Abstract
Study Objectives:
(1) To determine whether facial phenotype, measured by quantitative photography, relates to underlying craniofacial obstructive sleep apnea (OSA) risk factors, measured with magnetic resonance imaging (MRI); (2) To assess whether these associations are independent of body size and obesity.
Design:
Cross-sectional cohort.
Setting:
Landspitali, The National University Hospital, Iceland.
Participants:
One hundred forty patients (87.1% male) from the Icelandic Sleep Apnea Cohort who had both calibrated frontal and profile craniofacial photographs and upper airway MRI. Mean ± standard deviation age 56.1 ± 10.4 y, body mass index 33.5 ± 5.05 kg/m2, with on-average severe OSA (apnea-hypopnea index 45.4 ± 19.7 h-1).
Interventions:
N/A.
Measurements and Results:
Relationships between surface facial dimensions (photos) and facial bony dimensions and upper airway soft-tissue volumes (MRI) was assessed using canonical correlation analysis. Photo and MRI craniofacial datasets related in four significant canonical correlations, primarily driven by measurements of (1) maxillary-mandibular relationship (r = 0.8, P < 0.0001), (2) lower face height (r = 0.76, P < 0.0001), (3) mandibular length (r = 0.67, P < 0.0001), and (4) tongue volume (r = 0.52, P = 0.01). Correlations 1, 2, and 3 were unchanged when controlled for weight and neck and waist circumference. However, tongue volume was no longer significant, suggesting facial dimensions relate to tongue volume as a result of obesity.
Conclusions:
Significant associations were found between craniofacial variable sets from facial photography and MRI. This study confirms that facial photographic phenotype reflects underlying aspects of craniofacial skeletal abnormalities associated with OSA. Therefore, facial photographic phenotyping may be a useful tool to assess intermediate phenotypes for OSA, particularly in large-scale studies.
Citation:
Sutherland K, Schwab RJ, Maislin G, Lee RW, Benedikstdsottir B, Pack AI, Gislason T, Juliusson S, Cistulli PA. Facial phenotyping by quantitative photography reflects craniofacial morphology measured on magnetic resonance imaging in icelandic sleep apnea patients. SLEEP 2014;37(5):959-968.
Keywords: craniofacial morphology, obstructive sleep apnea, phenotyping
INTRODUCTION
Obstructive sleep apnea (OSA) is a common sleep disorder characterized by the repetitive collapse of the upper airway during sleep that results in sleep fragmentation and periods of hypoxia.1 This sleep related upper airway obstruction results from functional abnormalities that promote airway collapse and anatomic abnormalities that compromise the pharyngeal space.2 Obesity is the most recognized anatomical risk factor for OSA; however, craniofacial morphology is also a key predisposing factor in the development of OSA.3 Anatomic risk factors for OSA include restriction of the craniofacial skeleton, regional adiposity, and enlarged upper airway soft tissues.3–5 Such anatomic abnormalities have previously been identified using complex or sophisticated imaging techniques such as cephalometry (primarily for skeletal structures) or magnetic resonance imaging (MRI; primarily for upper airway soft tissues) in case-control studies.5 Although these imaging modalities and analyses are able to provide detailed information about anatomical mechanisms of OSA, they are costly and labor intensive and therefore are not suitable for routine clinical assessment or large population studies.
We have previously demonstrated that surface facial measurements obtained by simple digital photography differ between subjects with and without OSA in a sleep clinic population.6 Furthermore, photographic facial measurements, such as face width, were used to classify subjects with OSA in a prediction model that performed better than known anthropometric and clinical risk predictors in correctly classifying patients.7 This finding suggests that facial phenotype conveys important information related to OSA risk.
Relationships between facial phenotype and upper airway anatomy likely exist as a result of shared embryological origins of craniofacial components, and evidence exists that there is a reciprocal influence on soft and hard tissue growth by both skeletal and upper airway soft- tissue dimensions.8–10 Supporting this relationship, we have previously shown that a combination of bony facial widths predicted tongue size of patients with OSA more accurately than body mass index (BMI) or neck circumference in an MRI study.5 Surface facial dimensions also reflect obesity5,6 and therefore facial phenotype is a composite measure of regional adiposity, skeletal dimensions, and enlarged upper airway soft tissues. Although previous studies suggest facial photography may be a useful phenotyping strategy for OSA, the information that is obtained about specific aspects of craniofacial structure is not known. Therefore, the objective of this study was to assess relationships between photographic facial dimensions and underlying craniofacial structures measured with MRI in patients with OSA. We hypothesized that (1) there are significant relationships between facial photographic metrics and craniofacial morphology (MRI); and (2) these relationships are not simply explained by differences patient characteristics such as body size and obesity. This study, therefore, represents an important step in understanding the relationship between facial phenotype and underlying anatomical risk factors for OSA.
METHODS
Subjects
OSA subjects were members of the Icelandic Sleep Apnea Cohort (ISAC). The cohort is composed of 822 patients (666 males, 156 females) with a new diagnosis of moderate-severe OSA referred for continuous positive airway pressure treatment to Landspitali, The National University Hospital of Iceland, Reykjavik between September 2005 to December 2009. The cohort has been described in detail in recent publications.11–14 At entry into the study, all patients underwent anthropometric measures (height, weight, and neck and waist circumferences) and MRI of the upper airway (imaging completed in n = 653). Two years after treatment initiation, participants were invited for a follow-up visit (n = 741 completed, 90.1% of original sample), which included repeated anthropometric measurements and calibrated digital craniofacial photographs (n = 430). MRI was not repeated at the follow-up visit. Time between baseline and follow-up visit was 774 ± 135 days (mean ± standard deviation).
The ISAC participants included in the current analysis were those who had both upper airway MRI at baseline and craniofacial photographs taken at the follow-up visit. To help address the potential differences between photographic and MRI measurements related to the extended time period between MRI and facial photography image acquisition, the sample was restricted to those patients who had less than 2% weight change between these two time points. The total number of ISAC patients who had MRI at entry, facial photography at follow-up, and less than 2% difference in weight between visits was 140 (122 males, 18 females).
Craniofacial Photographic Technique
Standardized frontal and profile digital photos of the head and neck were performed to obtain quantitative facial measurements.6 Photography was performed with a consumer compact digital camera. Prior to photography, gonion (lateral posterior point on the angle of the mandible) was identified on the subject's right side and marked using a skin pen. A calibration marker (circular nylon washer) of known diameter (3 cm) was fixed to the subject's forehead or side of the face for the frontal and profile photographs, respectively. Subjects were photographed standing upright while assuming the natural head position by being asked to ‘look straight ahead as if looking into a mirror.’15 During the photograph, patients were instructed to keep a neutral facial expression with the mouth closed and teeth and lips lightly touching and to breathe quietly through the nose. Frontal position was achieved by ensuring that both ears were equally visible before taking the photograph. The subject was instructed to turn 90° to the left from the frontal position and visibility of the full profile was assessed before the profile image was captured.
