Abstract
To determine if augmenting podocyte injury promotes the development of advanced diabetic nephropathy (DN), we created mice that expressed the enzyme cytosine deaminase (CD) specifically in podocytes of diabetic Akita mice (Akita-CD mice). In these mice, treatment with the prodrug 5-flucytosine (5-FC) causes podocyte injury as a result of conversion to the toxic metabolite 5-fluorouracil (5-FU). We found that treatment of 4-5 week old Akita mice with 5-FC for 5 days caused robust albuminuria at 16 and 20 weeks of age compared to 5-FC treated Akita controls, which do not express CD (Akita CTLs). By 20 weeks of age, there was a significant increase in mesangial expansion in Akita-CD mice compared to Akita CTLs, which was associated with a variable increase in glomerular basement membrane (GBM) width and interstitial fibrosis. At 20 weeks of age, podocyte number was similarly reduced in both groups of Akita mice, and was inversely correlated with the albuminuria and mesangial expansion. Thus, enhancing podocyte injury early in the disease process promotes the development of prominent mesangial expansion, interstitial fibrosis, increased GBM thickness and robust albuminuria. These data suggest that podocytes play a key role in the development of advanced features of diabetic kidney disease.
Keywords: diabetes mellitus, diabetic nephropathy, podocyte
Introduction
Diabetic nephropathy (DN) is a serious complication of diabetes mellitus [1]. The economic consequences of this disorder are significant because the incidence of both diabetes mellitus and DN has reached epidemic proportions in developed countries [2,3]. Indeed, DN is the most common cause of end-stage renal disease (ESRD) in the United States [1]. While current strategies slow disease progression [4,5], approximately 20-30% of patients with diabetes ultimately develop ESRD requiring renal replacement therapy [3]. As a result, much effort has been devoted to understanding the mechanisms that promote glomerular damage in diabetic kidney disease with the hope of identifying new therapeutic targets and treatment strategies.
While mesangial cells were the initial focus of research into the molecular mechanisms of DN, more recent studies have concentrated on glomerular podocytes in disease pathogenesis [6]. These highly differentiated cells are important for maintaining the integrity of the glomerular filtration barrier [7,8]. In diabetes, podocyte injury is a common feature of both experimental and human diabetic kidney disease [6,7]. For example, foot process widening and loss of glomerular nephrin expression are observed in the early stages of diabetic kidney disease [6,7]. In the later stages of the disease, a reduction in the number of glomerular podocytes is characteristic of both human diabetic nephropathy and animal models of diabetic kidney disease [9,10,11]. Because podocytes are terminally differentiated cells with a limited capacity for replication [8,12], sufficient loss of podocytes leads to instability of the glomerular tuft and glomerulosclerosis [8]. In support of this hypothesis, urinary albumin excretion rates correlate negatively with podocyte number in patients with type 1 diabetes mellitus [11]. Similarly, podocyte number is a strong predictor of progressive renal disease in diabetic Pima Indians with microalbuminuria [10]. Podocyte injury might, therefore, promote the development of the characteristic functional and histopathologic features of both type 1 and type 2 diabetic kidney disease.
To investigate the role of glomerular podocytes in the pathogenesis of DN, we examined the effect of augmenting podocyte injury on the severity of diabetic kidney disease using the FVB/NJ Akita model of diabetes mellitus [13] and transgenic (TG) mice developed in our laboratory [14]. Akita mice are a genetic model of type 1 diabetes mellitus commonly utilized to study diabetic kidney disease [13,15,16]. These mice develop sustained and durable hyperglycemia associated with early features of DN in humans [13,15,16]. To selectively induce podocyte injury, we crossed Akita mice with TG mice expressing the yeast enzyme CD specifically in glomerular podocytes [14]. CD catalyzes the conversion of the prodrug 5-FC to 5-FU [17], a metabolite that inhibits both DNA and RNA synthesis and promotes death in cells that are not actively dividing. After targeting CD to podocytes, we selectively enhanced podocyte injury by treating mice with a short course of 5-FC. We found that augmenting podocyte injury at the onset of hyperglycemia promoted the development of some features of advanced diabetic kidney disease later in the disease process. These data support the notion that podocyte injury plays a critical role in the pathogenesis of diabetic kidney disease.
