Summary
Background
Stress hormones have been hypothesized to contribute to the social patterning of cardiovascular disease but evidence of differences in hormone levels across social groups is scant.
Purpose
To examine the associations of socioeconomic and psychosocial factors with urinary levels of cortisol and catecholamines and determine whether these associations are modified by race/ethnicity.
Methods
Measures of cortisol, epinephrine, norepinephrine and dopamine were obtained on 12-h overnight urine specimens from 942 White, African American and Hispanic participants in the Multi-Ethnic Study of Atherosclerosis (MESA). Linear regression was used to examine associations of income-wealth index, education, depression, anger, anxiety and chronic stress with the four hormones after adjustment for covariates.
Results
Higher income-wealth index was associated with lower levels of urinary cortisol, epinephrine, norepinephrine and dopamine, after adjustment for age, sex, race/ethnicity, medication use, body mass index, smoking, and alcohol use. Education and psychosocial factors were not associated with urinary stress hormone levels in the full sample. However, there was some evidence of effect modification by race: SES factors were more strongly inversely associated with cortisol in African Americans than in other groups and anger was inversely associated with catecholamines in African Americans but not in the other groups.
Conclusions
Lower SES as measured by income-wealth index in a multi-ethnic sample is associated with higher levels of urinary cortisol and catecholamines. Heterogeneity in these associations by race/ethnicity warrants further exploration.
Keywords: Urinary cortisol, Urinary catecholamines, Social factors, Psychological factors, Race/ethnicity, Multi-Ethnic of Atherosclerosis
1. Introduction
It has long been known that socioeconomically disadvantaged persons are at higher risk of developing cardiovascular disease (CVD), and tend to have worse outcomes once they develop it, compared to those who are less disadvantaged (Marmot et al., 1991; Kaplan and Keil, 1993; Diez Roux et al., 2001; Steenland et al., 2004; Williams and Jackson, 2005). Many studies have shown that behavioral and biomedical cardiovascular risk factors (Ramsay et al., 2011), such as smoking (Novotny et al., 1988), hypertension (Brummett et al., 2011), physical activity (Cauley et al., 1991), diet (Galobardes et al., 2001), obesity (Sobal and Stunkard, 1989), and diabetes and blood lipids (Manuck et al., 2010; Wild et al., 2010) are associated with measures of socioeconomic status (SES). However, behavioral and biomedical cardiovascular risk factors do not fully explain the association of CVD with SES (Kaplan and Keil, 1993; Diez Roux et al., 2001; Marmot, 2001). Some researchers have therefore hypothesized that psychosocial stress and its biological consequences may also contribute to the social patterning of CVD (Adler et al., 1994; Steptoe and Marmot, 2002; Gebreab et al., 2012).
Among the most widely recognized biomarkers of stress are cortisol and catecholamines, which are the effectors of the two major systems mediating most components of the stress response. Cortisol levels in plasma and urine reflect the functioning of the hypothalamic-pituitary axis (HPA) (Tsigos and Chrousos, 2002), while catecholamine concentrations in plasma and urine reflect activation of the sympathetic nervous system (SNS) (Axelrod and Reisine, 1984). A small but relatively constant proportion of the circulating levels of epinephrine and norepinephrine in the blood is excreted into the urine (Frankenhaeuser, 1971). Urinary measurements of both cortisol and epinephrine and norepinephrine have previously been used to assess the activity of the HPA axis and the SNS in laboratory and population studies (Lundberg and Frankenhaeuser, 1980; Seeman et al., 1994, 2001; Janicki-Deverts et al., 2007).
Dopamine is both a neurotransmitter and a precursor in the synthesis of norepinephrine, which is both a hormone and a neurotransmitter. To our knowledge, only two prior studies of stress and socioeconomic status and psychosocial factors have included dopamine; both studies combined dopamine measures into a multi-measure index of the allostatic load (Seplaki et al., 2004, 2006; Glei et al., 2007).
Dysregulation of the HPA or SNS or both has been hypothesized to contribute to the development of atherosclerosis or to cardiovascular events through a number of mechanisms (Manuck et al., 1995). Excessive cortisol production has been associated with development of the metabolic syndrome and visceral obesity, insulin resistance, dyslipidemia, and hyper-tension (Chrousos and Gold, 1998; Chrousos, 2000). An attenuated decline in norepinephrine and epinephrine excretion during nighttime sleep has been observed among “nondippers” (persons whose systolic blood pressure drop less than 10% during nighttime sleep) (Sherwood et al., 2002). Catecholamines may influence the development of atherosclerosis through their metabolic effects on glucose and insulin or through hemodynamic effects on the arterial wall (Pickering, 1999). Cortisol and catecholamines also initiate a cytokine response characterized by expression of adhesion molecules on the endothelium and a local inflammatory reaction in the vascular wall (Black and Garbutt, 2002). Stress hormones may also activate nuclear factor kappa (NF-κB) in macrophages, with consequences for inflammation and apoptosis (Black, 2006). In addition, epinephrine and to a lesser degree norepinephrine, are implicated in the activation and/or aggregation of platelets and may, therefore, promote thrombus formation (Markovitz and Matthews, 1991).
Although it is often hypothesized that stress hormones and related mechanisms could contribute to the social and psychosocial patterning of cardiovascular disease, few studies have directly examined the relationship between SES or psychosocial factors and stress hormones. A handful of studies have investigated associations of salivary cortisol measures obtained at varying times over the day with socioeconomic and psychosocial factors. For example, persons of low SES had lower salivary cortisol at wake up and less pronounce decline of salivary cortisol over the day than persons of high SES (Hajat et al., 2010). Higher levels of cynical hostility have also been linked to less pronounced daily declines in cortisol (Ranjit et al., 2009). Other studies have reported a relationship between elevated awakening salivary cortisol levels and depressive symptomatology (Pruessner et al., 2003).
Although it is often hypothesized that stress may also contribute to race and ethnic differences in cardiovascular disease (Williams and Jackson, 2005), very few studies have investigated differences in stress hormone levels by race/ethnicity. Persons of Black or Hispanic race/ethnicity have been found to have lower levels of wake up salivary cortisol and less pronounced daily cortisol decline over the day (Hajat et al., 2010) or higher values of bedtime salivary cortisol (Cohen et al., 2006b) than non-Hispanic whites. No studies of which we are aware have investigated whether associations of SES or psychosocial factors with stress hormones differ by race/ethnicity.
