Abstract
This methods chapter describes two methods for creating epithelial wounds in Drosophila larvae: pinch and puncture wounding. It also covers protocols for visualizing epithelial wounds, either in a dissected whole mount preparation or, using transgenic reporter larvae, in a live whole mount preparation. Finally, useful transgenic lines for live genetic screening of genes required for wound closure or inflammation are described.
Keywords: Drosophila, Genetic screening, Wound healing, Inflammation, Transgenic reporters
1 Introduction
Traditionally, wound healing has been studied using clinical patient samples or vertebrate models where the tissue architecture of the skin and dermis resembles more closely that of humans [1]. Further, these models have other substantial advantages, including similar phases of repair and diversity of wound-responsive cell types (especially in the immune system that mediates the wound-induced inflammatory response). Despite these advantages, rapid and unbiased discovery of new wound closure genes remains a challenge in vertebrate systems due to the cost and time involved in performing large-scale genetic screens with complicated physiological readouts [2].
Due to its substantial advantages for genetic analysis [3], in recent years a number of groups have begun to use the fruit fly, Drosophila melanogaster, to model various phases of both embryonic [4, 5] and postembryonic wound healing [6–9]. Here we focus on the use of Drosophila larvae to genetically dissect wound-induced epidermal cell migration and blood cell recruitment. Although the wormlike larvae are a provisional stage of the Drosophila life cycle (sandwiched between embryogenesis and the metamorphic events that shape the adult fly) they contain fully differentiated epidermal and innate immune cells and should thus be regarded as a useful model of postembryonic wound repair.
The Drosophila larval epidermis is composed of a monolayer of terminally differentiated epidermal cells that are functionally equivalent to vertebrate keratinocytes in that they form the barrier epidermis and secrete a cuticle that is molecularly distinct from, but functionally similar to, the stratum corneum of vertebrate skin. These epidermal cells are separated from the underlying hemolymph (blood) of the larval open circulatory system by a basal lamina. This hemolymph contains three major types of blood cells that comprise a robust innate immune system [10]. Each blood cell type is wound responsive [6, 7, 11, 12]. Although the tissue architecture of fly larvae is substantially different from vertebrates (there is no dermis, the epidermis is a non-proliferative monolayer, and there are fewer blood cell types and no lymphocyte-mediated adaptive immunity) the Drosophila system trades a somewhat less familiar set of wound-responsive cells for power and ease of genetic dissection. Furthermore, the migration of epidermal cells to close the wound gap bears significant similarities to the same process observed during re-epithelialization in vertebrates.
Here, we describe two methods for creating physical wounds to the epidermis of Drosophila larvae: pinch and puncture wounding (for schematic of wounding and subsequent visualization, see Fig. 1a). Pinch wounding creates a gap in the epidermal sheet but leaves the overlying cuticle barrier intact, thus resulting in a sterile wound. These wounds provoke a robust inflammatory response and heal over a 24-h period through a process of directed cell migration (Fig. 1b). Puncture wounding, by contrast, breaches both the cuticle and epidermis. Although the puncture wounds are smaller and heal faster (~8 h), they are not sterile and result in a robust coagulation response and epidermal cell–cell fusion (Fig. 1c) at the puncture site. In practice, pinch wounds are substantially more useful for studying both epidermal cell migration and blood cell recruitment since there is no melanized scab to obscure one’s view of the epidermis. In addition to these wounding procedures we also describe genetic tools and mounting techniques appropriate for visualizing epidermal tissue damage and recruitment of circulating blood cells in both live animals and in dissected, fixed, and immunostained whole mounts. See Table 1 for summary of reporters and antibodies useful for visualizing epidermal wounds in Drosophila larvae.
