Abstract
The present experiments examined the effects of prazosin, a selective α1-adrenergic receptor antagonist, on the development of methamphetamine conditioned hyperactivity and context-specific sensitization. Mice received an injection of vehicle (distilled water) or prazosin (0.5, 1.0 or 2.0 mg/kg) 30 minutes prior to a second injection of vehicle (saline) or methamphetamine (1.0 mg/kg) during the conditioning sessions (Experiment 1). Following the conditioning sessions, mice were tested for conditioned hyperactivity and then tested for context-specific sensitization. In subsequent experiments, mice received an injection of vehicle (distilled water) or prazosin (2.0 mg/kg) immediately (Experiment 2) or 24 hours (Experiment 3) after the conditioning sessions and then tested for conditioned hyperactivity and context-specific sensitization. Prazosin dose-dependently blocked the development of methamphetamine conditioned hyperactivity and context-specific sensitization when administered prior to the methamphetamine during the conditioning phase; however nonspecific motor impairments also were observed (Experiment 1). Immediate (Experiment 2), but not the 24-hour delay (Experiment 3), post-session administration of prazosin attenuated the development of methamphetamine conditioned hyperactivity and context-specific sensitization. Nonspecific motor impairments were not observed in these latter experiments. Collectively, these results suggest that the α1-adrenergic receptor mediates the development of methamphetamine-conditioned hyperactivity and context-specific sensitization, perhaps by altering memory consolidation and/or reconsolidation processes.
Keywords: psychostimulants, methamphetamine, α1-adrenergic receptor, antagonist, memory consolidation, memory reconsolidation, prazosin, locomotor activity, conditioning, sensitization
1. Introduction
In rodents, repeated administration of amphetamine or methamphetamine results in a robust increase in locomotor activity, a phenomenon known as behavioral sensitization (see [1] for a review). In the typical behavioral sensitization paradigm, some rodents receive daily pairings of amphetamine in the locomotor activity chambers (i.e., paired rodents) while other rodents receive comparable amphetamine exposures in their home cages (i.e., unpaired rodents). At some later point in time (test session), all rodents are “challenged” with amphetamine while in the locomotor activity chambers and paired rodents will show an enhanced response (i.e., greater locomotor activity, context-specific sensitization) compared to unpaired rodents (context nonspecific sensitization). Behavioral sensitization reflects both the pharmacological action of the drug (i.e., the unconditioned drug effect) as well as non-pharmacological, associative learning processes (i.e., classical conditioning; see [2] for a discussion of the role of classical conditioning in behavioral sensitization). That is, with respect to the latter, after the repeated pairings of the locomotor activity chamber (conditioned stimulus - CS) with the locomotor-activating effects of the drug (e.g., methamphetamine; unconditioned stimulus - US), the chamber itself will elicit an increase in locomotor activity (i.e., a conditioned hyperactive response; conditioned response - CR) relative to a control group. Moreover, the enhanced pharmacological response observed in paired rodents when challenged with amphetamine on the test session compared to unpaired rodents demonstrates context-specific sensitization (see [3] for a recent demonstration of methamphetamine conditioned hyperactivity and context-specific sensitization) and also thought to reflect the contribution of associative learning processes [4, 5].
The mesolimbic dopaminergic system has been implicated in the development of amphetamine-produced context-specific sensitization and conditioned hyperactivity (see [6] for a review). Studies, employing a number of techniques, have supported this role. For example, neurochemical studies have shown that 6-hydroxydopamine lesions of the nucleus accumbens attenuate the development of the conditioned hyperactive response to amphetamine [7] and concentrations of a metabolite of dopamine, homovanilic acid, are higher in mesolimbic and caudate regions of the brain in conditioned compared to pseudo-conditioned rats [8]. Pharmacological studies have shown that selective dopaminergic subtype-1 or -2 (D1 or D2) receptor antagonists attenuate and/or block the development of conditioned hyperactivity to amphetamine, supporting a role of these receptor subtypes in the development of the response [9, 10]. The role of the D1-dopamine receptor is further supported by studies employing genetic manipulations (e.g., gene knockout). For example, D1-dopaminergic receptor knockout mice show enhanced context-specific sensitization and conditioned hyperactivity following repeated context-amphetamine pairings [11]. Finally, it has been shown that the partial D3-dopaminergic receptor agonist, BP 897, attenuates the expression, but not the development, of amphetamine-produced conditioned hyperactivity when injected systemically [12] into the basolateral amygdala or nucleus accumbens [13]. Collectively, these latter studies suggest that dopaminergic receptors differentially mediate the development of amphetamine-produced conditioned hyperactivity.
