Abstract
Introduction:
Obesity in children is assumed to serve as a major risk factor in pediatric obstructive sleep apnea syndrome (OSAS). However, the prevalence of OSAS in otherwise healthy obese children from the community is unknown.
Aim:
To determine the prevalence of OSAS in obese children identified and recruited from primary care centers.
Methods:
A cross-sectional, prospective, multicenter study. Spanish children ages 3–14 y with a body mass index (BMI) greater than or equal to the 95th percentile for age and sex were randomly selected, and underwent medical history, snoring, and Pediatric Sleep Questionnaire (PSQ) assessments, as well as physical examination, nasopharyngoscopy, and nocturnal polysomnography (NPSG) recordings.
Results:
Two hundred forty-eight children (54.4% males) with mean age of 10.8 ± 2.6 y were studied with a BMI of 28.0 ± 4.7 kg/m2 corresponding to 96.8 ± 0.6 percentile when adjusted for age and sex. The mean respiratory disturbance index (RDI), obstructive RDI (ORDI), and obstructive apnea-hypopnea index (OAHI) were 5.58 ± 9.90, 5.06 ± 9.57, and 3.39 ± 8.78/h total sleep time (TST), respectively. Using ≥ 3/h TST as the cutoff for the presence of OSAS, the prevalence of OSAS ranged from 21.5% to 39.5% depending on whether OAHI, ORDI, or RDI were used.
Conclusions:
The prevalence of obstructive sleep apnea syndrome (OSAS) in obese children from the general population is high. Obese children should be screened for the presence of OSAS.
ClinicalTrials.gov Identifier:
Citation:
Alonso-Álvarez ML, Cordero-Guevara JA, Terán-Santos J, Gonzalez-Martinez M, Jurado-Luque MJ, Corral-Peñafiel J, Duran-Cantolla J, Kheirandish-Gozal L, Gozal D, for the Spanish Sleep Network. Obstructive sleep apnea in obese community-dwelling children: the NANOS study. SLEEP 2014;37(5):943-949.
Keywords: adenotonsillar hypertrophy, children, obesity, prevalence, sleep apnea
INTRODUCTION
Sleep disordered breathing (SDB) is a large group of associated conditions that include habitual snoring, upper airway resistance syndrome, hypoventilation, and obstructive sleep apnea syndrome (OSAS), all of which reflect respiratory alterations during sleep that may lead to intermittent hypoxemia, hypercapnia, and disrupted sleep. SDB, and more specifically, OSAS, has been associated with a large spectrum of neuro-cognitive, behavioral, cardiovascular, and metabolic adverse consequences, which appear to be particularly prominent in obese children.1,2
In the past decade, multiple investigative groups have explored the prevalence of OSAS in pediatric populations, and have primarily relied on clinical symptoms as derived from either clinically based settings or using questionnaires-tools, even if many of the latter have not been specifically validated, such that the overall wide spectrum of the reported prevalence findings is quite predictably heterogeneous.1 Among the major contributing factors to OSAS, adenotonsillar hypertrophy (ATH) emerges as the most important condition associated with OSAS in children. However, a large body of evidence in adults,3 and to a lesser extent in children,4,5 suggests that obesity also plays an important contributory role to the pathophysiology of OSAS and its consequences. Expanded and more accurate knowledge about the interactions between obesity and OSAS is all the more important when considering the increases in the rates of obesity in childhood. In Spain, the enKID study has clearly established a high frequency of obesity in the age group of 6–13 y, as evidenced by 15.6% and 12% rates in males and females, respectively.5 Furthermore, in the ALADINO study, which was conducted in children between 6 and 9.9 y of age, the prevalence of obesity was 20.9% in boys and 15.5% in girls.6 However, the risk of OSAS in these cohorts was not examined. Therefore, we conducted the current two-phase study with the aim to assess the contributions of obesity and ATH to OSAS. This report focuses on the first phase that assessed the prevalence and risk factors of OSAS in obese children recruited from the community.
SUBJECTS AND METHODS
The cohort included in this study is a prospectively recruited random sample of 334 subjects, age 3–14 y, from all obese children identified via standard, well-child visits at Primary Pediatric Care Centers in Burgos, Santander, Vitoria, Barcelona, and Cáceres during 2007–2010. The study was approved by the local human subject committees of the institutions of the various participating cities in which pediatric sleep laboratories are available, is in accordance with STROBE guidelines, and the study was registered at ClinicalTrials.gov under NCT01322763. Informed consent was obtained from every parent or legal caretaker, and assent was obtained from children older than 12 y. To guarantee the confidentiality of the data, coding of all data was performed, such that personal information was not available to the investigator-based network.
In Spain, the national health care system includes a program of well-child visits from birth to 14 y of age, in which systematic assessments of physical and mental development are conducted at predetermined ages, and include among many other items, measurement of height and weight. Based on this initial assessment, obesity in the cohort was defined as the presence of a body mass index (BMI) corresponding to ≥ 95th percentile for age and sex using national reference values and standard definitions.7,8 To avoid potential referral bias, participating pediatricians provided a list of 1,283 potential candidates presented as deidentified numbers, and the research coordinators for the study proceeded with a random selection of 334 numbers from the list, followed by contact and recruitment of these potential subjects by the investigators. Thus, of all children identified as fulfilling the obesity criteria during the compulsory Spanish Healthcare System well-child visit, 334 randomly selected parents were initially invited by either mail or telephone to participate in the study. Of these, 86 children were not included in the final cohort: 39 refused to participate, 33 had provided the wrong mailing address or telephone numbers and could not be located after at least three attempts, and 14 did not fulfill the inclusion criteria (see subsequent paragraphs). Thus, the final cohort included 248 obese children.
Inclusion criteria were age between 3–14 y, BMI ≥ 95% for age and sex, and informed consent. Exclusion criteria were failure to fulfill inclusion criteria, and presence of known genetic syndromes or any chronic debilitating disease.
