Abstract
Study Objectives:
To evaluate the relationship between sleep disordered breathing (SDB) in early infancy and outcomes at 3 years of age in children with cleft lip and/or palate (CL/P).
Design:
Observational follow-up study.
Setting:
Multidisciplinary CL/P clinic, tertiary centre.
Participants:
Children with CL/P who participated in a study of sleep and breathing in infancy.
Measurements and Results:
The families of 52 children were approached for this follow-up study. The children underwent neurocognitive (Bayley Scales of Infant and Toddler Development, Third Edition; BSID-III), quality of life (Infant/Toddler Quality of Life Questionnaire; ITQOL), and growth assessments at 3 years. The families of 33 children (66%) completed follow-up at 36.7 ± 1.4 months. The apnea-hypopnea index (AHI) in infancy was 23.9 ± 18.0 events/h. Mean group BSID-III scores fell within the standardized normal range (10 ± 3) for all domains; however, language scores were lower than control children. Quality of life scores and growth parameter z-scores were similar to published control data. PSG variables in infancy showed significant relationships with outcomes at 3 years of age; lower percentage of AS/REM sleep was associated with lower cognition score; more obstructive events were associated with lower global behavior ITQOL score; and higher number of respiratory events in infancy was associated with lower weight z-score.
Conclusion:
Neurocognition, quality of life, and growth measures from children with CL/P fall within a normal range; however, scores in the language domain are lower than controls. Sleep and respiratory elements of SDB in infancy appear to modify these outcomes at 3 years of age.
Citation:
Smith CB, Walker K, Badawi N, WAters KA, MacLean JE. Impact of sleep and breathing in infancy on outcomes at three years of age for children with cleft lip and/or palate. SLEEP 2014;37(5):919-925.
Keywords: cleft lip and/or palate, longitudinal, Bayley Scales of Infant and Toddler Development, Infant/Toddler Quality of Life Questionnaire
INTRODUCTION
Structural and functional changes present in the upper airway of infants with cleft lip and/or palate (CL/P) confer an increased risk of sleep disordered breathing (SDB).1 The changes in facial structures include decreased length and height of the maxilla, basal maxillary retrognathia, decreased length of the mandible, and mandibular retrognathia, which all result in a reduced size of the upper airway.2 Disturbance of the structure of the palate and altered insertions of the palatal muscles leads to dis-coordination of palatal movement with laryngeal muscles and also predisposes to airway obstruction.3,4 In both animal models and studies of newborn infants, these structural and functional changes are associated with a spectrum of airway compromise from life threatening airway obstruction to the intermittent airway obstruction during sleep of obstructive sleep apnea (OSA).5–8 In a study of 52 infants with CL/P who underwent PSG at a mean age of 2.7 ± 2.3 months, we demonstrated that 40% of infants had more than 10 obstructive respiratory events/h of sleep, while 14% had more than 20 central events/h of sleep with a mean apnea-hypopnea index (AHI) of 22.8 ± 18.4 events/h.9
SDB in childhood is associated with a range of health consequences including poor growth, neurocognitive compromise, and lower quality of life. There is, however, less information on the effects of OSA in early infancy. Animal models of OSA in early infancy, using exposure to intermittent hypoxia, have demonstrated increased baseline ventilation and decreased response to acute hypoxia—effects which persist after return to normoxia and into adulthood—as well as cognitive impairment.10,11 These effects are seen even with relatively brief exposure to intermittent hypoxia.12 Studies in infants with SDB show lower amounts of REM sleep, poor growth, delayed development, and a higher risk of sudden infant death syndrome (SIDS).13–15 While studies of SDB in childhood show reversal of some of the effects of SDB with treatment,16,17 there is limited information available on the long-term outcomes of SDB in infancy. Treatment studies of infants with CL/P demonstrate that a range of modalities can relieve airway obstruction,18–24 improve feeding skills,24 and improve growth velocity,18,21 but few studies examine treatment outcomes, and none have included long-term follow-up. One study of children age 4-11 years demonstrated significant differences in cognitive and psychosocial development between healthy controls and children with a history of Pierre Robin Sequence, a select group of infants with cleft palate at high risk of OSA.25
SDB is recognized as an important comorbidity in infants with CL/P; however, the long-term sequelae of SDB in this group are currently unknown. The aim of this study was to investigate the relationship between SDB during early infancy and neurocognitive, quality of life, and growth outcomes at 3 years of age in a group of infants with CL/P. The results from this study will provide clinicians and families with information about the consequences of SDB in infants with CL/P.
METHODS
All families of newborn infants referred to the cleft team were approached for participation in a longitudinal prospective study of sleep and breathing in infants with CL/P. Following their first contact with the cleft team, families were consented for participation and the infant was scheduled for initial polysomnography (PSG). Infants were excluded if a cleft was not confirmed, if they were medically unstable, or if they required medical interventions that precluded the performance of a diagnostic PSG prior to palate surgery. The study protocol was approved by the institution's research ethics committee.
All treatment decisions were the responsibility of the multidisciplinary cleft team. Standard protocols for cleft care included repair of cleft lips at approximately 3 months and primary palate closure between 9 and 12 months. Surgical procedures for primary palate closure included Langenbeck's bipedical flaps, Veau-Wardill-Kilner V-Y pushback, Furlow double-reversing Z-palatoplasty, and intervelar veloplasty. No children underwent procedures intended to leave a residual cleft. Decisions with regard to treatment for sleep disordered breathing (SDB) were the responsibility of the Sleep Medicine physicians.
The study consisted of data collection in infancy and follow-up at 3 years of age. A total of 52 infants were enrolled in infancy with the protocol and results of infant PSG previously reported.6 All infants underwent a diagnostic PSG as soon as possible after identification of their CL/P. At the time of the PSG, demographic information was collected from the medical chart, a parent or guardian completed a sleep and breathing research questionnaire and facial and growth measurements were collected. Families were then invited for follow-up by the research team just before their child's third birthday.
The main outcome measure collected at 3 years of age was a neurocognitive assessment using the Bayley Scales of Infant and Toddler Development, Third Edition (BSID-III). Testing was completed predominantly by 2 nurses, with occupational therapists completing testing when needed. All assessors had completed the Bayley course and were experienced in administering the assessment. The BSID-III consists of 5 subscales including cognitive, receptive language, expressive language, fine motor, and gross motor. Scores which fall outside ± 1 standard deviation (SD) are considered abnormal. BSID-III scores were compared to locally collected data from term born healthy control children participating in a follow-up study of infants who underwent general surgery or cardiac surgery in infancy.26 Additional outcome measures included the Infant/ Toddler Quality of Life Questionnaire (ITQOL; 97 items) and growth parameters. The ITQOL measures quality of life across 9 multi-item scales for infants and toddlers 2 months to 5 years of age (healthactchq.com). ITQOL scores were compared to previously published data collected from control samples,27–29 where possible scores range from 0 to 100 and higher scores indicate better quality of life. Z-scores for growth parameters were calculated using the CDC 2000 Growth Reference for children 2-19 years old (www.cdc.gov/growthcharts).