Craniofacial Photography
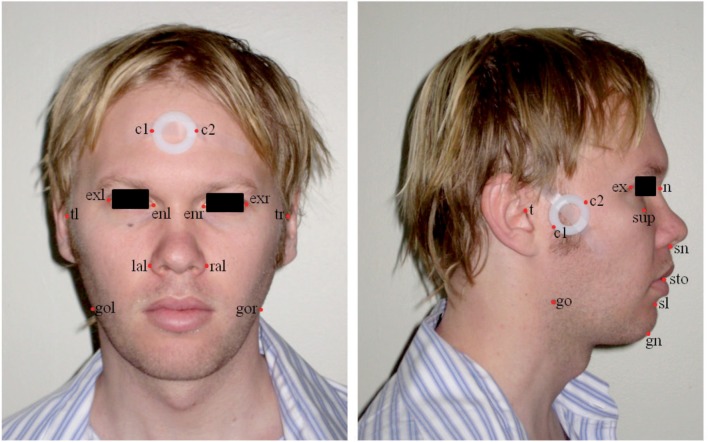
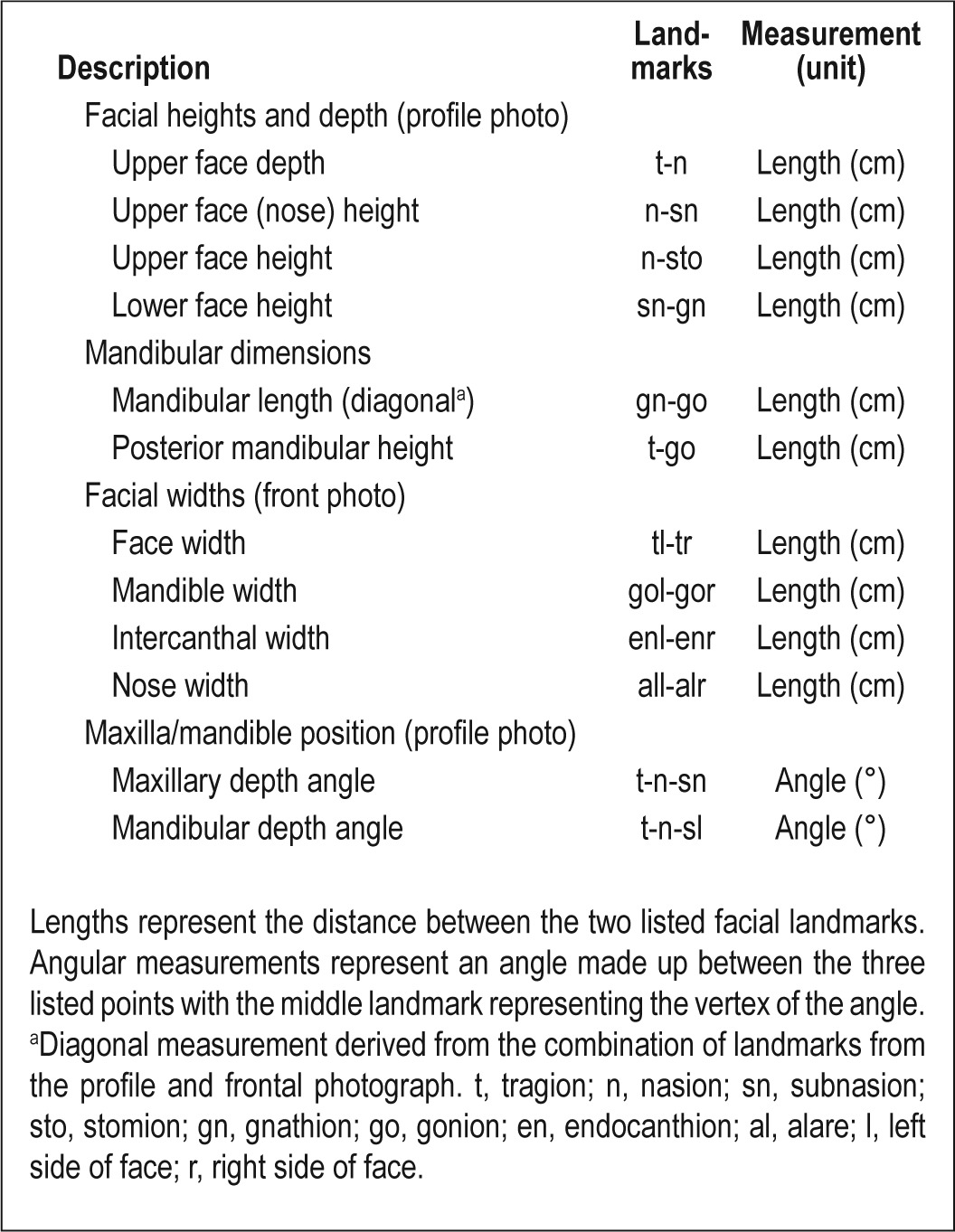
Frontal and profile digital photographs were analyzed using image analysis software (Image J, v1.42q, NIH, Bethesda, MD, USA). Craniofacial surface landmarks on the front and profile photos were identified and marked (Figure 1). The pixel coordinates (x, y) of these points, as well as additional points marking the maximum width of the calibration marker, were transferred to a custom-programmed spreadsheet for the computation of craniofacial linear and angular measurements. Pixel measurements were converted to metric dimensions (52 pixels/cm). These photo measurements represent dimensions and relationships of the various craniofacial regions including the face, mandible, maxilla, eyes, and nose and are defined in Table 1. Facial photographic measurements were found to be highly reproducible (intraclass correlation coefficient > 0.98).
Figure 1.
Quantitative craniofacial photography with calibration marker placement and identified surface landmarks. Pixel coordinates (x, y) of each landmark are obtained for computation of craniofacial measurements. Landmark definitions: al, alare; en, endocanthion; ex, exocanthion; gn, gnathion; go, gonion; n, nasion; sl, sublabiale; sn, subnasion; sto, stomion; t, tragion; 1, left side of face; r, right side of face; c1, calibration point 1; c2, calibration point 2.
Table 1.
Definitions of facial measurements obtained from craniofacial photographic analysis using identified landmarks
Magnetic Resonance Imaging
Imaging was performed in the supine position using a 1.5 Tesla MRI scanner (Siemens Avanto, Germany) with a neck coil for imaging of the upper airway, according to previously published protocols.3,4,14,16 Head position was standardized by aligning the Frankfort plane perpendicular to horizontal and stabilizing with foam padding. During scanning, subjects were instructed to keep the mouth closed with teeth touching and tongue touching the front teeth, breathe normally through the nose, and refrain from swallowing. An initial sagittal localization scan was performed, followed by contiguous axial T1-weighted spin echo imaging of 3.0 mm thick slices from the superior aspect of the mandibular rami to the vocal cords.
MRI Measurements
MRI analysis was performed in the Sleep Imaging Center at the University of Pennsylvania. The MRI datasets were used to obtain tissue volumes and bony dimensions as previously described.3,4,14,16 Analysis was performed using image and volumetric analysis software (Amira 4.1.2; Visage Imaging, San Diego, CA).
Craniofacial Skeletal Structures
Cephalometric measurements were obtained using midsagittal MRIs for the identification of the following cephalometric points: sella (S), nasion (N), anterior nasal spine (ANS), subspinale (A), supramentale (B), and menton (Me) (Figure 2A). These points were used to obtain measurements for angles (°) of maxillary and mandibular positions: SNA (sella-nasionsubspinale), SNB (sella-nasion-supramentale) and ANB (difference between SNA and SNB angles), and upper and lower face heights (cm). Axial slices were used to identify left and right gonion (Go, the most posterior and inferior points of the mandible), gnathion (Gn, the most anterior-inferior point of the mandible), and the centroid of the condyle, the superior point of the mandibular ramus.4 Mandibular width (cm) was measured as the distance between the left and right gonion and mandibular condyle width as the distance between the left and right condyle. Mandibular length (cm) was measured as the distance from gnathion to gonion and ramus height (cm) as gonioncondyle distance. MRI skeletal measurements are illustrated in Figure 2B. Skeletal MRI measurements have previously been shown to have good reproducibility on repeated measurements (intraclass correlation coefficient > 0.98).4
Figure 2.