All participating children underwent a general assessment of their medical and sleep history, and filled out a questionnaire about snoring and an abbreviated version of the Pediatric Sleep Questionnaire (PSQ) for sleep disturbances in its previously validated version from Spain.9 A score of 0.33 or higher was considered suggestive of the presence of SDB. Every child was subjected to a comprehensive physical examination as well as an otolaryngology assessment that included an awake nasopharyngoscopy under topical sedation, and children then underwent an overnight sleep study (nocturnal polysomnography, NPSG) followed by a blood draw in fasting conditions in the morning after the NPSG. Figure 1 illustrates the diagrammatic representation of study flow.
Figure 1.
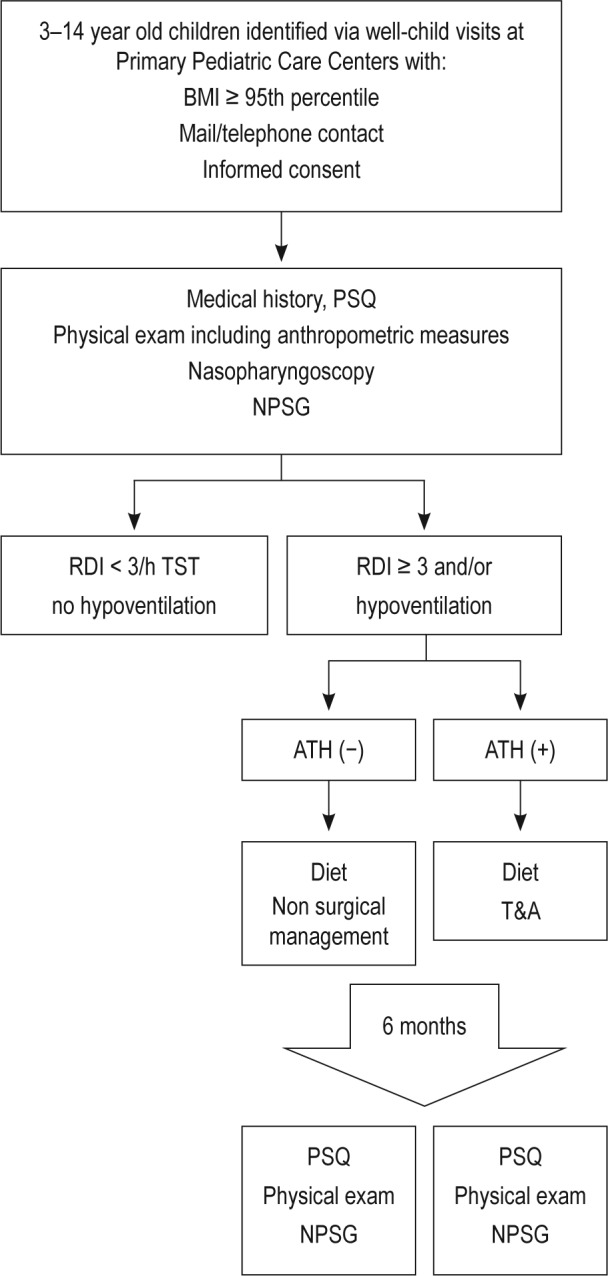
Schematic flow diagram of the study. BMI, body mass index; PSQ, Pediatric Sleep Questionnaire; NPSG, nocturnal polysomnography; RDI, respitory disturbance index; ATH, adenotonsillar hypertrophy; T&A, adenotonsillectomy.
Important medical history items that were sought included family antecedents of OSAS and obesity, previous adenotonsillar surgery, history of asthma, allergic rhinitis or bronchitis, and any medications previously or currently being administered.
For assessment of snoring frequency, three categorical groups were defined: (1) < 2 nights/w; (2) 2–4 nights/w; and (3) > 4 nights/w.
In addition to height in meters, weight in kilograms, and BMI z-score,7 the neck circumference was measured at the level of the cricopharyngeal membrane, and both waist and abdominal circumferences were measured with a measuring tape. Arterial blood pressure measurements were obtained in bed the morning immediately after the completion of the NPSG upon awakening.
All potential participating centers were identified prior to initiation of the study, and every center that was selected complied with the necessary technical and skilled personnel requirements. Furthermore, 6 mo prior to the initiation of the study, all centers underwent a meticulous process of standardization regarding the recording and scoring of all sleep related measures based on the 2007 American Academy of Sleep Medicine (AASM) Guidelines, as well as standardization of the nasopharyngoscopic findings.
Flexible nasopharyngoscopy was performed, and choanal obstruction was classified based on the degree of adenoid hypertrophy: 75–100%, 50–75%, 25–50%, 0–25%. Tonsillar size was also visually quantified as: grade 0: no tonsils, grade I: 0–25% of the airway at the tonsillar level; grade II: 25–50%; grade III: 50–75%; and grade IV: 75–100%.10
An overnight polysomnographic study (NPSG) was performed in the laboratory using standard techniques in the presence of one of the caretakers throughout the study. Children arrived accompanied by one of their parents to the sleep laboratory at approximately 19:30–20:00, and a lights-off routine was implemented at 21:00 with discontinuation of the sleep recordings at 08:00. After removal of movement and technical artifacts, the studies were scored according to standard criteria as defined by the AASM.11 The proportion of time spent in each sleep stage was expressed as percentage of total sleep time (%TST).11 The apnea index (AI) and the apnea-hypopnea index (AHI) were defined as the number of apnea and hypopneas per hour of TST. The AHI, obstructive AHI (OAHI), and the respiratory disturbance index (RDI) also were calculated. Furthermore, the flow limitation index was calculated based on all events in which flow limitation was identified.11
Data Analysis
Data are reported as means ± standard deviation unless indicated otherwise. Descriptive statistics, chi-square analyses for trends, and odd ratios as well as 95% confidence intervals (CIs) were calculated. The prevalence of OSA for the whole sample studied was the primary endpoint. The prevalence with its corresponding 95% CIs was calculated. Bivariate and multivariate analyses were conducted to assess potential significant variables contributing to the variance of the desired outcome measure, and followed by post hoc tests as appropriate using SPSS software (version 14.0; SPPS Inc., Chicago, IL). For all comparisons, a two-tailed P < 0.05 was considered to define statistical significance.