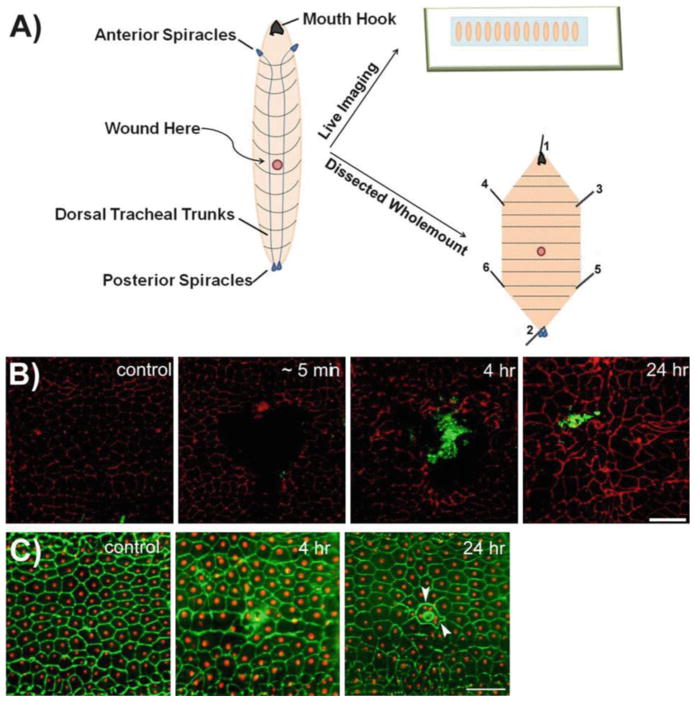
Fig. 1.
Two methods for epidermal wounding of Drosophila larvae. (a) Schematic representation of wounding and mounting of Drosophila larvae. The left illustration depicts the dorsal side of the Drosophila larvae, where the dorsal tracheal trunks connecting the anterior and posterior spiracles are clearly visible. Typically, the A4 segment of the Drosophila larva is chosen for wounding. The epidermal wounds can be visualized either by live imaging (upper right ) when reporter lines are used or by dissecting the larval epidermis and immunostaining for appropriate markers (lower right ). Numbers on dissected larva indicate the order in which pins should be inserted during dissections (see Subheading 3.6). (b) Dissected epidermal whole mounts of control or pinch-wounded larvae stained for Fasciclin III (red) at the indicated times after wounding. Larvae are of genotype w;;Pxn-Gal4, UAS-GFP/+. The GFP-expressing hemocytes are stained with anti-GFP (green ) antibody and the rapid accumulation of hemocytes at the wound site can be seen. At 24 h after wounding, the wound gap is completely closed as observed through Fasciclin III staining. Scale bar 100 μm (adapted from Babcock et al. [17], Copyright © 2008 by The National Academy of Sciences of the USA). (c) Dissected epidermal whole mounts of control or puncture-wounded w; e22c-Gal4, UAS DsRed2-Nuc/+ larvae stained with anti-Fasciclin III (green). Scab formation and epidermal cell–cell fusion around the scab (arrowhead ) can be seen. Scale bar 100 μm
Table 1.
Transgenic lines and antibodies used to label blood cells and epidermis in Drosophila larvae
| Tissue of interest | Antibodies | Direct labels | Gal4/UAS labels |
|---|---|---|---|
| Blood cells (plasmatocytes) | α-Pla |
Pxn-Gal4>GFPe HmlΔ-Gal4>GFPf |
|
| Epidermis | α-Fas IIIb |
Fas III-GFP
c Nrg-GFPd |
A58-Reporterg e22c-Reporterg |
| Both |
Nrg-GFP Pxn-Gal4, UAS-GFPh |
Kurucz E et al., Nimrod a putative phagocytosis receptor with EGF repeats in Drosophila plasmatocytes. Curr Biol. 2007 Apr 3;17(7):649–54
Patel NH et al., Characterization and cloning of fasciclin III: a glycoprotein expressed on a subset of neurons and axon pathways in Drosophila. Cell. 1987 Mar 27;48(6):975–88
Hudson AM et al., Mononuclear muscle cells in Drosophila ovaries revealed by GFP protein traps. Dev Biol. 2008 Feb 15;314(2):329–40
Morin X et al., A protein trap strategy to detect GFP-tagged proteins expressed from their endogenousloci in Drosophila. Proc Natl Acad Sci U S A. 2001 Dec 18;98(26): 15050–5
Stramer B et al., Live imaging of wound inflammation in Drosophila embryos reveals key roles for small GTPases during in vivo cell migration. J Cell Biol. 2005 Feb 14;168(4):567–73
Chew SK et al., The apical caspase drone govems programmed and unprogrammed cell death in Drosophila. Dev Cell. 2004 Dec; 7(6):897–907
Lesch C et al., A targeted UAS-RNAi screen in Drosophila larvae identifies wound closure genes regulating distinct cellular processes. Genetics. 2010 Nov;186(3):943–57
Babcock DT et al., Circulating blood cells function as a surveillance system for damaged tissue in Drosophila larvae. Proc Natl Acad Sci U S A. 2008 Jul 22;105(29):10017–22