Materials and Methods
Results
Development of the diabetic CD model
In previous studies [14], we developed a doxycycline inducible approach to target expression of CD to renal or extrarenal tissues using available Tet-On or Tet-Off mice [18]. For podocyte specific expression, two TG mice are required. The first TG animal expresses the rtTA under the control of the human podocin promoter [19]. The second TG mouse expresses CD under the control of tet operator sequence (tetO) and a minimal CMV promoter (PminCMV) [18]. By breeding the two TG mice, animals are obtained that express both transgenes. In these “double” TG mice, treatment with doxycycline induces CD expression. For the studies, we utilized “double” TG Akita mice, which express CD in the presence of doxycycline (Akita CD mice) as well as “single” TG and non-TG Akita controls (Akita CTLs), which do not express CD in the presence of doxycycline. Additional CTLs included wild type “single” TG and non-TG mice. At 4 weeks of age, mice were treated with doxycycline for 1 week and then received 5 doses of 500mg/kg 5-FC for 5 consecutive days while the doxycycline was continued, as previously described [14]. Mice were then studied as outlined in the Methods Section.
Effect of the diabetes on blood glucose levels, kidney weight, systemic BP and heart weight
Hyperglycemia and systemic blood pressure are important determinants of the severity of kidney disease in diabetes mellitus [3]. We, therefore, first examined blood glucose levels in the Akita mice. As shown in Figure 1A, blood glucose levels were similarly elevated in Akita-CD mice and Akita CTLs at 12, 16 and 20 weeks of age. Hyperglycemia was associated with a significant and similar increase in urine output in both groups of Akita mice (Supplementary Figure 1A). We next evaluated the diabetic milieu on systemic blood pressure (BP), heart weight and kidney weight. As shown in Figure 1B, BP tended to be elevated in Akita-CD mice and Akita CTLs compared to CTLs at 12- and 20-weeks of age, but these differences did not reach statistical significance. Heart weight was also increased in both groups of Akita mice compared to CTLs at 20 weeks of age, but these differences were only significant for the Akita CTLs compared to CTL animals (Supplementary Figure 1B). Lastly, consistent with the known effects of the diabetic milieu on kidney hypertrophy, kidney weight was similarly enhanced in both Akita-CD mice and Akita CTLs compared to CTL animals at 20 weeks of age (Supplementary Figure 1C).
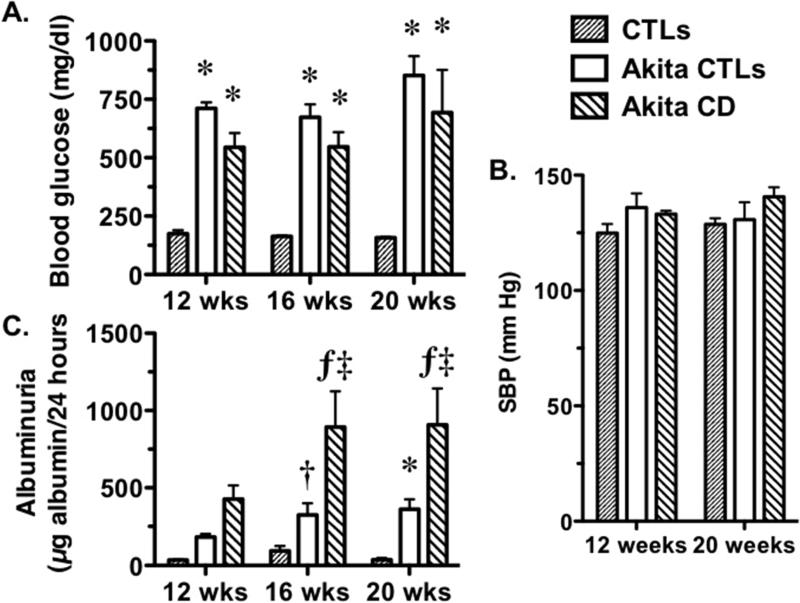
Figure 1.
Fasting blood glucose levels, systolic BP (SBP) and urinary albumin excretion (UAE) in Akita mice. (A) Akita CD and Akita CTLs mice developed sustained hyperglycemia, which was significantly increased in both groups of Akita amice compared to CTLs and persisted throughout the duration of the study. (B) SBP tended to be elevated in both groups of Akita mice compared to controls at 12 and 20 weeks of age but these differences were not statistically significant. (C) UAE was significantly elevated in both groups of Akita mice compared with CTL animals at 16 and 20 weeks of age. UAE was also elevated in Akita CD mice compared to Akita CTLs at 16 and 20 weeks of age. Eight Akita CTLs, 9 CTLs and 5-6 Akita CD mice were studied (one mouse died during the study). *P<0.05, †P<0.01 ƒP<0.005 vs. CTLs, ‡P<0.01 vs Akita CTLs.