RESULTS
A total of 248 obese children (135 boys, 113 girls), aged between 3 and 14 y (10.8 ± 2.6 y), were recruited into the study out of 320 eligible children identified as obese during the screening process. As indicated previously, the reasons for non-participation included the inability to contact these subjects via repeated telephone or mail attempts, and parental refusal in a minority of cases. Mean BMI was 28.0 ± 4.7, with a mean BMI percentile of 96.8 ± 0.6, corresponding to a BMI z-score of 1.28 ± 0.53. There were no statistically significant differences in age, sex, and BMI among the 72 children who did not participate and the 248 participants.
Maternal history of OSAS was present in 5.5%, and of paternal OSAS in 15.1%, with a prevalence of OSAS history of 3.1% among siblings. Obesity was present in 45.8% of mothers, 40.9% of fathers, 45.4% in both father and mother, and 22.1% of siblings. Approximately 19.2% of participants were taking medications on a regular basis, with bronchodilators, inhaled or nasal corticosteroids, leukotriene modifiers, and antihistaminic medications being the most frequent.
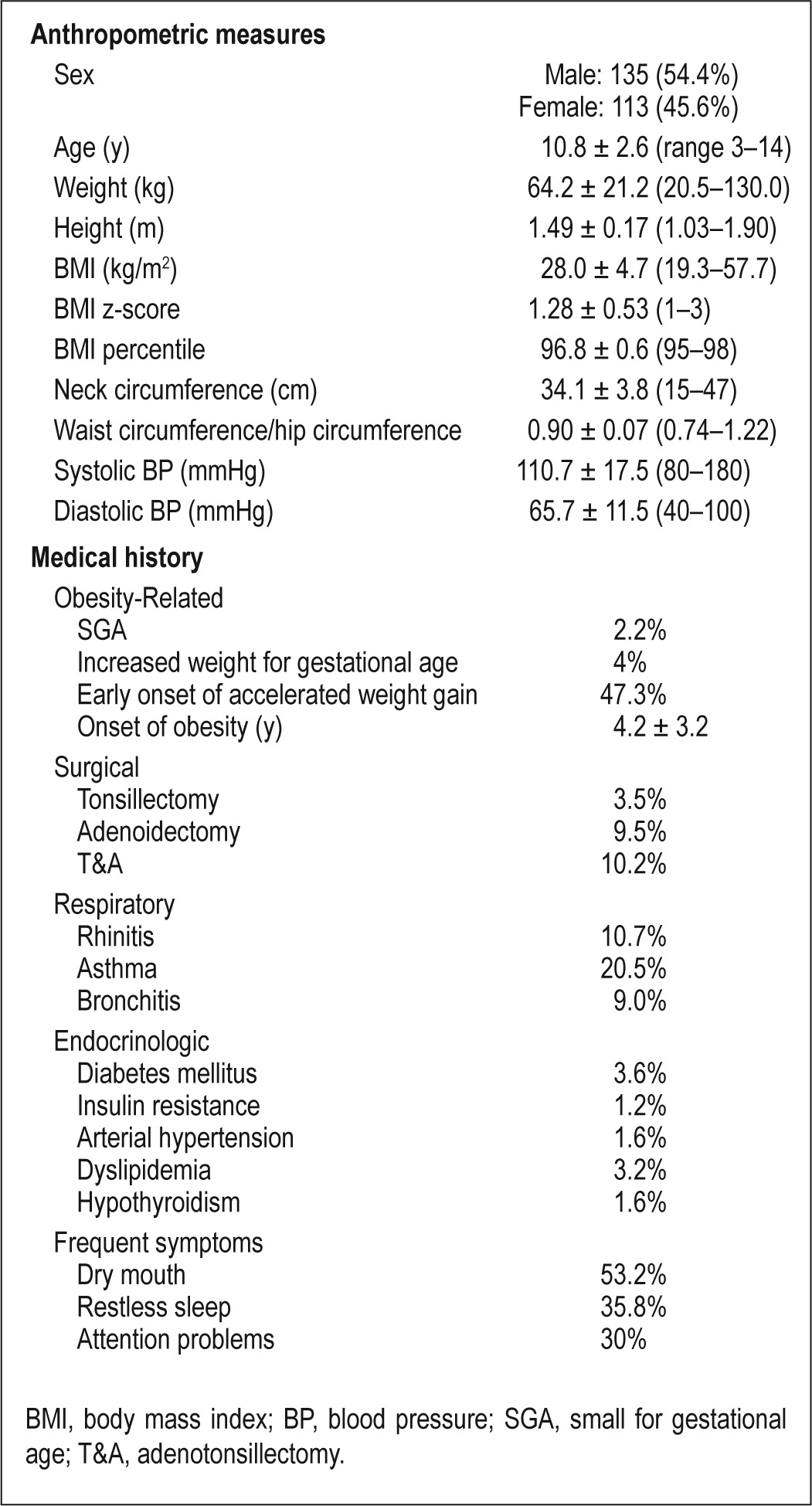
The mean abbreviated PSQ score was 6.62 ± 4.16. Based on such a questionnaire, there was a 37.1% prevalence of OSAS. Based on a RDI ≥ 3/h TST as previously defined in the Spanish Consensus Guidelines,4 the sensitivity and specificity of the PSQ in this cohort were 54.5% and 69.1%, respectively. The clinical and demographic characteristics of the current cohort are shown in Table 1.
Table 1.
