Abstract
Skin sensitization remains a major environmental and occupational health hazard. Animal models have been used as the gold standard method of choice for estimating chemical sensitization potential. However, a growing international drive and consensus for minimizing animal usage have prompted the development of in vitro methods to assess chemical sensitivity. In this paper, we examine existing approaches including in silico models, cell and tissue based assays for distinguishing between sensitizers and irritants. The in silico approaches that have been discussed include Quantitative Structure Activity Relationships (QSAR) and QSAR based expert models that correlate chemical molecular structure with biological activity and mechanism based read-across models that incorporate compound electrophilicity. The cell and tissue based assays rely on an assortment of mono and co-culture cell systems in conjunction with 3D skin models. Given the complexity of allergen induced immune responses, and the limited ability of existing systems to capture the entire gamut of cellular and molecular events associated with these responses, we also introduce a microfabricated platform that can capture all the key steps involved in allergic contact sensitivity. Finally, we describe the development of an integrated testing strategy comprised of two or three tier systems for evaluating sensitization potential of chemicals.
Keywords: Immunobiology, Skin sensitization, In silico approaches, QSAR, 2D cell based models, 3D skin tissue models, Integrated Testing Strategies, Microfabrication, Microfluidics
INTRODUCTION
Approximately 15–20% of the population in the Western world and ~1% of the worldwide population is allergic to one or more environmental chemicals.70 According to the National Center for Health Statistics (NCHS), in the United States alone, approximately 9% of the population in the 0–18 year age range suffers from allergic contact dermatitis, (ACD) with even higher risks in the adult population. While ACD is not a medical emergency, the risks associated with these conditions can be very distressing, causing great discomfort, emotional stress and feelings of hopelessness, and carry a huge economic burden due to the costs of medical care and lost productivity.34
ACD is a delayed hypersensitivity reaction (Type IV) mediated by antigen-specific T lymphocytes in sensitized patients exposed to contact allergens such as nickel, nickel sulfate, 2,4-dinitrochlorobenzene, paraphenylenediamine, cinnamic alcohol and formaldehyde. 87 Typically, a chemical is classified as a sensitizer allergen if it induces allergic contact sensitivity, or an irritant if it does not elicit a cytokine secretion profile that is responsible for T cell proliferation within the draining lymph node, but evokes non-specific skin inflammation.4 Metals such as nickel and gold used in medical implants are the most commonly known sensitizers 148 that elicit an inflammation mediated cytokine release that is pivotal for allergen specific T cell responses. Nickel sulfate has also been shown to induce secretion of cytokines such as IL12p40 that play a role in activating allergen or sensitizer specific T-cell response.6 2,4-dinitrofluorobenzene and nickel sulfate have been shown to upregulate surface markers such as CD40 and IL-12 receptor that are implicated in the sensitizer mediated T cell response in fetal dendritic cell lines163 while 2,4-dichloronitrobenzene, an irritant shows no effect. Previous research work has also been focused on generation of nickel specific T-cell lines that evoked a proliferative response specific to haptens.105
Paraphenylenediamine, a permanent hair-dye product highly susceptible to oxidation, has been shown to selectively bind to cysteine residues in skin peptides and proteins prior to eliciting a T-cell response.67 Moreover, the chemical, either alone or in combination with an oxidant induces B cell and T cell infiltration within the draining lymph nodes in mice.21 Cinnamic alcohol is a weak sensitizer, but, upon contact with skin, an epidermal enzyme mediated metabolic conversion to cinnamaldehyde elicits an allergen response.11
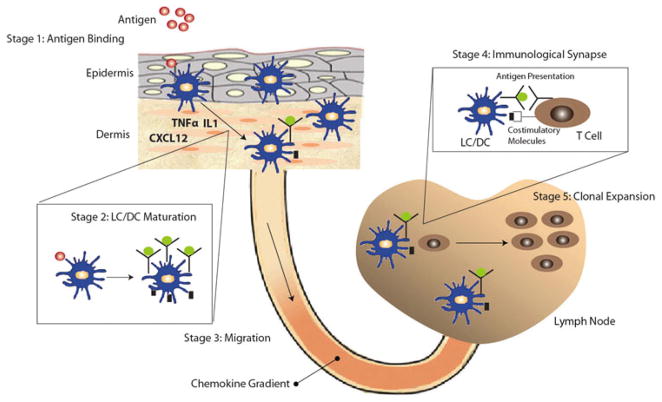
The overall immune response to allergens is comprised of a complex cascade of events as shown in Fig. 1. Topical application leads to diffusive penetration and distribution of allergens within skin wherein it reacts with extracellular or cell membrane bound proteins to form immunogenic complexes capable of inducing T cell responses.38 Exposure of allergen leads to maturation and migration of skin-resident Langerhans cells (LCs) and dermal dendritic cells (DCs) to the localized lymph node. The activation and migration of DCs is regulated by multiple factors including chemokines and cytokines secreted by various resident skin cells.75,76 The maturation results in the LC/DCs acquiring antigen, expressing co-stimulatory molecules, and expressing chemokine receptors such as CCR7.141 The migration of LC/DC is believed to be regulated by chemokine gradient of CCL19 and/or CCL21 across the lymphatic vessel.129 DCs play a central role in transporting the antigen to the localized lymph node, where they initially form immunological synapses with T cells, ultimately leading to T cell activation. The activated T cells undergo differentiation and proliferation, which results in the selective expansion of clonal populations of allergen-specific T cells. The activation of Th1 and/or Th2 clonal T cell populations ultimately results in allergen-specific sensitivity.
FIGURE 1.
Schematic illustrating the complex cascade of events associated with the immune response to an allergen. The exposure to an allergen leads to the maturation of LCs/DCs and migration of LC/DC to the localized lymph node. The activation and migration of DCs is regulated by multiple factors including chemokines and cytokines such as TNFα, IL1, and CXCL12 secreted by various resident skin cells. Allergen induced maturation results in the LC/DCs acquiring antigen and up regulation of co-stimulatory molecules, and chemokine receptors such as CCR7. The migration of LC/DC is believed to be regulated by chemokine gradient of CCL19 and/orCCL21 across the lymphatic vessel. The migrated LC/DC forms an immunological synapse with the T cells, which leads to the activation of T cells and their clonal expansion.
In order to both predict skin allergy responses and unravel the complexity of these responses, the murine local lymph node assay (LLNA) was developed using US FDA and the Organization for Economic Cooperation and Development (OECD) guidelines. Although in vivo assays like the murine LLNA have been extensively used to predict sensitization potential, both the pressing social need to replace animal sensitization models, and the scientific need to more precisely control and evaluate the mechanistic progression of hypersensitivity have spawned interest in the development of in vitro models. Moreover, animal models are expensive and require large animal numbers for rigorous chemical testing. In the US, there are rough estimations that about 10 million animals per year are used in toxicity testing of chemicals and drugs. In the EU, as of 2005, approximately 1 million animals were utilized for end-point toxicological studies.94 In the frame of the new EU-Chemicals directive Registration, Evaluation, Authorization of Chemicals (REACh),119 there are estimates that about 40–50 million additional animals may be required in order to fulfill all related obligations in toxicological characterization and risk assessment of the so called 30,000 “existing chemicals” that have entered the market before 1981.48,53 Moreover, the recent 7th Amendment to the European Union Cosmetics Directive (2003/15/EC) is set to ban all in vivo testing of toiletry and cosmetic ingredients and the marketing of such products within the European Union (EU) by 2013. Furthermore, this ban is likely to be effected in the US in subsequent years, as animal tested products may be at a distinct marketing disadvantage. Moreover, while the LLNA is a robust method to capture the complexity of skin allergies, the translation of animal model data to human systems is not entirely predictive. All of these factors necessitate the development of non-animal alternatives for chemical testing.
Current non-animal alternatives for predicting sensitization potential include in silico approaches involving the incorporation of experimental information into sophisticated computational modules to generate Quantitative Structure Activity Relationships (QSAR) i.e. skin sensitizer activity as a function of the chemical structure. These QSAR based models include expert systems such as the Deductive Estimation of Risk from Existing Knowledge (DEREK), Toxicity Prediction by Komputer Assisted Technology (TOPKAT) and Times Metabolism Simulator platform used for predicting Skin Sensitization (TIMESSS) systems.71 Other approaches include a mechanism based read-across model (that takes into account compound electrophilicity for sensitization potential assessment) and assessment of simple sensitization endpoints based on peptide-binding potential of chemicals. However, these assays do not recapitulate the cellular elements of the more complex in vivo events that take place in the skin and the draining lymph nodes such as maturation and migration of DCs from the skin to the lymph node where they present antigens to naive T cells. In order to address some of these issues, cell and tissue based assays have been developed that include a variety of mono and co-culture cell systems in conjunction with 3D skin models. Given the complexity of allergen triggered immune reactions, and the limited ability of existing systems to capture the entire gamut of cellular and molecular events associated with these immune responses, we also describe a microfabricated platform that can capture all the key steps involved in allergic contact sensitivity. Finally, we describe the development of an integrated testing strategy comprised of two or three tier systems for evaluating sensitization potential of chemicals.
In the paragraphs below, we discuss in detail, the approaches mentioned above that have been developed to recapitulate the sensitization process (Figs. 1 and 2) and future implications for an integrated microfluidic system to clearly mimic the in vivo ACD phenomena using non-animal alternatives.
FIGURE 2.
Overview of non-animal alternative methods for sensitization testing. There are four classes of non-animal alternative methods. (1) In silico approaches. (2) cell based models. (3) 3D skin tissue based models and (4) an integrated microfluidic platform.
IN SILICO APPROACHES
Many in silico approaches exist that predict the degree of skin sensitization based upon the chemical structure of chemical entities. For example QSAR as well as its derivatives, such as DEREK and TOPKAT systems, and alternatives such as mechanism based read-across models, have had similar impacts in predicting potential skin sensitization. In the following sections we will briefly describe these approaches.
(Quantitative) Structure Activity Relationships (QSAR)
Hansch and Fujita were the first to introduce the concept of Quantitative Structure Activity Relationship (QSAR). The research highlighted the relationship between the molecular structure and biological activity of a molecule. The basic assumption for all molecule based hypotheses was that similar molecules have similar activities. This principle is also called Structure-Activity Relationship (SAR). QSAR was primarily developed to estimate the boiling points of chemicals based on structure of alkenes. This methodology was further advanced for utilization in fields such as small molecule drug discovery,40 lead optimization147 and bioresponse data from combinatorial libraries of biomaterials. 145 This approach was derived in the pharmaceutical industry as a means of predicting the solubility, bioavailability, clearance, and cellular uptake of a compound relative to its dose dependant final efficacy and toxicity.19,23,27,28,71,106,112,122,151,162,164,170 Moreover, QSAR was utilized in combination with Principal Component Analysis (PCA) to predict the mechanism of action of anti-cancer drugs.85 QSAR has wide applicability in neuroscience for predicting the ability of chemicals to penetrate the Blood Brain Barrier (BBB) based on molecular size and lipophilicity.24
For predicting skin sensitization potency of chemicals, various QSAR models have been developed based on heterogeneous diverse chemical data-sets based on experimental conclusions from Local Lymph Node Assay (LLNA), Bundesinstitut für Gesundheitlichen Verbraucherschutz Und Veterinärmedizin (BgVV) or Guinea Pig Maximization Test (GPMT).
The induction phase of skin sensitization, specifically T-cell proliferation within the draining lymph nodes is the central event captured in the most widely utilized and accepted LLNA. In this assay groups of mice are treated with various concentrations of the test chemical applied topically to each ear, and the stimulation of proliferative responses in the draining lymph nodes is measured and compared with the response in control animals. The primary murine method provides a quantitative estimate of the concentration of chemical required to induce a stimulation index of three quantified as the extent of thymidine incorporation in lymph nodes from dosed animals relative to concurrent vehicle-treated controls using linear interpolation. This index is referred to as the EC3 value.13–17,54
The BgVV list of 264 chemicals was compiled and evaluated by a group of experts including dermatologists from universities and representatives of the chemical industry and from regulatory authorities that was established by the German Federal Institute for Health Protection of Consumers and Veterinary Medicine (BgVV) in 1985.143 Data from the literature on substances with documented contact allergenic properties in humans (from clinical data and experimental studies) and from animal experiments were evaluated resulting in a publication where chemicals were listed as belonging to one of 3 categories (A–C) where category A represented significant contact allergens, B. a solid based indication for contact allergenic potential and C. insignificant questionable contact allergenic potential.
The guinea pig maximization test (GPMT) of Magnusson and Kligman was published in 1969. There are some issues associated with this test. New information is required with regards to the interpretation of challenge results. In particular, overestimation of allergenicity owing to false-positive reactions is common. The control group should be exposed to a chemical insult at induction which provokes an inflammatory reaction comparable to the test substance. Allergic reactions should persist on rechallenge weeks later, while nonspecific irritant reactions generally do not persist and are irreproducible in particular animals. Finally, when a chemical is identified as a contact sensitizer in the GPMT, that result is simply a categorical statement of a theoretical hazard with no risk assessment. In the GPMT, a test substance is defined as a sensitizer if >30% of animals shows a positive response.
The chemical data-sets obtained from in vivo tests in animals have been rigorously tested and formulated over the past several years. The LLNA test is unique and comprehensive in providing a quantitative estimate of the sensitization potency of chemicals with relatively fewer animals and less expense as compared to the GPMT. Moreover, this test has been confirmed to have ~70% accuracy as compared to human test data.59 The positive predictivity of LLNA has been estimated to be >90%. However, the poor performance (<20%) in predicting non-sensitizers reduces the overall accuracy of LLNA. The approach based on chemical data-sets is more prevalent in the recent decades which entails statistical methods such as linear discriminant analysis or linear regression models that link the EC3 (estimated concentration of a chemical required to produce a 3-fold stimulation of draining lymph node cell proliferation compared with concurrent controls) values from LLNA data-sets as biological end-points to structural, topological, electronic or physiochemical descriptors of the molecules.
