Abstract
Objective
To evaluate and identify factors predictive for morbidity after radical nephrectomy in patients with metastatic renal cell carcinoma (mRCC).
Patients and methods
We identified patients with mRCC who underwent nephrectomy at Memorial Sloan-Kettering Cancer Center (MSKCC) between 1989 and 2009.
Postoperative complications were characterised using a modified version of the Clavien-Dindo classification system.
Patient and disease characteristics, including a previously validated MSKCC risk-stratification system using calcium, haemoglobin (Hb), lactate dehydrogenase, and Karnofsky Performance Status (KPS), were evaluated as predictors of postoperative complications using univariate and multivariable logistic regression models.
The area under the receiver operating characteristic curve (AUC) was calculated for each model to assess predictive accuracy and corrected for overfit using 10-fold cross validation.
Results
Over the study period, 195 patients with mRCC underwent nephrectomy; 53 (27%) developed grade ≥2 complications within 8 weeks of surgery.
Pulmonary, thromboembolic events and anaemia requiring transfusion were the most common types of complications after nephrectomy in the metastatic setting.
In univariate analysis, age, low albumin, low KPS, high corrected serum calcium, low serum Hb, and unfavourable MSKCC risk score were predictive of complications.
Patients who sustained postoperative complications were less likely to receive systemic therapy within 56 days (odds ratio [OR] 0.32; 95% confidence interval [CI] 0.12–0.86; P = 0.024).
A multivariable model containing KPS (OR 14.5; 95%CI 4.34–48.6; P < 0.001) and age (OR 1.04; 95%CI 1.01–1.08; P = 0.014) showed the greatest predictive accuracy (corrected AUC 0.72; 95%CI 0.63–0.80) for postoperative complications.
Conclusion
Postoperative complications after radical nephrectomy in the setting of mRCC are common and occur frequently in older patients and those with worse KPS.
These complications are important because they may delay or deny receipt of subsequent systemic therapy.
Keywords: renal cell carcinoma, metastatic, nephrectomy, sunitinib, complications, Clavien
Introduction
RCC is diagnosed in > 58 000 Americans annually and results in the death of nearly 13 000 each year [1]. Despite the increasing incidental detection of smaller renal masses the absolute number of patients presenting with metastatic RCC (mRCC) has not changed significantly over the past 20 years [2]. However, in the same time frame, there has been a paradigm shift in the management of these patients.
For patients with low metastatic burden and good performance status, nephrectomy followed by interferon-α showed improved survival compared with interferon-α alone [3, 4]. However, nephrectomy is an invasive surgical intervention and may not benefit all patients due to limited survival and risk of significant postoperative morbidity.
Tyrosine kinase inhibitors are more effective than interferon-α in prolonging overall survival [5, 6]. This has led to questions about the role and timing of nephrectomy, as patients with significant postoperative morbidity may experience delay or never receive these agents [7–9]. These patients might be more likely to benefit from targeted therapy with their primary tumour in situ. The objective of the present study was to characterise complications among patients undergoing nephrectomy in the setting of metastatic disease, and to identify predictors of complications to better direct early treatment decisions.
Patients and methods
After Institutional Review Board approval was obtained, we performed a multi-surgeon, single institution retrospective review of a prospectively collected database and identified 195 patients with known metastatic disease at the time of surgery who underwent nephrectomy between 1989 and 2009 at Memorial Sloan-Kettering Cancer Center (MSKCC). All charts, outpatient notes, billing records, and correspondence with local physicians were reviewed for complications. All complications within 56 days of surgery were recorded, defined, and graded. The best time to begin postoperative systemic therapy is not entirely clear and our time frame was selected to be all encompassing and capture both early and late complications. Complications were systematically reported using a modified version of the Clavien-Dindo classification system, a widely used scoring system based on the level of intervention required to treat the complication [10, 11].
