Abstract
Study Objectives:
A recent trend in increasing rates of severe maternal morbidity and mortality despite quality improvements has been noted. The goal of this study is to estimate the national prevalence of obstructive sleep apnea (OSA) in pregnant women and examine associations between OSA and pregnancy-related morbidities, including in-hospital maternal mortality.
Design:
A retrospective, cross-sectional analysis.
Setting:
A nationally representative sample of maternal hospital discharges from 1998-2009.
Patients or Participants:
The analytic sample included 55,781,965 pregnancy-related inpatient hospital discharges.
Interventions:
N/A.
Measurements and Results:
The Nationwide Inpatient Sample (NIS) database was used to identify hospital stays for women who were pregnant or gave birth. Among these women, we determined length of hospital stay, in-hospital mortality, and used International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes to identify OSA and other outcome measures. Multivariable logistic regression modeling was used to calculate adjusted odds ratios (OR) and 95% confidence intervals (CI) for the associations between OSA and each outcome. The overall rate of OSA was 3.0 per 10,000; however, the rate climbed substantially from 0.7 in 1998 to 7.3 in 2009, with an average annual increase of 24%. After controlling for obesity and other potential confounders, OSA was associated with increased odds of pregnancy-related morbidities including preeclampsia (OR, 2.5; 95% CI, 2.2–2.9), eclampsia (OR, 5.4; 95% CI, 3.3–8.9), cardiomyopathy (OR, 9.0; 95% CI, 7.5–10.9), and pulmonary embolism (OR, 4.5; 95% CI, 2.3–8.9). Women with OSA experienced a more than fivefold increased odds of in-hospital mortality (95% CI, 2.4–11.5). The adverse effects of OSA on selected outcomes were exacerbated by obesity.
Conclusions:
Obstructive sleep apnea is associated with severe maternal morbidity, cardiovascular morbidity, and in-hospital death. Targeted interventions may improve pregnancy outcomes in this group.
Citation:
Louis JM, Mogos MF, Salemi JL, Redline S, Salihu HM. Obstructive sleep apnea and severe maternal-infant morbidity/mortality in the United States, 1998-2009. SLEEP 2014;37(5):843-849.
Keywords: maternal mortality, obstructive sleep apnea, preeclampsia, pregnancy, pulmonary embolus
INTRODUCTION
In developed countries, recent trends indicate an increase in severe maternal morbidity and, to a lesser extent, maternal mortality.1–4 The obesity epidemic has garnered considerable attention and has been implicated as a potential cause of this increased morbidity.5,6 Obesity is a risk factor for the development or exacerbation of some of the most common causes of maternal mortality in the United States, including hemorrhage, hypertensive disorders of pregnancy, cardiovascular conditions, cardiomyopathy, infection, and thrombotic pulmonary embolism.4,7–9
One of the proposed pathways linking maternal obesity to poor pregnancy outcomes is through obstructive sleep apnea (OSA), a condition characterized by recurrent episodes of partial or complete airway obstruction, nocturnal hypoxemia, and sleep fragmentation.10,11 Cardiovascular morbidity, all-cause mortality, and a diminished quality of life are associated with OSA, which is an increasingly common condition.12–14 The prevalence of OSA among reproductive age women is 0.7–7%, rises to 11–20% among pregnant women, with the highest prevalence observed among obese gravidas.15–19 Despite increasing trends and potential deleterious effects, the effects of OSA on pregnancy are underinvestigated. Smaller cross-sectional and prospective studies have reported associations between OSA and adverse pregnancy outcomes such as preeclampsia, fetal growth restriction, and preterm delivery, which are similar to the initial published case reports.19–21 The largest of the most recent studies, a large population-based study from Taiwan, confirmed the association between OSA and pregnancy complications including gestational diabetes, gestational hypertension, and preeclampsia.21 However, only one study commented on severe maternal morbidity. This study, a prospective study of 175 obese women who underwent portable polysomnography, found a higher-than-expected occurrence of severe morbidity, including maternal death and venous thromboembolism, among the obese women in the cohort.19 However, the small size of the study limited the ability to evaluate the effect of OSA on less common morbidities.
Therefore, the primary aims of this study were to leverage a large, nationally representative database to estimate the prevalence of OSA among pregnant women in the United States over an 11-y period, and to examine associations between OSA and severe clinical and pregnancy-related morbidities, including in-hospital mortality. Considering the common co-occurrence of OSA and obesity, a secondary objective was to assess whether clinically diagnosed maternal obesity modified the effect of OSA on each outcome. We hypothesized that the joint effect of OSA and obesity on pregnancy-related outcomes would be worse than the effect of OSA in the absence of obesity.
METHODS
Study Design and Data Source
We conducted an analysis of pregnancy-related hospital discharges using 1998-2009 annual data from the Nationwide Inpatient Sample (NIS), the largest all-payer, publicly available inpatient database in the United States.22 The NIS is part of a family of administrative databases developed as part of the Healthcare Cost and Utilization Project (HCUP), sponsored by the Agency for Healthcare Research and Quality (AHRQ). Each year the NIS stratifies all non-federal community hospitals from participating states into groups based on five major hospital characteristics: rural/urban location, number of beds, geographic region, teaching status, and ownership. Within each stratum, a 20% sample of hospitals is drawn using a systematic random sampling technique.22 HCUP includes hospital strata and discharge-level sampling weights with the database so that national frequency and prevalence estimates can be generated while accounting for the complex sampling design of the NIS. In 2010, the NIS had data from 1,051 hospitals in 45 states.
