Abstract
Study Objectives:
Obesity alters the therapeutic window of sedative/hypnotic drugs and increases the probability of respiratory complications. The current experiments used an established rodent model of obesity to test the hypothesis that the sedative/hypnotic drugs eszopiclone and dexmedetomidine alter ventilation differentially in obese rats compared with lean/fit rats.
Design:
This study used a within-groups/between-groups experimental design.
Setting:
University of Michigan.
Participants:
Experiments were conducted using lean/fit rats (n = 21) and obese rats (n = 21) that have features of metabolic syndrome.
Interventions:
Breathing was measured with whole-body plethysmography after systemic administration of vehicle (control), the nonbenzodiazepine, benzodiazepine site agonist eszopiclone, or the alpha-2 adrenergic receptor agonist dexmedetomidine.
Measurements and Results:
Data were analyzed using two-way analysis of variance and appropriate post hoc comparisons. At baseline, the obese/metabolic syndrome rats had increased respiratory rates (21.6%), lower tidal volumes/body weight (-24.1%), and no differences in minute ventilation compared to lean/fit rats. In the obese rats, respiratory rate was decreased by dexmedetomidine (-29%), but not eszopiclone. In the lean and the obese rats, eszopiclone decreased tidal volume (-12%). Both sedative/hypnotic drugs caused a greater decrease in minute ventilation in the obese (-26.3%) than lean (-18%) rats. Inspiratory flow rate (VT / TI) of the obese rats was decreased by dexmedetomidine (-10.6%) and eszopiclone (-18%). Duty cycle (TI / TTOT) in both rat lines was decreased by dexmedetomidine (-16.5%) but not by eszopiclone.
Conclusions:
Dexmedetomidine, in contrast to eszopiclone, decreased minute ventilation in the obese/metabolic syndrome rats by depressing both duty cycle and inspiratory flow rate. The results show for the first time that the obese phenotype differentially modulates the respiratory effects of eszopiclone and dexmedetomidine. These differences in breathing are consistent with previously documented differences in sleep between lean/fit and obese rats. These findings also encourage future studies of obese/metabolic syndrome rats that quantify the effect of sedative/hypnotic drugs on respiratory mechanics as well as hypoxic and hypercapnic ventilatory responses. Continued findings of favorable homology between obese humans and rodents will support the interpretation that these obese rats offer a unique animal model for mechanistic studies.
Citation:
Filbey WA, Sanford DT, Baghdoyan HA, Koch LG, Britton SL, Lydic R. Eszopiclone and dexmedetomidine depress ventilation in obese rats with features of metabolic syndrome. SLEEP 2014;37(5):871-880.
Keywords: sedatives, alpha-2 adrenergic agonist, non-benzodiazepine, benzodiazepine site agonist, metabolic syndrome
INTRODUCTION
Obesity can alter the therapeutic window and incidence of unwanted side effects caused by sedative/hypnotic drugs. Administering sedative/hypnotic drugs based on total body weight can lead to overdose in the morbidly obese patient.1 It has been estimated that 42% of American adults will be obese by 2030.2 Obesity increases the risk for respiratory problems caused by sedative/hypnotic drugs,3–5 especially among obese patients with concomitant sleep apnea.6,7 Sleep disordered breathing is a common comorbidity in obese patients, yet it is generally under-diagnosed, even at the time of surgery.6
We have previously documented significant differences in sleep8 between lean/fit rats and obese rats with features of metabolic syndrome.9,10 The goal of the current study was to test the hypothesis that eszopiclone and dexmedetomidine alter ventilation differentially in obese/metabolic syndrome and lean/fit rats. The benzodiazepine binding site agonist eszopiclone and the alpha-2 receptor agonist dexmedetomidine were chosen specifically because they have different mechanisms of action.
Eszopiclone is a nonbenzodiazepine hypnotic drug that functions as a partial allosteric modulator at the benzodiazepine-binding site of the gamma-aminobutyric acid A (GABAA) receptor. Eszopiclone received Food and Drug Administration approval in 2004 for treatment of insomnia in patients 18 y of age and older. The efficacy and safety of eszopiclone have been supported by randomized, double-blind, placebo-controlled studies.11,12
Dexmedetomidine is an alpha-2 adrenergic receptor agonist that received Food and Drug Administration approval in 1999 as a sedative for intubated and mechanically ventilated patients. Off-label experience with dexmedetomidine,13 and subsequent studies14,15 documenting safe sedation of nonintubated patients, led to approval of dexmedetomidine for additional indications. Dexmedetomidine causes sedation and analgesia,16 with minimal effects on ventilation in healthy, normal-weight individuals.16,17 The finding that these sedative/hypnotic drugs, acting via two different mechanisms, caused respiratory depression supports the interpretation that the respiratory depression was attributed to the obesity/metabolic syndrome phenotype. Earlier results from these studies have been reported in abstracts.18,19
MATERIALS AND METHODS
Animals
Rats were housed in a 12-h light/12-h dark cycle (lights on from 08:00 to 20:00) with ad libitum access to food and water. Procedures were reviewed and approved by the University of Michigan Committee on the Use and Care of Animals. Every phase of this study adhered to the Guide for the Care and Use of Laboratory Animals, 8th Edition (National Academy of Sciences Press, Washington, DC, 2011).
The rat model of obesity with features of metabolic syndrome used in this study was created by a divergent breeding program that selected for differences in intrinsic (i.e., not trained) aerobic capacity.20 After 28 generations, rats bred for high aerobic capacity (HCR or lean/fit) ran an average of 500% farther than their low aerobic capacity (LCR or obese/metabolic syndrome) counterparts.21 In the current study, lean/fit rats (n = 21) had a mean ± standard deviation (SD) body weight of 393 ± 41 g. Obese rats (n = 21) had a mean ± SD weight of 495 ± 92 g. When compared with lean/fit rats, the rats with poor aerobic fitness showed a higher prevalence of obesity,9 cardiovascular disease,10 and insulin resistance, with significantly decreased longevity.22 These data support the interpretation that the rats with poor aerobic fitness provide a rodent model of obesity with features of metabolic syndrome.9
Behavioral Conditioning and Quantification of Ventilation
Rats were habituated to the handling required for intraperitoneal (i.p.) injections, and conditioned to being placed unrestrained in whole-body plethysmograph chambers (Buxco Electronics, Inc., Wilmington, NC). The 1-h conditioning trials were repeated daily for 1 week prior to injections. Each chamber held one rat. A continuous flow of room air (1 L/min) through the plethysmograph chamber maintained constant temperature and humidity (Figure 1A). Breathing variables were recorded using BioSystem XA software (Buxco Engineering, Wilmington, NC). Dependent measures included respiratory rate (f), tidal volume (VT), minute ventilation (VE = f × VT), inspiratory time (TI), expiratory time (TE), total respiratory cycle time (TTOT), duty cycle (TI / TTOT), and inspiratory flow rate (VT / TI).
Figure 1.