The Table 2 lists various QSAR models described using linear regression, discriminant analysis, neural networks etc., the number of chemicals tested as sensitizer or non-sensitizer and prominent features of the models. As shown in Table 2, the most comprehensive QSAR model (TIMES-SS) has been developed by incorporating the skin metabolism component into the model by introduction of weight parameters in the model system based on skin enzyme transformation capacity of the sensitizer. TIMES-SS developed at Bourgas University, incorporates skin metabolism and sensitizer structure–activity relationships in a single platform. Using TIMES-SS, there have been significantly consistent relationships between the simulations and certain experimental data for prediction of chemical sensitization potential. However, the methodology is not completely developed and requires further improvements such as inclusion of diverse reaction chemistry pathways. As indicated, the prediction capability for sensitizer is 65% while for non-sensitizer, it is 72% from a dataset of 634 chemicals (Table 2).37 The low predictability of the sensitizers is due to 34% prediction of weak sensitizer that results in reduction of the 80% prediction of moderate, strong and extreme sensitizers.
TABLE 2.
In silico QSAR and in vitro models for prediction of sensitization capacity of chemicals.
| Assay | Significant features | Number of compounds | % Prediction of sensitizer | % Prediction of non-sensitizer | Model system | References |
|---|---|---|---|---|---|---|
| In silico | ||||||
| LDA QSAR | 14 parameter model; HOMO-LUMO energy gap; Shannon index; discriminant analysis | 259 | 76.5 | 87.9 | GPMT | [33,35] |
| Neural network QSAR | 14 parameter model; HOMO-LUMO energy gap; Shannon index | 259 | 83.2 | 94.3 | GPMT | [35] |
| SAR | Skin metabolism; electrophilic interactions; molecular structure and reactivity; steric effects | 106 | Qualitative | Qualitative | LLNA | [7] |
| QSAR | 2D or 3D molecular r structure descriptors; topological indices; autocorrelation functions; logical regression | 54 | 72 | 93 | LLNA | [49] |
| DEREK, TOPKAT and linear regression | TOPKAT is GPMT based; DEREK and LR model is based on LLNA and GPMT | 178 | 82.9, 73.3, 87.6 | 82.9, 73.3, 87.6 | GPMT | [48] |
| DEREK and linear regression | DEREK and LR model is based on LLNA and GPMT | 178 | 73, 83.2 | 73, 83.2 | LLNA | [48] |
| TIMES-SS | Skin metabolism model; COREPA 3D-QSAR | 634 | 65 | 72 | LLNA, GPMT, BgVV | [37] |
| 4D-QSAR | Two-2-state (sensitizer, non-sensitizer) categorical model; 3D structure of molecules | 196 | 91.5 | 93.9 | LLNA | [91] |
| QSPR | Literature and structural descriptors | 358, 307, 251 | 90, 95, 90 | ND | LLNA, GPMT, BgVV | [59] |
| TOPSMODE-QSAR | Tonnage amount, cluster analysis; prediction of hair dye substances; only 10% of chemicals were pre-tested using LLNA and/or human tests | 229 | Qualitative | Qualitative | LLNA and human tests | [146] |
| Physiolab | Epidermal inflammation; LC maturation markers; Ag specific T cell proliferation | 8 | 100 | 0 | Entelos | |
| DEREK/METEOR | Expert system/metabolic fate predictor | Not disclosed | Not disclosed | Not disclosed | Not disclosed | LHASA limited |
| TOXHUNTER | Genomic, proteomic and metabolomic compound signatures | Not disclosed | Not disclosed | Not disclosed | Not disclosed | Genego |
| BioEpisteme | 2D or 3D molecular descriptors | Not disclosed | Not disclosed | Not disclosed | Not disclosed | Prous Institute |
| In chemico | ||||||
| GSH/Cys/Lys PRA | Six models; different ratios of GSH, Cys, or Lys to chemicals; recursive partitioning model based tree classification to compare LLNA EC3 | 82 | 89 | 89 | LLNA | [58] |
| DPRA | Different ratios of GSH, Cys, or Lys to chemicals; GSH (1:100), Lys (1:50), Cys (1:10), His(1:50) | 38 | 66, 67, 84, 36 | 66, 67, 84, 36 | LLNA | [57] |
| DPRA | Horseradish peroxidase and hydrogen peroxide assay; highly sensitive HPLC tandem mass spectrometry | 32 | 100 | Not Determined | LLNA | [56] |
| Cell based | ||||||
| h-CLAT | 3 rounds of trial for inter-laboratory [5 labs] consistency testing; CD54 and CD86 expression of THP-1 sensitized cells | 27 | 84 | 84 | LLNA | [135] |
| h-CLAT | CD54 and CD86 expression of THP-1 sensitized cells | 100 | 85 | 85 | LLNA | [8] |
| MUSST | CD86 expression of U-937 sensitized cells | 99 | 85 | Not determined | Human data | [1] |
| Keratinosens | Trial for inter-laboratory [5 labs] consistency testing; ARE expression of HaCat sensitized cells | 28 | 91 | 91 | LLNA | [5] |
| ARE luciferase assay | ARE gene expression in AREc32 cells; seeded in 96-well plates at a density of 50,000 cells | 102 | 83 | 83 | LLNA | [108] |
| Integrated testing strategy | ||||||
| Peptide/ARE/TIMES-SS | ARE expression of HaCat sensitized cells, TIMES-SS and peptide reactivity; data integration from multiple systems using regression analysis | 116 | 87.9 | 87.9 | LLNA | [109] |
QSAR models based on the guinea pig maximization test predicted skin sensitization for 76.5% of chemicals correctly by taking into account molecular descriptors and 27 structural alerts including the HOMO-LUMO energy gap (that represents the excitability of the molecule based on gap in energy of molecules between the lowest occupied and highest occupied molecular orbits), the Shannon index that accounts for molecular size and structural alerts that relate chemical reactivity sites causing skin sensitization (Table 2).33 Devillers, 2000 used artificial neural networks to predict accuracy of 83 and 94% for the sensitizers and non-sensitizers, respectively with a non-linear analysis model (Table 2).35 Extended database of chemicals (up to 300) is also included in Kern et al.73
More recently, an excellent QSPR model system has been established that takes into account the chemical data-sets from the LLNA, GPMT and BgVV tests.59 The QSPR model system was developed based on combination of literature and structural descriptors and resulted in 90% accuracy for the LLNA dataset This robust QSPR methodology was also tested against LLNA, GPMT and BgVV databases to obtain a comprehensive output of predictive capacity for each data-set.
TOPKAT as mentioned in Table 2 is multi-stage QSAR model system involved in the identification of sensitizers. The first stage searches for unknown fragments in the chemical to conclude if the molecule is present in the training set. The second stage determines if the structure of chemical is in the prediction space of the model. The third stage develops a probability value of the compound to be a sensitizer or a non-sensitizer. As indicated in Table 2, TOPKAT resulted in 83% prediction of sensitizers for a set of 178 chemicals for the GPMT database.
DEREK for Windows is an expert system that takes into account the toxicity and metabolism of the unknown molecule by comparison with similar molecular structure compounds in the training set. These lead to structural alerts that result in information processing that includes species based endpoint sensitization model (i.e. human, mouse, guinea pig etc.) and physicochemical properties of the molecules. The method provides end-users with a degree of confidence for the chemical to be a sensitizer or not. Multiple computer automated structure evaluation (MULTICASE) is a similar learning platform that further takes into account the mechanistic details of the compound transformations. As indicated in Table 2, DEREK resulted in 73.3% prediction of sensitizers for a set of 178 chemicals for the GPMT database and LLNA database.
For further details on the applicability domain of SAR and QSAR models for skin sensitizers based on chemical sensitizer data-sets using LLNA, GPMT and BgVV, please refer to literature reviews.37,55,59,120,160 Various companies have also been developed that provide QSAR based models as listed in Table 2.
While considerable effort has been expended into developing reliable predictive QSAR models, the following considerations may required to be incorporated:
Skin metabolic transformation mechanisms are not included in the validation or training datasets of most QSAR models. Incorporation of a step-wise QSAR model domain based on different descriptors could work for predicting sensitization potential of chemicals.37
The statistical correlation between biological end-points and chemical descriptors is a quantitative formulation that lacks development of multiple hypotheses from a scientific perspective that is critical for accurate prediction of chemicals.68 For e.g.: the models do not take into account mechanistic details such as electrophilicity and hydrophilicity.133 As a result, a mechanistic description of the molecular interaction of the sensitizer with a target nucleophilic skin peptide or protein cannot be estimated using these approaches. In a separate section below, we discuss a read-across method that takes into account the action of sensitizers through specific mechanisms.
Mechanism Based Read-across Models
Read-across is a qualitative method based on the concept that the activity of sensitizers, via a Michael addition mechanism, can be utilized to generate an electrophilicity index to categorize sensitization potential using quantitative methods. This approach has numerous advantages over existing QSAR based methods, i.e. ease of understanding and transparency, compliance with OECD regulations for QSAR validation due to a simple mechanistic approach, and ability to make computational interpolations of chemical toxicity in risk assessment platforms.
In a mechanism-based read-across method, the chemical to be tested is screened for structural alerts in accordance with a sensitization mode of action. If a Michael addition mechanism is involved, the global electrophilicity index can be determined as a function of chemical potential and chemical hardness for the specific chemical.
A common metric for distinguishing chemical sensitization potential is the Effect Concentration (EC3) value determined by the classical LLNA. EC3 is the test concentration of a chemical that elicits a 3-fold increase in lymph node T cell number as compared to control sets. Based on plots of the pEC3 (log of the molar EC3 concentration) values for known chemicals vs. the electrophilicity indices, the EC3 value for the unknown chemical can be estimated and compared to existing data using mechanism based read-across.45 The major limitation of mechanism based read-across is that the methodology requires upper and lower bounds of electrophilicity values of at least two known chemicals for the EC3 of the unknown chemical to be determined. This might not be available in the case of all sensitizers and hence limits the applicability of the method. Also, similar to QSAR models, the mechanism based read-across does not take into account pro-electrophilicity, i.e. the ability of chemicals to be metabolically activated by skin enzymes prior to becoming electrophiles.
IN VITRO APPROACHES
While in silico approaches are promising, a variety of in vitro systems also exist to investigate the effect that sensitizers have at the molecular, cellular and tissue levels. In the following sections we will review these approaches.
Protein Binding Assays
Landsteiner and Jacobs83 developed the first method to correlate simple chemical compounds to their sensitization potential based on formation of covalent adducts with protein molecules.83 Formation of macromolecular immunogen after chemical reaction of amino acid residues of nucleophilic skin proteins with electrophilic chemical sensitizers is the hallmark of sensitization.53 Human albumin is the model protein for most of the peptide reactivity assays53; approximately 40% of albumin not in vascular tissues is prevalent in the skin.
The peptide reactivity assay is based on the formation of a covalent bond between the sensitizer chemical and keratinocyte peptides or other protein structures in the skin (Fig. 3).53 This binding event is considered to be a determining step in the initiation of the sensitization process. The assay measures the percent depletion of electron rich Cysteine and Lysine as a function of sensitization potential of the chemicals. The reaction between electrophilic sensitizer chemicals and nucleophilic peptides along with glutathione serves as a platform to predict peptide reactivity of different chemicals.58 Direct peptide reactivity assay (DPRA) has been tested for 82 chemicals with 89% prediction of sensitizers and non-sensitizers (Table 2).
FIGURE 3.
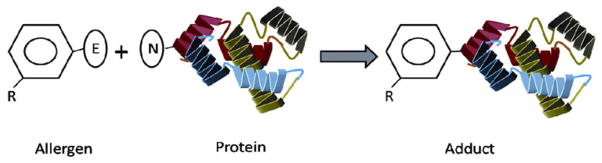
Schematic of direct peptide reactivity assay. The assay is based on evaluating covalent bond formation between nucleophilic protein and electrophilic allergen. The reaction is assessed by measuring either nucleophile depletion or adduct formation.
However, it is estimated that up to 33% of known chemicals as sensitizers are not reactive and require activation prior to interaction with skin peptides to sensitize skin tissue.56 DPRA does not take into account the skin-derived metabolic component that is clearly imperative for pro-haptens. In this regard, recent work has been focused on inclusion of poorly soluble chemicals and pro-haptens that undergo metabolic activation prior to interaction with skin peptides. The data clearly shows significant increase in peptide depletion (a biological endpoint defined as percent peptide depletion) for pro-haptens using the HRP/HP assay system as compared to routine DPRA.56 Overall, there is still an urgent need to test a tabulated list of chemicals from various datasets including complex molecular structure and functional groups, skin metabolic transformation capability and unknown reactivity based on standard mechanistic applicability domains. A comprehensive DPRA that tests a wide array of chemicals will provide the final outcome for accurate prediction of sensitizers. Further details of peptide reactivity assays is elucidated in the literature.80,160
2D Cell Based Models
A fundamental process in contact allergy is the activation and migration of LCs and dermal DCs followed by T-cell activation. Several investigative groups have targeted primary cells and cell lines in their considerations of appropriate in vitro 2D surrogates, whereby they monitor the increased expression of specific surface markers and signal transduction proteins, cytokine secretion profiles, chemokine induced DC migration, T cell activation and gene expression patterns of DCs amongst others in response to allergen treatment.
In the case of primary cells, human DCs are derived either from CD14+ peripheral monocytes (moDCs)131 or from CD34+ cord blood cells (CD34+ DCs). Maturation of DCs via sensitizers is measured by expression of IL1βmRNA,43,44 plus a variety of cell surface DC maturation markers such as CD86, CD83, CD207, CCR7 and CD54.3,4,32,64,155,156 In addition to measuring cell surface marker expression and cytokine production, identifying signal transduction pathways and gene expression patterns that change upon sensitizer exposure is another avenue for assessing sensitization potential.107,127 Also, the effect of reactive oxygen species on skin sensitization mediated by chemicals has been recently discussed in the literature. 98,101 While primary cells may provide the most suitable cell source, donor to donor variability and their limited availability may preclude their use in high throughput studies employing multiple allergens.