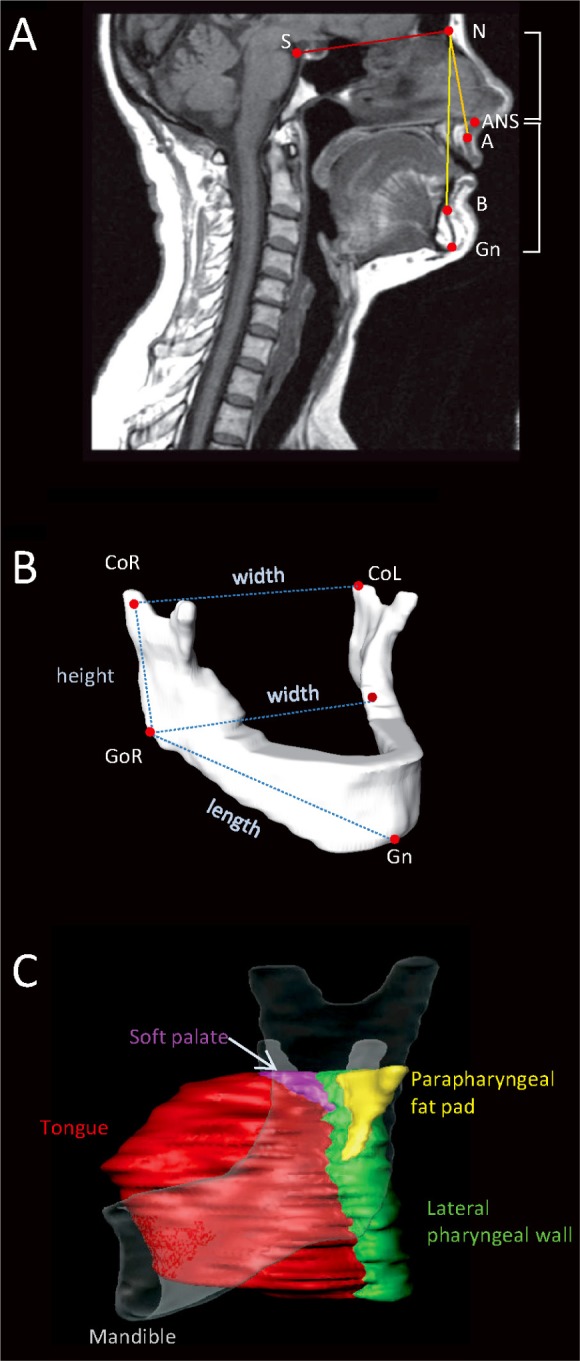
Magnetic resonance imaging (MRI) analysis of craniofacial skeletal measurements and upper airway soft tissues. (A) Midsagittal MRI slice with cephalometric points: nasion (N), sella (S), anterior nasal spine (ANS), subspinale (A), supramentale (B), Gnathion (Gn). These points were used to obtain measures of sella-nasion-subspinale (SNA) and sella-nasion-supramentale (SNB) of maxillary and mandibular position and upper (N-ANS) and lower (ANS-Me) facial heights. (B) Three-dimensional reconstruction of a mandible from MRI illustrating mandibular measurements used for analysis. Right and left gonion (GoR, GoL) and condylion (CoR, CoL) and gnathion (Gn) points were identified on axial MRI slices. Three-dimensional measurements of mandibular length (Go-Gn), ramus height (Co-Go), and mandibular widths at the levels of the gonion and condyle were calculated from these landmarks and are represented by the dotted lines in the image. (C) Volumetric soft-tissue analysis. Three-dimensional reconstructions of the tongue (red), soft palate (purple), parapharyngeal fat pads (yellow), and lateral pharyngeal walls (green). The mandible is shown transparently to give perspective. Airway space volume also was calculated.
Upper Airway and Soft Tissues
Axial images were used to identify and manually segment upper airway soft-tissue structures as previously described.3,16 Tongue (specifically genioglossus muscle, excluding base of tongue muscles such as the geniohyoid and myohyoid), soft palate, lateral pharyngeal walls, and parapharyngeal fat pad areas were identified on each axial slice and traced with a brush tool for manual segmentation. The volume (cm3) of each soft-tissue structure was then calculated from the segmented areas on each slice (Figure 2C). The upper airway space was segmented from the hard palate to the tip of the epiglottis using automated thresholding for pixel values corresponding to air to obtain the upper airway volume (cm3).
Statistical Analyses
Statistical analysis was performed using SAS statistical software (Version 9.3, Cary, NC, USA). Canonical correlation analysis was chosen to investigate relationships between MRI and photographic craniofacial variables. Canonical correlation is a multivariate statistical procedure for assessing relationships between variables; however, this procedure allows relationships between sets of variables to be explored.17 By comparison, multiple regression analysis aims to predict a single dependent variable from a linear function derived from its relationship to a set of multiple independent variables. Canonical correlation enables the assessment of interrelationships between two sets of multiple dependent and multiple independent variables (in the current analysis these two variable sets are photo and MRI craniofacial measurements). The procedure identifies a combination of variables from one variable set with maximum statistical correlation to a combination of variables from the other set. These combinations of dependent and independent variables are termed canonical variates. The strength of the relationship between canonical variates is indicated by the canonical correlation coefficient (r). The contribution of individual variables to these relationships can be inferred by the strength of their individual standardized coefficients. Individual variables with the highest coefficients are those that are primarily driving the relationship between the two variable sets. Canonical correlation analysis has increasingly been used in biomedical research over the past 10 y (for results by year, search ‘canonical correlation analysis’, PubMed, www.ncbi.nlm.nih.gov/pubmed) and has previously been used to assess craniofacial structures in OSA.18
During initial analysis, canonical correlation was used to assess interrelationships between variables from the two craniofacial imaging methods (MRI and photography). During secondary analysis, the canonical correlation procedure was repeated using adjusted MRI and photographic variables in which variance explainable by the influence of individual patient factors was removed. This was done by performing a regression analysis for each craniofacial variable using an a priori selected set of patient factors as independent variables. These factors included height, weight, and neck and waist circumference measurements obtained at baseline or 2 y, as appropriate for MRI and photo variables, as well as sex and baseline apneahypopnea index (AHI). In this way, secondary canonical correlation focused on the portion of each craniofacial measurement not related to these factors. An additional adjusted canonical correlation analysis was performed controlling only for weight and neck and waist circumference to determine the relative importance of obesity-related versus nonobesity-related patient factors.