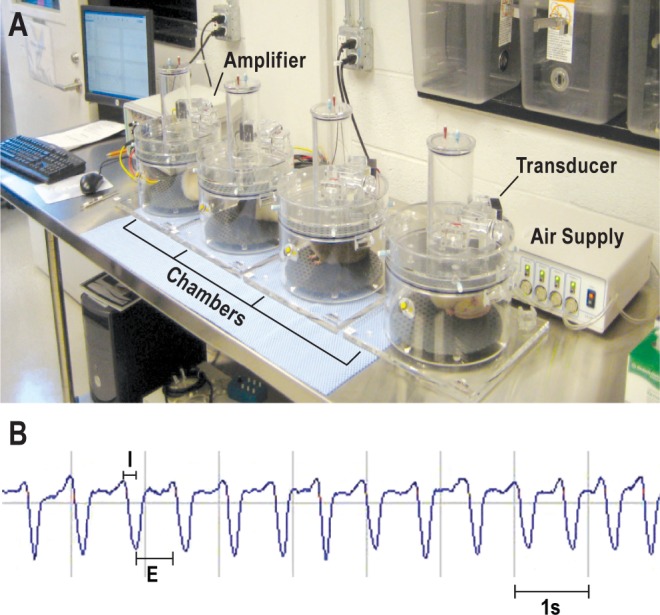
Plethysmographic measures of ventilatory behavior. (A) One unrestrained rat was placed in each of the four whole-body plethysmograph chambers for measuring breathing. Changes in air pressure and volume were detected by transducers, then amplified and digitized for computer analysis (left). Behind the rightmost chamber, the instrument labeled “Air Supply” provided fresh air to chambers at a rate of 1 L/min. (B) Screen shot of plethysmographic output from one chamber. System software calculated respiratory rate, tidal volume, minute ventilation, respiratory timing, duty cycle and inspiratory flow rate. E, expiratory phase; I, inspiratory phase.
Dexmedetomidine Administration
Before measuring breathing, initial experiments determined the dose of dexmedetomidine (0.01 mg/kg) that induced sedation with an average latency of 9.1 min, as judged by the loss of righting response. This response parallels clinical data indicating that a 0.01 mg/kg loading dose infused intravenously provides an onset of procedural sedation within 10 to 15 min. The pH of the injected solution was adjusted to 6.0 ± 0.2. Based on these preliminary studies, a dose of 0.01 mg/kg dexmedetomidine dissolved in saline was used to sedate obese (n = 10) and lean/fit (n = 10) rats. Following a 15-min acclimation period inside the plethysmograph chambers (Figure 1A), rats were injected with vehicle or dexmedetomidine and breathing was recorded (Figure 1B) for 15 min.
Eszopiclone Administration
Doses of eszopiclone were based on previous studies showing that systemic administration of 3 mg/kg significantly increased electroencephalographic delta power, eliminated rapid eye movement sleep, and significantly decreased acetylcholine release in the pontine reticular formation of Sprague-Dawley rats.23 Additional data indicated that systemic administration of 3 mg/kg and 10 mg/kg eszopiclone to Sprague-Dawley rats caused a significant increase in nonrapid eye movement sleep.24 On separate days, drug naïve obese (n = 11) and lean/fit (n = 11) rats received an i.p. injection of vehicle (50 mM acetate buffer) or eszopiclone (3 mg/kg or 10 mg/kg). The pH of each solution was adjusted to 5.0 ± 0.2. The higher dose of eszopiclone caused sedation of longer duration; thus, breathing was recorded for 48 min.
Statistical Analysis
Analyses were conducted by a statistician at the University of Michigan Center for Statistical Consultation and Research using Statistical Analysis System v9.1.3 (SAS Institute, Inc., Cary, NC). Measures of breathing were averaged for each rat and data were expressed as animal means. Each of the respiratory variables was analyzed using two-way analysis of variance (ANOVA) for repeated measures followed by post hoc t-tests with Bonferroni correction. These analyses made it possible to evaluate statistically significant alterations in breathing as a function of drug, rat line (lean vs. obese), and rat line-by-drug interactions. Volume-based measures of breathing were normalized per 100 g body weight (animal weight / 100 × dependent measure).
RESULTS
Dexmedetomidine Depressed Breathing More in Obese than Lean/Fit Rats
Figure 2 summarizes respiratory frequency, tidal volume, and minute ventilation in the two lines of rats, measured after administration of vehicle (0 mg/kg) and sedative/hypnotic drugs. The top row of Figure 2 displays measures of breathing after administering saline or dexmedetomidine in obese and lean/fit rats. Figure 2A shows respiratory frequency in the obese and lean/ fit lines. Two-way ANOVA revealed a significant rat line effect (P = 0.0021), drug effect (P < 0.0001), and rat line-by-drug interaction (P = 0.0022) on rate of breathing. Post hoc t-tests showed that frequency was significantly greater (P = 0.0005) in the obese rats after saline injections than in the lean/fit rats (asterisk in Figure 2A). Dexmedetomidine significantly decreased (P < 0.0001) respiratory rate in the obese (number sign in Figure 2A) but not in the lean/fit rats. After dexmedetomidine, there was no significant difference in respiratory rate between obese and lean/fit rats. Dexmedetomidine-induced changes in tidal volumes are summarized in Figure 2B. Two-way ANOVA revealed a significant (P = 0.0004) rat line effect, and no drug effect, on tidal volume. Post hoc t-test showed that tidal volume (per 100 g body weight) of the obese rats was significantly smaller (P = 0.0051) than observed in the lean/fit rats after vehicle injections (asterisk in Figure 2B). Dexmedetomidine did not significantly alter tidal volume. Figure 2C shows that there were no significant differences in minute ventilation between obese and lean/fit rats following saline injections. Two-way ANOVA revealed a rat line effect approaching significance (P = 0.064) and a significant (P < 0.0001) drug effect on minute ventilation. Compared to measures collected after saline injection, dexmedetomidine caused a significant depression in minute ventilation for both obese (P = 0.0026; number sign in Figure 2C) and lean/ fit (P = 0.0234; plus sign in Figure 2C) lines.
Figure 2.
Effects of sedative/hypnotic drugs on respiratory rate, tidal volume, and minute ventilation in obese/metabolic syndrome and lean/fit rats. A-C plot breathing data after control and dexmedetomidine (DMED) injections. (A) Respiratory frequency in obese rats was greater than that measured in the lean/fit rats following control injections. Dexmedetomidine depressed respiratory rate in the obese rats. (B) Tidal volume was smaller in the obese than lean/fit rats after control injections. (C) Dexmedetomidine decreased minute ventilation in both the obese and lean/fit rats. D-F illustrate breathing variables measured before and after administration of eszopiclone (ESZ). (D) Obese rats had a greater respiratory rate than lean/fit rats after control (0 mg/kg) injections. (E) Tidal volume was smaller in obese than lean/fit rats after receiving control and eszopiclone (3 and 10 mg/kg). Eszopiclone (10 mg/kg) decreased tidal volume in both rat lines. (F) Eszopiclone (3 and 10 mg/kg) decreased minute ventilation in the obese rats. G-I display percent changes from control for measures of frequency, tidal volume, and minute ventilation. (G) Dexmedetomidine and eszopiclone (10 mg/kg) caused a greater decrease in respiratory rate in the obese rats than the lean/fit rats. (H) There were no differences in percent change of tidal volume between rat lines. (I) Eszopiclone (3 and 10 mg/kg) caused a greater decrease in minute ventilation in the obese than lean/fit rats. An asterisk indicates statistically significant differences between lines or columns. The number sign indicates significant drug effects in the obese rats. The plus sign indicates significant drug effects in lean/fit rats.