Clinical and anthropometric characteristics of the study cohort (n = 248)
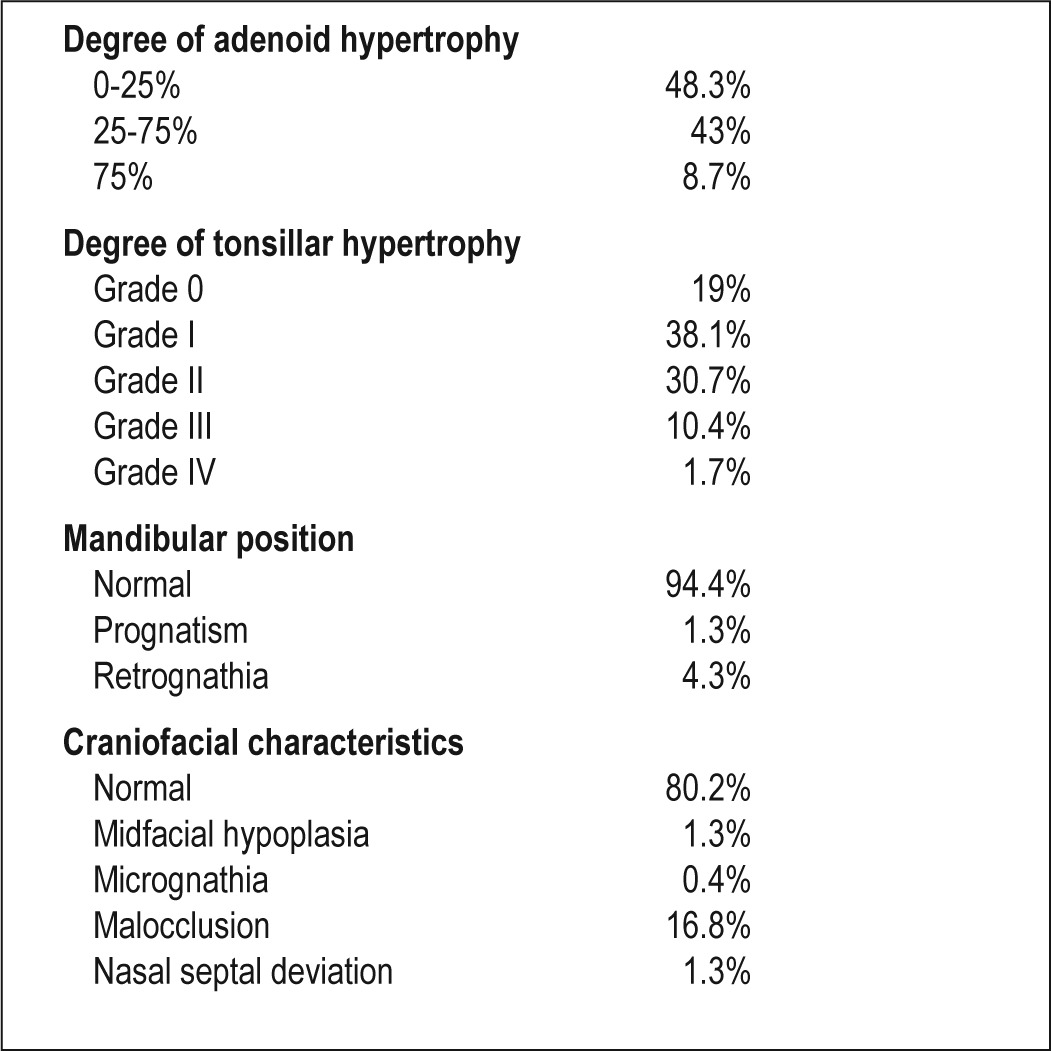
Nasopharyngoscopic assessments showed that 48.3% of the children had no evidence of choanal obstruction from enlarged adenoids, and that 38.1% had grade I tonsillar hypertrophy. A detailed description of the distribution of the assessment findings is shown in Table 2.
Table 2.
Otolaryngological examination including nasopharyngoscopy
Snoring prevalence as derived from the questionnaire was 50.8%, with 18.7% snoring fewer than 2 nights/w, 34.1% snoring 2–4 nights/w, and 47.2% snoring more than 4 nights/w. The prevalence of habitual snoring defined as more than 3 nights/w was 40.3%. Snoring was objectively confirmed in 235 of the 248 children, such that the prevalence of objectively determined snoring was 64.7%. Thus, objective snoring and parentally reported snoring were concurrently present in 74.1%. In 20.1% of patients, snoring was recorded during the sleep study but was not reported by parents. We should also note that 3.4% of the subjects had undergone tonsillectomy prior to enrollment in the study (usually several years before enrollment), with 9.5% having undergone adenoidectomy, and 10.2% adenotonsillectomy.
Mean RDI was 5.6 ± 9.9/h TST, with a mean obstructive RDI (ORDI) of 5.1 ± 9.6/h TST and a mean obstructive AHI (OAHI) of 3.4 ± 8.8/h TST. Mean nadir saturation level of oxygen (SaO2) was 90.3% ± 5.1%, and the total time spent with SaO2 < 90% (T90%) was 1.10% ± 6.64% of TST (Table 3). Using a sleep respiratory disturbance index (SRDI) < 3/h TST as the cutoff for the absence of OSAS and ≥ 3/h TST for the presence of OSAS, the prevalence of OSAS ranged from 21.5% to 39.5% depending on OAHI, ORDI, or RDI as the selected index. Based on a classification of OSAS as mild (SRDI: ≥ 3 and < 5/h TST), moderate (SRDI: ≥ 5 and < 10/h TST) or severe (SRDI: > 10/h TST), severe OSAS was present in 8.1–16.1% of the children based on OAHI-, ORDI-, or RDI-based cutoff values (Table 4). Based on the presence or absence of snoring and the severity of OSAS as described previously, the cohort was subdivided in five severity categories, with the prevalence of OSAS being lowest when OAHI was used as the diagnostic criterion (Table 5). Interestingly, the prevalence of OSAS was similar in girls (42.5%) and in boys (37%; P = 0.434). No differences in the prevalence of OSAS emerged based on age (39.1% for children younger than 7 y, 41% for children ages 7–10 y, and 38.4% in children older than 10 y; P = 0.796).
Table 3.
