Abstract
Background
Natural Killer (NK) cells have been implicated in the development of allergic airway inflammation. However the in vivo role of NK cells has not been firmly established due to the lack of animal models with selective deficiencies in NK cells.
Objective
To determine the specific contribution of NK cells in a murine model of allergic airway disease (AAD).
Methods
The role of NK cells in AAD was studied using NK-deficient (NKD) mice, perforin−/− mice, and mice depleted of Ly49A/D/G+ NK cell subsets in an ovalbumin-induced model of allergic airway disease (OVA-AAD).
Results
Induction of OVA-AAD in C57BL/6 wild-type (WT) mice resulted in the expansion of airway NK cells and the development of pronounced airway eosinophilia. In the absence of NK cells or specific subsets of NK cells, either in NKD mice, or after the depletion of Ly49A/D/G+ NK cells, the development of OVA-AAD was significantly impaired as seen by decreased airway inflammation and eosinophilia, decreased secretion of the Th2 cytokines IL-4, IL-5 and IL-13 and diminished OVA-specific antibody production. Furthermore, while OVA-exposure induced a dramatic expansion of dendritic cells (DCs) in WT mice, their induction was significantly attenuated in NKD mice. Development of OVA-AAD in perforin−/− mice suggested that the proinflammatory role of NK cells is not dependent on perforin-mediated cytotoxicity. Lastly, induction of allergic disease by OVA-specific CD4 T cells from WT but not NK-depleted or NKD mice in RAG−/− recipients, demonstrates that NK cells are essential for T cell priming.
Conclusions and Clinical Relevance
Our data demonstrate that conventional NK cells play an important and distinct role in the development of AAD. The presence of activated NK cells has been noted in patients with asthma. Understanding the mechanisms by which NK cells regulate allergic disease is therefore an important component of treatment approaches.
INTRODUCTION
Asthma is a chronic inflammation of the airways manifested as reversible airway obstruction, increased eosinophilic inflammation and airway hyperreactivity. T lymphocytes of the Th2 subset and their cytokines IL-4, IL-5 and IL-13 are pivotal in the development of asthma pathogenesis [1–7]. However, other types of immune cells including NK and NKT cells may also contribute to allergic inflammation [8–11].
NK cells participate at various levels in the generation of immune responses. This includes cytotoxic effector functions against virally infected and transformed cells [12, 13], the ability to modulate cytokine and chemokine environments [14], and induction of DC maturation [15]. These activities are mediated by cognate interactions via inhibitory and stimulatory receptors [16]. NKT cells, a subset of cells bearing T cell receptors with restricted heterogeneity and expressing NK cell markers (NK1.1 in C57BL/6 mice) [17, 18] can also play similar roles [19, 20]. In light of the various immunomodulatory effects exhibited by NK cells, we sought to examine whether these cells play a role in the development of allergic airway disease (AAD) in mice. Previous studies have suggested a role for NK cells in allergic inflammation in patients with asthma [21–23]. Similarly, depletion of NK and NKT cells using the pan-NK1.1 specific antibody, suggested that these cells can regulate the development of airway eosinophilia in C57BL/6 mice [9]. However, both NK and NKT cells were depleted in the above study, and due to the lack of animals with selective deficiencies in NK cells as well as observations that NKT cells can also regulate allergic inflammation [8, 10, 24], the specific contribution of NK cells has not been well-established.
In order to specifically address the role of NK cells in AAD, we studied the development of OVA-induced AAD in mice with selective deficiencies in the NK cell compartment (NKD mice), and in mice depleted of specific NK cell subsets using monoclonal antibodies reactive against Ly49 receptors. NKD mice are transgenic mice expressing the Ly49A inhibitory receptor under control of the granzyme A promoter [25, 26]. While these mice have functionally normal T, B and NKT cells, they have a profound deficiency in NK cells in peripheral organs, which translates into a functional impairment of NK cells in vitro and in vivo [27–29]. Expression of the transgene does not have endogenous functional consequences, since the ligand for Ly49A is H-2Dd, which is expressed in BALB/c mice. We show that the development of OVA-AAD was significantly inhibited in NKD mice as evidenced by an overall decrease in inflammation and eosinophilia in the BAL and lungs, decrease of OVA-specific IgE antibodies, and decreased production of Th2 cytokines in the airways. Similarly, Ly49A/D/G-depleted mice, a model that preferentially depletes specific subsets of conventional NK cells, also showed an inhibition of features of OVA-AAD. Exposure to OVA sensitization and challenge induced a dramatic expansion in the numbers of spleen and airway DCs, which was significantly attenuated in NKD mice. Furthermore, inhibition of airway inflammation in this model was not dependent on perforin-mediated NK cell cytotoxicity. Lastly, adoptive transfer experiments confirm the requirement for NK cells during OVA-AAD, and establish their effects during T cell priming. Our observations thus elucidate for the first time the specific role of conventional NK cells in the OVA model of AAD and demonstrate a pro-inflammatory role for NK cells in the development of airway inflammation and eosinophilia.
MATERIALS AND METHODS
Animals
Dr. W. Yokoyama (Washington University, St. Louis, MO) provided NKD mice; C57BL/6J and perforin-deficient mice were purchased (Jackson Laboratory, Bar Harbor, ME). All mice were bred extensively at the animal facility prior to performing experiments. Six to 8 weeks old mice were used. Mice were maintained at the Center for Laboratory Animal Care of the University of Connecticut Health Center, housed in sterile micro isolators at no more than 5 mice per cage and given water and rodent chow ad libitum. All animal experiments were fully approved by the Institutional Animal Care and Use Committee at the University of Connecticut Health Center (animal protocol numbers 97-044 and 2000-005-03).
Animal model of AAD
The OVA-induced model of murine AAD has been previously described [30, 31]. Mice were sensitized with three weekly i.p. injections of 25 μg OVA, grade V (Sigma, St. Louis, MO) in 2 mg of alum. One week after the last injection, animals were placed in a nose only exposure chamber and challenged with a 1% OVA aerosol generated by a Lovelace nebulizer (In-Tox Products, Albuquerque, NM) for 1 hr. for 3 or 7 days. We set the following experimental groups: OVA-OVA mice (sensitized and aerosol challenged with OVA); SAL-OVA (sensitized with saline and aerosol challenged with OVA), OVA i.p. (sensitized with OVA but not aerosol challenged with OVA) and naïve mice. For immunodepletion, mice were injected i.p. with 100 μg of rat anti-Ly49 A, D, G mAb (clone LAK 6/99) 2 or 7 days before the beginning of the priming regimen, and every 8 days thereafter until sacrifice. The efficacy of the depleting antibody was established using flow cytometry. NK depletion was monitored by screening NK1.1 and Ly49 positive cells in peripheral blood, spleen, lung and liver. Control mice received equivalent amounts of non-specific rat IgG (Sigma). Animals were sacrificed 24 hours after the last OVA-aerosol challenge.
Isolation of Cells
Animals were anesthetized and euthanized by cardiac exsanguination and/or perfusion. BAL was obtained by lavaging the lungs with five 1 ml aliquots of PBS [30]. Whole lungs were minced after BAL isolation and treated with 1.3 mM EDTA in Hank’s balanced salt solution (HBSS) at 37°C for 30 min, followed by digestion with 100 U/ml collagenase (Life Technologies, Rockville, MD) in RPMI containing 5% FCS, at 37°C for 1 h. Cell suspensions were washed with PBS, erythrocytes were lysed by hypotonic shock and leukocytes were resuspended in HBSS containing 10mM HEPES buffer pH 7.2 and 2% FCS (SM). Leukocytes were counted and viability was assessed by trypan blue exclusion.
Cell Surface Phenotypic Analysis
Leukocytes were stained with the following mAbs conjugated with either FITC, PE or APC: NK1.1, CD3, DX5, CD4, CD8, CD45R, CD11c, MHCII, CD80, CD86, CD40, IL-4R, CD11b, Gr-1 (BD Pharmingen, San Diego, CA) and Ly 49 A, D, G, C, E and I, and incubated on ice for 30 minutes. Cells were washed and resuspended in SM containing propidium iodide to exclude dead cells. Fluorescence was evaluated using a FACSCalibur and data was processed using CellQuest Software (BD Biosciences, San Jose, CA).