RESULTS
Patient Characteristics
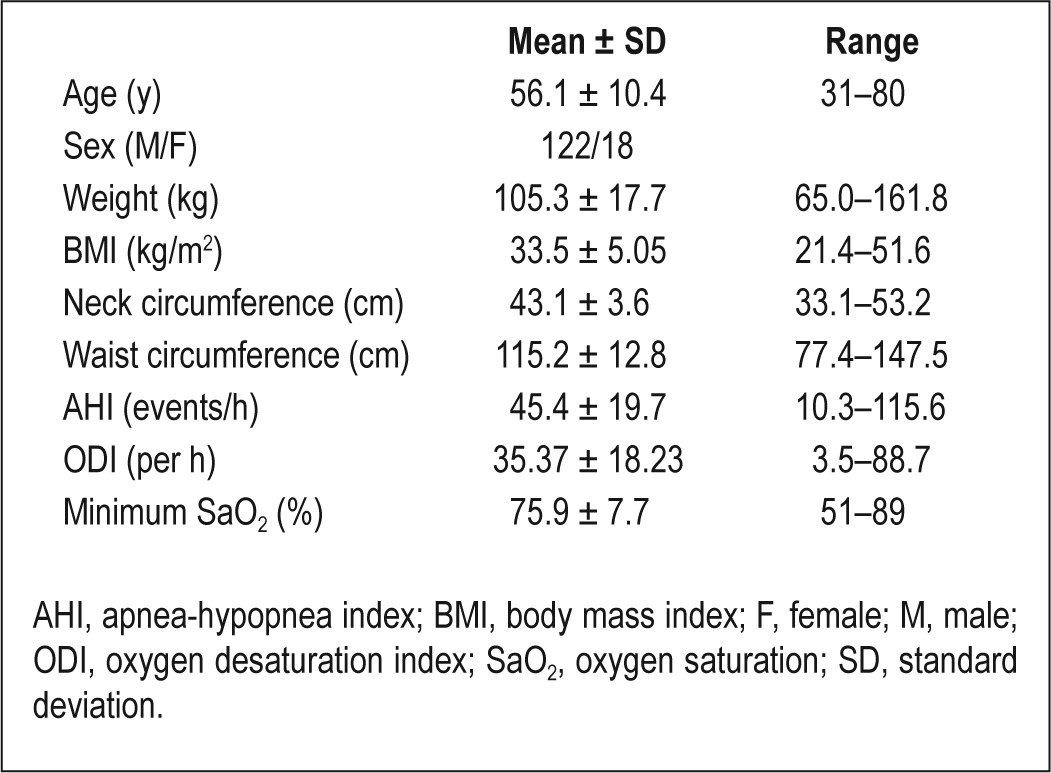
One hundred forty patients from the cohort had both craniofacial photography and MRI performed with less than 2% weight change between the two imaging time points. Therefore, only these 140 patients were included in this analysis. Patient characteristics are provided in Table 2. These 140 patients in the current analysis did not differ from the other 682 patients in the cohort in terms of baseline characteristics of BMI, neck and waist circumferences, AHI, or oxygen desaturation index (ODI; data not shown), although the mean age was slightly higher than that of the rest of the cohort (56.1 versus 54.1 y, P < 0.05).
Table 2.
Patient characteristics (n = 140)
Relationship Between Facial Photographic Measurements and MRI Variables
Canonical correlation analysis confirmed a significant multivariate relationship between the photo and MRI sets of craniofacial variables (Wilks Lambda F value = 2.87, degrees of freedom (df) = 156, 891.81, P < 0.0001). Four significant canonical correlations between the two variable sets were identified. These correlations as well as the coefficients of the individual MRI and photo variables that most strongly contribute to these relationships are shown in Table 3. These four canonical variates were named according to the craniofacial variables that most strongly contributed to the shared relationship; 1, maxilla/ mandible relationship; 2, lower face height; 3, mandible length; 4, tongue volume.
Table 3.
Canonical correlation analysis between original craniofacial variables measured from facial photographs and magnetic resonance imaging
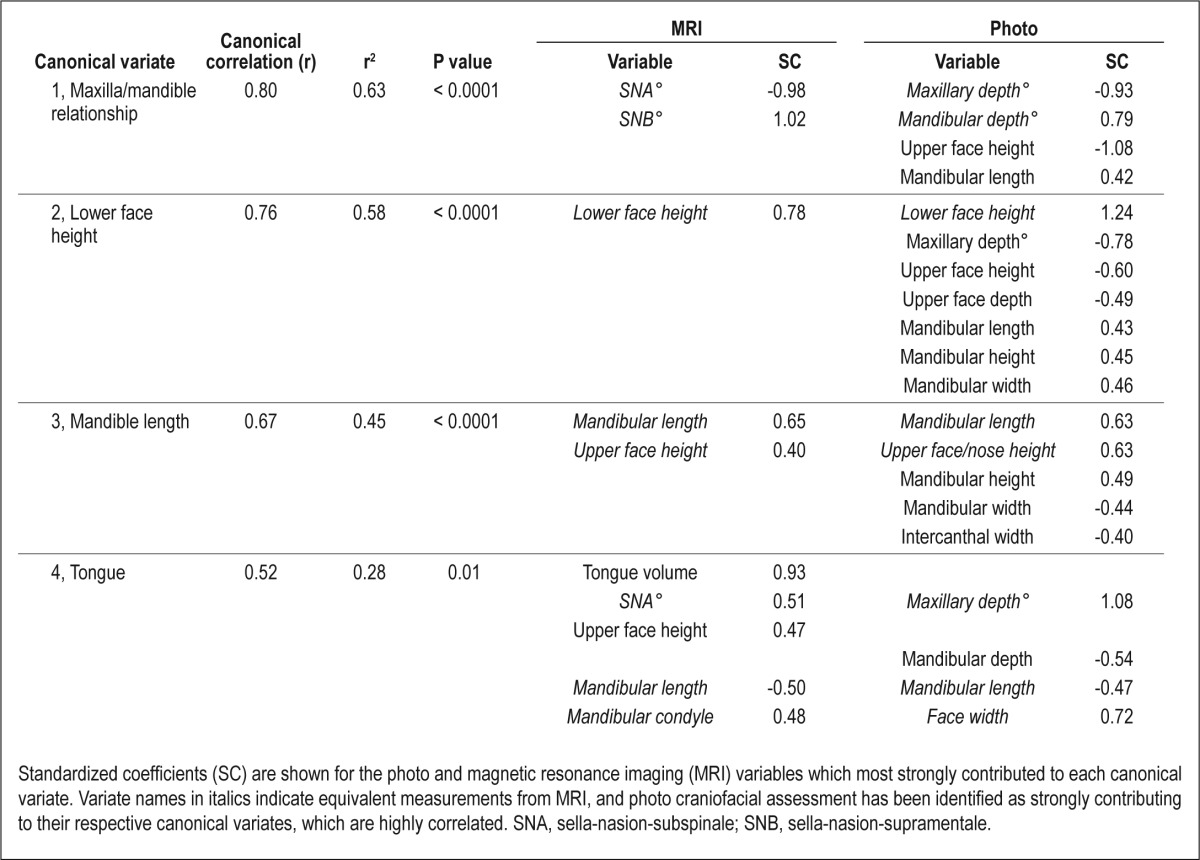
The first and strongest canonical correlation (r = 0.80, P < 0.0001) primarily reflected the angles of maxilla and mandible position (SNA° and SNB°) from MRI and their equivalent photo measurements. The signs of the coefficients of the maxillary and mandibular values are opposite (positive for SNA° and negative for SNB°), suggesting that the difference between these measurements drives this relationship. The maxillary and mandibular relationship was also reflected in upper face height and mandibular length measurements from the photographs. The variables comprising the second canonical correlation are constructed to be statistically independent to the variables comprising the first canonical correlation, thereby identifying a different set of relationships. This canonical correlation (r = 0.76, P < 0.0001) was primarily driven by lower face height measures, which was the highest weighted variable from both the MRI and photo data (Table 3). Photo measurements relating to the mandible in length, height, and width were also associated with this lower face dimension. This canonical variate also identified a number of photo variables relating to the upper face (maxillary depth angle, upper face height, and depth) as contributing to the relationship but with coefficients of opposite signs, suggesting a difference in upper and lower face proportions. The third canonical variate (r = 0.67, P < 0.0001) was most strongly influenced by mandibular length measurements from both the MRI and photo measurement, with a weaker contribution from upper face height (n-ans) measurements. Mandibular length was, to a lesser extent, differentially related to facial width measures (between the eyes and at the level of the mandible) on photographs.