In order to generate DCs for simulating contact hypersensitivity phenomena, a variety of cell lines of the myeloid lineage have also been used including THP-1, KG-1, U-937, K-562 and HL-60.130,137–140,149,150 In particular THP-1 and U-937 cell lines have been widely utilized in predicting the sensitization potential of chemical allergens based on Human Cell Line Activation Test (h-CLAT),8,135 and myeloid U937 skin sensitization test (MUSST),126 respectively. An inter-laboratory study assessing the ability of this test to distinguish sensitizers from non-sensitizers was also initiated (Table 2).
h-CLAT entails exposure of THP-1 cells to sensitizer or irritant for 48–72 h. Following this, the cells are tested for surface marker expression of CD54 and CD86. Inter-laboratory variation is included to identify a metric wherein if 2 out of 3 independent experiments show CD54>200% and CD86>150%, then the chemical is a sensitizer. Table 2 lists the number of chemicals that have been predicted as sensitizers using the h-CLAT or MUSST assays. While h-CLAT can predict sensitizers at a reasonable accuracy of 85% for 100 chemicals, MUSST assays predict sensitizers at accuracy of 85% for 99 chemicals.
However, these cell lines have certain limitations. For example, maturation of DCs derived from these cell lines is not necessarily correlative with increased antigen uptake and lymph node migratory capacity. Moreover, these cells do not undergo a DC-like differentiation pathway and hence are not necessarily good model systems to simulate the developmental process. Thus, although, these cell lines are available in potentially unlimited quantities, they may lack certain critical phenotypic characteristics necessary for assessing sensitization.
A promising cell line is the Mutz3 cell line, which was derived from the peripheral blood of a patient with acute myelomonocytic leukemia and exhibits morphological and phenotypical characteristics of monocytes. 22,84,128,137–140 These cells can be induced by cytokines and growth factors to differentiate into LC and DC lineages with close resemblance, from a functional marker expression profile, to epidermal LC25,51,158 and DCs.89 These studies suggest that Mutz3 cells is a reliable cell source for generating both LCs and DCs.70 More importantly, Mutz3 cells can present antigen and can migrate into lymph channels in response to CCL19 chemokine.117 In response to strong sensitizers such as dinitrochlorobenzene (DNCB), 2,4,6-trinitrochlorobenzene (TNCB) and moderate sensitizers such as eugenol, increased expression of HLADR, CD54 and CD86 has been stimulated on Mutz3 cells9 and Mutz3 derived LCs.119
In addition to maturation and migration of DCs, the activation and clonal expansion of allergen responsive T-lymphocytes represent other downstream events in the demonstration of skin sensitization, and are attractive targets for development of alternative approaches. Past studies have employed co-culture of activated LCs or DCs with allogeneic naïve T-cells, and then measured proliferation by incorporation of radioactive tritium104 or measurement of IFNγ.94 An extensive review of T cell based priming assays is elucidated in the literature.94
Further details of cell based priming assays elucidated in the literature39,160 conclude that IL-8,116,126 CD86,119,153,165,168,169 and P38 MAP kinase6,52,81,95–97,102,103,114,154 are the three commonly used metrics to distinguish sensitizer from irritant in various DC primary cells and cell line models.
The testing of chemotactic migration of cells in response to sensitizer or irritant in 2D model systems has not been reviewed previously in the literature. The effect of these chemicals on migration of cells in response to chemokines is a key determinant in the subsequent activation of T cells as part of the contact hypersensitivity phenomena. Thus, there have been few reports in the literature regarding migration as a metric to distinguish sensitizer from irritant treated LCs in response to a chemokine gradient.
There is at least one report that the number of LCs migrating post-sensitization in vivo is significantly higher for sensitizer vs. irritant treated cells irrespective of T cell number in the lymph node compartment.63 Recent work also highlights the effect of chemokines on migration of cells in vitro. To simulate hapten-induced migration of LCs from the epidermis to the dermis, a dual chamber experiment was designed. This migration depends on 2 fibroblast-derived chemokines, i.e. CXCL12 and CCL5. Pre-treatment of fluorescently labeled (CSFE) MUTZ-LC (upper compartment) with sensitizers, but not with irritants, induces the expression of a CXCL12 receptor and, hence, enhances migration towards CXCL12 (lower compartment). For every chemical, the index of migration directed towards CXCL12 vs. that directed towards CCL5 can be determined. An index of CXCL12:CCL5>1, therefore, indicates sensitizers, and values <1 indicate non-sensitizers.119
Literature evidence also shows the utilization of CD34+ mononuclear peripheral cells for migration assessment in response to CCL19. The literature evidence for CCL19 based migration in presence of NiSO4 or DNCB reveals significant migration thus confirming that sensitized mononuclear peripheral cells undergo migration in response to strong and extreme sensitizers.20 From a mechanistic perspective, there is also literature evidence of PGE2, a lipid mediator and its effect on migration of CCR7 positive cells in response to a chemokine.141 Thus, based on recent literature evidence, the identification of migration of DCs in response to chemokines could be a determinant metric to distinguish sensitizers from irritants.
However, the lack of sufficient data on a diverse chemical data-set and the lack of extensive validation in terms of inter and intra-laboratory variability reduces enthusiasm towards this concept for accurate prediction of skin sensitization of chemicals unless rigorously pursued.
In the studies mentioned above, monocultures of LCs and DCs were directly activated by the allergens. For example one group assessed the effect of a panel of known human contact allergens (1-fluoro-2,4-dinitrobenzene, DNFB; paraphenylenediamine PPD; methylchloroisothiazolinone/methylisothiazolinone, CMIT), as well as the skin irritant benzalkonium chloride and of the mitogen phorbol myristate acetate (PMA). It was found that a distinct response, as ascertained through Il-1B mRNA measurements, was elicited through treatment with each of the compounds.121 An interesting finding, though, was that there is a potential for donor lot-to-lot variation, which is one important when assessing the sensitization potential of new compounds and formulations, and thus being able to tease out population dynamics through the use of multiple lots is crucial. Another interesting finding was that the degree of cellular response in this study was not directly related to the severity of the sensitizer. Thus, in these cases, the allergens had no exposure to skin cells which is an important prerequisite for LC and DC activation,161 and also important transport dynamics were available within a purely single cell type based assay. Towards this end, one recent study describes a loose fit co-culture model of activated keratinocytes and DCs. This model is composed of a single layer of human non-differentiating keratinocytes and of allogeneic floating monocytes, which are co-cultured in presence of IL-4, GMCSF and TGF-β. This model uses the expression of CD86 as an endpoint, and was able to predict sensitization potential of both weak and strong sensitizers.161
Finally, none of the studies mentioned above takes into account the role that the skin structure plays in the sensitization process. Moreover, the effect of the sensitizers in direct monoculture with DCs is variable and not sufficient to induce significant enhanced expression of phenotypical markers.110 Therefore, there would seem to be a real need for the development of suitable in vitro systems that incorporate human skin equivalents in co-culture with human DCs that can mimic the in vivo ACD phenomena more accurately.
3D Skin Tissue Based Systems
While the 2D cell models described above may be useful tools in certain studies, they certainly do not accurately simulate the in vivo condition, as skin is a complex three dimensional tissue with considerable barrier and metabolic function. To address this limitation, DCs derived from blood have been successfully integrated into a 3D cell culture model and used this model to predict sensitization in response to known allergens and UV radiation, where the end point metric was measurement of IL1β and CD86 mRNA, and the reduction in LCs, presumably due to LC emigration in response to the allergens.46 These 3D skin models offer the benefit of testing whether a particular chemical can overcome the stratum corneum barrier and penetrate into the viable epidermis below. This step is particularly important for classification because potency prediction based solely on 2D cell based models may not be an accurate depiction of bioavailability of the compound in vivo. This was found to be the case where an in vitro assay measuring CD86 expression on DCs showed very different sensitizing properties of p-toluylenediamine (PTD) and hydroxyethyl-p-phenylenediamine (HE-PPD) when compared to in vivo data.2 This discrepancy could be due to the differential skin penetration and haptenation profiles of these chemicals yielding different end-point sensitization outcomes. Metabolic activity of skin has been tested recently based on cytochrome P450 enzyme activity in presence of pro-haptens.10,61 Another advantage of 3D models is the ability to test chemicals that have low aqueous solubility by topical application. In the following paragraphs, we describe the different skin models and their utilization in simulating the sensitization phenomena.
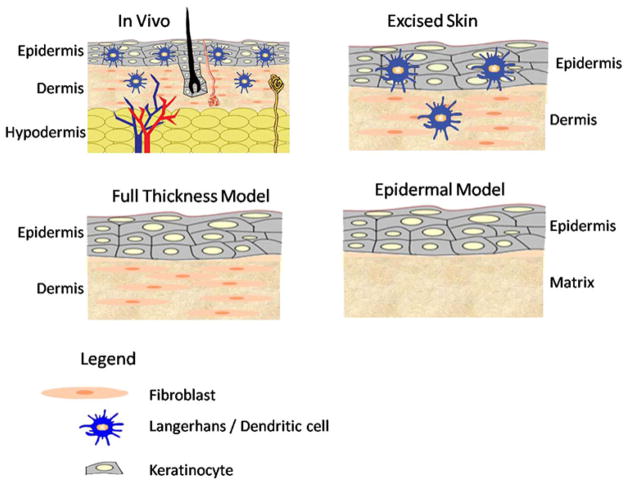
Three broad classes of 3D skin models include excised skin, engineered epidermal models, and full thickness skin equivalents (Fig. 4).
FIGURE 4.
Schematic of various skin models that can be potentially utilized for sensitization experiments. The excised skin model consists of the full complement of cells ranging from epidermal LCs, dermal DCs, keratinocytes, and fibroblasts. The engineered skin constructs are epidermal and full thickness models. The epidermal model is comprised of keratinocytes, while the full thickness model also includes fibroblasts.
Freshly excised human skin offers many advantages over engineered models since it retains a more in vivo like barrier and also features the full complement of accessory structures and cell types such as keratinocytes, fibroblasts, melanocytes, LCs and endothelial cells.124 Contrary to expectations, human skin explant studies show that there is a higher percentage of CD1a+ CD83+ LCs post migration for toxic concentration of irritant vs. non-toxic concentration of sensitizer in an in vitro 3D model system.66,88 It seems that all cells migrating in response to chemokine are CCR7+ in general. Due to the relatively sparse LCs and DCs in the epidermal and dermal layers, monitoring and quantifying migration rates of LCs and DCs in response to sensitizer remains challenging. The other major limitation of this model is large donor variability due to differences in lipid composition, skin thickness, hydration, age, sex and location of harvest. 42,82,86,123 Furthermore, due to its relatively short shelf life, it is not a practical model for allergen testing.
Engineered epidermal models are an attractive alternative since the epidermis is the site of entry and the location where hapten binding occurs. Keratinocytes in the epidermis also play a critical role in initiating and modulating the inflammatory reaction during sensitization. There are several commercially available epidermal equivalents that have been used for testing a variety of chemicals. Of these, three particular models (Episkin, Epiderm and SkinEthic’s Re-constructed Human Epidermis (RHE)) were recently accepted by the European Commission for skin irritation testing.
SkinEthic’s Episkin model consists of type I collagen matrix, representing the dermis, surfaced with a film of type IV human collagen upon which differentiated second passage keratinocytes (derived from mammary/abdominal samples obtained from healthy consenting donors during plastic surgery) are layered. 111 This skin model has been widely used in the literature for screening a wide variety of cosmetic formulations and irritants using metrics such as IL1α, IL-8 secretion and MTT for viability.31,136 The MTT assay is a colorimetric assay for measuring the activity of enzymes that reduce MTT to formazan dyes, giving a purple color. A main application allows assessing the viability and the proliferation of cells. It can also be used to determine effect of cytotoxic chemicals on stimulation or inhibition of cell viability and growth. Epiderm is composed of keratinocytes from a single donor cultured to form a multi-layered, highly differentiated model of the human epidermis on permeable culture inserts. Like Episkin, this model was also widely used in screening for a variety of sensitizers, and irritants using metrics such as MTT conversion for viability and testing for various secretion markers such as IL1α, IL-8, IL-1ra and PGE2 47; SkinEthic’s RHE model is described as an epidermis reconstructed by air lifted culture of normal human keratinocytes for 17 days in chemically defined medium on inert poly-carbonate filters. The RHE model has been shown to release IL-1α and IL-8 in a manner that corresponds with the MTT assay to distinguish between irritants and sensitizers.30,125
The inclusion of fibroblasts in the skin model may be important because fibroblasts and keratinocytes have been shown to synergistically perform during irritation and sensitization to produce secondary cytokines and chemokines such as IL-6, GM-CSF, CXCL12, CCL2 and CCL5.18,29,117,118 Furthermore, there is evidence that fibroblasts aid in the maturation of dermal DCs via direct cell contact and through soluble factors such as TNF-α.134 Thus, since full thickness models contain keratinocytes and fibroblasts, these models possess properties that reflect more physiologically relevant conditions. There are several commercially available full thickness models such as TestSkin, Apligraf, Skin,34 AST-2000 and RealSkin. TestSkin and Apligraf are both products from Organogenesis Inc. and consist of keratinocytes on top of a fibroblast populated bovine collagen type I matrix. Both products have been used to screen a range of chemicals such as sodium dodecyl sulfate (SDS), Vaseline and calcipotriol, where MTT was used for viability testing and secretion of PGE2, IL1α and IL-8 were assessed.115 However, Apligraf is commonly used in the context of wound healing and may not be an ideal candidate for the purpose of irritant and sensitizer screening. Skin34 is a skin equivalent from Advanced Tissue Sciences and consists of human keratinocytes and dermal fibroblasts co-cultured on a nylon mesh. A variety of surfactants and cosmetic formulations were tested on Skin34 where MTT conversion was assessed in addition to measuring IL1α, IL1ra, IL6, IL-8, GM-CSF and PGE2 levels.98 AST 2000 is a full thickness model from Cell Systems where keratinocytes are seeded on top of a dermal equivalent. This model was used to test sensitizers oxazolone, 1-fluoro-2,4-dinitrobenzene (DNFB) and irritants SDS and TritonX-100, where p38 and JNK1/2 were activated by phosphorylation exclusively with sensitizer treated conditions and ERK1/2 was only activated by irritant conditions.78 RealSkin is a full thickness model from SkinEthic consisting of a dermal equivalent where a lattice with acid-soluble collagen and normal human adult fibroblasts is overlaid by a stratified, well differentiated epidermis derived from normal human adult keratinocytes. Since this is a newer product, it has not been studied extensively for irritation or sensitization. However, mRNA expression levels for metabolic enzymes in RealSkin were found to be comparable to that of in vivo human skin.92
Overall, in terms of ease of availability, minimal batch to batch variability, ease of investigating functional outputs and ability to recapitulate in vivo like conditions, the engineered full thickness skin models are convenient and practical platforms for sensitization of DCs in co-culture model systems.
