Abstract
Study Objectives:
Sleep is known to increase as an acute response to infection. However, the function of this behavioral response in host defense is not well understood. To address this problem, we evaluated the effect of acute sleep deprivation on post-infection sleep and immune function in Drosophila.
Setting:
Laboratory.
Participants:
Drosophila melanogaster.
Methods and Results:
Flies were subjected to sleep deprivation before (early DEP) or after (late DEP) bacterial infection. Relative to a non-deprived control, flies subjected to early DEP had enhanced sleep after infection as well as increased bacterial clearance and survival outcome. Flies subjected to late DEP experienced enhanced sleep following the deprivation period, and showed a modest improvement in survival outcome. Continuous DEP (early and late DEP) throughout infection also enhanced sleep later during infection and improved survival. However, improved survival in flies subjected to late or continuous DEP did not occur until after flies had experienced sleep. During infection, both early and late DEP enhanced NFκB transcriptional activity as measured by a luciferase reporter (κB-luc) in living flies. Early DEP also increased NFκB activity prior to infection. Flies that were deficient in expression of either the Relish or Dif NFκB transcription factors showed normal responses to early DEP. However, the effect of early DEP on post-infection sleep and survival was abolished in double mutants, which indicates that Relish and Dif have redundant roles in this process.
Conclusions:
Acute sleep deprivation elevated NFκB-dependent activity, increased post-infection sleep, and improved survival during bacterial infection.
Citation:
Kuo TH, Williams JA. Acute sleep deprivation enhances post-infection sleep and promotes survival during bacterial infection in Drosophila. SLEEP 2014;37(5):859-869.
Keywords: sleep deprivation, bacterial infection, Drosophila, NFkappaB, immune response
INTRODUCTION
In a wide range of mammalian species, immune challenge increases non-REM sleep in a manner that is dependent on experimental circumstances, such as the microorganism used and the time of day of the infection.1 Increased sleep during an infection is known to be due to increased expression and activity of cytokines such as interleukin-1 (IL-1) and tumor necrosis factor α (TNFα) which subsequently activate an NFκB transcription factor.2 Although the effect of immune signaling on sleep in mammals has been established, the other arm of this relationship, the function of sleep in host defense, is not well understood. An approach that has been commonly used to address the impact of sleep on the immune response is to sleep deprive animals and evaluate immune parameters induced by infection, such as the expression of cytokines or the number of circulating lymphocytes which include NK cells, neutrophils, and monocytes.3 However, findings from these studies have been inconsistent. Some studies suggest that sleep deprivation impairs the immune response,4,5 but this is clearly a controversial issue.6–9 Renegar et al. demonstrated that short term sleep deprivation (6 h) in mice enhanced mucosal antiviral host defenses,8 a finding that is in agreement with our observation that 4 h sleep deprivation in flies enhances resistance to bacterial infection.10 Contradictory findings in the literature may be attributed to the duration of sleep deprivation or to other experimental circumstances such as the type of pathogen or mode of infection used in each of the studies.
Nonetheless, chronic sleep loss is associated with increased risks to human health.11 Thus, understanding the relationship between sleep and immune function will have important implications for clinical outcome. Our approach to this problem is to develop the Drosophila genetic model to elucidate a molecular link between sleep and the immune system. An interaction between sleep and the immune response in flies was first indicated by several genome-wide studies showing that many immune related genes increase expression with sleep deprivation (DEP).10,12,13 Our previous work demonstrated that inducing an immune response by infection or injury also promotes sleep in Drosophila.14 Similar to mammals, the amount of sleep that increased with infection or injury was time-of-day dependent and required expression of an NFκB transcription factor, Relish.
In this manuscript, we evaluated the effects of acute sleep deprivation on post-infection sleep and immune function in flies. Wild-type flies were subjected to DEP at different times relative to infection with Gram-negative bacteria. Behavioral responses to DEP and infection were measured, along with immune response parameters, which include NFκB activity, bacterial clearance, and survival outcome. DEP enhanced all of these parameters during infection, including post-infection sleep. However, we found that in double mutant flies lacking both the Relish and Dif NFκB genes, DEP failed to enhance post-infection sleep and likewise did not improve survival during infection. We conclude from these findings that the enhanced recovery sleep during infection as a result of prior DEP contributes to improved immune function.
MATERIALS AND METHODS
Fly Stocks
Flies were grown on standard cornmeal media. Stocks used in these studies include Canton-Special (CS), cantonized RelE20/TM3,sb mutants,10 and κB-luc transgenic flies.14 yw,DD1; Dif1,cn, bw; yw,DD1; Dif2,cn, bw and the control strain yw,DD1; cn,bw flies were back-crossed to CS for at least 8 generations with the use of a cantonized Cyo/sco balancer stock. The second chromosome which contains the Dif1, Dif2 and cn,bw alleles were carried through from the original background while the first and third chromosomes are derived from CS wild-type flies. Dif1/Dif2;RelE20/RelE20 double mutants were generated by crossing Dif1/cyo;RelE20/TM6B and Dif2/cyo;RelE20/TM6B flies, each of which was generated from cantonized Dif and Relish lines.
Behavioral Assays
All experiments were performed in females kept in constant light at 25°C unless otherwise indicated. Constant light was used to eliminate the circadian influence on the immune response14–16 and on the effects of recovery sleep associated with DEP.10,17 Sleep was measured by monitoring locomotor activity in flies using the Trikinetics Drosophila Activity Monitoring System (DAM2; Waltham, MA). Flies 1-4 days in age were loaded individually into glass tubes containing 5% sucrose and 2% agar medium. Activity counts correspond to the number of times a fly crosses an infrared light beam in the tube. Sleep in this assay is defined as an activity count of zero for a minimum of 5 consecutive minutes.17 Sleep parameters were analyzed using custom Matlab-based software (Insomniac2, written by Dr. Lesley Ashmore, or an updated version, Insomniac3, written in MSVC6, by Thomas Coradetti). Post-infection sleep was measured and reported only in flies that survived beyond the analysis period. We therefore used a 24 h cut-off in CS, Dif1, and cn,bw flies. In severely affected mutants, a lower 8 h cut-off was necessary in RelE20 flies, and a 12 h cut-off was used for Dif1/Dif2;RelE20 flies. To examine post-infection sleep in flies subjected to 16 h late DEP or continuous DEP, flies that survived a minimum of 36 h after infection were included in the analysis.
Sleep deprivation (DEP) was performed by attaching activity monitors to a multi-tube vortexer (Corning). The vortexer was controlled by software (Trikinetics, Waltham MA) that turned on the vortexer for 1-sec durations at random intervals ranging from 2-20 sec. The strength of vortexer was set to the minimal level that was required to keep flies awake ≥ 16 h. For behavioral and survival assays, early DEP (DEP before infection) was performed 2 or 3 days after loading flies into monitors and late DEP was performed immediately after infection.
To determine the arousal threshold, we used a modified version of a previously described method.18 A thin metal rod (bottle brush handle) was used to scrape activity tubes that were in the DAM2 monitor. One gentle stroke was used for mild stimulation, and 6 strong successive strokes were used for strong stimulation. Inter-stimulus intervals were a minimum of 30 min, such that one stimulus (either mild or strong) was applied at 2 h post-infection, and the other was applied either at 2.5 h or 3 h post-infection. Another stimulus set was applied at 10 h and 10.5 h or 11 h post-infection. The % flies responding corresponded to the number of sleeping flies that were awakened by the stimulation divided by the total number of flies that were sleeping prior to stimulation. Sleep was defined by a fly having 0 activity counts ≥ 5 min prior to the stimulation. Awakening or arousal was defined by having any activity counts occurring within 2 min following stimulation. Flies that were already awake at the time of stimulation were excluded from the analysis.