The advantage of immunostaining is that it affords a clearer visualization of the epidermal morphology and is applicable to virtually any mutant genotype. Immunostaining, however, is a fairly tedious method involving manual dissection of the larval epidermis and a multiday staining procedure. Therefore, it would be difficult to employ this technique for high- or medium-throughput genetic screening. An alternative approach is to use wound reporter lines to accelerate and simplify wound visualization. These reporters mostly employ the Gal4/UAS system for tissue-specific ectopic transgene expression [13] (Fig. 2a) to express membrane- and nuclear-localized fluorescent proteins in either the epidermis or the blood cells (Table 1) and can be used to quickly visualize wound morphology in live larvae (Fig. 2b, c). Due to the rapidity of visualization and the fact that they can be combined with near-whole- genome screening technologies [14, 15], the wound reporter lines can be used for medium-throughput genetic screening. The tradeoff is that the visualization of wound morphology is not as detailed in the live preparation. Regardless of the visualization method used, the larvae can be analyzed 24 h after epidermal wounding (when the epidermal wound gaps are typically closed in controls) or can be analyzed at intermediate time points to observe the dynamics of cell migration/elongation as closure progresses.
Fig. 2.
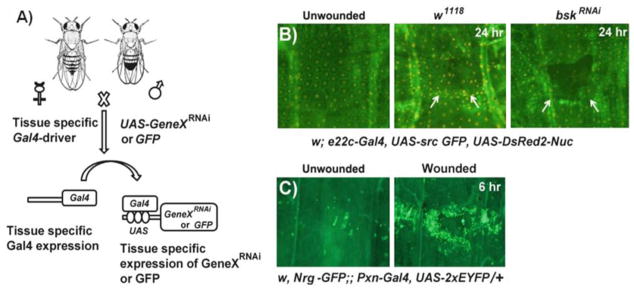
Application of the Gal4-UAS system for live imaging of wound responses and genetic screening. (a) A schematic overview of the Gal4-UAS system in Drosophila. When flies (females shown here) carrying a tissue-specific Gal4 driver are crossed to flies (males shown here) with a transgene under UAS control (for example, UAS-GeneX RNAi or UAS-GFP), progeny containing both genetic elements are produced leading to expression of the UAS-transgene in the tissues that express Gal4. (b ) Live whole mounts of the pinch-wounded larval progeny obtained after mating w; e22c-Gal4, UAS src-GFP, UAS DsRed2-Nuc/+ (e22c-reporter) flies with either w1118 (middle panel) or UAS-bskRNAi (right panel: to silence jun N-terminal kinase (JNK)) flies. The unwounded w; e22c-Gal4, UAS src-GFP, UAS DsRed2-Nuc/+ larvae are used as controls (left panel). Twenty-four hours after wounding the w1118 larvae show complete wound gap closure, but disorganized epidermal tissue (indicated by arrows) compared to the unwounded control. The larvae with epidermal JNK knockdown show open wounds indicated by the dark gap in the epidermal sheet (arrows). (c) Live larval whole mounts of the reporter line w, Nrg-GFP;; PxnGal4, UAS-2xEYFP/+ can be used to study the wound-induced inflammatory response, where a robust accumulation of hemocytes (green) labeled with YFP at the wound site can be seen 6 h after pinch wounding (right panel, compare to control)
We expect that these detailed protocols for both wounding and wound visualization will be useful for vertebrate wound healing researchers who wish to quickly test the function of orthologs of their favorite genes (or interactors with these genes) in the Drosophila system. Such a strategy allows a rapid assessment of whether the gene is important, either for epidermal wound closure or recruitment of blood cells, without the time and expense commitment of making a mouse knockout or transgenic model. A positive result in Drosophila larvae could provide the extra motivation to commit to a more clinically relevant model.
