Abstract
The use of high-intensity sweeteners has been proposed as a method to combat increasing rates of overweight and obesity in the human population. However, previous work with male rats suggests that consumption of such sweeteners might contribute to, rather than ameliorate, weight gain. The goals of the present experiments were to assess whether intake of high-intensity sweeteners is associated with increased food intake and body weight gain in female rats; to evaluate whether this effect depends on composition of the maintenance diet (i.e., standard chow compared to diets high in energy, fat and sugar [HE diets]); and to determine whether the phenotype of the rats with regard to propensity to gain weight on HE diets affects the consequences of consuming high-intensity sweeteners. The data demonstrated that female rats fed a low-fat, standard laboratory chow diet did not gain extra weight when fed yogurt dietary supplements sweetened with saccharin compared to those fed glucose-sweetened dietary supplements. However, female rats maintained on a “Westernized” diet high in fat and sugar (HE diet) showed significant increases in energy intake, weight gain and adiposity when given saccharin-sweetened compared to glucose-sweetened yogurt supplements. These differences were most pronounced in female rats known to be prone to obesity prior to the introduction of the yogurt diets. Both selectively-bred Crl:OP[CD] rats, and outbred Sprague-Dawley rats fed an HE diet showing high levels of weight gain (DIO rats) had increased weight gain in response to consuming saccharin-sweetened compared to glucose-sweetened supplements. However, in male rats fed an HE diet, saccharin-sweetened supplements produced extra weight gain regardless of obesity phenotype. These results suggest that the most negative consequences of consuming high-intensity sweeteners may occur in those most likely to use them for weight control, females consuming a “Westernized” diet and already prone to excess weight gain.
Keywords: obesity, learning, saccharin, sex differences, body weight, energy balance
Introduction
Obesity and its co-morbidities (e.g., Type II diabetes, hypertension, cardiovascular and pulmonary diseases) are serious threats to the health and well-being of both men and women across a wide variety of demographic subdivisions (e.g., Mokdad et al., 2003; Ogden, Carroll, Kit, & Flegal, 2012; Ogden, Yanovski, Carroll, & Flegal, 2007). However, neither the degree of risk nor the origins of these afflictions need be the same for both sexes. In both human and nonhuman animals, males and females are known to differ with respect to a number of processes implicated in energy balance and body weight regulation. For example, consuming a high energy (HE) or “western” diet (i.e., a diet high in saturated fat and refined sugar) produces less severe peripheral insulin resistance and dyslipidemia, and later onset of these conditions, in female rats and humans compared to male rats and humans, respectively (Meyer, Clegg, Prossnitz, & Barton, 2011). Moreover, rates of obesity in women are higher compared to men at all income levels except the highest (Ogden, Lamb, Carroll, & Flegal, 2010), and rates of severe obesity (BMI>35) are greater in women compared to men (Ogden et al., 2012).
Further, relative to male rats, female rats have been reported to show increased preferences for sweet solutions, differences that may relate to levels of ovarian hormones (e.g., Asarian & Geary, 2006; Atchley, Weaver, & Eckel, 2005; Curtis, Stratford, & Contreras, 2005; Kenney & Redick, 1980; Valenstein, Kakolewski, & Cox, 1967; Wade & Zucker, 1969). There have also been reports that women show some variation in sweet-taste thresholds, sweet preference, and intake of sweet-tasting foods associated with changes in ovarian hormones, for example across the menstrual cycle and during pregnancy (Bowen, 1992; Bowen & Grunberg, 1990; Tepper & Seldner, 1999; Than, Delay, & Maier, 1994). Such findings make it clear that different factors could promote weight gain and excess energy intake for females compared to males.
Previously we reported that male rats given high intensity sweeteners exhibit increased energy intake and body weight gain (for review, see Swithers, Martin, & Davidson, 2010) compared to those given the caloric sweetener glucose. We have provided evidence that these deficits in regulating energy balance are based on the disruption of a learned signaling relationship between sweet tastes and caloric or energetic outcomes (Davidson, Martin, Clark, & Swithers, 2011). Our experiment showed that the ability of the sweet taste of glucose to compete with a novel flavor for association with a nutritive postingestive US was weakened by prior experience consuming saccharin. This effect was specific to sweet taste, since prior experience with saccharin did not interfere with ability of the nonsweet taste of polycose to compete for association with the US. A second study showed that similar exposure to saccharin was followed by increased food intake and body weight gain for rats maintained on a high-fat diet that was sweetened with glucose, but not for rats maintained on an equicaloric diet with polycose added or for rats that received the plain high-fat diet. Thus, a manipulation that weakened the association between sweet taste and an energetic outcome also selectively promoted excessive intake and body weight gain by rats maintained on a sweetened high-energy diet.
To date, research examining the negative consequences of disrupting these types of sweet taste-calorie associations on long-term food intake and body weight gain has focused on effects in lean male rats. As described above, factors that determine energy balance in lean male rats may not fully represent the factors that affect energy balance in females. The goals of the present experiments were therefore to (a) assess whether intake of high-intensity sweeteners is associated with increased food intake and body weight gain in female rats; (b) evaluate whether this effect depends on composition of the maintenance diet (HE vs. standard chow) and (c) determine whether the phenotype of the rats with regard to propensity to gain weight on HE diets affects the consequences of consuming high-intensity sweeteners.
It is important to pursue these goals for several reasons. First, in addition to the male-female differences noted above, the available data indicate that the most likely consumers of “diet” foods and beverages are women, many of whom are overweight or obese (Duffey & Popkin, 2006; Fowler et al., 2008). Thus, women may be more likely to be exposed to the adverse effects of consuming high-intensity sweeteners. Second, while low-fat diets are frequently employed in laboratory studies, they are not representative of the HE diets most typically consumed in the U.S. The negative consequences of high-intensity sweeteners appear to be exacerbated when male rats are fed HE diets compared to standard low-fat, low energy chow (e.g., Davidson et al., 2011; Swithers, Laboy, Clark, Cooper, & Davidson, 2012); it is unknown whether or not the effects of consuming high intensity sweeteners will also be worsened by HE diets in female rats. Finally, it is well-known that Sprague-Dawley rats given access to HE diets show large variations in weight gain; animals termed DIO (diet-induced obese) gain large amounts of excess weight when given HE diets relative to both weight gain on a standard low-fat chow diet and to animals termed DR (diet resistant), whose body weight gain on HE diets remains low and similar to that observed when maintained on a low-fat chow diet (Levin & Dunn-Meynell, 2006; Levin, Dunn-Meynell, Balkan, & Keesey, 1997; Levin, Magnan, Migrenne, Chua, & Dunn-Meynell, 2005; Madsen et al., 2010; Paulsen et al., 2010).
It has been argued that these rodent DIO and DR phenotypes represent a useful model of human energy regulation because while many people have responded to the current obesogenic environment with significant increases in weight, some people remain resistant to these effects. In addition, through selective breeding of rats identified as DIO and DR, commercially-available lines of rats have been developed that are known to have consistent tendencies to exhibit diet-induced obesity when given HE diets. The line Crl:OP-CD (OP rats) is prone to obesity on HE diets, while the control line, Crl:OR-CD (OR rats) is resistant (Levin et al., 1997). Given data indicating that consumption of high-intensity sweeteners is the greatest in women with the highest tendency towards overweight and obesity, it is also important to determine if differences in the effects of consuming high intensity sweeteners on weight gain are related to pre-existing phenotypic differences toward weight gain in a female rat model.
Using procedures similar to those previously demonstrated to produce significant effects on body weight gain and food intake in male rats, Experiment 1 examined whether consuming a yogurt dietary supplement sweetened with non-caloric saccharin resulted in increased body weight gain compared to a yogurt supplement sweetened with the caloric sweetener glucose in female rats maintained on a standard low-fat laboratory chow diet. Experiments 2 and 3 employed diets high in energy, fat and sugar along with sweetened yogurt dietary supplements to determine whether consumption of “Westernized diets” in addition to sweetened yogurt dietary supplements exacerbated body weight gain in female rats and whether phenotypic differences in propensity to obesity on a HE diet interacted with the effects of exposure to diets which disrupt the predictive relation between sweet taste and calories. In Experiments 2 and 3, outbred Sprague-Dawley female rats were given exposure to an HE diet prior to the introduction of the yogurt supplements, and weight gain during this exposure period was used to identify animals as DIO or DR prior to the introduction of the sweetened yogurt supplements. Experiment 2 employed a diet high in sugar in which the fat source was lard, while Experiment 3 employed a different HE diet high in sugar in which the fat source was peanut oil. In Experiment 4, outbred Sprague-Dawley male rats were given exposure to the HE diet employed in Experiment 3 and identified as DIO or DR prior to the introduction of the yogurt supplements. Finally, in Experiment 5, subjects were females from selectively-bred OP and OR lines, and the effects of consuming sweetened yogurt supplements were determined both prior to the introduction of the HE diet employed in Experiments 3 and 4 and after HE diet was available.