Patient, treatment and disease characteristics, as well as performance measures were evaluated as predictors of postoperative complications using univariate logistic regression. Additionally, the MSKCC criteria, a previously validated risk-scoring system that has been shown to predict prognosis in patients with advanced RCC, was evaluated as a predictor of complications. This system assigns 1 point to each of the following: lactate dehydrogenase (LDH) of >1.5-times the upper limit of normal, serum haemoglobin (Hb) less than the lower limit of normal, corrected serum calcium (CCa) of >10 mg/mL, Karnofsky Performance Status (KPS) < 80%, or prior nephrectomy [12]. We used a modified version, which excluded prior nephrectomy, so that it could be applied as a preoperative predictive test, a score of 0 is favourable, and >0 is unfavourable. To evaluate the effect of grade ≥2 complications on receipt of systemic therapy after nephrectomy, we performed univariate logistic regression between receipt of systemic therapy within 56 days of nephrectomy and complications.
Three multivariable models were constructed; the first used all patient characteristics and performance measures found to be significant in the univariate model, with the exception of the MSKCC risk score. The second model included the modified risk score but excluded each of the factors included within this system (LDH, Hb, CCa, KPS). The third, a simplified model, included only KPS and age. The area under the receiver operating characteristic curve (AUC) was calculated for each model to assess predictive accuracy and corrected for overfit using 10-fold cross validation.
Results
Characteristics of the study population are listed in Table 1. In all, 195 patients met inclusion criteria; the median (interquartile range, IQR) age of patients was 62 (54–69); 31% were female and 93% were Caucasian. In all, 50 patients (26%) died ≤ 1 year of nephrectomy. In all, 46 patients (24%) had a metastasectomy performed at the same time as nephrectomy, 32 (16%) had a metastasectomy before nephrectomy and 23 (12%) after nephrectomy. Of the patients who had metastasectomy after nephrectomy, 10 were within 56 days and five were within 30 days. Most patients (66%) received systemic therapy at some time (14 patients before and 114 patients after nephrectomy); 86 (44%) ≤ 1 year of nephrectomy.
Table 1.
Summary of patient and disease characteristics for patients presenting with mRCC who underwent nephrectomy (n = 195). Data are median (interquartile range) or frequency (percentage)
| Patient characteristics | Disease characteristics | ||
|---|---|---|---|
| Median (IQR) age at surgery, years | 62 (54–69) | Median (IQR) maximum diameter of tumour, cm | 9 (6–11) |
| Gender, Female, n (%) | 61 (31) | No. of initial metastases, n (%) | |
| Race, Caucasian, n (%) | 181 (93) | 1 | 141 (72) |
| Median (IQR) BMI, kg/m2 | 27 (24–30) | ≥2 | 54 (28) |
| Preoperative eGFRa, mL/min/1.73m2 | 67 (55–78) | Location of metastases, n (%) | |
| Type of symptoms, n (%) | multiple sites | 54 (28) | |
| none | 40 (21) | lung | 65 (33) |
| local | 53 (27) | bone | 37 (19) |
| metastatic | 62 (32) | adrenal | 12 (6) |
| systemic | 40 (21) | brain | 6 (3) |
| Low serum Hbb, n (%) | 99 (51) | other | 21 (11) |
| High CCa (> 10 mg/dL), n (%) | 13 (7) | Clear cell histology, n (%) | 179 (92) |
| Low albumin (<4g/dL), n (%) | 49 (25) | Positive surgical margins, n (%) | 27 (15) |
| High LDHc, n (%) | 6 (3) | Positive lymph nodes, n (%) | 37 (29) |
| High white blood cell count (> 4–11 K/μL), n (%) | 30 (15) | Pathological T-stage (≥T3a), n (%) | 137 (70) |
|
| |||
| Performance measures | Treatment characteristics | ||
|
| |||
| Charlson comorbidity index, n (%) | Neoadjuvant treatment, n (%) | 14 (7) | |
| = 6 | 148 (76) | Postoperative systemic therapy, n (%) | 114 (58) |
| > 6 | 47 (24) | Receipt of any systemic therapyd, n (%) | 120 (62) |
| Age-adjusted Charlson score, n (%) | Procedure type, n (%) | ||
| ≤8 | 131 (67) | Radical nephrectomy | 176 (90) |
| ASA score, n (%) | Partial nephrectomy | 19 (10) | |
| 1 | 4 (2) | Laparoscopic approach | 12 (6) |
| 2 | 108 (55) | Side of procedure, n (%) | |
| 3 | 81 (42) | right | 95 (49) |
| missing | 2 (1) | left | 98 (50) |
| Low KPS (< 80%), n (%) | 18 (9) | bilateral | 2 (1) |
| Modified MSKCC criteria, n (%) | Curative metastasectomye, n (%) | 87 (45) | |
| favourable | 86 (44) | before nephrectomy | 32 (16) |
| unfavourable | 102 (52) | concurrent | 46 (24) |
| unknown | 7 (4) | after nephrectomy | 23 (12) |
Chronic Kidney Disease Epidemiology Collaboration;
male < 13 g/dL, female < 11.5 g/dL;
> 1.5 times the upper limit of normal;
eight patients had systemic before and after nephrectomy;
14 patients had more than one metastasectomy; BMI, body mass index; eGFR, estimated GFR; ASA, American Society of Anesthesiologists.