2 Materials
2.1 Fly Husbandry
Cornmeal dextrose media (for media preparation, refer to the Bloomington Drosophila Research Center [http://flystocks.bio.indiana.edu/Fly_Work/media-recipes/dextrosefood.htm], Indiana University) (see Note 1).
Plastic vials and cotton plugs.
25 °C incubator, preferably with humidity control to ~50 %.
2.2 Pinch and Puncture Wounding
Diethyl ether.
Coplin jar (Fig. 3a).
10 ml glass beaker, stuffed with a cotton ball (Fig. 3b).
Wounding vials: 1.0 dram glass vials with 1.0 ml of cornmeal dextrose media and sealed with a small cotton plug (Fig. 3c).
Paintbrush (Fig. 3e).
Perforated steel spoon (Fig. 3f).
Wounding pad (a thin vinyl sheet, preferably black to contrast the white larvae) (Fig. 3g).
2.0 ml Microcentrifuge tubes (Fig. 3h).
Nitex nylon mesh (pore size: 630 μm).
Dissecting stereomicroscope with brightfield gooseneck illumination (Zeiss Stemi 2000 microscope or equivalent).
Nitex Nylon mesh (pore size: 100 μm).
For Pinch wounding: Blunted dissecting forceps (Fig. 3i).
For puncture wounding: 0.1 mm steel needle held in needle or pin holder (Fig. 3l).
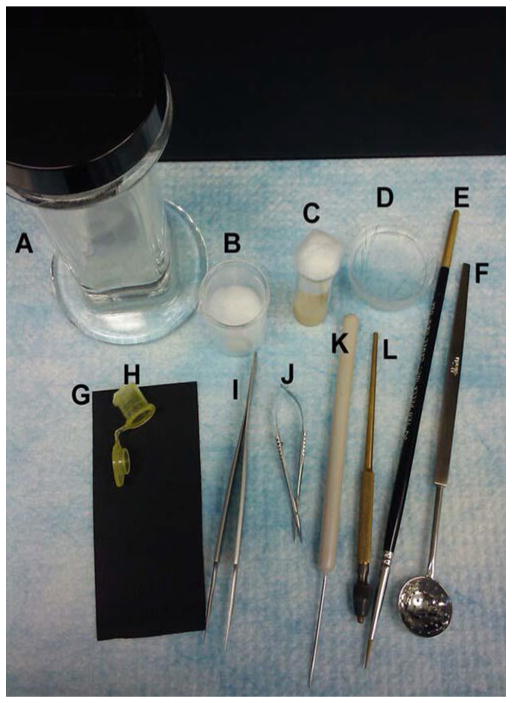
Fig. 3.
The instruments used for epidermal wounding and dissection of Drosophila larvae. (a, b) Coplin jar (a) and 10 ml beaker with a cotton ball (b, for etherization). (c ) Wounding vial. (d ) Sylgard plate with pins for dissections. (e) Paintbrush. (f ) Perforated spoon. (g ) Vinyl sheet (wounding pad). (h ) Etherization cage, cut from a 2 ml microcentrifuge tube. (i) Forceps. (j) Dissecting scissors. (k) Needle to soften the fly food. (l) Pin holder
2.3 Live Scoring of Epidermal Morphology or Inflammation After Pinch or Puncture Wounding
Epidermal reporter line chosen from among options in Table 1: w1118;; UAS-src-GFP,UASDsRed2-Nuc,A58-Gal4/TM6B (A58-reporter) or w1118;e22c-Gal4,UAS-src-GFP,UAS-DsRed2Nuc/CyO (e22c-reporter) or blood cell-specific reporter line: w, Nrg-GFP;; Pxn-Gal4, UAS2xEYFP.
70 % Glycerol.
Micro glass slides (3in. × 1 in.) and coverslips (24 × 60 mm). Double-sided clear tape.
Stereomicroscope equipped with dual filter capable of simultaneous visualization of GFP and RFP channels (Leica MZ16FA or equivalent) and color digital camera (Leica DFC300 FX or equivalent).
2.4 Dissection of Larval Epidermis
35× 10 mm plastic petri dishes.
Sylgard elastomer base and curing agent (Dow Corning, Catalog no. 3555-47-3 and 68988-896, respectively).