Polysomnographic measures in 248 obese children
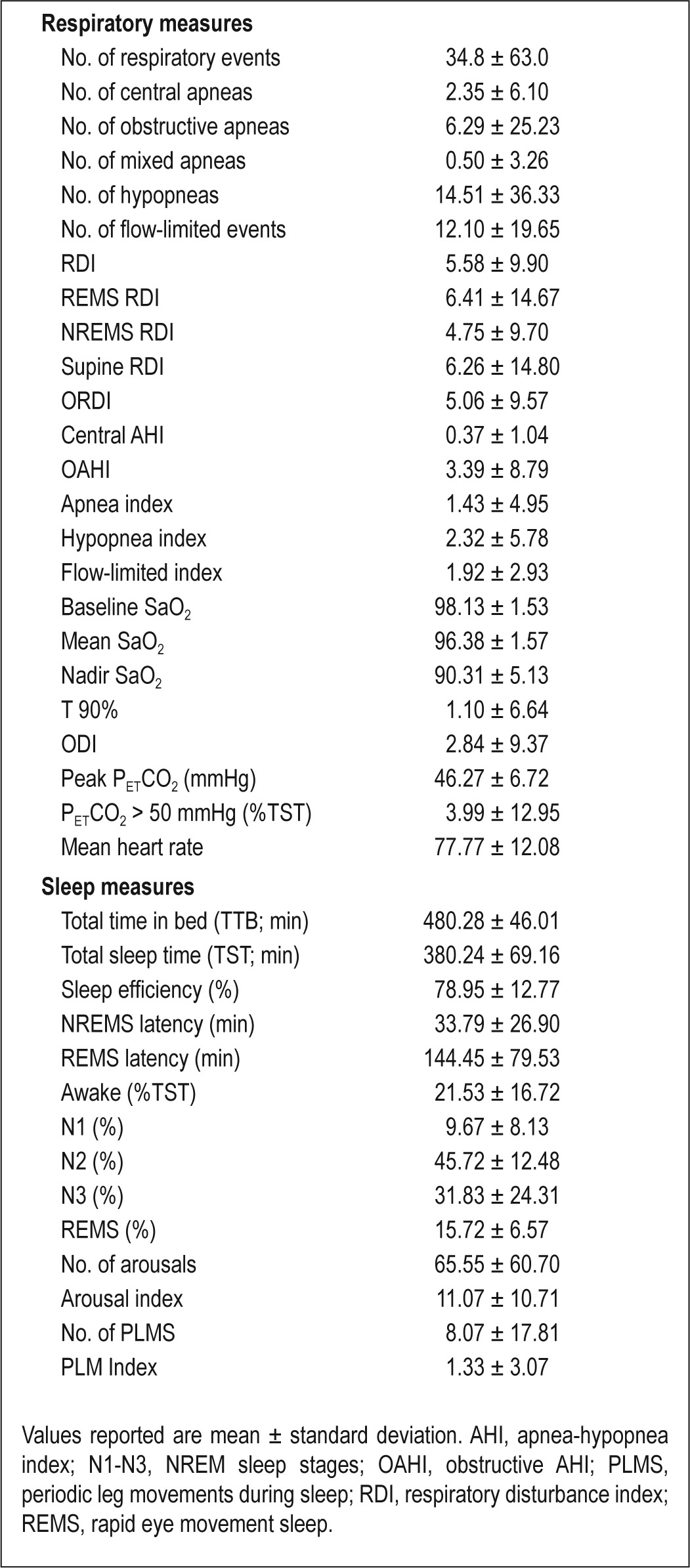
Table 4.
Prevalence and severity of OSAS based on different cutoff criteria in 248 obese children
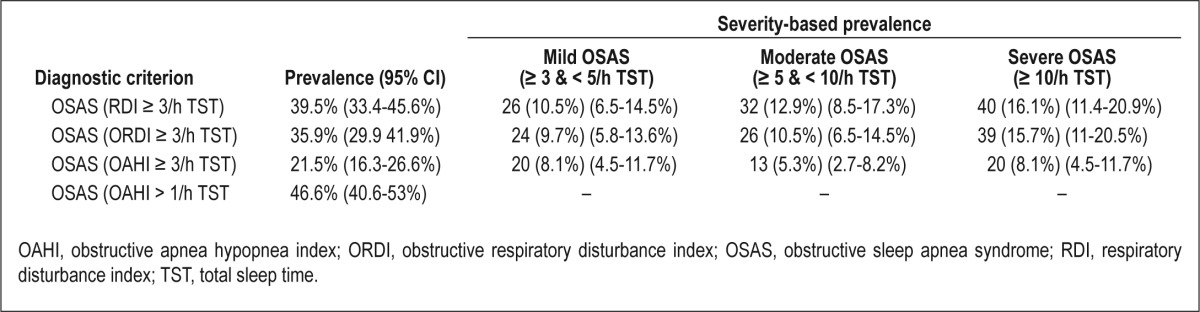
Table 5.
Categorical distribution of sleep disordered breathing in 235 obese children
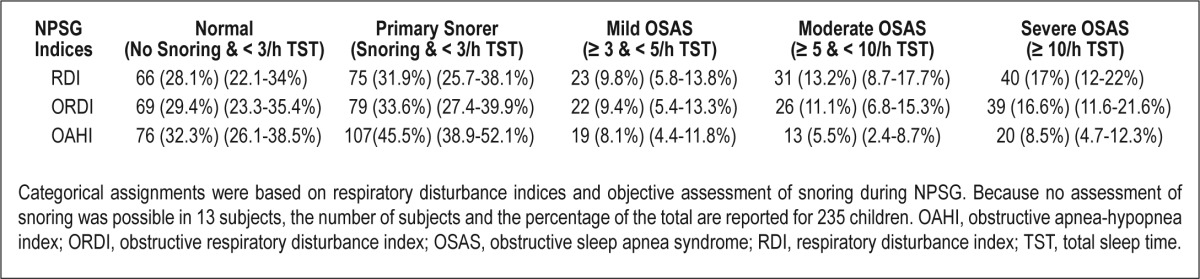
To assess potential associations between OSAS and tonsillar hypertrophy, we dichotomized tonsillar hypertrophy into two groups, namely G0-II and GIII-IV, with the prevalence being much higher in the GIII-IV group (36.5% versus 71.4%; odds ratio (OR): 4.35; 95% CI: 1.83–10.38; P = 0.001). Although to a lesser extent, the prevalence of OSAS in children with evidence of adenoid hypertrophy was higher (48.7% versus 32,4%; OR: 1.98; 95% CI: 1.16–3.38; P = 0.016).