Measurement of the Immune Response
Methods for measuring immune response parameters are as previously described.19 Briefly, Serratia marcescens (ATCC, #8100) and Pseudomonas aeruginosa (both are gifts from Dr. Jung-Eun Lee) were grown overnight in LB medium or LB medium containing 50 μg/mL gentamycin, respectively. Bacteria were diluted into phosphate buffered saline (PBS) and 1% food coloring (Brilliant Blue FCF) solution to achieve final concentrations at OD600 of 0.1 for S. marcescens and 2 × 10-4 for P. aeruginosa. The Dif1/Dif2; RelE20/RelE20 double mutants were infected with a lower concentration of S. marcescens, at an OD600 of 1 × 10-4. Glass capillary needles (WPI) were used for delivering bacterial solutions 3-4 days after flies were loaded in DAM2 systems (at 4-8 days of age). A control group of flies was subjected to injury by injection with equivalent dilutions of LB medium and food coloring in PBS.
Survival rate was determined by using activity data derived from the Trikinetics system. Custom software (Drosonex, gift of Thomas Coradetti) was used to measure survival in hours following infection or injury. Flies were considered dead when all activity counts reached zero for the remainder of the experiment. The Drosonex software was validated by visual inspection (data not shown).
To measure bacterial clearance, flies were infected with S. marcescens, and then homogenized in groups of up to 10 flies immediately or 24 h post-inoculation. Colony forming units (cfu) per fly were obtained from an average of 2 duplicates per condition for each experiment. For flies harvested 48 h post-inoculation, results were obtained from 3 sets of flies (n = 5-10 per group) for each condition across 2 independent experiments. Flies were homogenized in 400 μL LB medium and plated onto LB plates with appropriate dilutions (1:400 to 1:400000). Plates were incubated at 37°C, and colony numbers were counted the next day to calculate cfu/fly.
Luciferase Reporter Assay
One- to 4-day-old female κb-luc flies maintained in constant light for 3-4 days were loaded into vials containing 5% sucrose, 2% agar medium and maintained for another 2 days. Flies were then transferred individually to a 96-well plate containing 2 mM luciferin (Gold Biotechnology Inc.), the substrate of luciferase, in 5% sucrose and 1% agar medium. The next day, flies were subjected to treatment such as sleep deprivation and/or infection. DEP was performed by attaching the 96-well plate containing flies to a vortexer (VWR) controlled by software as described above. Luciferase activity in living flies was measured with a Fusion Universal Microplate Analyzer (Packard). For each experiment, 8 flies per condition were loaded into the plates. Results are reported from living flies as determined by visual inspection at each time point across 2-3 replicate experiments.
Statistical Analyses
One-way ANOVA followed by Tukey post hoc comparison was used to evaluate changes in sleep after infection relative to a baseline value within a group of flies (either sleep deprived or non-deprived). The baseline value was sleep (in minutes) for the 4 h time increment immediately prior to the sleep deprivation period (for early DEP), or immediately prior to inoculation (for late DEP). The baseline value at the same time point was also used for the non-deprived control groups. Student's t-test was used to compare sleep per 4 h interval between sleep-deprived and non-deprived groups as shown in Figures 1A, 2A, 2C, 4A, 4C, 6A, 6D, and 7A. A Bonferroni correction for the number of comparisons was applied to the P-value to avoid false positives. For the arousal experiments, results are reported as percent sleeping flies responding to stimuli; Wilcoxon signed-rank test was used for pairwise comparisons. Paired t-tests were used to evaluate changes in cfu/fly between sleep deprived and non-deprived groups. Finally, for analysis of survival, data from multiple experiments were pooled and survival probability was calculated using the Kaplan-Meier estimator followed by a log-rank test. To justify pooling of data, Cox proportional hazard survival regression was used to evaluate effects of independent experiments on the hazard ratio. In most cases, independent experiments had no impact on the hazard ratio or significance values (Table S1, supplemental material). Cox modeling was performed using a publicly available online analysis tool http://statpages.org/prophaz.html. All other statistical analyses were performed using open-access software, PAlaeontological Statistics software (PAST: http://folk.uio.no/ohammer/past/)20.
Figure 1.
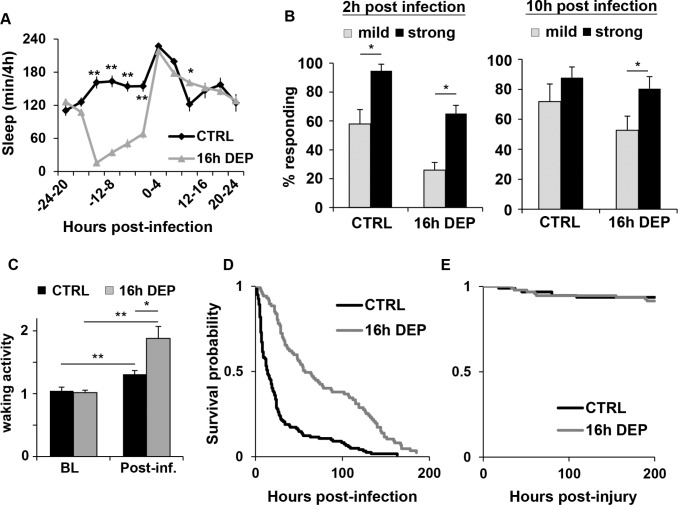
Early DEP prolongs sleep and enhances survival during infection with S. marcescens. (A) Mean ± SEM time sleeping in minutes per 4 h is plotted relative to time of infection with S. marcescens in wild-type CS flies; n = 32 for CTRL; n = 73 for the 16 h early DEP groups. Sleep is reported in flies that survived at least 24 h post-inoculation. Significant differences between CTRL and DEP groups for this and all other figures are indicated by asterisks, *P < 0.05 and **P < 0.005, t-test (Bonferroni corrected). (B) Arousal threshold is increased in flies subjected to 16 h early DEP. Mean ± SEM percent flies awakened by stimuli of indicated strength is plotted at 2 h and 10 h post-infection in non-sleep deprived control (CTRL), and 16 h early DEP groups. n = 93-195 flies from 5-7 independent experiments; *P < 0.05, Wilcoxon signed rank test. (C) Mean ± SEM activity rate (activity count per waking minute) per 12 h is plotted in flies with and without 16 h early DEP and infected with S. marcescens; *P < 0.05 and **P < 0.01, Student t-test. (D, E) Kaplan-Meier plots of flies surviving (D) infection with S. marcescens (P = 3.3 × 10-13, log rank test; n = 87 flies for CTRL and 122 flies for 16 h early DEP), and (E) aseptic injury (P = 0.58, log rank test; n = 94-95 flies).
Figure 2.
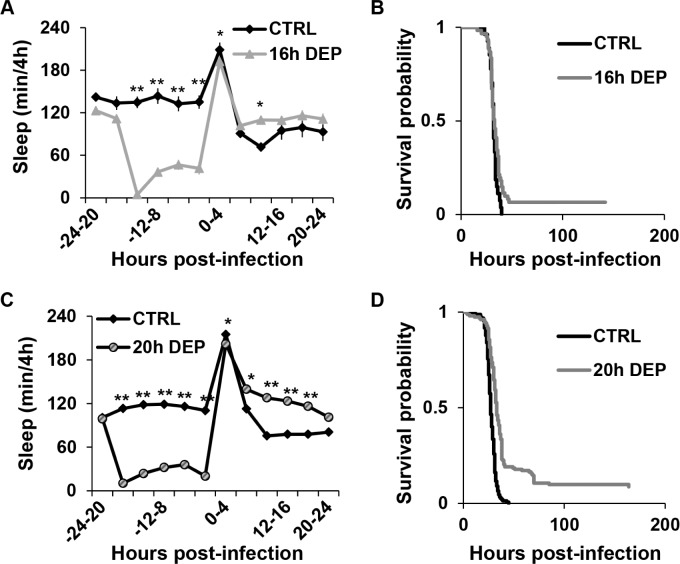
20 h early DEP increases post-infection sleep and improves survival during infection with P. aeruginosa. (A, C) Mean ± SEM percent time sleeping per 4 h is plotted relative to time of infection with P. aeruginosa in flies subjected to (A) 16 h early DEP; n = 61 for CTRL and n = 59 for 16 h early DEP (**P < 0.005; *P < 0.05), and (C) 20 h early DEP; n = 117 for CTRL and n = 139 for 20 h early DEP. (**P < 0.0001; *P < 0.005, Student t-test, Bonferroni corrected). (B, D) Kaplan-Meier plots of flies surviving infection with P. aeruginosa after (B) 16 h early DEP (P = 0.06, log rank test); n = 63 for CTRL and n = 61 flies for 16 h DEP, and (D) 20 h early DEP (P = 1.4 × 10-19, log rank test); n = 155 for CTRL and n = 152 flies for 20 h DEP.