Methods
In all experiments, animals were given daily access to 30 g plain or sweetened yogurt (Dannon low-fat plain yogurt; ∼0.6 kcal/g) along with ad lib access to their assigned maintenance diet and water. On half of the days in each experiment, yogurt was provided in unsweetened form (∼0.6kcal/g). On the other half of the days, yogurt was sweetened with 20% glucose (w/w; ∼1.2 kcal/g), or 0.3% saccharin (∼0.6 kcal/g). For the glucose group, sweet taste was associated with an increase in energetic content. For the saccharin group, sweet taste did not predict an increase in caloric content. The order of presentation of yogurt was semi-randomized with animals receiving no more than 2 days of the same yogurt (sweetened or plain) in a row; yogurts were presented in enamel camping cups fastened to the front of the cage. Because the interest was in comparing the consequences of equal exposure to the diets, animals that failed to consume at least 70% of the yogurts provided were excluded from analysis as described below. In all experiments including females, rats were gonadally-intact adult females; estrus status during experiments was not assessed to avoid potential stress associated with collecting vaginal cytology. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and was approved by the Animal Care and Use Committee at Purdue University.
Experiment 1
Subjects were 20 female Sprague-Dawley (Harlan, Indianapolis) rats weighing 200-225 g and given ad lib access to a standard low-fat laboratory chow (Harlan 2018) for 19 days in the laboratory after arrival. Animals were then assigned to one of two yogurt groups (Saccharin or Glucose), matched on body weight, and were then given yogurt dietary supplements 6 days per week for 4 weeks (12 days plain, 12 days sweetened), with a single day of chow and water alone provided each week.
Body weight gain was analyzed with a 2-Way (Sweetener X Day) repeated-measures ANOVA, with Day as a within-subjects factor and Sweetener as a between-subjects factor. Two animals were excluded from analysis due to tumor growth and consistent food spillage. After excluding these animals, body weights across the two groups at the start of yogurt exposure did not differ (253.6 + 2.8 g for Glucose and 255.5 + 3.2 g for Saccharin).
Experiment 2
Subjects were 36 adult female Sprague-Dawley rats purchased from Harlan. Following 1 week in the laboratory, on a standard chow diet (Harlan 2018), all animals were given ad lib access to a diet (Harlan, TD.04489) high in both saturated fat (17% lard by weight) and sugar (22 % glucose by weight) for 12 days. Body composition was then assessed using NMR (EchoMRI-900). A median split based on body weight gain during exposure to the HE diet was used to assign animals to DIO and DR phenotype groups based on weight. Within each phenotype, half of the animals were assigned to receive glucose-sweetened yogurt supplements while the remaining animals were assigned to receive saccharin-sweetened yogurt supplements. Yogurt supplements were provided 6 days per week for 4 weeks (12 days plain, 12 days sweetened), with one day of HE diet and water alone per week. Body composition was then assessed again using NMR. Body weight, yogurt intake, and chow intake were measured daily by weighing (corrected for spillage).
Three animals (2 DIO and 1 DR) failed to consume at least 70% of the yogurt offered and were excluded from analyses. After excluding these animals, body weight at the start of yogurt consumption was higher in DIO compared to DR animals, but did not differ within phenotype across the two sweeteners (Mean starting body weight +SEM = 270.1 + 2.5 g [n=9] and 271.9 + 2.8 g [n=7], for DIO Glucose and DIO Saccharin groups, respectively; and 259.3 + 2.6 g [n=8] and 256.7 + 2.5 g [n-9] for DR Glucose and DR Saccharin groups, respectively).
Body weight gain was analyzed with a 3-Way (Phenotype X Sweetener X Day) repeated measures ANOVA, with Phenotype and Sweetener as between-subjects factors and Day as a within-subjects factor, followed by 2-Way (Sweetener X Day) repeated measures ANOVAs as indicated. Total caloric intake, caloric intake from chow, total grams of yogurt and caloric intake from yogurt were analyzed with separate 2-Way (Phenotype X Sweetener) ANOVAs. To analyze changes in body composition, separate ANCOVAs for Fat mass and Lean Mass were conducted, using starting Fat mass and starting Lean mass as the covariates, respectively. Post-hoc tests were conducted with Newman-Keuls tests where indicated.
Experiment 3
Subjects were 48 adult female rats purchased from Harlan (Indianapolis) who were given ad lib access to a standard laboratory chow (Harlan 2018) for one week in the lab. All animals were then given ad lib access to an HE powdered chow diet (testdiet.com cat #25312) that was high in fat (16% peanut oil by weight) for 7 days and body composition was then assessed using NMR. A median split was performed on body weight gain during HE diet access and animals were then grouped into DIO and DR phenotypes. Half of the animals in each phenotype (matched for body weight within phenotype) were assigned to receive yogurt supplements sweetened with Glucose, while the remaining animals in each phenotype received yogurt supplements sweetened with Saccharin. At the same time, the maintenance diet was sweetened by the addition of 20% glucose by weight. Yogurt supplements were provided daily for a total of 16 days (8 days plain, 8 days sweetened), with two days of chow and water alone intervening. Yogurt intake and body weight were recorded daily and body composition was assessed using NMR at the end of the yogurt exposure. Due to excessive spillage of the powdered chow, chow intake was not measured.
Six animals (3 DIO and 3 DR) failed to consume at least 70% of the yogurt provided, and were excluded from the analyses. After excluding these animals, body weights at the start yogurt consumption were higher in DIO animals (Means + SEM = 257.8 + 3.3 g [n=11] and 253.5 + 3.5 g [n=11] for DIO Glucose and DIO Saccharin, respectively) than DR animals (Means + SEM = 242.1 + 3.1 [n=10] and 244.7 + 3.4 [n=10] for DR Glucose and DR Saccharin, respectively), but were matched within phenotype across the two sweeteners.
Body weight gain was analyzed with a 3-Way (Phenotype X Sweetener X Day) repeated measures ANOVA, with Phenotype and Sweetener as between-subjects factors and Day as a within-subjects factor, followed by 2-Way (Sweetener X Day) repeated measures ANOVAs as indicated. To analyze changes in body composition, separate ANCOVAs for Fat mass and Lean Mass were conducted, using starting Fat mass and starting Lean mass as the covariates, respectively. Post-hoc tests were conducted with Newman-Keuls tests where indicated.
Experiment 4
Subjects were 50 male Sprague-Dawley rats (Harlan, Indianapolis) weighing 300-325 g on arrival. Animals were placed on standard chow diet (Harlan 2018) for one week, then given ad lib access to the same high fat diet sweetened with 20% glucose used in Experiment 3 for 3 weeks. A median split based on body weight gain was then used to assign animals to DIO and DR phenotype groups. Within each phenotype, half of the animals were assigned to receive glucose-sweetened yogurt supplements while the remaining animals were assigned to receive saccharin-sweetened yogurt supplements. Yogurt supplements were provided daily for a total of 16 days (8 days plain, 8 days sweetened), with two days of chow and water alone intervening. Yogurt intake and body weight were recorded daily, and animals that failed to consume at least 70% of the yogurt provided were excluded from analysis. Due to large amounts of spillage of the powdered diet, chow intake was not measured.
Eight animals (4 DIO and 4 DR) failed to consume at least 70% of the yogurt After excluding these animals, body weights at the start yogurt presentation were higher in DIO animals (Means + SEM = 406.3 + 3.1 g [n=10] and 404.6 + 3.0 g [n=11] for DIO Glucose and DIO Saccharin, respectively) than in DR animals (Means + SEM = 388.2 + 2.9 [n=12] and 389.5 + 3.3 [n=9] for DR Glucose and DR Saccharin, respectively), but were matched within phenotype across the two sweeteners.
Body weight gain was analyzed with a 3-Way (Phenotype X Sweetener X Day) repeated measures ANOVA, with Phenotype and Sweetener as between-subjects factors and Day as a within-subjects factor, followed by 2-Way (Sweetener X Day) repeated measures ANOVAs as indicated. Post-hoc tests were conducted with Newman-Keuls tests where indicated.
Experiment 5
Subjects were 44 female offspring of adult Obesity Prone Crl:OP[CD] and Obesity Resistant Crl:OR[CD] male and female rats purchased from Charles River, and subsequently bred in our lab. Dams, sires and litters were maintained on a standard laboratory chow (Harlan 2018) throughout gestation, lactation and weaning. Litters were weaned at 23 days of age, and housed with same-sex littermates until approximately 90 days of age. Body composition was assessed with NMR, then half of the animals within each phenotype were assigned to receive saccharin- or glucose-sweetened yogurt. Sweetener groups were matched on body weight within each phenotype. Yogurt supplements were provided for 6 days (3 sweetened and 3 plain) along with one day of chow alone while animals remained on the standard chow diet (Harlan 2018). Animals were then given ad lib access to the sweetened HE powdered chow diet used in Experiments 3 and 4 while they continued to receive their assigned yogurt for 6 days (3 sweetened and 3 plain) per week for an additional 2 weeks. Body composition was assessed with NMR at the end of this 3-week yogurt exposure. Body weight and yogurt intake were measured daily; due to large amounts of spillage of the powdered diet, chow intake was not measured.