Effect of the diabetes on albuminuria and serum creatinine levels
Figure 1C shows the effects of diabetes mellitus on urinary albumin excretion (UAE). UAE was not significantly different at the 12-week time point but, was significantly increased in both groups of Akita mice compared to CTLs at 16- and 20-weeks of age. Moreover, UAE was significantly increased in Akita CD mice compared to Akita CTLs at these same time points. The findings were similar when data was expressed as micrograms albumin per milligram creatinine (Table 1). To evaluate renal function, we measured serum creatinine levels as described in the Methods Section. There was a modest but statistically insignificant increase in Akita CD mice (0.32 ± 0.08 mg/dl) and Akita CTLs (0.31 ± 0.11 mg/dl compared to CTL animals (0.20 ± 0.04 mg/dl).
Table 1.
Urinary albumin excretion (μg albumin/mg creatinine)
| Age |
|||
|---|---|---|---|
| 12 weeks | 16 weeks | 20 weeks | |
| CTLs | 34 ± 2.9 | 51 ± 11 | 36 ± 11 |
| Akita CTLs | 262 ± 44 | 358 ± 86† | 312 ± 67* |
| Akita CD | 902 ± 406 | 1552 ± 944‡ | 1576 ± 137‡ |
P<0.05
P<0.01 vs Akita CD
P<0.01 vs CTLs
Effect of the diabetes on renal histology
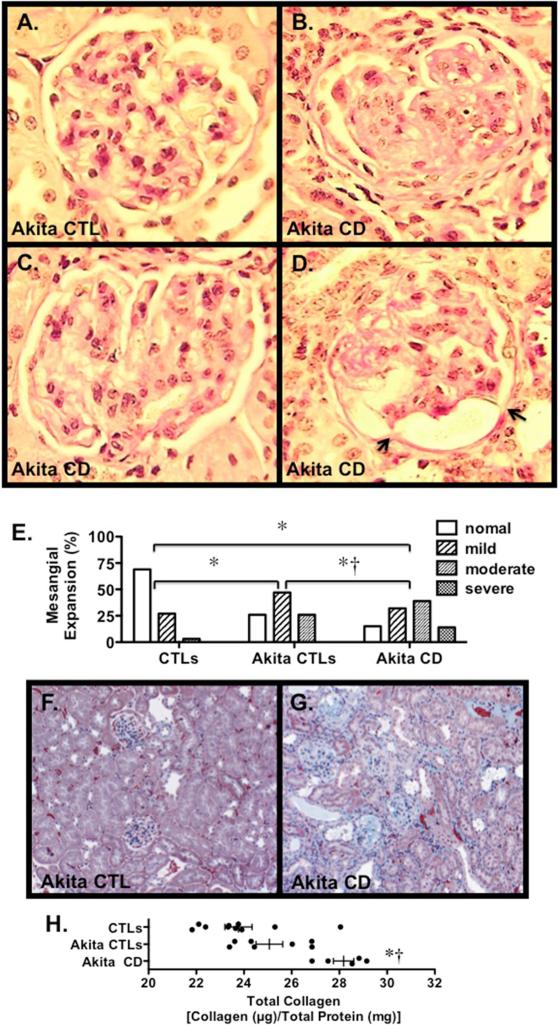
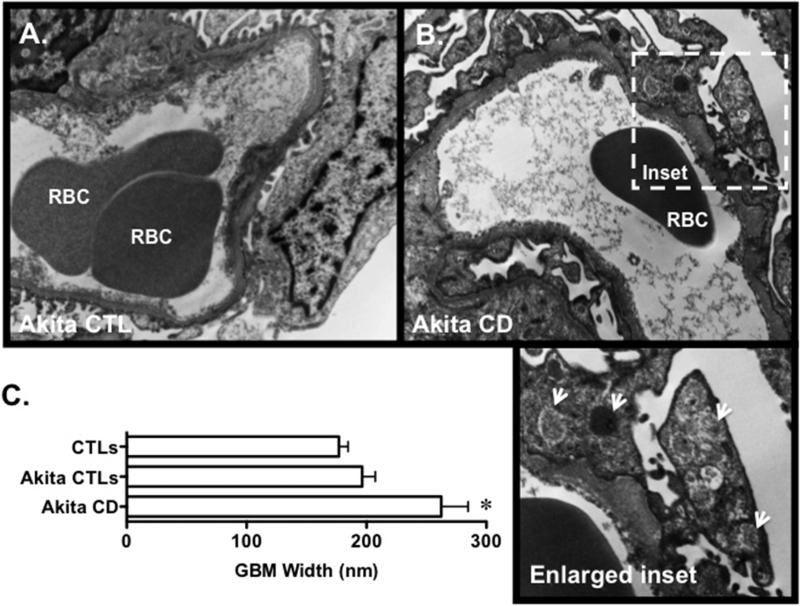
We next evaluated the effect of diabetes on renal histology. Figure 2A-D shows PAS stained sections from Akita CD mice and an Akita CTLs. Akita-CD mice developed marked mesangial expansion, which was associated with capsular adhesions in a few animals. Similar capsular adhesions have been reported in diabetic patients with high levels of proteinuria [20]. The severity of the mesangial expansion was graded using a semi-quantitative scale as described in the Methods Section. Results of these studies are shown in Figure 2E. There was a significant increase in semi-quantitative mesangial score in both Akita groups compared to controls. Moreover, the semi-quantitative score was significantly increased in Akita-CD mice compared to Akita CTLs. A few of the Akita-CD mice developed mild interstitial fibrosis as detected by the Masson-trichrome staining (Figure 2F and 2G). Quantitation of interstitial fibrosis using Sirius Red staining revealed a significant