Most existing work on socioeconomic, race/ethnic, and psychosocial differences in stress hormones has focused on salivary cortisol assessed during the day. Overnight urinary measures may capture features of the functioning of the HPA and SNS that are not reflected in daytime salivary measures (Seeman et al., 1997). However studies investigating associations of overnight urinary measures with SES, race/ethnicity, and psychosocial factors are scant. Two studies have reported associations of higher SES with lower levels of urinary catecholamines (Cohen et al., 2006a; Janicki-Deverts et al., 2007), but at least one population study has not (Dowd and Goldman, 2006). Janicki-Deverts et al. (2007) also investigated whether the association of SES with urinary catecholamines was modified by race and found no evidence of effect modification (Janicki-Deverts et al., 2007). Depression and anxiety have been linked to higher levels of 24-h urinary cortisol and catecholamines (Hughes et al., 2004; Otte et al., 2004), but other research on urinary catecholamine levels has been inconsistent with these findings (Hughes et al., 2004; Otte et al., 2005).
We used unique data from a subsample of MESA to examine associations of SES and selected psychosocial factors with urinary cortisol and catecholamines in a population-based sample. We hypothesized (1) that persons with low SES would have higher levels of urinary cortisol and catechola-mines than subjects with high SES; (2) that persons with higher levels of depression, anxiety, anger, and chronic stress burden would have higher levels of urinary cortisol and catecholamines than subjects who are less depressed, anxious, angry, or chronically burdened; and (3) that SES differences in psychosocial factors would at least partly contribute to SES differences in urinary stress hormones. We also examined whether the associations of SES or psychosocial factors with stress hormones were modified by race/ethnicity.
2. Methods and materials
2.1. Participants
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective longitudinal study of the predictors of subclinical cardiovascular disease and clinical events. MESA recruited 6814 men and women from six communities in the United States between 2000 and 2002 (Bild et al., 2002). At baseline, participants were between 45 and 84 years of age and free of clinically diagnosed cardiovascular disease. Participants self-identified as White, Black, Hispanic or Asian/Chinese. There have been five MESA exams, beginning in 2000—02. As part of MESA exams 3 and 4 (between July 2004 and October 2006), White, Black and Hispanic participants from the New York and Los Angeles sites were invited to participate in the MESA Stress 1, a MESA ancillary study. The goal of the recruitment of the MESA Stress substudy was to obtain a sample approximately proportional to the race distribution of White, Black and Hispanic participants at each of the two sites. Mesa Stress participants were enrolled at the time they attended their MESA Exam (either Exam 3 or Exam 4). Since the clinic visit order was generally random, MESA Stress 1 enrolled an approximately random sample of 1000 participants, approximately half from each of the two participating field centers.
The MESA Stress 1 protocol included collection of 12-hour overnight urine collected the night of the last day of the saliva collection. Participants also completed a Daily Questionnaire, which included items on perceived stress levels during the day and the wake-up time on that day.
Of the 1002 participants enrolled in the MESA Stress substudy, 57 were excluded from all analyses because of incomplete or no urine collection (urine volume less than 300 cc), leaving 945 participants for analysis. A small number of participants were excluded due to missing values of urinary hormones leaving 940 participants for cortisol analyses, 942 participants for norepinephrine and dopamine analyses, and 925 participants for epinephrine analyses. MESA and the MESA Stress study were approved by the Institutional Review Boards of the participating institutions, and all participants gave written informed consent for participation in the studies.
2.2. 12-hour urinary cortisol and catecholamines
During their clinic visit, participants received instructions on the collection of urine samples from trained staff. Following a protocol developed previously (Seeman et al., 1994), the staff calculated the anticipated start time of urine collection of the participant according to the participant's usual waking time and wrote the anticipated start and end time on a detailed instruction sheet. The participant was instructed to void at the start time and start collecting all urine until the stop time, which would be their last sample. Participants were instructed to pour the urine into the collection bottle immediately after each collection. The collection bottle included a preservative (sodium metabisulfate; NA2S205, crystal form; reagent ACS grade, Fisher Scientific No. S244). Participants were asked to store the urine collection bottle in a cool place and bring the sample to the clinic as soon as possible after the stop time. Participants recorded the medications they had taken during the 24 h preceding the stop time on the daily questionnaire.
Aliquots of urine for catecholamines essays were acidified with (~6 N) HCl to a pH ≤ 3 and frozen at -80 C until assayed. Catecholamine samples were analyzed for epinephrine, norepinephrine, and dopamine levels by high-performance liquid chromatography with electrochemical detection following the protocol of Macdonald and Lake, an improved technique for extracting catecholamines from body fluids (Macdonald and Lake, 1985). Cortisol was assayed at Northwestern University using the urinary cortisol EIA kit. Intra and interassay coefficients of variation were 3.2% and 9.99% for urinary cortisol, 3.2% and 6.7% for epinephrine, 2.3% and 2.6% for norepinephrine, and 2.8% and 3.2% for dopamine. All analyses adjusted urinary values for urine creatinine in order to reduce variability related to urine dilution.
2.3. Socioeconomic status (SES) and race/ethnicity
Education and a summary income-wealth index were examined as measures of socioeconomic status (SES). Information on educational attainment was collected at the MESA baseline visit. All questionnaires were available in English and Spanish, and both study sites had bilingual research assistants. Educational status was defined as the participant's highest level of education in the form of eight response categories ranging from “no schooling” to graduate or professional school. It was recategorized in three groups of main educational levels as follow: less than high school; completed high school, technical school certificate or associate degree; and completed college or more. Information on annual household income was obtained during Exam 3. The questionnaire provided 13 response categories ranging from less than $5,000 to $100,000 or more.
Family income was categorized, into quintiles, from zero to 4 (zero for those whose income was less than $12,000 and four for those whose income was greater than $75,000). As in prior work (Hajat et al., 2010), a wealth index was created by scoring across four wealth domains (forming a 5-level wealth index). The wealth measure was derived based on ownership of the following assets: owning a home or paying mortgage on a home, owning one or more car, owning land, or owning an investment (such as stocks, bonds, mutual funds, retirement investments). Participants received a zero score for wealth when they did not report any assets and four when families owned all four types of assets. As in prior work (Hajat et al., 2010), we created an income-wealth index by summing the five-category family income variable (zero to four) and the five point wealth index (zero to four), generating an income-wealth index of 9 points ranging from zero to eight. Those with an annual family income in the lowest quintile (<$12,000) and no assets received a score of zero, and those with income in the highest quintile (>$75,000) and all four assets received a score of eight. This score was analyzed as both categorical (in 4 categories) and continuous variable. Race/ethnicity was reported by participants in response to questions modeled on the year 2000 Census and was categorized into White, African American and Hispanic.