35× 10 mm petri dishes partially filled with polymerized Sylgard (preparation of Sylgard plates is described in detail methods Subheading 3.5 and [16]) (Fig. 3d).
0.1 mm thick dissecting pins.
Dissecting scissors (Fig. 3j).
Forceps.
Phosphate buffer saline (pH 7.2) (PBS).
3.7 % formaldehyde made in PBS.
2.5 Immunostaining and Mounting of Dissected Epidermis
PBS.
Goat serum. Inactivate the goat serum by incubating at 55 °C for 1 h.
Tween-20.
Anti-Fasciclin III antibody (Developmental Studies Hybridoma Bank, Catalog no. 7G10).
Fluorescein (FITC)-conjugated goat anti-mouse IgG.
BD Clay Adams Nutator.
Micro glass slides.
Coverslips.
Vectashield™ mounting medium.
3 Methods
3.1 Fly Husbandry and Harvesting Larvae
Culture appropriate wound reporter lines on standard cornmeal dextrose fly media at 25 °C. For genetic screening, cross the wound reporter lines to an appropriate UAS-RNAi line targeting your gene of interest (experimental condition) or to a w1118 control strain (closure should proceed normally) (see Notes 1 and 2).
Transfer the flies onto fresh food every other day (see Note 3).
Collect early-L3-stage larvae after 5 days of incubation at 25 °C. With a metal spatula, scoop out the soft food to a nylon mesh (630 μm pore size) and rinse gently but thoroughly with running tap water. Very little food should remain when done.
Using forceps, quickly select the green fluorescent larvae bearing the reporter using a fluorescence stereomicroscope (see Note 4).
3.2 Pinch and Puncture Wounding
Here we describe two techniques to physically wound larvae. Pinch wounding is recommended for studying the cell migration events [15, 17] (Fig. 1b) and puncture wounding is best suited for studying hemolymph coagulation and wound-induced epidermal cell–cell fusion [6] (Fig. 1c). Prepare a small etherization cage to anesthetize the larvae using a 2.0 ml microcentrifuge tube. Briefly, sever the tube with a razor blade above the taper. Save the part with the cap and melt the cut edge of the tube with a hot metal spatula. While the melted edge is still hot, attach a small square of nylon mesh (100 μm pore size) to the melted region. This will allow access to ether fumes but prevent the larvae from escaping.
Transfer rinsed L3 larvae into the cap of the etherization cage (see Note 5). To expose to ether, place a 10 ml glass beaker containing a cotton ball soaked in ~1.0 ml of diethyl ether in a Coplin jar. This should be done in a fume hood. Place the etherization cage on the cotton ball and close the lid of the Coplin jar. Wait for 2.5 min.
Remove etherization chamber from Coplin jar, open the cap, and then gently rinse larvae into a 35 × 10 mm petri dish using a water squirt bottle.
Using forceps, gently place up to 14 larvae onto the wounding pad with their dorsal sides up (see Note 6 and Fig. 1a).
Pinch wounding: While observing under the dissecting microscope, pinch wound each larva by grabbing the cuticle and epidermis with forceps and holding for 10 s (see Notes 7–9). The pinch wounds should be made in the smooth cuticle at the dorsal midline between the third and the fourth segmental boundaries marked by dorsal hairs (Fig. 1b).
Puncture wounding: Pierce the larval cuticle with a 0.1 mm steel needle at the dorsal midline between the third and the fourth dorsal segmental boundaries. Placing paper towels underneath the wounding pad will cushion both the larva and the pin during piercing (see Note 10 and Fig. 1c).
3.3 Recovery
After wounding, use a water squirt bottle to gently rinse the larvae off the pad and into a petri dish filled with water.
Gently transfer the larvae into wounding vials using forceps (see Note 11).
Incubate the larvae at 25 °C for the desired length of time. In control lines, wound closure is completed by 24 h.
3.4 Mounting for Live Imaging
After recovery for the desired length of time, use forceps to carefully remove the wounded larvae from the food within the wounding vial and rinse with water.
Anesthetize the larvae using ether for 5 min (see the above protocol).
Wash the larvae into a 35× 10 mm petri dish using a tap water squirt bottle.
Prepare the micro glass slide for mounting the larvae by sticking a length of double-sided tape sufficient to mount the number of larvae chosen for analysis.
