Abstract
Background:
Poor sleep, prevalent among cancer survivors, is associated with disrupted hormonal circadian rhythms and poor quality of life. Using a prospective research design, this study aimed to clarify the relationship between objective measures of sleep efficiency and sleep disruption with survival among women with advanced breast cancer.
Method:
We examined sleep quality and duration via wrist-worn actigraphy and sleep diaries for 3 days among 97 women in whom advanced breast cancer was diagnosed (age = 54.6 ± 9.8 years). Sleep efficiency was operationalized using actigraphy as the ratio of total sleep time to total sleep time plus wake after sleep onset.
Results:
As hypothesized, better sleep efficiency was found to predict a significant reduction in overall mortality (hazard ratio [HR], 0.96; 95% confidence interval [CI], 0.94–0.98; P < 0.001) at median 6 y follow-up. This relationship remained significant (HR, 0.94; 95% CI, 0.91–0.97; P < 0.001) even after adjusting for other known prognostic factors (age, estrogen receptor status, cancer treatment, metastatic spread, cortisol levels, and depression). Secondary hypotheses were also supported (after adjusting for baseline prognostic factors) showing that less wake after sleep onset (HR, 0.41; 95% CI, 0.25–0.67; P < 0.001), fewer wake episodes, (HR, 0.93; 95% CI, 0.88–0.98; P = 0.007); and shorter wake episode duration (HR, 0.29; 95% CI, 0.14–0.58; P < 0.001) also contributed to reductions in overall mortality.
Conclusions:
These findings show that better sleep efficiency and less sleep disruption are significant independent prognostic factors in women with advanced breast cancer. Further research is needed to determine whether treating sleep disruption with cognitive behavioral and/or pharmacologic therapy could improve survival in women with advanced breast cancer.
Citation:
Palesh O, Aldridge-Gerry A, Zeitzer JM, Koopman C, Neri E, Giese-Davis J, Jo B, Kraemer H, Nouriani B, Spiegel D. Actigraphy-measured sleep disruption as a predictor of survival among women with advanced breast cancer. SLEEP 2014;37(5):837-842.
Keywords: advanced breast cancer, sleep disruption, sleep efficacy, survival, wake after sleep onset
INTRODUCTION
Sleep disruption is a prevalent problem among cancer patients and survivors that adversely affects quality of life. Breast cancer is typically a disease of aging that is relatively rarely seen in younger women. Aging is associated with an increase in comorbid conditions including cancer diagnoses; cancer treatment causes side effects and late effects that place women at increased risk of cardiovascular events, diabetes, obesity, and secondary cancers. Metastatic disease remains the prevalent cause of death in women in whom metastatic breast cancer has been diagnosed. Eighty percent of patients undergoing chemo-therapy and more than two thirds of women with metastatic breast cancer experience poor sleep,1,2 which is associated with numerous negative physical and mental health outcomes.3,4 Sleep duration and disruption have been associated with all-cause mortality.5,6 The precise relationship, however, is likely complex because some studies have shown that short sleep duration is implicated in earlier mortality,7 whereas others suggest the relationship is quadratic, in which both shorter and longer sleep duration than normal are predictive of shorter survival.8
Although precipitating factors for the development of sleep disruption include stress associated with the diagnosis of cancer and its treatment9,10 and cytotoxic chemotherapy and its side effects,11 the mechanism behind the development of sleep disruption and how it might affect survival is not well understood.4 Prior studies found relationships between tumor characteristics and spread, receptor status, and treatment with survival in metastatic breast cancer.12 Other studies have shown that circadian dysregulation measured by flattened cortisol rhythms13 and depression14 are independent prognostic factors for survival in metastatic breast cancer. However, no studies to date have prospectively examined the effect of sleep duration and disruption on survival, specifically among patients with cancer in whom sleep disruption is so common. Given the data seen in the general population, our a priori hypothesis was that less disturbance of sleep among women with advanced breast cancer would lead to longer overall survival.
METHODS
Participants
Women included in this study had metastatic or locally advanced breast cancer, were at least 45 years or older, had scores of at least 70% on the Karnofsky Performance Scale, resided in the greater San Francisco Bay area, and were proficient in English. Women were excluded from the study if they reported other active cancers within the past 10 years (other than basal cell or squamous cell carcinomas of the skin or in situ cancer of the cervix), had positive supraclavicular lymph nodes as the only metastatic lesion, had a diagnosis of a concurrent medical condition likely to influence short-term survival, used corticosteroid(s) within the preceding month, or reported a history of major psychiatric illness requiring hospitalization or current alcohol or drug abuse/dependence.