The fourth and final canonical correlation was weaker (P = 0.52, P = 0.01), but was most strongly driven by tongue volume measured with MRI. Several skeletal measurements also contributed to the relationship but with a lesser weighting (Table 3). Both MRI and photo measurements showed tongue volume to be associated with maxillary depth angle (SNA°) and midface width (between the mandibular condyle points on MRI and tragion landmarks on the face). However, tongue volume was differentially related to mandibular length measures from both image sets, with the coefficients of these measurements having opposite signs.
Overall, the canonical correlation analysis has shown that there are strong relationships between sets of craniofacial measurements made with MRI and sets of facial phenotypic information acquired from simple digital photographs. These relationships primarily related to craniofacial bony dimensions; however, facial phenotype also seemed to capture some information about upper airway soft tissue in relationship to tongue volume (canonical variate 4). Within these four correlations between variable sets (MRI and photo), MRI bony measurements and what would be their equivalent on a photograph were consistently identified as the strongest contributors within each correlation (Table 3).
Descriptive statistics and correlations between all original facial photographic variables and MRI variables are shown in the supplemental material (Table S1).
Relationship Between Photographic and MRI Variables After Adjusting for Patient Characteristics
A relationship between facial photo measurements and aspects of craniofacial morphology measured from MRI may potentially exist through common dimensions related overall to confounding factors including body size, obesity, sex, age, severity, and OSA. Therefore, we additionally sought to confirm that facial phenotype conveys information about underlying craniofacial morphology beyond the influence of these patient characteristics, filtering variance explainable by these factors. An additional analysis was performed controlling only for obesity measures (weight, neck and waist circumferences).
All craniofacial measurements (photo and MRI) were converted to a form where the component relating to these patient factors was removed. This was done in a regression analysis for each craniofacial measurement (dependent variable) with patient factors as the independent variables (height, weight, neck and waist circumferences, age, AHI, sex). The relationships between patient factors and individual craniofacial measurements are shown in the supplemental material (Tables S2 and S3). The residuals from each analysis (the portion of each craniofacial measurement not accounted for by these factors) was used in lieu of the original craniofacial variable to repeat the canonical correlation analysis. In this way, the canonical correlation coefficients could be determined by filtering out shared variance explainable by these factors.
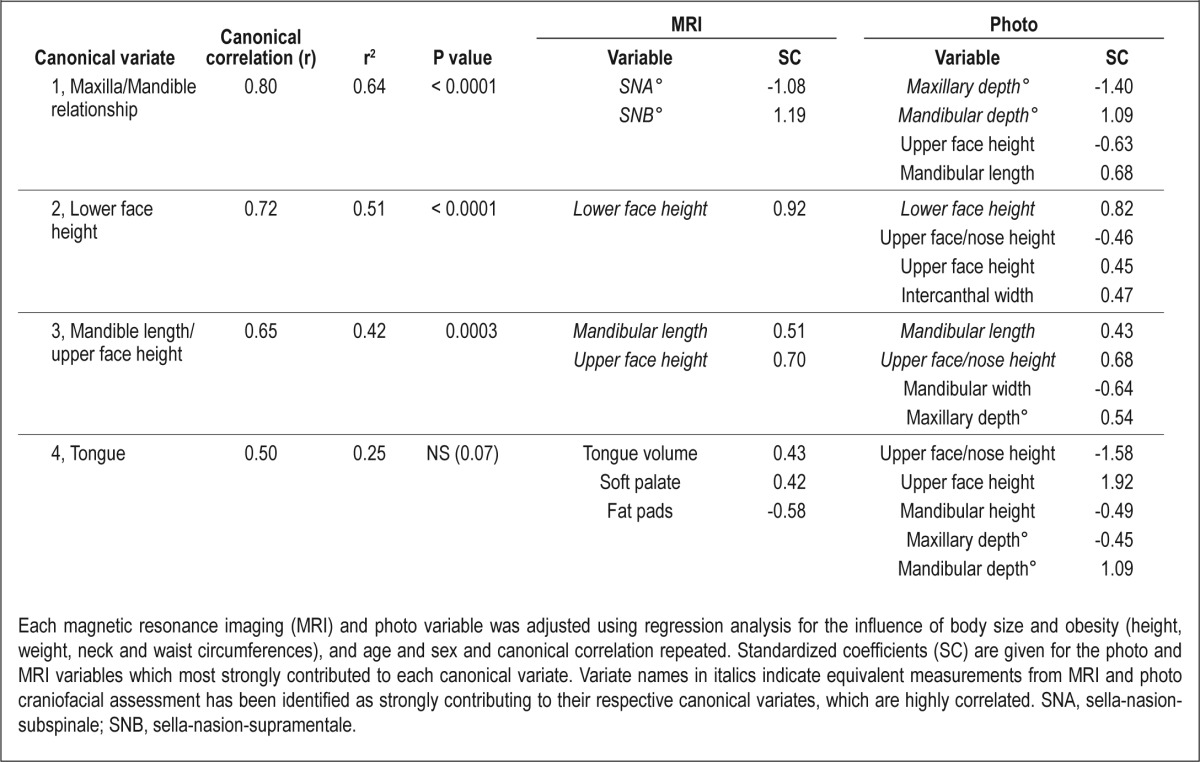
The adjusted canonical correlation analysis, with the influence of patient factors removed, showed equivalent results to the original analysis (Wilks Lambda F value = 2.52, df = 156, 856.26, P < 0.0001), although there were only three significant canonical correlations identified (Table 4). The three significant correlations between MRI and photo canonical variates were very similar in strength and relationships to the original analysis and again primarily identified relationships influenced by measures of maxillary/mandibular position (canonical variate 1), lower face height (canonical variate 2), and mandibular length/upper face height (canonical variate 3). In this adjusted analysis, the fourth canonical variate, primarily relating to tongue size, was no longer statistically significant (P = 0.07) and the strength of the tongue volume coefficient was greatly reduced (0.93 to 0.43). Tongue size was primarily influenced by patient factors of sex, weight, and neck circumference (Table S2). To determine the relative importance of the obesity-related variables in explaining the reduction in the tongue factor correlation, a final adjusted canonical correlation analysis was performed (results not shown), controlling only for the obesity-related covariates (weight, and waist and neck circumferences). The significance of the first three canonical correlations changed slightly (1, P < 0.0001; 2, P < 0.0001; 3, P < 0.0006). In contrast, the fourth canonical correlation and the tongue volume coefficient was close to zero (P = 0.20).Therefore, obesity alone appears sufficient to account for the shared variance between tongue volume related facial phenotype.