Identifying Maternal Cases and Clinical Conditions
In this study, we were interested in NIS records representing hospital stays for women who were pregnant or gave birth. To identify these discharges, we used a variable (NEOMAT) that HCUP includes with the NIS databases. This variable is used by HCUP to identify maternal and/or neonatal records on the basis of specific International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis and procedure codes.23 The NIS databases contain ICD-9-CM codes for a patient's principal diagnosis and up to 14 secondary diagnoses (up to 24 beginning in 2009). Maternal cases were those with one or more of the following diagnosis (630–677, 792.3, 796.5, V22.0–V24.2, V27.0–V28.2, V28.6, V61.6–V61.7, V65.11, V72.42) or procedure (72.0–75.37, 75.4–75.99) codes24 documented during their hospital stay. Among these women, we also used ICD-9-CM codes to identify the primary exposure, OSA, as well as a number of maternal and fetal outcomes including but not limited to gestational diabetes, gestational hypertension, preeclampsia, eclampsia, cardiomyopathy, early-onset delivery, poor fetal growth, and stillbirth. The complete list is presented in Table S1 (supplemental material). We also considered extended length of stay (defined as > 95th percentile, or > 5 days), and in-hospital mortality.
Demographics and Covariates
The NIS also collects information on a limited number of sociodemographic and hospital characteristics. Maternal age in years was classified into five categories: < 20, 20-24, 25-29, 30-34, and ≥ 35 y. Maternal race-ethnicity was first determined by self-reported ethnicity (Hispanic or non-Hispanic [NH]), with the NH group further subdivided by race (white, black, or other). Racial and ethnic disparities in medical comorbidities, severe maternal morbidity, and mortality have been consistently noted.7,25–27 We, therefore, chose to report these data because they represent a significant confounding variable.7 A proxy for socioeconomic status, we considered each woman's household income and her primary insurance type. Relative median household income was estimated using the documented ZIP code of residence, and ranked into quartiles by HCUP. We grouped primary payer for each hospital stay into government (Medicare/Medicaid), private (commercial carriers and private health maintenance organizations (HMOs) and preferred provider organization (PPOs), and other sources (including self-pay and no charge). We also considered several hospital characteristics including teaching status (teaching, in which the ratio of full-time equivalent interns and residents to nonnursing home beds is ≥ 0.25, vs. nonteaching), location (urban vs. rural), and region in the United States (Northeast, Midwest, South, or West). We also identified a number of prepregnancy and pregnancy-associated medical comorbidities that are associated with OSA and known or suspected risk factors for the clinical outcomes under study. These conditions were identified using ICD-9-CM codes documented during each hospital stay and include coronary heart disease, chronic renal disease, anemia, lipid metabolism (e.g., hyperlipidemia), hypothyroidism, and disorders of the adrenal glands (Table S1).
Data Analysis
Descriptive statistics were used to calculate the frequency and prevalence of OSA among pregnancy-related discharges. Because national frequency and rate estimates were desired, the discharges in the analyses were weighted to account for the complex sampling design of the NIS. We also calculated the distribution of sociodemographic, perinatal, behavioral, and hospital characteristics, and the rate of selected maternal-fetal-infant outcomes (per 1,000 pregnancy-related discharges) by OSA status.
Using the SURVEYLOGISTIC procedure in SAS (SAS software, version 9.3 (SAS Institute, Inc., Cary, NC), we constructed logistic regression models to calculate odds ratios (OR) and 95% confidence intervals (CI) for the associations between OSA and each outcome. For each association of interest, we constructed a crude (unadjusted) model and three multivariable (adjusted) models. We selected model covariates following a review of the literature, an assessment of biologic plausibility, and based on bivariate analyses. In the first multivariable model, we selected maternal age and race/ethnicity, household income, multiple gestation, tobacco, alcohol, and drug use, primary payer, and rural/urban status to control for variation in sociodemographic, perinatal, behavioral, and hospital-associated characteristics among the OSA groups. In the second model, we added clinically diagnosed obesity (based on selected ICD-9-CM codes, Table S1), a strong comorbidity, and in the third model we also included a composite variable coding for the aforementioned prepregnancy and pregnancy-associated comorbidities, to isolate the independent effect of OSA. In the final model, we assessed the joint effects of OSA and obesity on each outcome by including an interaction term into the model. Trends in rates of OSA and obesity during the study period were assessed using joinpoint regression. Joinpoint regression begins by modeling annual trend data by fitting a straight line (i.e., zero join-points).28 Using a Monte Carlo permutation test, it then examines whether adding one joinpoint is statistically significant, and if so, incorporates it into the model. This process is repeated until a model of best fit is specified with an optimal number of joinpoints. Each joinpoint in the final model corresponds to a significant increase or decrease in the trend and an annual percent change is calculated to describe how the rate changes within each time interval. The model also estimates the average annual percent change, which describes the trend over the entire study period, even when there are significant changes in the trend over time. Because the NIS sampling design has changed, we used the NIS-Trends files, supplied by HCUP, that consistently define trend weights and data elements over time.29
Statistical analyses were performed with SAS software, version 9.3 and the Joinpoint Regression Program (Join-point Regression Program, Version 4.0.1; Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute).30 This study has been approved by the institutional review board approval at the University of South Florida.
