of north-central Colorado. J Am Anim Hosp Assoc. 2003;39:52–56. doi: 10.5326/0390052. [DOI] [PubMed] [Google Scholar]
- Hald T, Vose D, Wegener HC, Koupeev T. A Bayesian approach to quantify the contribution of animal-food sources to human salmonellosis. Risk Anal. 2004;24:255–269. doi: 10.1111/j.0272-4332.2004.00427.x. [DOI] [PubMed] [Google Scholar]
- Heck KL, Van Belle G, Simberloff D. Explicit calculation of the rarefaction diversity measurement and the determination of sufficient sample size. Ecology. 1975;56:1459–1461. [Google Scholar]
- Jones K. Campylobacter in water, sewage and the environment. J Appl Microbiol. 2001;90:1–12. doi: 10.1046/j.1365-2672.2001.01355.x. [DOI] [PubMed] [Google Scholar]
- Jones K, Howard S, Wallace JS. Intermittent shedding of thermophilic campylobacters by sheep at pasture. J Appl Microbiol. 1999;86:531–536. doi: 10.1046/j.1365-2672.1999.00702.x. [DOI] [PubMed] [Google Scholar]
- Kapperud G, Espeland G, Wahl E, Walde A, Herikstad H, Gustavsen S, Tveit I, Natas O, Bevanger L, Digranes A. Factors associated with increased and decreased risk of Campylobacter infection: a prospective case-control study in Norway. Am J Epidemiol. 2003;158:234–242. doi: 10.1093/aje/kwg139. [DOI] [PubMed] [Google Scholar]
- Lee MK, Billington SJ, Joens LA. Potential virulence and antimicrobial resistance in Campylobacter jejuni isolates obtained from food and companion animals. Foodborne Pathog Dis. 2004;1:223–230. doi: 10.1089/fpd.2004.1.223. [DOI] [PubMed] [Google Scholar]
- Locking M, Browning L, Smith-Palmer A, Brownlie S. Gastro-intestinal and foodborne infections. Health Prot Scotl Wkly Rep. 2007;41:3–4. [Google Scholar]
- Luber P, Bartelt E. Enumeration of Campylobacter spp. on the surface and within chicken breast fillets. J Appl Microbiol. 2007;102:313–318. doi: 10.1111/j.1365-2672.2006.03105.x. [DOI] [PubMed] [Google Scholar]
- Madden RH, Murray KA, Gilmour A. Carriage of four bacterial pathogens by beef cattle in Northern Ireland at time of slaughter. Lett Appl Microbiol. 2007;44:115–119. doi: 10.1111/j.1472-765X.2006.02064.x. [DOI] [PubMed] [Google Scholar]
- Manly BFJ. The Statistics of Natural Selection on Animal Populations. Chapman and Hall; New York: 1985. [Google Scholar]
- Manly BFJ. Randomization, Bootsrap and Monte Carlo Methods in Biology. Chapman & Hall/CRC; Boca Raton, FL: 2007. [Google Scholar]
- Matthews L, McKendrick IJ, Ternent H, Gunn GJ, Synge B, Woolhouse ME. Super-shedding cattle and the transmission dynamics of Escherichia coli O157. Epidemiol Infect. 2006;134:131–142. doi: 10.1017/S0950268805004590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy ND, Colles FM, Dingle KE, Bagnall MC, Manning G, Maiden MCJ, Falush D. Host-associated genetic import in Campylobacter jejuni. Emerg Infect Dis. 2007;13:267–272. doi: 10.3201/eid1302.060620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffin PM, Tauxe RV. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud S, Menard S, Arbeit RD. Campylobacteriosis, eastern townships, Quebec. Emerg Infect Dis. 2004;10:1844–1847. doi: 10.3201/eid1010.040228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WG, Englen MD, Kathariou S, Wesley IV, Wang G, Pittenger-Alley L, Siletz RM, Muraoka W, Fedorka-Cray PJ, Mandrell RE. Identification of host-associated alleles by multilocus sequence typing of Campylobacter coli strains from food animals. Microbiology. 2006;152:245–255. doi: 10.1099/mic.0.28348-0. [DOI] [PubMed] [Google Scholar]
- Miller WG, Mandrell RE. Prevalence of Campylobacter in the food and water supply: incidence, outbreaks, isolation and detection. In: Ketley JM, Konkel ME, editors. Campylobacter: Molecular and Cellular Biology. Horizon Bioscience Press; Norfolk, UK: 2005. pp. 101–163. [Google Scholar]
- Moore JE, Corcoran D, Dooley JS, Fanning S, Lucey B, Matsuda M, McDowell DA, Megraud F, Millar BC, O’Mahony R, O’Riordan L, O’Rourke M, Rao JR, Rooney PJ, Sails A, Whyte P. Campylobacter. Vet Res. 2005;36:351–382. doi: 10.1051/vetres:2005012. [DOI] [PubMed] [Google Scholar]
- Mullner PG, Noble A, Spencer CEF, Hathaway S, French NP. Source attribution of food-borne zoonoses in New Zealand: a modified Hald model. Risk Anal. 2009;29:970–984. doi: 10.1111/j.1539-6924.2009.01224.x. [DOI] [PubMed] [Google Scholar]
- Nauta MJ, Jacobs-Reitsma WF, Havelaar AH. A risk assessment model for Campylobacter in broiler meat. Risk Anal. 2007;27:845–861. doi: 10.1111/j.1539-6924.2006.00834.x. [DOI] [PubMed] [Google Scholar]