Participants were recruited between 2002 and 2004 directly through oncologists at Stanford University Medical Center, study advertisements, and word of mouth. We screened 221 women: 25 were not eligible, 90 were eligible but declined participation, one had an accident that prevented participation, six did not complete actigraphy (see next paragraphs), and two died before the study began. We were, therefore, able to examine the data from 97 women (74.2% had metastatic, 18.6% had locally advanced, and 7.2% had either metastatic or locally advanced disease). The research protocol was approved by the Stanford Institutional Review Board and conformed to the principles described in the Declaration of Helsinki, and all participants provided written informed consent.
Demographic Data
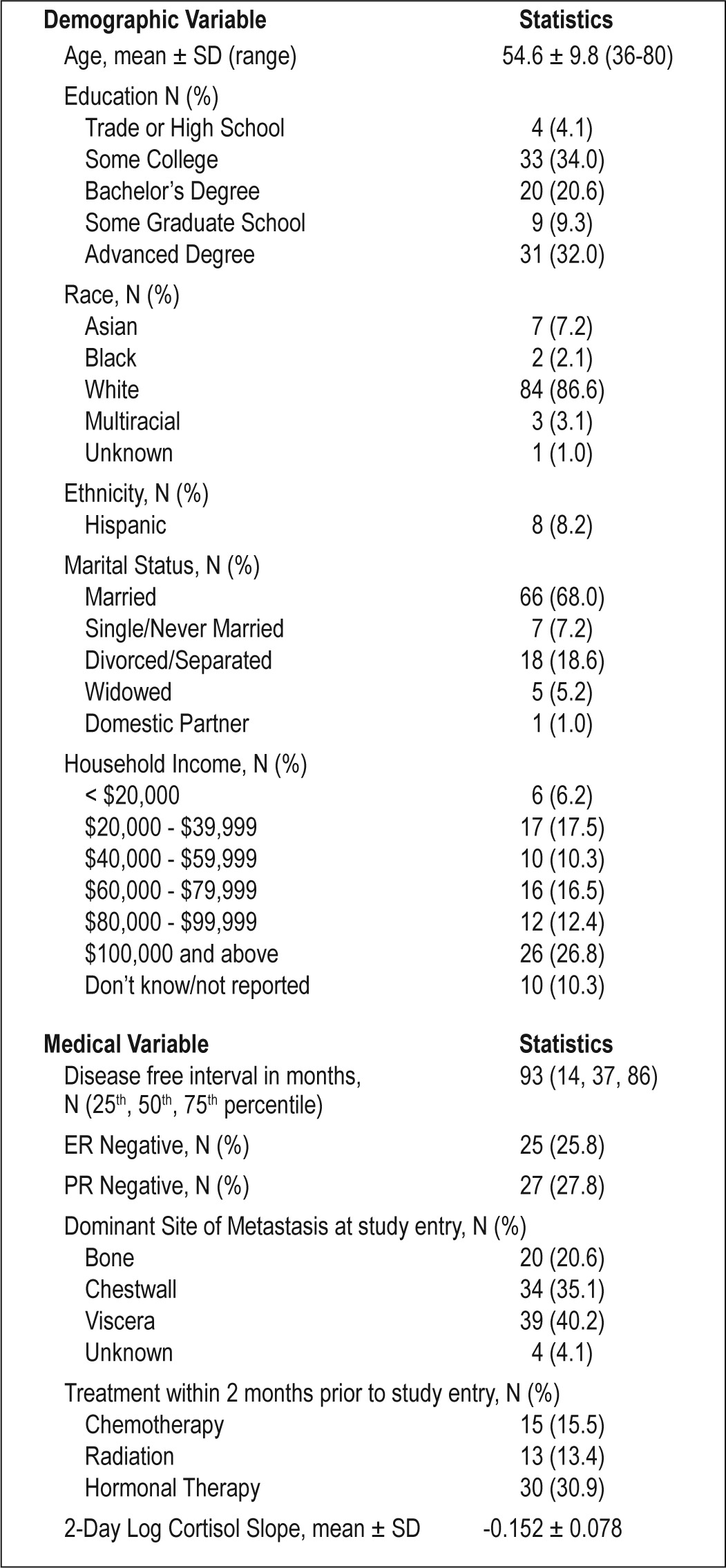
Demographic information and medical history are summarized in Table 1. At the baseline assessment, 41 participants (42.3%) took antidepressants and 19 (19.6%) took medication to treat sleep problems. Of those taking medication to treat their sleep problems, 10 took medication that acted on benzodiazepine receptors, 7 took over-the-counter sleep aids or supplements, one took an antidepressant, and one took a prescription antihistamine.
Table 1.