PSGs in infancy and at 3 years of age were completed using standard clinical laboratory procedures. This included determination of sleep state using an electroencephalography, electrooculogram, and submental electromyogram. Channels to evaluate respiratory status included pulse oximetry, nasal/oral air flow by thermistor, nasal pressure, chest and abdominal wall movement using respiratory inductance plethysmography, and diaphragm and abdominal muscle activity by trans-diaphragmatic electromyogram. Carbon dioxide (CO2) was monitored using transcutaneous CO2 (TcCO2). Cardiac monitoring included the pulse signal from the oximeter and electrocardiogram.
Analysis of PSG data was completed by a single experienced scorer using the criteria of the American Academy of Sleep Medicine (AASM).30 Sleep staging for infants < 6 months of age was completed using the criteria outline by Anders,31 with AASM criteria applied for those ≥ 6 months. Obstructive apnea was defined as the cessation of airflow (< 10% of baseline level) for a minimum duration of 2 missed breaths with evidence of ongoing respiratory efforts. Central apnea was defined as the cessation of airflow (< 10% of baseline level) for a minimum of 2 missed breaths if followed by an arousal, awakening, or ≥ 3% oxygen desaturation, or for ≥ 20 sec in the absence of any associated events. Mixed apneas included central and obstructive components in the same event. Hypopneas were defined based on a decrease in airflow of 10% to 50% of baseline associated with an arousal, awakening, or ≥ 3% oxygen desaturation. Apnea-hypopnoea index (AHI) was calculated based on the number of apneas and hypopneas during sleep divided by the total sleep time. The obstructive-mixed AHI (OMAHI) excluded central respiratory events. Oxygen desaturation index (ODI) was calculated based on the number of oxygen desaturation events ≥ 3% during sleep divided by the total sleep time. Oxygen saturation nadir represents the lowest oxygen saturation recorded during sleep. There is no accepted AHI cut-point to define an abnormal or pathological AHI in infancy; therefore, the median AHI of 15 events/h from the original study group (i.e., data from 50 infants previously reported)6 was used to define low (AHI < 15) and high (AHI ≥ 15) within this group.
Data were entered in a database (Microsoft Office Access 2003, Microsoft Corporation). Statistical analysis was performed using IBM SPSS 21.0.0.0 (IBM Corp, 1989, 2012). A p-value ≤ 0.05 was considered to indicate statistical significance. Descriptive analyses were used to examine demographic information, PSG parameters, and neurocognitive results. Logarithmic transformation to normalize distributions was applied to PSG variables including AHI, OMAHI, and ODI. Comparisons for continuous variables between groups were made with Student t-test, paired t-test, and ANOVA. A modified Bonferroni correction was made for multiple comparisons.
RESULTS
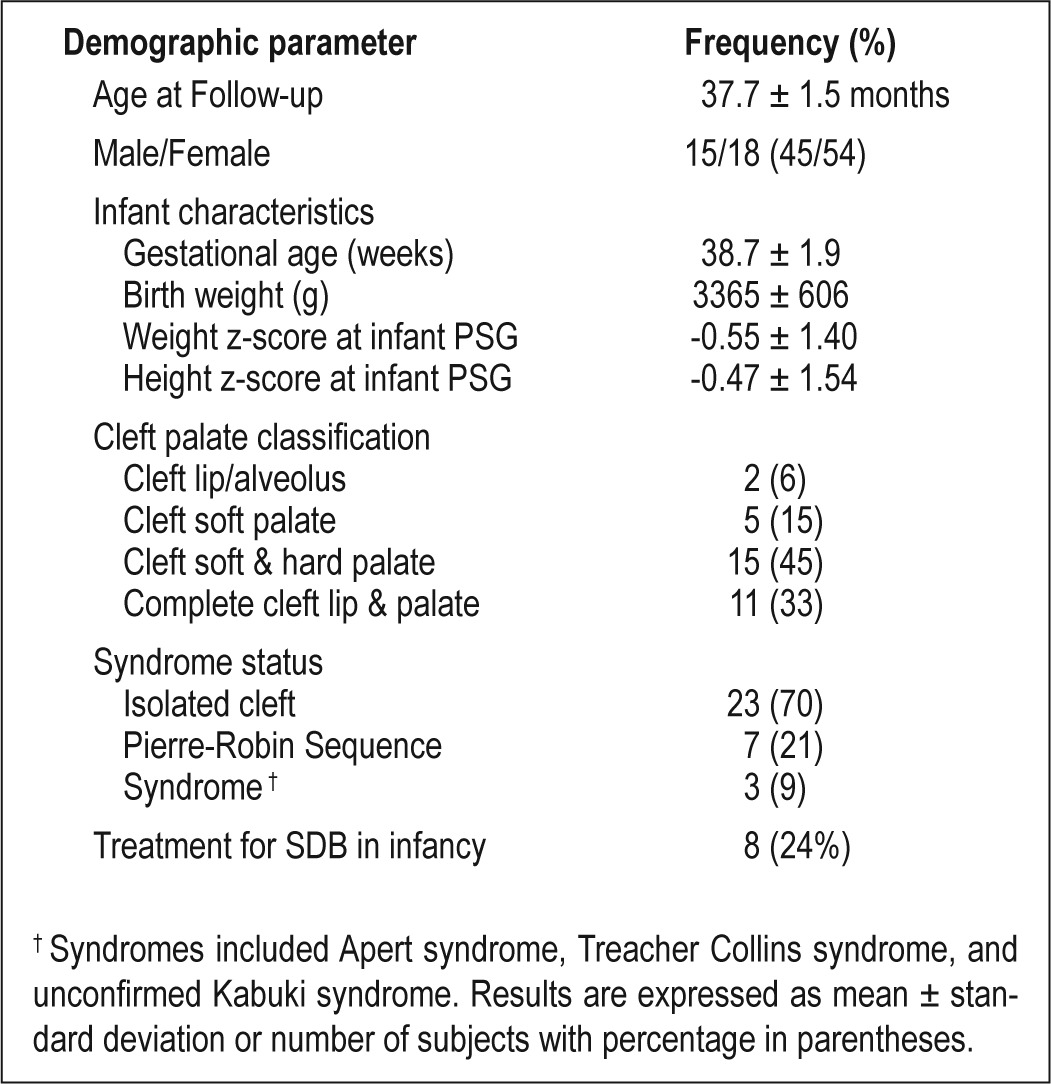
A full description of the infant characteristics of the original cohort is available elsewhere.6 Thirty-four children returned for follow-up at 3 years of age (68% response rate). Neurocognitive testing was unsuccessful for one child who was not willing to complete any of the tasks for the assessors. Therefore, results for 33 children are available for analysis across the spectrum of CL/P (Table 1). Compared to the original cohort, males were overrepresented in the follow-up cohort (45% vs 57%, P < 0.05), as were infants who were referred for clinical sleep medicine consultation prior to PSG (41% vs 51%, P < 0.05). There were no differences between the original and follow-up cohorts with respect to cleft classification, syndrome status, or infant PSG variables (data not shown).