increase in interstitial fibrosis in Akita-CD mice compared to Akita CTLs (Figure 2H). Lastly, we evaluated the effect of podocyte injury on glomerular ultrastructure. As shown in Figure 3A and 3B, focal areas of foot process effacement were seen in both groups of Akita mice that was qualitatively more severe in the Akita CD group compared to Akita CTLs. Akita CD mice also demonstrated frequent cytoplasmic vesicular inclusions, which may represent phagolysosomes, and a variable increase GBM width (200-900 nm). In contrast, vesicular inclusions and variable GBM thickening was not observed in Akita CTLs. Quantitation of GBM width revealed a significant increase in Akita-CD mice compared to CTL animals (Figure 5C). GBM width was not statistically different in Akita CTLs compared to CTL mice, consistent with previously published studies [13].
Figure 2.
Glomerular histopathology in Akita mice. (A-D) Photomicrographs from an Akita CTL (A) and Akita Cd mice (B-D). Mild to moderate mesangial expansion was detected in glomeruli from both groups of Akita mice, which was qualitatively more severe in the Akita CD animals. Rare capsular adhesions were detected in a few mice (arrows). (E) Mesangial expansion was graded using a semi-quantitative scale as described in the Methods Section. There was a significant increase in the distribution of the semi-quantitative mesangial scores of individual glomeruli in both groups of Akita mice compared to CTLs. In Akita CD mice, there was a significant increase in the mesangial score compared to Akita CTLs. (F-G) Kidney sections stained with Masson trichrome to highlight fibrotic areas (green). A few Akita CD mice demonstrated focal areas of interstitial fibrosis. (H) Total collagen content in kidney sections was quantitated using Sirius Red/Fast Green collagen staining. There was a significant increase in collagen content in Akita CD mice compared to either Akita CTLs or CTLS. (A-D) Tissue sections were stained with Periodic acid-Schiff (PAS). (F-G) Tissue sections were stained with Masson trichrome. For the routine histological studies, 7 Akita CTLs, 9 CTLs and 5 Akita CD mice were studied. *P<0.01 vs. CTLs, †P<0.01 vs. Akita CTLs
Figure 3.