Assessment of the association between BMI z-score and AHI revealed a mean AHI of 5.0 ± 7.37/h TST when the BMI z-score was less than 0, a mean AHI of 5.1 ± 8.28/h TST for a BMI z-score between 0 and 2, and a mean AHI of 19.1 ± 30.11/h TST if BMI z-score was higher than 2 (P < 0.001). A BMI z-score higher than 2 remained significantly associated with increased risk for OSAS after adjusting for all other potential confounders (age, sex, adenoid hypertrophy, tonsillar size). No significant associations were found between waist circumference or abdominal circumference and AHI, and no significant differences emerged between waist or abdominal circumference values among children with and without OSAS. Similarly, post hoc analysis for multiple comparisons showed that the group of children with higher degrees of obesity (BMI z-score higher than 2) had significant differences with respect to the other groups. However, the prevalence of OSAS was 38.7% in children with BMI z-scores less than 2 as compared to 60% in children with BMI z scores higher than 2 (OR: 2.38; 95% CI: 0.65–8.66; P = 0.200). A statistically significant association between BMI and SRDI emerged (r = 0.15; P = 0.018). However, no differences in BMI were apparent in the group with OSAS (28.53 ± 5.43 kg/m2) and the group without OSAS (27.67 ± 4.15/kg/m2; P = 0.164). OSAS family history, the presence of rhinitis, asthma, early onset of obesity (7 y of age or younger), neck circumference, and waist-to-hip circumference ratio did not provide any predictive risk for the presence or absence of OSAS.
In the multivariate logistic regression model using OSAS as a dichotomous outcome variable and age (7 y or older), sex (male), tonsillar hypertrophy (≤ 2), adenoid hypertrophy (< 25%), obesity (BMI z-score of 2 or less) and the interaction between tonsillar hypertrophy × BMI z-score and tonsillar hypertrophy × adenoid hypertrophy, the degree of tonsillar hypertrophy (OR: 5.15; 95% CI: 1.20–22.16; P = 0.028) and adenoid hypertrophy (OR:1.83; 95% CI: 1.01–3.32; P = 0.046), emerged as statistically significant variables contributing to the risk of OSAS. The degree of obesity showed an OR of 2.91, but failed to achieve statistical significance in the adjusted model (95% CI: 0.65–12.91; P = 0.161)
DISCUSSION
This study is the first to prospectively assess the actual prevalence of OSAS in a community-based cohort of children in whom obesity was diagnosed by their primary care physicians. In this nonreferral pediatric population, we found a very high prevalence of both snoring and OSAS, with the presence of adenoid hypertrophy and tonsillar hypertrophy during nasal pharyngoscopy emerging as the most important risk factors for the presence of polysomnographically demonstrated OSAS. Although in the single factorial analysis, the degree of obesity appeared to contribute to the risk of OSAS, the severity of obesity in this obese pediatric cohort was not retained as a major determinant of OSAS in the multivariate analysis.
Before we discuss the potential implications of our findings, some methodological issues deserve comment. First, the prospectively designed recruitment of this cohort was based on the initial diagnosis of obesity by the community pediatrician during routine well-child visits that are mandatory in the Spanish health care system, thereby preventing any selection bias. Therefore, none of these children had been previously evaluated for snoring or clinically suspected or treated for OSAS, even though a proportion underwent upper airway lymphoid tissue resection several years prior. Second, we not only obtained parental reports on snoring, but also ascertained the presence or absence of snoring during overnight PSG. Using a similar approach in nonobese community-based children, a relatively high sensitivity and specificity were reported between subjective and objective assessments of snoring.12 However, in the current study exclusively involving obese children, the presence of snoring was underestimated by approximately 20% of the caretakers, potentially contributing to a reduced rate of suspicion for the presence of SDB. These findings obviously raise the possibility that a specific set of questions may need to be specifically developed for obese children to increase the yield of a priori well-performing standardized screening survey tools.13 The prevalence of habitual snoring has ranged between 1.5–34.5% among the multitude of studies in the literature, with a mean prevalence of approximately 7.5% (95% CI: 5.7–9.6%).1 The large degree of variability among these reports probably reflects the use of different and mostly nonvalidated questionnaires,14 and the diverse age ranges and ethnic populations among the various studies. In addition, seasonal variation in snoring frequency may also have played a role, as well as the underlying presence of asthma.15 From the limited repertoire of prevalence studies in obese children, habitual snoring has also shown substantial degree of variability with prevalence rates all the way from zero to 15% of the children.1,16 In this context, our study also stresses not only the previously reported inability of questionnaires to serve as a OSAS diagnostic tool, but further provides evidence that questionnaires exhibit relatively robust screening capabilities if appropriate validation precautions are implemented. Here, we found a relatively weak performance for the use of the abbreviated PSQ in obese children despite the fact that it had undergone previous validation steps in a cohort of nonobese children.9 Indeed, the Spanish version of the PSQ identified a SDB prevalence of 37%, yielding a sensitivity and specificity of 54.5% and 69.1%, respectively. Third, we did not assess for pubertal status, and the earlier occurrence of puberty in obese children could play a role. However, we found no differences in the prevalence of OSAS across the three age strata, and furthermore the major risk factor for OSAS was ATH. Fourth, we used nasopharyngoscopic assessments of the degree of choanal obstruction by enlarged adenoids and for objective determination of tonsillar size. We opted for this approach to avoid the use of ionizing radiation as would be the case in lateral neck x-rays, and because the use of MRI approaches would be both impractical and excessively onerous.