Table 4.
Canonical correlation analysis for relationships between MRI and photo variables independent of body size
DISCUSSION
This study is the first to assess relationships between craniofacial variables measured by facial photographic phenotyping and MRI in a large sample of patients with OSA from a homogenous ethnic population. We found significant associations between the two sets of craniofacial variables, suggesting that facial phenotyping conveys key aspects of underlying craniofacial morphology that relate to OSA risk. We further confirmed that relationships between photo dimensions and craniofacial bony measurements existed when controlled for patient factors such as body size, age, and sex, indicating that facial phenotyping reflects OSA risk beyond what can be captured by basic anthropometry and demographics.
To investigate the relationship between facial photographic measurements and craniofacial MRI variables we chose canonical correlation analysis, a multivariate analysis procedure that examines relationships between multiple dependent and independent variables while assessing the contribution of individual variables to the relationship. We adopted this approach to the analysis because both methods of craniofacial imaging measure related structures, but the underlying craniofacial structure and overlying soft-tissue profile are inherently different and therefore single, absolute measurements are not likely directly comparable.19,20 Furthermore, although we have reason to believe that facial phenotype is related to upper airway soft tissues,5,8,10 no single facial measurement would be expected to directly reproduce these structures.
Our analysis identified three highly correlated canonical variates of photo and MRI craniofacial measurements. Interestingly, in each of these relationships, MRI bony dimensions, and what would be their photographic equivalent, were identified as the strongest contributing variables to the correlation. These canonical correlations related to (1) maxillary/mandibular position (SNA°, SNB° and their soft-tissue equivalents), (2) lower face height, and (3) mandibular length. These aspects of craniofacial morphology as identified by either MRI or photographs were highly related. Previous studies have found that linear and angular measurements from equivalent facial landmarks on lateral cephalometric x-rays and profile photographs do not necessarily correspond well in absolute dimensional terms.19,20 This is probably attributable to varying thicknesses of the soft-tissue profile and lower reproducibility of some surface chin landmarks on profile photos compared with cephalometry, and soft-tissue profile may additionally be affected by lip strain and posture.19 This is observable in our own data where correlations between individual measurements, even if constructed from equivalent soft and hard tissue landmarks, were relatively weak (particularly SNA° and SNB°). However, examining a composite of craniofacial morphology from both imaging data-sets identified strong relationships between similar underlying craniofacial aspects.
Craniofacial skeletal abnormalities are a known phenotype associated with OSA.21 Our analysis identified several associations with facial photographic variables that relate to known craniofacial risk factors for OSA. Our second canonical correlation identified lower face height as strongly related between MRI and photo craniofacial assessment. Elongation of the anterior face height is also reported in OSA,22 although this may reflect a secondary response to chronic airway obstruction rather than a primary craniofacial risk factor.21 We identified a strong relationship between photo and MRI data in assessing relative maxillary and mandibular position angles. Characteristics of maxillary and mandibular structure are among the most common skeletal differences reported between patients with OSA and nonapneic controls. Maxillary abnormalities of posterior positioning, shorter length and narrowing of the arch,23–25 mandibular retropositioning, and reduced overall mandibular dimensions and enclosure size4,26–28 have been associated with OSA. Maxillary and mandibular deficiencies are likely to contribute to upper airway collapsibility and OSA by reducing the pharyngeal airway space.29 The third canonical correlation between MRI and photo craniofacial variables identified a measure of mandibular dimension, mandibular length between the corner of the jaw and chin, as strongly associated between images supporting that craniofacial risk factors can be detected by facial phenotyping. Interestingly the relative positioning between the maxilla and mandible in terms of a relative posteriorly positioned mandible has previously been implicated as important in OSA pathogenesis.25,30 Our analysis found that the difference between the underlying maxillary and mandibular factors (SNA° and SNB°) was what drove the association between MRI and photo measurements.
Photographic facial width and height measurements showed differential associations (opposite signs) as contributors to the relationship with MRI variables in canonical variate 3. An association of lateral facial width with OSA has not been extensively explored because previous imaging studies have primarily involved analyses of lateral radiographs of the facial profile, and posterior-anterior imaging, allowing facial width measurement, has rarely been performed.31–33 One study using anthropo-metric measures of cranial and facial form (ratio of facial height to width) identified an association of brachycephalic head form (a wider and shorter facial form) with OSA in Caucasians.34 The differential association between facial width and height in the current analysis may indicate a similar phenotype.
The fourth identified relationship between MRI and photo variables in this study was primarily driven by tongue volume size, which related to a range of facial photo measurements reflecting overall facial dimensions. Interestingly, the coefficients suggest that tongue size shared an inverse relationship with lower mandibular measurements. Our sample only contained patients with OSA, so we are unable to determine if this association differs in subjects without OSA, but this relationship may reflect an imbalance in the relative sizes of upper airway soft tissues and the facial skeletal enclosure, which is thought to be relevant to the pathogenesis of OSA.35
In secondary analysis controlling for obesity measures (weight, waist and neck circumferences) the association between tongue volume and facial phenotype was no longer significant. This suggests that facial phenotype may only inform about tongue size through a shared relationship with obesity in deposition of fat within the tongue.36,37
Overall facial photographic phenotyping in this study identified elements that were strongly associated with skeletal craniofacial structures with a known association with OSA risk. Although direct measurements between individual photographic and MRI measurements may vary, it appears that important phenotypic associations can be gathered by this method. Therefore, this surrogate imaging technique is likely to adequately summarize key aspects of craniofacial morphology associated with OSA risk. Our previous work identified four facial photographic variables as predictive of OSA in a Caucasian sleep clinic population,7 and two of these facial measurements (face width and mandibular length) were confirmed to be associated with aspects of craniofacial morphology assessed with MRI. Facial photographs may prove to be a simple tool to stratify OSA risk as measurements appear to gather important phenotypic information about craniofacial dimensions and regional fat distribution. However, for wide applicability the influence of ethnicity and sex on facial phenotypes in OSA needs to be evaluated, as our study was of a homogenous Caucasian group. It is thought that the relative importance of skeletal and soft-tissue risk factors for OSA varies between ethnicities, with the limited available studies suggesting that skeletal restriction predominates in Asian patients and enlarged upper airway soft tissues in African Americans, whereas Caucasians may be somewhere in between with a large contribution of obesity.38,39 Therefore, facial dimensions that relate to OSA risk may predominantly reflect obesity in some populations and skeletal structure in others. However, interethnic studies of facial phenotype and relationship to underlying craniofacial risk factors are needed to explore this possibility. The only existing OSA facial photographic study in Caucasians and African Americans found the relationship between photo and cephalometric facial measurements to be similar within the two ethnicities, despite soft-tissue thickness being reported to be greater in African-Americans.20
In addition, facial photography represents a simple means to gather important phenotypic information about craniofacial risk factors in future studies of genetic risk factors of OSA. Craniofacial morphology is clearly an important intermediate phenotype in OSA pathophysiology, and there may be specific genes that influence craniofacial morphology and hence susceptibility to OSA.40,41 A recent genomewide association study in Europeans identified five candidate genes influencing facial morphology, obtained from eye and nose landmarks on surface-rendered MRI of the upper face, which related to aspects of facial shape such as the position of nasion.42 The facial analysis technique used in the current study involves acquisition of two-dimensional calibrated photos of the face and profile using a standard consumer digital camera for rapid computation of facial dimensions. Camera systems for acquisition of three-dimensional facial photographs are now in existence and allow measurement of the facial surface and shape that may more closely approximate reality.43 Further work is needed to determine if three-dimensional assessment with such camera systems provides additional facial information that would be of value. However, such systems are still relatively expensive and not widely available for large-scale studies, which is a strength of the current methodology in allowing facial assessment without the need for expensive and intensive imaging techniques.