Figure 4.
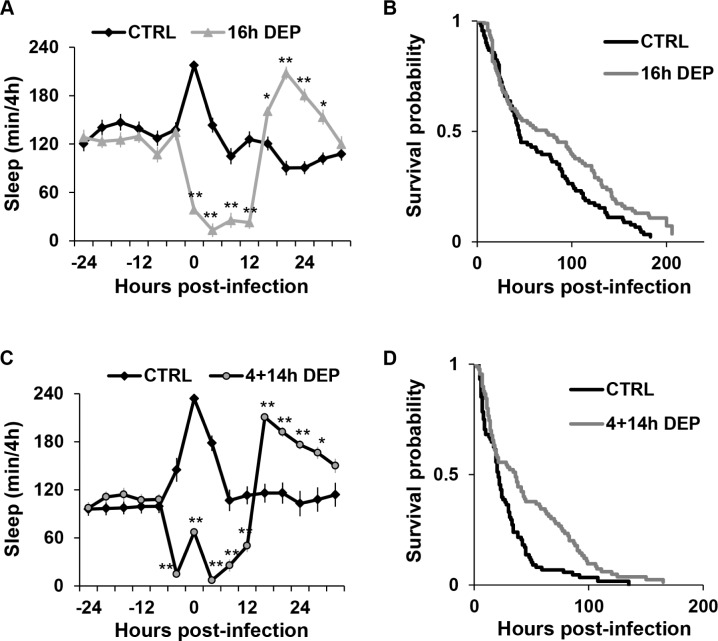
Late and continuous DEP delay post-infection sleep and enhance survival during infection. Mean ± SEM percent time sleeping per 4 h is plotted relative to time of infection in flies subjected to (A) 16 h late DEP (n = 34 and n = 33 for CTRL), and (C) 4 h DEP before and 14 h after infection (continuous DEP; n = 21 for CTRL and n = 44 for 4 + 14 h DEP groups). Sleep is reported in flies that survived at least 36 h post-inoculation. *P < 0.05 and **P < 0.0001, Student t-test (Bonferroni corrected). (B and D) Kaplan-Meier plots of flies surviving an infection with S. marcescens in non sleep-deprived controls or in those subjected to (B) 16 h late DEP (n = 93, and n = 91 flies for CTRL; P < 0.05, log rank test), and (D) continuous DEP (n = 90, and n = 88 for CTRL; P < 0.0004, log rank test).
Figure 6.
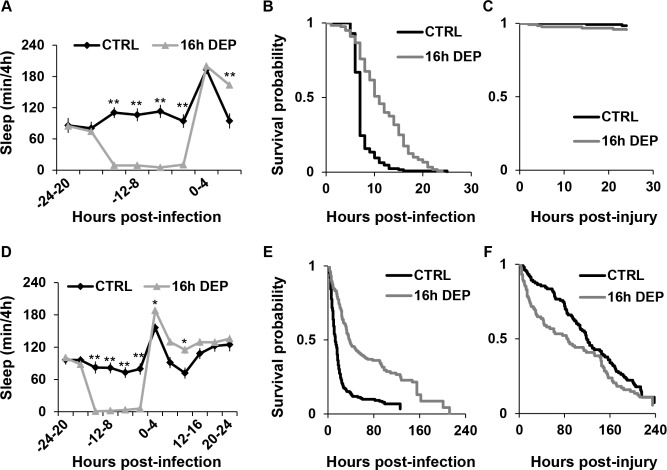
Early DEP in NFκB mutants prolongs sleep and enhances survival during infection. (A, D) Mean ± SEM percent time sleeping per 4 h is plotted relative to time of infection in (A) RelE20 flies subjected to 16 h early DEP (n = 80) or CTRL (n = 20); and (D) in Dif1 flies subjected to 16 h early DEP (n = 102) or CTRL (n = 35). *P < 0.05 and **P < 0.0001, Student t-test (Bonferroni corrected). (B, E) Kaplan-Meier plots of survival during infection with S. marcescens in (B) RelE20 flies subjected to 16 h early DEP (n = 120) and CTRL (n = 127; P = 3 × 10-15, log rank test); and for (E) Dif1 flies subjected to 16 h early DEP (n = 149) and CTRL (n = 152; P = 6 ×10-15, log rank test). (C and F) Kaplan-Meier plots of survival during aseptic injury in (C) RelE20 and (F) Dif1 flies with and without 16 h early DEP. For RelE20 flies, n = 127 for CTRL and n = 124 for 16 h early DEP, P > 0.23, log rank test. For Dif1 flies, n = 103 for CTRL and n = 92 for 16 h early DEP, P > 0.05, log rank test.
Figure 7.
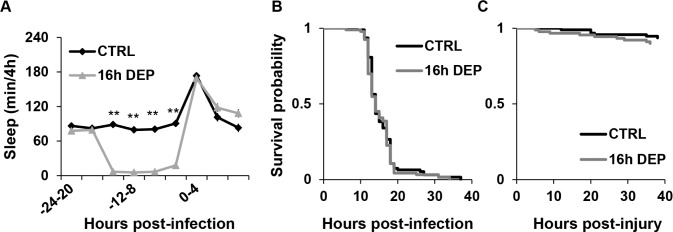
Dif; Relish double mutants abolish effects of early DEP on sleep and survival during infection. (A) Mean ± SEM percent time sleeping per 4 h is plotted relative to time of infection with S. marcescens in Dif1/Dif2;RelE20 flies; n = 73 for CTRL and n = 74 for 16 h early DEP. **P < 0.0001, Student t-test (Bonferroni corrected). (B, C) Kaplan-Meier plots of Dif1/Dif2;RelE20 flies surviving (B) an infection with S. marcescens (n = 93 for CTRL and n = 94 for 16 h early DEP, P = 0.6, log rank test); and (C) aseptic injury (n = 95 CTRL and n = 90 for 16 h early DEP; P = 0.35, log rank test).
RESULTS
Early Sleep Deprivation Increased Sleep During Bacterial Infection
To examine the role of sleep during infection, we first tested the effect of early DEP on sleep after an infection with pathogenic bacteria. To eliminate the influence of the circadian clock on sleep induced by infection,14 all experiments were conducted in constant light. Constant light in Drosophila degrades core clock proteins and renders flies arrhythmic.21 To ensure that DEP in constant light produces a compensatory increase in sleep, wild-type Canton-Special (CS) flies were subjected to DEP without infection. DEP in constant light produced a compensatory rebound sleep that lasted from hours (4 h DEP) to days (16 h DEP) (Figure S1, supplemental material). We therefore expected that early DEP would subsequently enhance sleep associated with infection relative to a non-deprived control.