Descriptive statistics for demographic and medical variables in advanced breast cancer participants (N = 97)
Measures
Sleep Measurement
A wrist actigraph (Micro Mini-Motionlogger, Ambulatory Monitoring Systems, Ardsley, NY) was worn for 3 consecutive days to monitor daily activity and nocturnal sleep. An actigraph is a device that detects and records arm movements through the use of a triaxial accelerometer; data from actigraphs commonly are used as a proxy for assessment of sleep and wake. The actigraphs were set to record movement in 60-sec epochs using a proprietary Proportional Integrating Mode (PIM) that captures information about the amplitude of movement. Actigraphy data were scored as sleep or wake time using the University of California San Diego (UCSD)15 PIM Sleep Scoring Algorithm available in the ACT Millennium (beta v3.8.8.9) and Action4 (v1.13) software (Ambulatory Monitoring Systems). While wearing the actigraph, each participant completed a daily log to document the time she went to bed, time at awakening, and any periods during which the actigraph was removed.
Sleep duration (time in bed, TIB) was calculated using a combination method of sleep logs and actigraphy such that the time in and out of bed, as recorded on the sleep logs, were confirmed or adjusted based on the actigraphy data. We found our subjective report of TIB that we had participants record when they went to bed and when they got out of bed to be highly correlated and statistically essentially the same measure with a variable that used latency of TIB (self-report) and sleep onset (actigraphy) in combination. Sleep latency was calculated using both self-report logs combined with sleep onset assessed with actigraphy.
Sleep efficiency (SE) was calculated as the ratio of TST, (which was derived from actigraph) to TST plus wake after sleep onset (WASO). The number and average length of nocturnal wake episodes and total duration of WASO also were derived from actigraph data. In addition, we calculated SE using either self-report and/or latency estimates (SE = TST / [TST + WASO + Latency] and SE = TST / TIB) and found these variables to be highly correlated (rhos of 0.98 and 0.98). The analyses showed that the three calculations are essentially the same. Thus, the variability of sleep latency has a minimal effect on the measure of SE, and given the more intuitive nature of SE and that WASO is not the same thing (given high variability in TIB), we are justified in using it.
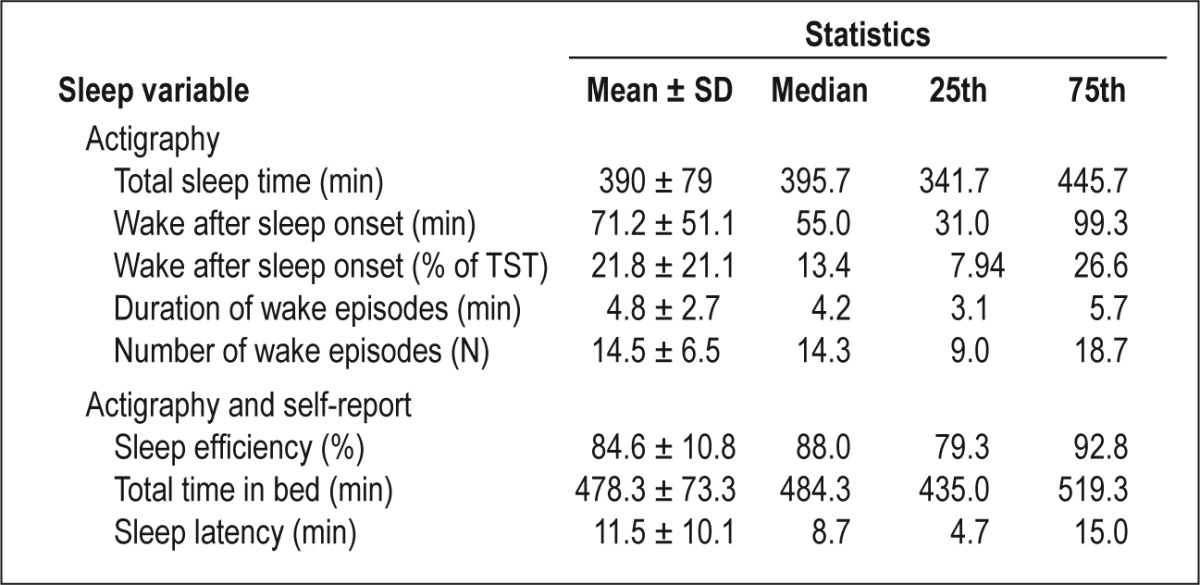
For non-normally distributed variables (latency, TIB, WASO, Average Wake Episode), we used appropriate transformations. To check on the reliability of key variables, we computed intra-class correlations (ICC). For actigraphy-derived TST over 3 days, ICC was 0.52 (95% confidence interval [CI] = 0.48–0.69). For WASO, ICC over 3 days was 0.58 (95% CI = 0.55– 0.74), suggesting moderate reliability. Descriptive statistics for sleep indices are presented in Table 2.
