Abstract
Evaluation of compound activity in vitro is crucial to drug discovery efforts and require that the compounds be accurately and reliably titrated and dispensed to the assay wells. The HP D300 dispenser utilizes inkjet technology to achieve small volume dispensing that allows concentration response testing using the direct dilution paradigm. While inkjet technology has been long in existence, it is new to the field of screening and drug development. We have evaluated the D300 dispenser in a biochemical assay, a cell-based reporter gene assay and a cytotoxicity assay. The software for this instrument is user-friendly and the compound dispensing process is streamlined. However, a limitation is that this dispenser is currently only applicable to 96-well and 384-well plate formats, and not to 1536-well high density plates. Our results indicate that the D300 generates clean and reproducible results that correlate with those produced with more commonly used instruments such as the pin tool. We found that the instrument is useful and can improve the throughput of compound dispensing in 96-well and 384-well plates.
Keywords: compound dispenser, liquid handler, compound screen, serial dilution, direct dilution
INTRODUCTION
Evaluation of in vitro compound activity and selectivity constitutes a key step in small molecule drug discovery. Accurate measurements of compound concentration responses guide the lead optimization process, in which hundreds and thousands of compounds are synthesized and tested before a final drug candidate is chosen. In these experiments, the ability to dispense compound solutions efficiently and accurately is critical for obtaining reliable and reproducible results of compound activities.
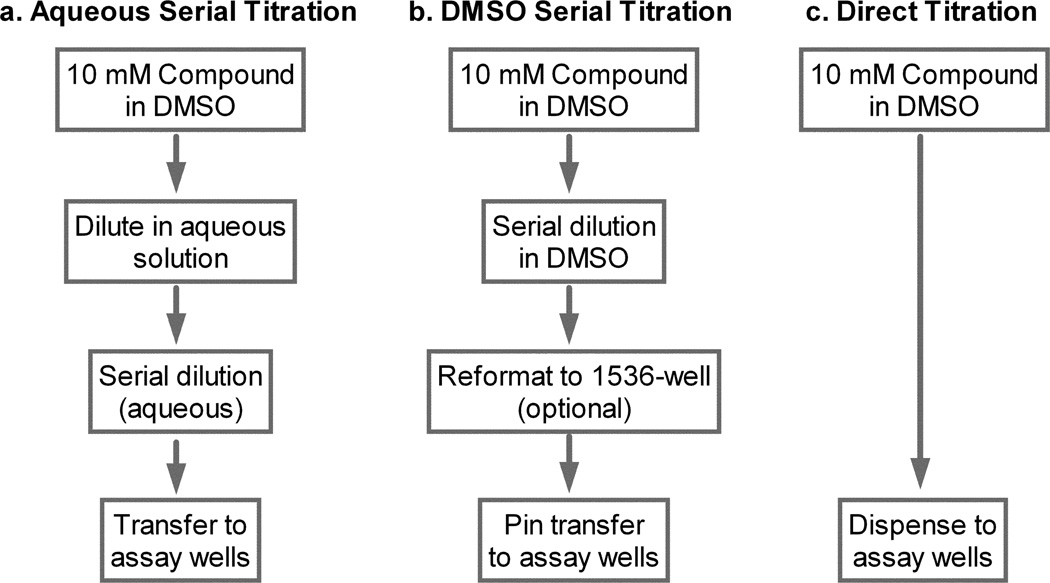
Stock compound solutions are commonly dissolved in DMSO for drug screening. Compound dilutions can be done using a variety of methods depending on the needs and capabilities of the laboratory. A traditional approach common in basic research laboratories is the use of manual dilution and dispensing (Fig. 1a). While this approach can be aided by the use of multi-channel and/or electronic pipettes, this method is error prone and limited in both throughput and miniaturizability. Due to the inability of pipettes to dispense volumes below 0.1 µl, compound stock solutions are typically serially diluted in aqueous buffer to minimize the concentration of DMSO in assay wells. However, this method causes compounds with poor aqueous solubility to precipitate out of buffer solutions that results in right-shifted dose response curves. In the last decade, a number of automated instruments capable of dispensing nanoliter and picoliter volumes of compounds have become available for compound handling including piezoelectric, pin tool and acoustic dispensers 1. The use of these instruments reduces human error through automated compound serial dilution and dispensing, and eliminates the aqueous intermediate dilution by allowing accurate low volume dispensing of compound DMSO solutions directly to assay wells. Therefore, these compound dispensing instruments greatly increase the accuracy of the measurement of compound potency, reduces compound consumption and increases screening throughput (Fig. 1b and c).