Table 1.
Demographic characteristics of the 33 children with a history of CL/P included in the analysis.
PSG in infancy was completed at 2.7 ± 2.1 months with an AHI of 24.3 ± 18.0 events/h (Table 2). All infants had an AHI > 1 event/h; all but 1 had an OMAHI > 1 event/h; and 75% had an OMAHI > 3 events/h. Infants with PRS had more severe SDB than infants with isolated cleft, but similar SDB severity compared to infants with syndromes (data not shown). Sleep medicine physicians determined that 30% of infants had normal PSG results. For the remaining infants, 33% were recommended for clinical follow-up without intervention, 6% side lying or prone positioning for sleep, 3% oxygen during sleep, and 27% non-invasive ventilation (NIV) therapy. For 3 infants recommended NIV, families declined starting the therapy.
Table 2.
Polysomnography (PSG) results from infancy and at follow-up. Results are expressed as mean ± standard deviation (SD).
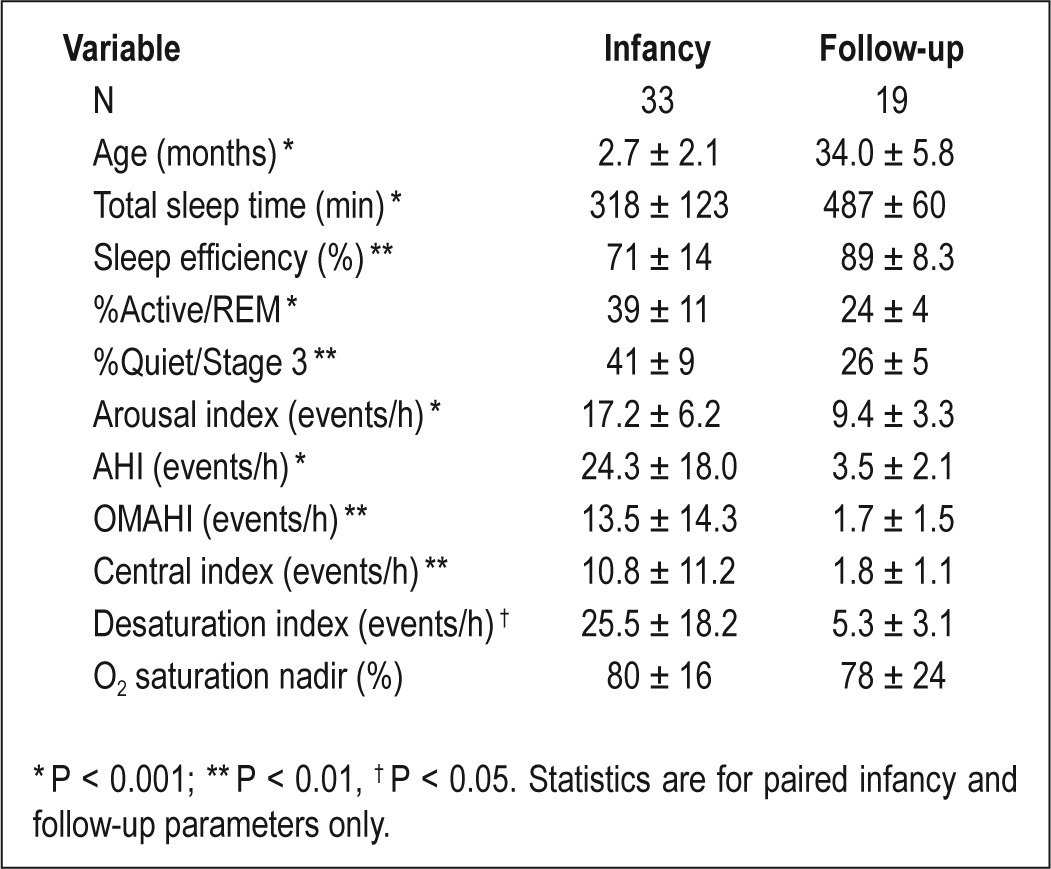
Among 19 children (57%) who completed PSG at follow-up, PSG parameters in infancy were not different from those children who declined follow-up PSG (data not shown). Comparison of paired infant and follow-up PSG results showed expected changes in sleep architecture, sleep efficiency, and arousal index between infancy and follow-up (Table 2). There was a significant decrease in all respiratory events but no change in minimum oxygen saturation. AHI decreased from infancy to follow-up for all children; however, 72% had AHI > 2 event/h at follow-up. Both OMAHI and central index was < 5 events/h for all children, consistent with mild SDB, at most, in those with residual respiratory events. Between the infant and follow-up assessments, 2 children were diagnosed with epilepsy (6%), and seizures were controlled with anti-epileptic medication. One child was diagnosed with global developmental delay. Four children (12%) were identified as having hearing deficits.
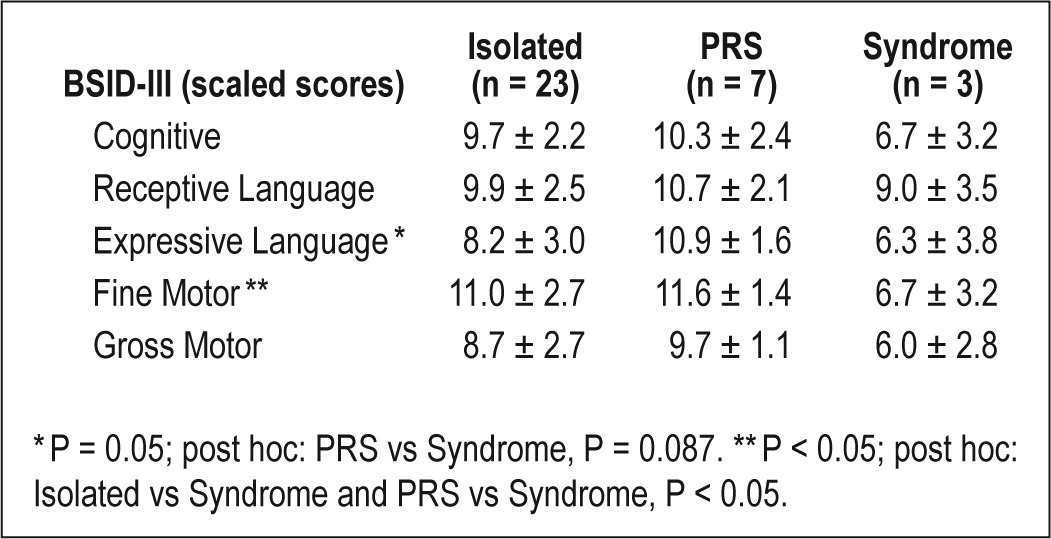
A summary of the outcome measures at 3 years of age is presented in Table 3. Mean BSID-III scaled scores at follow-up were within the normal range for all domains (Figure 1). Children with a history of CL/P had lower scores than control children on the receptive and expressive language domains (Table 4). BSID-III scaled scores also differed by syndrome status; children with isolated cleft and PRS had higher scores than children with syndromes (Table 5). ITQOL scores were within the range reported in previously published control samples,27–29 with means for physical ability, growth and development, general health perceptions, and parent impact-time below control mean scores. Weight z-scores were significantly higher at 3 years of age than in infancy (-0.17 vs -0.78, P < 0.001) with similar height z-scores at the 2 time points. At 3 years of age, 2 children had weight z-scores < 2; 3 children had height z-scores < 2; 2 had height z-scores > 2; and one child had a BMI z-score > 2. Quality of life scores and growth parameters did not differ by syndrome status.