Although this study is the first to confirm a relationship between facial phenotype assessed by quantitative photography to underlying craniofacial structure from MRI in a homogenous sample of Caucasian patients with OSA, there are some limitations to the data. Primarily, the OSA subjects included in this analysis were a convenience sample from the ISAC. This well-characterized cohort of patients with OSA in Iceland represented a unique opportunity to compare MRI to facial photography in a large group of patients with OSA for which these data were collected for phenotyping purposes. However, facial photography was only available at a follow-up assessment 2 y after upper airway MRI. Weight and age changes over this time period could significantly affect the relationships between surface facial dimensions and structures measured on MRI. To help circumvent this issue we restricted our analysis to those patients in the cohort who experienced less than 2% weight change between the two imaging time points. There is potential for changes in facial tissues over this time period unrelated to change in weight; however, we were able to identify significant relationships and suspect concurrent imaging would only strengthen these findings. Also, our skeletal measurements were obtained using novel methodology of MRI cephalometry,4 which provided a detailed analysis of upper airway soft-tissue and craniofacial structures. MRI is advantageous over lateral cephalometry in that a three-dimensional assessment of the mandible can be obtained. Other methodological differences between the photography and MRI, particularly the positional differences between upright (photo) and supine (MRI) imaging, may potentially alter craniofacial measurements; however, key aspects such as overall tissue volume and mandibular dimension from MRI analysis should not be affected by this difference in posture.
In conclusion, facial phenotype, assessed by quantitative photography, shows significant relationships with underlying aspects of craniofacial morphology measured by MRI. The relationships between photo and MRI variables are largely independent of body size, obesity, age, and sex. However, facial phenotype appears to primarily relate to tongue size through measures of obesity. This study confirms that facial phenotype is able to capture aspects of underlying craniofacial risk factors for OSA and may therefore be used as an objective tool to assess this phenotype in large-scale studies, including genetic studies. Craniofacial photography involves lower cost, greater simplicity and safety, and widespread availability compared with other methods of craniofacial assessment. Further investigation is needed to understand the influence of ethnicity and gender on the relationships between facial phenotype and craniofacial risk factors for OSA.
DISCLOSURE STATEMENT
This was not an industry supported study. Funding for this project was provided in part by NIH grant P01 HL094307. This study was done as part of the Sleep Apnea Genetics International Consortium (SAGIC).
Mr. Maislin is Principal Biostatistician of Biomedical Statistical Consulting (BSC). The research conducted for this manuscript is not within the scope of studies supported by BSC nor does BSC or Mr. Maislin have any interest, final or otherwise, in the results of this study. The other authors have indicated no financial conflicts of interest.
SUPPLEMENTAL MATERIAL
Descriptive statistics and correlations between facial photo dimensions and magnetic resonance imaging measurements.
Regression analysis to remove influence of patient factors from magnetic resonance imaging craniofacial variables
Regression analysis to remove influence of patient factors from photo craniofacial variables
REFERENCES
- 1.Cistulli PA, Sullivan CE. Pathophysiology of sleep apnea. In: Saunders NA, Sullivan CE, editors. Sleep and breathing. New York: Marcel Dekker Inc; 1994. pp. 405–48. [Google Scholar]
- 2.Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:144–53. doi: 10.1513/pats.200707-114MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwab RJ, Pasirstein M, Pierson R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168:522–30. doi: 10.1164/rccm.200208-866OC. [DOI] [PubMed] [Google Scholar]
- 4.Chi L, Comyn FL, Mitra N, et al. Identification of craniofacial risk factors for obstructive sleep apnoea using three-dimensional MRI. Eur Respir J. 2011;38:348–58. doi: 10.1183/09031936.00119210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee RW, Sutherland K, Chan AS, et al. Relationship between surface facial dimensions and upper airway structures in obstructive sleep apnea. Sleep. 2010;33:1249–54. doi: 10.1093/sleep/33.9.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee RW, Chan AS, Grunstein RR, Cistulli PA. Craniofacial phenotyping in obstructive sleep apnea--a novel quantitative photographic approach. Sleep. 2009;32:37–45. [PMC free article] [PubMed] [Google Scholar]
- 7.Lee RW, Petocz P, Prvan T, Chan AS, Grunstein RR, Cistulli PA. Prediction of obstructive sleep apnea with craniofacial photographic analysis. Sleep. 2009;32:46–52. [PMC free article] [PubMed] [Google Scholar]
- 8.Liu ZJ, Shcherbatyy V, Gu G, Perkins JA. Effects of tongue volume reduction on craniofacial growth: A longitudinal study on orofacial skeletons and dental arches. Arch Oral Biol. 2008;53:991–1001. doi: 10.1016/j.archoralbio.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maeda K, Tsuiki S, Isono S, Namba K, Kobayashi M, Inoue Y. Difference in dental arch size between obese and non-obese patients with obstructive sleep apnoea. J Oral Rehabil. 2012;39:111–7. doi: 10.1111/j.1365-2842.2011.02243.x. [DOI] [PubMed] [Google Scholar]
- 10.Yoo E, Murakami S, Takada K, Fuchihata H, Sakuda M. Tongue volume in human female adults with mandibular prognathism. J Dent Res. 1996;75:1957–62. doi: 10.1177/00220345960750120701. [DOI] [PubMed] [Google Scholar]
- 11.Arnardottir ES, Janson C, Bjornsdottir E, et al. Nocturnal sweating--a common symptom of obstructive sleep apnoea: the Icelandic sleep apnoea cohort. BMJ Open. 2013;3(5) doi: 10.1136/bmjopen-2013-002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnardottir ES, Maislin G, Jackson N, et al. The role of obesity, different fat compartments and sleep apnea severity in circulating leptin levels: the Icelandic Sleep Apnea Cohort study. Int J Obes (Lond) 2013;37:835–42. doi: 10.1038/ijo.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnardottir ES, Maislin G, Schwab RJ, et al. The interaction of obstructive sleep apnea and obesity on the inflammatory markers C-reactive protein and interleukin-6: the Icelandic Sleep Apnea Cohort. Sleep. 2012;35:921–32. doi: 10.5665/sleep.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maislin G, Ahmed MM, Gooneratne N, et al. Single slice vs. volumetric MR assessment of visceral adipose tissue: reliability and validity among the overweight and obese. Obesity. 2012;20:2124–32. doi: 10.1038/oby.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiu CSW, Clark RKF. Reproducibility of natural head position. J Dent. 1991;19:130–1. doi: 10.1016/0300-5712(91)90111-b. [DOI] [PubMed] [Google Scholar]