RESULTS
Among an estimated 55 million pregnancy-related discharges in the United States from 1998-2009, OSA was diagnosed at a rate of 3.0 (95% CI, 2.8–3.2) per 10,000 population. Over this time, the prevalence of OSA in the study population rose dramatically. In 1998, the rate was 0.7 per 10,000 population, and by 2009, it had increased tenfold to 7.3 per 10,000 population, with an average annual increase of 24.4% (95% CI, 22.1–26.8%).
Table 1 presents the distribution of maternal sociodemographic, behavioral, and perinatal characteristics by OSA status. Women in whom OSA had been diagnosed were more likely than other women to be older, in the lowest quartile of household income, have government-funded insurance (Medicare/Medicaid), and use tobacco, alcohol, and illicit drugs during pregnancy. Women with OSA also were more likely to be NH-black (versus NH-white) and have had a previous cesarean section. As hypothesized, women with OSA during pregnancy were more likely to experience adverse clinical conditions and pregnancy-related complications than women with no OSA diagnosis. After adjusting for known/suspected sociodemographic and clinical confounders available in the NIS database, OSA-related discharges had up to a ninefold increased odds of the outcomes under study (Table 2). The strongest associations with clinical conditions were observed for cardiomyopathy (adjusted OR, 9.0; 95% CI, 7.5–10.9), congestive heart failure (adjusted OR, 8.9; 95% CI, 7.5–10.7), and pulmonary edema (adjusted OR, 7.5; 95% CI, 4.6–12.2). Among pregnancy-related factors, OSA was associated with an adjusted OR of 5.4 (95% CI, 3.3-8.9) for eclampsia and 2.5 (95% CI, 2.2-2.9) for pre-eclampsia. OSA was also associated with an increased likelihood of gestational diabetes (adjusted OR, 1.9; 95% CI, 1.7–2.1) and gestational hypertension (adjusted OR, 1.3; 95% CI, 1.1–1.5), even after adjusting for clinically diagnosed obesity and common comorbidities.
Table 1.
Distribution of maternal sociodemographic, perinatal, behavioral, and hospital characteristics among pregnancy-related discharges, by obstructive sleep apnea status, Healthcare Cost and Utilization Project - Nationwide Inpatient Sample, 1998-2009
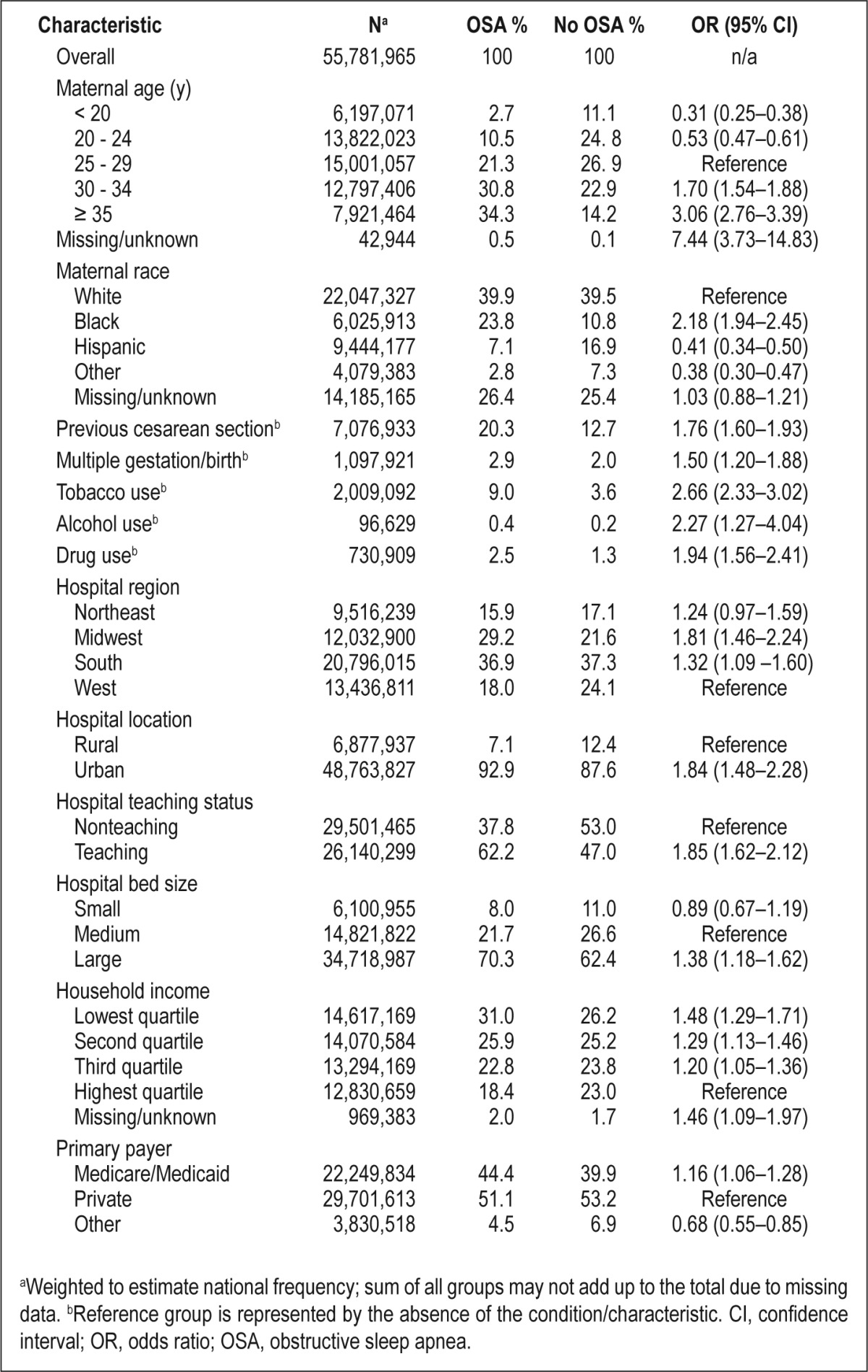
Table 2.