Evaluation of AAD parameters
Cell Counts
Percentages of eosinophils, macrophages, lymphocytes and neutrophils were enumerated using cytocentrifuged preparations stained with May-Grunwald/Giemsa.
Lung Histology
Unmanipulated, noninflated lung tissue was removed from animals, fixed in a 10% buffered formalin solution, and embedded in paraffin. Tissue sections were stained with hematoxylin and eosin (H&E) for general morphology.
Antibody Determination
ELISA kits (BD Pharmingen) were used to measure serum OVA-specific IgE and IgG1 antibodies.
Measurement of Cytokines
BAL fluid was concentrated 10-fold using a Centriplus YM-10 filtration device (Amicon. Beverly, MA) and examined by ELISA for the presence of IL-4 and IL-5 (BD Pharmingen) and IL-13 (R & D Systems, Minneapolis, MN). Cytokine concentrations are represented as total amount in the BAL fluid. Cells secreting IFN-γ and IL-4 in an Ag-specific manner were detected using the ELISPOT assay and the OVA-derived peptide TEWTSSNVMEERKIKV (OVA265–280) as previously described [32]. The peptide was obtained from Research Genetics (Huntsville, AL). Image analysis of colored spots was performed on an Immunospot Image Analyzer (Cellular Technology, Cleveland, OH). The background spots (effector cells plus feeders without peptide) were subtracted from wells with spots generated by the addition of peptide.
Isolation of DCs and Mixed Lymphocyte Proliferation (MLR) assays
Spleens and lungs from experimental mice were treated with 1mg/ml of collagenase (Roche Diagnostic Corporation, Indianapolis, IN) and 100 u/ml of lyophilized collagenase (Invitrogen Corporation, CA) and total cells were collected as described above. In addition spleen cells were also treated with Dnase I (Roche, Indianapolis, IN) to prevent cell aggregation. DCs were isolated by positive selection using CD11c microbeads (Miltenyi Biotec, Auburn, CA) and by running through LS columns (Miltenyi Biotec, Auburn, CA). Degassed SM was used for all magnetic sorting. DCs were irradiated at 2100R for 9 minutes and were set up in MLRs with naïve CD4 T cells isolated by positive selection using CD4 microbeads and LS columns (Miltenyi Biotec, Auburn, CA) from spleens of either B6 mice or allogeneic BALB/c mice, at DC:T cell ratios as indicated in the text. Cells were plated in triplicate in 96 well U-bottom plates and incubated for 48–72 hours. Twelve hours prior to harvest, they were pulsed with 1μCi of [3H]thymidine/well and the samples were harvested and counted by using a cell harvester (TomTec, Wallac, Gaithersburg, MD) and a 1450 Microbeta Trilux scintillation counter (Wallac).
Induction of AAD in RAG−/− recipients
WT or NKD mice were immunized with OVA-alum as described above. One group of WT mice was injected i.p. with anti-NK1.1 (clone PK136) prior to immunization with OVA-alum and throughout the immunization protocol to deplete NK cells as previously described [9]. Five days after the last injection, animals were sacrificed and spleen cells were isolated. Splenic cells were then incubated with a cocktail of PE-conjugated mAbs reactive against NK1.1, DX5, CD8, M170/CD11b, Gr-1, Ter119, F480 and B220 for 15 mins at 4°C. Cells were then washed and treated with anti-PE microbeads (Miltenyi Biotec) to isolate CD4 T cells by negative selection using an LS column. Isolated CD4 T cells were >95% CD3+CD4+. About 2–4 million cells were transferred intravenously (i.v.) into naïve RAG−/− recipients that had previously received a single OVA-aerosol challenge. One and half days after adoptive transfer, RAG−/− mice were challenged as usual with OVA-aerosol and sacrificed as indicated in the text.
Statistical Analysis
Experimental groups were compared using the Student’s t test. Values of p ≤ 0.05 were considered significant. All data are expressed as the mean ± SE.
RESULTS
Airway inflammation and eosinophilia are inhibited in NKD mice
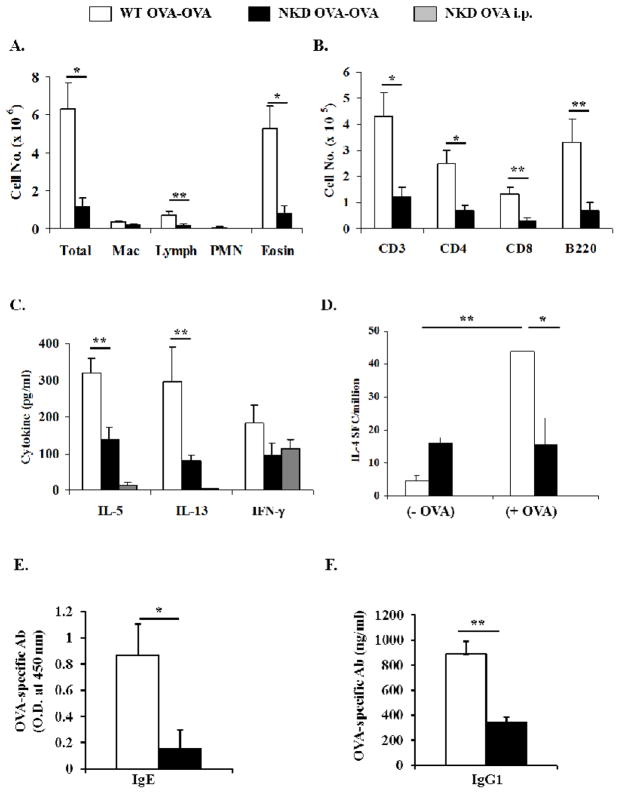
NKD mice have been previously described [25, 27]. They exhibit selective deficiencies in the conventional NK cell compartment in the spleen, lungs, liver and peripheral blood. In contrast, the numbers of peripheral T, B and NKT cells in these animals are comparable to those observed in WT mice. Since NKD mice have a significantly lower proportion of conventional NK cells in lung tissue (3.4 % ± 0.6 % of lymphocyte populations) as compared to WT mice (16.8% ± 0.35%), we hypothesized that they would be a suitable model for evaluating the role of NK cells in AAD. Seven days after daily OVA-aerosol challenge, OVA-primed WT (WT OVA-OVA) mice exhibited inflammation and pronounced airway eosinophilia in the BAL. In contrast, the degree of inflammation and eosinophilia in the BAL of NKD OVA-OVA mice was significantly reduced (Fig. 1A). Similar observations were made 3 days after OVA-aerosol challenge (data not shown). Decreased eosinophilia in BAL was accompanied by lower numbers of T and B cells (Fig. 1B). Cytokine levels were assessed 3 days after OVA-aerosol challenge (Fig 1C). IL-5 and IL-13 were detected in both groups, although at significantly reduced concentrations in the BAL of NKD OVA-OVA mice. Equivalent levels of IFN-γ were observed between the groups. Evaluation of IL-4 production by splenic T cells 7 days after OVA-aerosol challenge showed that basal levels of IL-4, without any antigen stimulation, were slightly elevated in NKD OVA-OVA mice as compared to WT OVA-OVA mice. However, after challenge with specific peptide WT OVA-OVA splenocytes produced elevated levels of IL-4 while NKD OVA-OVA mice did not present significant differences compared to unstimulated cells (Fig. 1D). Antibody responses were evaluated by quantifying the amounts of OVA-specific IgE and IgG1 in the serum (Figs. 1E&F). While elevated levels of OVA-specific IgE and IgG1 were observed in WT OVA-OVA, the production of both isotypes was significantly decreased in OVA-exposed NKD mice. Similarly, production of these antibodies could not be detected in naïve NKD mice and NKD mice immunized with OVA without subsequent challenge (OVA i.p. mice). These observations indicated that NKD mice were compromised in their ability to develop features of airway inflammation during AAD.
Figure 1. Inflammation and eosinophilia in NKD mice induced with OVA-AAD.