CS flies were sleep deprived for 4 or 16 h and infected with S. marcescens. A control group was not sleep-deprived, and also infected at the same time. In the non-sleep deprived control condition (CTRL), a one-way ANOVA indicated a significant effect of infection on sleep (P < 1.28 × 10-15). Sleep increased significantly for up to 8 h post-infection relative to baseline values (sleep per 4 h time increment) prior to infection (P < 8.1 × 10-5, Tukey post hoc). This finding is similar to our previously reported result,14 except that the induction of sleep in constant light was not dependent on the time of day of the infection. In the sleep-deprived conditions, infected flies also significantly increased sleep as compared to their own baseline (sleep per 4 h time increment immediately prior to DEP), but this enhanced sleep was extended to 12 h post-infection relative to baseline (P < 0.02 Tukey post hoc). Sixteen hour early DEP produced more sleep at 8-12 h post-infection than that in the non-deprived group (P < 0.03 t-test, Bonferroni corrected; Figure 1A). Four hour early DEP also produced more sleep than the non-deprived control at 8-12 h post-infection, but this fell short of significance with the Bonferroni correction (not shown). Because 16 h early DEP produced more robust effects on post-infection sleep, we focused on this duration of DEP to evaluate effects of early DEP on immune function and other measurements.
Infection in mammals is known to produce long periods of inactivity during wakefulness.22 To distinguish sleep from inactivity or quiet wakefulness in the locomotor assay, we evaluated arousal threshold after infection in both early DEP and CTRL flies (see methods). Two hours after infection, all flies that were sleeping by definition showed reduced responsiveness to a mild stimulation, indicating that flies both with and without early DEP were indeed sleeping (Figure 1B, left panel). However, at 10 h after infection, CTRL flies were equally responsive to both the mild and strong arousal stimuli, whereas the 16 h early DEP group remained more responsive to only the strong stimulus (Figure 1B, right panel). This result indicates that later during the infection, CTRL flies are awake but not moving, while those subjected to early DEP remain asleep. To ensure that the lack of responsiveness was not due to an inability to move, waking activity rates were determined before and after infection. Flies in all groups increased waking activity rates post infection relative to baseline (Figure 1C). Flies subjected to 16 h early DEP also had higher activity rates post-infection than the non-deprived controls. Thus locomotor ability was not negatively affected by infection in flies. Moreover, these results strengthen the finding that early DEP prolongs sleep during infection.
Early Sleep Deprivation Enhanced Immune Function
We next examined the effect of early DEP on survival outcome during infection. S. marcescens typically killed CS flies within approximately 6-8 days after inoculation. Flies that received 16 h early DEP had a survival outcome that was better than that in the non-deprived controls (Figure 1D). Additional early DEP and CTRL groups received injury by injection without bacteria. Most flies receiving injury survived for the duration of the experiment (Figure 1E), indicating that flies indeed died from infection.
To examine whether the effect of enhanced sleep on survival was specific to infection with S. marcescens, survival was also determined in flies that were sleep-deprived and infected with another strain of Gram-negative bacteria, P. aeruginosa. Most flies were killed by this strain within approximately 2 days after inoculation. The small percentage of flies that survived 2 days beyond the infection usually survived for the full duration of the experiment, up to 8 days post-inoculation. Non-sleep deprived flies infected with P. aeruginosa showed a transient increase in sleep immediately after infection, similar to that observed during an infection with S. marcescens. However, this period was followed by a significant decrease in sleep relative to their own baseline (P < 0.05 one-way ANOVA, Tukey post hoc; Figure 2A and 2C). Flies subjected to 16 h early DEP also showed a similar transient increase in sleep following infection that lasted for 4 h, but then returned to the baseline level. Differences in post-infection sleep between CTRL and 16 h early DEP groups were also transient and, at best, modest (Figure 2A); 16 h early DEP had no significant effect on survival during infection with P. aeruginosa (Figure 2B).
We next subjected flies to 20 h early DEP and infected with P. aeruginosa. Twenty hour early DEP not only produced elevated sleep as compared to its own baseline for up to 16 h post-inoculation (P < 0.008 one-way ANOVA, Tukey post hoc), but also in comparison to the CTRL group for up to 20 h post-infection (Figure 2C). Twenty hour early DEP also significantly improved survival relative to the non-deprived control (Figure 2D). This finding indicates that the lack of a substantial effect of 16 h early DEP on post-infection sleep may account for the lack of an effect on survival during infection with P. aeruginosa. In summary, early DEP prolongs post-infection sleep as well as survival during infection with Gram-negative bacteria S. marc-escens and P. aeruginosa.
We next determined a mechanism by which early DEP promotes survival. The outcome of host defense comes from the balance of two immune response parameters, resistance and tolerance. Resistance is the ability of host to limit the load of pathogens, while tolerance is the ability of host to limit the damage in response to pathogens.23 We determined resistance by measuring the number of colony forming units (cfu) remaining in flies after inoculation (Figure 3A). CS flies were sleep deprived for 16 h, infected with S. marcescens, and then harvested immediately or 24 h post-inoculation to measure cfu per fly (Figure 3B; see methods). No difference was observed in cfu/fly among groups right after inoculation, indicating that each group received an equal amount of bacterial cells (P > 0.98, t-test). While the number of cfu/fly in both deprived and non-deprived groups increased 24 h after inoculation (P < 0.0005, Student t-test), it was approximately 3-fold higher in the non-sleep deprived group (Figure 3B). These results indicate that early DEP increases the flies' ability to clear bacteria and likely accounts for the enhanced rate of survival.
Figure 3.
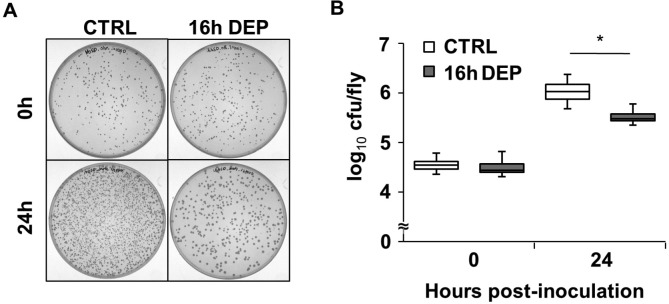
Early DEP increases bacterial clearance during infection with S. marcescens. (A) Representative bacterial culture from CS flies infected with S. marcescens. Flies were non-sleep deprived (CTRL) or sleep deprived for 16 h prior to infection with S. marcescens. Plates contain a 1:4000 dilution of LB medium in which flies were homogenized immediately after infection (upper panel), or 24 h after infection (lower panel). (B) Box-and-whisker plots of cfu/fly are plotted relative to the time of infection. The bottom, middle and top of the box represent the 25th, 50th (median) and 75th percentile, respectively. Error bars represent standard deviation. *P < 0.05 Student t-test; N = 4 independent experiments.
Late and Continuous Sleep Deprivation Delayed Sleep and Promoted Survival during Infection.
Based on the findings that early DEP prolongs post-infection sleep (Figure 1A and 2C) and promotes survival during infection (Figure 1D and 2D), we surmised that flies would show a decreased survival outcome if they were prevented from sleeping after infection (late DEP). To test this hypothesis, survival was measured in wild-type CS flies that were infected with S. marcescens and then sleep deprived for 16 h; 16 h late DEP significantly reduced sleep from 0-16 h post-infection (Figure 4A). However, sleep was significantly enhanced after the deprivation period, from 16-32 h post-infection. Thus late DEP failed to block post-infection sleep, and instead, delayed sleep to after the DEP period. Flies that were subjected to 16 h late DEP also had a small but significantly improved survival outcome (Figure 4B). Note that there was no difference in survival probability between sleep deprived and non-deprived groups within the first day after infection. Instead, the modest improvement in survival in the 16 h late DEP group occurred after these flies had experienced several hours of recovery sleep.