One animal was excluded from analysis for failing to consume at least 70% of the yogurt diet provided. Starting body weights were greater in OP than OR animals but did not differ between sweetener groups within each phenotype (Mean + SEM = 254.1 + 4.8 g [n=8] and 259.8 + 4.5 g [n=9] for OP Glucose and OP Saccharin groups, respectively; 198.8 + 3.7 g [n=13] and 199.2 + 3.7 g [n=13] for OR Glucose and OR Saccharin groups).
Body weight gain was analyzed using a 3-Way repeated-measures ANOVA (Phenotype X Sweetener X Day) with Phenotype and Sweetener as between-subjects factors and Day as a within-subjects factor. Lean mass and fat mass following yogurt exposure were analyzed with 2-Way ANCOVA (Phenotype X Sweetener), with starting lean mass and starting fat mass used as the covariates, respectively.
Results
Experiment 1
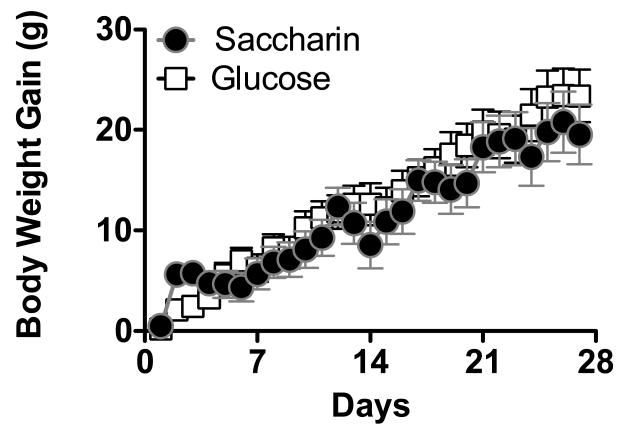
Weight gain in adult female rats on a standard powdered low-fat laboratory chow diet was not affected by the sweetener to which the animal was exposed for 28 days (Figure 1; Main effect of Day, F16, 416 = 43.5, p< .0000001; other F's =0.31 – 1.1, p's = .34 - .59).
Figure 1.
Body weight gain in female rats consuming low-fat standard laboratory chow diet was not affected by the type of sweetener provided in a daily yogurt dietary supplement.
Experiment 2
Prior to the introduction of the yogurt, weight gain on the HE diet was significantly greater for rats classified as DIO compared to DR animals (Main effect of Phenotype, F 1, 29 = 56.06, p < .000001; Means = 29.41 + 1.4 for DIO and 14.4 + 1.4 for DR).
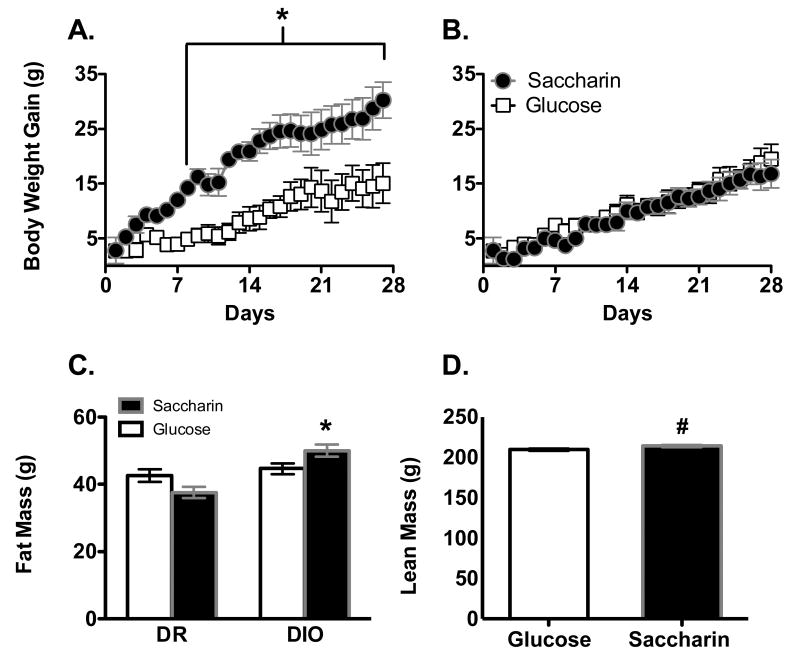
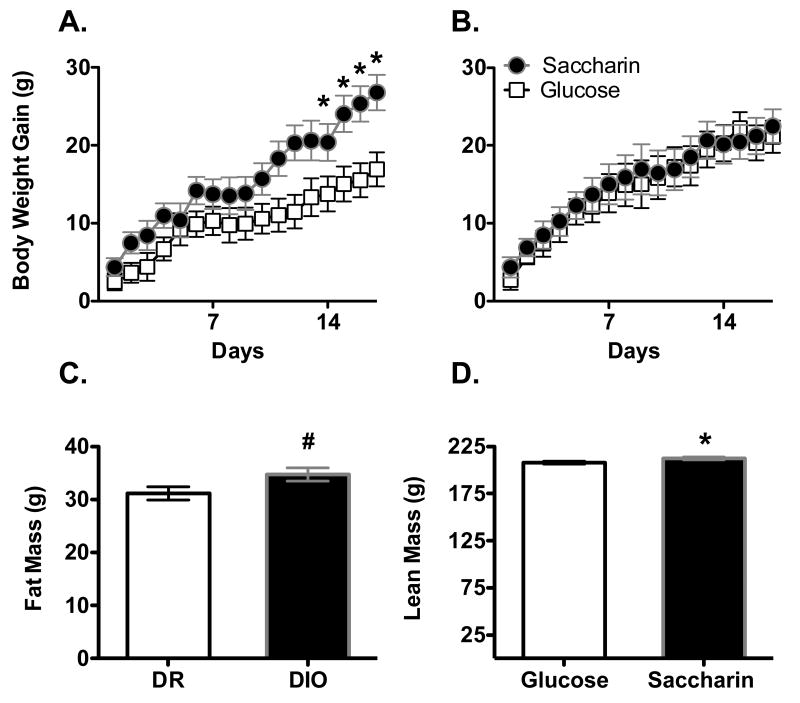
After the introduction of the yogurt supplements, body weight gain was affected by the sweetener used, phenotype and the day of testing (Figure 2A and 2B; Main effect of Phenotype, F1, 29 = 4.4, p =.046; Main effect of Sweetener, F1, 29 = 4.7, p =.038; Phenotype X Sweetener interaction, F1, 29 = 7.1, p = .012; Main effect of day, F26, 754 = 53.2, p < .0000001; Sweetener X Day interaction, F26, 754 = 1.9, p = .0042; Sweetener X Phenotype X Day interaction, F26, 754 = 1.71, p = .016). In DIO females, body weight gain was significantly greater in animals consuming the saccharin-sweetened yogurt compared to those consuming the glucose-sweetened yogurt beginning on Day 10 (Figure 2A; Main effect of Sweetener, F1, 14 = 5.12, p = .04; Main effect of Day, F 26, 364 = 19.7, p < .0000001). In contrast, weight gain was unaffected by the sweetener provided in the yogurt in DR animals (Figure 2B; Main effect of Day, F1, 15 =48.8, p < .0000001).
Figure 2.
Body weight gain in DIO female rats (panel A) was significantly greater when consuming Saccharin-sweetened yogurt along with a chow diet high in sugar and fat compared to those given Glucose-sweetened yogurt. In contrast, DR females (B) showed no differences in body weight gain based on the type of sweetener consumed. Filled circles = saccharin-sweetened yogurt; white squares = glucose-sweetened yogurt. Fat mass (C) was significantly higher in DIO animals given Saccharin-sweetened yogurt compared to DIO animals given Glucose-sweetened yogurt. Lean mass (D) was higher in females given Saccharin-sweetened yogurt compared to females given Glucose-sweetened yogurt across both phenotypes.
* p < .05 compared to DIO Glucose
# p < .05 between Glucose and Saccharin
Fat mass was affected by the sweetener consumed, the phenotype of the animals and the animal's starting fat mass (Figure 2C; Main effect of Starting Fat mass, F1, 28 = 77.8, p < .0000001; Phenotype X Sweetener interaction, F1, 28 = 8.7, p= .00065). Post-hoc ANCOVAs indicated that in DIO animals, fat mass was significantly greater in animals given the saccharin- compared to glucose-sweetened yogurt (Main effect of Sweetener, F1, 13 = 4.85, p = .046), while in DR animals, there were no significant differences between animals given saccharin- versus glucose-sweetened yogurt. Lean mass was affected by the sweetener consumed and the starting lean mass, but not the phenotype (Figure 2D; Main effect of Starting lean mass, F1, 28 = 35.7, p = .00002; Main effect of Sweetener, F1, 28 = 4.38, p = .046), with post-hoc analyses indicating that females given saccharin-sweetened yogurt had significantly greater lean mass than females given glucose-sweetened yogurt.