(A) BAL differential count indicating the total numbers of inflammatory cells as assessed by modified Giemsa stain. (B) Total numbers of T and B cells in BAL. (C) Measurement of total BAL IL-5, IL-13 and IFN-γ by ELISA 3 days after OVA-aerosol challenge. (D) IL-4 production by OVA peptide stimulated splenic cells measured by ELISPOT assay 7 days after OVA-aerosol challenge. (E) serum OVA-specific IgE and (F) IgG1 measured by ELISA. Three or more independent experiments were performed. n = 7–10 mice/group. * = p < 0.01, ** = p < 0.05.
NK and NKT cells are elevated in lung tissue of WT mice during allergic inflammation
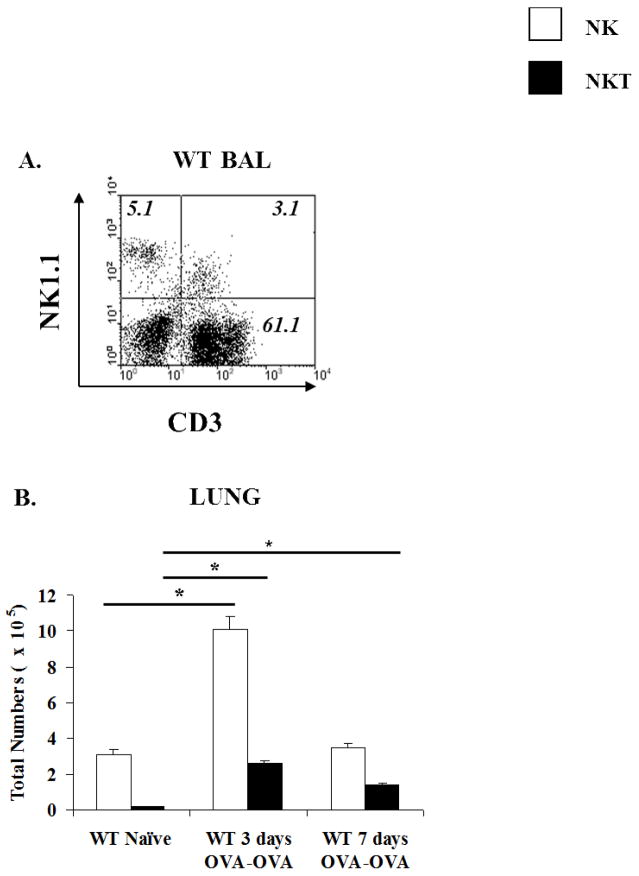
Significant numbers of NK and NKT cells were present in the BAL of WT OVA-OVA mice after OVA-aerosol challenge (Fig. 2A). Similarly, there was a marked elevation of NK cells (from 2.7×105 to 6×105 cells three days after OVA-aerosol challenge) in the lungs of WT OVA-OVA mice as compared to naïve animals during the progression of AAD (Fig. 2B). These numbers returned to baseline values (2×105) 7 days after challenge. Interestingly, NKT cells also increased in percentage (from 1.0 to 3% and 1.7%) and number (0.1×105 to 1.0×105 and 0.7×105), both at days 3 and 7 after OVA-aerosol challenge (Fig. 2B). This indicated that both populations were present and elevated in the lungs of WT OVA-OVA mice. To determine whether a similar pattern of NK and NKT cell distribution could also be observed in lungs of NKD mice, we examined the percentages and absolute numbers of these cells. Interestingly, although the percentages and numbers of NK cells in the lungs of NKD mice are significantly decreased, the induction of AAD modestly increased the numbers of NK cells (from 0.35×105 to 0.9×105) but significantly increased those of NKT cells (from 0.06×105 to 0.95×105) in NKD mice, indicating that the mechanisms of populating the lungs with NK cells are not defective in these mice. Sufficient numbers of BAL cells could not be obtained from naïve NKD mice to perform a similar analysis.
Figure 2. Elevated numbers of NK and NKT cells in the airways of WT OVA-OVA mice.
(A) BAL FACS profile of WT OVA-OVA mice depicting percentages of NK and NKT cells. Sufficient BAL cells could not be obtained from WT controls and NKD mice for FACS analysis. (B) Absolute numbers of NK and NKT cells in the lungs of naïve and OVA-OVA WT mice as enumerated by flow cytometry. Three or more independent experiments were performed. n=5 mice/group. *= p ≤ 0.0005.
Evidence of decreased OVA-AAD with lung histopathology in NKD mice
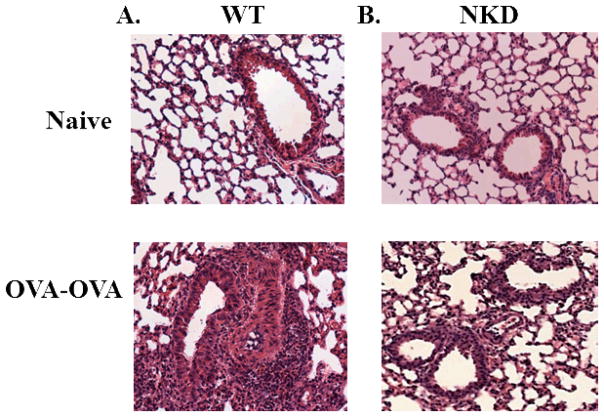
To determine whether the differences observed in BAL and lung correlated with lung inflammation in situ, we performed qualitative histological examinations of lung tissue. The lungs from control non-OVA exposed mice (Fig. 3A, top) appeared normal without any remarkable pathologic changes. As expected, the lungs of WT OVA-OVA mice had significant peribronchial and perivascular inflammation characterized by an infiltration of lymphocytes and eosinophils (Fig. 3A, bottom). In contrast, a significant reduction in the degree of inflammation was observed in the lungs of NKD OVA-OVA mice (Fig. 3B, bottom).
Figure 3. Lung histopathology of wildtype and NK deficient mice.
The top panels represent naïve non-OVA exposed control mice for (A) WT and (B) NKD mice. The bottom panels represent (A) WT and (B) NKD mice exposed to OVA 7 days post-OVA aerosol challenge. Three or more independent experiments were performed.
Inhibition of airway inflammation and eosinophilia in mice depleted of specific NK cell subsets
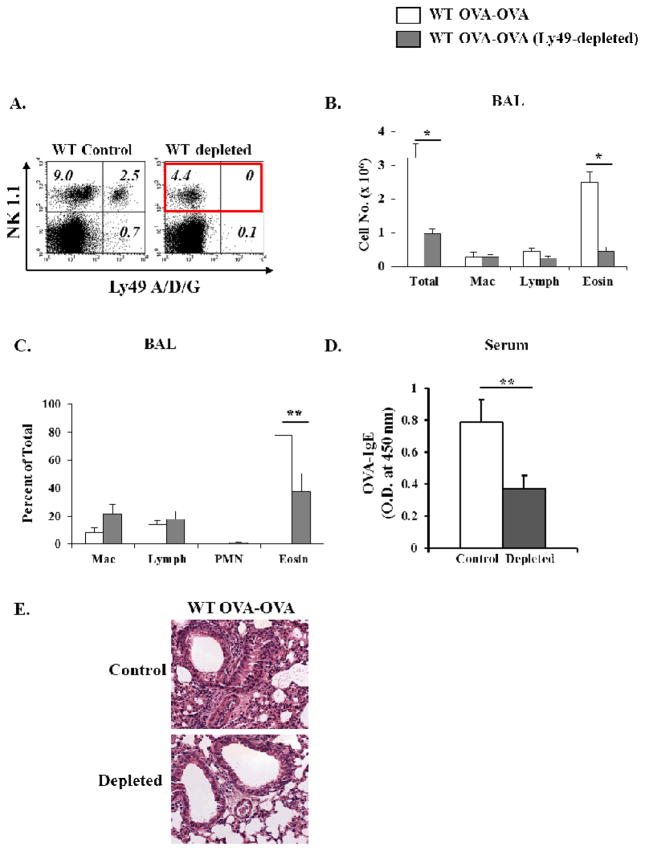
Individual NK cells express a repertoire of Ly49 receptors with the ability to differentially bind MHC Class I molecules. We generated a monoclonal antibody (LAK 6/99) reactive against a shared Ly49 epitope including an activating receptor Ly49 D and inhibitory receptors Ly 49 A and G. Intraperitoneal administration of this antibody resulted in depletion of NK cells expressing these receptors in the periphery (and lungs) of WT mice (Fig. 4A). We used this system to corroborate the results obtained in NKD mice. Seven days following OVA-aerosol challenge, animals treated with the LAK 6/99 antibody exhibited significantly decreased inflammation and eosinophilia in the BAL as compared with WT OVA-OVA mice (Fig. 4B&C). Similarly, a reduction of serum OVA-specific IgE antibody (Fig. 4D) was also detected. Furthermore, Ly49-depleted OVA-OVA exposed mice demonstrated substantial inhibition of airway inflammation (Fig. 4E, bottom) compared to WT OVA-OVA exposed mice treated with non-specific rat IgG (Fig. 4E, top). These data therefore suggest that depletion of specific subsets of NK cells is sufficient to inhibit airway inflammation and eosinophilia during AAD.