We next determined the effect of late DEP on resistance to infection with S. marcescens. Control flies were infected with S. marcescens and harvested immediately after inoculation to determine the bacterial load (log10 cfu/fly = 4.13 ± 0.16). Other flies were infected, sleep-deprived for 16 h or not, and then harvested 24 h after inoculation (log10 cfu/fly for CTRL = 6.04 ± 0.21, and for 16 h late DEP = 6.05 ± 0.26). The number of cfu/fly increased 24 h after inoculation (P < 0.01, Student t-test). However, no difference was observed in cfu/ fly between DEP and CTRL groups (P > 0.9 Student t-test), which was consistent with the finding that no change in survival was observed within this time frame. We therefore determined cfu/fly 48 h after infection. To ensure sufficient numbers of flies survived during this time frame, a lower concentration of bacteria was used. Flies were infected with an average of 3.45 ± 0.06 log10 cfu/fly. Forty-eight hour after inoculation, flies subjected to 16 h late DEP showed higher resistance to infection with 4.9 ± 0.11 log10 cfu/fly, which was significantly lower than the non-deprived control with 5.6 ± 0.25 log10 cfu/fly (P < 0.04, N = 3, see methods).
Because late DEP failed to block excess sleep associated with infection, we next evaluated the effect of continuous DEP before and after infection. Flies were subjected to 4 h early DEP, infected with S. marcescens, and then subjected to additional DEP for up to 14 h. Similar to late DEP, continuous DEP delayed sleep until the end of the sleep deprivation period (Figure 4C). Flies subjected to continuous DEP also showed improved survival with infection (P < 0.0004; Figure 4D). However, note that improved survival in sleep-deprived flies did not occur until at least 18 h after infection, after these flies had started sleeping.
Sleep Deprivation Increased NFκB Transcriptional Activity in Uninfected and Infected Flies
Our previous study showed that sleep deprivation increases mRNA expression of the NFκB Relish and its antimicrobial peptide gene targets.10 Importantly, increases in expression of Relish mRNA during DEP were more sensitive to mechanical stimulation applied at times of day when flies were expected to be sleeping rather than awake and active. To further confirm this result and to investigate a molecular mechanism by which DEP and post-infection sleep promote the immune response, the transcriptional activity of NFκB was measured in transgenic flies carrying a κB-luciferase reporter (κB-luc).14 This reporter contains an NFκB response element derived from the cecropin promoter and is sensitive to Relish activity in vivo.14 κB-luc flies were subjected to 4 h DEP in a 12:12 light: dark cycle either from zeitgeber time (ZT) 1-5, which corresponds to daytime hours when flies are expected to be awake, or at nighttime from ZT 15-19 when flies are expected to be sleeping. Mechanical stimulation at nighttime produced a significantly greater increase in κB-luc reporter activity than that in the daytime (P < 0.04; Student t-test; n = 24 flies each group; Figure S2, supplemental material). Similar to previous results,10 this finding indicates that increases in NFκB activity in flies are more sensitive to sleep loss than to mechanical stimulation alone. To evaluate effects of DEP on NFκB activity in constant light, κB-luc flies were sleep-deprived for 16 h in constant light and luciferase activity was measured for up to 48 h after the initiation of stimulation. Reporter activity steadily and significantly increased throughout the 16 h DEP period (P < 2.1 × 10-17, one-way ANOVA; Figure 5A). The increase in reporter activity peaked at the end of the 16 h DEP period, and recovered to baseline levels after 36 h (hour 48, Figure 5A, P = 0.6 Tukey post hoc). However, pairwise comparisons with the non-deprived group indicated that κB-luc reporter activity remained elevated at 48 h (P < 0.005; t-test, Bonferroni corrected). No increase in reporter activity was detected in the non-deprived group, although there was a significant decline in the signal by 48 h (P < 0.03, Tukey post hoc), which is due to depletion of the substrate.
Figure 5.
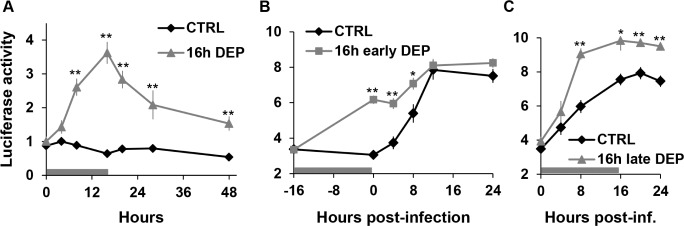
Sleep deprivation enhances NFκB activity during infection with S. marcescens. (A) Mean ± SEM Luciferase activity (arbitrary units adjusted to natural log values) is plotted against time in flies that were non sleep-deprived or sleep-deprived for 16 h without infection. Horizontal bar corresponds to the duration of DEP. The reading at hour 0 was performed immediately before the initiation of DEP. **P < 0.005, Student t-test (Bonferroni corrected). Mean ± SEM Luciferase activity (arbitrary units adjusted to natural log values) in infected flies that were subjected to (B) 16 h early DEP or (C) 16 h late DEP. Horizontal bars correspond to the DEP period. Readings at Hour 0 in B and C were obtained immediately before infection. Total n = 14-24 flies per group (see methods). *P < 0.05 and **P < 0.005, Student t-test (Bonferroni corrected) for the comparison between sleep-deprived and non-deprived groups.
We next tested whether early DEP alters NFκB activity during infection. NFκB activity was measured immediately before the initiation of sleep deprivation and up to 24 h post-inoculation. κB-luc reporter activity increased significantly during infection with S. marcescens in flies without DEP (Figure 5B and 5C; “CTRL”; ANOVA, P < 0.0005). Sixteen hour early DEP also significantly increased κB-luc reporter activity during infection (indicated by horizontal bar, Figure 5B, “16 h early DEP”; ANOVA, P < 2 × 10-25). In addition, the activity was significantly enhanced by early DEP at the time immediately before inoculation (compare “16 h early DEP” to “CTRL” at time 0, Figure 5B), and lasted for up to 8 h post-inoculation. Late DEP also resulted in enhanced NFκB activity during infection. Sixteen hour late DEP (indicated by horizontal bar, Figure 5C) increased κB-luc reporter activity relative to non-deprived controls that lasted beyond the DEP period, for at least 24 h post-inoculation (Figure 5C). In summary, while both manipulations of sleep enhanced κB-luc activity during the infection, early DEP was associated with a significant rise of NFκB activity prior to the infection.
The Effects of Early Sleep Deprivation on Sleep and Survival during Infection Persist in Relish Mutants
We reported previously that Relish was required for increased sleep during an immune response.14 Given the impact of DEP on NFκB activity during infection (Figure 5), we further investigated a role of NFκB in sleep and its effect on survival during infection by measuring sleep behavior and survival in NFκB mutants during infection. First, RelishE20 (RelE20) null mutants24 were subjected to 16 h early DEP and then infected with S. marcescens. RelE20 mutants are known to succumb quickly to infection with Gram-negative bacteria.24 In contrast to CS flies, which succumb 6-8 days post-infection, RelE20 mutants died within 24 h post-infection (compare Figure 6B to 1D). Infected flies increased sleep significantly 0-4 h after infection as compared to baseline (P < 9.6 × 10-5, one-way ANOVA, Tukey post hoc). In sleep-deprived conditions, infected flies also significantly increased sleep as compared to their baseline, but for a longer duration, up to 8 h after infection (P < 3.3 × 10-5, one-way ANOVA with Tukey post hoc comparison). In addition, sleep-deprived flies had significantly more sleep relative to the non-deprived group 4-8 h after infection (Figure 6A), indicating that early DEP produces a longer post-infection sleep in RelE20 mutants. RelE20 flies experiencing a longer post-infection sleep period also had an increased rate of survival as compared to the non-deprived control group (Figure 6B). RelE20 flies that received injury by injection without bacteria showed no differences in survival between sleep-deprived and non-deprived groups within 24 h (Figure 6C) indicating that flies indeed died from infection. In summary, early DEP enhances sleep and survival in RelE20 flies during infection with S. marcescens.