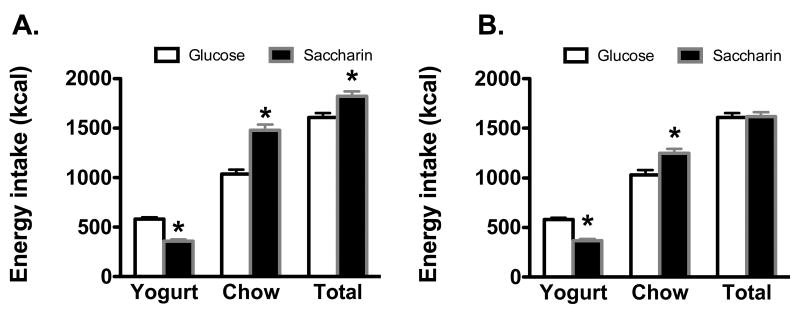
Total energy intake (chow plus yogurt) was affected by the Phenotype and the Sweetener (Main effect of Phenotype, F1, 29 = 5.06, p = .032; Main effect of Sweetener, F1, 29 = 6.4, p = .017; Phenotype X Sweetener interaction, F1, 29 = 5.16, p = .031). Post-hoc tests indicated that in DIO animals (Figure 3A), total caloric intake was significantly higher in animals receiving the saccharin-sweetened yogurt compared to the glucose-sweetened yogurt, while there were no differences in total caloric intake in DR animals receiving saccharin-sweetened versus glucose-sweetened yogurt (Figure 3B). There were no significant differences in total grams of yogurt consumed across sweeteners or phenotype (Means = 635 + 23 g and 598 + 27 g for DIO glucose and saccharin groups, respectively; 632 + 25 g and 627 + 23 g for DR glucose and saccharin groups; all Fs < 1). Since there were no differences in the quantity of yogurt consumed, the number of calories consumed from yogurt was significantly higher in animals given the glucose-sweetened yogurt, but there were no effects of phenotype (Main effect of Sweetener, F1, 29 = 147.02, p < .0000001, other Fs < 1). Since energy intake from the yogurt was lower in the DIO Saccharin group compared to the DIO Glucose group, but total energy intake was higher in the DIO Saccharin group compared to the DIO Glucose group, the difference in total energy was higher accounted for by increased intake of the HE diet DIO Saccharin group compared to the DIO Glucose group. This was confirmed by statistical analysis, with energy consumed from the HE diet affected by the sweetener as well as the phenotype (Main effect of Sweetener, F1, 29 = 4.6, p = .024; Main effect of Phenotype, F1, 29 = 43.4, p < .0000001; Sweetener X Phenotype interaction, F1, 29 = 5.1, p=.03). Post-hoc analysis revealed that in both DIO (Figure 3A) and DR (Figure 3B) groups, animals given the saccharin-sweetened yogurt consumed greater number of calories from the HE diet than animals given the glucose-sweetened yogurt. In addition, while there were no differences in calories consumed from the HE diet in DIO Glucose animals compared to DR Gucose animals, DIO Saccharin animals consumed significantly more than DR Saccharin animals.
Figure 3.
A) In DIO animals fed a diet high in saturated fat and sugar across 4 weeks of yogurt exposure, caloric intake from yogurt was significantly higher in female rats consuming glucose-sweetened yogurt compared to DIO rats consuming saccharin-sweetened yogurt, while both caloric intake from the HE diet and total caloric intake (from HE diet plus yogurt) was significantly greater in animals consuming the saccharin-sweetened yogurt. B) In DR females, energy intake from yogurt was higher in females given the glucose-sweetened yogurt and intake from the HE diet was significantly higher in females given the saccharin-sweetened yogurt. However, total caloric intake in DR female rats did not differ between saccharin and glucose groups.
* p < .05 compared to glucose group
Experiment 3
Prior to introduction of the yogurt supplements, weight gain on the HE diet was significantly greater in the DIO than DR females (Main effect of Phenotype, F1, 38 = 98.5, p < .0000001; Means = 14.8 + 1.5 for DIO and -5.8 + 1.5 for DR).
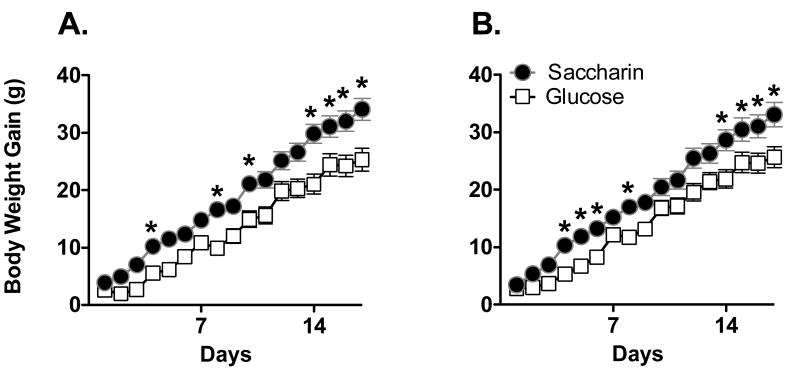
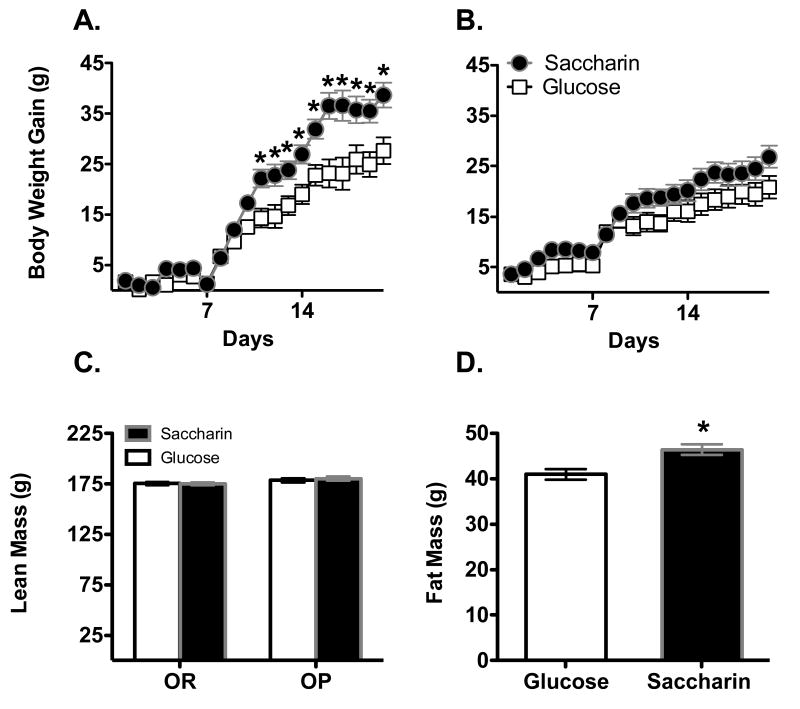
After the introduction of the yogurt supplements, weight gain was affected by the sweetener provided in the yogurt supplements, as well as by the phenotype of the animal (Figure 5; Main effect of Day, F16, 608 = 84.2, p < .0000001; Sweetener X Phenotype X Day interaction, F16, 608 = 1.85 p = .023). DIO females given the Saccharin-sweetened yogurt gained more weight than DIO females given Glucose-sweetened yogurt (Figure 4A; Main effect of Sweetener, F1, 19 = 5.65, p = 0.028; Main effect of Day, F16,304 = 38.9, p < .0000001; Sweetener X Day interaction, F16, 304 =2.60, p = .00083) with post-hoc tests revealing significant differences on the last 3 days of yogurt exposure. In contrast, there were no differences in body weight gain in DR animals given Saccharin-sweetened versus Glucose-sweetened yogurt (Figure 4B; Main effect of Day, F16, 304 = 46.9, p < .0000001; all other Fs < 1).
Figure 5.
Body weight gain in male rats was significantly greater when consuming Saccharin-sweetened yogurt along with a chow diet high in sugar and fat compared to those given Glucose-sweetened yogurt in both DIO (A) and DR (B) phenotypes.
*p < .05 compared to glucose-sweetened group
Figure 4.
Body weight gain in DIO female rats (panel A) was significantly greater when consuming Saccharin-sweetened yogurt along with a chow diet high in sugar and fat compared to those given Glucose-sweetened yogurt. In contrast, DR females (B) showed no differences in body weight gain based on the type of sweetener consumed. Fat mass (C) was significantly greater in female DIO rats compared to female DR rats, while lean mass (D) was significantly greater in females that consumed Saccharin-sweetened yogurt compared to females that consumed Glucose-sweetened yogurt.