(A-B) Glomerular ultrastructure was evaluated using TEM. Focal areas of foot process effacement were observed in both groups of Akita mice, which was qualitatively more severe in the Akita CD group compared to Akita CTLs. Akita CD mice also demonstrated frequent cytoplasmic vesicular inclusions (arrows, enlarged inset) and a variable increase GBM width (200-900 nm). In contrast, vesicular inclusions and GBM thickening was not observed in Akita CTLs. (C) Quantitation of GBM width revealed a significant increase in Akita-CD mice compared to CTL animals. Three mice were studied in each group. *P<0.01 vs. CTLs and Akita CTLs
Effect of diabetes on podocyte number
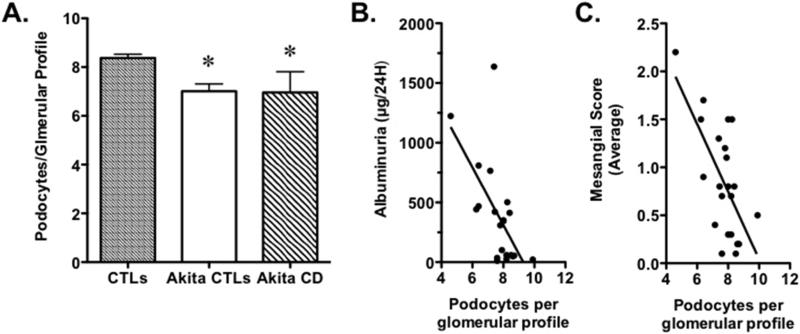
Podocyte number was quantitated in CTLs, Akita-CTLs and Akita-CD at 20 weeks of age. As shown in Figure 4, podocytes per glomerular profile were decreased in both groups of Akita mice compared to CTLS. A similar pattern was observed when the data was expressed as Nv(P/Glom), although these differences in podocyte density were largely due to increased VGlom in Akita CTLs and Akta-CD mice compared to controls and not N(P,Glom) (Table 2). To determine the relationship between podocytes per glomerular profile and the severity of functional and histologic features of diabetic kidney disease, we correlated podocytes per glomerular profile with albuminuria and the average semi-quantitative mesangial score for each animal. There was a significant inverse correlation between the average number of podocytes per glomerular profile and both albuminuria and the semi-quantitative mesangial score (Figure 4B and 4C).
Figure 4.
Relationship between podocyte number and albuminuria and mesangial expansion. (A) Podocyte number per glomerular profile was significantly decreased in both groups of Akita mice compared to CTL animals. (B) There was a significant inverse correlation between podocyte number per glomerular profile and UAE. (C) There was a significant inverse correlation between podocyte number per glomerular profile and the mesangial semi-quantitative score. Eight Akita CTLs, 9 CTLs and 5 Akita CD mice were studied. Eight Akita CTLs, 9 CTLs and 5 Akita CD mice were studied. *P< vs CTLs, P<0.01 for the regression analyses
Table 2.
Podocyte density, glomerular volume and podocyte number
| Nv(P/Glom) (×10−5/μm3) | VGlom (×105 μm3) | N(P,Glom) | |
|---|---|---|---|
| CTLs | 53.2 ± 11 | 2.31 ± .09 | 120 ± 2.5 |
| Akita CTLs | 33.6 ± 31* | 3.18 ± 23† | 107 ± 9.9 |
| Akita CD | 34.6 ± 19* | 3.29± 33† | 113 ± 9.9 |
P<0.001 vs CTLs
P<0.01 vs Akita CD
Discussion
It postulated that podocytes are the critical cellular element responsible for the progressive loss of kidney function characteristic of glomerular disease processes [8,12] including diabetic nephropathy [6]. In support of this hypothesis, strategies that inhibit podocyte injury in diabetic kidney disease preserve podocyte numbers, reduce albuminuria and improve glomerular histopathology [21]. In the present studies, we induced podocyte injury early in the disease process at the onset of albuminuria and hyperglycemia in 4-5 week old Akita mice [13]. This strategy caused a robust albuminuric response and some features of advanced diabetic kidney disease in Akita mice. While podocyte number was similar in Akita CD mice and Akita CTLs at the 20-week time point, there was a significant correlation between the number of glomerular podocytes and either albuminuria or mesangial expansion. Moreover, Akita CD mice developed increased GBM thickness and enhanced interstitial fibrosis compared to Akita CTLs. These data suggest that albuminuria, mesangial expansion, increased GBM thickness and interstitial fibrosis may be driven, at least in part, by podocyte injury in diabetes mellitus. Indeed, the podocyte plays a key role in maintaining filtration barrier integrity, contributes to formation of the GBM [8,22] and may play a role in the development of mesangial expansion, perhaps through paracrine mechanisms such as generation of vascular endothelial growth factor [23]. Loss of glomerular filtration barrier integrity may also indirectly contribute to the development to interstitial fibrosis by promoting protein overload [24]. Excessive urinary protein levels may activate renal tubular cells to produce pro-inflammatory cytokines and, in turn, promote an inflammatory fibrotic response [24]. In contrast, injury to other cell types in the glomerulus may be required for the development of additional features of advanced diabetic nephropathy. In this regard, mesangiolysis is seen in animals models of mesangial injury [25] suggesting that mesangial cell injury plays an important role in the development of this pathologic abnormality. Similarly, arteriolar hyalinosis is thought to result from endothelial cell injury and, in turn, loss of the integrity of the vascular endothelium [26]. While additional studies will be required to further define the role other cell types in the development of the characteristic functional and histopathologic lesions of diabetic nephropathy, these data are consistent with the notion that the glomerular podocyte contributes to the development of some features of advanced diabetic kidney disease.