Eszopiclone Depressed Breathing More in Obese than Lean/Fit Rats
The middle row in Figure 2 plots measures of breathing after administering vehicle (0 mg/kg) and eszopiclone (3 mg/kg or 10 mg/kg) to the obese and lean/fit rats. Figure 2D illustrates that respiratory frequency was significantly greater (P = 0.025) in the obese than the lean/fit rats after administration of vehicle. Two-way ANOVA revealed a significant (P = 0.007) rat line-by-drug interaction for both doses of eszopiclone. Neither dose of eszopiclone significantly altered rate of breathing in the obese or lean/fit rats. When comparing frequency between lines after equivalent doses of eszopiclone, respiratory frequency in the obese rats did not differ significantly from breathing rate in the lean/fit rats. Figure 2E displays body weight-adjusted tidal volume for the obese and lean/fit rats. Two-way ANOVA revealed significant rat line (P = 0.0002) and drug (P < 0.0001) effects of eszopiclone on tidal volume. Obese rats had a significantly smaller tidal volume following injections of vehicle (P = 0.0012), 3 mg/kg eszopiclone (P = 0.0091), and 10 mg/kg eszopiclone (P = 0.0448) than did the lean/fit rats (asterisks in Figure 2E). Compared with the vehicle injections, eszopiclone (10 mg/kg) significantly decreased tidal volume in both the obese (P = 0.0287; number sign in Figure 2E) and the lean/fit (P < 0.0001; plus sign in Figure 2E) rats. Figure 2F shows that minute ventilation was not significantly different between rat lines following vehicle injections. Two-way ANOVA revealed a rat line effect approaching significance (P = 0.0567), a significant (P = 0.0056) concentration effect of eszopiclone, and a significant (P = 0.0025) rat line-by-eszopiclone interaction on minute ventilation. Minute ventilation in obese rats was significantly decreased by 3 mg/kg (P = 0.0289; number sign in Figure 2F) and 10 mg/kg (P = 0.0003; number sign in Figure 2F) eszopiclone relative to vehicle. Minute ventilation in the lean/fit rats was not significantly altered by either dose of eszopiclone.
Effect Size Comparison of Respiratory Depression Caused by Sedative/Hypnotics
The bar graphs in the bottom row of Figure 2 compare the percent change from control for measures of respiratory frequency, tidal volume, and minute ventilation between obese and lean/fit rats caused by dexmedetomidine and eszopiclone. Figure 2G shows that dexmedetomidine caused a significantly greater decrease (P < 0.0041) in respiratory frequency in obese rats (-29%) than in lean/fit rats (-11%). Eszopiclone (10 mg/kg) caused a significantly greater decrease (P = 0.0068) in respiratory rate of obese rats than in lean/fit rats. Figure 2H demonstrates no significant differences between obese and lean/fit rats in tidal volume caused by dexmedetomidine or eszopiclone. Figure 2I shows that dexmedetomidine did not cause significant differences in minute ventilation as a function of rat line. Post hoc analysis showed that eszopiclone caused a significantly greater decrease in minute ventilation in the obese than in the lean/fit rats at the 3 mg/kg (P = 0.0219) and 10 mg/kg (P = 0.0019) doses.
Dexmedetomidine and Eszopiclone Altered Respiratory Cycle Timing
Data were also obtained for measures of inspiratory time (TI), expiratory time (TE), and the duration of the total respiratory cycle (TTOT). The raw data (not shown) were analyzed using the same methods as described for Figures 2A-F in the previous section. Figure 3 summarizes the percent change data for duration of inspiratory, expiratory, and total respiratory time. Figure 3A demonstrates that dexmedetomidine caused a significantly greater (P = 0.0023) percent increase in inspiratory time of obese (15.5%) than lean/fit (-4.9%) rats. Eszopiclone (3 mg/kg) caused a significantly greater (P = 0.0224) percent increase in inspiratory duration of obese (17.3%) than lean/fit (-7.7%) rats. Eszopiclone (10 mg/kg) also caused a significantly (P = 0.0112) greater percent increase in inspiratory duration of obese (17.6%) than lean/fit (-10.1%) rats. Figure 3B shows that dexmedetomidine caused a significantly greater (P = 0.0225) percent increase in expiratory time in the obese (52.1%) than in the lean/fit (26.4%) rats. Administering eszopiclone (3 mg/kg) caused a significantly greater (P = 0.0211) percent increase in expiratory time in obese rats (17.2%) than in lean/fit (-12.7%). Figure 3C illustrates the significantly greater (P = 0.003) percent increase in total respiratory time caused by dexmedetomidine in the obese rats (38.9%) compared to the lean/fit rats (15.5%). Effect size for total respiratory time differed significantly (P = 0.0122) between rat lines after administering eszopiclone (10 mg/kg). Total respiratory cycle time was increased by eszopiclone (10 mg/kg) in obese rats (9.4%) and decreased in the lean/fit rats (-16.6%).
Figure 3.

Respiratory timing in obese and lean/fit rats plotted as a function of percent change from vehicle control. (A) Percent change of inspiratory time was greater in the obese than lean/fit rats following administration of dexmedetomidine (DMED) and eszopiclone (ESZ, 3 and 10 mg/kg). (B) Expiratory time was increased more in the obese than the lean/fit rats following administration of dexmedetomidine and eszopiclone (3 mg/kg). (C) Total respiratory cycle time was increased more in the obese than the lean/fit line following dexmedetomidine and eszopiclone (10 mg/kg). The asterisk indicates significant differences between rat lines.
Duty Cycle (TI / TTOT) was Altered by Dexmedetomidine and Eszopiclone
Duty cycle reflects the proportion of the total respiratory timing spent in the active, inspiratory phase of respiration. Figure 4 plots the effect of sedative/hypnotic drug injections on the duty cycle of obese and lean/fit rats. Figure 4A displays the dexmedetomidine-induced decrease in the duty cycle. Two-way ANOVA revealed a significant (P < 0.0001) drug effect on the duty cycle of obese and lean/fit rats caused by dexmedetomidine. There was no difference in duty cycle between obese and lean/fit rats after injection of saline. Post hoc analysis showed that dexmedetomidine caused a significant decrease in the duty cycle of the lean/fit (P = 0.0005; plus sign in Figure 4A) and the obese (P = 0.0006; number sign in Figure 4A) rats.
Figure 4.