# p < .05 compared to DR
* p < .05 compared to Glucose
Analysis of body fat mass indicated that fat mass at the end of yogurt exposure was affected by the starting fat mass and by the phenotype, but not by the type of sweetener consumed (Main effect of Starting Fat mass, F1, 37 = 9.7, p = .0035; Main effect of Phenotype, F1, 37 = 4.17, p = .048). Post-hoc analysis revealed that DIO animals had significantly higher fat mass than DR animals at the end of yogurt exposure (Figure 4C). Lean mass at the end of yogurt was affected by Starting Lean mass and the Sweetener consumed but not by Phenotype, with animals given saccharin-sweetened yogurt having greater lean mass than animals given glucose-sweetened yogurt (Figure 4D; Main effect of Starting Lean mass, F1, 37 = 31.1, p = .000002; Main effect of Sweetener, F1, 37 = 5.29, p = .029).
Experiment 4
Body weight gain on the HE diet prior to the introduction of the yogurt supplements was significantly higher in DIO male than DR males (Main effect of Phenotype, F1, 38 = 68.2, p < .0000001; Means + SEM = 77.8 + 1.4 for DIO and 61.3 + 1.4 for DR) but did not differ across sweeteners within each phenotype.
After the introduction of the yogurt, body weight gain in male rats was significantly affected by the sweetener provided but not the phenotype (Figure 5; Main effect of Sweetener, F1, 38 = 20.7, p = .000054; Main effect of Day, F16, 608 = 534.7, p < .0000001; Day X Sweetener interaction, F16, 608 = 5.4, p < .0000001; other F's < 1). Post-hoc analyses indicated that male rats given the saccharin-sweetened yogurt gained significantly more weight on days 4-16 of the yogurt exposure, with no significant differences between DIO and DR animals.
Experiment 5
Body weight gain was affected by the sweetener, phenotype of the animal, and the day of testing (Figure 6; Main effect of Sweetener, F1, 39 = 9.7, p = .0035; Main effect of day, F19, 741 = 254.6, p < .0000001; Sweetener X Day interaction, F19, 741 = 7.1, p < .0000001; Phenotype X Day interaction, F19, 741 = 22.1, p < .0000001; Sweetener X Phenotype X Day interaction, F19, 665 = 2.30, p = .0013). Post-hoc analyses indicated that in OP animals, weight gain was affected by the sweetener provided, as well as by the day of testing (Figure 6A; Main effect of Sweetener, F1, 14 = 11.5, p = .0044; Main effect of Day, F19, 266 = 143.3, p < .0000001; Sweetener X Day interaction, F19, 266 = 6.44, p < .0000001), with female OP rats given saccharin-sweetened yogurt showing significantly greater body weight gain compared to OP rats given glucose-sweetened yogurt beginning on Day 11. In female OR animals, body weight gain was not significantly affected by the sweetener provided, although a trend for increased weight gain in OR females given saccharin-sweetened yogurt was observed (Figure 6B; Main effect of Day, F19, 399 = 87.3, p < .0000001; Sweetener X Day interaction, F19, 399 = 1.59, p = .055).
Figure 6.
Body weight gain in offspring of selectively bred OP rats (A) and OR rats (B) given saccharin-sweetened yogurt did not differ from same-phenotype females during the first week of yogurt exposure when a standard low-fat laboratory chow was provided along with the sweetened yogurt. However, body weight gain was significantly higher during the last 2 weeks of yogurt when OP rats were given Saccharin-sweetened yogurt and an HE diet compared to glucose-sweetened yogurt and the HE diet. These effects were more pronounced in OP females compared to OR females, where only a trend towards increased weight gain in the animals consuming the saccharin-sweetened diet was observed. C) Fat mass was significantly greater in female OP and OR rats that consumed Saccharin-sweetened yogurt compared to females that consumed Glucose-sweetened yogurt, while lean mass (D) did not differ significantly based on sweetener or phenotype.
* p < .05 compared to Glucose-sweetened yogurt
Fat mass at the end of yogurt exposure was significantly affected by the starting body fat mass and the sweetener to which animals had been exposed (Figure 6C; Main effect of Starting fat mass, F1, 38 = 105.91, p < .0000001; Main effect of Sweetener, F1, 38 = 11.3, p = .0018). Animals given the saccharin-sweetened yogurt had significantly greater fat mass than animals given the glucose-sweetened yogurt. Lean mass was affected by starting lean mass, but there were no significant effects of sweetener or phenotype on lean mass at the end of yogurt exposure (Figure 6D; Main effect of Starting lean mass, F1, 38 = 143.2, p < .0000001; trend for Main effect of Phenotype, F1, 38 = 2.89, p =.097).
Discussion
Our previous results with male rats provided evidence that, compared to consuming caloric sweeteners, consuming non-caloric sweeteners produces excess energy intake and weight gain, not reduced energy intake and weight loss (e.g., Davidson et al., 2011; Davidson & Swithers, 2004; Swithers, Baker, & Davidson, 2009; Swithers & Davidson, 2008; Swithers et al., 2012; Swithers, Martin, Clark, Laboy, & Davidson, 2010; Swithers, Martin, & Davidson, 2010). Our previous data also provided evidence for a physiologically-relevant mechanism that describes how consumption of non-caloric sweeteners can interfere with the normal controls of energy regulation by interfering with a typically predictive relation between sweet taste and calories (Davidson et al., 2011; Davidson & Swithers, 2004; Swithers & Davidson, 2008; Swithers et al., 2012; Swithers, Martin, Clark, et al., 2010; Swithers, Martin, & Davidson, 2010). The results of the present experiments with female rats indicate that the negative effects of consuming noncaloric sweeteners on intake and body weight may be the most severe for females that are most prone to developing high body weight and adiposity.
For example, female rats maintained on a standard, low-fat laboratory chow diet (Experiment 1) are relatively resistant to the negative consequences dissociating sweet taste from energetic consequences. However, when female rats eat a high fat, sweetened diet similar to that presently consumed by large segments of the U.S. population, saccharin-sweetened yogurts in fact stimulate food intake (Experiment 2), promote excess weight gain (Experiments 2, 3 and 5) and may result in increased adiposity (Experiments 2 and 5) compared to glucose-sweetened yogurts. Most strikingly, these negative effects of consuming a HE diet along with saccharin-sweetened yogurt were noted in animals already prone to excess weight gain prior to the introduction of the yogurt diets (i.e., the DIO and OP phenotypes). In female rats with a phenotype already resistant to HE diet-induced weight gain, the addition of a saccharin-sweetened yogurt to the HE diet had minimal effects on energy balance.
The design employed here provided animals with a fixed amount (30 g) of a dietary supplement (low-fat yogurt) in plain, unsweetened form on some days, and a form to which a sweetener has been added on other days. For one set of animals, the sweetener provides additional calories; for example, addition of 20% glucose by weight results in the sweetened yogurt providing approximately twice as many calories per gram as the unsweetened yogurt (1.2 kcal/g compared to 0.6 kcal/g). These animals are therefore provided with explicit exposure to a typical relation in which sweet taste consistently predicts the delivery of energy. For the second group, the addition of 0.3% saccharin increased the sweetness of the yogurt without changing the energy. This group was therefore provided with explicit exposure to a relation in which sweet taste no longer served as a reliable predictor of the delivery of energy.
Two features of this design approach merit discussion. First, animals in the two groups consume identical, fixed amounts of yogurt. That is, increases in food intake, body weight gain and adiposity in animals given the saccharin-sweetened yogurt do not result from one group of animals over-consuming the sweetened yogurt supplements. Instead, they appear to result from overconsumption of the maintenance chow provided along with the yogurt in animals given the saccharin-sweetened yogurt (Experiment 2 and Swithers & Davidson, 2008; Swithers, Martin, Clark, et al., 2010; Swithers, Martin, & Davidson, 2010). Second, the design does not require that the saccharin-sweetened yogurt and the glucose-sweetened yogurt be identical to one another in terms of sweetness per se. Determining which sweetener, saccharin or glucose, at the concentrations provided in these studies, actually tastes sweeter when provided in yogurt is not a simple matter. For example, some data suggest that rats may prefer solutions of glucose to solutions of saccharin at these concentrations (e.g., Fernstrom et al., 2012; Sclafani & Nissenbaum, 1985), suggesting that the glucose-sweetened yogurt might be perceived as sweeter than the saccharin-sweetened yogurt. However, preferences are affected not just by taste properties but also by post-ingestive effects, so a preference for glucose does not necessarily indicate that the glucose-sweetened yogurt tastes sweeter. In addition, other data suggest that saccharin solutions can produce stronger electrophysiological responses in the chorda tympani than glucose (Miyasaka & Imoto, 1995), suggesting that the saccharin-sweetened yogurt might in fact be sweeter than the glucose-sweetened yogurt. In either case, it is not immediately clear how differences in the intensity of the two sweeteners could produce the pattern of results observed.