2.4. Psychosocial factors
Depressive symptoms, anger and anxiety, and chronic psychological stress (chronic burden of stress) were examined. Depression was assessed using the 20 item Center for Epidemiology Studies-Depression (CES-D) scale (Radloff, 1977). The possible scores of this scale range from 0 to 60, with higher scores indicating higher levels of depressive symptoms. Anger and anxiety were assessed using the Spielberg trait anger and the Spielberg trait anxiety scales (Spielberger, 1980), respectively. The ranges for both scales are from 10 to 40; higher scores indicate higher levels of anger and anxiety. CES-D, anger and anxiety scores were analyzed as continuous variables after standardized them. Chronic psychological stress was assessed using the chronic burden scale (Bromberger and Matthews, 1996). This scale asks participants to report ongoing difficulties in five domains: health of self, health of others, job or ability to work, finances, and relationships. Participants were coded as having a difficulty in the domain in question if they reported a moderately stressful or severely stressful ongoing problem that had been present for 6 months or more. The chronic burden score was the number of items for which participants reported an ongoing difficulty (range, 0 - 4). Participants were classified into three groups (0, 1, and ≥2 ongoing difficulties) for analysis. Chronic burden was specified both as categorical and as a continuous variable.
2.5. Covariates
Key covariates included age (at the time of the ancillary study), race/ethnicity, and gender. Based on prior work, we also investigated behavioral factors including smoking, body mass index (BMI), alcohol use, and use of medications (beta blockers, diuretics and oral hypoglycemic agents) that could affect steroid or catecholamine levels as potential confounders or mediators. Smoking was categorized as current, past or never. BMI was calculated as weight in kilograms divided by height in meters squared.
2.6. Statistical analysis
Because the levels of cortisol, epinephrine, norepinephrine and dopamine were skewed, they were natural logarithm transformed for analyses. We used multiple linear regression analyses to investigate the associations of socioeconomic and psychosocial variables with the outcomes of interest (urinary levels of cortisol, epinephrine, norepinephrine and dopa-mine). Each socioeconomic and each psychosocial predictor was initially examined in separate models. Two models were fit for each outcome: model 1 adjusted for age (continuous variable), gender (male or female), race/ethnicity (White, African American or Hispanic), and use of medications (whether they were using beta-blockers, diuretics and/or oral hypoglycemic). Model 2 adjusted for all the previous covariates plus BMI, current smoking and alcohol use.
To determine whether the associations of the different SES measures with the outcomes were independent of each other we also fit models that included race/ethnicity, education and income-wealth index in the same model (model 3). In addition, we investigated whether psychosocial factors contributed to the associations of socioeconomic factors with urinary hormone levels by adding psychosocial variables to the models including the socioeconomic indicators and examining changes in the coefficients associated with the SES variables. These models were also fit before and after adjustment for other covariates (models 4 and 5).
Because the health implications of SES or psychosocial factors have sometimes been shown to vary by race/ethnicity (Williams et al., 2003; Williams and Jackson, 2005), we tested for effect modification by race/ethnicity by including appropriate interaction terms in the fully adjusted model (including SES factors, psychosocial factors and covariates) and through stratified analyses.
3. Results
A total of 940 participants were included in the analyses for cortisol, 942 participants had information on norepinephrine and dopamine. Of these, 925 had information on epinephrine. Table 1 shows the distribution of key covariates and stress hormones by race/ethnicity for these 942 participants, of whom 19% were White, 28% African American, and 53% Hispanic. Whites were older than African Americans and Hispanics. Levels of income, wealth and education were highest in Whites and lowest in Hispanics. Anger and anxiety scores were lower for African Americans than for Whites of Hispanics. There were no differences in chronic burden or depression among the three racial/ethnic groups. Cortisol was significantly higher in Whites than in African Americans and Hispanics, whereas dopamine was lower in Whites than in African Americans and Hispanics.
Table 1.
Distribution of socioeconomic and psychosocial factors and urinary stress hormones by race and ethnicity in the MESA stress study.
| Distribution | Non-Hispanic White N = 176 | Non-Hispanic Black N = 263 | Hispanic N = 503 | p-Value¶ |
|---|---|---|---|---|
| Age (mean, SD) | 66.9 (10.3) | 64.8 (9.8) | 64.9 (9.4) | <0.05 |
| Gender (% male) | 48.90 | 45.30 | 47.90 | 0.700 |
| Education (%) | ||||
| ≤Completed high school | 17.61 | 33.08 | 66.20 | <.0001 |
| Completed HS, technical | 21.59 | 45.25 | 24.45 | |
| ≥Bachelor degree | 60.80 | 21.67 | 9.34 | |
| Income (%) | ||||
| <12,000 | 2.91 | 13.73 | 21.22 | <.0001 |
| 12,000–24,999 | 9.30 | 16.86 | 32.04 | |
| 25,000–39,999 | 16.28 | 31.37 | 22.65 | |
| 40,000–74,999 | 34.20 | 23.92 | 16.94 | |
| ≥75,000 | 37.21 | 14.12 | 7.14 | |
| Wealth-index (%) | ||||
| Index score 0 | 3.98 | 17.87 | 25.50 | <.0001 |
| Index score 1 | 21.59 | 26.24 | 26.10 | |
| Index score 2 | 18.18 | 22.05 | 26.49 | |
| Index score 3 | 32.95 | 20.53 | 14.34 | |
| Index score 4 | 23.30 | 13.31 | 7.57 | |
| Income-wealth index (%) | ||||
| 0–1 | 1.74 | 14.51 | 26.73 | <.0001 |
| 2–3 | 15.70 | 30.20 | 33.67 | |
| 4–6 | 50.00 | 40.00 | 31.02 | |
| 7–8 | 32.56 | 15.29 | 8.57 | |
| Chronic burden (%) | ||||
| None | 38.29 | 34.36 | 36.58 | 0.387 |
| 1 | 30.86 | 25.87 | 29.22 | |
| 2 or more | 30.86 | 39.77 | 34.19 | |
| Median anger (Q1,Q3) | 14 (12,17) | 13 (11,16) | 14 (11,17) | 0.005 |
| Median anxiety (Q1,Q3) | 16 (12,20) | 14 (11,18) | 15 (12,19) | 0.004 |
| Median depression (Q1,Q3) | 5 (2,12) | 6 (3,12) | 7 (3,13) | 0.138 |
| Median cortisola (Q1,Q3) | 18.9 (11.9,26.6) | 15.6 (9.0,23.8) | 15.5 (9.8,23.4) | 0.013 |
| Median norepinephrinea (Q1,Q3) | 2.5 (1.6,3.8) | 2.4 (1.4,3.7) | 2.3 (1.4,3.7) | 0.528 |
| Median epinephrinea (Q1,Q3) | 26.4 (19.8,33.2) | 25.8 (19.4,35.4) | 27.4 (20.3,36.5) | 0.384 |
| Median dopaminea (Q1,Q3) | 180 (149,244) | 219 (172,286) | 225 (182,286) | <0.0001 |
ng/mg creatinine.
p-Values calculated using chi-square tests for categorical and Kruskal–Wallis test for non-normally distributed continuous variables.