Table 2.
Sleep among women with advanced breast cancer over 3 nights (N = 97)
Covariates
Participants and their oncologists completed a medical status questionnaire that assessed their disease status, medication, and treatment. Participants completed the Beck Depression Inventory to assess depression. Concomitant with the actigraphy data collection, participants were asked to collect their saliva five times a day for 2 days (waking, wake +30 min, 12:00, 17:00, and 21:00). Saliva samples were immediately frozen (-20°C) and transported to a -70 °C freezer at the end of the 2 days of collection. Saliva samples were stored at -70°C before centrifugation and duplicate assessment of salivary cortisol concentrations using a luminescence immunoassay (Immuno-Biological Laboratories, Hamburg, Germany) was conducted. Assay sensitivity was 0.015 μg/dL. Intra-assay variations on low, medium, and high controls averaged 2.78%, 10.45%, and 4.79%, respectively. The mean values of the low, medium, and high controls were 0.054 μg/dL, 0.228 μg/dL, and 0.863 μg/dL, respectively. Interassay coefficients of variation for low, medium, and high controls were 10.9%, 10.5%, and 5.5%, respectively.16
Survival Data
Participants, their caregivers, their treating physicians, and the Social Security Death Index (SSDI) were contacted to obtain survivorship data used in the current study. We extracted cause of death from either the death certificate or the participant's medical records. The last death occurred in February 2012.
We located the death certificates for 55 of 58 participants who died. For one of the three patients for whom we could not locate a death certificate, we found an obituary listing, with the information matching the demographic data that she provided during enrollment. The obituary said the patient “battled breast cancer” but did not cite the specific cause of death. The other two participants were found in the Social Security database. Of 55 death certificates, 33 listed metastatic breast cancer as primary cause of death. The remaining certificates listed respiratory arrest (n = 9), cardiac/cardiopulmonary arrest (n = 4), hepatic failure (n = 3), renal failure (n = 1), pneumonia (n = 1), septic shock (n = 1), and disseminated intravascular coagulopathy (n = 1). With the exception of one participant whose cardiac arrest was attributed to lung cancer, all of these causes of death (n = 19) were listed as secondary to breast metastatic disease. There were two participants whose cause of death was listed as colon cancer (n = 1), and another participant's cause of death was listed as brain metastases secondary to lymphoma. Thus, of 55 death certificates, 33 listed metastatic breast cancer as the primary source of death, 19 causes of death were directly attributable to metastatic breast cancer disease progression, and three were attributed to other cancers (lung, colon, and lymphoma).
Data Analyses
Cox Proportional Hazards Models were used to analyze the survival data. We conducted Cox regression analysis with and without adjusting for the known prognostic factors of survival (age, estrogen receptor status, treatment [chemotherapy, radiation therapy, hormonal treatment], dominant site of metastatic disease spread [viscera, bone, and chest wall], depression, and cortisol levels). Data are presented as mean ± standard deviation and/or median with interquartile distance as appropriate. All analyses were conducted using SAS (Version 9.3, SAS Institute Inc., Cary, NC). The SAS PROC PHREG procedure was used for Cox Proportional Hazards modeling. Analyses for Graph 2 were conducted using OriginPro 8.0 software (Origin-Labs, Northampton, MA).
RESULTS
Primary Analysis
Sleep Efficiency (SE)
Higher SE in women with advanced breast cancer was significantly associated with lower mortality over the ensuing 6 years (N = 97, hazard ratio [HR], 0.96; 95% CI, 0.94–0.98; P < 0.001). Using the Cox model parameter estimate, we found that a 10% increase in SE reduced the hazard of subsequent mortality by 32%.
Because 85% SE is the generally used cutoff score indicating good sleep,17 we also conducted the analysis using the 85% cutoff rate. We found that mean survival for the efficient sleeper group of 85% or higher (n = 60) was 68.9 ± 4.0 months compared with 33.2 ± 4.3 months for the poor SE group (< 85%; n = 37; log-rank test, χ = 23.13; P < 0.001; Figure 1).
Figure 1.