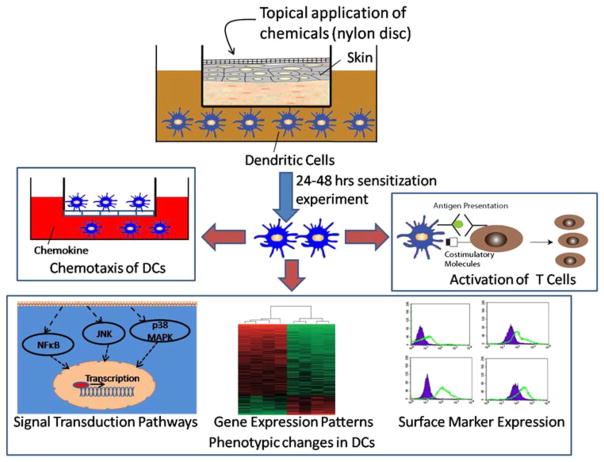
Once an engineered skin model is identified, sensitization studies can be conducted using co-cultures of skin and DCs as shown in Fig. 5. For example, skin can be mounted in a transwell, while DCs reside in the bottom chamber. In a typical experiment, a chemical is applied topically onto skin, generally via some type of absorbent material placed on top of the skin. After the chemical has penetrated into the skin and activated the DCs in the bottom chamber, a number of assays can be performed for assessing allergen potential of the chemical. The various assays include measuring chemotactic migration capacity of DCs, assessing expression of surface markers, gene expression patterns and relevant signal transduction pathways of activated DCs as indicators of DC maturation and DC induced stimulation of T-cell activation.
FIGURE 5.
Schematic of a typical sensitization experiment. A chemical is applied topically to the skin, which is in co-culture with the DCs. After the chemical has penetrated into the skin and activated the DCs, various functional assays are preformed that include measuring chemotactic migration capacity of DCs, assessing expression of surface markers, gene expression patterns and signal transduction pathways of activated DCs as indicators of dendritic cell maturation, and DC induced stimulation of T-cell activation.
Microfabricated Platforms
As described above, all the candidate in vitro assays typically include as their endpoint, only one or two of the hallmark events that occur during the demonstration of skin sensitization. Although consideration of one endpoint in isolation may provide some information regarding the sensitization potential of a chemical, they do not achieve the same predictive accuracy as in vivo methods such as LLNA (Table 2) and do not provide mechanistic insights into the contact hypersensitivity phenomena. It is likely that the development of a platform that successfully brings together all the inductive events involved in skin allergy will potentially provide investigators with a tool for studying the mechanistic aspects of these complex phenomena as well as prediction of sensitizers using this “LLNA-like” assay system. The disciplines of microscale engineering in conjunction with tissue engineering can potentially provide such a platform and revolutionize the in vitro allergy test field.
Integration of microscale technologies with cell and tissue culture is emerging as a powerful approach for creating advanced cell culture models.41,100 Microtechnology, originally developed in the semiconductor industry, provided the basis for fabrication of microdevices with a characteristic feature size ranging from microns to millimeters. Initial approaches for fabrication relied on photolithography and etching based techniques.62 With advances in soft lithography, fabrication of microdevices in elastomeric materials such as poly (dimethylsiloxane) became relatively easier.166 The ease of fabrication in combination with other desirable properties such as gas permeability, optical transparency, and compatibility with biological assays further paved the way for utilizing elastomer based microdevices for cell culture. There are several advantages in utilizing microdevices for cell culture. Microfluidics facilitates exquisite spatial152 and temporal 77 control over the cellular microenvironment, which were previously unattainable using conventional cell culture methods. In addition, scaling down to the micron level permits significant reagent and raw material saving, and has the added benefit of the possibility of evaluating responses at a single cell level.132 Microdevices can also be used to develop interconnected cell culture chambers that mimic the interconnectivity found in vivo, thus providing further relevance and presumed predictability.26,74,93,113,144 For example, multi-tissue device have been developed that allow for the assessment of drug metabolism (clearance), as well as volume of distribution for new chemical entities. These chips have the potential to act as a surrogate for animal testing, in that they can be integrated with human cell types thereby reducing the necessity for animal models, or even animal derived cell types.26,93,113 In addition to the advantage of miniaturized cell and multi-tissue culture models, chemotaxis (another cell and tissue level process important in allergic immune response) has been successfully adapted to the microfluidic format.60,65,72,90 In comparison to traditional methods, microfluidics can reproduce in vivo like channel sizes, and the establishment of more precise spatially and temporally stable chemokine gradients.90 Fast liquid switching capability provided by microfluidics further facilitates realization of user-defined chemokine gradients.72 Additionally, controlling and quantifying gradients at the cellular level together with microscopic monitoring of the movement of single cells, offers the potential to establish quantitative correlations between cell migration and response to a chemokine gradient at a single cell level.
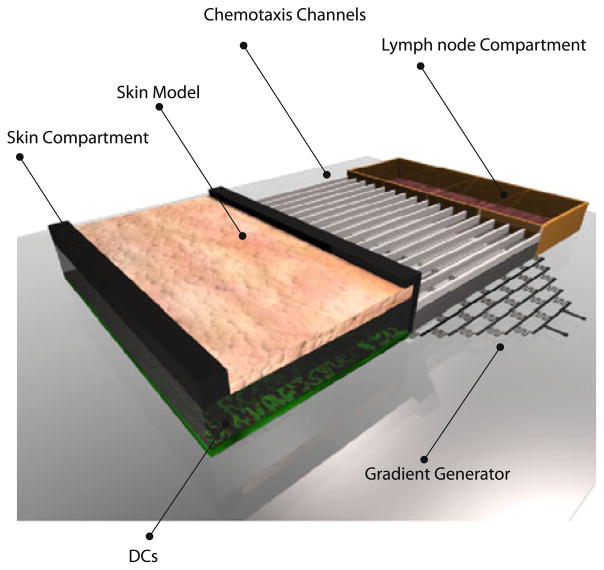
Given the flexibility of microdevices in designing interconnected tissue compartments which can integrate chemotaxis, our laboratory begun developing a microfabricated platform that has the potential to essentially capture the majority of the key steps involved in the sensitizer-induced immune response. As shown in Fig. 6, a microdevice can include both skin and lymph node compartments connected via microchannels. 167 The skin compartment can contain the engineered skin model with DCs either integrated in the skin model or residing directly underneath the skin. In response to an allergen, DCs in the skin compartment can become activated by sensitizers, leading to their migration to the lymph node compartment where they can activate T cells.79 The microfabricated system has the potential to mimic the essential elements of LLNA, including topical application of chemical on skin, intercellular communication between skin resident cells, maturation and chemotactic migration of DCs, and T cell activation by DCs. This system offers a comprehensive set of in vitro assays with the collective potential of developing into an alternative to LLNA for animal free testing of chemicals.142 However, this assay system, due to its nascent technological development status, needs validation.
FIGURE 6.
Schematic of microdevice that can potentially be used for performing the LLNA on chip. The microdevice consists of the skin and the lymph node compartments connected via microchannels. The skin compartment can contain the engineered skin model with DCs either integrated in the model or residing directly underneath the skin. In response to an allergen, DCs in the skin compartment can get activated leading to their migration to the lymph node compartment where they can activate the T cells.
HUMAN REPEATED INSULIN PATCH TEST (HRIPT)
HRIPT has a 50 year history and was first developed in 1944 by Schwartz and Peck. Quantitative risk assessment of sensitizers utilizes HRIPT in combination with LLNA50 though recent literature mentions the ethical concerns associated with HRIPT.159 Human tests for assessment of chemicals as sensitizers is ethical based on written informed consent of patients and is conducive as a comparison of the skin sensitization challenge to improve robustness of historical or novel control approaches.12 Detail protocols and factors implicated in HRIPT have been cited in the literature. 99 There have also been recent reports on the genetics of contact allergy and its relationship to polysensitization.157 The cumbersome and lengthy procedure to test chemicals in human volunteers and the ethical concerns associated with the protocol dampens enthusiasm towards the large scale implementation of this method for skin sensitization studies. However, for validation of compounds especially of complex molecular structure, unknown mechanistic applicability domains and pro-haptens, the human patch test can be a fairly robust and conclusive endpoint assay.
INTEGRATED TESTING STRATEGIES
As described above, all the candidate assays do not achieve the same predictive accuracy as in vivo methods such as LLNA (Table 2). Integrated testing systems comprising of a combination of the above discussed approaches could be the most optimal solution for validation of chemicals categorized as sensitizer/non-sensitizer based on the LLNA, human or GPMT datasets 69,107 and/or prediction of new chemicals.
Examples of multi-tier systems from the literature are described:
Incorporation of a step-wise QSAR model domain based on different descriptors could work for predicting sensitization potential of chemicals.37
Another example is inclusion of compounds that have failed positive testing using Natsch and Emter method108 as sensitizer, though the chemical is identified as a sensitizer using the QSAR based models (e.g.: aldehydes and ketones). The Natsch and Emter methodology is an elaborate and very promising method that is based on antioxidant response gene expression changes (Keap1) in presence of sensitizer for AREc32 cell line (CXR Biosciences) that uniquely links metabolic mechanism of cells to sensitizer-protein binding. Also, currently, CXR Biosciences is working with collaborators to introduce adenoviral vectors that lead to expression of multiple cytochrome P450 isozymes in response to specific pro-haptens in these cell lines. Recently, the Natsch approach has been integrated with peptide reactivity assay and TIMES-SS platform with 88% prediction for 116 chemicals in comparison to the LLNA database (Table 2). This set of chemical classification is further scored by each tier of the system according to proposed guidelines.69
In a recent study an integrated approach that combines in chemico coupling (peptide-binding) assay) with T-cell activation was described.36 The chemicals that form adduct with the skin relevant protein such as human serum albumin, or directly modified cellular proteins in dendritic cells induced T cell response as estimated by measuring T cell proliferation and interferon gamma production. The two step approach further underscored the difficulty associated with interpretation of results obtained in single assay in isolation. For example direct treatment of immature DCs with the sensitizer DNCB failed to generate robust T cell response. The failed T cell response was explained based on the peptide binding assay that revealed inefficient modification of peptide by DNCB.
As described above, the major limitations of integrated systems are the conflicting results in the multitier approach and they have not been extensively tested for large chemical data-sets.
To develop an optimal integrated testing platform, it is imperative to firstly generate a formal list of chemicals that cover the entire range of mechanistic reaction domains, complex chemistry and metabolic transformation capacity.43,58 Further validation of each tier of the integrated system utilizing chemical data-sets such as LLNA and categorically scoring chemicals based on sensitization potency is essential.69 The development of a pre-validated multi-tier integrated system with multiple biological end-points can then be utilized for predicting sensitization potency of unknown chemicals with reasonable confidence.
SUMMARY
In the current paper, we have discussed key technologies that can potentially replace the animal-based gold standards, such as the LLNA, for assessing sensitization potential of chemicals. These in vitro and in silico approaches described above offer alternatives that can potentially limit or even eliminate the use of animal models for testing of sensitizers. While the in silico QSAR based models have been extensively tested for a large chemical data-set, the in vitro models, such as the DPRA and 2D based models, while are promising, however, do lack extensive validation. The 3D based models and the microfluidic platform are interesting solutions to the underlying problem of capturing the complexity of ACD associated events in vitro but they lack validation.
Overall, despite their utility, these entire model systems display some inherent limitations as summarized in Table 1. Also, as shown in Table 2, there is some percentage of chemicals that cannot be predicted accurately as sensitizers or non-sensitizers in comparison to the animal tests derived experimental data-sets.
TABLE 1.