Table 3.
Summary of group outcome measures including neurocognition as measured by the Bayley Scale of Infant and Toddler Development III (BSID III), quality of life as measured by the Infant/Toddler Quality of Life Questionnaire (ITQOL), and growth parameters.
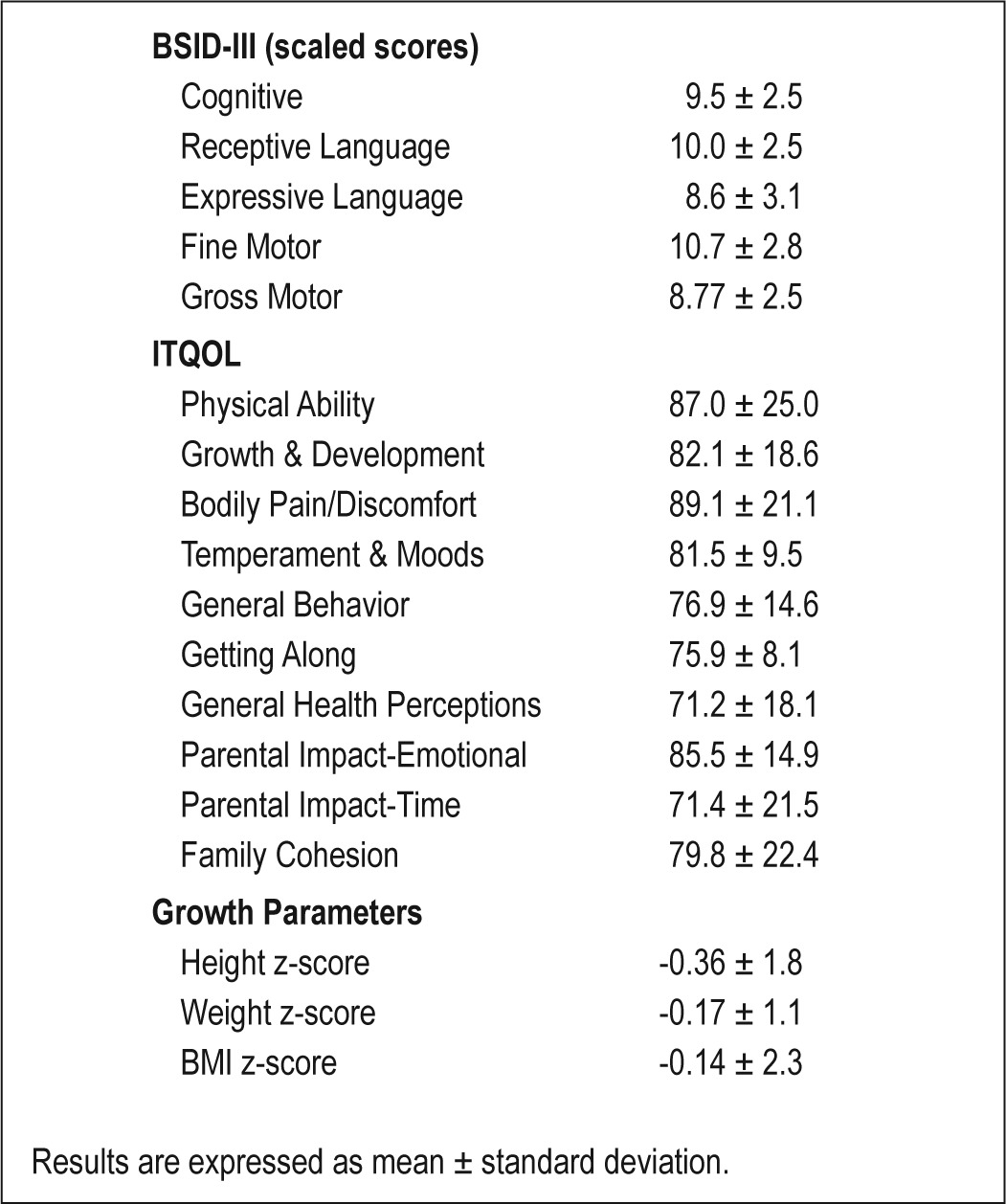
Figure 1.
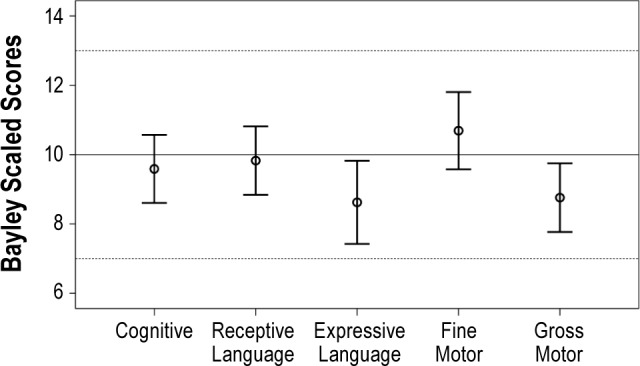
Bayley scaled scores distribution by Domain. Bayley scaled scores have a range of 1-19, with a mean of 10 (SD 3). Error bars represent the 95% confidence interval around the mean group score.
Table 4.
Comparison of neurocognitive outcomes between children with a history of CL/P and control children.
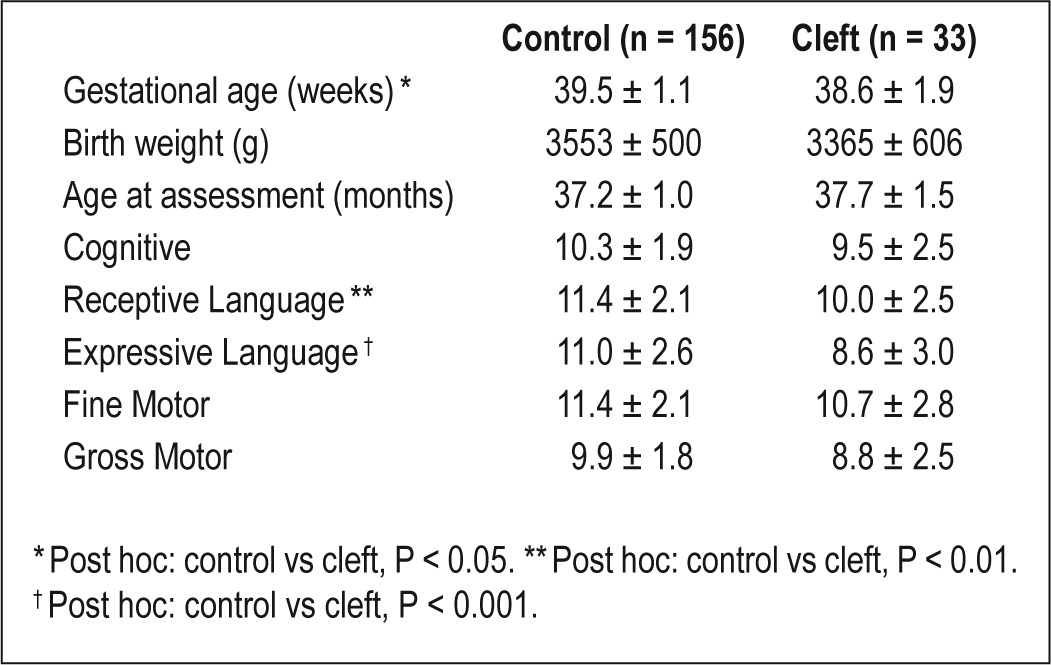
Table 5.