All but one of the event-free patients had at least 8 weeks of follow-up after nephrectomy, unless they died within that time frame, and 53 patients (27%) had grade ≥ 2 complications. One patient had 55 days of follow-up. The median (IQR) time to grade ≥ 2 complication was 5 (2–14) days. Table 2 stratifies patients according to highest complication grade at 56 days postoperatively; most (70%) complications were moderate (grade 2), 16 patients (8% of all patients) experienced major (grade ≥ 3) complications. Table 3 lists the type of complications. The most frequent was pulmonary complications, which occurred in 10 patients, 19% of the 53 patients with moderate or major complications. Nine patients had grade 5 complications (death) ≤ 56 days of surgery, four of these patients died from progression of disease while the remaining five died from cardiac, pulmonary, metabolic and neurological complications (Table 3).
Table 2.
Patients’ complications by 56 days after nephrectomy in the setting of mRCC
| Maximum grade of complications | Level of severity of complication | Patients with complications, n (%) | Proportion of complications |
|---|---|---|---|
| 1 | Minor | 19 (10) | Not included |
| 2 | Moderate | 37 (19) | 70 |
| 3 | Major | 7 (4) | 13 |
| 4 | Major | 0 (0) | 0 |
| 5 | Major | 9 (5) | 17 |
Table 3.
Patients’ complications of grade ≥ 2 by 56 days after nephrectomy by type of highest grade complicationin the setting of mRCC
| Type of complication | Patients, n(%) | Proportion of complications, % | Range of complication grades |
|---|---|---|---|
| Pulmonary | 10 (5) | 19 | 2–5 |
| Thromboembolic | 8 (4) | 15 | 2–3 |
| Bleeding/Anemia | 7 (4) | 13 | 2 |
| Metabolic | 5 (3) | 9 | 2–5 |
| Infectious | 5 (3) | 9 | 2–3 |
| Gastrointestinal | 4 (2) | 8 | 2 |
| Death from progression of disease | 4 (2) | 8 | 5 |
| Cardiac | 3 (2) | 6 | 2–5 |
| Neurologic | 3 (2) | 6 | 2–5 |
| Wound | 2 (1) | 4 | 2 |
| Genitourinary | 2 (1) | 4 | 2–3 |
On univariate analysis (Table 4) the patient characteristics that predicted grade ≥ 2 complications were age at surgery (P = 0.035), low albumin (P = 0.042), high CCa (P = 0.008), and low Hb (P = 0.024). Additionally, the performance measures, low KPS (P < 0.001) and unfavourable modified MSKCC risk score (P = 0.003) also predicted complications. Disease and treatment characteristics, e.g. number or location of metastatic disease or surgical approach (open vs laparoscopic), were not significant predictors of complications. Patients with grade ≥ 2 complications ≤ 8 weeks of nephrectomy were less likely to receive systemic therapy within 56 days of nephrectomy (odds ratio [OR] 0.32; 95% CI 0.12–0.86; P = 0.024), which supports our hypothesis that complications are associated with forgoing systemic therapy.
Table 4.