Figure 4. Inhibition of airway inflammation in mice depleted of Ly49 A/D/G+ NK cells.
(A) WT mice were injected with rat anti-Ly49 A/D/G every 8 days. Percentages of Ly49+ NK cells in lung tissue by flow cytometry is shown: (left) undepleted; (right) depleted. Control mice were either untreated or treated with nonspecific rat IgG. (b,c) BAL cell distribution in OVA-OVA mice 7 days after OVA-aerosol challenge is shown. (B) total numbers of individual inflammatory cells, and (C) relative abundance of the respective populations as assessed by modified Giemsa stain. (D) serum OVA-specific IgE determined by ELISA. (E) The top panel represents control WT OVA-OVA exposed mice treated with non-specific rat IgG. The bottom panel represents Ly49 A/D/G depleted groups 7 days after OVA- aerosol challenge. Two independent experiments were performed. n=7–10. *=p<0.01, **=p<0.05.
Sensitization with OVA-alum results in activation of NKT cells during OVA-AAD induction
To assess the contribution of NK cells to the priming and challenge phases of OVA-AAD, phenotypic analysis of NK cell subsets in various organs including the spleen, lung, liver, bone marrow and thymus was performed. NK cell subsets expressing Thy1.2, B220, M170 or Ly49 A,D,G,C,E,I were enumerated 12, 24, 36, 48, 72, 96, 120, 144 and 168 hours after OVA-alum sensitization as well as after 3 and 7 days of OVA-aerosol challenge. These studies demonstrated no significant changes in expression of these markers on NK cells during either the priming or challenge phases of OVA-AAD (data not shown).
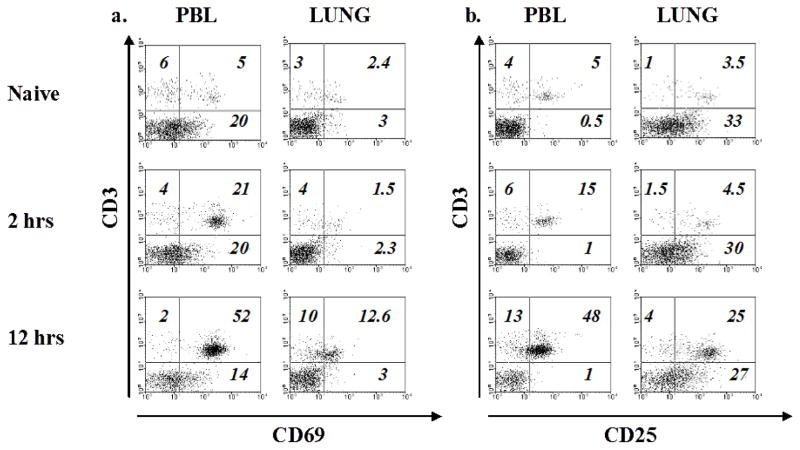
To further determine whether NK cells demonstrate an activated phenotype after sensitization with OVA-alum and during OVA-aerosol challenge, we analyzed lung and peripheral blood of WT mice for expression of CD69 and CD25 on NK and NKT cells, 2 and 12 hours after OVA-alum sensitization, and 3 and 7 days after OVA-aerosol challenge. NK cells did not express higher quantities of either CD69 or CD25 at any time point in comparison with saline sensitized mice. In contrast, NKT cells showed high expression of both markers in the blood and the lungs 2 and 12 hours after OVA sensitization (Fig. 5A&B). To determine whether the lack of NK cells conferred any change on NKT cell activation, we assessed expression of the above antigens on NKT cells in NKD mice. Interestingly, NKT cells demonstrated enhanced expression of these markers in these animals as well (data not shown). These data indicated that NKT cells are rapidly activated after OVA-alum sensitization and have the capacity to modulate NK cell activation as previously reported [33].
Figure 5. NKT cells are activated after sensitization with OVA-alum.
(A&B) WT mice were injected with OVA-alum i.p. and 2 and 12 hrs. later CD69+ NK and NKT cells were evaluated by flow cytometry. Gated NK1.1+ cells are shown in the peripheral blood (PBL) and lung respectively. Two or more independent experiments were performed.
NKD mice fail to induce splenic and airway dendritic cells during OVA-AAD
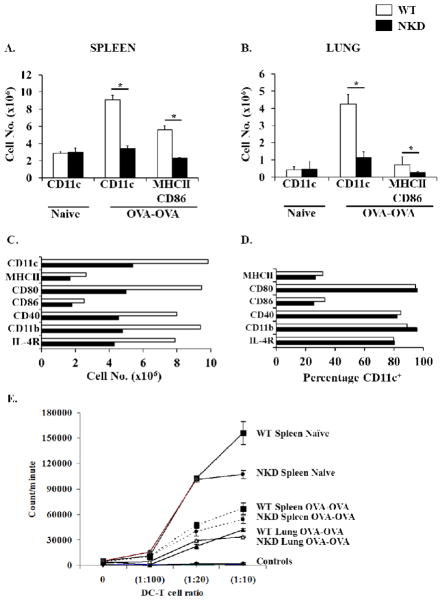
The ability of NK cells to modulate immune responses may also be linked to their capacity to influence DC function. Activated NK cells have been shown to induce both maturation and lysis of immature DC in vitro. Additionally, NK cells have also been associated with DC activation in vivo during MCMV infection [34, 35]. We therefore hypothesized that NK cells may similarly impact DC function during the course of development of OVA-AAD in allergic mice. Initial studies demonstrated an equivalent number of CD11c+ DCs in the spleen and lung tissues of both WT and NKD mice, suggesting that a deficiency of peripheral NK cells does not impact the numbers of endogenous DCs in naïve NKD mice as previously reported. Three days after OVA-aerosol challenge, a dramatic increase in the total numbers of CD11c+ DCs was observed in the spleens and lungs of OVA-challenged WT animals in comparison to naïve controls (Fig. 6A&B). Furthermore, a significant number of these cells in the spleen, and a fair number in the lung, were positive for MHCII and CD86. In contrast, expansion of CD11c+ cells was not observed in the spleens and lungs of NKD OVA-OVA mice, suggesting that NK cell deficiency constrains the induction of DCs during OVA challenge in allergic animals (Fig. 6A&B). Similar findings were observed in the lungs and spleen of WT and NKD mice 7 days after OVA-aerosol challenge (Fig. 6C and data not shown). These data therefore suggest that exposure to allergen induces the expansion of spleen and airway DCs during allergic inflammation, and that the influx of DCs into immune compartments is severely attenuated in the absence of peripheral NK cells. While these data strongly implicate NK cells in the expansion of allergen-specific DCs in vivo, the effects of NK cell deficiency on DC function is not clear. Hence, to further determine whether the absence of NK cells impairs DC function during allergic inflammation, we examined the expression of co-stimulatory molecules on spleen and lung DCs from experimental animals. Similarly, the capacity of these cells to induce the activation of naïve CD4 T cells was also assessed. These studies demonstrated that DCs from both WT and NKD mice expressed equivalent levels of co-stimulatory molecules (Fig. 6D) and they were both able to induce the proliferation of naïve CD4 T cells (Fig. 6E). These data therefore suggest that while NKD mice fail to induce DC expansion during OVA-AAD, they retain the ability to express costimulatory molecules and induce T cell activation.