Fig. 1.
Workflow schematic of three compound dilution and dispensing protocols. (a) Aqueous serial dilution. (b) DMSO serial dilution. (c) Direct dilution.
Recently, a thermal inkjet dispensing technology has been applied to compound dispensing. The HP D300 dispenser, utilizing this dispensing technology, is now available for compound dilution and dispensing in 96- and 384-well plate formats. This instrument, similar to those described above, is capable of compound dispensing in picoliter and nanoliter scales. Thus, it can be used for a direct dilution compound dispensing paradigm without the intermediate compound dilution plate as those dispensing technologies described above. A single compound stock solution (usually in DMSO) can be dispensed at different volumes to achieve a range of compound concentrations in the assay wells (Fig. 1c). This instrument has a wide range of compound dispensing dynamics starting from 13 pl up to micro liter with 1 nl increment for the compounds dissolved in DMSO solution. We report here an evaluation and comparison of the HP D300 dispenser for compound dilution in three types of common screening assays including a biochemical enzyme assay, a cell-based reporter gene assay and a cytotoxicity assay. Our results demonstrate that the HP D300 dispenser generates accurate and reproducible IC50s that correlate with those determined using pin tool transfer.
MATERIALS AND METHODS
Materials
Dexamethasone, hydrocortisone, prednisolone, tamoxifen, tetraoctylammonium bromide (TOAB), green coffee bean α-galactosidase A (GLA), 4-methylumbelliferyl α-D-galactopyranoside (4MU-α-galc), 1-deoxygalactonojirimycin (DGJ), lansoprazole, and phenylmercuric acetate (PMA) were purchased from Sigma-Aldrich. Daunorubicin was purchased from Tocris. CCF2-AM, CellSensor® MMTV-bla HeLa, all cell culture media, and Dulbecco phosphate-buffered saline (PBS) were purchased from Life Technologies. CellTiter-Glo® Luminescent Cell Viability Assay was purchased from Promega (Madison, WI). All compound and assay plates were purchased from Greiner Bio-One (Monroe, NC).
Instrumentation
Cells were dispensed using the Multi-drop Combi (Thermo Scientific). Enzyme and detection reagents were dispensed using the BioRAPTR FRD (Beckman Coulter). In 384-well format varying concentrations of compounds were dispensed using the HP D300 Digital Dispenser (Tecan, Durham, NC). The CyBi-Well dispensing station (Cybio, Bedford, MA), was used to reformat compound dilutions from 384-well to 1536-well plates. Compound dispensing in 1536-well format used the pintool station (Wako Automation USA, San Diego, CA). Signal detection was done with the EnVision and ViewLux plate readers (PerkinElmer, Waltham, MA).
Compound dilution and pin transfer
Stock DMSO solutions for each compound were serially diluted manually 1:3 in DMSO in a 384-well polypropylene plate (Greiner Bio-One), then reformated to a 1536-well polypropylene plate (Greiner Bio-One) with the CyBi-well dispensing station. For serial dilution, 12 µl of the compound solution was transferred into 24 µl DMSO in subsequent dilution wells, and each solution was mixed by pipetting 3 times. Then, the compounds were reformatted to a 1536-well plate with the CyBi-well dispensing station by aspirating 20 µl of each solution from the 384-well plate and dispensing 5 µl into 4 replicate wells in a 1536-well plate. The compound solutions in DMSO were dispensed at 23 nL volumes directly to buffer or medium in assay plates with a pin tool station.
Enzyme assay
The α-galactosidase (GLA) enzyme assay was previously reported 2. In brief, 13 μl/well of 2 nM GLA enzyme in citric acid buffer (50 mM citric acid, 115 mM K2PO4, 110 mM KCl, 10 mM NaCl, 1 mM MgCl2, and 0.01% Tween-20 at pH 5) was dispensed into a solid-bottom, black 384-well plate with the BioRAPTR FRD microfluidic workstation. The compounds were dispensed in a 10-point 1:3 dilution with the HP D300. After a 5 min room temperature incubation, the reaction was initiated by dispensing 13 μl/well of 20 µM 4-methylumbelliferyl-α-D-galactopyranoside (4MU-α-galc) in citric acid buffer. The reaction was allowed to proceed for 20 min at room temperature before 26 µl/well of stop solution (1 M glycine, 1 M NaOH) was added and the signal was read with an EnVision plate reader.