Outcome rates, adjusted odds ratios, and 95% confidence intervals for the association between obstructive sleep apnea and maternal-infant morbidity and mortality, Healthcare Cost and Utilization Project - Nationwide Inpatient Sample, 1998-2009
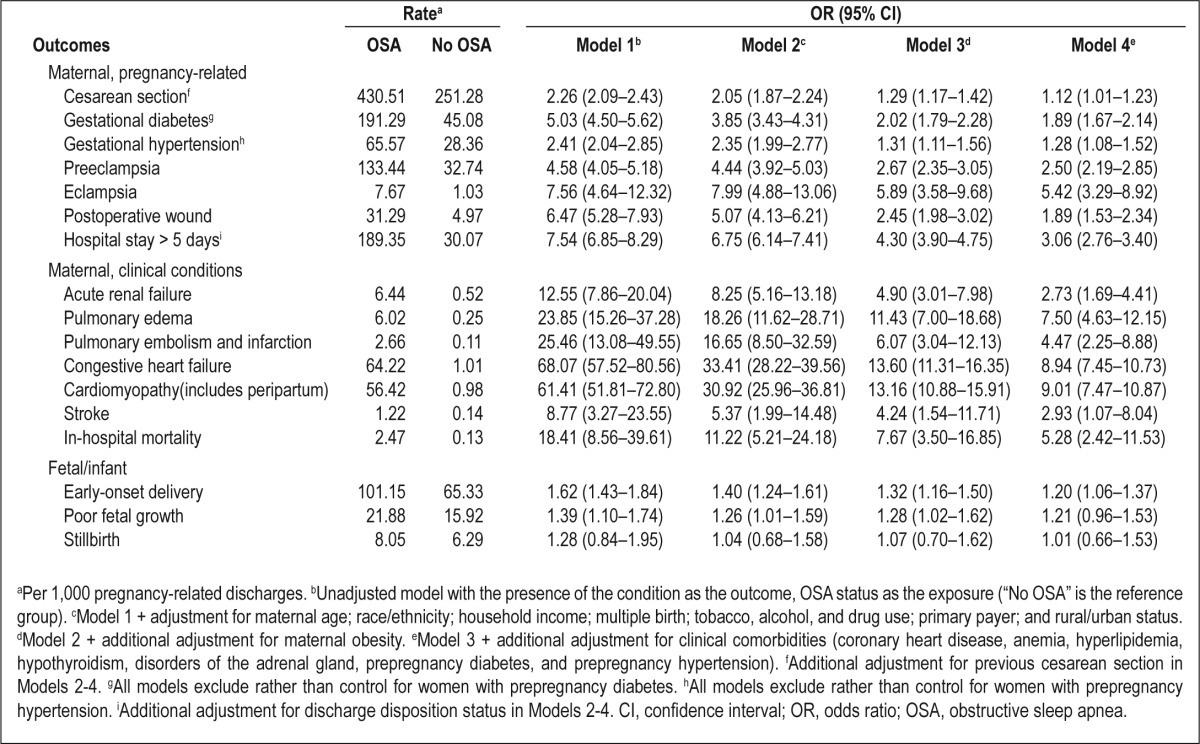
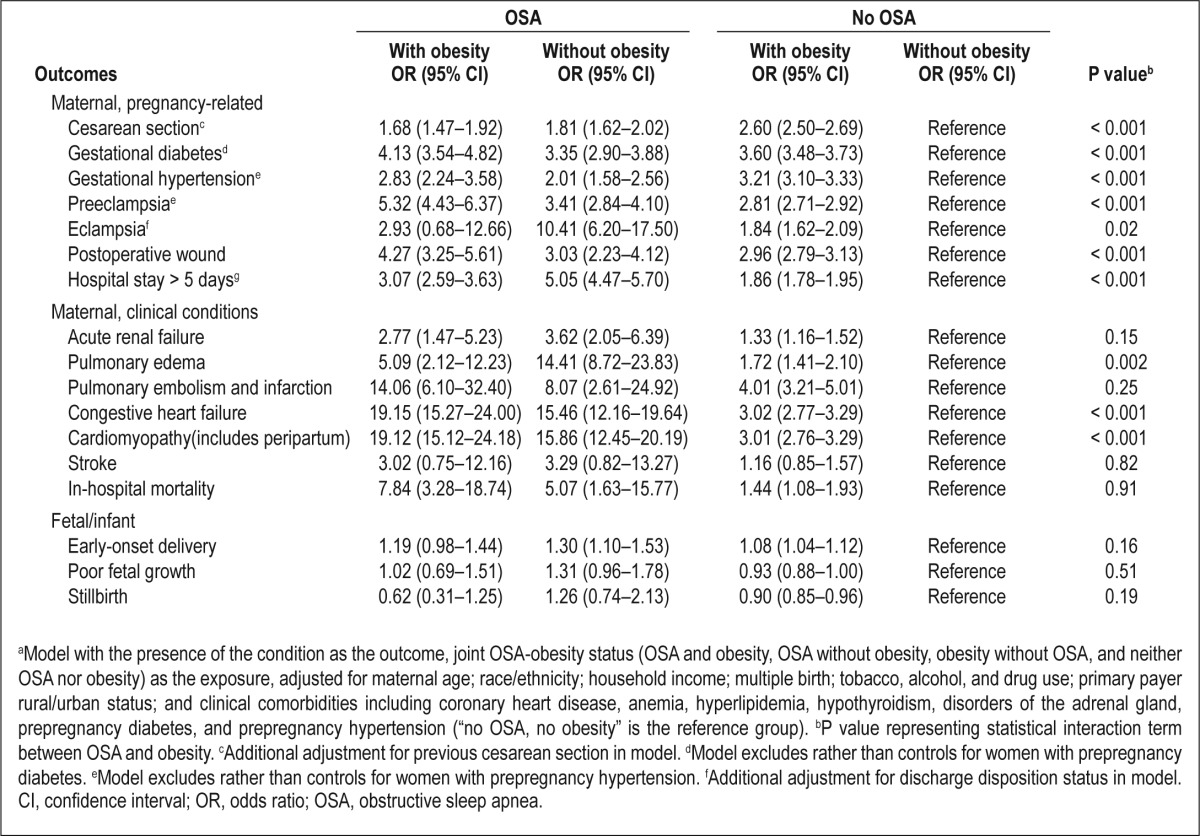
Pregnant women with an OSA diagnosis were three times as likely as women without an OSA diagnosis to have an extended length of stay (> 5 days) in the hospital. During this time, women with OSA had moderately increased odds of early-onset delivery (adjusted odds ratio, 1.2; 95% CI, 1.1–1.4); however, we did not find any statistically significant increase in the likelihood of poor fetal growth or stillbirth. The most remarkable finding was the association between OSA and maternal mortality. Compared with women without OSA, women with OSA had approximately fivefold higher odds of dying prior to discharge from the hospital, even after adjusting for serious cardiovascular, renal, and metabolic conditions that are known to affect mortality. The estimated 24% annual increase in OSA rates during the study period was mirrored by an average 20% annual increase in clinically diagnosed obesity rates (data not shown). Table 3 presents the results of the analysis for the joint effect of obesity and OSA on severe maternal-infant morbidity/mortality. With the exceptions of cesarean delivery, gestational hypertension, and stillbirth, the adjusted OR of any severe maternal morbidity and mortality were higher among women with OSA than without OSA, regardless of obesity status. Furthermore, the associations between OSA and outcomes, particularly preeclampsia and severe cardiovascular morbidities (congestive heart failure and cardiomyopathy), were stronger in women with comorbid obesity than in women without comorbid obesity. However, the associations between OSA and stroke, pulmonary embolism, acute renal failure, and in-hospital mortality were not significantly increased by a joint diagnosis of obesity.
Table 3.
Adjusted odds ratiosa and 95% confidence intervals for the joint effect of obstructive sleep apnea and obesity on selected clinical outcomes, Healthcare Cost and Utilization Project - Nationwide Inpatient Sample, 1998-2009