Comparison of non-animal alternative methods for assessing sensitization potential of chemicals.
| Model systems | Description | Advantages | Disadvantages |
|---|---|---|---|
| In silico approaches | |||
| DEREK | Uses structural alerts for sensitization and physiochemical properties of chemicals and provides degree of confidence of chemical as sensitizer | User-friendly and takes into account type of animal model used for sensitizer | Does not take into account electrophilicity of chemicals |
| TOPKAT | Uses structural alerts present in prediction space and provides probability score of chemical as sensitizer | Takes into account large scale chemical datasets | Does not take into account electrophilicity of chemicals |
| TIMES-SS | Incorporates skin metabolism and interaction of chemical with reactive proteins | Incorporates skin metabolism of pro-haptens for assessing sensitization potential | Requires testing large data-sets for validation; does not take into account reaction chemistry pathways |
| Mechanism Based Read-Across | Incorporates reaction between electrophilic sensitizer and nucleophilic skin peptides | Incorporates electrophilicity index of chemical for sensitizer classification purposes | Requires a priori EC3 data |
| In vitro approaches | |||
| Peptide binding | Chemical reacts with proteins containing Cys and Lys forming stable covalent bonds | High-throughput, simple assay | Not accurate with weak sensitizers and pro-haptens |
| 2D cell based models | Incorporates keratinocytes, primary DCs and DC cell lines (Mutz-3, THP-1, U937, KG-1, K-562, HL-60) | Incorporates dendritic cell-sensitizer interaction | Lacking skin tissue |
| 3D Skin tissue based models | Epidermal equivalents, full thickness models, excised skin in co-culture with DCs | Topical application, Incorporates skin-DC interaction | Lacks dynamic interaction between skin, DC and T cells |
| Microfluidic platform | Microfabricated system integrating skin and lymph node tissues | Integrated approach; reduced cell and reagents requirements | Requires validation; relatively nascent technology |
At one end, there is a requirement for developing novel in vitro techniques that target specific chemicals of complex cases based on reaction mechanistic domains, structural alerts and difficult chemistry.58 At the other end, there is an urgent need to utilize already existing robust approaches and novel in vitro techniques under development to be implemented in an integrated platform based on existing experimental data to validate set of chemicals.
ABBREVIATIONS
- ACD
Allergic Contact Dermatitis
- BBB
Blood Brain Barrier
- BgVV
Bundesinstitut für Gesundheitlichen Verbraucherschutz Und Veterinärmedizin
- DC
Dendritic Cells
- DEREK
Deductive Estimation of Risk from Existing Knowledge
- DNCB
DiNitroChloroBenzene
- DNFB
1-Fluoro-2,4-dinitrobenzene
- DPRA
Direct Peptide Reactivity Assay
- GPMT
Guinea Pig Maximization Test
- h-CLAT
human Cell Line Activation Test
- HE-PPD
Hydroxyethyl-p-phenylenediamine
- HRIPT
Human Repeated Insulin Patch Test
- HRP/HP
Horseradish Peroxidase/Hydrogen Peroxide
- ITS
Integrated Testing Strategies
- LC
Langerhans Cells
- LR
Linear Regression
- LLNA
Local Lymph Node Assay
- MUSST
Myeloid U937 Skin Sensitisation Test
- MULTICASE
Multiple Computer Automated Structure Evaluation
- NCHS
National Center for Health Statistics
- OECD
Organization for Economic Cooperation and Development
- PCA
Principal Component Analysis
- PTD
p-toluylenediamine
- QSAR
Quantitative Structure Activity Relationship
- QSPR
Quantitative Structure Property Relationship
- REACh
Registration, Evaluation, Authorization of Chemicals
- SAR
Structure Activity Relationship
- TIMES-SS
TImes MEtabolism Simulator platform used for predicting Skin Sensitization
- TNCB
2,4,6-Trinitrochlorobenzene
- TOPKAT
Toxicity Prediction by Komputer Assisted Technology
References
- 1.Aeby P, Ashikaga T, Bessou-Touya S, Schepky A, Gerberick F, Kern P, Marrec-Fairley M, Maxwell G, Ovigne JM, Sakaguchi H, et al. Identifying and characterizing chemical skin sensitizers without animal testing: Colipa’s research and method development program. Toxicol In Vitro. 2010;24:1465–1473. doi: 10.1016/j.tiv.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Aeby P, Wyss C, Beck H, Griem P, Scheffler H, Goebel C. Characterization of the sensitizing potential of chemicals by in vitro analysis of dendritic cell activation and skin penetration. J Invest Dermatol. 2004;122:1154–1164. doi: 10.1111/j.0022-202X.2004.22402.x. [DOI] [PubMed] [Google Scholar]
- 3.Aiba S, Manome H, Yoshino Y, Tagami H. In vitro treatment of human transforming growth factor-beta1-treated monocyte-derived dendritic cells with haptens can induce the phenotypic and functional changes similar to epidermal Langerhans cells in the initiation phase of allergic contact sensitivity reaction. Immunology. 2000;101:68–75. doi: 10.1046/j.1365-2567.2000.00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aiba S, Terunuma A, Manome H, Tagami H. Dendritic cells differently respond to haptens and irritants by their production of cytokines and expression of co-stimulatory molecules. Eur J Immunol. 1997;27:3031–3038. doi: 10.1002/eji.1830271141. [DOI] [PubMed] [Google Scholar]
- 5.Andreas N, Caroline B, Leslie F, Frank G, Kimberly N, Allison H, Heather I, Robert L, Stefan O, Hendrik R, et al. The intra- and inter-laboratory reproducibility and predictivity of the KeratinoSens assay to predict skin sensitizers in vitro: results of a ring-study in five laboratories. Toxicol In Vitro. 2010 doi: 10.1016/j.tiv.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Antonios D, Rousseau P, Larange A, Kerdine-Romer S, Pallardy M. Mechanisms of IL-12 synthesis by human dendritic cells treated with the chemical sensitizer NiSO4. J Immunol. 2010;185:89–98. doi: 10.4049/jimmunol.0901992. [DOI] [PubMed] [Google Scholar]
- 7.Ashby J, Basketter DA, Paton D, Kimber I. Structure activity relationships in skin sensitization using the murine local lymph node assay. Toxicology. 1995;103:177–194. doi: 10.1016/0300-483x(95)03132-y. [DOI] [PubMed] [Google Scholar]
- 8.Ashikaga T, Sakaguchi H, Sono S, Kosaka N, Ishikawa M, Nukada Y, Miyazawa M, Ito Y, Nishiyama N, Itagaki H. A comparative evaluation of in vitro skin sensitisation tests: the human cell-line activation test (h-CLAT) versus the local lymph node assay (LLNA) Altern Lab Anim. 2010;38:275–284. doi: 10.1177/026119291003800403. [DOI] [PubMed] [Google Scholar]
- 9.Azam P, Peiffer JL, Chamousset D, Tissier MH, Bonnet PA, Vian L, Fabre I, Ourlin JC. The cytokine-dependent MUTZ-3 cell line as an in vitro model for the screening of contact sensitizers. Toxicol Appl Pharmacol. 2006;212:14–23. doi: 10.1016/j.taap.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 10.Baron JM, Wiederholt T, Heise R, Merk HF, Bickers DR. Expression and function of cytochrome p450-dependent enzymes in human skin cells. Curr Med Chem. 2008;15:2258–2264. doi: 10.2174/092986708785747535. [DOI] [PubMed] [Google Scholar]
- 11.Basketter DA. Skin sensitization to cinnamic alcohol: the role of skin metabolism. Acta Derm Venereol. 1992;72:264–265. [PubMed] [Google Scholar]
- 12.Basketter DA. The human repeated insult patch test in the 21st century: a commentary. Cutan Ocul Toxicol. 2009;28:49–53. doi: 10.1080/15569520902938032. [DOI] [PubMed] [Google Scholar]
- 13.Basketter DA, Balikie L, Dearman RJ, Kimber I, Ryan CA, Gerberick GF, Harvey P, Evans P, White IR, Rycroft RJ. Use of the local lymph node assay for the estimation of relative contact allergenic potency. Contact Dermatitis. 2000;42:344–348. doi: 10.1034/j.1600-0536.2000.042006344.x. [DOI] [PubMed] [Google Scholar]
- 14.Basketter DA, Evans P, Fielder RJ, Gerberick GF, Dearman RJ, Kimber I. Local lymph node assay—validation, conduct and use in practice. Food Chem Toxicol. 2002;40:593–598. doi: 10.1016/s0278-6915(01)00130-2. [DOI] [PubMed] [Google Scholar]
- 15.Basketter DA, Gerberick GF, Kimber I, Loveless SE. The local lymph node assay: a viable alternative to currently accepted skin sensitization tests. Food Chem Toxicol. 1996;34:985–997. doi: 10.1016/s0278-6915(96)00059-2. [DOI] [PubMed] [Google Scholar]
- 16.Basketter DA, Gilmour NJ, Briggs D, Ullmann LG, Gerberick GF, Ryan CA, Dearman RJ, Kimber I. Utility of historical vehicle-control data in the interpretation of the local lymph node assay. Contact Dermatitis. 2003;49:37–41. doi: 10.1111/j.0105-1873.2003.00162.x. [DOI] [PubMed] [Google Scholar]
- 17.Basketter DA, Lea LJ, Cooper K, Stocks J, Dickens A, Pate I, Dearman RJ, Kimber I. Threshold for classification as a skin sensitizer in the local lymph node assay: a statistical evaluation. Food Chem Toxicol. 1999;37:1167–1174. doi: 10.1016/s0278-6915(99)00112-x. [DOI] [PubMed] [Google Scholar]
- 18.Bernhofer LP, Seiberg M, Martin KM. The influence of the response of skin equivalent systems to topically applied consumer products by epithelial– mesenchymal interactions. Toxicol In Vitro. 1999;13:219–229. doi: 10.1016/s0887-2333(98)00087-3. [DOI] [PubMed] [Google Scholar]
- 19.Bhhatarai B, Gramatica P. Per- and polyfluoro toxicity (LC(50) inhalation) study in rat and mouse using QSAR modeling. Chem Res Toxicol. 2010;23:528–539. doi: 10.1021/tx900252h. [DOI] [PubMed] [Google Scholar]
- 20.Boisleve F, Kerdine-Romer S, Rougier-Larzat N, Pallardy M. Nickel and DNCB induce CCR7 expression on human dendritic cells through different signalling pathways: role of TNF-alpha and MAPK. J Invest Dermatol. 2004;123:494–502. doi: 10.1111/j.0022-202X.2004.23229.x. [DOI] [PubMed] [Google Scholar]
- 21.Bonefeld CM, Larsen JM, Dabelsteen S, Geisler C, White IR, Menne T, Johansen JD. Consumer available permanent hair dye products cause major allergic immune activation in an animal model. Br J Dermatol. 2010;162:102–107. doi: 10.1111/j.1365-2133.2009.09417.x. [DOI] [PubMed] [Google Scholar]
- 22.Bontkes HJ, Ruizendaal JJ, Kramer D, Santegoets SJ, Scheper RJ, de Gruijl TD, Meijer CJ, Hooijberg E. Constitutively active STAT5b induces cytokine-independent growth of the acute myeloid leukemia-derived MUTZ-3 cell line and accelerates its differentiation into mature dendritic cells. J Immunother. 2006;29:188–200. doi: 10.1097/01.cji.0000197095.00359.67. [DOI] [PubMed] [Google Scholar]
- 23.Buist HE, van Burgsteden JA, Freidig AP, Maas WJ, van de Sandt JJ. New in vitro dermal absorption database and the prediction of dermal absorption under finite conditions for risk assessment purposes. Regul Toxicol Pharmacol. 2010;57:200–209. doi: 10.1016/j.yrtph.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Burns J, Weaver DF. A mathematical model for prediction of drug molecule diffusion across the blood-brain barrier. Can J Neurol Sci. 2004;31:520–527. doi: 10.1017/s0317167100003759. [DOI] [PubMed] [Google Scholar]
- 25.Caux C, Valladeau J, Dieu MC, Ravel O, Vanbervliet B, Vicari A, Saeland S, Lebecque S. Langerhans cells have unique features illustrating selective migration, antigen uptake and routage capacities. J Invest Dermatol. 2000;114:207–207. [Google Scholar]
- 26.Chao P, Maguire T, Novik E, Cheng KC, Yarmush ML. Evaluation of a microfluidic based cell culture platform with primary human hepatocytes for the prediction of hepatic clearance in human. Biochem Pharmacol. 2009;78:625–632. doi: 10.1016/j.bcp.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cleuvers M. Mixture toxicity of the anti-inflammatory drugs diclofenac, ibuprofen, naproxen, and acetylsalicylic acid. Ecotoxicol Environ Saf. 2004;59:309–315. doi: 10.1016/S0147-6513(03)00141-6. [DOI] [PubMed] [Google Scholar]
- 28.Cooper AE, Potter T, Luker T. Prediction of efficacious inhalation lung doses via the use of in silico lung retention QSAR models and in vitro potency screens. Drug Metab Dispos. 2010;38(12):2218–2225. doi: 10.1124/dmd.110.034462. [DOI] [PubMed] [Google Scholar]
- 29.Coquette A, Berna N, Vandenbosch A, Rosdy M, De Wever B, Poumay Y. Analysis of interleukin-1alpha (IL-1alpha) and interleukin-8 (IL-8) expression and release in in vitro reconstructed human epidermis for the prediction of in vivo skin irritation and/or sensitization. Toxicol In Vitro. 2003;17:311–321. doi: 10.1016/s0887-2333(03)00019-5. [DOI] [PubMed] [Google Scholar]