In 1536-well format, 2 ul/well of 2 nM GLA enzyme in citric acid buffer was dispensed into a solid-bottom, black plate with a BioRAPTR FRD microfluidic workstation. The compounds were dispensed as 23 nl/well volumes via pin tool transfer with 3 pin dips in the assay plate for mixing. After a 5 min room temperature incubation, the reaction was initiated by dispensing 2 μl/well of 20 µM 4MU-α-galc in citric acid buffer. The reaction was allowed to proceed for 20 min at room temperature before the addition of 2 µl/well stop solution and the signal was read with a ViewLux plate reader.
Reporter gene assay
Life Technologies MMTV-βLa HeLa cells were cultured in growth media (DMEM 10% FBS, 2.5 µg/ml blasticidin, 100 µM nonessential amino acids, 1mM pyruvate, 50 U penicillin, 50 µg/ml streptomycin) using standard cell culture conditions. The beta-lactamase assay was performed according to manufacturer’s instructions. In brief, the cells were dispensed at 4000 cells/well in 26 µl of assay media (OPTI-MEM, 1% charcoal-stripped FBS, 100 µM nonessential amino acids, 1mM pyruvate, 50 U penicillin, 50 µg/ml streptomycin) into 384-well, black, clear-bottom plates with the Multidrop Combi dispenser. Cells were allowed to attach during a 16–20 hr incubation at 37°C, 5% CO2. The test compounds were dispensed using the HP D300 Digital Dispenser in 10-point 1:3 dilution. The cells were stimulated with compounds during a 4 hr incubation at 37°C, 5% CO2. Subsequently, the detection reagent CCF2-AM was reconstituted according to manufacturer’s instructions and dispensed at 6 µl/well. The detection reagent was incubated for 1 hr at room temperature before the signal was read using the bottom read protocol on an EnVision plate reader.
In 1536-well plate format, the cells were dispensed at 1000 cells/well in 4 μl of assay media, into black, clear-bottom plates. Following a 16–20 hr incubation at 37°C, 5% CO2, 23 nl of compound dilution was dispensed via pin tool transfer with 3 pin dips in the assay plate to mix the reagents. The cells were stimulated for 4 hours with the compounds before the addition of 1 µl/well CCF2-AM detection reagent. After a 1hr room temperature incubation, the plates were read using the EnVision plate reader.
Cytotoxicity assay
Life Technologies MMTV-βLa HeLa cells were cultured in growth media (DMEM 10% FBS, 2.5 µg/ml blasticidin, 100 µM nonessential amino acids, 1 mM pyruvate, 50 U penicillin, 50 µg/ml streptomycin) using standard cell culture conditions. The cells were dispensed at a density of 1000 cells/ well in 26 µl of growth media, into 384-well, white, solid-bottom plates. After an initial attachment period of 16–20 hr at 37°C, the compounds were dispensed using the HP D300 and incubated with the cells for 72 hr under standard cell culture conditions. The cytotoxic effects of these compounds were evaluated with the addition of CellTiter-Glo® ATP content detection reagent at 20 µl/well. After a 10 min incubation at room temperature, the luminescence signal was read on a ViewLux plate reader.
In the 1536-well format, the cells were plated at 250 cells/well, in 6 µl of growth media, into 1536-well, white, solid-bottom plates, and incubated for 16–20 hr at 37°C. The compound dilutions were dispensed as 23 nl/well pin tool transfer with 3 pin dips in the assay plate to mix the reagents, and were incubated with the cells for 72 hr. The ATP levels were evaluated by dispensing 4 µl/well of CellTiter-Glo® and the signal was read on the ViewLux plate reader after a 5 min room temperature incubation.
Data analysis
Concentration-response curves were analyzed and EC50 values, reported as mean ± standard deviation (SD), calculated using Prism software (GraphPad). Results in figures are expressed as mean of triplicates ± SD. Correlation of variation (CV) values are calculated from 12 wells treated with top concentration of each compound dispensed by the HP D300 in 384-well plate format and 16 wells treated with top concentration of each compound dispensed with the pin tool in 1536-well format.