Correlations among the four measures of socioeconomic status ranged from 0.32 for education and wealth index to 0.89 for wealth index and income-wealth index (p < 0.0001). Income and education were correlated R = 0.50, p < 0.0001; income-wealth index and education were correlated R = 0.46, p < 0.0001; wealth index and income were correlated R = 0.60, p < 0.000; and income-wealth index and income were correlated R = 0.88, p < 0.0001. Psychosocial factors were also correlated (range 0.17 anger and chronic burden to 0.71 for anxiety and depression; p < 0.0001). Levels of the four stress hormones studied were also correlated (range of 0.37 for cortisol and epinephrine to 0.69 for norepinephrine and dopamine; p < 0.0001).
3.1. Associations of SES and psychosocial factors with urinary stress hormones in separate models
Table 2 shows the associations of SES and psychosocial factors with urinary stress hormone levels. Education level was not associated with any of the four stress hormone levels. Higher income-wealth index score was consistently associated with lower cortisol, epinephrine, norepinephrine, and dopamine after adjustment for age, gender, race/ethnicity and medications. Associations with cortisol and epinephrine were clearly graded. The associations of the income-wealth index with urinary stress hormone levels were largely unchanged after additional adjustment for BMI, alcohol use and current smoking. The chronic burden index, anxiety, anger, and depression were not associated with stress hormone levels. Similar results were obtained for chronic burden when health of self was dropped from the scale.
Table 2.
Mean differences in log transformed values of urinary hormones (in ng hormone per mg creatinine) associated with socioeconomic indicators, before and after adjustment for covariates.
| Predictors | Cortisol |
Epinephrine |
Norepinephrine |
Dopamine |
||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| Education | ||||||||
| Completed HS or less | Ref | |||||||
| Technical or associate | –0.14* (.06) | –0.12* (.06) | –0.05 (.06) | –0.05 (.06) | –0.01 (.04) | –0.00 (.04) | –0.01 (.04) | –0.01 (.04) |
| Bachelor or more | –0.09 (.07) | –0.07 (.07) | 0.01 (.07) | –0.00 (.07) | 0.01 (.05) | 0.01 (.05) | 0.01 (.04) | 0.02 (.04) |
| p-Trend | 0.09 | 0.15 | 0.91 | 0.82 | 0.91 | 0.92 | .92 | .76 |
| Income-wealth index | ||||||||
| Index low | Ref | |||||||
| Index low-mid | –0.08 (.07) | –0.08 (.07) | –0.12 (.07) | –0.13 (.07) | –0.10* (.05) | –0.10* (.05) | –0.09* (.04) | –0.09* (.05) |
| Index mid-high | –0.12 (.07) | –0.13 (.07) | –0.21** (.07) | –0.20** (.07) | –0.07 (.05) | –0.08 (.05) | –0.08 (.04) | –0.07 (.04) |
| Index high | –0.20* (.09) | –0.21* (.09) | –0.24** (.09) | –0.24* (.09) | –0.15* (.06) | –0.15* (.06) | –0.11* (.06) | –0.10 (.06) |
| p-Trend | 0.01 | <0.01 | <0.01 | <0.01 | 0.02 | 0.01 | 0.04 | 0.06 |
| Chronic burden | ||||||||
| None | Ref | |||||||
| 1 | –0.06 (.06) | –0.05 (0.06) | –0.01 (.06) | –0.02 (.06) | –0.02 (.04) | –0.02 (.04) | –0.03 (.04) | –0.03 (.03) |
| 2 or more | 0.04 (.06) | 0.03 (0.06) | –0.01 (.06) | –0.01 (.06) | 0.06 (.04) | 0.06 (.04) | 0.03 (.03) | 0.03 (.03) |
| p-Trend | 0.16 | 0.22 | 0.97 | 0.85 | 0.09 | 0.11 | 0.30 | 0.27 |
| Anxiety (per SD) | –0.01 (.02) | –0.01 (.02) | 0.02 (.02) | 0.00 (.02) | 0.01 (.02) | 0.01 (.02) | –0.01 (.01) | –0.01 (.01) |
| Anger (per SD) | 0.02 (.02) | 0.01 (.02) | –0.00 (.02) | –0.01 (.02) | 0.01 (.02) | 0.01 (.02) | 0.01 (.01) | 0.01 (.01) |
| Depression (per SD) | 0.01 (.02) | 0.01 (.02) | 0.01 (.02) | 0.01 (.02) | 0.01 (.02) | 0.01 (.02) | –0.01 (.01) | –0.01 (.01) |
Model 1: adjusted for age, gender, race/ethnicity and medications, Model 2: adjusted for age, gender, race/ethnicity, medications, BMI, alcohol use and current smoking.
Standard Errors are shown in parentheses next to each coefficient.
p ≤ .05.
p ≤ .01.
3.2. Associations of SES and psychosocial factors with urinary stress hormones in mutually adjusted models
The income-wealth index was consistently and inversely associated with all four urinary stress hormones after adjustment for education (model 3, Table 3), after additional adjustment for psychosocial factors (model 4), and after further adjustment for BMI, smoking and alcohol use (model 5). Socioeconomic and psychosocial factors were entered into these models as continuous variables because there was no clear evidence of thresholds when categories were examined.
Table 3.
Mean differences in logged values for Cortisol, epinephrine, norepinephrine and dopamine (in ng hormone per mg creatinine) associated with socioeconomic and psychosocial variables simultaneously adjusted for each other and before and after adjustment for other covariates.