DISCUSSION
In this study, we found that, in the United States from 1998-2009, the rate of OSA among pregnancy-related discharges increased significantly, and coincided with the rise in obesity rates. We observed strong associations between OSA and increased likelihood not only of morbidities that have an adverse effect on pregnancy outcomes, including preeclampsia, eclampsia, and early-onset delivery, but also of maternal mortality. These associations persisted even after statistical adjustment for potential confounders including clinically diagnosed obesity.
The findings of this investigation are consistent with other studies reporting a higher prevalence of chronic cardiovascular and metabolic medical conditions in subjects with OSA.19,31 These women are at higher risk of gestational diabetes and hypertensive disorders complicating pregnancy, and are more likely to undergo cesarean delivery.31 These events/conditions provide a basis for increased severe maternal and infant morbidity and an increased likelihood of maternal death.4,32,33 There are plausible pathophysiological mechanisms by which OSA can lead to maternal morbidity. OSA is associated with increased inflammation, sympathetic traffic, insulin resistance, and oxidative stress.34,35 Repetitive forced inspiration against a closed upper airway generates a negative intrathoracic pressure gradient which, when transmitted to the heart, can result in significant cardiac dysfunction.13,14,18,36 Over time, the recurrent hemodynamic stress, hypoxemia, and adrenergic activation lead to chronic systemic inflammation and oxidative vascular injury. These events may have a direct effect on maternal vasculature, resulting in increased endothelial injury and cardiovascular disease. These changes, paired with the physiological demands of pregnancy, increase the risk of morbidity for the pregnant patient. Adverse fetal outcomes may be secondary to worsening maternal health or may be a direct consequence of OSA's effect on placental function via similar pathophysiological mechanisms.