RESULTS
Operation of HP D300 dispenser
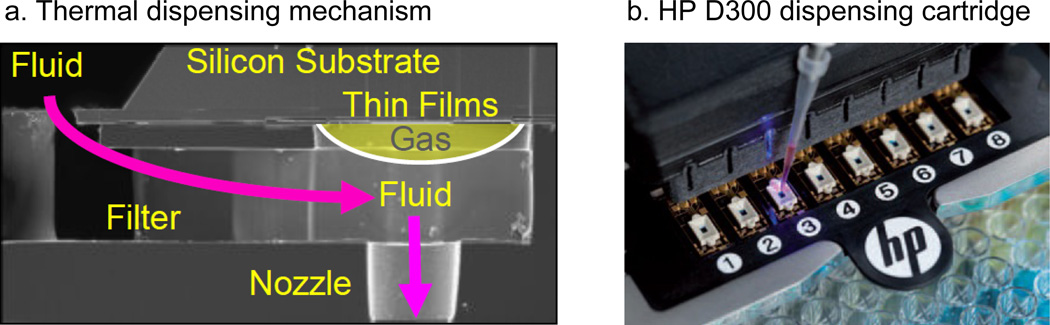
The HP D300 instrument utilizes inkjet thermal dispensing technology, in which force is generated by gas bubble expansion to drive fluid through a nozzle (Fig. 2a). The gas bubble is created by a liquid to vapor phase conversion at the surface of a thin film resistor fabricated on silicon. After droplet ejection, fluid is replaced from the reservoir through a slot in the silicon, and this is repeated thousands of times per second. Compound dispense is controlled by the digital dispensing software, which interfaces with the instrument. The digital dispensing software first prompts the appropriate volumes of DMSO or compounds to be loaded into each dispense head (Fig. 2b). The compound reservoirs and nozzles are arrayed in 8-channel dispense heads mounted on a disposable dispense cartridge (Fig. 2b). The next step is to design a plate map for compound dispensing using the software that dictates dilution and volumes of each compound with the lowest dispense volume of 13 pl. The compound in DMSO stock solution is loaded at 2 to 6 μl to one of the 8 reservoir on a dispense cartridge that is inserted into the instrument. After loading the compound cartridge and an assay plate (either dry or with aqueous solution) to the instrument, the compound dispensing can be initiated that is typically completed in 1 minute in a 384-well plate, depending on dispensing volume. Compound dilution concentrations in the assay plate generated from this instrument can span four orders of magnitude with a single compound stock solution (typically at 1 or 10 mM). There is an option to backfill the assay wells with DMSO to equalize the final amount of DMSO in each well. For the following experiments, a 10-point 1:3 dilution was generated from one DMSO stock per compound at varying starting concentration with dispense volumes ranging from 13 pl to 0.26 µl and DMSO was backfilled in order to keep the equal volume of final DMSO in each well. The final DMSO concentration in the assays was 1 %. We also tried the same experiments with compound dispensing without DMSO backfill (normalization) option, in which the final amount of DMSO ranged from 0.00005 to 1%.
Fig. 2.
(a) Cross sectional micrograph of fluidic architecture depicting gas drive bubble formation. Build in filtration reduces clogging and insolubles. (b) HP dispensehead array with one of eight dispense heads being filled with two microliters of compound on the HP D300 Digital Dispenser.