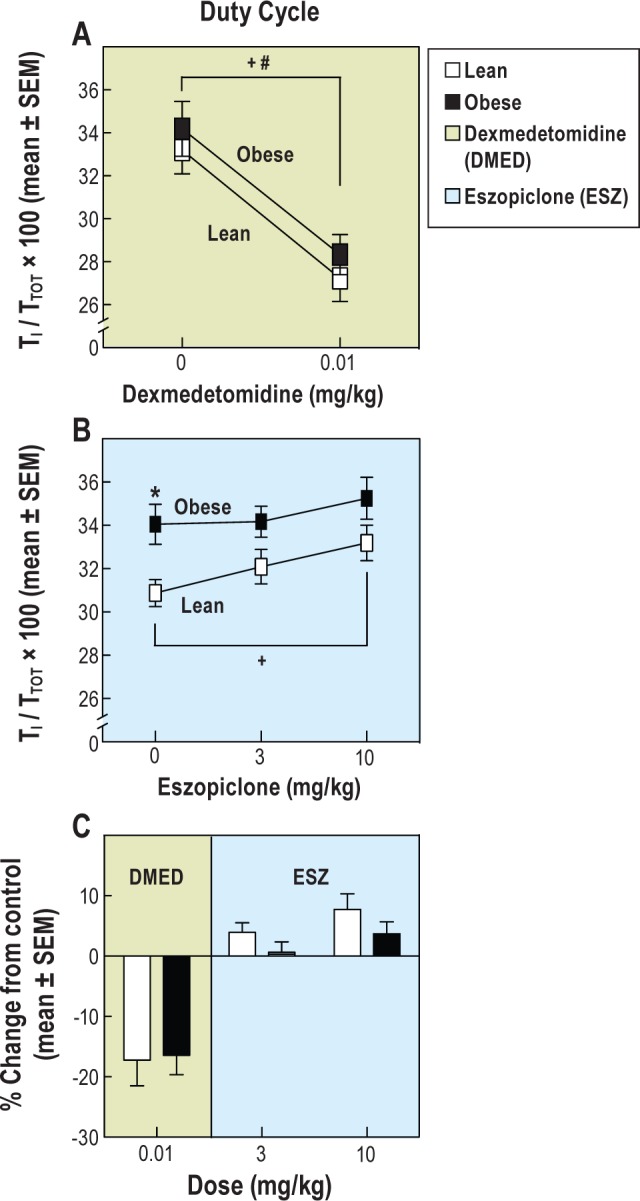
Duty cycle (TI / TTOT) of obese/metabolic syndrome and lean/fit rats before and after sedative/hypnotic drug administration. (A) Dexmedetomidine (DMED) decreased the duty cycle in obese and lean/ fit rats. (B) Obese rats had a greater duty cycle than lean/fit rats before receiving control injections. Eszopiclone (ESZ, 10 mg/kg) caused an increased duty cycle in lean/fit rats compared to control injections. (C) Dexmedetomidine decreased the duty cycle to the same extent in the obese and lean/fit rats. The duty cycle was not significantly altered by eszopiclone (3 and 10 mg/kg). The asterisk indicates significant differences between lines. The number sign indicates significant drug effects in the obese rats. The plus sign indicates significant drug effects in lean/fit rats.
Eszopiclone caused a significant rat line (P = 0.0249) and drug (P = 0.0035) effect on duty cycle (Figure 4B). The duty cycle of obese rats was significantly greater (P = 0.0089) than that of lean/fit rats at baseline (asterisk in Figure 4B). Compared with controls (0 mg/kg), eszopiclone (10 mg/kg) caused a significant increase (P = 0.0018) in duty cycle of lean/fit rats (plus sign in Figure 4B).
Figure 4C summarizes the percent change data for the duty cycle in obese and lean/fit rats following administration of dexmedetomidine and eszopiclone. The duty cycle was depressed to the same extent by dexmedetomidine in both rat lines. Percent change in the duty cycle was not significantly different between obese and lean/fit rats following either dose of eszopiclone.
Dexmedetomidine and Eszopiclone Decreased Inspiratory Flow Rate (VT / TI) in Obese Rats
Measures of inspiratory flow rate (mL/s/100 g body weight) provide an indirect index of ventilatory drive. Figure 5 summarizes inspiratory flow rate for both rat lines after administration of vehicle, dexmedetomidine, and eszopiclone. Obese and lean/fit rats expressed similar levels of inspiratory flow after saline injection (Figure 5A). Two-way ANOVA showed a significant (P = 0.0117) rat line effect on inspiratory flow rate. Post hoc t-test revealed that compared with controls, dexmedetomidine caused a significant decrease in the inspiratory flow rate of obese (P = 0.017; number sign in Figure 5A) but not lean/fit rats. Inspiratory flow rate was significantly less (P = 0.0108) in the obese than lean/fit rats after dexmedetomidine (asterisk in Figure 5A).
Figure 5.

Inspiratory flow rate (VT / TI) was decreased by sedative/ hypnotic drugs. (A) Dexmedetomidine (DMED) caused a decrease in inspiratory flow rate in the obese rats. Obese rats had a decreased inspiratory flow rate caused by dexmedetomidine (0.01 mg/kg) compared with lean/fit rats. (B) Eszopiclone (ESZ, 3 and 10 mg/kg) decreased inspiratory flow rate in the obese rats relative to their control injections. Eszopiclone did not significantly alter inspiratory flow rate in the lean/fit rats. (C) The percent change in inspiratory flow rate was similar in both rat lines. The percent change in inspiratory flow rate was significantly decreased in the obese rats by eszopiclone (3 and 10 mg/kg). The asterisk indicates significant differences between lines. The number sign indicates significant drug effects in the obese rats. The plus sign indicates significant drug effects in lean/fit rats.
Figure 5B shows similar measures of inspiratory flow rate between obese and lean/fit rats after vehicle injections. Two-way ANOVA revealed that eszopiclone caused a significant rat line (P = 0.0012) and drug (P = 0.0003) effect, as well as a significant rat line-by-drug interaction (P = 0.0052) on inspiratory flow rate. Post hoc t-test revealed significant decreases in inspiratory flow rate relative to 0 mg/kg of obese rats caused by 3 mg/kg (P = 0.0026) and 10 mg/kg (P < 0.0001) eszopiclone (number sign in Figure 5B). Inspiratory flow rate in lean/fit rats was not significantly altered by eszopiclone. Inspiratory flow rate in the obese rats was significantly less than in the lean/ fit rats after 3 mg/kg (P = 0.0002) and 10 mg/kg (P = 0.0003) eszopiclone (asterisk in Figure 5B).
Figure 5C summarizes percent change in inspiratory flow rate of obese and lean/fit rats. Effect size on inspiratory flow rate was not significantly different between rat lines after administering dexmedetomidine. Post hoc t-test showed that eszopiclone (3 mg/kg) caused a significantly greater (P = 0.0135) depression of inspiratory flow rate in obese rats (-12.2 %) compared with the lean/fit rats (5.1%). Eszopiclone (10 mg/kg) also caused a significantly greater (P = 0.0043) depression of inspiratory flow rate in the obese (-21.1%) than in the lean/fit rats (-3.3%).