- 30.Coquette A, Berna N, Vandenbosch A, Rosdy M, Poumay Y. Differential expression and release of cytokines by an in vitro reconstructed human epidermis following exposure to skin irritant and sensitizing chemicals. Toxicol In Vitro. 1999;13:867–877. doi: 10.1016/s0887-2333(99)00076-4. [DOI] [PubMed] [Google Scholar]
- 31.Cotovio J, Grandidier MH, Portes P, Roguet R, Rubinstenn G. The in vitro skin irritation of chemicals: optimisation of the EPISKIN prediction model within the framework of the ECVAM validation process. Altern Lab Anim. 2005;33:329–349. doi: 10.1177/026119290503300403. [DOI] [PubMed] [Google Scholar]
- 32.Coutant KD, Ulrich P, Thomas H, Cordier A, Brugerolle de Fraissinette A. Early changes in murine epidermal cell phenotype by contact sensitizers. Toxicol Sci. 1999;48:74–81. doi: 10.1093/toxsci/48.1.74. [DOI] [PubMed] [Google Scholar]
- 33.Cronin MT, Basketter DA. Multivariate QSAR analysis of a skin sensitization database. SAR QSAR Environ Res. 1994;2:159–179. doi: 10.1080/10629369408029901. [DOI] [PubMed] [Google Scholar]
- 34.del Savio B, Sherertz EF. Is allergic contact dermatitis being overlooked? Arch Fam Med. 1994;3:537–543. doi: 10.1001/archfami.3.6.537. [DOI] [PubMed] [Google Scholar]
- 35.Devillers J. New trends in (Q)SAR modeling with topological indices. Curr Opin Drug Discov Devel. 2000;3:275–279. [PubMed] [Google Scholar]
- 36.Dietz L, Esser PR, Schmucker SS, Goette I, Richter A, Schnolzer M, Martin SF, Thierse HJ. Tracking human contact allergens: from mass spectrometric identification of peptide-bound reactive small chemicals to chemical-specific naive human T-cell priming. Toxicol Sci. 2010;117:336–347. doi: 10.1093/toxsci/kfq209. [DOI] [PubMed] [Google Scholar]
- 37.Dimitrov S, Dimitrova G, Pavlov T, Dimitrova N, Patlewicz G, Niemela J, Mekenyan O. A stepwise approach for defining the applicability domain of SAR and QSAR models. J Chem Inf Model. 2005;45:839–849. doi: 10.1021/ci0500381. [DOI] [PubMed] [Google Scholar]
- 38.Divkovic M, Pease CK, Gerberick GF, Basketter DA. Hapten-protein binding: from theory to practical application in the in vitro prediction of skin sensitization. Contact Dermatitis. 2005;53:189–200. doi: 10.1111/j.0105-1873.2005.00683.x. [DOI] [PubMed] [Google Scholar]
- 39.dos Santos GG, Reinders J, Ouwehand K, Rustemeyer T, Scheper RJ, Gibbs S. Progress on the development of human in vitro dendritic cell based assays for assessment of the sensitizing potential of a compound. Toxicol Appl Pharmacol. 2009;236:372–382. doi: 10.1016/j.taap.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Ebalunode JO, Zheng W, Tropsha A. Application of QSAR and shape pharmacophore modeling approaches for targeted chemical library design. Methods Mol Biol. 2011;685:111–133. doi: 10.1007/978-1-60761-931-4_6. [DOI] [PubMed] [Google Scholar]
- 41.El-Ali J, Sorger PK, Jensen KF. Cells on chips. Nature. 2006;442:403–411. doi: 10.1038/nature05063. [DOI] [PubMed] [Google Scholar]
- 42.Elias PM, Cooper ER, Korc A, Brown BE. Percutaneous transport in relation to stratum corneum structure and lipid composition. J Invest Dermatol. 1981;76:297–301. doi: 10.1111/1523-1747.ep12526137. [DOI] [PubMed] [Google Scholar]
- 43.Enk AH, Katz SI. Early events in the induction phase of contact sensitivity. J Invest Dermatol. 1992;99:39S– 41S. doi: 10.1111/1523-1747.ep12668608. [DOI] [PubMed] [Google Scholar]
- 44.Enk AH, Katz SI. Contact sensitivity as a model for T-cell activation in skin. J Invest Dermatol. 1995;105:80S– 83S. doi: 10.1111/1523-1747.ep12316112. [DOI] [PubMed] [Google Scholar]
- 45.Enoch SJ, Cronin MT, Schultz TW, Madden JC. Quantitative and mechanistic read across for predicting the skin sensitization potential of alkenes acting via Michael addition. Chem Res Toxicol. 2008;21:513–520. doi: 10.1021/tx700322g. [DOI] [PubMed] [Google Scholar]
- 46.Facy V, Flouret V, Regnier M, Schmidt R. Langerhans cells integrated into human reconstructed epidermis respond to known sensitizers and ultraviolet exposure. J Invest Dermatol. 2004;122:552–553. doi: 10.1046/j.0022-202X.2004.22209.x. [DOI] [PubMed] [Google Scholar]
- 47.Faller C, Bracher M, Dami N, Roguet R. Predictive ability of reconstructed human epidermis equivalents for the assessment of skin irritation of cosmetics. Toxicol In Vitro. 2002;16:557–572. doi: 10.1016/s0887-2333(02)00053-x. [DOI] [PubMed] [Google Scholar]
- 48.Fedorowicz A, Singh H, Soderholm S, Demchuk E. Structure-activity models for contact sensitization. Chem Res Toxicol. 2005;18:954–969. doi: 10.1021/tx0497806. [DOI] [PubMed] [Google Scholar]
- 49.Fedorowicz A, Zheng LY, Singh H, Demchuk E. QSAR study of skin sensitization using local lymph node assay data. Int J Mol Sci. 2004;5:56–66. [Google Scholar]
- 50.Fluhr J, Lademann J. Penetration properties and safety aspects of topically applied products. Skin Pharmacol Physiol. 2008;21:293. doi: 10.1159/000162290. [DOI] [PubMed] [Google Scholar]
- 51.Fukunaga A, Khaskhely NM, Sreevidya CS, Byrne SN, Ullrich SE. Dermal dendritic cells, and not Langerhans cells, play an essential role in inducing an immune response. J Immunol. 2008;180:3057–3064. doi: 10.4049/jimmunol.180.5.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galbiati V, Mitjans M, Lucchi L, Viviani B, Galli CL, Marinovich M, Corsini E. Further development of the NCTC 2544 IL-18 assay to identify in vitro contact allergens. Toxicol In Vitro. 2010 doi: 10.1016/j.tiv.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 53.Gerberick F, Aleksic M, Basketter D, Casati S, Karlberg AT, Kern P, Kimber I, Lepoittevin JP, Natsch A, Ovigne JM, et al. Chemical reactivity measurement and the predicitve identification of skin sensitisers. The report and recommendations of ECVAM Workshop 64. Altern Lab Anim. 2008;36:215–242. doi: 10.1177/026119290803600210. [DOI] [PubMed] [Google Scholar]
- 54.Gerberick GF, Ryan CA, Dearman RJ, Kimber I. Local lymph node assay (LLNA) for detection of sensitization capacity of chemicals. Methods. 2007;41:54–60. doi: 10.1016/j.ymeth.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 55.Gerberick GF, Ryan CA, Kern PS, Schlatter H, Dearman RJ, Kimber I, Patlewicz GY, Basketter DA. Compilation of historical local lymph node data for evaluation of skin sensitization alternative methods. Dermatitis. 2005;16:157–202. [PubMed] [Google Scholar]
- 56.Gerberick GF, Troutman JA, Foertsch LM, Vassallo JD, Quijano M, Dobson RL, Goebel C, Lepoittevin JP. Investigation of peptide reactivity of prohapten skin sensitizers using a peroxidase-peroxide oxidation system. Toxicol Sci. 2009;112:164–174. doi: 10.1093/toxsci/kfp192. [DOI] [PubMed] [Google Scholar]
- 57.Gerberick GF, Vassallo JD, Bailey RE, Chaney JG, Morrall SW, Lepoittevin JP. Development of a peptide reactivity assay for screening contact allergens. Toxicol Sci. 2004;81:332–343. doi: 10.1093/toxsci/kfh213. [DOI] [PubMed] [Google Scholar]
- 58.Gerberick GF, Vassallo JD, Foertsch LM, Price BB, Chaney JG, Lepoittevin JP. Quantification of chemical peptide reactivity for screening contact allergens: a classification tree model approach. Toxicol Sci. 2007;97:417–427. doi: 10.1093/toxsci/kfm064. [DOI] [PubMed] [Google Scholar]
- 59.Golla S, Madihally S, Robinson RL, Jr, Gasem KA. Quantitative structure-property relationship modeling of skin sensitization: a quantitative prediction. Toxicol In Vitro. 2009;23:454–465. doi: 10.1016/j.tiv.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 60.Haessler U, Kalinin Y, Swartz MA, Wu M. An agarose-based microfluidic platform with a gradient buffer for 3D chemotaxis studies. Biomed Microdevices. 2009;11:827– 835. doi: 10.1007/s10544-009-9299-3. [DOI] [PubMed] [Google Scholar]
- 61.Hagvall L, Baron JM, Borje A, Weidolf L, Merk H, Karlberg AT. Cytochrome P450-mediated activation of the fragrance compound geraniol forms potent contact allergens. Toxicol Appl Pharmacol. 2008;233:308–313. doi: 10.1016/j.taap.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 62.Harrison DJ, Fluri K, Seiler K, Fan Z, Effenhauser CS, Manz A. Micromachining a miniaturized capillary electrophoresis-based chemical analysis system on a chip. Science. 1993;261:895–897. doi: 10.1126/science.261.5123.895. [DOI] [PubMed] [Google Scholar]
- 63.Hill S, Edwards AJ, Kimber I, Knight SC. Systemic migration of dendritic cells during contact sensitization. Immunology. 1990;71:277–281. [PMC free article] [PubMed] [Google Scholar]
- 64.Hulette BA, Ryan CA, Gerberick GF. Elucidating changes in surface marker expression of dendritic cells following chemical allergen treatment. Toxicol Appl Pharmacol. 2002;182:226–233. doi: 10.1006/taap.2002.9447. [DOI] [PubMed] [Google Scholar]
- 65.Irimia D, Charras G, Agrawal N, Mitchison T, Toner M. Polar stimulation and constrained cell migration in microfluidic channels. Lab Chip. 2007;7:1783–1790. doi: 10.1039/b710524j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jacobs JJ, Lehe CL, Hasegawa H, Elliott GR, Das PK. Skin irritants and contact sensitizers induce Langerhans cell migration and maturation at irritant concentration. Exp Dermatol. 2006;15:432–440. doi: 10.1111/j.0906-6705.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 67.Jenkinson C, Jenkins RE, Maggs JL, Kitteringham NR, Aleksic M, Park BK, Naisbitt DJ. A mechanistic investigation into the irreversible protein binding and antigenicity of p-phenylenediamine. Chem Res Toxicol. 2009;22:1172–1180. doi: 10.1021/tx900095r. [DOI] [PubMed] [Google Scholar]
- 68.Johnson SR. The trouble with QSAR (or how I learned to stop worrying and embrace fallacy) J Chem Inform Model. 2008;48:25–26. doi: 10.1021/ci700332k. [DOI] [PubMed] [Google Scholar]
- 69.Jowsey IR, Basketter DA, Westmoreland C, Kimber I. A future approach to measuring relative skin sensitising potency: a proposal. J Appl Toxicol. 2006;26:341–350. doi: 10.1002/jat.1146. [DOI] [PubMed] [Google Scholar]
- 70.Karlberg AT, Bergstrom MA, Borje A, Luthman K, Nilsson JL. Allergic contact dermatitis–formation, structural requirements, and reactivity of skin sensitizers. Chem Res Toxicol. 2008;21:53–69. doi: 10.1021/tx7002239. [DOI] [PubMed] [Google Scholar]
- 71.Katritzky AR, Girgis AS, Slavov S, Tala SR, Stoyanova-Slavova I. QSAR modeling, synthesis and bioassay of diverse leukemia RPMI-8226 cell line active agents. Eur J Med Chem. 2010;45:5183–5199. doi: 10.1016/j.ejmech.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 72.Keenan TM, Frevert CW, Wu A, Wong V, Folch A. A new method for studying gradient-induced neutrophil desensitization based on an open microfluidic chamber. Lab Chip. 2010;10:116–122. doi: 10.1039/b913494h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kern PS, Gerberick GF, Ryan CA, Kimber I, Aptula A, Basketter DA. Local lymph node data for the evaluation of skin sensitization alternatives: a second compilation. Dermatitis. 2010;21:8–32. [PubMed] [Google Scholar]
- 74.Kidambi S, Yarmush RS, Novik E, Chao P, Yarmush ML, Nahmias Y. Oxygen-mediated enhancement of primary hepatocyte metabolism, functional polarization, gene expression, and drug clearance. Proc Natl Acad Sci USA. 2009;106:15714–15719. doi: 10.1073/pnas.0906820106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kimber I, Cumberbatch M, Dearman RJ, Bhushan M, Griffiths CE. Cytokines and chemokines in the initiation and regulation of epidermal Langerhans cell mobilization. Br J Dermatol. 2000;142:401–412. doi: 10.1046/j.1365-2133.2000.03349.x. [DOI] [PubMed] [Google Scholar]
- 76.Kimber I, Dearman RJ, Cumberbatch M, Huby RJ. Langerhans cells and chemical allergy. Curr Opin Immunol. 1998;10:614–619. doi: 10.1016/s0952-7915(98)80078-2. [DOI] [PubMed] [Google Scholar]
- 77.King KR, Wang S, Jayaraman A, Yarmush ML, Toner M. Microfluidic flow-encoded switching for parallel control of dynamic cellular microenvironments. Lab Chip. 2008;8:107–116. doi: 10.1039/b716962k. [DOI] [PubMed] [Google Scholar]
- 78.Koeper LM, Schulz A, Ahr HJ, Vohr HW. In vitro differentiation of skin sensitizers by cell signaling pathways. Toxicology. 2007;242:144–152. doi: 10.1016/j.tox.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 79.Kremen R. Technology Review. 2010. MIT; Jan 20, 2010. Cosmetics testing without animals. [Google Scholar]