Enzyme assay
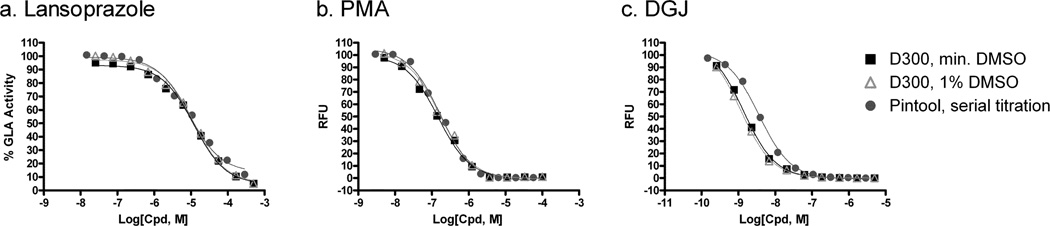
The α-galactosidase (GLA) is a glycoside hydrolase enzyme that hydrolyses the terminal alpha-galactosyl moieties from glycolipids and glycoproteins. A fluorogenic enzyme assay for GLA was previously developed and optimized for compound screens 2. Lansoprazole, phenylmercuric acetate (PMA) and 1-deoxygalactonojirimycin (DGJ) are three known GLA inhibitors that were used for measurement of their inhibitory activities in this experiment 3, 4. To assess the well to well variation, we measured the enzyme activities in eight wells with high concentrations of inhibitors. We found that the coefficient of variation (CV) to be 2.4, 4.7 and 4.9 %, and the Z factor to be 0.93, 0.95 and 0.96 for lansoprazole, PMA and DGJ, respectively (Table 1). These values indicate small well-to-well variation and robust compound dispensing. As shown in Figure 3, the concentration-response curves and their IC50 values of all three GLA inhibitors dispensed by HP D300 dispenser were similar to these dispensed by the pin tool workstation. The variation for replicate number (n=3) in each compound concentration were quite small, indicating high quality compound dispensing. The different concentrations of each compound in wells were achieved by dispensing varied amount of compound in one DMSO stock solution ranging from 13 pl to 260 nl to each well. To normalize the amount of DMSO in each well, the D300 dispenser allows automated backfill of DMSO solvent to these wells with lower volume of compounds to a total of 260 nl in each well. We found that the normalization of DMSO concentration in the GLA enzyme assay did not affect the results, which may be explained by the high DMSO tolerance (1%) of this enzyme assay. The IC50 values for DGJ and lansoprazole also correlated well with that of previously published values 4. However, PMA was >50-fold more potent in our assay than previously reported. This could be due to differences in compound purity and/or age of compound DMSO solution at the time of testing.
Table 1.
Comparison of EC50 values of known compounds dispensed by the HP D300 instrument with traditional pin tool dispensing in three assays. 95% confidence intervals for EC50 values are shown in parentheses.
| Compound | HP D300 - without DMSO backfill |
HP D300 - with DMSO backfill |
Pin tool – 1536-well |
|---|---|---|---|
| α-galactosidase enzyme assay | |||
| Lansoprazole | 1.20×10−5 (1.07–1.34×10−5) | 1.15×10−5 (0.99–1.32×10−5) | 8.95×10−6 (7.40–10.8×10−6) |
| PMA | 1.47×10−7 (1.30–1.60×10−7) | 1.67×10−7 (1.47–1.88×10−7) | 1.69×10−7 (1.53–1.86×10−7) |
| DGJ | 1.38×10−9 (1.27–1.48×10−9) | 1.69×10−7 (1.53–1.86×10−7) | 3.64×10−9 (3.36–3.92×10−9) |
| GR reporter gene assay | |||
| Prednisolone | 3.74×10−9 (2.90–4.82×10−9) | 9.77×10−9 (6.20–1.53×10−9) | 1.09×10−8 (8.92–1.33×10−8) |
| Dexamethasone | 3.85×10−10 (2.90–5.09×10−10) | 3.11×10−10 (2.71–3.57×10−10) | 5.37×10−10 (3.75–7.69×10−10) |
| Cortisol | 6.74×10−9 (5.10–8.90×10−9) | 4.37×10−9 (3.49–5.47×10−9) | 2.55×10−8 (2.09–3.10×10−8) |
| Cytotoxicity assay (ATP content) | |||
| Tamoxifen | 9.00×10−6 (3.60–2.24×10−6) | 8.77×10−6 (4.55–1.68×10−6) | 3.21×10−5 (1.70–6.04×10−5) |
| Daunorubicin | 2.52×10−7 (2.34–2.70×10−7) | 1.89×10−7 (1.73–2.05×10−7) | 3.80×10−7 (3.32–4.34×10−7) |
| TOAB | 2.50×10+ (1.81–3.45×10−7) | 1.66×10−7 (1.36–2.04×10+) | 1.75×10−7 (1.37–2.23×10−7) |
Fig. 3.
Enzyme assay. Three known inhibitors (a) lansoprazole, (b) PMA, and (c) DGJ were tested in the GLA assay. Compounds were dispensed with the D300 in 384-well format without and with DMSO backfill, and via pin tool transfer in 1536-well format.