The ability of investigators to study the pathogenesis and treatment of glomerular diseases has been significantly enhanced by the use of genetically manipulated animals [18,27]. Preeminent among these genetically manipulated animals are mice, which have become the animals of choice for performing genetic manipulations in vertebrates [27,28]. Unfortunately, mice have proven resistant to many of the glomerular disease models developed in rodents [27,29,30] including diabetic kidney disease [29]. In this regard, current mouse models of diabetic kidney disease are limited by modest levels albuminuria, mild histopathologic findings and a lack of a significant decline in glomerular filtration rate (GFR). Our data suggests that promoting podocyte injury in Akita mice using our TG model promotes the development of robust albuminuria, advanced mesangial matrix accumulation, increased GBM thickness and modest levels of interstitial fibrosis. Both groups of Akita mice also had similar, modest elevations in serum creatinine levels suggesting a decrease in GFR in both groups. Whether or not this increase in serum creatinine was related to intrinsic renal disease or hemodynamic factors due to the massive urine outputs of the Akita mice cannot be determined from the present studies. Nevertheless, the decrease in GFR in our Akita mice was minimal. Moreover, we did not find evidence of additional histological features of advanced diabetic nephropathy such as mesangiolysis or arteriolar hyalinosis. Taken together, the these data that suggest that enhancing podocyte injury may be a strategy to promote the development of some features of advanced features of diabetic kidney disease and, in turn, a mouse model that more completely recapitulates human diabetic nephropathy.
Lastly, we have previously characterized both the TG CD mice and the FVB/NJ Akita model of diabetes [13,14]. In this Akita model the onset of hyperglycemia occurs at 4 weeks of age and is associated with the onset of podocyte apoptosis [13,31]. High rates of podocyte apoptosis are observed early in the disease process in Akita mice but, in later stages of the disease, podocyte apoptosis is difficult to detect [13,31]. In CD model, robust albuminuria occurs promptly after treatment with 5-FC in mice expressing CD but not in control animals [14]. The onset of albuminuria is associated with podocyte apoptosis and a ≈40% decrease in podocyte number per glomerular profile by the 1-2 week time point. By 8-10 week time point, however, albuminuria returns to baseline levels. By combining 5-FC treatment with the onset of hyperglycemia and podocyte apoptosis in Akita CD mice, we attempted to enhance podocyte loss at 20-week time point in Akita CD mice compared to Akita CTLs. Podocyte number, however, was similar in Akita CD mice and Akita CTLs at 20 weeks of age. The inability of 5-FC treatment to enhance podocyte loss may reflect differential sensitivity of podocyte subsets to apoptotic stimuli. Alternatively, there may be some capacity of kidney to regenerate podocytes after a podocyte depleting injury as suggested by some investigators [32,33,34,35]. Further studies will be necessary to investigate these possibilities.
In summary, we found that enhancing podocyte injury early in the disease process promoted the development of robust albuminuria, increased GBM thickness, mesangial expansion and mild interstitial fibrosis. Podocyte number was significantly correlated with albuminuria and mesangial expansion suggesting that the development of these abnormalities may be, at least partially, dependent on podocyte damage. Taken together, these data are consistent with the notion that podocyte injury plays a key role in the development of some features of advanced diabetic kidney disease in humans.
Supplementary Material
Highlights.
➢ Augmenting podocyte injury promotes robust albuminuria in diabetic mice
➢ Podocyte injury causes advanced histologic features of diabetic kidney disease
➢ Podocyte number correlates inversely with albuminuria and mesangial expansion
Acknowledgements
These studies were supported by grants RO1-DK075688 (R.F.S.) and R01DK087707 (R.F.S.) from the National Institutes of Health as well as BX000791 (R.F.S) from the Veterans Administration Merit Review Program. The results presented in this paper have not been published previously, in whole or in part, except in abstract format.