Figure 6. Evaluation of DC numbers and antigen presentation capacity in allergic WT and NKD mice.
Total number of CD11c+ DCs in (A) the spleen and (B) the lungs of WT and NKD mice 3 days after OVA-aerosol challenge. (C) Total numbers of airway DCs 7 days after OVA-aerosol challenge. Lung tissue from several mice was pooled and DCs were isolated using CD11c magnetic beads. (D) Expression of surface molecules on airway DCs shown in C. (E) Proliferation of naïve CD4 T cells from BALB/c mice to spleen or lung DCs from naïve or 7 day OVA-OVA WT and NKD mice is shown. Allogeneic T cells were isolated using magnetic beads and positive selection. Controls are syngeneic CD4 T cells from WT and NKD OVA-OVA spleen and lungs stimulated with corresponding DCs. Two independent experiments were performed. n=4–5 mice/group. *=p<0.01.
NK cell cytotoxicity is dispensable for the modulation of AAD
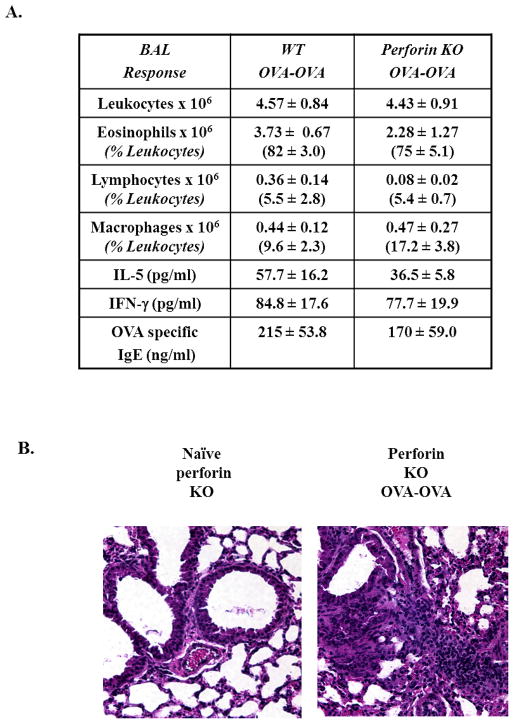
NKD mice are compromised in their ability to generate effective NK cell-mediated cytotoxicity [25]. NK cell cytotoxicity is not only involved in the killing of virally-infected cells and tumors, but also in DC lysis and maturation, thus having the potential to regulate the numbers of DCs during immune responses [36–38]. To determine whether NK cell cytotoxicity was essential in OVA-AAD, we examined perforin-deficient mice, which cannot mediate granule-mediated cytotoxicity [39] but have normal levels of NK cells. Exposure to OVA challenge in OVA-sensitized perforin-deficient mice resulted in marked airway inflammation and the development of eosinophilia comparable with similarly treated WT animals (Fig. 7A). In addition, these mice produced equivalent levels of the Th2 cytokine IL-5 and OVA-specific IgE antibodies. Evaluation of lung histology showed the presence of airway inflammation and vasculitis in both WT and perforin-deficient OVA-OVA mice (Fig. 7B). These data therefore suggest that NK cell-mediated perforin-dependent cytotoxicity is not required for the development of OVA-AAD, and that failure to induce DC expansion in NKD mice is unrelated to defective NK cell cytotoxic function.
Figure 7. Perforin-deficient mice develop AAD similar to WT mice.
(A) BAL responses of WT and perforin-deficient OVA-OVA mice 7 days after OVA-aerosol challenge. Differential counts and relative abundance of individual populations are as assessed by modified Giemsa stain. Cytokines (IL-5 and IFN-γ) and serum OVA-specific IgE were measured by ELISA 3 and 7 days after OVA-aerosol challenge respectively. (n=7) (B) Lung histology of perforin-deficient mice: the Left panel represents non-OVA exposed control perforin-deficient mice. The right panel represents perforin-deficient OVA-OVA mice after 7 days of OVA-aerosol challenge.
Adoptive transfer of OVA-specific T cells from WT but not NK-depleted or NKD mice induces the development of OVA-AAD in RAG−/− recipients
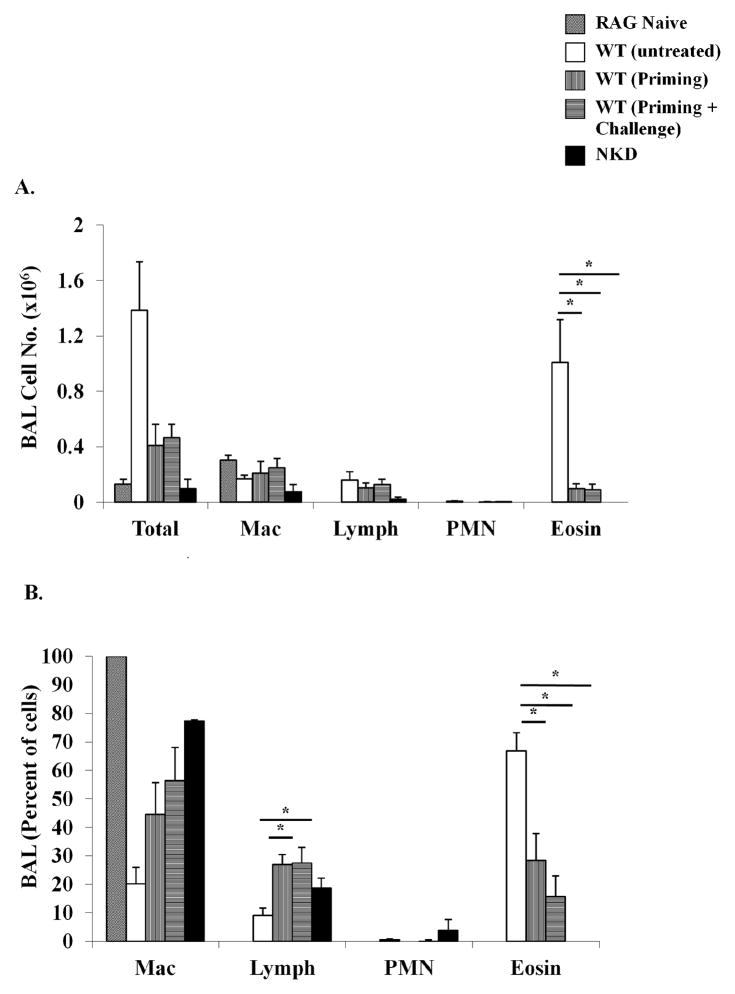
The effects of NK cells on DC induction during OVA-AAD suggest that in the absence of NK cells, T cell activation and effector function may be impaired in NKD mice during the generation of allergic responses. To further assess the effects of NK cells on T cell activation, and to determine whether NK cells are essential during the priming or challenge phases of OVA-AAD, OVA-specific CD4 T cells were isolated from OVA-alum sensitized WT mice (WT, UNTREATED), WT mice depleted of NK cells (using anti-NK1.1) prior to priming with OVA-alum, and NKD mice, and transferred to naïve RAG−/− recipients. Additionally, OVA-specific CD4 T cells from the NK-depleted group were transferred into two groups of RAG−/− recipients: (i) naïve RAG−/− recipients that had a normal endogenous NK cell compartment, and (ii) RAG−/− mice treated with anti-NK1.1 monoclonal antibody to deplete NK cells before transfer. The first group therefore represents T cells from mice that were depleted of NK cells during the priming phase, but were exposed to a normal NK cell compartment during the challenge phase and are termed as WT, PRIMING, while the second group represents T cells that were not exposed to NK cells during both the priming and challenge phases and are termed as WT, PRIMING + CHALLENGE. Two days after transfer, RAG−/− recipients were challenged as usual for 7 days with OVA-aerosol. Challenged recipients that had received CD4 T cells from WT OVA-sensitized mice (WT, untreated) became sick and developed BAL eosinophilia as compared with naïve RAG−/− mice that did not receive any T cells (Fig. 8A&B). In contrast, the development of BAL eosinophilia was significantly attenuated in RAG−/− recipients receiving CD4 T cells from either WT mice depleted of NK cells before the priming phase (WT, priming) or from NKD mice (Fig. 8A&B). Similarly, depletion of NK cells during both the priming and challenge phases (WT, priming + challenge) also resulted in diminished BAL eosinophilia in OVA-challenged recipients. These data therefore demonstrate that NK cells are critical during T cell priming and influence the outcome of Th2-mediated OVA-AAD in allergen-sensitized and challenged mice.