Reporter gene assay
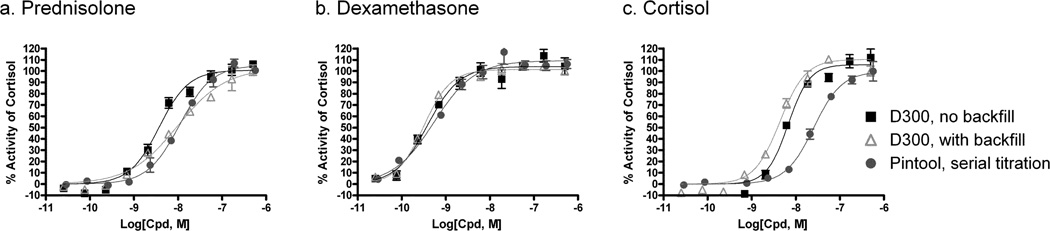
We then examined the compound activities in a cell based glucocorticoid receptor (GR) β-lactamase reporter gene assay with compounds dispensed by the HP D300 dispenser. The β-lactamase reporter is expressed once GR signaling is activated in this transformed HeLa cell line 5. This cell line tolerates up to 1 % DMSO. Cortisol, the natural GR ligand, as well as two corticosteroid drugs, prednisolone and dexamethasone, were tested for their ability to activate β-lactamase reporter signal in this experiment. The three agonists were either dispensed with the HP D300 dispenser with or without DMSO backfill to normalize the total DMSO concentration in 384-well format, or via pin tool transfer in 1536-well format. The curve shapes and EC50 values of three GR agonists were essentially identical when compounds were dispensed by HP D300 dispenser with or without DMSO backfill (Fig. 4a, b). The EC50 values for the prednisolone and dexamethasone matched well between the different compound dispensing methods (Table 1). However, cortisol was 4- to 6-fold more potent in the 384-well format, which might be due to differences in cell biological responses between different plate formats rather than compound dispensing mechanisms. Nevertheless, the CV values for compound activities obtained with the HP D300 dispenser were under 10%, indicating good accuracy and reproducibility.
Fig. 4.
Reporter gene assay. Three known GR agonists (a) prednisolone, (b) dexamethasone, and (c) cortisol were evaluated in the MMTV-βLa HeLa cell line. Compounds were dispensed with the D300 in 384-well format without and with DMSO backfill, and via pin tool transfer in 1536-well format.
Cytotoxicity assay
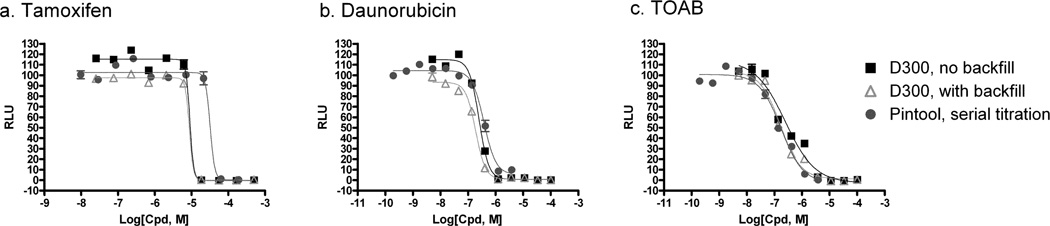
An ATP content assay was used to measure compound cytotoxicity for evaluation of compound dispensing by the HP D300 dispenser as an example of a signal-decrease cell-based assay. Three known cytotoxic compounds were used to compare their cytotoxicity between the compound dispensing methods, including tamoxifen, daunorubicin and TOAB 6, 7. Concentration response curves were nearly identical whether or not the DMSO backfill option was used in the HP D300 dispenser (Fig.5a, b). The compound potencies also matched between the different dispensing paradigms and plate formats for both TOAB and daunorubicin. For tamoxifen, a 3-fold more potent IC50 value in HP D300 dispensing method was obtained with an EC50 value of 8.8 and 9.0 µM in the presence and absence of DMSO normalization, respectively, whereas the EC50 was 32.1 µM in 1536-well pin tool transfer format (Table 1).
Fig. 5.