Neurocognition as measured by Bayley Scales of Infant and Toddler Development III (BSID-III; scaled scores) by syndrome status
The percentage for AS/REM sleep in infancy showed a positive correlation with the cognitive domain on the BSID-III (Pearson 0.35, P < 0.05). No other PSG variables in infancy— including sleep efficiency, arousal index, AHI, and ODI— showed relationships with neurocognition at 3 years of age. The 3 children who were recommended treatment for SDB but whose families declined treatment had lower scores on the cognitive domain than the rest of the group (6.33 ± 3.06 vs 9.87 ± 2.2, P < 0.05); one of these children had PRS and one had a syndrome. OMAHI in infancy showed a negative relationship with ITQOL Global Behaviour domain (Pearson -0.43, P < 0.05). There were no significant correlations between PSG variables and growth parameters at 3 years of age. Children with low AHI (< 15) in infancy did not differ from those with high AHI on any outcome variable except for weight, where children with high AHI in infancy had a lower weight z-score at 3 years than children with a low AHI in infancy (-0.54 vs 0.40, P < 0.05).
DISCUSSION
This is the first study to report on long-term outcomes of SDB in infants with CL/P. The results demonstrate that, as a group, children with a history of CL/P are doing well at 3 years of age with respect to neurocognition, quality of life, and growth, despite high AHI in infancy. Sleep and breathing differences in infancy, as measured by PSG prior to intervention, demonstrate significant relationships with outcomes at 3 years of age including; (1) higher percentage of AS/REM sleep in infancy correlating with higher cognitive score on the BSID-III; (2) higher OMAHI correlating with lower global behaviour score on the ITQOL; and (3) high AHI in infancy associated with lower weight z-scores at 3 years of age. Children with CL/P do show important deficits in receptive and expressive language compared to control children. Syndrome status influences neurocognitive but not quality of life and growth outcomes.
Limitations of the study must be acknowledged. Follow-up was completed for 66% of the original cohort raising the possibility of selection bias. Males and children referred for sleep medicine consultation prior to initial PSG were overrepresented in the follow-up cohort. The potential effect of more males on outcomes is difficult to anticipate. The higher rate of children referred in the follow-up cohort could bias towards worse outcomes, as these infants may have been identified as at risk for reason other than sleep related symptoms. The original and follow-up cohorts did not differ, however, by syndrome status or infant PSG variables. Families who had concerns about how their children were doing may have been motivated to participate in follow-up.
Common comorbidities associated with CL/P, in addition to airway obstruction, include feeding difficulties, growth failure, speech abnormalities, and developmental delay. Feeding difficulties and failure to thrive are more common in newborns with palatal clefts and those with CL/P in the context of syndromes.24,32 Both feeding and growth concerns may persist into childhood with catch-up growth often seen after surgical repair of the cleft.33–35 Pre-speech and speech difficulties are also common and can continue beyond palate repair into adolescence.36–40 Children with cleft palate are at increased risk of delayed development with a decline in mental development noted over the first two years of life.41,42 Previous studies using the BSID have documented lower cognitive performance in infants with cleft compared to control infants.43,44 A recent meta-analysis and review article both demonstrate that while infants, children, and adults with cleft have cognitive function within a normal range, cognitive performance is lower than controls, with more significant deficits in the language domains, including reading skills.45,46 The results from the current study add to this literature by demonstrating an association between SDB in infancy and long-term neurocognitive, growth, and quality of life outcomes.
While the current observational study cannot argue a causative relationship between SDB in infancy and outcomes at 3 years of age, the results are in agreement with experimental animal models that demonstrate a positive relationship between early exposure to intermittent hypoxia (IH), and later alterations in brain structure, neurocognitive function, and growth. For example, mice pups exposed to IH between postnatal days 2 and 10, equivalent to the perinatal period in human infants, show region selective hypomyelination in the corpus callosum, striatum, fornix, and cerebellum.47 Myelination is a developmental process that is important for normal cognitive development.48 In addition, these findings in infant animals parallel changes in susceptible brain regions associated with IH exposure in adult animals including the hippocampus and cortex.49 Several studies have confirmed that chronic IH impairs memory, with a number of proposed mechanisms accounting for this effect including alterations in glutamate levels,50 increased iNOS (inducible nitric oxide synthase),51 and decreases in brain-derived neurotrophic factor (BDNF).52 IH exposure in postnatal rats resulted in the same pattern of growth seen in the current infant study—growth restriction during IH with subsequent recovery when IH is removed.53
Sleep disruption, independent of IH, also plays an important role in learning, primarily through its impact on memory. Though the exact role of specific sleep states remains controversial, stage 2 sleep, SWS, and REM sleep have been implicated in sleep related memory processing54–56; SWS is postulated to reactivate circuits activated by learning experiences with REM sleep responsible for consolidation of learning into long-term memory. Experimental sleep deprivation in animal models demonstrates changes in multiple memory systems resulting in cognitive deficits. The cellular and molecular mechanisms are unclear with several proposed mechanisms under investigation including the role of extracellular adenosine,57 astrocyte-derived adenosine,58 and altered NMDA receptor function.59 REM-specific sleep deprivation alters cytoskeleton reorganization in the hippocampus.60 While less work has been done investigating sleep deprivation in infants, experiments in rats at postnatal day 2 and 8 show that sleep disruption results in increased sleep pressure (number of times an arousing stimulus presented to maintain wakefulness) and sleep rebound (increase in sleep duration or intensity following sleep deprivation),61 showing infants are also vulnerable to sleep deprivation.