Figure 8. Adoptive transfer of OVA-specific T cells from WT, NK-depleted and NKD mice into RAG−/− recipients.
Numbers and percentages of BAL cells in OVA-aerosol challenged RAG−/− mice adoptively transferred with T cells from either naïve WT mice or OVA-alum sensitized (primed) WT and NKD mice are shown. Donor OVA-alum sensitized mice were either treated with anti-NK1.1 monoclonal antibodies before the priming phase (WT, Priming) or untreated (WT, untreated). The WT, Priming + Challenge group represents RAG−/− mice that received T cells from NK depleted OVA-alum sensitized mice and that were continuously depleted of NK cells during OVA-aerosol challenge. CD4 T cells were isolated using magnetic beads and negative selection. (A) BAL differential count indicating the total numbers of individual inflammatory cells, and (B) the relative distribution of macrophages (Mac), lymphocytes (lymph), neutrophils (PMN), and eosinophils (eosin) as assessed by modified Giemsa stain. **=p<0.05 compared to WT (untreated). n = 5 mice/group.
DISCUSSION
In this study, we demonstrate the specific contribution of conventional NK cells in the development of airway allergic disease in C57BL/6 mice. Using mice with a predominant deficiency in airway NK cells (but not NKT cells), we show that the development of airway inflammation and eosinophilia is inhibited in the absence of physiological levels of NK cells. A role for NK cells in allergic inflammation has previously been postulated [9]. However, their specific contribution could not be assessed, since the investigators used depleting antibodies that eliminated both NK and NKT cells in the model. Additionally, subsequent studies have also demonstrated impairment of allergic disease in the absence of NKT cells, further confounding the results obtained in the Korsgren et al. study [8, 10]. Our data highlight for the first time the specific contributions of conventional NK cells during allergic inflammation, and emphasize their distinct role in contributing to the pathogenesis of the disease.
NKD mice, which exhibit significant deficiencies in peripheral NK cells (in the spleen, lungs and blood), but have normal numbers of DCs as well as T, B and NKT cells, displayed a marked inhibition of airway inflammation and eosinophilia during exposure to OVA challenge. Additionally, these mice were unable to produce OVA-specific IgE and IgG1 antibodies in amounts comparable to those seen in WT animals. Similarly, they also produced decreased levels of Th2 cytokines and exhibited reduced inflammation in lung tissue in response to OVA challenge. These data therefore demonstrate a pro-inflammatory role for NK cells during the development of airway inflammation, and suggest that these cells have the capacity to modulate the Th2-mediated inflammatory process during the development of OVA-AAD.
NK cells are a heterogeneous population of lymphocytes [13]. The existence of diverse subsets of NK cells that can differentially regulate the immune response has been confirmed challenging traditional concepts of NK cells as simple effectors of viral and tumor defense. NK cells can produce Th1 cytokines like IFN-γ and TNF-α [14], as well as Th2 cytokines like IL-4, IL-5, IL-13 and IL-10 [40–43], and chemokines like MIP-1α and MIP-1β [44]. This ability allows NK cells to regulate immune responses at various stages. This may include regulation of T cell differentiation from naïve precursors and contribution to inflammation during the effector phases. Similarly, unique subsets of NK cells have also been shown to affect the maturation of immature DCs through a selective process requiring both cytokines and cell- cell contact interactions [34, 36]. In this context, interestingly, the Korsgren et al. study showed that elimination of NK (and NKT) cells prior to priming but not challenge resulted in inhibition of airway inflammation, suggesting that NK cells are required at priming [9].
In light of these observations as well our own results, we therefore examined NK cells during both the priming and challenge phases of OVA-AAD to determine whether exposure to OVA induces phenotypic changes in the NK cell compartment in WT mice. While no changes in the phenotype or numbers of NK cells was observed in OVA i.p. mice during priming, we noted the presence of elevated numbers of NK cells during OVA challenge in the lungs of OVA-OVA WT mice, suggesting that NK cells are recruited to the airways during the development of OVA-AAD. To further investigate the phenotype of NK cells, NK cell activation was assessed in OVA-exposed mice 2 and 12 hours after OVA i.p. immunization. Interestingly, our experiments revealed that NKT, but not NK cells exhibited an activated phenotype immediately after sensitization with OVA-alum. This is consistent with studies that show an important role for NKT cells in AAD [8, 10]. Furthermore, activated NKT cells have also been shown to further activate NK cells, which may also be important during AAD development [33]. In this context, our observation that activated NKT cells were also present in NKD mice, raises important questions about how these cell types interact during the development of immune responses. CD69 and CD25 are transiently expressed on NK cells and our analysis may not accurately reflect the activation status of NK cells during the development of AAD. Also, other markers of NK cell activation such as the production of IFN-γ and TNF-α, may be more important during OVA-AAD in our model. This is supported by the presence of activated NK cells in the peripheral blood of allergic patients in various human studies [21–23].
The ability of NK cells to influence the immune response has also been attributed to their ability to influence DC maturation in in vitro studies [37, 45, 46]. Conversely, DCs have also been shown to reciprocally induce the activation of NK cells under these situations. Our observations that the number of splenic and airway DCs is significantly attenuated in OVA-exposed NKD mice suggests that the induction of DC activation and the capacity for effective antigen presentation may be impaired in the absence of NK cells. While these DCs are functional in terms of their ability to upregulate costimulatory molecules and induce T cell priming, significantly fewer DCs are available in NKD mice to induce antigen presentation. This suggests that NK cells may modulate the outcome of Th2-mediated responses by stimulating DC maturation and/or function during AAD and regulating the magnitude of antigen presentation.
Our experiments using NKD mice were confirmed using a separate model in which NK cells were depleted by injecting an antibody reactive against Ly49 receptors. The specificity and effectiveness of this antibody has been previously determined in our laboratory ([47] and data not shown). This antibody not only depletes cells bearing the Ly49 receptors associated with antibody recognition, but also cells expressing other Ly49 molecules due to the co-expression of multiple Ly49 receptors by individual NK cells [48, 49]. As observed in NKD mice, immunodepleted mice manifested suppression of inflammation and eosinophilia during AAD. Because this antibody does not deplete all NK cells, but only subsets of NK cells that express the respective receptors, the data suggest that depletion of certain NK cell types expressing select NK receptors is sufficient to affect the outcome of the overall inflammation.
The definition of NK cell receptor specificity as a requirement for modulation of AAD development should be an important topic of future research. NK cells expressing specific activating or inhibitory receptors have been shown to play crucial roles during MCMV infection and tumor rejection [27, 50, 51], and thus may also contribute to the pathogenesis of AAD. Our current data do not provide answers to this question due to the fact that our experiments were conducted in C57BL/6 mice, which do not express MHC class I ligands for some of the receptors (Ly49 A and Ly49 D) recognized by the depleting mAb. However, this kind of treatment not only deplete cells bearing the Ly49 specificities associated with antibody recognition, but also cells expressing other Ly49 molecules due to the co-expression of multiple Ly49 receptors by individual cells [48, 49].
In addition to selective deficiencies in the NK cell compartment, NKD mice are also compromised in their ability to mount cytotoxic responses that are dependent on NK cells. We therefore examined the development of OVA-AAD in perforin-deficient mice, which are unable to mount NK and T-cell mediated cytotoxic responses. Evidence of airway inflammation and eosinophilia in perforin-deficient mice demonstrated that perforin-mediated cytotoxic activity by NK cells is not essential for their ability to modulate AAD, suggesting that other effects of activated NK cells such as the production of cytokines or receptor-dependent activities may play a role. However, other cytotoxic mechanisms like Fas and TRAIL mediated killing could be involved.