Results of univariate logistic regression of patient and disease characteristics predicting grade ≥ 2 complications within 8 weeks among patients presenting with mRCC who underwent nephrectomy (n = 195)
| Patient characteristics | Disease characteristics | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| OR | 95% CI | P | OR | 95% CI | P | ||
| Age at surgery | 1.03 | 1.00–1.07 | 0.035 | Diameter of tumour | 0.98 | 0.90–1.07 | 0.7 |
| Gender, Female | 0.73 | 0.36–1.47 | 0.4 | No. of initial metastases | |||
| Race, Caucasian | 0.65 | 0.21–2.04 | 0.5 | 1 | Reference | ||
| BMI | 0.94 | 0.88–1.01 | 0.078 | ≥2 | 1.04 | 0.52–2.10 | 0.9 |
| Preoperative eGFRa | 1.00 | 0.98–1.01 | 0.6 | Location of metastases | |||
| Type of symptoms | multiple sites | Reference | |||||
| none | Reference | lung | 0.85 | 0.37–1.93 | 0.7 | ||
| local | 1.12 | 0.42–2.96 | 0.8 | bone | 0.96 | 0.38–2.46 | 0.9 |
| metastatic | 1.30 | 0.51–3.29 | 0.6 | adrenal | 1.30 | 0.34–4.96 | 0.7 |
| systemic | 1.85 | 0.69–4.97 | 0.2 | brain | 1.30 | 0.22–7.86 | 0.8 |
| Low serum Hbb | 2.12 | 1.11–4.07 | 0.024 | other | 1.04 | 0.34–3.18 | 0.9 |
| High CCac | 4.84 | 1.51–15.5 | 0.008 | Clear cell histology | 0.81 | 0.27–2.44 | 0.7 |
| Low albumin (<4g/dL) | 2.05 | 1.03–4.09 | 0.042 | Positive surgical margins | 1.19 | 0.48–2.93 | 0.7 |
| High LDHd | 1.41 | 0.25–7.98 | 0.7 | Positive lymph nodes | 0.94 | 0.39–2.28 | 0.9 |
| High white blood cell count e | 1.18 | 0.50–2.77 | 0.7 | Pathological T-stage (≥T2) | 1.25 | 0.62–2.54 | 0.5 |
|
| |||||||
| Performance measures | Treatment characteristics | ||||||
|
| |||||||
| OR | 95% CI | P | OR | 95% CI | P | ||
|
| |||||||
| Charlson index: | Neoadjuvant treatment | 1.08 | 0.32–3.60 | 0.9 | |||
| = 6 | Reference | Nephrectomy type: | |||||
| > 6 | 1.12 | 0.53–2.36 | 0.8 | partial | Reference | ||
| Age-adjusted Charlson score: | radical | 1.05 | 0.36–3.07 | 0.9 | |||
| ≤8 | 0.66 | 0.34–1.28 | 0.2 | Procedure type: | |||
| ASA score: | open | Reference | |||||
| 1 | Reference | laparoscopic | 1.37 | 0.39–4.74 | 0.6 | ||
| 2 | 0.95 | 0.09–9.54 | >0.9 | Side of procedure: | |||
| 3 | 1.26 | 0.13–12.8 | 0.8 | right | Reference | ||
| missing | left | 0.91 | 0.48–1.71 | 0.8 | |||
| Low KPS (<80%) | 12.3 | 3.83–39.5 | <0.001 | bilateral | 1.00 | 1.00–1.00 | >0.9 |
| Modified MSKCC criteria: | Curative metastasectomy | 1.04 | 0.55–1.96 | 0.9 | |||
| favourable | Reference | ||||||
| unfavourable | 2.93 | 1.45–5.90 | 0.003 | ||||
Chronic Kidney Disease Epidemiology Collaboration, ml/min per 1.73m2;
male < 13g/dL, female < 11.5 g/dL;
> 1.5-times the upper limit of normal;
> 10 mg/dL;
> 4–11 K/μL; ASA, American Society of Anesthesiologists; BMI, body mass index; eGFR, estimated GFR.