- 80.Lalko JF, Kimber I, Dearman RJ, Gerberick GF, Sarlo K, Api AM. Chemical reactivity measurements: potential for characterization of respiratory chemical allergens. Toxicol In Vitro. 2011;25:433–445. doi: 10.1016/j.tiv.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 81.Lambert S, Frankart A, Poumay Y. p38 MAPK-regulated EGFR internalization takes place in keratinocyte monolayer during stress conditions. Arch Dermatol Res. 2010;302:229–233. doi: 10.1007/s00403-009-1020-0. [DOI] [PubMed] [Google Scholar]
- 82.Lampe MA, Burlingame AL, Whitney J, Williams ML, Brown BE, Roitman E, Elias PM. Human stratum corneum lipids: characterization and regional variations. J Lipid Res. 1983;24:120–130. [PubMed] [Google Scholar]
- 83.Landsteiner K, Jacobs J. Studies on the sensitization of animals with simple chemical compounds. Ii. J Exp Med. 1936;64:625–639. doi: 10.1084/jem.64.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Larsson K, Lindstedt M, Borrebaeck CA. Functional and transcriptional profiling of MUTZ-3, a myeloid cell line acting as a model for dendritic cells. Immunology. 2006;117:156–166. doi: 10.1111/j.1365-2567.2005.02274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lauria A, Ippolito M, Almerico AM. Combined use of PCA and QSAR/QSPR to predict the drugs mechanism of action. An application to the NCI ACAM database. Qsar Combinatorial Sci. 2009;28:387–395. [Google Scholar]
- 86.Lavrijsen AP, Bouwstra JA, Gooris GS, Weerheim A, Bodde HE, Ponec M. Reduced skin barrier function parallels abnormal stratum corneum lipid organization in patients with lamellar ichthyosis. J Invest Dermatol. 1995;105:619–624. doi: 10.1111/1523-1747.ep12323752. [DOI] [PubMed] [Google Scholar]
- 87.Lee HK, Alarie Y, Karol MH. Induction of formaldehyde sensitivity in guinea pigs. Toxicol Appl Pharmacol. 1984;75:147–155. doi: 10.1016/0041-008x(84)90085-1. [DOI] [PubMed] [Google Scholar]
- 88.Lehe CL, Jacobs JJ, Hua CM, Courtellemont P, Elliott GR, Das PK. Subtoxic concentrations of allergenic haptens induce LC migration and maturation in a human organotypic skin explant culture model: a novel method for identifying potential contact allergens. Exp Dermatol. 2006;15:421–431. doi: 10.1111/j.0906-6705.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 89.Lenz A, Heine M, Schuler G, Romani N. Human and murine dermis contain dendritic cells. Isolation by means of a novel method and phenotypical and functional characterization. J Clin Invest. 1993;92:2587–2596. doi: 10.1172/JCI116873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li Jeon N, Baskaran H, Dertinger SK, Whitesides GM, Van de Water L, Toner M. Neutrophil chemotaxis in linear and complex gradients of interleukin-8 formed in a microfabricated device. Nat Biotechnol. 2002;20:826–830. doi: 10.1038/nbt712. [DOI] [PubMed] [Google Scholar]
- 91.Li Y, Pan D, Liu J, Kern PS, Gerberick GF, Hopfinger AJ, Tseng YJ. Categorical QSAR models for skin sensitization based upon local lymph node assay classification measures part 2: 4D-fingerprint three-state and two-2-state logistic regression models. Toxicol Sci. 2007;99:532–544. doi: 10.1093/toxsci/kfm185. [DOI] [PubMed] [Google Scholar]
- 92.Luu-The V, Duche D, Ferraris C, Meunier JR, Leclaire J, Labrie F. Expression profiles of phases 1 and 2 metabolizing enzymes in human skin and the reconstructed skin models Episkin and full thickness model from Episkin. J Steroid Biochem Mol Biol. 2009;116:178–186. doi: 10.1016/j.jsbmb.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 93.Maguire TJ, Novik E, Chao P, Barminko J, Nahmias Y, Yarmush ML, Cheng KC. Design and application of microfluidic systems for in vitro pharmaco-kinetic evaluation of drug candidates. Curr Drug Metab. 2009;10:1192–1199. doi: 10.2174/138920009790820093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martin SF, Esser PR, Schmucker S, Dietz L, Naisbitt DJ, Park BK, Vocanson M, Nicolas JF, Keller M, Pichler WJ, et al. T-cell recognition of chemicals, protein allergens and drugs: towards the development of in vitro assays. Cell Mol Life Sci. 2010;67:4171–4184. doi: 10.1007/s00018-010-0495-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Matos TJ, Duarte CB, Goncalo M, Lopes MC. Role of oxidative stress in ERK and p38 MAPK activation induced by the chemical sensitizer DNFB in a fetal skin dendritic cell line. Immunol Cell Biol. 2005;83:607–614. doi: 10.1111/j.1440-1711.2005.01378.x. [DOI] [PubMed] [Google Scholar]
- 96.Matos TJ, Duarte CB, Goncalo M, Lopes MC. DNFB activates MAPKs and upregulates CD40 in skin-derived dendritic cells. J Dermatol Sci. 2005;39:113–123. doi: 10.1016/j.jdermsci.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 97.Matos TJ, Jaleco SP, Goncalo M, Duarte CB, Lopes MC. Release of IL-1beta via IL-1beta-converting enzyme in a skin dendritic cell line exposed to 2,4-dinitrofluorobenzene. Mediators Inflamm. 2005;2005:131–138. doi: 10.1155/MI.2005.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Matsue H, Edelbaum D, Shalhevet D, Mizumoto N, Yang C, Mummert ME, Oeda J, Masayasu H, Takashima A. Generation and function of reactive oxygen species in dendritic cells during antigen presentation. J Immunol. 2003;171:3010–3018. doi: 10.4049/jimmunol.171.6.3010. [DOI] [PubMed] [Google Scholar]
- 99.McNamee PM, Api AM, Basketter DA, Frank Gerberick G, Gilpin DA, Hall BM, Jowsey I, Robinson MK. A review of critical factors in the conduct and interpretation of the human repeat insult patch test. Regul Toxicol Pharmacol. 2008;52:24–34. doi: 10.1016/j.yrtph.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 100.Meyvantsson I, Beebe DJ. Cell culture models in microfluidic systems. Annu Rev Anal Chem (Palo Alto Calif) 2008;1:423–449. doi: 10.1146/annurev.anchem.1.031207.113042. [DOI] [PubMed] [Google Scholar]
- 101.Migdal C, Foggia L, Tailhardat M, Courtellemont P, Haftek M, Serres M. Sensitization effect of thimerosal is mediated in vitro via reactive oxygen species and calcium signaling. Toxicology. 2010;274:1–9. doi: 10.1016/j.tox.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 102.Miyazawa M, Ito Y, Kosaka N, Nukada Y, Sakaguchi H, Suzuki H, Nishiyama N. Role of TNF-alpha and extracellular ATP in THP-1 cell activation following allergen exposure. J Toxicol Sci. 2008;33:71–83. doi: 10.2131/jts.33.71. [DOI] [PubMed] [Google Scholar]
- 103.Miyazawa M, Ito Y, Kosaka N, Nukada Y, Sakaguchi H, Suzuki H, Nishiyama N. Role of MAPK signaling pathway in the activation of dendritic type cell line, THP-1, induced by DNCB and NiSO. J Toxicol Sci. 2008;33:51–59. doi: 10.2131/jts.33.51. [DOI] [PubMed] [Google Scholar]
- 104.Moulon C, Peguet-Navarro J, Courtellemont P, Redziniak G, Schmitt D. In vitro primary sensitization and restimulation of hapten-specific T cells by fresh and cultured human epidermal Langerhans’ cells. Immunology. 1993;80:373–379. [PMC free article] [PubMed] [Google Scholar]
- 105.Moulon C, Wild D, Dormoy A, Weltzien HU. MHC-dependent and -independent activation of human nickel-specific CD8+ cytotoxic T cells from allergic donors. J Invest Dermatol. 1998;111:360–366. doi: 10.1046/j.1523-1747.1998.00306.x. [DOI] [PubMed] [Google Scholar]
- 106.Naik PK, Singh T, Singh H. Quantitative structure-activity relationship (QSAR) for insecticides: development of predictive in vivo insecticide activity models. SAR QSAR Environ Res. 2009;20:551–566. doi: 10.1080/10629360903278735. [DOI] [PubMed] [Google Scholar]
- 107.Natsch A. The Nrf2-Keap1-ARE toxicity pathway as a cellular sensor for skin sensitizers—functional relevance and a hypothesis on innate reactions to skin sensitizers. Toxicol Sci. 2010;113:284–292. doi: 10.1093/toxsci/kfp228. [DOI] [PubMed] [Google Scholar]
- 108.Natsch A, Emter R. Skin sensitizers induce antioxidant response element dependent genes: application to the in vitro testing of the sensitization potential of chemicals. Toxicol Sci. 2008;102:110–119. doi: 10.1093/toxsci/kfm259. [DOI] [PubMed] [Google Scholar]
- 109.Natsch A, Emter R, Ellis G. Filling the concept with data: integrating data from different in vitro and in silico assays on skin sensitizers to explore the battery approach for animal-free skin sensitization testing. Toxicol Sci. 2009;107:106–121. doi: 10.1093/toxsci/kfn204. [DOI] [PubMed] [Google Scholar]
- 110.Nelissen I, Selderslaghs I, Heuvel RV, Witters H, Verheyen GR, Schoeters G. MUTZ-3-derived dendritic cells as an in vitro alternative model to CD34+ progenitor-derived dendritic cells for testing of chemical sensitizers. Toxicol In Vitro. 2009;23:1477–1481. doi: 10.1016/j.tiv.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 111.Netzlaff F, Lehr CM, Wertz PW, Schaefer UF. The human epidermis models EpiSkin, SkinEthic and EpiDerm: an evaluation of morphology and their suitability for testing phototoxicity, irritancy, corrosivity, and substance transport. Eur J Pharm Biopharm. 2005;60:167– 178. doi: 10.1016/j.ejpb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 112.Niederlehner BR, Cairns J, Jr, Smith EP. Modeling acute and chronic toxicity of nonpolar narcotic chemicals and mixtures to Ceriodaphnia dubia. Ecotoxicol Environ Saf. 1998;39:136–146. doi: 10.1006/eesa.1997.1621. [DOI] [PubMed] [Google Scholar]
- 113.Novik E, Maguire TJ, Chao P, Cheng KC, Yarmush ML. A microfluidic hepatic coculture platform for cell-based drug metabolism studies. Biochem Pharmacol. 2010;79:1036–1044. doi: 10.1016/j.bcp.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nukada Y, Miyazawa M, Kosaka N, Ito Y, Sakaguchi H, Nishiyama N. Production of IL-8 in THP-1 cells following contact allergen stimulation via mitogenactivated protein kinase activation or tumor necrosis factor-alpha production. J Toxicol Sci. 2008;33:175–185. doi: 10.2131/jts.33.175. [DOI] [PubMed] [Google Scholar]
- 115.Osborne R, Perkins MA. An approach for development of alternative test methods based on mechanisms of skin irritation. Food Chem Toxicol. 1994;32:133–142. doi: 10.1016/0278-6915(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 116.Ott H, Wiederholt T, Bergstrom MA, Heise R, Skazik C, Czaja K, Marquardt Y, Karlberg AT, Merk HF, Baron JM. High-resolution transcriptional profiling of chemical-stimulated dendritic cells identifies immunogenic contact allergens, but not prohaptens. Skin Pharmacol Physiol. 2010;23:213–224. doi: 10.1159/000313897. [DOI] [PubMed] [Google Scholar]
- 117.Ouwehand K, Santegoets SJ, Bruynzeel DP, Scheper RJ, de Gruijl TD, Gibbs S. CXCL12 is essential for migration of activated Langerhans cells from epidermis to dermis. Eur J Immunol. 2008;38:3050–3059. doi: 10.1002/eji.200838384. [DOI] [PubMed] [Google Scholar]
- 118.Ouwehand K, Scheper RJ, de Gruijl TD, Gibbs S. Epidermis-to-dermis migration of immature Langerhans cells upon topical irritant exposure is dependent on CCL2 and CCL5. Eur J Immunol. 2010;40:2026–2034. doi: 10.1002/eji.200940150. [DOI] [PubMed] [Google Scholar]
- 119.Ouwehand K, Spiekstra SW, Reinders J, Scheper RJ, de Gruijl TD, Gibbs S. Comparison of a novel CXCL12/CCL5 dependent migration assay with CXCL8 secretion and CD86 expression for distinguishing sensitizers from non-sensitizers using MUTZ-3 Langerhans cells. Toxicol In Vitro. 2010;24:578–585. doi: 10.1016/j.tiv.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 120.Patlewicz G, Aptula AO, Roberts DW, Uriarte E. A minireview of available skin sensitization (Q)SARs/expert systems. Qsar Combinatorial Sci. 2008;27:60– 76. [Google Scholar]
- 121.Pichowski JS, Cumberbatch M, Dearman RJ, Basketter DA, Kimber I. Allergen-induced changes in interleukin 1 beta (IL-1 beta) mRNA expression by human blood-derived dendritic cells: inter-individual differences and relevance for sensitization testing. J Appl Toxicol: JAT. 2001;21:115–121. doi: 10.1002/jat.742. [DOI] [PubMed] [Google Scholar]
- 122.Piclin N, Pintore M, Wechman C, Roncaglioni A, Benfenati E, Chretien JR. Ecotoxicity prediction by adaptive fuzzy partitioning: comparing descriptors computed on 2D and 3D structures. SAR QSAR Environ Res. 2006;17:225–251. doi: 10.1080/10659360600636212. [DOI] [PubMed] [Google Scholar]
- 123.Pilgram GS, Vissers DC, van der Meulen H, Pavel S, Lavrijsen SP, Bouwstra JA, Koerten HK. Aberrant lipid organization in stratum corneum of patients with atopic dermatitis and lamellar ichthyosis. J Invest Dermatol. 2001;117:710–717. doi: 10.1046/j.0022-202x.2001.01455.x. [DOI] [PubMed] [Google Scholar]