Finally we used adoptive transfer systems to confirm the pro-inflammatory role of NK cells in our model of OVA-AAD. Since it is very difficult to maintain sustained populations of adoptively transferred NK cells in recipient mice, we followed an established system of T cell transfer to determine the role of NK cells [52–55]. Our results confirm the pro-inflammatory role of NK cells during the development of OVA-AAD. T cells derived from OVA-alum sensitized WT mice could fully induce eosinophilia in RAG−/− recipients, while T cells derived from mice that were depleted of NK cells could not induce eosinophilia in RAG−/− recipients in the presence or absence of NK cells. This was also corroborated by observations that T cells from NKD mice could also not induce eosinophilia in RAG−/− recipients. Our results therefore further establish the role of NK cells in AAD and also confirm the observations by Korsgren et al., that NK cells are required before the priming regimen for the development of OVA-AAD [9].
Despite the attenuation of airway eosinophilia and Th2-mediated disease factors during OVA-AAD in NKD mice, the presence of mucus secreting cells and reactivity to methacholine were also noted (unpublished observations). While these observations may have been affected by the presence of residual populations of NK cells in NKD mice, they also suggest that NK cells regulate certain aspects of AAD but not all. This is consistent with reports that suggest an absence of correlation between eosinophilic inflammation and reactivity to bronchoconstrictors [56, 57]. Alternatively, since reactivity to methacholine was observed in naïve NKD mice, it is possible that this is a phenotypic characteristic inherent to the mice, and unrelated to the deficiency in NK cells.
In conclusion, we show that conventional NK cells play a pro-inflammatory role in the development of allergic disease, and regulate the development of airway eosinophilia and Th2 cytokine production. Future studies are needed to elucidate the exact mechanisms by which NK cells regulate the outcome of disease. These will enrich our basic understanding of the disease and may provide novel approaches for treatment.
Acknowledgments
This work was supported by grants from the American Asthma Foundation (Sandler Program For Asthma Research), NIH-AI46078, NIH-HL068692 and NIH-AI43573.
We would like to thank Adrienne Zuwallach for expert technical assistance, and Drs. Lynn Puddington, Craig Schramm and T.V. Rajan for discussion and comments.
ABBREVIATIONS USED
- OVA-AAD
ovalbumin-induced model of allergic airway disease
- AAD
allergic airway disease
- NKD
NK cell-deficient
References
- 1.Azzawi M, Bradley B, Jeffery PK, Frew AJ, Wardlaw AJ, Knowles G, Assoufi B, Collins JV, Durham S, Kay AB. Identification of activated T lymphocytes and eosinophils in bronchial biopsies in stable atopic asthma. Am Rev Respir Dis. 1990;142:1407–13. doi: 10.1164/ajrccm/142.6_Pt_1.1407. [DOI] [PubMed] [Google Scholar]
- 2.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, Corry DB. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–3. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lukacs NW, Strieter RM, Chensue SW, Kunkel SL. Interleukin-4-dependent pulmonary eosinophil infiltration in a murine model of asthma. Am J Respir Cell Mol Biol. 1994;10:526–32. doi: 10.1165/ajrcmb.10.5.8179915. [DOI] [PubMed] [Google Scholar]
- 5.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 6.Walker C, Kaegi MK, Braun P, Blaser K. Activated T cells and eosinophilia in bronchoalveolar lavages from subjects with asthma correlated with disease severity. J Allergy Clin Immunol. 1991;88:935–42. doi: 10.1016/0091-6749(91)90251-i. [DOI] [PubMed] [Google Scholar]
- 7.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–61. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 8.Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9:582–8. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 9.Korsgren M, Persson CG, Sundler F, Bjerke T, Hansson T, Chambers BJ, Hong S, Van Kaer L, Ljunggren HG, Korsgren O. Natural killer cells determine development of allergen-induced eosinophilic airway inflammation in mice. J Exp Med. 1999;189:553–62. doi: 10.1084/jem.189.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lisbonne M, Diem S, de Castro Keller A, Lefort J, Araujo LM, Hachem P, Fourneau JM, Sidobre S, Kronenberg M, Taniguchi M, Van Endert P, Dy M, Askenase P, Russo M, Vargaftig BB, Herbelin A, Leite-de-Moraes MC. Cutting edge: invariant V alpha 14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J Immunol. 2003;171:1637–41. doi: 10.4049/jimmunol.171.4.1637. [DOI] [PubMed] [Google Scholar]
- 11.Schramm CM, Puddington L, Yiamouyiannis CA, Lingenheld EG, Whiteley HE, Wolyniec WW, Noonan TC, Thrall RS. Proinflammatory roles of T-cell receptor (TCR)gammadelta and TCRalphabeta lymphocytes in a murine model of asthma. Am J Respir Cell Mol Biol. 2000;22:218–25. doi: 10.1165/ajrcmb.22.2.3620. [DOI] [PubMed] [Google Scholar]
- 12.Biron CA. Activation and function of natural killer cell responses during viral infections. Curr Opin Immunol. 1997;9:24–34. doi: 10.1016/s0952-7915(97)80155-0. [DOI] [PubMed] [Google Scholar]
- 13.Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Annu Rev Immunol. 2004;22:405–29. doi: 10.1146/annurev.immunol.22.012703.104711. [DOI] [PubMed] [Google Scholar]
- 14.Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moretta A. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat Rev Immunol. 2002;2:957–64. doi: 10.1038/nri956. [DOI] [PubMed] [Google Scholar]
- 16.Yokoyama WM, Plougastel BF. Immune functions encoded by the natural killer gene complex. Nat Rev Immunol. 2003;3:304–16. doi: 10.1038/nri1055. [DOI] [PubMed] [Google Scholar]
- 17.Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–90. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 18.Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol. 2002;2:557–68. doi: 10.1038/nri854. [DOI] [PubMed] [Google Scholar]
- 19.Biron CA, Brossay L. NK cells and NKT cells in innate defense against viral infections. Curr Opin Immunol. 2001;13:458–64. doi: 10.1016/s0952-7915(00)00241-7. [DOI] [PubMed] [Google Scholar]
- 20.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198:267–79. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kusaka Y, Sato K, Zhang Q, Morita A, Kasahara T, Yanagihara Y. Association of natural killer cell activity with serum IgE. Int Arch Allergy Immunol. 1997;112:331–5. doi: 10.1159/000237476. [DOI] [PubMed] [Google Scholar]
- 22.Lin SJ, Chang LY, Yan DC, Huang YJ, Lin TJ, Lin TY. Decreased intercellular adhesion molecule-1 (CD54) and L-selectin (CD62L) expression on peripheral blood natural killer cells in asthmatic children with acute exacerbation. Allergy. 2003;58:67–71. doi: 10.1034/j.1398-9995.2003.t01-1-23697.x. [DOI] [PubMed] [Google Scholar]
- 23.Vesterinen E, Timonen T. Natural killer cell activity in specific and non-specific bronchial challenge. Ann Allergy. 1988;60:247–9. [PubMed] [Google Scholar]
- 24.Akbari O, Faul JL, Hoyte EG, Berry GJ, Wahlstrom J, Kronenberg M, DeKruyff RH, Umetsu DT. CD4+ invariant T-cell-receptor+ natural killer T cells in bronchial asthma. N Engl J Med. 2006;354:1117–29. doi: 10.1056/NEJMoa053614. [DOI] [PubMed] [Google Scholar]