Place the etherized larvae on the glass side adjacent to the tape with the dorsal side facing up (see Note 12). Now, hold the larva with forceps near the posterior spiracles and attach the mouth hook to the tape. Gently stretch the larva without breaking the cuticle and attach the posterior spiracle to the tape as well. Flatten the larva to the extent possible to obtain better quality images.
Add approximately 100 μl of 70 % glycerol onto the coverslip and spread it in a line sufficient to cover the larvae. Take the slide with the larvae affixed to it and invert it onto the coverslip to cover the larvae uniformly in glycerol (see Note 13).
3.5 Dissections and Immunostaining of Dissected Samples
Dissection of the larval epidermis allows for a clearer visualization of the epidermal wound morphology. See Fig. 1a for layout of a properly dissected larva. In addition to anti-Fasciclin III which labels epidermal cell membranes, whole mounts can be immunostained for other cellular markers if desired.
Prepare polymerized Sylgard plates by mixing 1 part of elastomer with 10 parts of base. Pour ~3 ml of the mixture into a 35 × 10 mm petri dish and allow the gel to set by incubating the plates at room temperature for 2 days.
Gently transfer the larvae from wounding vials and rinse with water or PBS. Transfer a larva to a Sylgard plate containing enough PBS to cover the surface. Using one pair of forceps, grab and stabilize the larva. Using a second pair of forceps, grab a 0.1 mm pin and pin the larva to the soft Sylgard surface. The pin should pierce the anterior of the larva near the mouth hooks and the ventral epidermis should be facing up.
Grab the posterior of the larva and pin near the posterior spiracles while gently stretching the larva along the anteroposterior axis.
Using dissecting scissors make a small incision at the midbody along the anteroposterior axis and cut the ventral epidermis/cuticle both anteriorly and posteriorly towards the head and tail pins. Take care not to nick the underlying dorsal epidermis while cutting.
Using one pair of forceps, stretch the right-hand anterior epidermal flap just shy of breaking. Using a second pair of forceps, grab an insect pin and pin the right epidermal flap to the Sylgard. Similarly, pin the left-hand anterior flap, the right-hand posterior flap, and the left-hand posterior flap in that order (Fig. 1a). These steps of the dissection will take practice to perfect (see Note 14).
Eviscerate by gently grabbing the internal organs at the posterior and gently pulling upward and anteriorward. If one grabs between the epidermis and the tracheal dorsal trunks, one can usually pull up all of the viscera (gut, fat bodies, malpighian tubules, and associated trachea) at once. Use a second pair of forceps to sever the connection between the pulled organs and the anterior dorsal epidermis (avoid poking the dorsal epidermis). Residual internal organs such as brain and fat body should be gently picked off the epidermis by forceps.
Fix in a fume hood for 1 h at room temperature by replacing the PBS with 3.7 % formaldehyde in PBS.
Using forceps gently unpin each larva and transfer the epidermis to a 1.5 ml microcentrifuge tube containing PBS (see Note 15).
3.6 Immunostaining of Dissected Larval Epidermis with Anti-Fasciclin III
The epidermal wounds can be studied by immunostaining the larval epidermis for markers such as Fasciclin III. The antibody stains epidermal cell membranes (Fig. 1b, c) and the pinch and puncture wounds can be clearly visualized. The staining protocol described here can be adapted to any marker of interest.
Block fixed larvae for at least 1 h at room temperature in PHT by rotating on an Adams nutator, but any equivalent will do.
Dilute anti-Fasciclin III 1:50 in PHT, which is PBS containing 5 % heat-inactivated normal goat serum and 0.3 % Tween-20.
Replace block solution with sufficient diluted primary antibody to cover all of the larvae being stained.
Incubate on a nutator overnight at 4 °C.
Remove and save the primary antibody and wash the larvae at least six times for at least 30 min with 1.0 ml PHT.
Replace final wash with diluted secondary antibody: Goat anti- mouse IgG conjugated to the fluor of your choice. Incubate overnight at 4 °C.
Replace secondary antibody with PHT and wash again as in step 5.
Add a drop of Vectashield™ to a glass microscope slide and place the epidermal whole mount into the drop. Slowly mount a coverslip onto the drop without creating air bubbles. Seal the coverslip to the glass slide using nail polish.