Multivariable logistic regression models were constructed to predict the risk of complications (Table 5). The first model included all significant univariate predictors, except modified MSKCC risk criteria. Low KPS (OR 11.2; 95%CI 3.19–39.4; P < 0.001) and increasing age (OR 1.05; 95%CI 1.01–1.09; P = 0.007) remained statistically significant predictors of complications (AUC 0.74). A second model including the modified MSKCC risk score, albumin and age, showed that the risk score (OR 2.96; 95% CI 1.40–6.24; P = 0.005) and age (OR 1.03; 95% CI 1.00–1.07; P = 0.033) were significant albeit with lower predictive accuracy (AUC 0.69). A third, simplified model was created using only age and KPS; this model had an AUC of 0.73.
Table 5.
Results of multivariable logistic regression predicting grade ≥ 2 complications within 8 weeks among patients presenting with mRCC who underwent nephrectomy
| Model 1, n = 193 | Model 2, n = 188 | Model 3, n = 194 | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Age at surger | 1.05 | 1.01–1.09 | 0.007 | 1.03 | 1.00–1.07 | 0.033 | 1.04 | 1.01–1.08 | 0.014 |
| Low KPS (< 80%) | 11.2 | 3.19–39.4 | <0.001 | 14.5 | 4.34–48.6 | <0.001 | |||
| Albumin (< 4 g/dL) | 0.87 | 0.36–2.12 | 0.8 | 1.17 | 0.54–2.55 | 0.7 | |||
| Modified MSKCC risk criteria (unfavourable) | 2.96 | 1.40–6.24 | 0.005 | ||||||
| High CCa (>10 mg/dL) | 3.45 | 0.85–14.1 | 0.084 | ||||||
| Low serum Hba | 1.93 | 0.90–4.15 | 0.092 | ||||||
|
| |||||||||
| AUC | 0.74 | 0.69 | 0.73 | ||||||
|
| |||||||||
| Corrected AUC (95% CI) | 0.71 (0.62–0.80) | 0.63 (0.54–0.72) | 0.72 (0.63–0.80) | ||||||
Male < 13g/dL, female <11.5 g/dL.
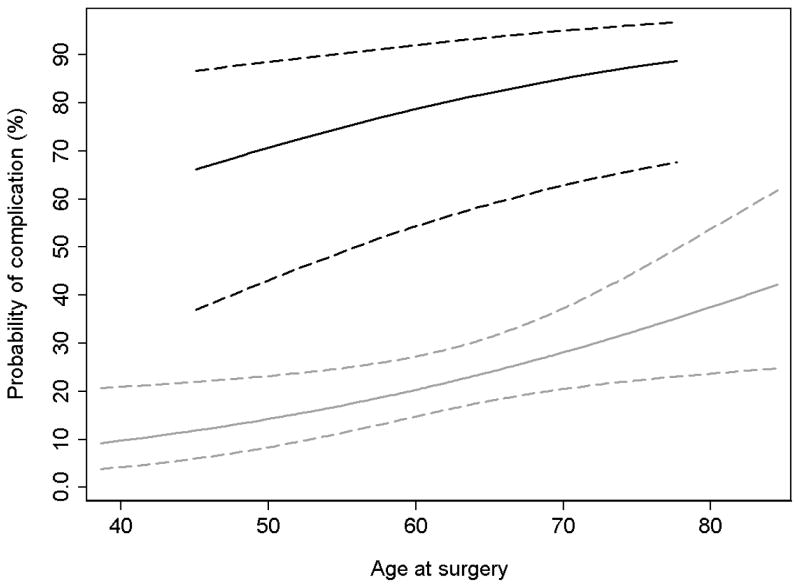
To account for overfit, we calculated corrected AUCs using 10-fold cross validation. Based on uncorrected AUCs, model 1 appears to have better discrimination than model 3; this however, is due to overfit. Once adjusted, the simple model is the most accurate and parsimonious model (corrected AUCs, model 3: 0.72; Model 1: 0.71). Based on model 3, we constructed Figure 1 to show the relationship between age and probability of complications, by KPS. For both low (<80), and higher KPS (>80) the risk of complications increases with age, but for any given age the risk of complications are much greater with low KPS.
Figure 1. Probability of complication by age.
Black lines represent KPS of < 80, grey, KPS ≥ 80. Dashed lines indicate CIs.