The results from this study cannot be used to determine which infants with SDB will benefit from treatment and may not generalize to infants with CL/P receiving care in a different context. There are no accepted criteria for defining SDB in infancy. Previous studies in infants have chosen different cut-offs for defining abnormality including: a respiratory disturbance index (RDI) > 5 events/h of sleep62; mixed plus obstructive apnea index > 2 events/h of sleep13; and AHI > 2 events/h unless > 25% of events were central.63 In the current study, SDB was identified by experienced pediatric sleep physicians rather than by defined PSG or clinical criteria. It would be unethical to withhold treatment from infants with SDB given the data supporting a negative impact of SDB on neurobehavioral function and quality of life in children.17,64–71 In addition, several studies have demonstrated important comorbidities in infants with SDB, including growth and feeding problems,21,72 neurological and behavioral abnormalities73–75 and evidence that treatment of SDB improves short-term outcomes.21,72 Given this context, the current study demonstrates that infants with CL/P receiving clinical care for SDB by pediatric sleep physicians through a multidisciplinary cleft clinic have overall normal neurocognitive function, quality of life, and growth at 3 years of age compared to reference standards but with some impairment compared to control children. Further work is needed to develop criteria to aid in defining clinically significant abnormalities in infants with SDB and determining whether earlier intervention can improve long-term outcomes.
In summary, the results from the present study support an association between SDB in early infancy and neurocognitive, quality of life, and growth outcomes at 3 years of age. This information supports the need for greater attention to sleep and breathing abnormalities in infants with CL/P. In addition, there is a need for further work examining SDB across infancy to better understand the implications for treatment choices with respect to long-term outcomes.
DISCLOSURE STATEMENT
This was not an industry supported study. Financial support was received through the J Christian Gillin M.D. Research Program, Sleep Research Society Foundation.
The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.MacLean JE, Hayward P, Fitzgerald DA, Waters K. Cleft lip and/or palate and breathing during sleep. Sleep Med Rev. 2009;13:345–54. doi: 10.1016/j.smrv.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Hermann NV, Kreiborg S, Darvann TA, Jensen BL, Dahl E, Bolund S. Early craniofacial morphology and growth in children with unoperated isolated cleft palate. Cleft Palate Craniofac J. 2002;39:604–22. doi: 10.1597/1545-1569_2002_039_0604_ecmagi_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 3.Mortimore IL, Douglas NJ. Palatal muscle EMG response to negative pressure in awake sleep apneic and control subjects. Am J Respir Crit Care Med. 1997;156:867–73. doi: 10.1164/ajrccm.156.3.9608008. [DOI] [PubMed] [Google Scholar]
- 4.Malhotra A, Trinder J, Fogel R, et al. Postural effects on pharyngeal protective reflex mechanisms. Sleep. 2004;27:1105–12. doi: 10.1093/sleep/27.6.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacLean JE, Fitzsimons D, Hayward P, Waters KA, Fitzgerald DA. The identification of children with cleft palate and sleep disordered breathing using a referral system. Pediatr Pulmonol. 2008;43:245–50. doi: 10.1002/ppul.20763. [DOI] [PubMed] [Google Scholar]
- 6.MacLean JE, Fitzsimons D, Fitzgerald DA, Waters KA. The spectrum of sleep-disordered breathing symptoms and respiratory events in infants with cleft lip and/or palate. Arch Dis Child. 2012;97:1058–63. doi: 10.1136/archdischild-2012-302104. [DOI] [PubMed] [Google Scholar]
- 7.Kadowaki S, Sakamoto M, Kamiishi H, Tanimura T. Embryologic features of term fetuses and newborns in CL/Fr mice with special reference to cyanosis. Cleft Palate Craniofac J. 1997;34:211–7. doi: 10.1597/1545-1569_1997_034_0211_efotfa_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 8.Li HY, Lo LJ, Chen KS, Wong KS, Chang KP. Robin sequence: review of treatment modalities for airway obstruction in 110 cases. Int J Pediatr Otorhinolaryngol. 2002;65:45–51. doi: 10.1016/s0165-5876(02)00131-3. [DOI] [PubMed] [Google Scholar]
- 9.MacLean JE, Fitzsimons D, Fitzgerald DA, Waters KA. The spectrum of sleep-disordered breathing symptoms and respiratory events in infants with cleft lip and/or palate. Arch Dis Child. 2012;97:1058–63. doi: 10.1136/archdischild-2012-302104. [DOI] [PubMed] [Google Scholar]
- 10.Eden GJ, Hanson MA. Maturation of the respiratory response to acute hypoxia in the newborn rat. J Physiol (Lond) 1987;392:1–9. doi: 10.1113/jphysiol.1987.sp016765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Decker MJ, Hue GE, Caudle WM, Miller GW, Keating GL, Rye DB. Episodic neonatal hypoxia evokes executive dysfunction and regionally specific alterations in markers of dopamine signaling. Neuroscience. 2003;117:417–25. doi: 10.1016/s0306-4522(02)00805-9. [DOI] [PubMed] [Google Scholar]
- 12.Waters KA, Beardsmore CS, Paquette J, Meehan B, Cote A, Moss IR. Respiratory responses to rapid-onset, repetitive vs. continuous hypoxia in piglets. Respir Physiol. 1996;105:135–42. doi: 10.1016/0034-5687(96)00046-1. [DOI] [PubMed] [Google Scholar]
- 13.McNamara F, Sullivan CE. Sleep-disordered breathing and its effects on sleep in infants. Sleep. 1996;19:4–12. doi: 10.1093/sleep/19.1.4. [DOI] [PubMed] [Google Scholar]
- 14.Greenfeld M, Tauman R, DeRowe A, Sivan Y. Obstructive sleep apnea syndrome due to adenotonsillar hypertrophy in infants. Int J Pediatr Otorhinolaryngol. 2003;67:1055–60. doi: 10.1016/s0165-5876(03)00182-4. [DOI] [PubMed] [Google Scholar]
- 15.Kahn A, Groswasser J, Rebuffat E, et al. Sleep and cardiorespiratory characteristics of infant victims of sudden death: a prospective case-control study. Sleep. 1992;15:287–92. doi: 10.1093/sleep/15.4.287. [DOI] [PubMed] [Google Scholar]
- 16.Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics. 1998;102:616–20. doi: 10.1542/peds.102.3.616. [DOI] [PubMed] [Google Scholar]
- 17.Chervin RD, Ruzicka DL, Giordani BJ, et al. Sleep-disordered breathing, behavior, and cognition in children before and after adenotonsillectomy. Pediatrics. 2006;117:e769–e778. doi: 10.1542/peds.2005-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cozzi F, Totonelli G, Frediani S, Zani A, Spagnol L, Cozzi DA. The effect of glossopexy on weight velocity in infants with Pierre Robin syndrome. J Pediatr Surg. 2008;43:296–8. doi: 10.1016/j.jpedsurg.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 19.Denny AD, Kalantarian B, Hanson PR. Rotation advancement of the midface by distraction osteogenesis. Plast Reconstr Surg. 2002;111:1789–99. doi: 10.1097/01.PRS.0000055467.06355.0E. [DOI] [PubMed] [Google Scholar]