| Cortisol |
Epinephrine |
|||||
|---|---|---|---|---|---|---|
| Model 3 β (SE) | Model 4 β (SE) | Model 5 β (SE) | Model 3 β (SE) | Model 4 β (SE) | Model 5 β (SE) | |
| African-Americans | –0.17* (.08) | –0.18* (.08) | –0.19* (.08) | –0.02 (.08) | –0.01 (.08) | –0.05 (.08) |
| Hispanics | –0.24** (.08) | –0.25** (.08) | –0.23** (.08) | –0.12 (.08) | –0.12 (.08) | –0.08 (.08) |
| Income-wealth index | –0.03* (.01) | –0.03* (.01) | –0.03* (.01) | –0.04** (.01) | –0.04** (.01) | –0.04** (.01) |
| Education | –0.01 (.01) | –0.01 (.01) | –0.01 (.01) | 0.01 (.01) | 0.01 (.01) | 0.01 (.01) |
| Anger (per SD) | 0.03 (.03) | 0.03 (.03) | –0.02 (.03) | –0.01 (.03) | ||
| Anxiety (per SD) | –0.05 (.04) | –0.05 (.04) | 0.02 (.04) | –0.01 (.04) | ||
| Depression (per SD) | 0.03 (.04) | 0.02 (.04) | –0.00 (.04) | 0.01 (.04) | ||
| Chronic burden | 0.02 (.02) | 0.02 (.02) | –0.01 (.02) | 0.00 (.02) | ||
| Norepinephrine |
Dopamine |
|||||
|---|---|---|---|---|---|---|
| Model 3 β (SE) | Model 4 β (SE) | Model 5 β (SE) | Model 3 β (SE) | Model 4 β (SE) | Model 5 β (SE) | |
| African–Americans | 0.02 (.05) | 0.02 (.05) | 0.02 (.05) | 0.18** (.05) | 0.18** (.05) | 0.19** (.05) |
| Hispanics | 0.03 (.05) | 0.03 (.05) | 0.03 (.05) | 0.16** (.05) | 0.16** (.05) | 0.17** (.05) |
| Income-wealth index | –0.02** (.01) | –0.02* (.01) | –0.02** (.01) | –0.02* (.01) | –0.02* (.01) | –0.02* (.01) |
| Education | 0.01 (.01) | 0.01 (.01) | 0.01 (.01) | 0.01 (.01) | 0.01 (.01) | 0.01 (.01) |
| Anger (per SD) | 0.01 (.02) | 0.01 (.02) | 0.02 (.02) | 0.02 (.02) | ||
| Anxiety (per SD) | –0.00 (.03) | 0.00 (.03) | –0.03 (.02) | –0.03 (.02) | ||
| Depression (per SD) | –0.01 (.03) | –0.02 (.03) | –0.00 (.02) | –0.00 (.02) | ||
| Chronic burden | 0.02 (.01) | 0.02 (.01) | 0.02 (.01) | 0.02 (.01) | ||
Model 3 includes age gender, medications, race/ethnicity and SES variables.
Model 4 includes age, gender, medications, race/ethnicity, SES and psychosocial factors.
Model 5, includes age, gender, medications, race/ethnicity, SES, psychosocial factors, BMI, alcohol use, and current smoking.
Income-wealth is included as a continuous score variable ranging from 0 to 8. Education is included as a continuous variable ranging from no schooling to graduate or professional school representing the 8 educational categories. Estimates shown correspond to a unit increase in the predictor.
Standard errors are shown in parentheses next to each coefficient p ≤ .05.
Standard errors are shown in parentheses next to each coefficient p ≤ .01.
3.3. Effect modification by race/ethnicity
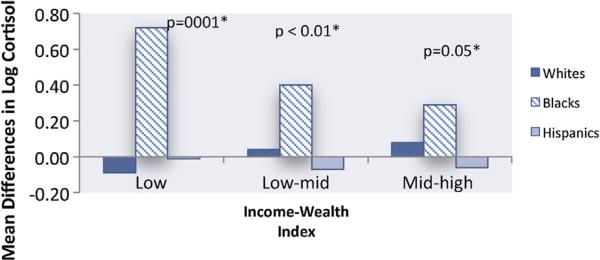
African American and Hispanic race/ethnicity were associated with lower level of urinary cortisol in all models, but no differences by race/ethnicity were observed for catecholamines (Table 3). There was evidence of heterogeneity in the associations of income-wealth index and education with cortisol by race/ethnicity in the fully adjusted model such that the inverse associations of income-wealth index and education with cortisol were stronger in African Americans than in other race/ethnic groups (mean differences in log cortisol by income-wealth index –0.068 in African Americans compared to –0.01 in Whites and Hispanics, p value for heterogeneity 0.004; mean differences in log cortisol by education were –0.073 in African Americans compared to 0.01 in Whites and Hispanics, p value for heterogeneity 0.003). The associations of the income-wealth index with urinary cortisol levels in African Americans, after adjustment for age, medications, BMI, alcohol use and current smoking were clearly graded and statistically significant (Fig. 1).
Figure 1.
Mean differences in log Cortisol Associated with Income-Wealth Index adjusted for Age and Behavioral Factors. *Reference group = High Income-Wealth Index.
There was also evidence of effect modification of the associations of anger with catecholamine levels by race/ethnicity such that higher anger was associated with lower urinary catecholamine levels in African Americans but not in the other race/ethnic groups (mean differences in log epinephrine: –0.15 in African Americans and 0.02 in Whites and Hispanics, p for heterogeneity 0.007; mean differences in log norepinephrine –0.10 in African Americans and 0.03 in Whites and Hispanics p for heterogeneity 0.003). No other interactions between race/ethnicity and the SES or psychosocial indicators were observed.
4. Discussion
The major finding of our study is the consistent inverse association of an income-wealth index with lower levels of urinary measures of the stress hormones cortisol, epinephrine, norepinephrine and dopamine, in a large and diverse population-based sample. These associations persisted after adjustment for age, gender, race/ethnicity, medication use, body mass index, smoking, and alcohol use. In contrast, education was not associated with the urinary stress hormone levels, and neither were depressive symptoms, levels or anger or anxiety, or measures of chronic burden. There was some evidence of heterogeneity in these associations by race/ethnicity such that stronger associations of SES and anger with hormone levels were observed in African Americans than in Whites or Hispanics. Our analyses add to existing work by investigating associations of a range of SES and psychosocial measures with the four major stress hormones in a large and diverse population based sample and by exploring heterogeneity in these associations by race/ethnicity.
Studies relating socioeconomic and psychosocial factors to urinary measures of stress hormones are still scarce, and their results are not consistent. Among 193 men and women aged 21—55 years, Cohen et al. (2006a) reported statistically significant inverse associations of composite SES (an index based on standardized scores of income and education) with urinary epinephrine obtained from two 24-h urine samples. No associations of composite SES with norepinephrine were observed (Cohen et al., 2006a). In a study of 672 participants from the CARDIA study, Janicki-Deverts et al. (2007) found higher composite SES (scored based on standardized education, income and occupation) associated with lower levels of urinary epinephrine and norepinephrine obtained from a 12-h overnight collection. (Janicki-Deverts et al., 2007). In contrast, in a study of 972 middle-aged and elderly men and women in Taiwan, Dowd and Goldman (2006) found no association of education or income with levels of urinary cortisol, epinephrine or norepinephrine obtained from a 12-h overnight collection (Dowd and Goldman, 2006).
To our knowledge, ours is the largest study of the socioeconomic patterning of urinary stress hormones in the US to date. An important advantage of our study is the race and ethnically diverse sample and the availability of multiple measures of SES, multiple measures of urinary hormones, and relevant information on important covariates. We found robust associations of a higher income-wealth index with lower levels of urinary cortisol and catecholamines. In contrast, education was not associated with the hormones.