The results of this study support a number of significant differences in the responses of female rats compared to male rats when high-intensity sweeteners are consumed. For example, male rats tested with a standard laboratory chow diet, like the one used in the present Experiment 1, show increased weight gain when given saccharin-sweetened compared to glucose-sweetened yogurt supplement (Swithers et al., 2009; Swithers & Davidson, 2008; Swithers et al., 2012; Swithers, Martin, & Davidson, 2010), while female rats failed to demonstrate such effects. Further, the consequences of a predisposition to gain excess weight appeared limited to female rats, as male rats in Experiment 4 showed similar tendencies to gain excess weight when given saccharin-sweetened diets compared to glucose-sweetened diets whether they were prone to diet-induced obesity or resistant to it prior to the introduction of the sweetener. While slightly different procedures were used to phenotype males compared to females in these experiments, taken together the results are consistent with significant sexual diergism in responding to disruption of the relation between sweet tastes and calories.
Mechanisms that underlie such sexually divergent and phenotypic responses are not known, but might include differences in the perception of the sweet tastes (e.g., Asarian & Geary, 2006; Atchley et al., 2005; Curtis et al., 2005; Kenney & Redick, 1980; Valenstein et al., 1967; Wade & Zucker, 1969); differences in Pavlovian conditioning (e.g., Ackroff & Sclafani, 2004; Andreano & Cahill, 2009; Chambers, 1985; de Beun et al., 1991); and/or basic metabolic systems underlying energy balance (e.g., Asarian & Geary, 2006; Clegg, Brown, Woods, & Benoit, 2006; Eckel, 2011; Flanagan-Cato, Grigson, & King, 2001; Shi, Seeley, & Clegg, 2009; Wade, 1972; Wade & Gray, 1979; Woods, Gotoh, & Clegg, 2003). Elucidating these mechanisms will require additional study.
Our previous research showed that in addition to increased weight gain, male rats that previously consumed saccharin exhibited higher levels of blood glucose, lower levels of GLP-1, and a weakened thermogenic response following intake of sweet caloric foods or fluids (Swithers & Davidson, 2008; Swithers et al., 2012). Whether these changes are causes or effects of weight gain is the subject of continued research interest. However, our findings and conceptual framework suggest that both weight gain and changes in hormonal or metabolic profiles may be based, at least in part, on the operation of a different set of factors.
Specifically, we showed that consuming saccharin selectively weakens the ability of sweet taste to signal caloric outcomes and selectively reduces the ability of rats to regulate their intake of sweetened high-energy food (Davidson et al., 2011). These data suggest that, in addition to investigating the potential metabolic or hormonal processes that may contribute to obesity, research attention should also be directed at elucidating the neural mechanisms that underlie the effects of high-intensity sweeteners on learning and memory. Recent research on “taste memory” and the processing of taste information has produced results that are consistent with our general Pavlovian model (Nunez-Jaramillo, Ramirez-Lugo, Herrera-Morales, & Miranda, 2010). For example, a variety of evidence shows that for animals as diverse as fruit flies and rodents, the taste and nutritive consequences of consuming sweet food are encoded independently (Wright, 2011).
Furthermore, other findings indicate that little may be learned or remembered about sweet tastes that are not paired with nutritive outcomes (Burke & Waddell, 2011). It has been proposed that the function of sweet taste is to enable animals to identify substances in the environment that are sources of energy (e.g., Small, 2010). Findings that this signaling ability is not an inherent property of sweet taste but depends on experiencing both sweet taste and caloric consequences concurrently is at the core of our model and makes more plausible our notion that separating sweet taste from nutritive consequences reduces the ability for animals to use sweet taste to help regulate intake.
This analysis suggests that to advance understanding of energy and body weight regulation greater research attention should be directed toward determining how and where sweet tastes are associated with calories in the brain. In the rodent, there is evidence that the nucleus accumbens contains neural populations that encode information independently about taste and about changes in metabolic status that may arise from detection of nutrients in the gastrointestinal tract (de Araujo et al., 2008). In humans, the insula is thought to be a brain region where orosensory information, including taste, is represented (Small, 2010). One imaging study (Rudenga, Green, Nachtigal, & Small, 2010) reported that oral stimulation regardless of modality (chemesthetic or gustatory) or physiological relevance (nutritive or harmful) evoked a response in the anterior ventral insula. However, connectivity between this area and hypothalamic and striatal circuits involved with feeding was observed when subjects sampled potentially nutritive sweet or salty tastes. The authors suggested that the anterior ventral insula preferentially interacts with brain systems that underlie feeding behaviors and homeostasis when the oral stimulus is potentially nutritive.
Another neuroimaging study (Rudenga & Small, 2012) compared responses to sweet, caloric, sucrose solutions by humans that differed in their self-reported use of noncaloric sweeteners. This study found significant negative correlations between frequency of artificial sweetener use and response in the insula and amygdala. A further imaging study in humans also documented altered activation in the amygdala, as well as the ventral tegmental area, in self-reported diet soda drinkers compared to those that did not consume diet sodas (Green & Murphy, 2012). Previous studies with rats indicate that different areas of amygdala may be involved with the acquisition and extinction of learning about tastes. For example, lesions of the central nucleus of the amygdala impaired acquisition, but not extinction, of a taste aversion, whereas lesions of the basolateral amygdala (BLA) had the opposite effect (Bahar, Samuel, Hazvi, & Dudai, 2003). Impaired extinction of responding to a visual cue associated with food following BLA lesions has also been reported (e.g., Lindgren, Gallagher, & Holland, 2003). If intake of high-intensity sweeteners involves at least partial extinction of an association between tastes and a nutritive outcome, it would be of interest to determine if interference with BLA function would retard or block the effect of experience with those sweeteners on food intake and weight gain. One important implication of the present findings is that identification of psychological and physiological substrates involved with the formation and modification of sweet taste-calorie relations will need to consider potential sex differences.
The use of high-intensity sweeteners has been considered to be a useful tool to control body weight, body adiposity and metabolic disorders because it is expected to allow individuals to limit caloric intake without sacrificing the pleasure of consuming sweet foods and beverages. Indeed, many nutrition and health authorities have condoned, and even encouraged, the immense rise in the availability and consumption of artificially-sweetened products (American Dietetic Association, 2004; Bellisle & Drewnowski, 2007; Duffey, Steffen, Van Horn, Jacobs, & Popkin, 2012; Mattes & Popkin, 2009; Tate et al., 2012). This enthusiasm is based in large part on two assumptions; first, that holding energy expenditure constant, weight loss will result as a consequence of reduced caloric intake and second that replacing caloric sugars with non-caloric sweeteners will reduce caloric intake. The first assumption appears to be undeniably correct. We believe that the second assumption may be dangerously flawed. In fact, an important implication of our findings for humans is that the potential adverse effects of consuming high intensity sweeteners on energy and body weight regulation may be exacerbated in females who are most likely to consume those products; namely, women who are obese or prone to obesity and who are consuming a westernized diet high in fat and sugar.
Generalizing from these laboratory data to results from correlational or interventional studies in humans can be complicated by a number of factors, not least the variability in results from human studies attempting to understand the relationship between consuming high-intensity sweeteners and energy balance. For example, while some epidemiological studies indicate that consuming artificially-sweetened products is associated with increased risk of negative outcomes, including weight gain, diabetes, heart disease and metabolic syndrome (e.g., Dhingra et al., 2007; Fowler et al., 2008; Gardener et al., 2012; Laska, Murray, Lytle, & Harnack, 2012; Ludwig, 2009; Lutsey, Steffen, & Stevens, 2008; Nettleton, Polak, Tracy, Burke, & Jacobs, 2009; Yang, 2010), other data suggest that no such association exists, or that consumption of sweeteners is associated with decreased risk (e.g., Berkey, Rockett, Field, Gillman, & Colditz, 2004; de Koning, Malik, Rimm, Willett, & Hu, 2011; Fung et al., 2009; Ludwig, Peterson, & Gortmaker, 2001; Schulze et al., 2004). Further, while some interventional studies demonstrate that consumption of high-intensity sweeteners is associated with reduced weight gain, weight loss or improved metabolic parameters (de Ruyter, Olthof, Seidell, & Katan, 2012; Raben et al., 2011; Raben, Vasilaras, Moller, & Astrup, 2002), other studies show no benefit from consumption of high-intensity sweeteners (Blackburn, Kanders, Lavin, Keller, & Whatley, 1997; Duffey et al., 2012; Tate et al., 2012). One advantage of animal studies such as those reported here is that they may identify factors contributing to the variability observed in human studies. Based on the results of the present data, among the factors that may demand special attention are the composition of the diet consumed along with the high-intensity sweeteners, the sex of the individual consuming the sweetener and phenotypic differences related to propensity to gain weight before sweeteners are introduced. Thus, to the extent that women are more prone to overweight and obesity (e.g., Ogden et al., 2010) and may be more likely to consume non-caloric sweeteners (e.g., Duffey & Popkin, 2006; Fowler et al., 2008), data such as those obtained in our rat experiments suggest that the biggest negative impact of high-intensity sweeteners may actually occur in those individuals most likely to use them as a means of weight reduction, weight maintenance or improving metabolic health.