Abbreviations
- DN
Diabetic nephropathy
- ESRD
end-stage renal disease
- SBP
systolic blood pressure
- H&E
hematoxylin and eosin
- PAS
periodic acid Schiff
- SEM
error of the mean
- ANOVA
one way analysis of variance
- tetO
tet operator sequence
- PminCMV
minimal CMV promoter
- BP
blood pressure
- CTLs
controls
- UAE
urinary albumin excretion
- CD
cytosine deaminase
- Nv(P/Glom)
Podocyte density
- [N(P,Glom)]; Nv(P/Glom)
podocytes per glomerulus
- Vglom
glomerular volume
- TEM
Transmission electron microscopy
- GBM
glomerular basement membrane
- TG
transgenic
- rtTA
reverse tetracycline transactivator
- tTA
tetracycline transactivator
- GFR
glomerular filtration rate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have no conflicts of interest.
References
- 1.Collins AJ, Foley RN, Chavers B, et al. United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis. 2012;59:A7, e1–420. doi: 10.1053/j.ajkd.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Ritz E, Rychlik I, Locatelli F, et al. End-stage renal failure in type 2 diabetes: A medical catastrophe of worldwide dimensions. Am J Kidney Dis. 1999;34:795–808. doi: 10.1016/S0272-6386(99)70035-1. [DOI] [PubMed] [Google Scholar]
- 3.Rodby RA. Type II diabetic nephropathy: its clinical course and therapeutic implications. Semin Nephrol. 1997;17:132–147. [PubMed] [Google Scholar]
- 4.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 5.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 6.Wolf G, Chen S, Ziyadeh FN. From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes. 2005;54:1626–1634. doi: 10.2337/diabetes.54.6.1626. [DOI] [PubMed] [Google Scholar]
- 7.Jefferson JA, Shankland SJ, Pichler RH. Proteinuria in diabetic kidney disease: a mechanistic viewpoint. Kidney Int. 2008;74:22–36. doi: 10.1038/ki.2008.128. [DOI] [PubMed] [Google Scholar]
- 8.Wiggins RC. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 2007;71:1205–1214. doi: 10.1038/sj.ki.5002222. [DOI] [PubMed] [Google Scholar]
- 9.Dalla Vestra M, Masiero A, Roiter AM, et al. Is podocyte injury relevant in diabetic nephropathy? Studies in patients with type 2 diabetes. Diabetes. 2003;52:1031–1035. doi: 10.2337/diabetes.52.4.1031. [DOI] [PubMed] [Google Scholar]
- 10.Meyer TW, Bennett PH, Nelson RG. Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia. 1999;42:1341–1344. doi: 10.1007/s001250051447. [DOI] [PubMed] [Google Scholar]
- 11.White KE, Bilous RW, Marshall SM, et al. Podocyte number in normotensive type 1 diabetic patients with albuminuria. Diabetes. 2002;51:3083–3089. doi: 10.2337/diabetes.51.10.3083. [DOI] [PubMed] [Google Scholar]
- 12.Kriz W, Gretz N, Lemley KV. Progression of glomerular diseases: is the podocyte the culprit? Kidney Int. 1998;54:687–697. doi: 10.1046/j.1523-1755.1998.00044.x. [DOI] [PubMed] [Google Scholar]
- 13.Chang JH, Paik SY, Mao L, et al. Diabetic kidney disease in FVB/NJ Akita mice: temporal pattern of kidney injury and urinary nephrin excretion. PLoS One. 2012;7:e33942. doi: 10.1371/journal.pone.0033942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L, Tang Y, Howell DN, et al. A novel mouse model of podocyte depletion. Nephron Exp Nephrol. 2012;121:e10–22. doi: 10.1159/000342369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gurley SB, Clare SE, Snow KP, et al. Impact of genetic background on nephropathy in diabetic mice. Am J Physiol Renal Physiol. 2006;290:F214–222. doi: 10.1152/ajprenal.00204.2005. [DOI] [PubMed] [Google Scholar]
- 16.Gurley SB, Mach CL, Stegbauer J, et al. Influence of genetic background on albuminuria and kidney injury in Ins2(+/C96Y) (Akita) mice. Am J Physiol Renal Physiol. 298:F788–795. doi: 10.1152/ajprenal.90515.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vermes A, Guchelaar HJ, Dankert J. Flucytosine: a review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J Antimicrob Chemother. 2000;46:171–179. doi: 10.1093/jac/46.2.171. [DOI] [PubMed] [Google Scholar]