Previously reported findings from the MESA study, focusing on salivary rather than urinary stress hormone levels, also found wealth to be a stronger predictor of cortisol level than education (Hajat et al., 2010). In our analyses, the wealth-income index was a stronger and more consistent predictor than income alone (data not shown). These findings may be related to the multiethnic composition of our sample; other investigators have reported that measures of income and education alone may not fully reflect the influence of socioeconomic circumstances on race/ethnic groups other than Whites (Karlamangla et al., 2005). Wealth may be an especially important SES indicator in older adults, and 50% of the MESA Stress study participants were 65 years or older at the time of the MESA Stress study. Hence, in this multiethnic older sample the income-wealth index may better capture the range of potentially stress-producing exposures than do measures such as education and income alone. Despite the advantages of including a wealth measure, the wealth measures obtained were relatively crude in that they did not account for debts or include quantitative information on the amount of wealth. In addition, the index we used is arbitrary. Future studies should investigate more complete measures of wealth.
Our results regarding the SES patterning of stress hormone levels are generally consistent with the studies of Cohen et al. (2006a) and Janicki-Deverts et al. (2007) but these studies were smaller in size, included less diverse samples, did not examine dopamine, and did not incorporate measures of wealth. The absence of socioeconomic patterning in Taiwan (Dowd and Goldman, 2006) may reflect differential distribution of stressors by SES across countries. Other studies have also shown that the SES patterning of cardiovascular risk is quite heterogeneous across countries (Subramanian et al., 2013).
Our results suggest that the inverse association of higher income-wealth index with lower urinary cortisol level is stronger in African Americans than in Hispanics or Whites. In addition, higher education was associated with lower cortisol in African Americans but not in other race/ethnic groups. These findings suggest that the stress consequences of lower income or education may be greater in African Americans than in other groups. These differences need to be confirmed in other samples and their causes explored further before firm conclusions can be drawn.
Few studies have investigated associations of psychosocial factors with urinary stress hormones. In a cross-sectional study of 693 patients with coronary heart disease who provided 24-h urine samples for the Heart and Soul Study, depression was linked to higher levels of cortisol and norepinephrine (Otte et al., 2004) but not epinephrine or dopamine (Otte et al., 2005). Among 91 women ages 47—55 years, depression and anxiety were positively correlated with 24-h norepinephrine excretion and urinary cortisol, but not with urinary epinephrine (Hughes et al., 2004). Male veterans with posttraumatic stress disorder (PTSD) had higher levels of 24-h urinary dopamine, norepinephrine, and epinephrine than other male veterans (Yehuda et al., 1992), and among females, those with abuse-related PTSD had higher levelsof 24-h urinary dopamine, norepinephrine, epinephrine and cortisol than women without PTSD (Lemieux and Coe, 1995). We found no evidence that psychosocial factors (depressive symptoms, anxiety, anger, or a measure of chronic stress burden) were related to urinary hormone measures in the full sample. However, there was some evidence that higher anger was associated with lower urinary catecholamine level specifically in African Americans. The reasons for this unexpected association as well as the reasons for the lack of associations of psychosocial factors with the stress hormones in other race/ethnic groups need further investigation with more refined psychosocial and hormone measurement. Since psychosocial factors were not consistently related to urinary stress hormones, they did not contribute to the SES patterning that we observed. This was confirmed by the fact that further adjustment for the psychosocial factors did not modify the coefficients for the SES variables.
A novel aspect of our study is the investigation of urinary dopamine, a precursor of norepinephrine, which appears to have independent peripheral actions (Christensen et al., 1975). Some authors have evaluated dopamine as a component of scores used to measure allostatic load along with epinephrine, norepinephrine and cortisol but have not reported separate results for dopamine (Glei et al., 2007). Animal studies have shown activation of the mesolimbic dopaminergic system in response to acute stress (Puglisi-Allegra et al., 1991). We found that a higher income-wealth index was associated with lower level of urinary dopamine after adjustment for age, gender and medication use. These associations remained largely unchanged after additional adjustment for covariates. Future studies are needed to better understand the causes and consequences of this association.
Important strengths of our study include the large and diverse population sample and the availability of multiple socioeconomic and psychosocial measures. Limitations include the cross-sectional design, a limitation our study shares with other studies in this field. A second limitation is that we performed a 12-h as opposed to 24-h urine collection. The collection of even 12-h samples is challenging in large populations. Although the 24-h urine analysis may sometimes be more informative, the 12-h overnight collection period has better compliance than the 24-h urine collection (Seeman et al., 1994, 1997) and the 12-h overnight measure is itself of interest as a basal measure of the function of the HPA and SNS axis (Seeman et al., 1994, 2004). In addition, Seeman et al. (1994) found strong correlations for cortisol (.81) and catecholamines (.95 for epinephrine and .80 for norepinephrine) between 12- and 24-h urine measures (Seeman et al., 1994). Nevertheless, measurement error related to variability in the 12-h overnight collection may have biased our results toward the null.
In summary, our results support the hypothesis that lower SES, as measured by an index of wealth and income, is associated with higher levels of urinary stress hormones, in a multi-ethnic sample. Our results are compatible with the idea that stress is a mediator of the effect of SES on health. Our findings also suggest that heterogeneities in the effects of SES and psychosocial factors on stress biomarkers by race/ethnicity need further investigation.
Acknowledgements
We thank the participants, the staff, and the other investigators of the MESA study for their valuable contributions.
Role of funding source
This work was supported by R01 HL076831 (PI: Ana V. Diez Roux). MESA was supported by contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169 from the National Heart, Lung, and Blood Institute (NHLBI), and by GCRC grant UL1 TR000040 (formerly UL1 RR024156). NHLBI and GCRC had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit this paper for publication.
Footnotes
Conflicts of interest statement
All authors declare they have no conflicts of interest.