Acknowledgments
We thank Melissa McCurley, Kiely Clark, Stephanie Cooper, Ethan Flint, Natalie Rappaport and Catherine Harlan for technical assistance. Supported by NIH grants R01 DK076078 and P01 HD052112.
References Cited
- Ackroff K, Sclafani A. Fructose-conditioned flavor preferences in male and female rats: effects of sweet taste and sugar concentration. Appetite. 2004;42(3):287–297. doi: 10.1016/j.appet.2004.01.001. [DOI] [PubMed] [Google Scholar]
- American Dietetic Association. Position of the American Dietetic Association: use of nutritive and nonnutritive sweeteners. Journal of the American Dietetic Association. 2004;104(2):255–275. doi: 10.1016/j.jada.2003.12.001. Erratum appears in J Am Diet Assoc. 2004 Jun;104(6):1013. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Sex influences on the neurobiology of learning and memory. Learn Mem. 2009;16(4):248–266. doi: 10.1101/lm.918309. [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci. 2006;361(1471):1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchley DP, Weaver KL, Eckel LA. Taste responses to dilute sucrose solutions are modulated by stage of the estrous cycle and fenfluramine treatment in female rats. Physiol Behav. 2005;86(3):265–271. doi: 10.1016/j.physbeh.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Bahar A, Samuel A, Hazvi S, Dudai Y. The amygdalar circuit that acquires taste aversion memory differs from the circuit that extinguishes it. European Journal of Neuroscience. 2003;17(7):1527–1530. doi: 10.1046/j.1460-9568.2003.02551.x. [DOI] [PubMed] [Google Scholar]
- Bellisle F, Drewnowski A. Intense sweeteners, energy intake and the control of body weight. Eur J Clin Nutr. 2007;61(6):691–700. doi: 10.1038/sj.ejcn.1602649. [DOI] [PubMed] [Google Scholar]
- Berkey CS, Rockett HR, Field AE, Gillman MW, Colditz GA. Sugar-added beverages and adolescent weight change. Obes Res. 2004;12(5):778–788. doi: 10.1038/oby.2004.94. [DOI] [PubMed] [Google Scholar]
- Blackburn GL, Kanders BS, Lavin PT, Keller SD, Whatley J. The effect of aspartame as part of a multidisciplinary weight-control program on short- and long-term control of body weight. Am J Clin Nutr. 1997;65(2):409–418. doi: 10.1093/ajcn/65.2.409. [DOI] [PubMed] [Google Scholar]
- Bowen DJ. Taste and food preference changes across the course of pregnancy. Appetite. 1992;19(3):233–242. doi: 10.1016/0195-6663(92)90164-2. [DOI] [PubMed] [Google Scholar]
- Bowen DJ, Grunberg NE. Variations in food preference and consumption across the menstrual cycle. Physiol Behav. 1990;47(2):287–291. doi: 10.1016/0031-9384(90)90144-s. [DOI] [PubMed] [Google Scholar]
- Burke CJ, Waddell S. Remembering nutrient quality of sugar in Drosophila. Curr Biol. 2011;21(9):746–750. doi: 10.1016/j.cub.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers KC. Sexual dimorphisms as an index of hormonal influences on conditioned food aversions. Ann N Y Acad Sci. 1985;443:110–125. doi: 10.1111/j.1749-6632.1985.tb27067.x. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55(4):978–987. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- Curtis KS, Stratford JM, Contreras RJ. Estrogen increases the taste threshold for sucrose in rats. Physiol Behav. 2005;86(3):281–286. doi: 10.1016/j.physbeh.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Martin AA, Clark K, Swithers SE. Intake of high-intensity sweeteners alters the ability of sweet taste to signal caloric consequences: implications for the learned control of energy and body weight regulation. Q J Exp Psychol (Hove) 2011;64(7):1430–1441. doi: 10.1080/17470218.2011.552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Swithers SE. A Pavlovian approach to the problem of obesity. International Journal of Obesity & Related Metabolic Disorders. 2004;28(7):933–935. doi: 10.1038/sj.ijo.0802660. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA, Simon SA. Food reward in the absence of taste receptor signaling. Neuron. 2008;57(6):930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- de Beun R, Jansen E, Smeets MA, Niesing J, Slangen JL, van de Poll NE. Estradiol-induced conditioned taste aversion and place aversion in rats: sex- and dose-dependent effects. Physiol Behav. 1991;50(5):995–1000. doi: 10.1016/0031-9384(91)90427-p. [DOI] [PubMed] [Google Scholar]
- de Koning L, Malik VS, Rimm EB, Willett WC, Hu FB. Sugar-sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. Am J Clin Nutr. 2011;93(6):1321–1327. doi: 10.3945/ajcn.110.007922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruyter JC, Olthof MR, Seidell JC, Katan MB. A trial of sugar-free or sugar-sweetened beverages and body weight in children. N Engl J Med. 2012;367(15):1397–1406. doi: 10.1056/NEJMoa1203034. [DOI] [PubMed] [Google Scholar]
- Dhingra R, Sullivan L, Jacques PF, Wang TJ, Fox CS, Meigs JB, D'Agostino RB, Gaziano JM, Vasan RS. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation. 2007;116(5):480–488. doi: 10.1161/CIRCULATIONAHA.107.689935. [DOI] [PubMed] [Google Scholar]
- Duffey KJ, Popkin BM. Adults with healthier dietary patterns have healthier beverage patterns. J Nutr. 2006;136(11):2901–2907. doi: 10.1093/jn/136.11.2901. [DOI] [PubMed] [Google Scholar]
- Duffey KJ, Steffen LM, Van Horn L, Jacobs DR, Jr, Popkin BM. Dietary patterns matter: diet beverages and cardiometabolic risks in the longitudinal Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr. 2012;95(4):909–915. doi: 10.3945/ajcn.111.026682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel LA. The ovarian hormone estradiol plays a crucial role in the control of food intake in females. Physiol Behav. 2011;104(4):517–524. doi: 10.1016/j.physbeh.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernstrom JD, Munger SD, Sclafani A, de Araujo IE, Roberts A, Molinary S. Mechanisms for sweetness. J Nutr. 2012;142(6):1134S–1141S. doi: 10.3945/jn.111.149567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan-Cato LM, Grigson PS, King JL. Estrogen-induced suppression of intake is not mediated by taste aversion in female rats. Physiol Behav. 2001;72(4):549–558. doi: 10.1016/s0031-9384(01)00411-5. [DOI] [PubMed] [Google Scholar]
- Fowler SP, Williams K, Resendez RG, Hunt KJ, Hazuda HP, Stern MP. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity (Silver Spring) 2008;16(8):1894–1900. doi: 10.1038/oby.2008.284. [DOI] [PubMed] [Google Scholar]
- Fung TT, Malik V, Rexrode KM, Manson JE, Willett WC, Hu FB. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr. 2009;89(4):1037–1042. doi: 10.3945/ajcn.2008.27140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardener H, Rundek T, Markert M, Wright CB, Elkind MS, Sacco RL. Diet Soft Drink Consumption is Associated with an Increased Risk of Vascular Events in the Northern Manhattan Study. J Gen Intern Med. 2012 doi: 10.1007/s11606-011-1968-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green E, Murphy C. Altered processing of sweet taste in the brain of diet soda drinkers. Physiol Behav. 2012 doi: 10.1016/j.physbeh.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney NJ, Redick JH. Effects of ovariectomy and subsequent estradiol replacement on intake of sweet solutions. Physiol Behav. 1980;24(4):807–809. doi: 10.1016/0031-9384(80)90418-7. [DOI] [PubMed] [Google Scholar]
- Laska MN, Murray DM, Lytle LA, Harnack LJ. Longitudinal associations between key dietary behaviors and weight gain over time: transitions through the adolescent years. Obesity (Silver Spring) 2012;20(1):118–125. doi: 10.1038/oby.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA. Differential effects of exercise on body weight gain and adiposity in obesity-prone and -resistant rats. Int J Obes (Lond) 2006;30(4):722–727. doi: 10.1038/sj.ijo.0803192. [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol. 1997;273(2 Pt 2):R725–730. doi: 10.1152/ajpregu.1997.273.2.R725. [DOI] [PubMed] [Google Scholar]
- Levin BE, Magnan C, Migrenne S, Chua SC, Jr, Dunn-Meynell AA. F-DIO obesity-prone rat is insulin resistant before obesity onset. Am J Physiol Regul Integr Comp Physiol. 2005;289(3):R704–711. doi: 10.1152/ajpregu.00216.2005. [DOI] [PubMed] [Google Scholar]