DISCUSSION
This study addressed a gap in knowledge concerning the influence of obesity on changes in breathing caused by sedative/ hypnotic drugs. Baseline measures consistently showed that the obese/metabolic syndrome rats had elevated respiratory rates, lower tidal volumes, and no differences in minute ventilation compared with lean/fit rats. In the obese/metabolic syndrome rats, dexmedetomidine, but not eszopiclone, decreased respiratory rate. In the lean/fit rats and the obese/metabolic syndrome rats, eszopiclone decreased tidal volume. Both drugs caused a greater decrease in minute ventilation in the obese/metabolic syndrome rats than lean/fit rats. Inspiratory flow rate (VT / TI) of the obese rats was decreased by both drugs. Duty cycle (TI / TTOT) in both rat lines was decreased by dexmedetomidine but not by eszopiclone. These results are discussed in relation to (1) limitations involving the barometric measurement of ventilation; (2) potential clinical relevance; and (3) rodent models of polygenic human disease.
Whole Body Plethysmography: Strengths and Limitations
The barometric method has long been appreciated as a tool for measuring ventilation from unrestrained animals25 and newborn infants.26 Complex thermodynamic interactions between pressure, water vapor, and temperature, however, limit measurement accuracy.27–29 For example, tidal volume estimation is critically dependent on chamber integrity, chamber size/configuration, the reference chamber, choice of bias airflow through the chamber, and the temperature of both the chamber and the animal. The absolute accuracy of tidal volume is suspect even with the use of calibration volumes created by injecting a syringe of air. There can be differences in the pressure wave that are detected as tidal volume created by spontaneous breathing or by a given calibrating syringe at a given calibrating frequency. At best, differences of up to 10% may occur between tidal volume measured by plethysmograph and directly from an anesthetized animal. Correlation coefficients between direct and plethysmographic measures remain high enough (> 0.75) to show correlations even under the more extreme conditions. In the current study, unanesthetized animals were tested under environmentally similar circumstances (chamber size, material, temperature, and humidity being equal). We chose not to computationally adjust for temperature changes for several reasons. First, such an approach would need to correct for humidity, yet these corrections would introduce other uncertainties. Second, computational adjustments would also imply a level of measurement accuracy that cannot be justified. The foregoing limitations apply to tidal volume, whereas frequency is an excellent signal that is not altered as a signal by body temperature or chamber temperature and humidity. All limitations apply to the control conditions as well as the drug conditions and to the comparisons of the two lines of rat. Therefore, the limitations of plethysmography do not confound the current results.
Potential Clinical Relevance
Obese “patients are a major challenge for all anaesthetic techniques” and “trends in population obesity mean that the number of patients at risk is almost certain to increase.”30 Current dosing and safety guidelines for dexmedetomidine are based on studies of healthy individuals of normal weight. Available safety and efficacy data14,15 do not address the use of dexmedetomidine in obese patients. We are unaware of data determining whether dexmedetomidine and eszopiclone shift obese patients with apnea to a steeper portion of the oxygen saturation curve, which would result in greater desaturation during sedation. Thus, little is known about the effects of eszopiclone or dexmedetomidine on respiratory control in obese patients.
Clinical data show that “the propensity for respiratory depression during general anaesthesia and sleep are related.”31 This association, and the recognized importance of sleep for anesthesia care,32,33 encouraged the selection of a sleeping aid, eszopiclone, for investigating the effect of obesity on changes in breathing.
Studies of the prevalence with which sedatives are prescribed show that approximately 8% of the general population is prescribed sedating medications, whereas 30% of patients with previously undiagnosed obstructive sleep apnea are prescribed sedatives.34 Data from 2011 confirm the increased use of sedatives by obese individuals in comparison with the general population.35 The factors linking obesity with increased use of sedative/hypnotic drugs are not understood. This lack of understanding contributes to the fact that “specialty societies and government agencies have published 12 conflicting sets of guidelines for sedation.”36 Clinical practice guidelines for sedation cannot recommend specific drugs for specific types of patients.37 We are aware of no systematic studies of sedative/hypnotic-induced respiratory depression in nonintubated obese humans compared to normal-weight controls. Therefore, the current results provide the first comparison of breathing in obese versus lean/fit rats before and after systemic administration of two different sedative/hypnotic drugs that are widely used in clinical practice.
Sedative/Hypnotics Differentially Alter Ventilatory Behavior in Obese/Metabolic Syndrome and Lean/Fit Rats
Increases in body weight cause increased metabolic rate and body oxygen consumption that increase frequency of breathing and breathlessness.38 The respiratory rate of the obese rats was greater than that of lean/fit rats in control (0 mg/kg) recordings (Figures 2A and 2D). In the obese rats, dexmedetomidine, but not eszopiclone, significantly decreased respiratory rate. Obese/ metabolic syndrome rats have increased abdominal obesity that is homologous to this characteristic of human metabolic syndrome.9 The distribution of eszopiclone and dexmedetomidine into body fat of the lean versus obese rats has not been quantified. It is known, however, that the low aerobic capacity, obese rats have disrupted sleep8 and that sleep fragmentation alters transcriptional regulation of visceral adipocytes in mice.39 Characterizing potential differences in drug distribution will be an important study for future comparison of sedative effects on arousal and breathing. Reduced tidal volume occurs in obese humans,40 and the obese rats had a significantly smaller tidal volume per 100 g body weight (Figures 2B and 2E) than the lean/fit rats. Tidal volume was significantly decreased only by the highest dose of eszopiclone (Figure 2E). Minute ventilation was decreased more in the obese than the lean/fit rats by both dexmedetomidine and eszopiclone (Figure 2I). Studies using systemic drug administration cannot directly address the mechanisms underlying the observed changes in respiratory rate, tidal volume, or minute ventilation. Whole-body plethysmography can, however, provide novel data characterizing the effects of sedatives on breathing.41 Previous findings42–44 emphasize that rat line, more than obesity, exerts a significant effect on ventilatory behavior. Data emphasizing the robust effect of rat line may account, in part, for some species-specific differences in ventilatory behavior.45 The previous and current findings support the interpretation that unidentified genetic factors are potent regulators of ventilatory behavior. Genetics also modulate obesity that results from increased energy intake and/or decreased energy expenditure. The divide for obesity between the low aerobic capacity and high aerobic capacity rats is multifaceted. For example, the high aerobic capacity rats are lean, in part, because they have more spontaneous motor activity than the low aerobic capacity rats.46 Obesity within the context of metabolic syndrome is undoubtedly influenced by mitochrondial dysfunction in the low aerobic capacity rats and in humans. The ubiquitious influence of mitochondrial function47 means that mitochondrial impairment is intertwined at some level with essentiallly all complex disease traits.
Direct comparison of the current results with previous studies that measured the effects of dexmedetomidine on breathing in rat are complicated by the significant differences in dexmedetomidine dose and by differences in the rat strain studied.48 These earlier studies used Wistar rats and concluded that dexmedetomidine administered i.p. (250 μg/kg/h) followed by intravenous infusion (0.5 μg/kg/h) caused respiratory depression.48 The previous study48 and the current results indicate that dexmedetomidine decreased minute ventilation primarily by decreasing respiratory frequency, rather than by altering tidal volume (Figures 2G-2I).