To our knowledge, this is the largest study in the United States to assess the temporal trends in OSA diagnoses during pregnancy, and the only one to analyze the associated perinatal morbidity using nationally representative data. There has been increasing attention paid to the putative adverse effects of sleep disorders on pregnancy. Early literature on the effects of OSA on pregnancy outcomes were limited to case reports and small cohorts.31 Larger studies have analyzed associations using symptoms of OSA such as habitual snoring or excessive daytime sleepiness instead of a clinical diagnosis of OSA.36,37 The largest study in the literature used a population-based cohort of 759 Chinese women with confirmed OSA and a comparative group of women without the diagnosis, and the authors reported an increased risk of gestational hypertension (OR, 3.2; 95% CI, 2.1–4.7) and preeclampsia (OR, 1.6; 95% CI, 2.2–11.3) among women with OSA. In that study, OSA also was associated with an increased risk of small-for-gestational age infants.21 Several small, prospective, observational cohort studies used unattended sleep recordings to identify OSA. Those studies reported significant associations between OSA and preeclampsia, but they were statistically underpowered to evaluate certain severe maternal or fetal outcomes.19,38,39 The findings of this study support some of that earlier work, but significantly extends the literature by analyzing less common, but clinically relevant (and potentially devastating) outcomes such as eclampsia, stillbirth, and most importantly, maternal mortality.
The magnitude of the observed associations between OSA and grave cardiovascular disease outcomes is significant when examining trends in maternal mortality. Although the causes of maternal mortality have remained the same, there has been a noted increase in cardiovascular disease as a underlying cause of maternal deaths.4 In our study, the association of cardiovascular disease with OSA was magnified by the presence of obesity. Therefore, in addition to OSA being associated with maternal mortality, it was also associated with cardiovascular disease, a significant contributor to severe maternal morbidity and mortality. If confirmed, these findings may have significant implications for the prenatal and intrapartum care of those affected with OSA as we continue to strive for safer deliveries, and ultimately, healthy mothers and babies.
Among fetal outcomes, OSA was associated with early-onset delivery, but not fetal growth restriction or stillbirth. The NIS database does not have a link between maternal and infant birth/delivery hospitalizations, which precludes further examination of the etiology for preterm birth and other infant outcomes. However, this mirrors findings previously observed and may be related to medically indicated preterm birth related to comorbid conditions or pregnancy complications.31 The presence of these conditions would predispose them to medically indicated preterm birth from preeclampsia or suspected fetal compromise.40
Strengths and Limitations
The results of this study should be considered in light of several noteworthy limitations. Despite the extensive use of the NIS and other HCUP databases to estimate national prevalence rates of conditions and to investigate potential exposure-outcome associations, the identification of most conditions still relied exclusively on ICD-9-CM codes. As such, these administrative data are subject to errors in coding, which increase false-positive and false-negative diagnoses. For example, the prevalence of obesity in this study was extremely low (1.5%) in comparison with published literature that defines obesity using prepregnancy body mass index. Although cases of mild to moderate obesity were likely misclassified because of this finding, the use of the ICD-9-CM code allowed us to control for and investigate a more severe form of clinically diagnosed obesity that has a significant potential to be associated with OSA and to affect pregnancy outcomes.41 Also, we expect measurement error associated with covariates included in our models because we used ICD-9-CM codes in hospital records to identify clinical comorbidities (e.g., coronary heart disease, anemia, chronic renal disease) as well as risky behaviors during pregnancy (use of tobacco, alcohol, and drugs). Differential misclassification may exist if knowledge of a woman's OSA status results in a more thorough clinical assessment and documentation of conditions and behaviors in the medical record, relative to women without OSA. However, because these factors are most often positively associated with both OSA and the outcomes investigated in this study, overdiagnosis of behaviors and comorbidities among women with OSA would serve to bias estimates conservatively toward the null in multivariable analyses.
A related concern when relying exclusively on ICD-9-CM codes is that they are updated annually and, thus, change over time. For example, some codes included in the diagnostic definitions for OSA (e.g., 327.23) and obesity (e.g., V85) were introduced in the middle of the study period (October 2005). To assess any effect of differential diagnoses over time on the observed associations, we performed a sensitivity analysis that was restricted to 2006-2009 data during which coding for important study variables was more consistent. Although some measures of effect were reduced slightly, there were no appreciable differences from the analysis on the complete 1998-2009 dataset.
Another limitation relates to the collection of race-ethnicity data in the NIS. Not all states report race-ethnicity data, and there is wide variation in reporting for those that do. Thus, although HCUP attempts to standardize state-specific reports into a single race-ethnicity variable, we were still missing race-ethnicity for one fourth of the study population. Although racial-ethnic disparities were not considered in this study, one's ability to control for the potential confounding effects of maternal race-ethnicity on the OSA-outcome associations was limited. Finally, it is possible that some of the increased prevalence of OSA among pregnancy-related hospitalizations is attributable to heightened clinician awareness of the OSA as opposed to a genuine increasing trend of the magnitude we observed. Despite these limitations, common to nearly every study using HCUP data, the large size and representativeness of the NIS database provided us with the ability to generate national estimates for the frequency and prevalence of OSA among pregnancy-related discharges. We also had the statistical power to evaluate uncommon pregnancy outcomes as well as assess effect modification between OSA and obesity.