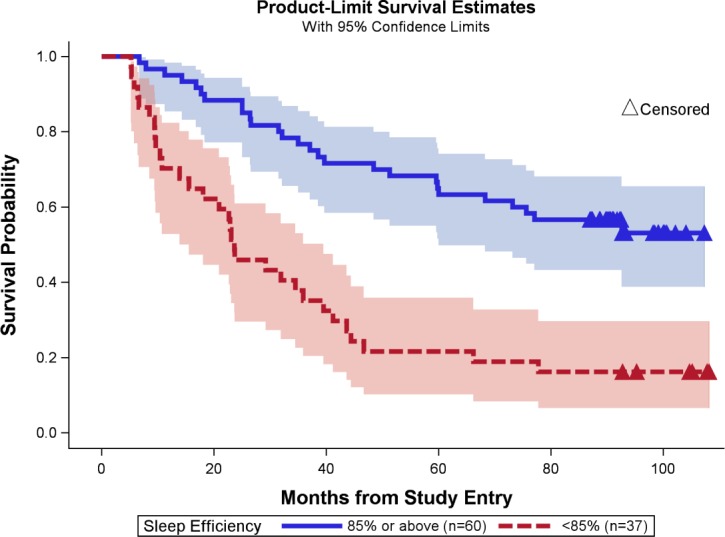
Sleep efficiency and survival in women with advanced breast cancer. Sleep efficiency of 85% or above is shown in dark blue, associated 95% confidence interval is shown in light blue. Sleep efficiency of less than 85% is shown in red and the associated confidence interval is shown in light red. Censored data are shown in triangles.
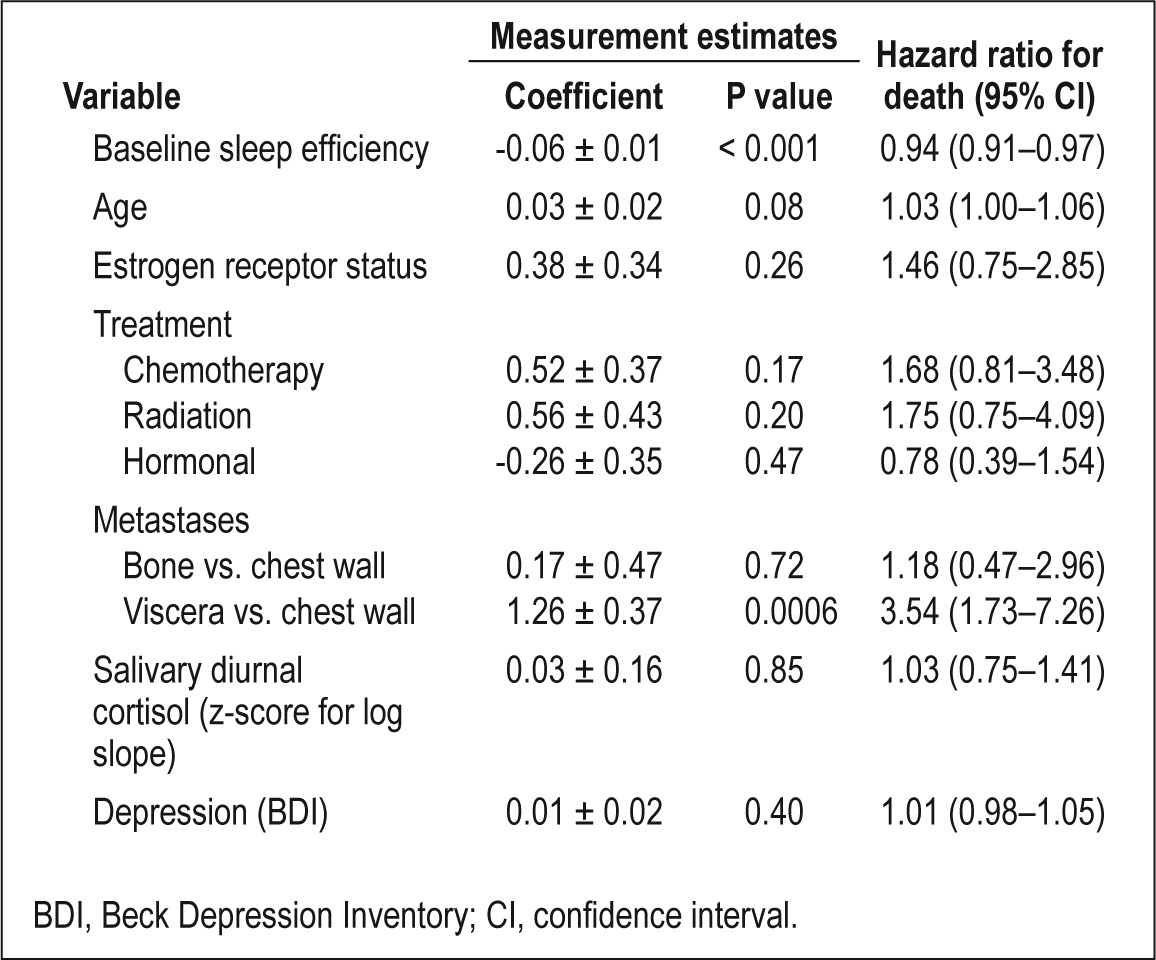
The effect of SE on overall survival remained after adjusting for baseline prognostic factors (age, estrogen receptor status, treatments received, metastatic disease spread [dominant site], depression, and cortisol levels), as shown in Table 3 in a multivariate Cox Proportional Hazards analysis (HR, 0.94; CI, 0.91– 0.97, P < 0.001).