- 18.Gingrich JR, Roder J. Inducible gene expression in the nervous system of transgenic mice. Annu Rev Neurosci. 1998;21:377–405. doi: 10.1146/annurev.neuro.21.1.377. [DOI] [PubMed] [Google Scholar]
- 19.Shigehara T, Zaragoza C, Kitiyakara C, et al. Inducible podocyte-specific gene expression in transgenic mice. J Am Soc Nephrol. 2003;14:1998–2003. doi: 10.1681/ASN.V1481998. [DOI] [PubMed] [Google Scholar]
- 20.Najafian B, Kim Y, Crosson JT, et al. Atubular glomeruli and glomerulotubular junction abnormalities in diabetic nephropathy. J Am Soc Nephrol. 2003;14:908–917. doi: 10.1097/01.asn.0000057854.32413.81. [DOI] [PubMed] [Google Scholar]
- 21.Zheng S, Carlson EC, Yang L, et al. Podocyte-specific overexpression of the antioxidant metallothionein reduces diabetic nephropathy. J Am Soc Nephrol. 2008;19:2077–2085. doi: 10.1681/ASN.2007080967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.St John PL, Abrahamson DR. Glomerular endothelial cells and podocytes jointly synthesize laminin-1 and -11 chains. Kidney Int. 2001;60:1037–1046. doi: 10.1046/j.1523-1755.2001.0600031037.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H, Schin M, Saha J, et al. Podocyte-specific overexpression of GLUT1 surprisingly reduces mesangial matrix expansion in diabetic nephropathy in mice. Am J Physiol Renal Physiol. 2010;299:F91–98. doi: 10.1152/ajprenal.00021.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eddy AA. Proteinuria and interstitial injury. Nephrol Dial Transplant. 2004;19:277–281. doi: 10.1093/ndt/gfg533. [DOI] [PubMed] [Google Scholar]
- 25.Schlondorff D, Banas B. The mesangial cell revisited: no cell is an island. J Am Soc Nephrol. 2009;20:1179–1187. doi: 10.1681/ASN.2008050549. [DOI] [PubMed] [Google Scholar]
- 26.Gamble CN. The pathogenesis of hyaline arteriolosclerosis. Am J Pathol. 1986;122:410–420. [PMC free article] [PubMed] [Google Scholar]
- 27.Anders H, Schlondorff D. Murine models of renal disease: possibilities and problems in studies using mutant mice. Exp Nephrol. 2000;8:181–193. doi: 10.1159/000020667. [DOI] [PubMed] [Google Scholar]
- 28.Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129–1155. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- 29.Breyer MD, Bottinger E, Brosius FC, 3rd, et al. Mouse models of diabetic nephropathy. J Am Soc Nephrol. 2005;16:27–45. doi: 10.1681/ASN.2004080648. [DOI] [PubMed] [Google Scholar]
- 30.Fogo AB. Animal models of FSGS: lessons for pathogenesis and treatment. Semin Nephrol. 2003;23:161–171. doi: 10.1053/snep.2003.50015. [DOI] [PubMed] [Google Scholar]
- 31.Susztak K, Raff AC, Schiffer M, et al. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. [PubMed] [Google Scholar]
- 32.Appel D, Kershaw DB, Smeets B, et al. Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol. 2009;20:333–343. doi: 10.1681/ASN.2008070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ronconi E, Sagrinati C, Angelotti ML, et al. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol. 2009;20:322–332. doi: 10.1681/ASN.2008070709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sagrinati C, Netti GS, Mazzinghi B, et al. Isolation and characterization of multipotent progenitor cells from the Bowman's capsule of adult human kidneys. J Am Soc Nephrol. 2006;17:2443–2456. doi: 10.1681/ASN.2006010089. [DOI] [PubMed] [Google Scholar]
- 35.Pichaiwong W, Hudkins KL, Wietecha T, et al. Reversibility of structural and functional damage in a model of advanced diabetic nephropathy. J Am Soc Nephrol. 2013;24:1088–1102. doi: 10.1681/ASN.2012050445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.