CONCLUSIONS
The findings of this study reveal a dramatic increase in a condition that is tied to significant maternal morbidity and mortality, and that also may be detrimental to the developing fetus. The morbidity associated with OSA among its delivery-related hospital discharges implicates OSA as a potential factor in maternal and neonatal health, independently of morbid obesity. Treatment of obesity may potentially decrease this morbidity while also contributing to improvement in OSA-related morbidity. As the pregnant population becomes older and more obese, the rates are likely to rise further. Therefore, future research of perinatal OSA should focus on interventions that improve both OSA and obesity.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Louis has received compensation from Hutton & Hutton, LLC for expert testimony. Dr. Redline's institution received a grant from ResMed Inc., and ResMed Foundation and equipment from both ResMed and Philips Respironics for use in clinical trials. The other authors have indicated no financial conflicts of interest. All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. This work includes no discussion of off-label or investigational use of any products. This work was performed at the University of South Florida, Tampa, FL.
SUPPLEMENTAL MATERIAL
List of International Classification of Diseases, Ninth Edition, Clinical Modification codes used to identify selected clinical and behavioral conditions
REFERENCES
- 1.Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012;120:1029–36. doi: 10.1097/aog.0b013e31826d60c5. [DOI] [PubMed] [Google Scholar]
- 2.Roberts C, Ford J, Algert C, Bell J, Simpson J, Morris J. Trends in adverse maternal outcomes during childbirth: a population-based study of severe maternal morbidity. BMC Pregnancy Childbirth. 2009;9:7. doi: 10.1186/1471-2393-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuklina EV, Meikle SF, Jamieson DJ, et al. Severe obstetric morbidity in the United States: 1998–2005. Obstet Gynecol. 2009;113:293–9. doi: 10.1097/AOG.0b013e3181954e5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998 to 2005. Obstet Gynecol. 2010;116:1302–9. doi: 10.1097/AOG.0b013e3181fdfb11. [DOI] [PubMed] [Google Scholar]
- 5.Schutte JM, Steegers EAP, Schuitemaker NWE, et al. Rise in maternal mortality in the Netherlands. BJOG. 2010;117:399–406. doi: 10.1111/j.1471-0528.2009.02382.x. [DOI] [PubMed] [Google Scholar]
- 6.Zwart JJ, Richters JM, Öry F, De Vries JIP, Bloemenkamp KWM, Van Roosmalen J. Severe maternal morbidity during pregnancy, delivery and puerperium in the Netherlands: a nationwide population-based study of 371 000 pregnancies. BJOG. 2008;115:842–50. doi: 10.1111/j.1471-0528.2008.01713.x. [DOI] [PubMed] [Google Scholar]
- 7.Goffman D, Madden RC, Harrison EA, Merkatz IR, Chazotte C. Predictors of maternal mortality and near-miss maternal morbidity. J Perinatol. 2007;27:597–601. doi: 10.1038/sj.jp.7211810. [DOI] [PubMed] [Google Scholar]
- 8.Marshall NE, Guild C, Cheng YW, Caughey AB, Halloran DR. Maternal superobesity and perinatal outcomes. Am J Obstet Gynecol. 2012;206:417, e411–417.e416. doi: 10.1016/j.ajog.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catalano PM, Ehrenberg HM. Review article: The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG. 2006;113:1126–33. doi: 10.1111/j.1471-0528.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 10.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 11.Quan S, Gillin JC, Littner M, Shepard J. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. editorials. Sleep. 1999;22:662–89. [PubMed] [Google Scholar]
- 12.Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA. 2012;307:2169–76. doi: 10.1001/jama.2012.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campos-Rodriguez F, Martinez-Garcia MA, de la Cruz-Moron I, Almeida-Gonzalez C, Catalan-Serra P, Montserrat JM. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: a cohort study. Ann Intern Med. 2012;156:115. doi: 10.7326/0003-4819-156-2-201201170-00006. [DOI] [PubMed] [Google Scholar]
- 14.Baldwin CM, Ervin A-M, Mays MZ, et al. Sleep disturbances, quality of life, and ethnicity: the Sleep Heart Health Study. J Clin Sleep Med. 2010;6:176. [PMC free article] [PubMed] [Google Scholar]
- 15.Bixler EO, Vgontzas AN, Lin H-M, et al. Prevalence of Sleep-disordered Breathing in Women Effects of Gender. Am J Respir Crit Care Med. 2001;163:608–13. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 16.Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2003;167:1181–5. doi: 10.1164/rccm.200209-1055OC. [DOI] [PubMed] [Google Scholar]
- 17.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 18.Olivarez SA, Maheshwari B, McCarthy M, et al. Prospective trial on obstructive sleep apnea in pregnancy and fetal heart rate monitoring. Am J Obstet Gynecol. 2010;202:552.e1–7. doi: 10.1016/j.ajog.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Louis J, Auckley D, Miladinovic B, et al. Perinatal outcomes associated with obstructive sleep apnea in obese pregnant women. Obstet Gynecol. 2012;120:1085–92. doi: 10.1097/AOG.0b013e31826eb9d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Facco FL, Liu CS, Cabello AA, Kick A, Grobman WA, Zee PC. Sleep-Disordered Breathing: A Risk Factor for Adverse Pregnancy Outcomes? Amer J Perinatol. 2012;29:277–82. doi: 10.1055/s-0031-1295658. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y-H, Kang J-H, Lin C-C, Wang IT, Keller JJ, Lin H-C. Obstructive sleep apnea and the risk of adverse pregnancy outcomes. Am J Obstet Gynecol. 2012;206:136.e1–5. doi: 10.1016/j.ajog.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Health Care Cost and Utilization Project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality; 2011. Introduction to the NIS:2009, HCUP NIS related reports. [Google Scholar]
- 23.Merrill C, Owens PL. Statistical Brief #33: Reasons for being admitted to the hospital through the emergency department for children and adolescents, 2004. 2007. Jun, http://www.hcup-us.ahrq.gov/reports/statbriefs/sb33.pdf. [PubMed]
- 24.HCUP. HCUP quality control procedures. 2008. http://www.hcup-us.ahrq.gov/db/quality.pdf.