References
- Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kahn RL, Syme SL. Socioeconomic status and health: the challenge of the gradient. American Psychologist. 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- Axelrod J, Reisine TD. Stress hormones: their interaction and regulation. Science. 1984;224:452–459. doi: 10.1126/science.6143403. [DOI] [PubMed] [Google Scholar]
- Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacobs DR, Jr., Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. American Journal of Epidemiology. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- Black PH. The inflammatory consequences of psychologic stress: relationship to insulin resistance, obesity, atherosclerosis and diabetes mellitus, type II. Medical Hypotheses. 2006;67:879–891. doi: 10.1016/j.mehy.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Black PH, Garbutt LD. Stress, inflammation and cardiovascular disease. Journal of Psychosomatic Research. 2002;52:1–23. doi: 10.1016/s0022-3999(01)00302-6. [DOI] [PubMed] [Google Scholar]
- Bromberger JT, Matthews KA. A longitudinal study of the effects of pessimism, trait anxiety, and life stress on depressive symptoms in middle-aged women. Psychology and Aging. 1996;11:207–213. doi: 10.1037//0882-7974.11.2.207. [DOI] [PubMed] [Google Scholar]
- Brummett BH, Babyak MA, Siegler IC, Shanahan M, Harris KM, Elder GH, Williams RB. Systolic blood pressure, socioeconomic status, and biobehavioral risk factors in a nationally representative US young adult sample. Hypertension. 2011;58:U161–U185. doi: 10.1161/HYPERTENSIONAHA.111.171272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauley JA, Donfield SM, Laporte RE, Warhaftig NE. Physical activity by socioeconomic status in two population based cohorts. Medicine and Science in Sports and Exercise. 1991;23:343–351. [PubMed] [Google Scholar]
- Christensen NJ, Neubauer B, Brandsborg O, Mathias CJ, Frankel HL. Letter: dopamine and the peripheral autonomic nervous system.?215. Lancet. 1975;1:1084–1085. doi: 10.1016/s0140-6736(75)91849-8. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: neuro-endocrine and target tissue-related causes. International Journal of Obesity & Related Metabolic Disorders. 2000;24:S50. doi: 10.1038/sj.ijo.0801278. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. A healthy body in a healthy mind — and vice versa — the damaging power of uncontrollable stress. Journal of Clinical Endocrinology and Metabolism. 1998;83:1842–1845. doi: 10.1210/jcem.83.6.4908. [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Baum A. Socioeconomic status is associated with stress hormones. Psychosomatic Medicine. 2006a;68:414–420. doi: 10.1097/01.psy.0000221236.37158.b9. [DOI] [PubMed] [Google Scholar]
- Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic status, race, and diurnal cortisol decline in the coronary artery risk development in young adults (CARDIA) study. Psychosomatic Medicine. 2006b;68:41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, Sorlie P, Szklo M, Tyroler HA, Watson RL. Neighborhood of residence and incidence of coronary heart disease. New England Journal of Medicine. 2001;345:99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- Dowd JB, Goldman N. Do biomarkers of stress mediate the relation between socioeconomic status and health? Journal of Epidemiology and Community Health. 2006;60:633–639. doi: 10.1136/jech.2005.040816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenhaeuser M. Behavior and circulating catecholamines. Brain Research. 1971;31:241–262. doi: 10.1016/0006-8993(71)90180-6. [DOI] [PubMed] [Google Scholar]
- Galobardes B, Morabia A, Bernstein MS. Diet and socioeconomic position: does the use of different indicators matter? International Journal of Epidemiology. 2001;30:334–340. doi: 10.1093/ije/30.2.334. [DOI] [PubMed] [Google Scholar]
- Gebreab SY, Diez-Roux AV, Hickson DA, Boykin S, Sims M, Sarpong DF, Taylor HA, Wyatt SB. The contribution of stress to the social patterning of clinical and subclinical CVD risk factors in African Americans: the Jackson Heart Study. Social Science & Medicine. 2012;75:1697–1707. doi: 10.1016/j.socscimed.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glei DA, Goldman N, Chuang Y-L, Weinstein M. Do chronic stressors lead to physiological dysregulation? Testing the theory of allostatic load. Psychosomatic Medicine. 2007;69:769–776. doi: 10.1097/PSY.0b013e318157cba6. [DOI] [PubMed] [Google Scholar]
- Hajat A, Diez-Roux A, Franklin TG, Seeman T, Shrager S, Ranjit N, Castro C, Watson K, Sanchez B, Kirschbaum C. Socioeconomic and race/ethnic differences in daily salivary cortisol profiles: the multi-ethnic study of atherosclerosis. Psychoneuroendocrinology. 2010;35:932–943. doi: 10.1016/j.psyneuen.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JW, Watkins L, Blumenthal JA, Kuhn C, Sherwood A. Depression and anxiety symptoms are related to increased 24-h urinary norepinephrine excretion among healthy middle-aged women. Journal of Psychosomatic Research. 2004;57:353–358. doi: 10.1016/j.jpsychores.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Janicki-Deverts D, Cohen S, Adler NE, Schwartz JE, Matthews KA, Seeman TE. Socioeconomic status is related to urinary catecholamines in the coronary artery risk development in young adults (CARDIA) study. Psychosomatic Medicine. 2007;69:514–520. doi: 10.1097/PSY.0b013e3180f60645. [DOI] [PubMed] [Google Scholar]
- Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation. 1993;88:1973–1998. doi: 10.1161/01.cir.88.4.1973. [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Singer BH, Williams DR, Schwartz JE, Matthews KA, Kiefe CI, Seeman TE. Impact of socioeconomic status on longitudinal accumulation of cardiovascular risk in young adults: the CARDIA study (USA). Social Science & Medicine. 2005;60:999–1015. doi: 10.1016/j.socscimed.2004.06.056. [DOI] [PubMed] [Google Scholar]
- Lemieux A, Coe C. Abuse-related posttraumatic stress disorder: evidence for chronic neuroendocrine activation in women. Psychosomatic Medicine. 1995;57:105–115. doi: 10.1097/00006842-199503000-00002. [DOI] [PubMed] [Google Scholar]
- Lundberg U, Frankenhaeuser M. Pituitary-adrenal and sympathetic-adrenal correlates of distress and effort. Journal of Psychosomatic Research. 1980;24:125–130. doi: 10.1016/0022-3999(80)90033-1. [DOI] [PubMed] [Google Scholar]
- Macdonald IA, Lake DM. An improved technique for extracting catecholamines from body fluids. Journal of Neuroscience Methods. 1985;13:239–248. doi: 10.1016/0165-0270(85)90072-x. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Marsland AL, Kaplan JR, Williams JK. The pathogenicity of behavior and its neuroendocrine mediation: an example from coronary artery disease. Psychosomatic Medicine. 1995;57:275–283. doi: 10.1097/00006842-199505000-00009. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Phillips JE, Gianaros PJ, Flory JD, Muldoon MF. Subjective socioeconomic status and presence of the metabolic syndrome in midlife community volunteers. Psychosomatic Medicine. 2010;72:35–45. doi: 10.1097/PSY.0b013e3181c484dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovitz JH, Matthews KA. Platelets and coronary heart disease: potential psychophysiologic mechanisms. Psychosomatic Medicine. 1991;53:643–668. doi: 10.1097/00006842-199111000-00006. [DOI] [PubMed] [Google Scholar]