Standardized PSG assessments have seldom been applied to nonclinical obese pediatric cohorts, and particular care was exerted to standardize the NPSG methodology across the various participating centers. As such, it is not surprising that in other studies in which either only one center was involved, or in which the use of NPSG recordings and scoring was not uniform, the prevalence of OSAS was extremely varied and ranged from 0.7% to 66%,1,16–18 with the additional caveat that most studies evaluated clinical referral populations. In addition, every one of these studies in obese children used different diagnostic criteria. Here, we used our previously established consensus guidelines in Spain using a RDI ≥ 3/h TST,3 and such an approach resulted in a OSAS prevalence of 39.5%. However, even if we used alternative criteria based on the literature (e.g., AHI ≥ 3/h TST, OAHI ≥ 3/h TST, or AHI > 1/h TST), the prevalence would still be very high (i.e., 21.5% to 46.6% for AHI > 1).1,2 To enable more universal comparisons across the pediatric centers, we also examined the distribution of OSAS according to widely accepted severity cutoff values.1,2 In this largest cohort of obese children ever evaluated using PSG, the prevalence of mild OSAS (8.1–10.5%), moderate OSAS (5.3–12.9%), and severe OSAS (8.1–16.1%) were all markedly elevated compared to previous reports of OSAS prevalence in children,1 and clearly establish the presence of pediatric obesity as a major risk factor for the occurrence of OSAS. In a study by Verhulst and colleagues16 that included 64 obese children from a clinical referral sample, the prevalence of OSAS was 11% for mild OSAS, and 8% for moderate OSAS. Similarly, among the various studies available in general populations, the Penn State Child Cohort19 assigned a 4.59-fold increase in the risk for OSAS when obesity was present, whereas Rosen et al. reported a 2.1-fold increase in risk.20 In the current study, neither age nor sex assigned any risk modification to the presence of OSAS. However, the statistically significant risk factors retained from the univariate analyses showed ORs of 2.91 for the degree of obesity, 1.83 for adenoid hypertrophy, and 5.15 for tonsillar hypertrophy, thereby confirming the coordinated contributions of these three well known risk factors to OSAS risk.21 Thus, the significant interactions between obesity and upper airway lymphadenoid tissue hypertrophy shown here as well as in previous studies16,17 strongly support surgical extirpation of these tissues in obese children with OSAS who also have evidence of ATH. Based on the current findings, we postulate that the development of ATH constitutes the major and primary driver for the occurrence of OSAS in obese children, and that the concurrent presence of obesity facilitates the emergence of OSAS, and potentially also contributes to the differential phenotype that appears to characterize this disorder in obese children when compared with nonobese children.22,23 Of note, particularly when considering the high proportion of children who had already undergone upper airway surgery (i.e., tonsil-lectomy, adenoidectomy, or both), is the potential detrimental effect of obesity on adenotonsillectomy outcomes,24–27 and the higher risk for OSAS recurrence after adenotonsillectomy, particularly when weight gain is excessive during the year following surgery.28
In summary, the current study reports on the uniquely elevated prevalence of OSAS in a nonreferral cohort of obese children being evaluated in a general pediatric clinic during well-child routine visits. Our findings further stress the need for improved screening questionnaires that are specifically tailored for the obese pediatric population, as well as the potentially problematic concordance between subjectively reported and objectively verified snoring. When taking into consideration the increased risk for OSAS-associated morbidities when obesity is concurrently present,29–33 it will be important to proceed with programs that increase the awareness of OSAS in the context of pediatric obesity, and to develop effective screening tools that encompass both clinical and biomarker-based phenotypic risk assessments.2,34,35
DISCLOSURE STATEMENT
This study was funded by the Spanish Respiratory Society (SEPAR) and Mutua Madrileña. Dr. Leila Kheirandish-Gozal and Dr. David Gozal are supported by grant HL-65270 from the National Institutes of Health. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Other Members of the Spanish Sleep Network: Estrella Ordax Carbajo, MD (Hospital Universitario de Burgos); Ana Isabel Navazo-Egüia, MD (Hospital Universitario de Burgos); Marian Martínez Martínez, MD (Hospital Universitario Valdecilla, Santander); Odile Romero Santo-Tomas, MD (Hospital Val D′Hebron); Fernando Masa-Jimenez, MD (Hospital San Pedro de Alcantara, Caceres); Cristina Martinez Null (Hospital Universitario Araba, Vitoria); Antonia Barcelo-Bennassar, PhD (Hospital Son Dureta, Palma de Mallorca)
The authors are grateful to pediatricians Jesús Rodrigo, Elsa Ramila, Isabel Cubillo, Pilar Sanmartin, Carmen Aguado, Ana Gutierrez, Ana Elvira, Estrella Trabada, Carmen Bermejo, Juana Acera, Soledad Estebanez, Ana Camino, María José Martin, Jose Colinas, and Fabiola Lorenzo for facilitating the recruitment process. We also thank all the sleep technologists of the participating sleep units for their efforts and enthusiasm in making this work possible. A special thanks to the children, parents, and caretakers for their willingness to participate in this study.
REFERENCES
- 1.Marcus CL, Brooks LJ, Draper KA, et al. American Academy of Pediatrics. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:e714–55. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- 2.Luz Alonso-Álvarez M, Canet T, Cubell-Alarco M, Estivill E, Fernández-Julián E, Gozal D, et al. Documento de Consenso del Síndrome de Apneas Hipopneas durante el sueño en niños. Arch Bronconeumol. 2011;47:1–18. doi: 10.1016/S0300-2896(11)70026-6. [DOI] [PubMed] [Google Scholar]
- 3.Bonsignore MR, McNicholas WT, Montserrat JM, et al. Adipose tissue in obesity and obstructive sleep apnoea. Eur Respir J. 2012;39:746–67. doi: 10.1183/09031936.00047010. [DOI] [PubMed] [Google Scholar]
- 4.Gozal D, Kheirandish-Gozal L. Childhood obesity and sleep: relatives, partners, or both?--a critical perspective on the evidence. Ann N Y Acad Sci. 2012;1264:135–41. doi: 10.1111/j.1749-6632.2012.06723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serra Majem L, Ribas Barba l, Aranceta B, et al. Obesidad infantil y juvenil en España. Resultados del Estudio enKid (1998-2000) Med Clin Barc. 2003;121:725–32. doi: 10.1016/s0025-7753(03)74077-9. [DOI] [PubMed] [Google Scholar]
- 6.Estudio Aladino. www.naos.aesan.msps.es/naos/investigacion/aladino.