- 25.Kuklina EV, Meikle SF, Jamieson DJ, et al. Severe obstetric morbidity in the United States: 1998–2005. Obstet Gynecol. 2009;113:293. doi: 10.1097/AOG.0b013e3181954e5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown HL, Small M, Taylor YJ, Chireau M, Howard DL. Near miss maternal mortality in a multiethnic population. Ann Epidemiol. 2011;21:73–7. doi: 10.1016/j.annepidem.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Lyndon A, Lee HC, Gilbert WM, Gould JB, Lee KA. Maternal morbidity during childbirth hospitalization in California. J Matern Fetal Neonatal Med. 2012;25:2529–35. doi: 10.3109/14767058.2012.710280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–51. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 29.Houchens RL, Elixhauser A. Using the HCUP nationwide inpatient sample to estimate trends (updated for 1988-2004). HCUP methods series Report #2006-05 online. http://www.hcup-us.ahrq.gov/reports/methods/2006_05_NISTrendsReport_1988-2004.pdf.
- 30. Joinpoint Regression Program, Version 4.0.1 - January 2013; Statistical Methodology and Applications Branch and Data Modeling Branch, Surveillance Research Program National Cancer Institute [computer program]2013.
- 31.Louis JM, Auckley D, Sokol RJ, Mercer BM. Maternal and neonatal morbidities associated with obstructive sleep apnea complicating pregnancy. Am J Obstet Gynecol. 2010;202:261.e261–5. doi: 10.1016/j.ajog.2009.10.867. [DOI] [PubMed] [Google Scholar]
- 32.Silver RM, Landon MB, Rouse DJ, et al. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet Gynecol. 2006;107:1226–32. doi: 10.1097/01.AOG.0000219750.79480.84. [DOI] [PubMed] [Google Scholar]
- 33.Betran A, Wojdyla D, Posner S, Gulmezoglu A. National estimates for maternal mortality: an analysis based on the WHO systematic review of maternal mortality and morbidity. BMC Public Health. 2005;5:131. doi: 10.1186/1471-2458-5-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arnardottir ES, Mackiewicz M, Gislason T, Teff KL, Pack AI. Molecular signatures of obstructive sleep apnea in adults: a review and perspective. Sleep. 2009;32:447. doi: 10.1093/sleep/32.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jelic S, Lederer DJ, Adams T, et al. Vascular inflammation in obesity and sleep apnea. Circulation. 2010;121:1014–21. doi: 10.1161/CIRCULATIONAHA.109.900357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Brien LM, Bullough AS, Owusu JT, et al. Pregnancy-onset habitual snoring, gestational hypertension, and preeclampsia: prospective cohort study. Am J Obstet Gynecol. 2012;207:487.e481–9. doi: 10.1016/j.ajog.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bourjeily G, El Sabbagh R, Sawan P, et al. Epworth sleepiness scale scores and adverse pregnancy outcomes. Sleep Breath. 2013:1–8. doi: 10.1007/s11325-013-0820-9. [DOI] [PubMed] [Google Scholar]
- 38.Sahin FK, Koken G, Cosar E, et al. Obstructive sleep apnea in pregnancy and fetal outcome. Int J Gynecol Obstet. 2008;100:141–6. doi: 10.1016/j.ijgo.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 39.Facco FL, Ouyang DW, Zee PC, Grobman WA. Development of a pregnancy-specific screening tool for sleep apnea. J Clin Sleep Med. 2012;8:389. doi: 10.5664/jcsm.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong AE, Grobman WA. Medically indicated—iatrogenic prematurity. Clin Perinatol. 2011;38:423–39. doi: 10.1016/j.clp.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Elixhauser A, Steiner C. Statistical Brief #20: Obese patients in U.S. hospitals, 2004. 2006. Dec, http://www.hcup-us.ahrq.gov/reports/statbriefs/sb20.pdf. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of International Classification of Diseases, Ninth Edition, Clinical Modification codes used to identify selected clinical and behavioral conditions