- Neimann J, Engberg J, Molbak K, Wegener HC. A case-control study of risk factors for sporadic Campylobacter infections in Denmark. Epidemiol Infect. 2003;130:353–366. doi: 10.1017/s0950268803008355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson SK, Ethelberg S, van Pelt W, Tauxe RV. Epidemiology of Campylobacter jejuni infections in industrialised nations. In: Nachamkin I, Szymanski CM, Blaser MJ, editors. Campylobacter jejuni: Current and Future Trends. ASM Press; Herndon, VA: 2008. pp. 163–190. [Google Scholar]
- Oporto B, Esteban JI, Aduriz G, Juste RA, Hurtado A. Prevalence and strain diversity of thermophilic campylobacters in cattle, sheep and swine farms. J Appl Microbiol. 2007;103:977–984. doi: 10.1111/j.1365-2672.2007.03328.x. [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues LC, Cowden JM, Wheeler JG, Sethi D, Wall PG, Cumberland P, Tompkins DS, Hudson MJ, Roberts JA, Roderick PJ. The study of infectious intestinal disease in England: risk factors for cases of infectious intestinal disease with Campylobacter jejuni infection. Epidemiol Infect. 2001;127:185–193. doi: 10.1017/s0950268801006057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosef O, Gondrosen B, Kapperud G, Underdal B. Isolation and characterization of Campylobacter jejuni and Campylobacter coli from domestic and wild mammals in Norway. Appl Environ Microbiol. 1983;46:855–859. doi: 10.1128/aem.46.4.855-859.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria J, Toranzos GA. Enteric pathogens and soil: a short review. Int Microbiol. 2003;6:5–9. doi: 10.1007/s10123-003-0096-1. [DOI] [PubMed] [Google Scholar]
- Schonberg-Norio D, Takkinen J, Hanninen ML, Katila ML, Kaukoranta SS, Mattila L, Rautelin H. Swimming and Campylobacter infections. Emerg Infect Dis. 2004;10:1474–1477. doi: 10.3201/eid1008.030924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard SK, Dallas JF, Strachan NJ, MacRae M, McCarthy ND, Wilson DJ, Gormley FJ, Falush D, Ogden ID, Maiden MC, Forbes KJ. Campylobacter genotyping to determine the source of human infection. Clin Infect Dis. 2009;48:1072–1078. doi: 10.1086/597402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard SK, McCarthy ND, Falush D, Maiden MC. Convergence of Campylobacter species: implications for bacterial evolution. Science. 2008;320:237–239. doi: 10.1126/science.1155532. [DOI] [PubMed] [Google Scholar]
- Skelly C, Weinstein P. Pathogen survival trajectories: an eco-environmental approach to the modeling of human campylobacteriosis ecology. Environ Health Perspect. 2003;111:19–28. doi: 10.1289/ehp.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley K, Cunningham R, Jones K. Isolation of Campylobacter jejuni from groundwater. J Appl Microbiol. 1998;85:187–191. doi: 10.1046/j.1365-2672.1998.00494.x. [DOI] [PubMed] [Google Scholar]
- Stanley K, Jones K. Cattle and sheep farms as reservoirs of Campylobacter. J Appl Microbiol. 2003;94(Suppl):104S–113S. doi: 10.1046/j.1365-2672.94.s1.12.x. [DOI] [PubMed] [Google Scholar]
- Strachan NJ, Gormley FJ, Rotariu O, Ogden ID, Miller G, Dunn GM, Sheppard SK, Dallas JF, Reid TM, Howie H, Maiden MC, Forbes KJ. Attribution of Campylobacter infections in northeast Scotland to specific sources by use of multilocus sequence typing. J Infect Dis. 2009;199:1205–1208. doi: 10.1086/597417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellinga A, Van Loock F. The dioxin crisis as experiment to determine poultry-related Campylobacter enteritis. Emerg Infect Dis. 2002;8:19–22. doi: 10.3201/eid0801.010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler JG, Sethi D, Cowden JM, Wall PG, Rodrigues LC, Tompkins DS, Hudson MJ, Roderick PJ. Study of infectious intestinal disease in England: rates in the community, presenting to general practice, and reported to national surveillance. The Infectious Intestinal Disease Study Executive. BMJ. 1999;318:1046–1050. doi: 10.1136/bmj.318.7190.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DJ, Gabriel E, Leatherbarrow AJ, Cheesbrough J, Gee S, Bolton E, Fox A, Fearnhead P, Hart CA, Diggle PJ. Tracing the source of campylobacteriosis. PLoS Genet. 2008;4:e1000203. doi: 10.1371/journal.pgen.1000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman SN, Mathison GE, Lavoie MC. Pet dogs and chicken meat as reservoirs of Campylobacter spp. in Barbados. J Clin Microbiol. 2005;43:2642–2650. doi: 10.1128/JCM.43.6.2642-2650.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.