Table 3.
Sleep and subsequent hazard of death in advanced breast cancer: multivariate analysis
Sleep Duration (TIB)
We found no linear or quadratic (where low or high sleep duration would be detrimental to survival) association between sleep duration and survival. As shown in Figure 2, SE rather than total sleep time (TST) was associated with longer subsequent overall survival. Shorter time in bed (TIB) was not predictive of mortality (HR, 0.99; 95% CI, 0.97–1.00; P = 0.09).
Figure 2.
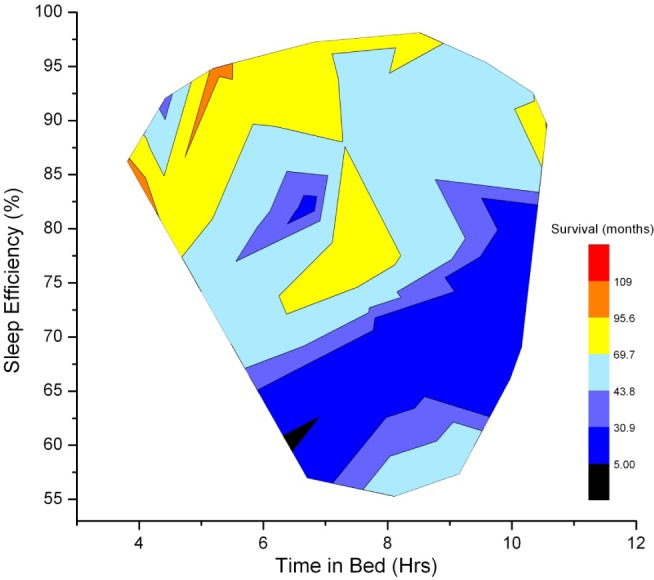
The relationships among time in bed (x-axis), percent sleep efficiency (y-axis), and survival. Heat map shows the relationships among time in bed (x-axis), percent sleep efficiency (y-axis), and survival (color coded, see legend). As the statistical analyses indicate, individuals with poorer sleep efficiency have shorter survival times (color range from black to blue). This heat map indicates that it is good sleep efficiency, rather than time in bed, that is associated with longer survival times (color range from yellow to red). Graphing boundaries were set by the extant data range, and contours were smoothed with a thin plate spline parameter of 0.01 (OriginPro 8.0, OriginLabs, Northampton MA).
Exploratory Analyses Using Other Actigraphy or Self-Report Derived Sleep Variables
Wake after Sleep Onset (WASO in min)
Lower WASO measured in minutes in participants with advanced breast cancer was significantly associated with lower mortality over the ensuing 6 years (HR, 0.48; 95% CI, 0.33–0.71; P < 0.0001). The effect of WASO on overall mortality was maintained after adjusting for baseline prognostic factors as shown in a multivariate analysis (HR, 0.41; 95% CI, 0.25–0.67, P < 0.001).
Wake after Sleep Onset (WASO % of TST)
Lower WASO as a percentage of TST in participants with advanced breast cancer was significantly associated with lower mortality over the ensuing 6 years (HR, 0.98; 95% CI, 0.97–0.99, P < 0.001). The effect of WASO (% of TST) on overall mortality was maintained after adjusting for baseline prognostic factors as shown in a multivariate analysis (HR, 0.97; 95% CI, 0.96–0.98, P < 0.001).
Mean Number of Wake Episodes
Fewer wake episodes were significantly associated with lower mortality over 6 years (HR, 0.94; 95% CI, 0.90–0.98; P = 0.003). The effect of the mean number of wake episodes on overall mortality was also maintained after adjusting for baseline prognostic factors in multivariate analysis (HR, 0.93; 95% CI = 0.88–0.98; P = 0.007).
Mean Wake Episode Duration
Shorter wake episodes during each nocturnal awakening were also predictive of lower mortality over 6 years (HR, 0.46; 95% CI, 0.29–0.75; P = 0.002). The effect of mean wake episode duration on overall mortality was maintained after adjusting for baseline prognostic factors as shown in a multivariate analysis (HR, 0.29; 95% CI, 0.14–0.58; P < 0.001).
Sleep Latency
Shorter sleep latency was not associated with lower mortality over 6 years (HR, 0.89; 95% CI, 0.75–1.06; P = 0.19).