Cytotoxicity assay. Three known cytotoxic agents (a) tamoxifen, (b) daunorubicin, and (c) TOAB were tested in an ATP-content assay against HeLa cells. Compounds were dispensed with the D300 in 384-well format without and with DMSO backfill, and via pin tool transfer in 1536-well format.
DISCUSSION
We have applied the HP D300 dispenser for direct compound dilution to determine compound biological activities and cytotoxicity using an enzyme assay, a reporter gene assay, and a cytotoxicity assay. Overall, the compound dilution in these experiments using HP D300 dispenser produced comparable EC50 values for most of 9 compounds tested with low CV values, except for two compounds that were 4- and 6-fold (cortisol) and 3-fold (tamoxifen) less active via pin tool compound transfer. These discrepancies could be caused by physical differences between thermo inkjet dispensing to 384-well plates and pin tool transfer to 1536-well plates. The two plate formats have different depth of medium and well geometry. The pin tool transfer protocol used 3 pin dips into the assay plate to dispense as well as mix the assay reagents, while the HP D300 dispensed DMSO droplets onto the surface of the assay medium. These differences could have affected the mixing efficiency and therefore affect apparent compound potencies. In addition, differences in well geometry between the two plate formats, with the culture surface area being 10 mm2/well in 384-well plates and the 2.3 mm2/well in 1536-well plates, exposes different percentage of cells to the edge of the well, which could also affect apparent compound potencies.
The ultra-low volume dispensing capabilities of the HP D300 dispenser allows direct dilution of compounds from the DMSO solution in assay plates without using an intermediate compound dilution plate, which streamlines the experimental process for determination of compound dose response. The main advantages of direct dilution using the HP D300 dispenser versus intermediate dilution and pin tool transfer method are (1) elimination of compound serial dilution step, (2) reduction of compound consumption, (3) reducing consumables such as plates and tips used for compound dilution, and (4) prevention of compound cross contamination due to the contact-free nature of dispensing. Direct dilution requires the ability to accurately dispense picoliter volumes and until now, the only other technology capable of such low volume dispensing is acoustic dispensing 8, 9 While both acoustic and inkjet technologies are capable of direct dilution, the HP D300 dispenser has two advantages over acoustic dispensing technology. The HP D300 dispenser has a small footprint that will easily fit into bio-safety hoods to allow compound dispensing into cell plates under a sterile condition. This feature may be particularly useful for cell based assays in which longer incubation time is needed and a relatively large amount of compounds needed to be screened in 96-well plate format. Unlike the acoustic compound dispending that requires inversion of the assay plate to face a compound plate, the assay plate is held upright in during the dispensing process. The late feature of HP D300 dispenser allows direct dispensing of compounds to an assay plate in either a 96-well or 384-well format assays with high liquid volumes. In addition, the software in HP D300 dispenser that controls compound dilution and dispensing is user-friendly as it is similar to that of Microsoft Excel. The current limitation for HP D300 dispenser is that it is not applicable to 1536-well plates, although in principle, the technology should be capable of dispensing in higher density plates.
The traditional method for compound dilution in the biological experiments in 96-well and 384-well plates involves an intermediate compound dilution plate. For example, a compound in DMSO solution is first diluted 10-fold with assay buffer in the 96-well dilution plate followed by another 10 to 20 fold dilution after the compound solution is added to assay wells (Fig. 1a). An obvious disadvantage is that the lipophilic compounds may precipitate in the aqueous intermediate dilution plate due to high final compound concentration in intermediate dilution plates. This can right shift the compound concentration response and falsely reduce the apparent compound potency. This issue can be avoided with the use of direct transfer methods, where the compounds serially diluted in DMSO are directly transferred at nanoliter volume to assay plates using instruments such as a pin tool station or a piston-based dispensers with small volume tips (e.g. Cybi-well dispenser) (Fig. 1b). With advances in acoustic and inkjet dispensing technologies, the intermediate dilution step can be eliminated in the experiments with 96-well and 384-well plates to streamline workflow and reduce consumables and cross contamination (Fig. 1c).