- 7.Sobradillo B, Aguirre A, Aresti U, et al. Madrid: Ergon; 2004. Curvas y tablas de crecimiento (estudio longitudinal y trasversal). En Fundación F. Orbegozo, editor. Patrones de crecimiento y desarrollo en España. Atlas de gráficas y tablas. [Google Scholar]
- 8.Cole T, Bellizzi M, Flegal K, et al. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–3. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomás Vila M, Miralles Torres A, Beseler Soto B. Versión Española del Pediatric Sleep Questionnaire. Un instrumento útil en la investigación de los trastornos del sueño en la infancia. Análisis de su fiabilidad. Ann Pediatr. 2007;66:121–8. doi: 10.1157/13098928. [DOI] [PubMed] [Google Scholar]
- 10.Brodsky L, Moore L, Stanievich J. A comparison of tonsillar size and oropharyngeal, dimensions in children with obstructive adenotonsillar hypertrophy. Int J Pediatr Otorhinolaryngol. 1987;13:149–56. doi: 10.1016/0165-5876(87)90091-7. [DOI] [PubMed] [Google Scholar]
- 11.Iber C, Ancoli-Israel S, Chesson A, Quan SF for the American Academy of Sleep Medicine. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 12.Montgomery-Downs HE, O'Brien LM, Holbrook CR, et al. Snoring and sleep-disordered breathing in young children: subjective and objective correlates. Sleep. 2004;27:87–94. doi: 10.1093/sleep/27.1.87. [DOI] [PubMed] [Google Scholar]
- 13.Spruyt K, Gozal D. Screening of pediatric sleep-disordered breathing: a proposed unbiased discriminative set of questions using clinical severity scales. Chest. 2012;142:1508–15. doi: 10.1378/chest.11-3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spruyt K, Gozal D. Pediatric sleep questionnaires as diagnostic or epidemiological tools: a review of currently available instruments. Sleep Med Rev. 2011;15:19–32. doi: 10.1016/j.smrv.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gozal D, Shata A, Nakayama M, Spruyt K. Seasonal variability of sleep-disordered breathing in children. Pediatr Pulmonol. 2011;46:581–6. doi: 10.1002/ppul.21408. [DOI] [PubMed] [Google Scholar]
- 16.Verhulst SL, Schrauwen N, Haentjens D, et al. Sleep-disordered breathing in overweight and obese children and adolescents: Prevalence, Characteristics and the role of fat distribution. Arch Dis Child. 2007;92:205–8. doi: 10.1136/adc.2006.101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wing YK, Hui SH, Pack WM, et al. A controlled study of sleep related disordered breathing in obese children. Arch Dis Child. 2003;88:1043–7. doi: 10.1136/adc.88.12.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loreto GS, Storfer-Isser A, Rosen CL, et al. Associations of obesity, sleep-disordered breathing, and wheezing in children. Am J Respir Crit Care Med. 2005;171:659–64. doi: 10.1164/rccm.200403-398OC. [DOI] [PubMed] [Google Scholar]
- 19.Bixler EO, Vgontzas AN, Lin HM, et al. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009;32:731–6. doi: 10.1093/sleep/32.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosen CL, Larkin EK, Kirchner HL, et al. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. J Pediatr. 2003;142:383–9. doi: 10.1067/mpd.2003.28. [DOI] [PubMed] [Google Scholar]
- 21.Kheirandish-Gozal L. Fat and lymphadenoid tissues: a mutually obstructive combination. Am J Respir Crit Care Med. 2011;183:694–5. doi: 10.1164/rccm.201010-1681ED. [DOI] [PubMed] [Google Scholar]
- 22.Dayyat E, Kheirandish-Gozal L, Gozal D. Childhood obstructive sleep apnea: one or two distinct disease entities? Sleep Med Clin. 2007;2:433–44. doi: 10.1016/j.jsmc.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhattacharjee R, Kim J, Kheirandish-Gozal L, et al. Obesity and obstructive sleep apnea syndrome in children: a tale of inflammatory cascades. Pediatr Pulmonol. 2011;46:313–23. doi: 10.1002/ppul.21370. [DOI] [PubMed] [Google Scholar]
- 24.Tauman R, Gulliver TE, Krishna J, et al. Persistence of obstructive sleep apnea syndrome in children after adenotonsillectomy. J Pediatr. 2006;149:803–8. doi: 10.1016/j.jpeds.2006.08.067. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell R, Kelly J. Outcome of adenotonsillectomy for obstructive sleep apnea in obese and normal-weight children. Otolaryngol Head Neck Surg. 2007;137:43–8. doi: 10.1016/j.otohns.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 26.Alonso-Alvarez ML, Navazo-Egüia AI, Cordero-Guevara JA, et al. Respiratory polygraphy for follow-up of obstructive sleep apnea in children. Sleep Med. 2012;13:611–5. doi: 10.1016/j.sleep.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med. 2010;182:676–83. doi: 10.1164/rccm.200912-1930OC. [DOI] [PubMed] [Google Scholar]
- 28.Amin R, Anthony L, Somers V, et al. Growth velocity predicts recurrence of sleep-disordered breathing 1 year after adenotonsillectomy. Am J Respir Crit Care Med. 2008;177:654–9. doi: 10.1164/rccm.200710-1610OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gozal D, Khalyfa A, Capdevila OS, et al. Cognitive function in prepubertal children with obstructive sleep apnea: A modifying role for NADPH Oxidase p22 subunit gene polymorphisms? Antioxid Redox Signal. 2012;16:171–7. doi: 10.1089/ars.2011.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spruyt K, Gozal D. A mediation model linking body weight, cognition, and sleep-disordered breathing. Am J Respir Crit Care Med. 2012;185:199–205. doi: 10.1164/rccm.201104-0721OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kheirandish-Gozal L, Bhattacharjee R, Kim J, et al. Endothelial progenitor cells and vascular dysfunction in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2010;182:92–7. doi: 10.1164/rccm.200912-1845OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhattacharjee R, Kim J, Alotaibi WH, et al. Endothelial dysfunction in children without hypertension: potential contributions of obesity and obstructive sleep apnea. Chest. 2012;141:682–91. doi: 10.1378/chest.11-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gozal D, Capdevila OS, Kheirandish-Gozal L. Metabolic alterations and systemic inflammation in obstructive sleep apnea among nonobese and obese prepubertal children. Am J Respir Crit Care Med. 2008;177:1142–9. doi: 10.1164/rccm.200711-1670OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gozal D. Serum, urine, and breath-related biomarkers in the diagnosis of obstructive sleep apnea in children: is it for real? Curr Opin Pulm Med. 2012;18:561–7. doi: 10.1097/MCP.0b013e328358be2d. [DOI] [PubMed] [Google Scholar]
- 35.Kheirandish-Gozal L, Gozal D. Genotype-phenotype interactions in pediatric obstructive sleep apnea. Respir Physiol Neurobiol. 2013;189:338–43. doi: 10.1016/j.resp.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