- 16.Welch KC, Foster GD, Ritter CT, et al. A novel volumetric magnetic resonance imaging paradigm to study upper airway anatomy. Sleep. 2002;25:532–42. [PubMed] [Google Scholar]
- 17.Hair JF, Anderson RE, Tatham RL, Black WC. 5th ed. Prentice-Hall International; 1998. Canonical correlation anlysis. Multivariate data analysis. [Google Scholar]
- 18.Tangugsorn V, Krogstad O, Espeland L, Lyberg T. Obstructive sleep apnea: a canonical correlation of cephalometric and selected demographic variables in obese and nonobese patients. Angle Orthod. 2001;71:23–35. doi: 10.1043/0003-3219(2001)071<0023:OSAACC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 19.Moate SJ, Geenty JP, Shen G, Darendeliler MA. A new craniofacial diagnostic technique: the Sydney diagnostic system. Am J Orthod Dentofacial Orthop. 2007;131:334–42. doi: 10.1016/j.ajodo.2005.04.047. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Hans MG, Graham G, Kirchner HL, Redline S. Correlations between cephalometric and facial photographic measurements of craniofacial form. Am J Orthod Dentofacial Orthop. 2007;131:67–71. doi: 10.1016/j.ajodo.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 21.Lee RW, Sutherland K, Cistulli PA. Craniofacial morphology in obstrructive sleep apnea: A review. Clin Pulm Med. 2010;17:189–95. [Google Scholar]
- 22.Lyberg T, Krogstad O, Djupesland G. Cephalometric analysis in patients with obstructive sleep apnoea syndrome. I. Skeletal morphology. J Laryngol Otol. 1989;103:287–92. doi: 10.1017/s0022215100108734. [DOI] [PubMed] [Google Scholar]
- 23.Dempsey JA, Skatrud JB, Jacques AJ, et al. Anatomic determinants of sleep-disordered breathing across the spectrum of clinical and nonclinical male subjects. Chest. 2002;122:840–51. doi: 10.1378/chest.122.3.840. [DOI] [PubMed] [Google Scholar]
- 24.Seto BH, Gotsopoulos H, Sims MR, Cistulli PA. Maxillary morphology in obstructive sleep apnoea syndrome. Eur J Orthod. 2001;23:703–14. doi: 10.1093/ejo/23.6.703. [DOI] [PubMed] [Google Scholar]
- 25.Lowe AA, Santamaria JD, Fleetham JA, Price C. Facial morphology and obstructive sleep apnea. Am J Orthod Dentofacial Orthop. 1986;90:484–91. doi: 10.1016/0889-5406(86)90108-3. [DOI] [PubMed] [Google Scholar]
- 26.Okubo M, Suzuki M, Horiuchi A, et al. Morphologic analyses of mandible and upper airway soft tissue by MRI of patients with obstructive sleep apnea hypopnea syndrome. Sleep. 2006;29:909–15. doi: 10.1093/sleep/29.7.909. [DOI] [PubMed] [Google Scholar]
- 27.Sakakibara H, Tong M, Matsushita K, Hirata M, Konishi Y, Suetsugu S. Cephalometric abnormalities in non-obese and obese patients with obstructive sleep apnoea. Eur Respir J. 1999;13:403–10. doi: 10.1183/09031936.99.13240399. [DOI] [PubMed] [Google Scholar]
- 28.Shelton KE, Gay SB, Hollowell DE, Woodson H, Suratt PM. Mandible enclosure of upper airway and weight in obstructive sleep apnea. Am Rev Respir Dis. 1993;148:195–200. doi: 10.1164/ajrccm/148.1.195. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe T, Isono S, Tanaka A, Tanzawa H, Nishino T. Contribution of body habitus and craniofacial characteristics to segmental closing pressures of the passive pharynx in patients with sleep-disordered breathing. Am J Respir Crit Care Med. 2002;165:260–5. doi: 10.1164/ajrccm.165.2.2009032. [DOI] [PubMed] [Google Scholar]
- 30.Tangugsorn V, Krogstad O, Espeland L, Lyberg T. Obstructive sleep apnoea: multiple comparisons of cephalometric variables of obese and non-obese patients. J Craniomaxillofac Surg. 2000;28:204–12. doi: 10.1054/jcms.2000.0147. [DOI] [PubMed] [Google Scholar]
- 31.Coltman R, Taylor DR, Whyte K, Harkness M. Craniofacial form and obstructive sleep apnea in polynesian and caucasian men. Sleep. 2000;23:943–50. [PubMed] [Google Scholar]
- 32.Finkelstein Y, Wexler D, Horowitz E, et al. Frontal and lateral cephalometry in patients with sleep-disordered breathing. Laryngoscope. 2001;111:634–41. doi: 10.1097/00005537-200104000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Poirrier AL, Pire S, Raskin S, Limme M, Poirrier R. Contribution of postero-anterior cephalometry in obstructive sleep apnea. Laryngoscope. 2012;122:2350–4. doi: 10.1002/lary.23458. [DOI] [PubMed] [Google Scholar]
- 34.Cakirer B, Hans MG, Graham G, Aylor J, Tishler PV, Redline S. The relationship between craniofacial morphology and obstructive sleep apnea in whites and in African-Americans. Am J Respir Crit Care Med. 2001;163:947–50. doi: 10.1164/ajrccm.163.4.2005136. [DOI] [PubMed] [Google Scholar]
- 35.Tsuiki S, Isono S, Ishikawa T, Yamashiro Y, Tatsumi K, Nishino T. Anatomical balance of the upper airway and obstructive sleep apnea. Anesthesiology. 2008;108:1009–15. doi: 10.1097/ALN.0b013e318173f103. [DOI] [PubMed] [Google Scholar]
- 36.Brennick MJ, Pack AI, Ko K, et al. Altered upper airway and soft tissue structures in the New Zealand Obese mouse. Am J Respir Crit Care Med. 2009;179:158–69. doi: 10.1164/rccm.200809-1435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nashi N, Kang S, Barkdull GC, Lucas J, Davidson TM. Lingual fat at autopsy. Laryngoscope. 2007;117:1467–73. doi: 10.1097/MLG.0b013e318068b566. [DOI] [PubMed] [Google Scholar]
- 38.Lee RW, Vasudavan S, Hui DS, et al. Differences in craniofacial structures and obesity in Caucasian and Chinese patients with obstructive sleep apnea. Sleep. 2010;33:1075–80. doi: 10.1093/sleep/33.8.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med. 1997;155:186–92. doi: 10.1164/ajrccm.155.1.9001310. [DOI] [PubMed] [Google Scholar]
- 40.Redline S, Tishler PV. The genetics of sleep apnea. Sleep Med Rev. 2000;4:583–602. doi: 10.1053/smrv.2000.0120. [DOI] [PubMed] [Google Scholar]
- 41.Schwab RJ. Genetic determinants of upper airway structures that predispose to obstructive sleep apnea. Respir Physiol Neurobiol. 2005;147:289–98. doi: 10.1016/j.resp.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 42.Liu F, van der Lijn F, Schurmann C, et al. A genome-wide association study identifies five loci influencing facial morphology in Europeans. PLoS Genet. 2012;8:e1002932. doi: 10.1371/journal.pgen.1002932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kau CH, Richmond S, Incrapera A, English J, Xia JJ. Three-dimensional surface acquisition systems for the study of facial morphology and their application to maxillofacial surgery. Int J Med Robot. 2007;3:97–110. doi: 10.1002/rcs.141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Descriptive statistics and correlations between facial photo dimensions and magnetic resonance imaging measurements.
Regression analysis to remove influence of patient factors from magnetic resonance imaging craniofacial variables
Regression analysis to remove influence of patient factors from photo craniofacial variables