- Marmot M. Inequalities in health. New England Journal of Medicine. 2001;345:134–136. doi: 10.1056/NEJM200107123450210. [DOI] [PubMed] [Google Scholar]
- Marmot MG, Smith GD, Stansfeld S, Patel C, North F, Head J, White I, Brunner E, Feeney A. Health inequalities among British civil servants: the Whitehall II study. Lancet. 1991;337:1387–1393. doi: 10.1016/0140-6736(91)93068-k. [DOI] [PubMed] [Google Scholar]
- Novotny TE, Warner KE, Kendrick JS, Remington PL. Smoking by blacks and whites: socioeconomic and demographic differences. American Journal of Public Health. 1988;78:1187–1189. doi: 10.2105/ajph.78.9.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte C, Marmar CR, Pipkin SS, Moos R, Browner WS, Whooley MA. Depression and 24-h urinary cortisol in medical outpatients with coronary heart disease: the heart and soul study. Biological Psychiatry. 2004;56:241–247. doi: 10.1016/j.biopsych.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte C, Neylan TC, Pipkin SS, Browner WS, Whooley MA. Depressive symptoms and 24-h urinary norepinephrine excretion levels in patients with coronary disease: findings from the heart and soul study. American Journal of Psychiatry. 2005;162:2139–2145. doi: 10.1176/appi.ajp.162.11.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering T. Cardiovascular pathways: socioeconomic status and stress effects on hypertension and cardiovascular function. Annals of the New York Academy of Sciences. 1999;896:262–277. doi: 10.1111/j.1749-6632.1999.tb08121.x. [DOI] [PubMed] [Google Scholar]
- Pruessner M, Hellhammer DH, Pruessner JC, Lupien SJ. Self-reported depressive symptoms and stress levels in healthy young men: associations with the cortisol response to awakening. Psychosomatic Medicine. 2003;65:92–99. doi: 10.1097/01.psy.0000040950.22044.10. [DOI] [PubMed] [Google Scholar]
- Puglisi-Allegra S, Imperato A, Angelucci L, Cabib S. Acute stress induces time-dependent responses in dopamine mesolimbic system. Brain Research. 1991;554:217–222. doi: 10.1016/0006-8993(91)90192-x. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale. A self report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Ramsay SE, Morris RW, Whincup PH, Papacosta AO, Thomas MC, Wannamethee SG. Prediction of coronary heart disease risk by Framingham and SCORE risk assessments varies by socioeconomic position: results from a study in British men. European Journal of Cardiovascular Prevention and Rehabilitation: Official Journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology. 2011;18:186–193. doi: 10.1177/1741826710389394. [DOI] [PubMed] [Google Scholar]
- Ranjit N, Diez-Roux AV, Sanchez B, Seeman T, Shea S, Shrager S, Watson K. Association of salivary cortisol circadian pattern with cynical hostility: multi-ethnic study of atherosclerosis. Psychosomatic Medicine. 2009;71:748–755. doi: 10.1097/PSY.0b013e3181ad23e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman TE, Berkman LF, Blazer D, Rowe JW. Social ties and support and neuroendocrine function: the MacArthur studies of successful aging. Annals of Behavioral Medicine. 1994;16:95–106. [Google Scholar]
- Seeman TE, Crimmins E, Huang M-H, Singer B, Bucur A, Gruenewald T, Berkman LF, Reuben DB. Cumulative biological risk and socio-economic differences in mortality: MacArthur studies of successful aging. Social Science & Medicine. 2004;58:1985–1997. doi: 10.1016/S0277-9536(03)00402-7. [DOI] [PubMed] [Google Scholar]
- Seeman TE, McEwen BS, Rowe JW, Singer BH. Allo-static load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4770–4775. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman TE, McEwen BS, Singer BH, Albert MS, Rowe JW. Increase in urinary cortisol excretion and memory declines: MacArthur studies of successful aging. Journal of Clinical Endocrinology and Metabolism. 1997;82:2458–2465. doi: 10.1210/jcem.82.8.4173. [DOI] [PubMed] [Google Scholar]
- Seplaki CL, Goldman N, Weinstein M. How are biomarkers related to physical and mental well-being? Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2004;59:B201–B217. doi: 10.1093/gerona/59.3.b201. [DOI] [PubMed] [Google Scholar]
- Seplaki CL, Goldman N, Weinstein M, Lin YH. Measurement of cumulative physiological dysregulation in an older population. Demography. 2006;43:165–183. doi: 10.1353/dem.2006.0009. [DOI] [PubMed] [Google Scholar]
- Sherwood A, Steffen PR, Blumenthal JA, Kuhn C, Hinderliter AL. Nighttime blood pressure dipping: the role of the sympathetic nervous system. American Journal of Hypertension. 2002;15:111–118. doi: 10.1016/s0895-7061(01)02251-8. [DOI] [PubMed] [Google Scholar]
- Sobal J, Stunkard AJ. Socioeconomic status and obesity: a review of the literature. Psychological Bulletin. 1989;105:260–275. doi: 10.1037/0033-2909.105.2.260. [DOI] [PubMed] [Google Scholar]
- Spielberger C. Preliminary manual for the state-trail personality inventory. Consulting Psychologist Pr; Palo Alto, CA: 1980. [Google Scholar]
- Steenland K, Hu S, Walker J. All-cause and cause-specific mortality by socioeconomic status among employed persons in 27 US states, 1984—1997. American Journal of Public Health. 2004;94:1037–1042. doi: 10.2105/ajph.94.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Marmot M. The role of psychobiological pathways in socio-economic inequalities in cardiovascular disease risk. European Heart Journal. 2002;23:13–25. doi: 10.1053/euhj.2001.2611. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Corsi DJ, Subramanyam MA, Davey Smith G. Jumping the gun: the problematic discourse on socioeconomic status and cardiovascular health in India. International Journal of Epidemiology. 2013;42:1410–1426. doi: 10.1093/ije/dyt017. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP. Hypothalamic—pituitary—adrenal axis, neuroendocrine factors and stress. Journal of Psychosomatic Research. 2002;53:865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Wild SH, McKnight JA, McConnachie A, Lindsay RS. Socioeconomic status and diabetes-related hospital admissions: a cross-sectional study of people with diagnosed diabetes. Journal of Epidemiology and Community Health. 2010;64:1022–1024. doi: 10.1136/jech.2009.094664. [DOI] [PubMed] [Google Scholar]
- Williams DR, Jackson PB. Social sources of racial disparities in health. Health Affairs. 2005;24:325–334. doi: 10.1377/hlthaff.24.2.325. [DOI] [PubMed] [Google Scholar]
- Williams DR, Neighbors HW, Jackson JS. Racial/ethnic discrimination and health: findings from community studies. American Journal of Public Health. 2003;93:200–208. doi: 10.2105/ajph.93.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Southwick S, Giller EL, Xiaowan Mason JW. Urinary catecholamine excretion and severity of PTSD symptoms in Vietnam combat veterans. The Journal of Nervous and Mental Disease. 1992;180:321–325. doi: 10.1097/00005053-199205000-00006. [DOI] [PubMed] [Google Scholar]