- 124.Pistoor FH, Rambukkana A, Kroezen M, Lepoittevin JP, Bos JD, Kapsenberg ML, Das PK. Novel predictive assay for contact allergens using human skin explant cultures. Am J Pathol. 1996;149:337–343. [PMC free article] [PubMed] [Google Scholar]
- 125.Poumay Y, Dupont F, Marcoux S, Leclercq-Smekens M, Herin M, Coquette A. A simple reconstructed human epidermis: preparation of the culture model and utilization in in vitro studies. Arch Dermatol Res. 2004;296:203–211. doi: 10.1007/s00403-004-0507-y. [DOI] [PubMed] [Google Scholar]
- 126.Python F, Goebel C, Aeby P. Assessment of the U937 cell line for the detection of contact allergens. Toxicol Appl Pharmacol. 2007;220:113–124. doi: 10.1016/j.taap.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 127.Python F, Goebel C, Aeby P. Comparative DNA microarray analysis of human monocyte derived dendritic cells and MUTZ-3 cells exposed to the moderate skin sensitizer cinnamaldehyde. Toxicol Appl Pharmacol. 2009;239:273–283. doi: 10.1016/j.taap.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 128.Quentmeier H, Duschl A, Hu ZB, Schnarr B, Zaborski M, Drexler HG. MUTZ-3, a monocytic model cell line for interleukin-4 and lipopolysaccharide studies. Immunology. 1996;89:606–612. doi: 10.1046/j.1365-2567.1996.d01-780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 130.Rasaiyaah J, Yong K, Katz DR, Kellam P, Chain BM. Dendritic cells and myeloid leukaemias: plasticity and commitment in cell differentiation. Br J Haematol. 2007;138:281–290. doi: 10.1111/j.1365-2141.2007.06622.x. [DOI] [PubMed] [Google Scholar]
- 131.Reuter H, Spieker J, Gerlach S, Engels U, Pape W, Kolbe L, Schmucker R, Wenck H, Diembeck W, Wittern KP, et al. In vitro detection of contact allergens: development of an optimized protocol using human peripheral blood monocyte-derived dendritic cells. Toxicol In Vitro. 2010 doi: 10.1016/j.tiv.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 132.Roach KL, King KR, Uygun BE, Kohane IS, Yarmush ML, Toner M. High throughput single cell bioinformatics. Biotechnol Prog. 2009;25:1772–1779. doi: 10.1002/btpr.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Roberts DW, Aptula AO, Patlewicz G, Pease C. Chemical reactivity indices and mechanism-based read-across for non-animal based assessment of skin sensitisation potential. J Appl Toxicol. 2008;28:443–454. doi: 10.1002/jat.1293. [DOI] [PubMed] [Google Scholar]
- 134.Saalbach A, Klein C, Sleeman J, Sack U, Kauer F, Gebhardt C, Averbeck M, Anderegg U, Simon JC. Dermal fibroblasts induce maturation of dendritic cells. J Immunol. 2007;178:4966–4974. doi: 10.4049/jimmunol.178.8.4966. [DOI] [PubMed] [Google Scholar]
- 135.Sakaguchi H, Ryan C, Ovigne JM, Schroeder KR, Ashikaga T. Predicting skin sensitization potential and inter-laboratory reproducibility of a human Cell Line Activation Test (h-CLAT) in the European Cosmetics Association (COLIPA) ring trials. Toxicol In Vitro. 2010;24:1810–1820. doi: 10.1016/j.tiv.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 136.Sandby-Moller J, Poulsen T, Wulf HC. Epidermal thickness at different body sites: relationship to age, gender, pigmentation, blood content, skin type and smoking habits. Acta Derm Venereol. 2003;83:410–413. doi: 10.1080/00015550310015419. [DOI] [PubMed] [Google Scholar]
- 137.Santegoets SJ, Bontkes HJ, Stam AG, Bhoelan F, Ruizendaal JJ, van den Eertwegh AJ, Hooijberg E, Scheper RJ, de Gruijl TD. Inducing antitumor T cell immunity: comparative functional analysis of interstitial versus Langerhans dendritic cells in a human cell line model. J Immunol. 2008;180:4540–4549. doi: 10.4049/jimmunol.180.7.4540. [DOI] [PubMed] [Google Scholar]
- 138.Santegoets SJ, Masterson AJ, van der Sluis PC, Lougheed SM, Fluitsma DM, van den Eertwegh AJ, Pinedo HM, Scheper RJ, de Gruijl TD. A CD34(+) human cell line model of myeloid dendritic cell differentiation: evidence for a CD14(+)CD11b(+) Langerhans cell precursor. J Leukoc Biol. 2006;80:1337–1344. doi: 10.1189/jlb.0206111. [DOI] [PubMed] [Google Scholar]
- 139.Santegoets SJ, Schreurs MW, Masterson AJ, Liu YP, Goletz S, Baumeister H, Kueter EW, Lougheed SM, van den Eertwegh AJ, Scheper RJ, et al. In vitro priming of tumor-specific cytotoxic T lymphocytes using allogeneic dendritic cells derived from the human MUTZ-3 cell line. Cancer Immunol Immunother. 2006;55:1480– 1490. doi: 10.1007/s00262-006-0142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Santegoets SJ, van den Eertwegh AJ, van de Loosdrecht AA, Scheper RJ, de Gruijl TD. Human dendritic cell line models for DC differentiation and clinical DC vaccination studies. J Leukoc Biol. 2008;84:1364– 1373. doi: 10.1189/jlb.0208092. [DOI] [PubMed] [Google Scholar]
- 141.Scandella E, Men Y, Legler DF, Gillessen S, Prikler L, Ludewig B, Groettrup M. CCL19/CCL21-triggered signal transduction and migration of dendritic cells requires prostaglandin E2. Blood. 2004;103:1595– 1601. doi: 10.1182/blood-2003-05-1643. [DOI] [PubMed] [Google Scholar]
- 142.Schaefer K. Cosmetics & Toiletries Magazine. 2010. Mar, 2010. Microfluidic Testing for LLNA Replacement. [Google Scholar]
- 143.Schlede E, Aberer W, Fuchs T, Gerner I, Lessmann H, Maurer T, Rossbacher R, Stropp G, Wagner E, Kayser D. Chemical substances and contact allergy—244 substances ranked according to allergenic potency. Toxicology. 2003;193:219–259. doi: 10.1016/s0300-483x(03)00266-x. [DOI] [PubMed] [Google Scholar]
- 144.Sin A, Chin KC, Jamil MF, Kostov Y, Rao G, Shuler ML. The design and fabrication of three-chamber microscale cell culture analog devices with integrated dissolved oxygen sensors. Biotechnol Prog. 2004;20:338–345. doi: 10.1021/bp034077d. [DOI] [PubMed] [Google Scholar]
- 145.Smith JR, Kholodovych V, Knight D, Welsh WJ, Kohn J. QSAR models for the analysis of bioresponse data from combinatorial libraries of biomaterials. Qsar Combinatorial Sci. 2005;24:99–113. [Google Scholar]
- 146.Sosted H, Rastogi SC, Andersen KE, Johansen JD, Menne T. Hair dye contact allergy: quantitative exposure assessment of selected products and clinical cases. Contact Dermatitis. 2004;50:344–348. doi: 10.1111/j.0105-1873.2004.00362.x. [DOI] [PubMed] [Google Scholar]
- 147.Sprous DG, Palmer RK, Swanson JT, Lawless M. QSAR in the pharmaceutical research setting: QSAR models for broad, large problems. Curr Top Med Chem. 2010;10:619–637. doi: 10.2174/156802610791111506. [DOI] [PubMed] [Google Scholar]
- 148.Stejskal V, Hudecek R, Stejskal J, Sterzl I. Diagnosis and treatment of metal-induced side-effects. Neuro Endocrinol Lett. 2006;27(Suppl 1):7–16. [PubMed] [Google Scholar]
- 149.Steube KG, Meyer C, Drexler HG. Constitutive protein expression of monocyte chemotactic protein-1 (MCP-1) by myelomonocytic cell lines and regulation of the secretion by anti- and proinflammatory stimuli. Leuk Res. 1999;23:843–849. doi: 10.1016/s0145-2126(99)00107-1. [DOI] [PubMed] [Google Scholar]
- 150.Steube KG, Meyer C, Drexler HG. Multiple regulation of constitutive and induced interleukin 8 secretion in human myelomonocytic cell lines. Cytokine. 2000;12:1236–1239. doi: 10.1006/cyto.2000.0702. [DOI] [PubMed] [Google Scholar]
- 151.t Hoen PA, Out R, Commandeur JN, Vermeulen NP, van Batenburg FH, Manoharan M, van Berkel TJ, Biessen EA, Bijsterbosch MK. Selection of antisense oligodeoxynucleotides against glutathione S-transferase Mu. RNA. 2002;8:1572–1583. [PMC free article] [PubMed] [Google Scholar]
- 152.Takayama S, Ostuni E, LeDuc P, Naruse K, Ingber DE, Whitesides GM. Subcellular positioning of small molecules. Nature. 2001;411:1016. doi: 10.1038/35082637. [DOI] [PubMed] [Google Scholar]
- 153.Tietze C, Blomeke B. Sensitization assays: monocyte-derived dendritic cells versus a monocytic cell line (THP-1) J Toxicol Environ Health A. 2008;71:965–968. doi: 10.1080/15287390801989168. [DOI] [PubMed] [Google Scholar]
- 154.Trompezinski S, Migdal C, Tailhardat M, Le Varlet B, Courtellemont P, Haftek M, Serres M. Characterization of early events involved in human dendritic cell maturation induced by sensitizers: cross talk between MAPK signalling pathways. Toxicol Appl Pharmacol. 2008;230:397–406. doi: 10.1016/j.taap.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 155.Tuschl H, Kovac R. Langerhans cells and immature dendritic cells as model systems for screening of skin sensitizers. Toxicol In Vitro. 2001;15:327–331. doi: 10.1016/s0887-2333(01)00030-3. [DOI] [PubMed] [Google Scholar]
- 156.Tuschl H, Kovac R, Weber E. The expression of surface markers on dendritic cells as indicators for the sensitizing potential of chemicals. Toxicol In Vitro. 2000;14:541–549. doi: 10.1016/s0887-2333(00)00051-5. [DOI] [PubMed] [Google Scholar]
- 157.Uter W, de Padua CM, Pfahlberg A, Nink K, Schnuch A, Lessmann H. Contact allergy to topical corticosteroids–results from the IVDK and epidemiological risk assessment. J Dtsch Dermatol Ges. 2009;7(34–41):34– 42. doi: 10.1111/j.1610-0387.2008.06844.x. [DOI] [PubMed] [Google Scholar]
- 158.Valladeau J, Ravel O, Dezutter-Dambuyant C, Moore K, Kleijmeer M, Liu Y, Duvert-Frances V, Vincent C, Schmitt D, Davoust J, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71–81. doi: 10.1016/s1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]
- 159.van Loveren H, Cockshott A, Gebel T, Gundert-Remy U, de Jong WH, Matheson J, McGarry H, Musset L, Selgrade MK, Vickers C. Skin sensitization in chemical risk assessment: report of a WHO/IPCS international workshop focusing on dose-response assessment. Regul Toxicol Pharmacol. 2008;50:155–199. doi: 10.1016/j.yrtph.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 160.Vandebriel RJ, van Loveren H. Non-animal sensitization testing: state-of-the-art. Crit Rev Toxicol. 2010;40:389–404. doi: 10.3109/10408440903524262. [DOI] [PubMed] [Google Scholar]
- 161.Vandebriel RJ, Van Och FM, van Loveren H. In vitro assessment of sensitizing activity of low molecular weight compounds. Toxicol Appl Pharmacol. 2005;207:142– 148. doi: 10.1016/j.taap.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 162.Venkatapathy R, Wang CY, Bruce RM, Moudgal C. Development of quantitative structure-activity relationship (QSAR) models to predict the carcinogenic potency of chemicals I. Alternative toxicity measures as an estimator of carcinogenic potency. Toxicol Appl Pharmacol. 2009;234:209–221. doi: 10.1016/j.taap.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 163.Vital AL, Goncalo M, Cruz MT, Figueiredo A, Duarte CB, Celeste Lopes M. The sensitizers nickel sulfate and 2,4-dinitrofluorobenzene increase CD40 and IL-12 receptor expression in a fetal skin dendritic cell line. Biosci Rep. 2004;24:191–202. doi: 10.1007/s10540-005-2580-7. [DOI] [PubMed] [Google Scholar]
- 164.Wilkes JG, Hass BS, Buzatu DA, Pence LM, Archer JC, Beger RD, Schnackenberg LK, Halbert MK, Jennings L, Kodell RL. Modeling and assaying dioxin-like biological effects for both dioxin-like and certain non-dioxin-like compounds. Toxicol Sci. 2008;102:187–195. doi: 10.1093/toxsci/kfm294. [DOI] [PubMed] [Google Scholar]
- 165.Williams EH, Williams CA, McLeod JD. Identification of PDL-1 as a novel biomarker of sensitizer exposure in dendritic-like cells. Toxicol In Vitro. 2010;24:1727– 1735. doi: 10.1016/j.tiv.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 166.Xia YN, Whitesides GM. Soft lithography. Annu Rev Mat Sci. 1998;28:153–184. [Google Scholar]
- 167.Yarmush ML, Freedman R. Immune system modeling devices and methods. Patent # 20110027804A1. 2011
- 168.Yoshida Y, Sakaguchi H, Ito Y, Okuda M, Suzuki H. Evaluation of the skin sensitization potential of chemicals using expression of co-stimulatory molecules, CD54 and CD86, on the naive THP-1 cell line. Toxicol In Vitro. 2003;17:221–228. doi: 10.1016/s0887-2333(03)00006-7. [DOI] [PubMed] [Google Scholar]
- 169.Zhang YB, Lin HF, Lv L, Hua WG, Tian F, Shen GZ, Xia ZL, Jin XP. In vitro evaluation of cutaneous allergic reaction induced by chemicals using dendritic cells. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2008;26:147–150. [PubMed] [Google Scholar]
- 170.Zhu H, Ye L, Richard A, Golbraikh A, Wright FA, Rusyn I, Tropsha A. A novel two-step hierarchical quantitative structure-activity relationship modeling work flow for predicting acute toxicity of chemicals in rodents. Environ Health Perspect. 2009;117:1257–1264. doi: 10.1289/ehp.0800471. [DOI] [PMC free article] [PubMed] [Google Scholar]