- 25.Kim S, Iizuka K, Aguila HL, Weissman IL, Yokoyama WM. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc Natl Acad Sci U S A. 2000;97:2731–6. doi: 10.1073/pnas.050588297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim S, Song YJ, Higuchi DA, Kang HP, Pratt JR, Yang L, Hong CM, Poursine-Laurent J, Iizuka K, French AR, Sunwoo JB, Ishii S, Reimold AM, Yokoyama WM. Arrested natural killer cell development associated with transgene insertion into the Atf2 locus. Blood. 2005 doi: 10.1182/blood-2005-04-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loh J, Chu DT, O’Guin AK, Yokoyama WM, Virgin HWt. Natural killer cells utilize both perforin and gamma interferon to regulate murine cytomegalovirus infection in the spleen and liver. J Virol. 2005;79:661–7. doi: 10.1128/JVI.79.1.661-667.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warfield KL, Perkins JG, Swenson DL, Deal EM, Bosio CM, Aman MJ, Yokoyama WM, Young HA, Bavari S. Role of natural killer cells in innate protection against lethal ebola virus infection. J Exp Med. 2004;200:169–79. doi: 10.1084/jem.20032141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whitman SC, Rateri DL, Szilvassy SJ, Yokoyama W, Daugherty A. Depletion of natural killer cell function decreases atherosclerosis in low-density lipoprotein receptor null mice. Arterioscler Thromb Vasc Biol. 2004;24:1049–54. doi: 10.1161/01.ATV.0000124923.95545.2c. [DOI] [PubMed] [Google Scholar]
- 30.Wu CA, Puddington L, Whiteley HE, Yiamouyiannis CA, Schramm CM, Mohammadu F, Thrall RS. Murine cytomegalovirus infection alters Th1/Th2 cytokine expression, decreases airway eosinophilia, and enhances mucus production in allergic airway disease. J Immunol. 2001;167:2798–807. doi: 10.4049/jimmunol.167.5.2798. [DOI] [PubMed] [Google Scholar]
- 31.Yiamouyiannis CA, Schramm CM, Puddington L, Stengel P, Baradaran-Hosseini E, Wolyniec WW, Whiteley HE, Thrall RS. Shifts in lung lymphocyte profiles correlate with the sequential development of acute allergic and chronic tolerant stages in a murine asthma model. Am J Pathol. 1999;154:1911–21. doi: 10.1016/S0002-9440(10)65449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marzo AL, Vezys V, Williams K, Tough DF, Lefrancois L. Tissue-level regulation of Th1 and Th2 primary and memory CD4 T cells in response to Listeria infection. J Immunol. 2002;168:4504–10. doi: 10.4049/jimmunol.168.9.4504. [DOI] [PubMed] [Google Scholar]
- 33.Carnaud C, Lee D, Donnars O, Park SH, Beavis A, Koezuka Y, Bendelac A. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–50. [PubMed] [Google Scholar]
- 34.Andrews DM, Scalzo AA, Yokoyama WM, Smyth MJ, Degli-Esposti MA. Functional interactions between dendritic cells and NK cells during viral infection. Nat Immunol. 2003;4:175–81. doi: 10.1038/ni880. [DOI] [PubMed] [Google Scholar]
- 35.Krug A, French AR, Barchet W, Fischer JA, Dzionek A, Pingel JT, Orihuela MM, Akira S, Yokoyama WM, Colonna M. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–19. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 36.Fernandez NC, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, Perricaudet M, Tursz T, Maraskovsky E, Zitvogel L. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405–11. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 37.Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med. 2002;195:335–41. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson JL, Heffler LC, Charo J, Scheynius A, Bejarano MT, Ljunggren HG. Targeting of human dendritic cells by autologous NK cells. J Immunol. 1999;163:6365–70. [PubMed] [Google Scholar]
- 39.Liu CC, Walsh CM, Eto N, Clark WR, Young JD. Morphologic and functional characterization of perforin-deficient lymphokine-activated killer cells. J Immunol. 1995;155:602–8. [PubMed] [Google Scholar]
- 40.Hoshino T, Winkler-Pickett RT, Mason AT, Ortaldo JR, Young HA. IL-13 production by NK cells: IL-13-producing NK and T cells are present in vivo in the absence of IFN-gamma. J Immunol. 1999;162:51–9. [PubMed] [Google Scholar]
- 41.Mehrotra PT, Donnelly RP, Wong S, Kanegane H, Geremew A, Mostowski HS, Furuke K, Siegel JP, Bloom ET. Production of IL-10 by human natural killer cells stimulated with IL-2 and/or IL-12. J Immunol. 1998;160:2637–44. [PubMed] [Google Scholar]
- 42.Walker C, Checkel J, Cammisuli S, Leibson PJ, Gleich GJ. IL-5 production by NK cells contributes to eosinophil infiltration in a mouse model of allergic inflammation. J Immunol. 1998;161:1962–9. [PubMed] [Google Scholar]
- 43.Warren HS, Kinnear BF, Phillips JH, Lanier LL. Production of IL-5 by human NK cells and regulation of IL-5 secretion by IL-4, IL-10, and IL-12. J Immunol. 1995;154:5144–52. [PubMed] [Google Scholar]
- 44.Dorner BG, Smith HR, French AR, Kim S, Poursine-Laurent J, Beckman DL, Pingel JT, Kroczek RA, Yokoyama WM. Coordinate expression of cytokines and chemokines by NK cells during murine cytomegalovirus infection. J Immunol. 2004;172:3119–31. doi: 10.4049/jimmunol.172.5.3119. [DOI] [PubMed] [Google Scholar]
- 45.Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Munz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195:343–51. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195:327–33. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schluns KS, Nowak EC, Cabrera-Hernandez A, Puddington L, Lefrancois L, Aguila HL. Distinct cell types control lymphoid subset development by means of IL-15 and IL-15 receptor alpha expression. Proc Natl Acad Sci U S A. 2004;101:5616–21. doi: 10.1073/pnas.0307442101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dorfman JR, Raulet DH. Acquisition of Ly49 receptor expression by developing natural killer cells. J Exp Med. 1998;187:609–18. doi: 10.1084/jem.187.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanke T, Takizawa H, Raulet DH. MHC-dependent shaping of the inhibitory Ly49 receptor repertoire on NK cells: evidence for a regulated sequential model. Eur J Immunol. 2001;31:3370–9. doi: 10.1002/1521-4141(200111)31:11<3370::aid-immu3370>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 50.Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413:165–71. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dokun AO, Kim S, Smith HR, Kang HS, Chu DT, Yokoyama WM. Specific and nonspecific NK cell activation during virus infection. Nat Immunol. 2001;2:951–6. doi: 10.1038/ni714. [DOI] [PubMed] [Google Scholar]
- 52.Cohn L, Herrick C, Niu N, Homer R, Bottomly K. IL-4 promotes airway eosinophilia by suppressing IFN-gamma production: defining a novel role for IFN-gamma in the regulation of allergic airway inflammation. J Immunol. 2001;166:2760–7. doi: 10.4049/jimmunol.166.4.2760. [DOI] [PubMed] [Google Scholar]
- 53.Finotto S, Neurath MF, Glickman JN, Qin S, Lehr HA. Green FH, Ackerman K, Haley K, Galle PR, Szabo SJ, Drazen JM, De Sanctis GT, Glimcher LH, Development of spontaneous airway changes consistent with human asthma in mice lacking T-bet. Science. 2002;295:336–8. doi: 10.1126/science.1065544. [DOI] [PubMed] [Google Scholar]
- 54.Mattes J, Yang M, Siqueira A, Clark K, MacKenzie J, McKenzie AN, Webb DC, Matthaei KI, Foster PS. IL-13 induces airways hyperreactivity independently of the IL-4R alpha chain in the allergic lung. J Immunol. 2001;167:1683–92. doi: 10.4049/jimmunol.167.3.1683. [DOI] [PubMed] [Google Scholar]
- 55.Mattes J, Yang M, Mahalingam S, Kuehr J, Webb DC, Simson L, Hogan SP, Koskinen A, McKenzie AN, Dent LA, Rothenberg ME, Matthaei KI, Young IG, Foster PS. Intrinsic defect in T cell production of interleukin (IL)-13 in the absence of both IL-5 and eotaxin precludes the development of eosinophilia and airways hyperreactivity in experimental asthma. J Exp Med. 2002;195:1433–44. doi: 10.1084/jem.20020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tomkinson A, Cieslewicz G, Duez C, Larson KA, Lee JJ, Gelfand EW. Temporal association between airway hyperresponsiveness and airway eosinophilia in ovalbumin-sensitized mice. Am J Respir Crit Care Med. 2001;163:721–30. doi: 10.1164/ajrccm.163.3.2005010. [DOI] [PubMed] [Google Scholar]
- 57.Wilder JA, Collie DD, Wilson BS, Bice DE, Lyons CR, Lipscomb MF. Dissociation of airway hyperresponsiveness from immunoglobulin E and airway eosinophilia in a murine model of allergic asthma. Am J Respir Cell Mol Biol. 1999;20:1326–34. doi: 10.1165/ajrcmb.20.6.3561. [DOI] [PubMed] [Google Scholar]