These analyses were repeated assessing the rates of complications ≤ 30 days of surgery; 46 patients (24%) had grade ≥ 2 complications. Results were similar in this analysis and there were no new factors that predicted complications. Age, albumin, and Hb were no longer predictive on univariate analysis; however, KPS and modified MSKCC criteria scores remained significant in both the univariate and multivariable models.
Discussion
In the present study we characterised postoperative morbidity using minor modifications to a well-established complication grading system. Furthermore, we developed a model to predict complications based on various clinical, oncological, and surgical factors [10]. Moderate or major complications (grade ≥ 2) occurred within 56 days in 27% of patients who underwent nephrectomy for mRCC. The most common types of complications after nephrectomy were pulmonary, thromboembolic events and acute blood loss anaemia requiring blood transfusion. The best predictors of increasing complications were age and KPS; various other performance measures were not independently associated with postoperative morbidity [13]. Additionally, a previously validated risk-stratification system based on KPS, CCa, Hb, and LDH also predicted complications in multivariate analysis, albeit with less accuracy than simply age and KPS. These findings are meaningful because as we show in the present study, complications after nephrectomy in the setting of mRCC may result in omission or delay of systemic treatment.
The role and timing of nephrectomy in the modern era of targeted therapy has not been fully elucidated and randomised trials comparing nephrectomy followed by sunitinib to sunitinib alone are currently accruing [7]. Imperative to defining the role of nephrectomy for patients with mRCC is clarifying the morbidity. Simple extrapolation of complication data from series based on localised disease is inaccurate as population-based studies have shown in-hospital mortality, complication rates and transfusion rates to be greater in patients with mRCC [14].
KPS is a widely accepted numerical scale used to characterise a patient with cancer’s ability to perform normal physical activities and care for themselves [15]. It is highly correlated with prognosis in various malignancies, including mRCC, and has been used to guide enrolment in clinical trials [12]. A KPS of 80% characterises a patient as: capable of normal activity, but with effort, and exhibiting some symptoms of disease. We have shown that a KPS of < 80% is highly correlated with postoperative complications. This is not an unexpected finding, as these patients have less reserve and are more likely to have a difficult recovery after surgical intervention. Decreased KPS has been shown to be correlated with increased risk of postoperative complications in other surgical settings [16, 17]. Perhaps not surprising, increasing age was also associated with higher complication rates and has been similarly shown in patients with localised kidney cancer [18].
Additionally, we evaluated a modified version of a categorical staging system previously used to predict survival in patients with mRCC and survival after RCC recurrence [12, 19]. A model including age, albumin and the modified MSKCC risk criteria has lower predictive accuracy (corrected AUC 0.63) than a model including age, albumin and all individual risk criteria components (corrected AUC 0.71). A model including only age and KPS, showed essentially equivalent predictive accuracy as the more complex model, emphasising the importance of age and KPS in predicting postoperative complications.
Considering the association between low KPS score and inferior overall survival [12], and increased risk of postoperative morbidity, careful consideration of treatments other than upfront nephrectomy are reasonable. If patients with mRCC elect to delay or forgo nephrectomy due to increased risk of postoperative complications, treatment with systemic agents can be initiated with the option to surgically intervene later (integrative cytoreductive nephrectomy) pending improvement in KPS score and the extent of disease. Preoperative treatment with targeted systemic agents has been shown to result in no increase in postoperative morbidity after nephrectomy [20].
Various factors associated with increased surgical complexity, e.g. large tumour diameter, positive surgical margins, high stage (≥T3a), positive lymph nodes, type of surgical intervention (radical vs partial nephrectomy) or surgical approach (laparoscopy vs open), or curative metastasectomy did not correspond with increased risk of postoperative morbidity. This may be due to the overall surgical complexity of the entire cohort, as exemplified by the mean tumour diameter of 9 cm, and 70% with pathological ≥T3a lesions. Additionally, the location of metastatic lesion(s) and number of metastatic lesions did not correspond with increased risk of postoperative morbidity.