The Effects of Early Sleep Deprivation on Sleep and Survival during Infection Persist in Dif Mutants
Since the effects of early DEP persisted in RelE20 flies, we next examined sleep and survival in flies lacking another NFκB transcription factor, Dif, during infection with S. marcescens. Dif is central to the Toll signaling pathway, which is typically responsive to Gram-positive bacterial or fungal infections. However, cross-talk between Toll and Imd signaling pathways during the immune response has been described,25,26 and in some instances, the Toll signaling pathway is responsive to infection with Gram-negative bacteria.27 Dif1 is a loss-of-function allele28 that is no more susceptible to infection with S. marcescens than wild-type controls. Indeed, we found that Dif1 mutants died out at a similar rate as CS wild-type flies (Figure 6E) as well as cantonized background control flies, cn,bw (Figure S3, supplemental material) during infection with S. marcescens. Sixteen hour early DEP also increased sleep in infected Dif1 mutants as compared to baseline (ANOVA, P < 1.8 × 10-5, one-way ANOVA, Tukey post hoc), but was prolonged relative to non-deprived controls for up to 12 h after infection (Figure 6D). Sixteen hour early DEP in Dif1 mutants also significantly improved survival outcome (Figure 6E). Early DEP also prolonged sleep (Figure S3A; P < 0.005, one-way ANOVA, Tukey post hoc) and improved survival (Figure S3B) in cantonized cn,bw control flies during infection. Surprisingly, we found that both Dif1 mutants as well as the cn,bw controls succumbed slowly to injury over the 6-day course of the experiment (Figure 6F and Figure S3C), which was mildly worsened by early DEP. Nonetheless, the acute effect of prolonged post-infection sleep that was produced by 16 h early DEP was associated with improved survival rates in both RelE20 and Dif1 mutants infected with S. marcescens.
The Effects of Early Sleep Deprivation on Sleep and Survival During Infection Are Abolished in Relish and Dif Double Mutants
We next measured sleep and survival during infection with S. marcescens in Dif; Relish double mutant flies. We initially found that the rate at which double mutants succumbed to infection with S. marcescens was extremely rapid—usually within 8-12 h of inoculation. Dif1/Dif2;RelE20 transheterozygous double mutants were therefore subjected to 16 h early DEP and infected with a much lower concentration of S. marc-escens (see methods). Most Dif1/Dif2;RelE20 flies were able to survive up to 12-16 h with infection, but afterward, succumbed rapidly and died within 24 h after the infection (Figure 7B). Sleep-deprived Dif1/Dif2;RelE20 double mutants showed no significant difference in post-infection sleep as compared to non-deprived controls (Figure 7A). Moreover, 16 h early DEP had no effect on survival during infection in Dif1/Dif2;RelE20 double mutants as compared to non-sleep deprived controls (Figure 7B). Dif1/Dif2;RelE20 mutants were not susceptible to aseptic injury within 40 h (Figure 7C). These findings indicate that the effect of early DEP on sleep and survival during infection is abolished in Dif/Rel double mutants, and suggest that Relish and Dif have redundant roles in the effect of early DEP on immune function.
DISCUSSION
Although sleep has been proposed to have a role in maintaining a robust immune system, studies that have directly tested this hypothesis in terms of survival outcome have been lacking. One study, which involved an analysis of findings in the literature, reported an association of longer durations of sleep in animals with longer survival times during infection.29 A recent study, based on findings reported in a wide range of mammalian species, concluded that longer daily sleep time is associated with enhanced immune function as indicated by the number of circulating immune cells and parasitic infection.30 We have also found that using a genetic approach to induce excess sleep prior to infection improves survival.31 We report here evidence that acute sleep deprivation prolonged sleep during an immune response and subsequently prolonged survival during bacterial infection.
Early DEP enhanced sleep and promoted survival during infection by increasing resistance to the infection. Contrary to expected results, late and continuous DEP failed to block excess sleep during infection and also resulted in an improved effect on immune function. Effects of DEP on sleep during immune challenge have been reported in rabbits32 and pigeons.33 In both cases, early and late DEP enhanced sleep post-inoculation with E. coli32 or LPS,33 which is consistent with the current findings. Together, these findings indicate that recovery sleep induced by DEP is additive to that associated with immune challenge. Furthermore, improved survival always occurred in flies that also showed enhanced post-infection sleep, but was absent in those that did not. We therefore propose that the enhanced post-infection sleep produced by DEP was a major contributing factor to an improved survival outcome.
This notion is supported by the following observations. First, improved survival during infection with P. aeruginosa was associated only with an early DEP duration (20 h) that substantially enhanced post-infection sleep (see Figure 2). A shorter duration of early DEP (16 h) did not enhance post-infection sleep relative to control in a manner that was sufficient to significantly improve survival during infection with this species. Second, late and continuous DEP also improved survival during infection, but the effect was not seen until several hours after the flies had started sleeping (compare Figure 4B and 4D to Figure 1D). Bacterial clearance was not affected in flies subjected to late DEP at 24 h post-infection, but was reduced as compared to the non-deprived control at 48 h post-infection, after a period of recovery sleep. Weaker effects of late DEP (as compared to those seen with early DEP) may be attributed to the delay of excess sleep during infection. By the time that sleep occurs, the infection may have progressed to an extent such that any amount of sleep is not sufficient to overcome pathogenic effects. Finally, early DEP did not improve survival in the Dif; Relish double mutants. Enhanced post-infection sleep was also abolished in these mutants. Together, these findings support the hypothesis that the acute sleep response during infection benefits immune function.
Acute stress has been reported to benefit immune function in mammals.34 An alternate interpretation of our findings is that stress from sleep loss itself or from the mechanical stimulation that was used to keep flies awake at the time of inoculation protected the flies from infection. Earlier work in mammals also showed beneficial effects of DEP before and after infection. However, effects of DEP on subsequent recovery sleep during infection were not investigated,8 leaving open the possibility that potentially enhanced post-infection sleep induced by DEP may have contributed to an improved immune function. Early, late, and continuous DEP may therefore influence immune function through multiple mechanisms that involve a combination of stress factors and restorative sleep. However, if stress from sleep loss was indeed the predominant contributing factor to improved immune function, a much stronger survival outcome would have been expected from both late as well as continuous DEP. As noted above, improved survival in these experimental conditions occurred later after infection, after flies experienced sleep. Furthermore, in cases where enhanced post-infection sleep induced by DEP was blocked by infectious species such as P. aeruginosa (discussed above), there was no change in survival outcome relative to a non-deprived control. Future studies that investigate the relationship of DEP to immune function should also consider the impact of DEP on post-infection sleep as well as the timing of measurements of immune response parameters relative to this sleep period.
NFκB is known to increase with prolonged wakefulness in both mammals35–39 and flies,10,13 and to promote sleep.40 Both early and late DEP augmented NFκB activity associated with infection in living flies, as well as the ensuing sleep. Given that Relish is necessary for the sleep promoting effect of infection,14 the augmented NFκB activity likely accounted for the enhanced sleep following DEP during infection. Although the effect of early DEP persisted in both Relish and Dif mutants, this effect was abolished in double mutants. This finding suggests that Relish and Dif have redundant roles in this process: when one of these genes is absent, the other increases with DEP and thereby promotes sleep during infection. Relish and Dif are NFκB family members of two distinct immune response pathways, Immune deficiency (Imd) and Toll, respectively.41 The expression of Relish, but not Dif, is required for fighting infection against S. marcescens. The mechanism by which Dif may be contributing to the improved survival with early DEP in infected Relish mutants (and vice versa) will be an interesting topic for future study. One possibility is that Dif is targeting the same pathway that is normally induced by Relish during infection, but to a much weaker extent.42 Alternatively, Relish and Dif may activate a signaling pathway induced by early DEP that is distinct from the canonical immune response.