- 20.Wilson AC, Moore DJ, Moore MH, Martin AJ, Staugas REM, Kennedy JD. Late presentation of upper airway obstruction in Pierre Robin sequence. Arch Dis Child. 2000;83:435–8. doi: 10.1136/adc.83.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heaf DP, Helms PJ, Dinwiddie R, Matthew DJ. Nasopharyngeal airways in Pierre Robin syndrome. J Pediatr. 1982;100:698–703. doi: 10.1016/s0022-3476(82)80567-2. [DOI] [PubMed] [Google Scholar]
- 22.Freed G, Pearlman MA, Brown AS, Barot LR. Polysomnographic indications for surgical intervention in Pierre Robin sequence: acute airway management and follow-up studies after repair and take-down of tongue-lip adhesion. Cleft Palate J. 1988;25:151–5. [PubMed] [Google Scholar]
- 23.Buchenau W, Urschitz MS, Sautermeister J, et al. A randomized clinical trial of a new orthodontic appliance to improve upper airway obstruction in infants with Pierre Robin sequence. J Pediatr. 2007;151:145–9. doi: 10.1016/j.jpeds.2007.02.063. [DOI] [PubMed] [Google Scholar]
- 24.Pandya AN, Boorman JG. Failure to thrive in babies with cleft lip and palate. Br J Plast Surg. 2001;54:471–5. doi: 10.1054/bjps.2001.3618. [DOI] [PubMed] [Google Scholar]
- 25.Drescher FD, Jotzo M, Goelz R, Meyer TD, Bacher M, Poets CF. Cognitive and psychosocial development of children with Pierre Robin sequence. Acta Paediatr. 2008;97:653–6. doi: 10.1111/j.1651-2227.2008.00742.x. [DOI] [PubMed] [Google Scholar]
- 26.Walker K, Badawi N, Halliday R, et al. Early developmental outcomes following major noncardiac and cardiac surgery in term infants: a population-based study. J Pediatr. 2012;161:748–52. doi: 10.1016/j.jpeds.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 27.Oostenbrink R, Jansingh-Piepers EM, Raat H, et al. Health-related quality of life of pre-school children with wheezing illness. Pediatr Pulmonol. 2006;41:993–1000. doi: 10.1002/ppul.20486. [DOI] [PubMed] [Google Scholar]
- 28.Klassen AF, Landgraf JM, Lee SK, et al. Health related quality of life in 3 and 4 year old children and their parents: preliminary findings about a new questionnaire. Health Qual Life Outcomes. 2003;1:81. doi: 10.1186/1477-7525-1-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raat H, Landgraf JM, Oostenbrink R, Moll HA, Essink-Bot ML. Reliability and validity of the Infant and Toddler Quality of Life Questionnaire (ITQOL) in a general population and respiratory disease sample. Qual Life Res. 2007;16:445–60. doi: 10.1007/s11136-006-9134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iber C, Ancoli-Israel S, Cheeson A, Quan SF for the American Academy of Sleep Medicine. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 31.Anders TR, Emde R, Parmelee A. Los Angeles: Brain Information Service/Brain Research Institute; 1971. A manual of standarized terminology: Techniques and criteria for scoring states of sleep and wakefulness in newborn infants. [Google Scholar]
- 32.Reid J, Kilpatrick N, Reilly S. A prospective, longitudinal study of feeding skills in a cohort of babies with cleft conditions. Cleft Palate Craniofac J. 2006;43:702–9. doi: 10.1597/05-172. [DOI] [PubMed] [Google Scholar]
- 33.Lipman TH, Rezvani I, Mitra A, Mastropieri CJ. Assessment of stature in children with orofacial clefting. MCN Am J Matern Child Nurs. 1999;24:252–6. doi: 10.1097/00005721-199909000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Lee J, Nunn J, Wright C. Height and weight achievement in cleft lip and palate. Arch Dis Child. 1997;76:70–2. doi: 10.1136/adc.76.1.70a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laitinen S, Heliovaara A, Pere A, Ranta R. Growth in children with Pierre Robin sequence and isolated cleft palate. Acta Paediatr. 1994;83:1161–4. doi: 10.1111/j.1651-2227.1994.tb18273.x. [DOI] [PubMed] [Google Scholar]
- 36.Chapman KL, Hardin-Jones M, Halter KA. The relationship between early speech and later speech and language performance for children with cleft lip and palate. Clin Linguist Phon. 2003;17:173–97. doi: 10.1080/0269920021000047864. [DOI] [PubMed] [Google Scholar]
- 37.Hutters B, Henningsson G. Speech outcome following treatment in cross-linguistic cleft palate studies: methodological implications. Cleft Palate Craniofac J. 2004;41:544–9. doi: 10.1597/02-164.1. [DOI] [PubMed] [Google Scholar]
- 38.Peterson-Falzone SJ. Speech outcomes in adolescents with cleft lip and palate. Cleft Palate Craniofac J. 1995;32:125–8. doi: 10.1597/1545-1569_1995_032_0125_soiawc_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 39.Paliobei V, Psifidis A, Anagnostopoulos D. Hearing and speech assessment of cleft palate patients after palatal closure. Long-term results. Int J Pediatr Otorhinolaryngol. 2005;69:1373–81. doi: 10.1016/j.ijporl.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 40.Broen PA, Devers MC, Doyle SS, Prouty JM, Moller KT. Acquisition of linguistic and cognitive skills by children with cleft palate. J Speech Lang Hear Res. 1998;41:676–87. doi: 10.1044/jslhr.4103.676. [DOI] [PubMed] [Google Scholar]
- 41.Neiman GS, Savage HE. Development of infants and toddlers with clefts from birth to three years of age. Cleft Palate Craniofac J. 1997;34:218–25. doi: 10.1597/1545-1569_1997_034_0218_doiatw_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 42.Swanenburg d V, Beemer FA, Mellenbergh GJ, Wolters WH, Heinemande Boer JA. An investigation of the relationship between associated congenital malformations and the mental and psychomotor development of children with clefts. Cleft Palate Craniofac J. 2003;40:297–303. doi: 10.1597/1545-1569_2003_040_0297_aiotrb_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 43.Speltz ML, Endriga MC, Hill S, Maris CL, Jones K, Omnell ML. Cognitive and psychomotor development of infants with orofacial clefts. J Pediatr Psychol. 2000;25:185–90. doi: 10.1093/jpepsy/25.3.185. [DOI] [PubMed] [Google Scholar]
- 44.Kapp-Simon KA, Krueckeberg S. Mental development in infants with cleft lip and/or palate. Cleft Palate Craniofac J. 2000;37:65–70. doi: 10.1597/1545-1569_2000_037_0065_mdiiwc_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 45.Roberts RM, Mathias JL, Wheaton P. Cognitive functioning in children and adults with nonsyndromal cleft lip and/or palate: a meta-analysis. J Pediatr Psychol. 2012;37:786–97. doi: 10.1093/jpepsy/jss052. [DOI] [PubMed] [Google Scholar]
- 46.Hardin-Jones M, Chapman KL. Cognitive and language issues associated with cleft lip and palate. Semin Speech Lang. 2011;32:127–40. doi: 10.1055/s-0031-1277715. [DOI] [PubMed] [Google Scholar]