In the present study, a threshold of 56 days was chosen to account for both early and late complications, both of which may influence the decision or ability to receive subsequent adjuvant treatment. Most postoperative complications occurred in the immediate postoperative setting, at a median (IQR) time to event of 5 (2–14) days. The best time to begin postoperative systemic therapy or enrolment in clinical trials has not been elucidated. In an ongoing randomised trial assessing the role of nephrectomy with targeted agents, patients are to begin systemic therapy ≤ 42 days of nephrectomy [7]. In the original trials showing survival benefit to nephrectomy in the setting of metastatic disease, patients received immunotherapy ≤ 30 days of nephrectomy [3, 4]. To account for potential differences in complications at different time points, a sensitivity analysis using a threshold of 30 days was performed and low KPS score and unfavourable modified risk score continued to predict a higher risk of complications in both univariate and multivariable models.
One important limitation of the present study is that the indications for receipt of adjuvant therapy were not uniform across all patients. Some patients underwent nephrectomy with the intent to receive immediate adjuvant therapy a priori, while for others, additional therapy was electively delayed until there was radiographic evidence of disease progression. This variability was due to both the multiple systemic therapies used over the many years of the present study, as well as the varied preferences of the physicians and patients. The lack of uniformity in both the type of systemic therapies received and the indications for their use limit the generalizability of the present findings for the timing of receipt of systemic therapy. Nevertheless some complications unquestionably limit or delay the ability to receive systemic therapy and the present data clearly showed that patients with grade ≥ 2 complications were less likely to receive systemic therapy ≤ 56 days.
Patients in the present study were deemed fit to undergo nephrectomy. Thus there is an important selection bias; however, our group has similar demographics and oncological characteristics to that of other published series of patients receiving nephrectomy in the metastatic setting [21]. Also the modified Clavien-Dindo classification system is based on the invasiveness of the intervention used to resolve the postoperative complication and this may not correlate with potential to receive systemic therapy. Despite these limitations, the Clavien-Dindo grading system is frequently cited and readily accepted as an accurate method of reporting complications [11]. Additionally, in the present study, patients with postoperative complications were less likely to receive systemic therapy (OR 0.32). Finally, receipt and timing of metastasectomy may have introduced a potential confounder; however, on univariate analysis we saw no evidence to suggest a relationship between metastasectomy and complications. We also performed sensitivity analyses omitting patients who received metastasectomy within the time frames that complications were measured (five patients ≤ 30 days and 10 ≤ 56 days) and this too had no impact on our outcomes.
In conclusion, in the era of targeted therapy the timing and role of nephrectomy for mRCC is not entirely clear, particularly when nephrectomy results in significant delay or inability to receive systemic treatment. We reviewed and classified all complications after nephrectomy in patients with mRCC and constructed a model to predict complications. Grade ≥ 2 complications ≤ 8 weeks of surgery, occurred in 27% of patients and the most common type of complications were pulmonary, thromboembolic and anaemia requiring blood transfusion. These were more likely to occur in older patients and those with lower KPS scores. Such complications may delay systemic treatment with targeted therapy or prevent entry into clinical trials. Caution should be taken in performing nephrectomy on these patients and consideration may be given to initiating targeted therapy before nephrectomy.
What’s known on the subject? and What does the study add?
It is known that radical nephrectomy for patients with metastatic disease is a more involved procedure and results in greater rates of complications than with radical nephrectomy for localised disease.
This is the first paper to systematically characterise complications associated with nephrectomy for metastatic renal cell carcinoma (mRCC). Additionally, our data suggest that various clinical and oncological factors may not be correlated with postoperative complications as some might believe. Importantly, increasing age and worsening performance status were both associated with increased probability of complications. Furthermore when complications were sustained patients were less likely to receive systemic therapy in a timely fashion. This suggests that for older patients and those with worse performance status, surgeons should be aware that these patients are at greatest risk of complications after nephrectomy for mRCC and these complications may result in delay or omission of important systemic medical therapy.
Acknowledgments
Supported by the Sidney Kimmel Center for Prostate and Urologic Cancers, by funds provided by David H. Koch through the Prostate Cancer Foundation, and by the National Cancer Institute (NCI) (U54CA137788 and U54CA132378).