- Lindgren JL, Gallagher M, Holland PC. Lesions of basolateral amygdala impair extinction of CS motivational value, but not of explicit conditioned responses, in Pavlovian appetitive second-order conditioning. European Journal of Neuroscience. 2003;17(1):160–166. doi: 10.1046/j.1460-9568.2003.02421.x. [DOI] [PubMed] [Google Scholar]
- Ludwig DS. Artificially sweetened beverages: cause for concern. JAMA. 2009;302(22):2477–2478. doi: 10.1001/jama.2009.1822. [DOI] [PubMed] [Google Scholar]
- Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet. 2001;357(9255):505–508. doi: 10.1016/s0140-6736(00)04041-1. [DOI] [PubMed] [Google Scholar]
- Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation. 2008;117(6):754–761. doi: 10.1161/CIRCULATIONAHA.107.716159. [DOI] [PubMed] [Google Scholar]
- Madsen AN, Hansen G, Paulsen SJ, Lykkegaard K, Tang-Christensen M, Hansen HS, Levin BE, Larsen PJ, Knudsen LB, Fosgerau K, Vrang N. Long-term characterization of the diet-induced obese and diet-resistant rat model: a polygenetic rat model mimicking the human obesity syndrome. J Endocrinol. 2010;206(3):287–296. doi: 10.1677/joe-10-0004. [DOI] [PubMed] [Google Scholar]
- Mattes RD, Popkin BM. Nonnutritive sweetener consumption in humans: effects on appetite and food intake and their putative mechanisms. Am J Clin Nutr. 2009;89(1):1–14. doi: 10.3945/ajcn.2008.26792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MR, Clegg DJ, Prossnitz ER, Barton M. Obesity, insulin resistance and diabetes: sex differences and role of oestrogen receptors. Acta Physiol (Oxf) 2011;203(1):259–269. doi: 10.1111/j.1748-1716.2010.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaka A, Imoto T. Electrophysiological characterization of the inhibitory effect of a novel peptide gurmarin on the sweet taste response in rats. Brain Res. 1995;676(1):63–68. doi: 10.1016/0006-8993(95)00086-6. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289(1):76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- Nettleton JA, Polak JF, Tracy R, Burke GL, Jacobs DR., Jr Dietary patterns and incident cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2009;90(3):647–654. doi: 10.3945/ajcn.2009.27597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez-Jaramillo L, Ramirez-Lugo L, Herrera-Morales W, Miranda MI. Taste memory formation: latest advances and challenges. Behav Brain Res. 2010;207(2):232–248. doi: 10.1016/j.bbr.2009.10.040. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll ME, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009-2010. NCHS Data Brief. 2012;(82):1–8. [PubMed] [Google Scholar]
- Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132(6):2087–2102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Lamb MM, Carroll MD, Flegal KM. Obesity and socioeconomic status in adults: United States 1988-1994 and 2005-2008. Hyattsville, MD: 2010. [PubMed] [Google Scholar]
- Paulsen SJ, Jelsing J, Madsen AN, Hansen G, Lykkegaard K, Larsen LK, Vrang N. Characterization of beta-cell mass and insulin resistance in diet-induced obese and diet-resistant rats. Obesity (Silver Spring) 2010;18(2):266–273. doi: 10.1038/oby.2009.245. [DOI] [PubMed] [Google Scholar]
- Raben A, Moller BK, Flint A, Vasilaris TH, Christina Moller A, Juul Holst J, Astrup A. Increased postprandial glycaemia, insulinemia, and lipidemia after 10 weeks' sucrose-rich diet compared to an artificially sweetened diet: a randomised controlled trial. Food Nutr Res. 2011;55 doi: 10.3402/fnr.v55i0.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raben A, Vasilaras TH, Moller AC, Astrup A. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2002;76(4):721–729. doi: 10.1093/ajcn/76.4.721. [DOI] [PubMed] [Google Scholar]
- Rudenga K, Green B, Nachtigal D, Small DM. Evidence for an integrated oral sensory module in the human anterior ventral insula. Chem Senses. 2010;35(8):693–703. doi: 10.1093/chemse/bjq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenga KJ, Small DM. Amygdala response to sucrose consumption is inversely related to artificial sweetener use. Appetite. 2012;58(2):504–507. doi: 10.1016/j.appet.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, Hu FB. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292(8):927–934. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Nissenbaum JW. On the role of the mouth and gut in the control of saccharin and sugar intake: a reexamination of the sham-feeding preparation. Brain Res Bull. 1985;14(6):569–576. doi: 10.1016/0361-9230(85)90106-6. [DOI] [PubMed] [Google Scholar]
- Shi H, Seeley RJ, Clegg DJ. Sexual differences in the control of energy homeostasis. Front Neuroendocrinol. 2009;30(3):396–404. doi: 10.1016/j.yfrne.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM. Taste representation in the human insula. Brain Struct Funct. 2010;214(5-6):551–561. doi: 10.1007/s00429-010-0266-9. [DOI] [PubMed] [Google Scholar]
- Swithers SE, Baker CR, Davidson TL. General and persistent effects of high-intensity sweeteners on body weight gain and caloric compensation in rats. Behav Neurosci. 2009;123(4):772–780. doi: 10.1037/a0016139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swithers SE, Davidson TL. A role for sweet taste: calorie predictive relations in energy regulation by rats. Behav Neurosci. 2008;122(1):161–173. doi: 10.1037/0735-7044.122.1.161. [DOI] [PubMed] [Google Scholar]
- Swithers SE, Laboy AF, Clark K, Cooper S, Davidson TL. Experience with the high-intensity sweetener saccharin impairs glucose homeostasis and GLP-1 release in rats. Behav Brain Res. 2012 doi: 10.1016/j.bbr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swithers SE, Martin AA, Clark KM, Laboy AF, Davidson TL. Body weight gain in rats consuming sweetened liquids. Effects of caffeine and diet composition. Appetite. 2010;55(3):528–533. doi: 10.1016/j.appet.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swithers SE, Martin AA, Davidson TL. High-intensity sweeteners and energy balance. Physiol Behav. 2010;100(1):55–62. doi: 10.1016/j.physbeh.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate DF, Turner-McGrievy G, Lyons E, Stevens J, Erickson K, Polzien K, Diamond M, Wang X, Popkin B. Replacing caloric beverages with water or diet beverages for weight loss in adults: main results of the Choose Healthy Options Consciously Everyday (CHOICE) randomized clinical trial. Am J Clin Nutr. 2012;95(3):555–563. doi: 10.3945/ajcn.111.026278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper BJ, Seldner AC. Sweet taste and intake of sweet foods in normal pregnancy and pregnancy complicated by gestational diabetes mellitus. Am J Clin Nutr. 1999;70(2):277–284. doi: 10.1093/ajcn.70.2.277. [DOI] [PubMed] [Google Scholar]
- Than TT, Delay ER, Maier ME. Sucrose threshold variation during the menstrual cycle. Physiol Behav. 1994;56(2):237–239. doi: 10.1016/0031-9384(94)90189-9. [DOI] [PubMed] [Google Scholar]
- Valenstein ES, Kakolewski JW, Cox VC. Sex differences in taste preference for glucose and saccharin solutions. Science. 1967;156(3777):942–943. doi: 10.1126/science.156.3777.942. [DOI] [PubMed] [Google Scholar]
- Wade GN. Gonadal hormones and behavioral regulation of body weight. Physiol Behav. 1972;8(3):523–534. doi: 10.1016/0031-9384(72)90340-x. [DOI] [PubMed] [Google Scholar]
- Wade GN, Gray JM. Gonadal effects on food intake and adiposity: a metabolic hypothesis. Physiol Behav. 1979;22(3):583–593. doi: 10.1016/0031-9384(79)90028-3. [DOI] [PubMed] [Google Scholar]
- Wade GN, Zucker I. Hormonal and developmental influences on rat saccharin preferences. J Comp Physiol Psychol. 1969;69(2):291–300. doi: 10.1037/h0028208. [DOI] [PubMed] [Google Scholar]
- Woods SC, Gotoh K, Clegg DJ. Gender differences in the control of energy homeostasis. Exp Biol Med (Maywood) 2003;228(10):1175–1180. doi: 10.1177/153537020322801012. [DOI] [PubMed] [Google Scholar]
- Wright GA. Appetitive learning: memories need calories. Curr Biol. 2011;21(9):R301–302. doi: 10.1016/j.cub.2011.03.055. [DOI] [PubMed] [Google Scholar]
- Yang Q. Gain weight by “going diet?” Artificial sweeteners and the neurobiology of sugar cravings: Neuroscience 2010. Yale J Biol Med. 2010;83(2):101–108. [PMC free article] [PubMed] [Google Scholar]