- 47.Cai J, Tuong CM, Zhang Y, et al. Mouse intermittent hypoxia mimicking apnoea of prematurity: effects on myelinogenesis and axonal maturation. J Pathol. 2012;226:495–508. doi: 10.1002/path.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361–70. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xie H, Yung WH. Chronic intermittent hypoxia-induced deficits in synaptic plasticity and neurocognitive functions: a role for brain-derived neurotrophic factor. [Review] Zhongguo Yao Li Xue Bao/Acta Pharmacologica Sinica. 2012;33:5–10. doi: 10.1038/aps.2011.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fung SJ, Xi MC, Zhang JH, et al. Apnea promotes glutamate-induced excitotoxicity in hippocampal neurons. Brain Res. 2007;1179:42–50. doi: 10.1016/j.brainres.2007.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li RC, Row BW, Kheirandish L, et al. Nitric oxide synthase and intermittent hypoxia-induced spatial learning deficits in the rat. Neurobiol Dis. 2004;17:44–53. doi: 10.1016/j.nbd.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 52.Xie H, Leung KL, Chen L, et al. Brain-derived neurotrophic factor rescues and prevents chronic intermittent hypoxia-induced impairment of hippocampal long-term synaptic plasticity. Neurobiol Dis. 2010;40:155–62. doi: 10.1016/j.nbd.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 53.Pozo ME, Cave A, Koroglu OA, et al. Effect of postnatal intermittent hypoxia on growth and cardiovascular regulation of rat pups. Neonatology. 2012;102:107–13. doi: 10.1159/000338096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diekelmann S, Wilhelm I, Born J. The whats and whens of sleep-dependent memory consolidation. Sleep Med Rev. 2009;13:309–21. doi: 10.1016/j.smrv.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 55.Ficca G, Salzarulo P. What in sleep is for memory. Sleep Med. 2004;5:225–30. doi: 10.1016/j.sleep.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 56.Cartwright RD. The role of sleep in changing our minds: a psychologist's discussion of papers on memory reactivation and consolidation in sleep. Learn Mem. 2004;11:660–3. doi: 10.1101/lm.75104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Halassa MM, Florian C, Fellin T, et al. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–9. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Florian C, Vecsey CG, Halassa MM, Haydon PG, Abel T. Astrocyte-derived adenosine and A1 receptor activity contribute to sleep loss-induced deficits in hippocampal synaptic plasticity and memory in mice. J Neurosci. 2011;31:6956–62. doi: 10.1523/JNEUROSCI.5761-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen C, Hardy M, Zhang J, LaHoste GJ, Bazan NG. Altered NMDA receptor trafficking contributes to sleep deprivation-induced hippocampal synaptic and cognitive impairments. Biochem Biophys Res Commun. 2006;340:435–40. doi: 10.1016/j.bbrc.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 60.Davis CJ, Meighan PC, Taishi P, Krueger JM, Harding JW, Wright JW. REM sleep deprivation attenuates actin-binding protein cortactin: a link between sleep and hippocampal plasticity. Neurosci Lett. 2006;400:191–6. doi: 10.1016/j.neulet.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 61.Todd WD, Gibson JL, Shaw CS, Blumberg MS. Brainstem and hypothalamic regulation of sleep pressure and rebound in newborn rats. Behav Neurosci. 2010;124:69–78. doi: 10.1037/a0018100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ednick M, Tinkle BT, Phromchairak J, Egelhoff J, Amin R, Simakajornboon N. Sleep-related respiratory abnormalities and arousal pattern in achondroplasia during early infancy. J Pediatr. 2009;155:510–5. doi: 10.1016/j.jpeds.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 63.Kasow KA, Stocks RM, Kaste SC, et al. Airway evaluation and management in 7 children with malignant infantile osteopetrosis before hematopoietic stem cell transplantation. J Pediatr Hematol Oncol. 2008;30:225–9. doi: 10.1097/MPH.0b013e318162c463. [DOI] [PubMed] [Google Scholar]
- 64.Key AP, Molfese DL, O'Brien L, Gozal D. Sleep-disordered breathing affects auditory processing in 5-7-year-old children: evidence from brain recordings. Dev Neuropsychol. 2009;34:615–28. doi: 10.1080/87565640903133608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jackman AR, Biggs SN, Walter LM, et al. Sleep-disordered breathing in preschool children is associated with behavioral, but not cognitive, impairments. Sleep Med. 2012;13:621–31. doi: 10.1016/j.sleep.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 66.Barnes ME, Gozal D, Molfese DL. Attention in children with obstructive sleep apnoea: an event-related potentials study. Sleep Med. 2012;13:368–77. doi: 10.1016/j.sleep.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Landau YE, Bar-Yishay O, Greenberg-Dotan S, Goldbart AD, Tarasiuk A, Tal A. Impaired behavioral and neurocognitive function in preschool children with obstructive sleep apnea. Pediatr Pulmonol. 2012;47:180–8. doi: 10.1002/ppul.21534. [DOI] [PubMed] [Google Scholar]
- 68.Miano S, Paolino MC, Urbano A, et al. Neurocognitive assessment and sleep analysis in children with sleep-disordered breathing. Clin Neurophysiol. 2011;122:311–9. doi: 10.1016/j.clinph.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 69.Marcus CL, Moore RH, Rosen CL, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368:2366–76. doi: 10.1056/NEJMoa1215881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Powell SM, Tremlett M, Bosman DA. Quality of life of children with sleep-disordered breathing treated with adenotonsillectomy. J Laryngol Otol. 2011;125:193–8. doi: 10.1017/S0022215110001635. [DOI] [PubMed] [Google Scholar]
- 71.Randhawa PS, Cetto R, Chilvers G, Georgalas C, Narula AA. Long-term quality-of-life outcomes in children undergoing adenotonsillectomy for obstructive sleep apnoea: a longitudinal study. Clin Otolaryngol. 2011;36:475–81. doi: 10.1111/j.1749-4486.2011.02383.x. [DOI] [PubMed] [Google Scholar]
- 72.Lidsky ME, Lander TA, Sidman JD. Resolving feeding difficulties with early airway intervention in Pierre Robin Sequence. Laryngoscope. 2008;118:120–3. doi: 10.1097/MLG.0b013e31815667f3. [DOI] [PubMed] [Google Scholar]
- 73.Einspieler C. Abnormal spontaneous movements in infants with repeated sleep apnoeas. Early Hum Dev. 1994;36:31–48. doi: 10.1016/0378-3782(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 74.Baroni MA. Bayley's infant behavior record ratings of infants with recurrent apnea: behavioral profile and correlates with apnea, age, and developmental status. J Dev Behav Pediatr. 1992;13:158–64. [PubMed] [Google Scholar]
- 75.Loscher WN, Einspieler C, Klug EM, et al. Neurological status, sleep apnea frequency and blood oxygenation in six weeks old infants. Early Hum Dev. 1990;24:119–30. doi: 10.1016/0378-3782(90)90142-6. [DOI] [PubMed] [Google Scholar]