Abbreviations
- mRCC
metastatic RCC
- MSKCC
Memorial Sloan-Kettering Cancer Center
- LDH
lactate dehydrogenase
- Hb
serum haemoglobin
- CCa
corrected serum calcium
- KPS
Karnofsky Performance Status
- AUC
area under the receiver operating characteristic curve
- IQR
interquartile range
- OR
odds ratio
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Sun M, Thuret R, Abdollah F, et al. Age-adjusted incidence, mortality, and survival rates of stage-specific renal cell carcinoma in North America: a trend analysis. Eur Urol. 2011;59:135–41. doi: 10.1016/j.eururo.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345:1655–9. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 4.Mickisch GH, Garin A, van Poppel H, de Prijck L, Sylvester R European Organisation for Research and Treatment of Cancer (EORTC) Genitourinary Group. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001;358:966–70. doi: 10.1016/s0140-6736(01)06103-7. [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–90. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russo P, O’Brien MF. Surgical intervention in patients with metastatic renal cancer: metastasectomy and cytoreductive nephrectomy. Urol Clin North Am. 2008;35:679–86. doi: 10.1016/j.ucl.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Russo P. Multi-modal treatment for metastatic renal cancer: the role of surgery. World J Urol. 2010;28:295–301. doi: 10.1007/s00345-010-0530-x. [DOI] [PubMed] [Google Scholar]
- 9.Russo P, Synder M, Vickers A, Kondagunta V, Motzer R. Cytoreductive nephrectomy and nephrectomy/complete metastasectomy for metastatic renal cancer. Scientific World Journal. 2007;7:768–78. doi: 10.1100/tsw.2007.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shabsigh A, Korets R, Vora KC, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol. 2009;55:164–74. doi: 10.1016/j.eururo.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 12.Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530–40. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 13.Schroeder RA, Marroquin CE, Bute BP, Khuri S, Henderson WG, Kuo PC. Predictive indices of morbidity and mortality after liver resection. Ann Surg. 2006;243:373–9. doi: 10.1097/01.sla.0000201483.95911.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdollah F, Sun M, Thuret R, et al. Mortality and morbidity after cytoreductive nephrectomy for metastatic renal cell carcinoma: a population-based study. Ann Surg Oncol. 2011;18:2988–96. doi: 10.1245/s10434-011-1715-2. [DOI] [PubMed] [Google Scholar]
- 15.Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol. 1984;2:187–93. doi: 10.1200/JCO.1984.2.3.187. [DOI] [PubMed] [Google Scholar]
- 16.Rabadan AT, Hernandez D, Eleta M, et al. Factors related to surgical complications and their impact on the functional status in 236 open surgeries for malignant tumors in a Latino-American hospital. Surg Neurol. 2007;68:412–20. doi: 10.1016/j.surneu.2006.11.050. [DOI] [PubMed] [Google Scholar]
- 17.Asano K, Nakano T, Takeda T, Ohkuma H. Risk factors for postoperative systemic complications in elderly patients with brain tumors. Clinical article J Neurosurg. 2009;111:258–64. doi: 10.3171/2008.10.17669. [DOI] [PubMed] [Google Scholar]
- 18.Lowrance WT, Yee DS, Savage C, et al. Complications after radical and partial nephrectomy as a function of age. J Urol. 2010;183:1725–30. doi: 10.1016/j.juro.2009.12.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eggener SE, Yossepowitch O, Pettus JA, Snyder ME, Motzer RJ, Russo P. Renal cell carcinoma recurrence after nephrectomy for localized disease: predicting survival from time of recurrence. J Clin Oncol. 2006;24:3101–6. doi: 10.1200/JCO.2005.04.8280. [DOI] [PubMed] [Google Scholar]
- 20.Margulis V, Matin SF, Tannir N, et al. Surgical morbidity associated with administration of targeted molecular therapies before cytoreductive nephrectomy or resection of locally recurrent renal cell carcinoma. J Urol. 2008;180:94–8. doi: 10.1016/j.juro.2008.03.047. [DOI] [PubMed] [Google Scholar]
- 21.Culp SH, Tannir NM, Abel EJ, et al. Can we better select patients with metastatic renal cell carcinoma for cytoreductive nephrectomy? Cancer. 2010;116:3378–88. doi: 10.1002/cncr.25046. [DOI] [PubMed] [Google Scholar]