DISCUSSION
We found sleep disruption as measured by actigraphy and sleep diary to be an independent predictor of overall survival among women with advanced breast cancer, even after accounting for other medical and demographic risk factors. Examination of the survival curves over the median 6-year period available (Figure 1) indicates that the finding was not due to acute, pre-terminal sleep disruption, but rather sleep disruption at baseline predicted the subsequent survival outcome at the median follow-up of 6 years, as the survival curves continued to diverge. Overall, these participants with advanced breast cancer spent about 8 hours in bed but slept for only about 6.5 hours, yielding an average SE of 84.6%, which is slightly below the clinical cutoff of 85% for clinically significant sleep disruption.17 Approximately 38% (n = 37) of the participants in our sample with especially poor SE (< 85%) had markedly shorter overall survival. Most of the participants in this study (52 of 58) died of their advanced breast cancer disease rather than of comorbid health conditions.
This is the first study to demonstrate the long-term detrimental effects of objectively quantified sleep on survival in women with advanced cancer. The literature is relatively sparse regarding actigraphy in early stages of breast cancer. A recent study by Garrett et al.18 conducted in 78 women in whom primary breast cancer was diagnosed (metastatic disease was excluded) reported that their actigraphy-measured SE was 85.17%, SE = 1.58, TST = 7.02 h, and TIB = 8.30 h. Wright et al.19 reported actigraphy-measured SE to be 86.3%, and TST to be 5.68 h in 39 women with a newly diagnosed disease who were scheduled to undergo surgery for breast cancer. Other studies that used actigraphy typically recruited women with some level of sleep problems (e.g., insomnia), thus making SE, TST, and TIB difficult to compare with our study.20 Thus, our findings on sleep disturbance in women with advanced breast cancer are either on par or slightly more concerning than those that have been reported in the literature in women with early breast cancer. Less WASO, lower mean number of wake episodes, and shorter wake duration all had lower HRs (more protective) compared with SE. It is unclear why might this be, but it is possible that the causes of reductions in these wake-specific variables have a different mechanism of action that affects overall mortality more directly. In this study, sleep duration was not a significant predictor of survival. Although this is a surprising finding, it suggests that given adequate amount of time in bed (average in our sample of 8 hours), it might be the quality rather than the quantity of sleep that matters for survival in this population.
How might sleep disruption be related to breast cancer progression? We accounted for factors that, in previous work, have been directly associated with shorter survival, including depression, cortisol level, and medical variables.12–14 Many of these, notably depression and cortisol level, also have shown significant effects on sleep.2,21,22 However, even after adjusting for these relationships, less disrupted sleep remained a significant predictor of lower mortality. Although not directly examined in this study, it is possible that sleep disruption leads to diminished immune function4 or impaired hormonal stress responses21 that are more directly responsible for the decrease in survival. It is notable that in this study, it is the quality of sleep rather than the quantity of sleep that predicts survival. This is in accordance with the cognitive behavioral treatment approach for sleep disruption that encourages patients to trade sleep duration for sleep quality via sleep restriction (restricting the number of hours spent in bed based on the formula that takes their actual sleep time into account), allowing patients to build up homeostatic pressure that enables better sleep.
Our study is, of course, not without limitations. Our objective measure of sleep (actigraphy) is a proxy measurement, with polysomnography as the gold standard. The generalizability of the study is also reduced by our sample's size and composition of predominantly non-Hispanic white women with relatively high education and socioeconomic status. Further, data were collected only for participants with advanced breast cancer; thus we cannot generalize our results to women in earlier stages of breast cancer, or to other individuals, including men, with advanced cancers.
CONCLUSION
Our findings reveal that women with advanced breast cancer who experience less objective sleep disturbance, specifically more efficient sleep (> 85%) have significantly lower mortality. Our data suggest that an improvement in SE by 10% among women with severe sleep disruption could potentially lead to a 32% increase in survival time. However, it will be important to conduct future research using a prospective controlled experimental design to further clarify the effect of improving SE on survival. There is a need for research on the effect of evidence-based sleep interventions–such as cognitive behavioral therapy for insomnia–to improve survival among patients with advanced breast cancer experiencing sleep disruption. Meanwhile, the current findings suggest the importance of careful assessment of sleep among women with advanced breast cancer and referral of those with sleep disturbance for appropriate treatment.
DISCLOSURE STATEMENT
This was not an industry supported study. This research was supported by NIA/NCI Program Project AG18784, CA118567, NCI RO1CA118567, and NCI K07CA132916. Additional support was received from NIH 5 M01 RR000070-44. There was no off-label or investigational use. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Sandra Sephton, PhD; Seymour Levine, PhD; and Dirk Hellhammer, PhD for consultation regarding cortisol measurement. We also thank Manijeh Parineh for recruitment, Mark Rothkopf for sample management, and Ben Varasteh and the General Clinical Research Center at Stanford for conducting cortisol assays.