Our previous work showed that Relish is not required for normal sleep rebound responses to sleep deprivation,10 but is required for the sleep promoting effect of the immune response.14 This latter finding may apparently contrast with the current study, which showed a mild increase in sleep in RelE20 mutants during infection (see Figure 6A). However, it is important to note that experiments in this study were conducted in constant light and that this apparent increase in sleep is partially attributed to handling or artifact due to removal of activity monitors from the incubator for up to one hour to inoculate flies. In our previous study,14 we subtracted these effects out with the use of an additional non-infected, non-injured group. Nonetheless, the duration of post-infection sleep of RelE20 flies in constant light was substantially shorter than that observed in wild-type flies.19 The Dif1/Dif2;RelE20 double mutants also showed reduced post-infection sleep. A lower concentration of bacteria used for infecting these flies allowed duration of survival up to 24 h. It is unlikely that this lower bacterial concentration was a factor in the lack of an effect of early DEP on post-infection sleep and survival in these mutants, because we previously showed that aseptic injury promotes sleep in a manner that is similar to that during infection.14 In some cases, infection limits the duration of the acute sleep response (see Figure 2), and has been reported to increase activity in other insect species.43
In conclusion, acute sleep deprivation produces complex effects on immune function. Acute DEP ultimately produces a recovery sleep that is additive to that produced by an immune response. This effect was abolished in NFκB double mutants, which suggests that increased activation of NFκB contributed to a stronger sleep promoting effect as well as improved survival. Given that enhancement of post-infection sleep by acute DEP has also been reported in rodent and avian species,32,33 the current findings in Drosophila indicate that this is a conserved behavioral response that has a beneficial function to the host during bacterial infection.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by the National Science Foundation under grant #IOS-1025627. The authors would like to thank Dr. Jung-Eun Lee and Dr. Isaac Edery for providing bacterial strains, Dr. Ken Irvine for access to a luminescence counter, Mr. Thomas Coradetti for survival analysis software, Brendan Keenan for advice on statistical analyses, and Dr. Arun Handa for early development of this project.
SUPPLEMENTAL MATERIAL
Recovery sleep in response to sleep deprivation in wild-type CS flies maintained in constant light. (A) Mean ± SEM time sleeping in minutes per 1 h is plotted for CS flies without sleep deprivation (CTRL), and for those with 4 h or 16 h DEP. Colored bars indicate the duration of sleep deprivation. n = 63 for CTRL; n = 60 for the 4 h DEP group and n = 62 for the 16 h DEP group.(B) Data from (A) is plotted as cumulative sleep lost during sleep deprivation as indicated by the corresponding colored bars, and gained during recovery in minutes per 1 h.
Sleep-depriving stimuli at night produces greater increases in κB-luc reporter activity than that in the daytime. Net changes in κB-luc reporter activity (arbitrary units) are shown for flies subjected to mechanical stimulation from ZT 1-5 (day) or from ZT 15-19 (night). Measurements were obtained immediately before and immediately after the 4 h stimulation period. Mechanical stimulation increased reporter activity at both times, but the net change was greater at night as compared to the daytime. *P < 0.04, n = 24 flies each group.
Early DEP prolongs sleep and enhances survival during infection in cn,bw flies. (A) Mean ± SEM percent time sleeping per 4 h is plotted relative to time of infection in cn,bw flies. n = 27 for CTRL and n = 72 for the 16 h early DEP group. Sleep is reported in flies that survived ≥ 24 h post-inoculation. *P < 0.05 and **P < 0.01, Student t-test (Bonferroni corrected). (B, C) Kaplan-Meier plots of cn,bw flies with and without 16 h early DEP during (B) infection with S. marcescens (P = 3.3 × 10-16, log rank test; n = 89 for CTRL and n = 82 flies for 16 h early DEP). And (C) aseptic injury (P < 0.05, log rank test; n = 93 for CTRL and n = 79 for 16 h early DEP).
Cox proportional hazard survival regression analyses.
REFERENCES
- 1.Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009;10:199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zielinski MR, Krueger JM. Sleep and innate immunity. Front Biosci (Schol Ed) 2011;3:632–42. doi: 10.2741/s176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bryant PA, Trinder J, Curtis N. Sick and tired: does sleep have a vital role in the immune system? Nat Rev Immunol. 2004;4:457–67. doi: 10.1038/nri1369. [DOI] [PubMed] [Google Scholar]
- 4.Everson CA. Sustained sleep deprivation impairs host defense. Am J Physiol. 1993;265:R1148–54. doi: 10.1152/ajpregu.1993.265.5.R1148. [DOI] [PubMed] [Google Scholar]
- 5.Everson CA, Toth LA. Systemic bacterial invasion induced by sleep deprivation. Am J Physiol Regul Integr Comp Physiol. 2000;278:R905–16. doi: 10.1152/ajpregu.2000.278.4.R905. [DOI] [PubMed] [Google Scholar]
- 6.Benca RM, Kushida CA, Everson CA, Kalski R, Bergmann BM, Rechtschaffen A. Sleep deprivation in the rat: VII. Immune function. Sleep. 1989;12:47–52. doi: 10.1093/sleep/12.1.47. [DOI] [PubMed] [Google Scholar]
- 7.Rechtschaffen A, Bergmann BM. Sleep deprivation and host defense. Am J Physiol Regul Integr Comp Physiol. 2001;280:R602–3. doi: 10.1152/ajpregu.2001.280.2.R602. [DOI] [PubMed] [Google Scholar]
- 8.Renegar KB, Crouse D, Floyd RA, Krueger J. Progression of influenza viral infection through the murine respiratory tract: the protective role of sleep deprivation. Sleep. 2000;23:859–63. [PubMed] [Google Scholar]
- 9.Bergmann BM, Rechtschaffen A, Gilliland MA, Quintans J. Effect of extended sleep deprivation on tumor growth in rats. Am J Physiol. 1996;271:R1460–4. doi: 10.1152/ajpregu.1996.271.5.R1460. [DOI] [PubMed] [Google Scholar]
- 10.Williams JA, Sathyanarayanan S, Hendricks JC, Sehgal A. Interaction between sleep and the immune response in Drosophila: a role for the NFkappaB relish. Sleep. 2007;30:389–400. doi: 10.1093/sleep/30.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luyster FS, Strollo PJ, Jr, Zee PC, Walsh JK. Sleep: a health imperative. Sleep. 2012;35:727–34. doi: 10.5665/sleep.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cirelli C, LaVaute TM, Tononi G. Sleep and wakefulness modulate gene expression in Drosophila. J Neurochem. 2005;94:1411–9. doi: 10.1111/j.1471-4159.2005.03291.x. [DOI] [PubMed] [Google Scholar]
- 13.Zimmerman JE, Rizzo W, Shockley KR, et al. Multiple mechanisms limit the duration of wakefulness in Drosophila brain. Physiol Genomics. 2006;27:337–50. doi: 10.1152/physiolgenomics.00030.2006. [DOI] [PubMed] [Google Scholar]
- 14.Kuo TH, Pike DH, Beizaeipour Z, Williams JA. Sleep triggered by an immune response in Drosophila is regulated by the circadian clock and requires the NFkappaB Relish. BMC Neurosci. 2010;11:17. doi: 10.1186/1471-2202-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JE, Edery I. Circadian Regulation in the Ability of Drosophila to Combat Pathogenic Infections. Curr Biol. 2008;18:195–9. doi: 10.1016/j.cub.2007.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stone EF, Fulton BO, Ayres JS, Pham LN, Ziauddin J, Shirasu-Hiza MM. The circadian clock protein timeless regulates phagocytosis of bacteria in Drosophila. PLoS Pathog. 2012;8:e1002445. doi: 10.1371/journal.ppat.1002445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huber R, Hill SL, Holladay C, Biesiadecki M, Tononi G, Cirelli C. Sleep homeostasis in Drosophila melanogaster. Sleep. 2004;27:628–39. doi: 10.1093/sleep/27.4.628. [DOI] [PubMed] [Google Scholar]