Measures of respiratory cycle timing (Figure 3) show that dexmedetomidine and eszopiclone significantly slowed inspiration (Figure 3A), expiration (Figure 3B), and total respiratory cycle time (Figure 3C) more in the obese/metabolic syndrome rats than in the lean/fit rats. These temporal measures made it possible to calculate the ratio of inspiratory time to total respiratory cycle time (TI / TTOT) (Figure 4), and inspiratory flow rate (Figure 5). The duty cycle (TI / TTOT) provides an index of the amount of time the inspiratory musculature is activated, whereas inspiratory flow rate (mL/s/100 g body weight) reflects ventilatory drive. The obese/metabolic syndrome and lean/fit rats had similar TI / TTOT ratios at baseline, and TI / TTOT was significantly decreased by dexmedetomidine in both rat lines (Figure 4A). The eszopiclone experiments revealed baseline (0 mg/kg) differences in TI / TTOT due to rat line (asterisk in Figure 4B) and, in the lean/fit rats, due to eszopiclone dose (plus sign in Figure 4B). Both dexmedetomidine (Figure 5A) and eszopiclone (Figure 5B) significantly depressed inspiratory flow rate in the obese/ metabolic syndrome rats. Minute ventilation (Figure 2) is the product of inspiratory flow rate and duty cycle.49 Therefore, the data in Figures 4 and 5 support the interpretation that, in contrast to eszopiclone, dexmedetomidine decreased minute ventilation in the obese/metabolic syndrome rats (Figure 2) by depressing both duty cycle and ventilatory drive.
Rodent Models of Complex Disease Traits and the Aerobic Hypothesis
Studies of rodent ventilatory behavior permit unique insights into the genetic50 and neuronal51 regulation of ventilation. Animal models of human disease continue to advance understanding of underlying cellular and molecular mechanisms.52 Both eszopiclone and dexmedetomidine caused a greater decrease in minute ventilation in the obese/metabolic syndrome rats than in the lean/fit rats. The similar effects on breathing stand in contrast to the two different mechanisms of drug action. Eszopiclone is a nonbenzodiazepine that acts as a partial allosteric modulator at the benzodiazepine-binding site of the GABAA receptor. Dexmedetomidine is an alpha-2 adrenergic receptor agonist. The respiratory depression caused by eszopiclone and dexmedetomidine may reflect decreases in central respiratory drive mediated by GABAA and alpha-2 adrenergic receptors.53,54 Although dexmedetomidine and eszopiclone have different mechanisms of action, both drugs diminish arousal and the wakefulness stimulus for breathing.55 Thus, alterations in arousal by these two sedative/hypnotic drugs may be the primary mechanism through which these drugs depressed breathing. Administering eszopiclone to patients with obstructive sleep apnea caused an increase in the respiratory arousal threshold and decreased the apnea/hypopnea index (AHI).56 Sleep apnea is a complex disease, however, and Figure 3 in the study by Eckert et al.56 shows that eszopiclone increased the AHI in approximately 24% of the patients.
The rat lines used in the current study satisfy four criteria for evaluating the adequate use of nonhuman animals that seek to model complex human disease. First, the animals must emulate an important clinical phenotype. The two lines of rats used in this study were from generation 28 and 29 of a divergent breeding program that selected for differences in intrinsic (not trained) aerobic capacity. Rats that were bred to have high aerobic capacity have a lower body weight and are healthy. In contrast, rats with low aerobic capacity are obese and have multiple features of metabolic syndrome.9 Second, the animals should exhibit traits that are polygenic in origin and, therefore, translatable to human disease. Aerobic capacity is polygenic, and low maximal oxygen consumption, along with low exercise endurance,57 are stronger predictors of human morbidity and mortality than risk factors such as diabetes or hypertension.58 Third, the animal model should exhibit reliable responses to health challenges. Compared with lean/fit rats, the obese/metabolic syndrome rats have diminished longevity,22 increased susceptibility to cardiac ventricular fibrillation,59 hepatic steatosis,60 and reduced capacity for oxidation of lipids in skeletal muscle,61 the liver,60 and the heart.9 Finally, the animal model should enable hypothesis testing that is guided by an established theoretical construct. The theoretical foundation of this selective breeding program is the aerobic hypothesis postulating that variation in aerobic energy metabolism is a central mechanistic determinant of the divide between health and disease.10,20 The current finding that both eszopiclone and dexmedetomidine caused a greater decrease in minute ventilation in obese/metabolic syndrome rats than in lean/fit rats is consistent with the aerobic hypothesis.
The obese/metabolic syndrome rats have been shown to differ from the lean/fit rats on measures of sleep,8 acute pain threshold,62 minimum alveolar concentration for isoflurane,63 recovery time from neuropathic pain,8 and postoperative cognitive impairment.64 The results encourage future studies of respiratory mechanics, respiratory muscle electromyographic activity, and central respiratory drive in obese/metabolic syndrome rats before and after sedative/hypnotic administration.
DISCLOSURE STATEMENT
This was not an industry supported study. Supported by grants HL65272 (RL) and MH45361 (HAB) from the National Institutes of Health, Bethesda, MD, and by the Department of Anesthesiology, University of Michigan, Ann Arbor, MI. The rat model system was funded by the National Center for Research Resources grant R24 RR017718 and is currently supported by the Office of Research Infrastructure Programs/OD grant R24OD010950 (to Dr. Koch and Dr. Britton) from the National Institutes of Health. National Institutes of Health grants RO1 DK077200 and R01GM104194 also supported Dr. Britton. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
For expert assistance, the authors thank Mary A. Norat, BS, from the Department of Anesthesiology, Kathy Welch, MA, MPH, Sha Jiang, BS, and Adam Saulles, PharmD, University of Michigan, Ann Arbor, MI.