REFERENCES
- 1.Palesh OG, Roscoe JA, Mustian KM, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. J Clin Oncol. 2010;28:292–8. doi: 10.1200/JCO.2009.22.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palesh OG, Collie K, Batiuchok D, et al. A longitudinal study of depression, pain, and stress as predictors of sleep disturbance among women with metastatic breast cancer. Biol Psychol. 2007;75:37–44. doi: 10.1016/j.biopsycho.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrington CB, Hansen JA, Moskowitz M, Todd BL, Feuerstein M. It's not over when it's over: long-term symptoms in cancer survivors—a systematic review. Int J Psychiatry Med. 2010;40:163–81. doi: 10.2190/PM.40.2.c. [DOI] [PubMed] [Google Scholar]
- 4.Palesh O, Peppone L, Innominato PF, et al. Prevalence, putative mechanisms, and current management of sleep problems during chemotherapy for cancer. Nat Sci Sleep. 2012;4:151–62. doi: 10.2147/NSS.S18895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen-Mansfield J, Perach R. Sleep duration, nap habits, and mortality in older persons. Sleep. 2012;35:1003–9. doi: 10.5665/sleep.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33:585–92. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 8.Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res. 2009;18:148–58. doi: 10.1111/j.1365-2869.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- 9.Costanzo ES, Stawski RS, Ryff CD, Coe CL, Almeida DM. Cancer survivors' responses to daily stressors: implications for quality of life. Health Psychol. 2012;31:360–70. doi: 10.1037/a0027018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spiegel D. Mind matters in cancer survival. Psychooncology. 2012;21:588–93. doi: 10.1002/pon.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cleeland CS, Allen JD, Roberts SA, et al. Reducing the toxicity of cancer therapy: recognizing needs, taking action. Nat Rev Clin Oncol. 2012;9:471–8. doi: 10.1038/nrclinonc.2012.99. [DOI] [PubMed] [Google Scholar]
- 12.Nicolini A, Giardino R, Carpi A, et al. Metastatic breast cancer: an updating. Biomed Pharmacother. 2006;60:548–56. doi: 10.1016/j.biopha.2006.07.086. [DOI] [PubMed] [Google Scholar]
- 13.Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- 14.Giese-Davis J, Collie K, Rancourt KM, Neri E, Kraemer HC, Spiegel D. Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: a secondary analysis. J Clin Oncol. 2011;29:413–20. doi: 10.1200/JCO.2010.28.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jean-Louis G, Kripke DF, Mason WJ, Elliott JA, Youngstedt SD. Sleep estimation from wrist movement quantified by different actigraphic modalities. J Neurosci Methods. 2001;105:185–91. doi: 10.1016/s0165-0270(00)00364-2. [DOI] [PubMed] [Google Scholar]
- 16.Spiegel D, Giese-Davis J, Taylor CB, Kraemer H. Stress sensitivity in metastatic breast cancer: analysis of hypothalamic-pituitary-adrenal axis function. Psychoneuroendocrinology. 2006;31:1231–44. doi: 10.1016/j.psyneuen.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487–504. [PMC free article] [PubMed] [Google Scholar]
- 18.Garrett K, Dhruva A, Koetters T, et al. Differences in sleep disturbance and fatigue between patients with breast and prostate cancer at the initiation of radiation therapy. J Pain Symptom Manage. 2011;42:239–50. doi: 10.1016/j.jpainsymman.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright CE, Schnur JB, Montgomery GH, Bovbjerg DH. Psychological factors associated with poor sleep prior to breast surgery: An exploratory study. Behav Med. 2010;36:85–91. doi: 10.1080/08964280903521305. [DOI] [PubMed] [Google Scholar]
- 20.Mustian KM, Sprod LK, Janelsins M, et al. Multicenter, randomized controlled trial of yoga for sleep quality among cancer survivors. J Clin Oncol. 2013;31:3233–41. doi: 10.1200/JCO.2012.43.7707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palesh O, Zeitzer JM, Conrad A, et al. Vagal regulation, cortisol, and sleep disruption in women with metastatic breast cancer. J Clin Sleep Med. 2008;4:441–9. [PMC free article] [PubMed] [Google Scholar]
- 22.Palesh OG, Mustian KM, Peppone LJ, et al. Impact of paroxetine on sleep problems in 426 cancer patients receiving chemotherapy: a trial from the University of Rochester Cancer Center Community Clinical Oncology Program. Sleep Med. 2012;13:1184–90. doi: 10.1016/j.sleep.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