- 18.Wu MN, Koh K, Yue Z, Joiner WJ, Sehgal A. A genetic screen for sleep and circadian mutants reveals mechanisms underlying regulation of sleep in Drosophila. Sleep. 2008;31:465–72. doi: 10.1093/sleep/31.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuo TH, Handa A, Williams JA. Quantitative measurement of the immune response and sleep in Drosophila. J Vis Exp. 2012:e4355. doi: 10.3791/4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammer Ø, Harper DAT, Ryan PD. PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica. 2001;4:4. [Google Scholar]
- 21.Devlin PF, Kay SA. Circadian photoperception. Annu Rev Physiol. 2001;63:677–94. doi: 10.1146/annurev.physiol.63.1.677. [DOI] [PubMed] [Google Scholar]
- 22.Toth LA, Rehg JE, Webster RG. Strain differences in sleep and other pathophysiological sequelae of influenza virus infection in naive and immunized mice. J Neuroimmunol. 1995;58:89–99. doi: 10.1016/0165-5728(94)00193-r. [DOI] [PubMed] [Google Scholar]
- 23.Schneider DS, Ayres JS. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nature Reviews Immunology. 2008;8:889–95. doi: 10.1038/nri2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hedengren M, Asling B, Dushay MS, et al. Relish, a central factor in the control of humoral but not cellular immunity in Drosophila. Mol Cell. 1999;4:827–37. doi: 10.1016/s1097-2765(00)80392-5. [DOI] [PubMed] [Google Scholar]
- 25.Tanji T, Yun EY, Ip YT. Heterodimers of NF-kappaB transcription factors DIF and Relish regulate antimicrobial peptide genes in Drosophila. Proc Natl Acad Sci U S A. 2010;107:14715–20. doi: 10.1073/pnas.1009473107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Gregorio E, Spellman PT, Tzou P, Rubin GM, Lemaitre B. The Toll and Imd pathways are the major regulators of the immune response in Drosophila. EMBO J. 2002;21:2568–79. doi: 10.1093/emboj/21.11.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lau GW, Goumnerov BC, Walendziewicz CL, et al. The Drosophila melanogaster toll pathway participates in resistance to infection by the gram-negative human pathogen Pseudomonas aeruginosa. Infect Immun. 2003;71:4059–66. doi: 10.1128/IAI.71.7.4059-4066.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rutschmann S, Jung AC, Hetru C, Reichhart JM, Hoffmann JA, Ferrandon D. The Rel protein DIF mediates the antifungal but not the antibacterial host defense in Drosophila. Immunity. 2000;12:569–80. doi: 10.1016/s1074-7613(00)80208-3. [DOI] [PubMed] [Google Scholar]
- 29.Toth LA, Tolley EA, Krueger JM. Sleep as a prognostic indicator during infectious disease in rabbits. Proc Soc Exp Biol Med. 1993;203:179–92. doi: 10.3181/00379727-203-43590. [DOI] [PubMed] [Google Scholar]
- 30.Preston BT, Capellini I, McNamara P, Barton RA, Nunn CL. Parasite resistance and the adaptive significance of sleep. BMC Evol Biol. 2009;9:7. doi: 10.1186/1471-2148-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuo TH, Williams JA. Excess sleep promotes survival during a bacterial infection in Drosophila. Sleep. 2014;37 doi: 10.5665/sleep.3764. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toth LA, Opp MR, Mao L. Somnogenic effects of sleep deprivation and Escherichia coli inoculation in rabbits. J Sleep Res. 1995;4:30–40. doi: 10.1111/j.1365-2869.1995.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 33.Lapshina KV, Ekimova IV. Effects of sleep deprivation on measures of the febrile reaction and the recovery of somatovisceral functions and sleep in endotoxemia. Neurosci Behav Physiol. 2010;40:381–8. doi: 10.1007/s11055-010-9268-6. [DOI] [PubMed] [Google Scholar]
- 34.Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009;16:300–17. doi: 10.1159/000216188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Irwin MR, Wang M, Ribeiro D, et al. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008;64:538–40. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basheer R, Rainnie DG, Porkka-Heiskanen T, Ramesh V, McCarley RW. Adenosine, prolonged wakefulness, and A1-activated NF-kappaB DNA binding in the basal forebrain of the rat. Neuroscience. 2001;104:731–9. doi: 10.1016/s0306-4522(01)00111-7. [DOI] [PubMed] [Google Scholar]
- 37.Ramesh V, Thatte HS, McCarley RW, Basheer R. Adenosine and sleep deprivation promote NF-kappaB nuclear translocation in cholinergic basal forebrain. J Neurochem. 2007;100:1351–63. doi: 10.1111/j.1471-4159.2006.04314.x. [DOI] [PubMed] [Google Scholar]
- 38.Chen Z, Gardi J, Kushikata T, Fang J, Krueger JM. Nuclear factor-kappaB-like activity increases in murine cerebral cortex after sleep deprivation. Am J Physiol. 1999;276:R1812–8. doi: 10.1152/ajpregu.1999.276.6.R1812. [DOI] [PubMed] [Google Scholar]
- 39.Brandt JA, Churchill L, Rehman A, et al. Sleep deprivation increases the activation of nuclear factor kappa B in lateral hypothalamic cells. Brain Res. 2004;1004:91–7. doi: 10.1016/j.brainres.2003.11.079. [DOI] [PubMed] [Google Scholar]
- 40.Kubota T, Kushikata T, Fang J, Krueger JM. Nuclear factor-kappaB inhibitor peptide inhibits spontaneous and interleukin-1beta-induced sleep. Am J Physiol Regul Integr Comp Physiol. 2000;279:R404–13. doi: 10.1152/ajpregu.2000.279.2.R404. [DOI] [PubMed] [Google Scholar]
- 41.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 42.Busse MS, Arnold CP, Towb P, Katrivesis J, Wasserman SA. A kappaB sequence code for pathway-specific innate immune responses. EMBO J. 2007;26:3826–35. doi: 10.1038/sj.emboj.7601798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lima-Camara TN, Bruno RV, Luz PM, et al. Dengue infection increases the locomotor activity of Aedes aegypti females. PLoS ONE. 2011;6:e17690. doi: 10.1371/journal.pone.0017690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Recovery sleep in response to sleep deprivation in wild-type CS flies maintained in constant light. (A) Mean ± SEM time sleeping in minutes per 1 h is plotted for CS flies without sleep deprivation (CTRL), and for those with 4 h or 16 h DEP. Colored bars indicate the duration of sleep deprivation. n = 63 for CTRL; n = 60 for the 4 h DEP group and n = 62 for the 16 h DEP group.(B) Data from (A) is plotted as cumulative sleep lost during sleep deprivation as indicated by the corresponding colored bars, and gained during recovery in minutes per 1 h.
Sleep-depriving stimuli at night produces greater increases in κB-luc reporter activity than that in the daytime. Net changes in κB-luc reporter activity (arbitrary units) are shown for flies subjected to mechanical stimulation from ZT 1-5 (day) or from ZT 15-19 (night). Measurements were obtained immediately before and immediately after the 4 h stimulation period. Mechanical stimulation increased reporter activity at both times, but the net change was greater at night as compared to the daytime. *P < 0.04, n = 24 flies each group.
Early DEP prolongs sleep and enhances survival during infection in cn,bw flies. (A) Mean ± SEM percent time sleeping per 4 h is plotted relative to time of infection in cn,bw flies. n = 27 for CTRL and n = 72 for the 16 h early DEP group. Sleep is reported in flies that survived ≥ 24 h post-inoculation. *P < 0.05 and **P < 0.01, Student t-test (Bonferroni corrected). (B, C) Kaplan-Meier plots of cn,bw flies with and without 16 h early DEP during (B) infection with S. marcescens (P = 3.3 × 10-16, log rank test; n = 89 for CTRL and n = 82 flies for 16 h early DEP). And (C) aseptic injury (P < 0.05, log rank test; n = 93 for CTRL and n = 79 for 16 h early DEP).
Cox proportional hazard survival regression analyses.