In conclusion, the HP D300 dispenser offers an alternative tool for directly dispensing compound stock solution to assay plates. The advantages of this instrument include low compound consumption, no compound dilution plates, direct compound and low-volume dispensing and compatible with biological hoods for 96-well and 384-well plates. We also identified several updates which could improve the utility of the D300 dispensers, such as (1) dispensing to 1536-well plates, (2) flexibility of single use or multiple uses of a dispense cartridge (only a single use of the cartridge is currently allowed), and (3) increase in the maximal amount of compound solution that can be loaded in the compound reservoir on the dispense cartridge (currently only 6 µl), (4) automation compatible for integration into a robotic platform, and (5) combination of compound storage and dispensing for compound libraries. In its current form, the HP D300 dispenser is useful in the HTS hit follow-up and lead optimization stages of drug discovery. We hope that the further development of this compound dispenser technology will improve the efficiency and screening throughput of compound dilution experiment in the drug discovery process.
Table 2.
Correlation of variation (CV) values of assays and compounds tested. The CV values are calculated from 12 wells treated with top concentration of each compound dispensed by the HP D300 and 16 wells treated with top concentration of each compound dispensed with the pin tool.
| Assays | CV (%) | |||
|---|---|---|---|---|
| Enzyme | Lansoprazole | PMA | DGJ | |
| HP D300 | 2.4 | 4.7 | 4.9 | |
| Pin tool | 4.9 | 9.1 | 2.6 | |
| Reporter Gene | Predisolone | Dexamethasone | Cortisol | |
| HP D300 | 5.7 | 6.8 | 6.0 | |
| Pin tool | 7.01 | 6.25 | 16.79 | |
| Cytotoxicity | Tamoxifen | Daunorubicin | TOAB | |
| HP D300 | 5.3 | 4.1 | 9.6 | |
| Pin tool | 10.35 | 27.12 | 25.15 | |
ACKNOWLEDGEMENTS
This work was supported by the Intramural Research Program of the Therapeutics for rare and neglected Diseases, National Center for Advancing Translational Sciences, National Institutes of Health.
REFERENCES
- 1.Zheng W, Chen CZ. A practical guide to assay development and high-throughput screening in drug discovery. Boca Raton: CRC Press; 2009. Screening Automation; pp. 183–192. [Google Scholar]
- 2.Shi ZD, Motabar O, Goldin E, Liu K, Southall N, Sidransky E, et al. Synthesis and characterization of a new fluorogenic substrate for alpha-galactosidase. Anal Bioanal Chem. 2009;394(7):1903–1909. doi: 10.1007/s00216-009-2879-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan JQ, Ishii S, Asano N, Suzuki Y. Accelerated transport and maturation of lysosomal alpha-galactosidase A in Fabry lymphoblasts by an enzyme inhibitor. Nat Med. 1999;5(1):112–115. doi: 10.1038/4801. [DOI] [PubMed] [Google Scholar]
- 4.Motabar O, Liu K, Southall N, Marugan JJ, Goldin E, Sidransky E, et al. High throughput screening for inhibitors of alpha-galactosidase. Curr Chem Genomics. 2010;4:67–73. doi: 10.2174/1875397301004010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nehme A, Lobenhofer EK, Stamer WD, Edelman JL. Glucocorticoids with different chemical structures but similar glucocorticoid receptor potency regulate subsets of common and unique genes in human trabecular meshwork cells. BMC Med Genomics. 2009;2:58. doi: 10.1186/1755-8794-2-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho MH, Niles A, Huang R, Inglese J, Austin CP, Riss T, et al. A bioluminescent cytotoxicity assay for assessment of membrane integrity using a proteolytic biomarker. Toxicol In Vitro. 2008;22(4):1099–1106. doi: 10.1016/j.tiv.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia M, Huang R, Witt KL, Southall N, Fostel J, Cho MH, et al. Compound cytotoxicity profiling using quantitative high-throughput screening. Environ Health Perspect. 2008;116(3):284–291. doi: 10.1289/ehp.10727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grant RJ, Roberts K, Pointon C, Hodgson C, Womersley L, Jones DC, et al. Achieving accurate compound concentration in cell-based screening: validation of acoustic droplet ejection technology. J Biomol Screen. 2009;14(5):452–459. doi: 10.1177/1087057109336588. [DOI] [PubMed] [Google Scholar]
- 9.Harris D, Olechno J, Datwani S, Ellson R. Gradient, contact-free volume transfers minimize compound loss in dose-response experiments. J Biomol Screen. 2010;15(1):86–94. doi: 10.1177/1087057109351027. [DOI] [PubMed] [Google Scholar]