3.7 Microscopy
Live or dissected larvae can be viewed with an appropriate fluorescent filter on a Leica MZ16FA stereomicroscope using Planapo 1.6× objective. Capture images using a color digital camera (Leica DFC300 FX or equivalent) and Image-Pro AMS v5.1 software (Media Cybernetics or equivalent).
Footnotes
Please refer to the Bloomington Stock Center Web site or to Ralph Greenspan’s book “Flypushing” for basics of fly husbandry.
We recommend 25 °C for Drosophila culture. Note that the Gal4/UAS system and Drosophila in general are temperature sensitive. Decreasing the temperature will give a longer generation time and lower transgene expression while increasing the temperature will give a shorter generation time and higher transgene expression although fecundity may be compromised. This is useful to keep in mind when encountering a transgene that is lethal at 25 °C or gives no phenotype. Lower temperatures may promote viability and higher temperatures may reveal a phenotype.
It is only necessary to brood the flies if the cross is going slowly (low number of embryo/larval progeny) and until a sufficient population of larvae is obtained for wounding.
After sorting the fluorescent larvae, it is advisable to leave the larvae on small moist pads of fly food in a 35 mm × 10 mm petri dish. This practice avoids starvation of larvae before wounding.
Larvae should be transferred to the etherization cage with as little accompanying water as possible since water will hinder the desired anesthetization.
The dorsal side is marked by the presence of the two prominent dorsal tracheal trunks and the absence of dark denticles that mark the segmental boundaries on the ventral side.
A good pair of dissecting forceps is very important for pinch wounding. The tips should be filed blunt and the edges made smooth so that the forceps do not puncture the larval cuticle. Forceps can be blunted by filing the tips with a sanding stone. Tips should be rounded when done but blades should still be flat and close flush.
Standardize the forceps used for pinching by checking for the wound morphology immediately after wounding. Occasional refiling or replacement of the wounding forceps may be necessary if the initial wound morphology drifts from the ideal that is a well-rounded wound that is centered in a single body segment (see Fig. 1a, b).
The amount of pressure applied while pinch wounding should be enough to create a gap in the epidermis but not so much as to puncture the cuticle and thus create a scab. The proper amount of pressure can be determined through practice.
During puncture wounding, it is typical to poke the needle through both the dorsal and ventral cuticle. This is because the cuticle is firm but the interior of the larva is filled with liquid hemolymph. Either the dorsal entry or ventral exit wound can be used for further analysis although the investigator has more control over the location of the dorsal wound.
A maximum of 14 larvae should be transferred to a single wounding vial to avoid overcrowding and excess competition for food. Likewise, a minimum of 5 or 6 larvae should be in each vial so that they can assist each other in working up the food. Pre-mashing the food (with the needle shown in Fig. 3k) before transferring the larvae to the wounding vial helps in quick revival of the larvae.
After transferring the etherized larvae onto the slide from water, there will be a small drop of water around the larvae. Remove this water droplet using a kimwipe before mounting onto the tape as they will stick better.
Live-mounted larvae should be observed immediately after mounting and photographed quickly. Occasionally they will wake up and wriggle free from the tape. This can be minimized by chilling them for approximately 30 min at 4 °C.
When pinning out the larva during dissection it is imperative that the pins be placed symmetrically to ensure that there are no stretch lines skewing across the epidermis (see Fig. 1a). The ventral cut edges should be parallel to each other, and the two anterior and two posterior pins should be on the same line perpendicular to the anteroposterior midline. Finally, all flaps should be fully stretched and form an angle of 45° with respect to the anteroposterior midline.
It is a good idea to use a separate pair of forceps for dissecting and for unpinning to avoid damage to the tips. In general, since the forceps are used to grab and place the pins, the tips will only last in good condition for up to a few months even if good care is taken.