REFERENCES
- 1.Ingrande J, Lemmens HJ. Dose adjustment of anaesthetics in the morbidly obese. Br J Anaesth. 2010;105:i16–23. doi: 10.1093/bja/aeq312. [DOI] [PubMed] [Google Scholar]
- 2.Finkelstein EA, Khavjou OA, Thompson H, et al. Obesity and severe obesity forecasts through 2030. Am J Prev Med. 2012;42:563–70. doi: 10.1016/j.amepre.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 3.Biring MS, Lewis MI, Liu JT, Mohsenifar Z. Pulmonary physiologic changes of morbid obesity. Am J Med Sci. 1999;318:293–7. doi: 10.1097/00000441-199911000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Candiotti KA, Sharma S, Shankar R. Obesity, obstructive sleep apnoea, and diabetes mellitus: anaesthetic implications. Br J Anaesth. 2009;103:i23–30. doi: 10.1093/bja/aep294. [DOI] [PubMed] [Google Scholar]
- 5.Casati A, Putzu M. Anesthesia in the obese patient: pharmacokinetic considerations. J Clin Anesth. 2005;17:134–45. doi: 10.1016/j.jclinane.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Finkel KJ, Searleman AC, Tymkew H, et al. Prevalence of undiagnosed obstructive sleep apnea among adult surgical patients in an academic medical center. Sleep Med. 2009;10:753–8. doi: 10.1016/j.sleep.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Ankichetty S, Wong J, Chung F. A systematic review of the effects of sedatives and anesthetics in patients with obstructive sleep apnea. J Anaesthesiol Clin Pharmacol. 2011;27:447–58. doi: 10.4103/0970-9185.86574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muncey AR, Saulles AR, Koch LG, Britton SL, Baghdoyan HA, Lydic R. Disrupted sleep and delayed recovery from chronic peripheral neuropathy are distinct phenotypes in a rat model of metabolic syndrome. Anesthesiology. 2010;113:1176–85. doi: 10.1097/ALN.0b013e3181f56248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wisloff U, Najjar SM, Ellingsen O, et al. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science. 2005;307:418–20. doi: 10.1126/science.1108177. [DOI] [PubMed] [Google Scholar]
- 10.Koch LG, Britton SL, Wisloff U. A rat model system to study complex disease risks, fitness, aging, and longevity. Trends Cardiovasc Med. 2012;22:29–34. doi: 10.1016/j.tcm.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krystal AD, Walsh JK, Laska E, et al. Sustained efficacy of eszopiclone over 6 months of nightly treatment: results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep. 2003;26:793–9. doi: 10.1093/sleep/26.7.793. [DOI] [PubMed] [Google Scholar]
- 12.Roth T, Walsh JK, Krystal A, Wessel T, Roehrs TA. An evaluation of the efficacy and safety of eszopiclone over 12 months in patients with chronic primary insomnia. Sleep Med. 2005;6:487–95. doi: 10.1016/j.sleep.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Wunsch H, Kahn JM, Kramer AA, et al. Dexmedetomidine in the care of critically ill patients from 2001 to 2007: An observational cohort study. Anesthesiology. 2010;113:386–94. doi: 10.1097/ALN.0b013e3181e74116. [DOI] [PubMed] [Google Scholar]
- 14.Bergese SD, Candiotti KA, Bokesch PM, Zura A, Wisemandle W, Bekker AY. A phase IIIb, randomized, double-blind, placebo-controlled, multicenter study evaluating the safety and efficacy of dexmedetomidine for sedation during awake fiberoptic intubation. Am J Ther. 2010;17:586–95. doi: 10.1097/MJT.0b013e3181d69072. [DOI] [PubMed] [Google Scholar]
- 15.Candiotti KA, Bergese SD, Bokesch PM, Feldman MA, Wisemandle W, Bekker AY. Monitored anesthesia care with dexmedetomidine: A prospective, randomized, double-blind multicenter trial. Anesth Analg. 2010;110:47–56. doi: 10.1213/ane.0b013e3181ae0856. [DOI] [PubMed] [Google Scholar]
- 16.Arcangeli A, D'Alo C, Gaspari R. Dexmedetomidine use in general anaesthesia. Curr Drug Targets. 2009;10:687–95. doi: 10.2174/138945009788982423. [DOI] [PubMed] [Google Scholar]
- 17.Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382–94. doi: 10.1097/00000542-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Sanford DT, Filbey WA, Koch LG, Britton SL, Baghdoyan HA, Lydic R. Systemic administration of eszopiclone depresses ventilation in a rat model of metabolic syndrome. Sleep. 2012;35:A44. doi: 10.5665/sleep.3650. (Abstract Supplement) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saulles AR, Filbey WA, Koch LG, Britton SL, Baghdoyan HA, Lydic R. Dexmedetomidine depresses breathing in a rat model of metabolic syndrome. Soc Neurosci Abstracts. 2011:502.01. [Google Scholar]
- 20.Koch LG, Britton SL. Aerobic metabolism underlies complexity and capacity. J Physiol. 2008;586:83–95. doi: 10.1113/jphysiol.2007.144709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirkton SD, Howlett RA, Gonzalez NC, et al. Continued artificial selection for running endurance in rats is associated with improved lung function. J Appl Physiol. 2009;106:1810–8. doi: 10.1152/japplphysiol.90419.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koch LG, Kemi OJ, Qi N, et al. Intrinsic aerobic capacity sets a divide for aging and longevity. Circ Res. 2011;109:1162–72. doi: 10.1161/CIRCRESAHA.111.253807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hambrecht-Wiedbusch VS, Gauthier EA, Baghdoyan HA, Lydic R. Benzodiazepine receptor agonists cause drug-specific and state-specific alterations in EEG power and acetylcholine release in rat pontine reticular formation. Sleep. 2010;33:909–18. doi: 10.1093/sleep/33.7.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar S, Alam MN, Rai S, Bashir T, McGinty D, Szymusiak R. Central nervous system sites of the sleep promoting effects of eszopiclone in rats. Neuroscience. 2011;181:67–78. doi: 10.1016/j.neuroscience.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Chapin JL. Ventilatory response of the unrestrained and unanesthetized hamster to CO2. Am J Physiol. 1954;179:146–8. doi: 10.1152/ajplegacy.1954.179.1.146. [DOI] [PubMed] [Google Scholar]
- 26.Drorbaugh JE, Fenn WO. The barometric method for measuring ventilation in newborn infants. Pediatrics. 1955;16:81–6. [PubMed] [Google Scholar]
- 27.Epstein MAF, Epstein RA. A theoretical analysis of barometric method for measurement of tidal volume. Respir Physiol. 1978;32:105–20. doi: 10.1016/0034-5687(78)90103-2. [DOI] [PubMed] [Google Scholar]
- 28.Jacky JP. Barometric measurement of tidal volume: effects of pattern and nasal temperature. J Appl Physiol. 1980;49:319–25. doi: 10.1152/jappl.1980.49.2.319. [DOI] [PubMed] [Google Scholar]
- 29.Chaui-Berlinck JG, Bicudo JEPW. The signal in total-body plethysmograph: errors due to adiabatic-isothermic differences. Respir Physiol. 1998;113:259–70. doi: 10.1016/s0034-5687(98)00060-7. [DOI] [PubMed] [Google Scholar]
- 30.Cook TM, Woodall N, Frerk C. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: anaesthesia. Br J Anaesth. 2011;106:617–31. doi: 10.1093/bja/aer058. [DOI] [PubMed] [Google Scholar]
- 31.Eastwood PR, Szollosi I, Platt PR, Hillman DR. Comparison of upper airway collapse during general anaesthesia and sleep. Lancet. 2002;359:1207–9. doi: 10.1016/S0140-6736(02)08224-7. [DOI] [PubMed] [Google Scholar]
- 32.Isono S. Obesity and obstructive sleep apnoea: mechanisms for increased collapsibility of the passive pharyngeal airway. Respirology. 2012;17:32–42. doi: 10.1111/j.1440-1843.2011.02093.x. [DOI] [PubMed] [Google Scholar]