References
- 1.Grose R, Werner S. Wound-healing studies in transgenic and knockout mice. Mol Biotechnol. 2004;28(2):147–166. doi: 10.1385/MB:28:2:147. [DOI] [PubMed] [Google Scholar]
- 2.Liu J, Johnson K, Li J, Piamonte V, Steffy BM, Hsieh MH, Ng N, Zhang J, Walker JR, Ding S, Muneoka K, Wu X, Glynne R, Schultz PG. Regenerative phenotype in mice with a point mutation in transforming growth factor β type I receptor (TGFBR1) Proc Natl Acad Sci. 2011;108(35):14560–14565. doi: 10.1073/pnas.1111056108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bier E. Drosophila, the golden bug, emerges as a tool for human genetics. Nat Rev Genet. 2005;6(1):9–23. doi: 10.1038/nrg1503. [DOI] [PubMed] [Google Scholar]
- 4.Wood W, Jacinto A, Grose R, Woolner S, Gale J, Wilson C, Martin P. Wound healing recapitulates morphogenesis in Drosophila embryos. Nat Cell Biol. 2002;4(11):907–912. doi: 10.1038/ncb875. [DOI] [PubMed] [Google Scholar]
- 5.Stramer B, Wood W, Galko MJ, Redd MJ, Jacinto A, Parkhurst SM, Martin P. Live imaging of wound inflammation in Drosophila embryos reveals key roles for small GTPases during in vivo cell migration. J Cell Biol. 2005;168(4):567–573. doi: 10.1083/jcb.200405120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galko MJ, Krasnow MA. Cellular and Genetic Analysis of Wound Healing in Drosophila Larvae. PLoS Biol. 2004;2(8):e239. doi: 10.1371/journal.pbio.0020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Babcock DT, Brock AR, Fish GS, Wang Y, Perrin L, Krasnow MA, Galko MJ. Circulating blood cells function as a surveillance system for damaged tissue in Drosophila larvae. Proc Natl Acad Sci. 2008;105(29):10017–10022. doi: 10.1073/pnas.0709951105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosch M, Serras F, Martín-Blanco E, Baguñà J. JNK signaling pathway required for wound healing in regenerating Drosophila wing imaginal discs. Dev Biol. 2005;280(1):73–86. doi: 10.1016/j.ydbio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Rämet M, Lanot R, Zachary D, Manfruelli P. JNK Signaling Pathway Is Required for Efficient Wound Healing in Drosophila. Dev Biol. 2002;241(1):145–156. doi: 10.1006/dbio.2001.0502. [DOI] [PubMed] [Google Scholar]
- 10.Evans CJ, Hartenstein V, Banerjee U. Thicker Than Blood: Conserved Mechanisms in Drosophila and Vertebrate Hematopoiesis. Dev Cell. 2003;5(5):673–690. doi: 10.1016/s1534-5807(03)00335-6. [DOI] [PubMed] [Google Scholar]
- 11.Bidla G, Dushay MS, Theopold U. Crystal cell rupture after injury in Drosophila requires the JNK pathway, small GTPases and the TNF homolog Eiger. J Cell Sci. 2007;120(7):1209–1215. doi: 10.1242/jcs.03420. [DOI] [PubMed] [Google Scholar]
- 12.Márkus R, Kurucz É, Rus F, Andó I. Sterile wounding is a minimal and sufficient trigger for a cellular immune response in Drosophila melanogaster. Immunol Lett. 2005;101(1):108–111. doi: 10.1016/j.imlet.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 13.Duffy JB. GAL4 system in drosophila: A fly geneticist’s swiss army knife. Genesis. 2002;34(1–2):1–15. doi: 10.1002/gene.10150. [DOI] [PubMed] [Google Scholar]
- 14.Dietzl G, Chen D, Schnorrer F, Su K-C, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, Couto A, Marra V, Keleman K, Dickson BJ. A genome- wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448(7150):151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- 15.Lesch C, Jo J, Wu Y, Fish GS, Galko MJ. A Targeted UAS-RNAi Screen in Drosophila Larvae Identifies Wound Closure Genes Regulating Distinct Cellular Processes. Genetics. 2010;186(3):943–957. doi: 10.1534/genetics.110.121822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brent JR, Werner KM, McCabe BD. Drosophila Larval NMJ Dissection. J Vis Exp. 2009;24:e1107. doi: 10.3791/1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Y, Brock AR, Wang Y, Fujitani K, Ueda R, Galko MJ. A Blood-Borne PDGF/VEGF-like Ligand Initiates Wound-Induced Epidermal Cell Migration in Drosophila Larvae. Curr Biol. 2009;19(17):1473–1477. doi: 10.1016/j.cub.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]