- 33.Chung F, Hillman D, Lydic R. Sleep medicine and anesthesia: A new horizon for anesthesiologists. Anesthesiology. 2011;114:1261–2. doi: 10.1097/ALN.0b013e318216e858. [DOI] [PubMed] [Google Scholar]
- 34.Lu B, Budhiraja R, Parthasarathy S. Sedating medications and undiagnosed obstructive sleep apnea: Physician determinants and patient consequences. J Clin Sleep Med. 2005;1:367–71. [PubMed] [Google Scholar]
- 35.Vozoris NT, Leung RS. Sedative medication use: prevalence, risks factors and associations with body mass index using population-level data. Sleep. 2011;34:869–74. doi: 10.5665/SLEEP.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krauss B, Green SM. Sedation and analgesia for procedures in children. N Engl J Med. 2000;342:938–45. doi: 10.1056/NEJM200003303421306. [DOI] [PubMed] [Google Scholar]
- 37.Jacobi J, Fraser GL, Coursin DB, et al. Task Force of the American College of Critical Care Medicine (ACCM) of the Society of Critical Care Medicine (SCCM), American Society of Health System Pharmacists (ASHP), American College of Chest Physicians: Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119–41. doi: 10.1097/00003246-200201000-00020. [DOI] [PubMed] [Google Scholar]
- 38.Gibson GJ. Obesity, respiratory function and breathlessness. Thorax. 2000;55:S41–4. doi: 10.1136/thorax.55.suppl_1.s41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gharib SA, Khalyfa A, Abdelkarim A, Bhushan B, Gozal D. Integrative miRNA-mRNA profiling of adipose tissue unravels transcriptional circuits induced by sleep fragmentation. PLoS One. 2012;7:e37669. doi: 10.1371/journal.pone.0037669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parameswaran K, Todd DC, Soth M. Altered respiratory physiology in obesity. Can Respir J. 2006;13:203–10. doi: 10.1155/2006/834786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eikermann M, Grosse-Sundrup M, Zaremba S, et al. Ketamine activates breathing and abolishes the coupling between loss of consciousness and upper airway dilator muscle dysfunction. Anesthesiology. 2012;116:35–46. doi: 10.1097/ALN.0b013e31823d010a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strohl KP, Thomas AJ, St Jean P, Schlenker EH, Koletsky RJ, Schork NJ. Ventilation and metabolism among rat strains. J Appl Physiol. 1997;82:317–23. doi: 10.1152/jappl.1997.82.1.317. [DOI] [PubMed] [Google Scholar]
- 43.Han F, Strohl KP. Inheritance of ventilatory behavior in rodent models. Respir Physiol Neurobiol. 2000;121:247–56. doi: 10.1016/s0034-5687(00)00132-8. [DOI] [PubMed] [Google Scholar]
- 44.Strohl KP, Thomas AJ. Ventilatory behavior and metabolism in two strains of obese rats. Respir Physiol Neurobiol. 2001;124:85–93. doi: 10.1016/s0034-5687(00)00190-0. [DOI] [PubMed] [Google Scholar]
- 45.Chlif M, Keochkerian D, Choquet D, Vaidie A, Ahmaidi S. Effects of obesity on breathing pattern, ventilatory neural drive and mechanics. Respir Physiol Neurobiol. 2009;168:198–202. doi: 10.1016/j.resp.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 46.Novak CM, Escande C, Burghardt PR, et al. Spontaneous activity, economy of activity, and resistance to diet-induced obesity in rats bred for high intrinsic aerobic capacity. Horm Behav. 2010;58:355–67. doi: 10.1016/j.yhbeh.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duchen MR. Contributions of mitochondria to animal physiology: from homeostatic sensor to calcium signalling and cell death. J Physiol. 1999;516:1–17. doi: 10.1111/j.1469-7793.1999.001aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernandes FC, Ferriera HC, Cagido VR, et al. Effects of dexmedetomidine on respiratory mechanics and control of breathing in normal rats. Respir Physiol Neurobiol. 2006;154:342–50. doi: 10.1016/j.resp.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 49.Milic-Emili J, Grunstein MM. Drive and timing components of ventilation. Chest. 1976;70:131–3. doi: 10.1378/chest.70.1_supplement.131. [DOI] [PubMed] [Google Scholar]
- 50.Schneider H, Patil SP, Canisius S, et al. Hypercapnic duty cycle is an intermediate physiological phenotype linked to mouse chromosome 5. J Appl Physiol. 2003;95:11–9. doi: 10.1152/japplphysiol.01144.2002. [DOI] [PubMed] [Google Scholar]
- 51.Feldman JL, Del Negro CA, Gray PA. Understanding the rhythm of breathing: So near, yet so far. Annu Rev Physiol. 2013;75:423–52. doi: 10.1146/annurev-physiol-040510-130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Min T, Chang K, editors. Animal models of human disease. New York: Academic Press; 2011. Progress in Molecular Biology and Translational Science, Vol. 100. [DOI] [PubMed] [Google Scholar]
- 53.Bol CJJG, Danhof M, Stanski DR, Mandema JW. Pharmacokineticpharmacodynamic characterization of the cardiovascular, hypnotic, EEG, and ventilatory responses to dexmedetomidine in the rat. J Pharmacol Exp Ther. 1997;283:1051–8. [PubMed] [Google Scholar]
- 54.Brown EN, Purdon PL, Van Dort CJ. General anesthesia and altered states of arousal: A systems neuroscience analysis. Annu Rev Neurosci. 2011;34:601–28. doi: 10.1146/annurev-neuro-060909-153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fink BR. Influence of cerebral activity in wakefulness on regulation of breathing. J Appl Physiol. 1961;16:15–20. doi: 10.1152/jappl.1961.16.1.15. [DOI] [PubMed] [Google Scholar]
- 56.Eckert DJ, Owens RL, Kehlmann GB, et al. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci (Lond) 2011;120:505–14. doi: 10.1042/CS20100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 58.Kokkinos P, Myers J, Nylen E, et al. Exercise capacity and all-cause mortality in African American and Caucasian men with type 2 diabetes. Diabetes Care. 2009;32:623–8. doi: 10.2337/dc08-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lujan HL, Britton SL, Koch LG, DiCarlo SE. Reduced susceptibility to ventricular tachyarrhythmias in rats selectively bred for high aerobic capacity. Am J Physiol Heart Circ Physiol. 2006;291:H2933–41. doi: 10.1152/ajpheart.00514.2006. [DOI] [PubMed] [Google Scholar]
- 60.Thyfault JP, Rector RS, Uptergrove GM, et al. Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. J Physiol. 2009;587:1805–16. doi: 10.1113/jphysiol.2009.169060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lessard SJ, Rivas DA, Chen ZP, et al. Impaired skeletal muscle beta-adrenergic activation and lipolysis are associated with whole-body insulin resistance in rats bred for low intrinsic exercise capacity. Endocrinology. 2009;150:4883–91. doi: 10.1210/en.2009-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Geisser ME, Wang W, Smuck M, Koch LG, Britton SL, Lydic R. Nociception before and after exercise in rats bred for high and low aerobic capacity. Neurosci Lett. 2008;443:37–40. doi: 10.1016/j.neulet.2008.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pal D, Walton ME, Lipinski WJ, et al. Determination of minimum alveolar concentration for isoflurane and sevoflurane in a rodent model of human metabolic syndrome. Anesth Analg. 2012;114:297–302. doi: 10.1213/ANE.0b013e31823ede22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feng X, Degos V, Koch LG, et al. Surgery results in exaggerated and persistent cognitive decline in a rat model of the metabolic syndrome. Anesthesiology. 2013;118:1098–105. doi: 10.1097/ALN.0b013e318286d0c9. [DOI] [PMC free article] [PubMed] [Google Scholar]