Recently, studies have shown that the noradrenergic system interacts with the mesocorticolimbic dopaminergic system, via the α1-adrenergic receptor, to modulate dopaminergic activity as well as the sensitizing (pharmacological) and conditioned rewarding effects of drugs of abuse (see [14] for a review). Neuroanatomical studies have shown that noradrenergic neurons, arising from the locus coeruleus, A1 and A2 nuclei, have excitatory projections to dopaminergic-containing neurons in the ventral tegmental area [15]. Furthermore, neurons that release norepinephrine project to and stimulate, through the α1-adrenergic receptor, neurons that release dopamine, leading to increased D1- and D2-dopaminergic receptor activity downstream [16]. Electrophysiological studies have shown that stimulation of α1-adrenergic receptors, typically from the locus coeruleus, directly increases the likelihood of action potentials in both the ventral tegmental area and substantia nigra pars compacta [17]. Conversely, antagonism of the α1-adrenergic receptors with the selective α1-adrenergic receptor antagonist, prazosin, inhibits bursts firing of ventral tegmental dopaminergic neurons [18]. The locus coeruleus also has dense projections to the prefrontal cortex, sending excitatory glutamatergic projections to the ventral tegmental area dopaminergic neurons [19, 20], and this projection is critical for dopamine release in the nucleus accumbens, as lesions of norepinephrine-containing prefrontal cortical neurons abolishes amphetamine-induced dopamine release [21]. Moreover, site-specific infusion of prazosin into prefrontal cortical neurons blocks release of dopamine into the nucleus accumbens, indicating that the α1-adrenergic receptor mediates this effect [22, 23]. With respect to amphetamine, behavioral studies further corroborate an interaction of the noradrenergic and dopaminergic systems via the α1-adrenergic receptor. For example, lesions of the locus coeruleus attenuate amphetamine-induced locomotor activity [24] and prazosin attenuates amphetamine-induced hyperactivity [23, 25]. Studies have shown that depletion of norepinephrine in the medial prefrontal cortex attenuates amphetamine-produced conditioned place preference and amphetamine-induced mesoaccumbens dopamine release in mice [21]. Finally, α1b-adrenergic receptor knockout mice are less sensitive to the locomotor-activating effects of amphetamine [26]. Collectively, these studies suggest that the noradrenergic system interacts with the dopaminergic system, via the α1-adrenergic receptor, to mediate the locomotor-activating and conditioned rewarding properties of amphetamine.
To date, no research has examined the interaction of the noradrenergic and dopaminergic systems in mediating the pharmacological or conditioned components of methamphetamine sensitization, particularly focusing on the α1-adrenergic receptor. Thus, in Experiment 1, prazosin was administered at various doses (0.5, 1.0 or 2.0 mg/kg) 30 minute (min) prior to mice receiving a dose of methamphetamine (1.0 mg/kg). Then, the mice were placed in a locomotor activity chamber for a 30-min conditioning session and their locomotor activity recorded. This experiment found that when the highest prazosin dose (2.0 mg/kg) was administered 30 min prior to the methamphetamine, then the development of conditioned hyperactivity and context-specific sensitization was attenuated. However, because the prazosin was administered prior to the conditioning event, this experiment failed to specify whether the prazosin disrupted the development of the conditioned hyperactive response and context-specific sensitization by altering acquisition processes (i.e., learning at the time of conditioning event) or consolidation processes (e.g., memory formation after the conditioning event). Research has shown that memory consolidation is temporally limited [27]. That is, after the conditioning event, the memory trace is malleable and susceptible to pharmacological manipulations during an experimenter-defined window of time (< less 24 hours, h). For example, previous research has found that immediate, but not delayed (24 h), administration of lidocaine into the amygdala, following the conditioning event, impaired recall on an inhibitory avoidance task [28]. Thus, in order to determine whether prazosin disrupted the development of methamphetamine conditioned hyperactivity and context-specific sensitization, Experiments 2 and 3 examined the effect of immediate (Experiment 2) vs. delayed (24 h; Experiment 3) post-session administration of prazosin on the development of methamphetamine conditioned hyperactivity and context-specific sensitization. If prazosin disrupts the development of conditioned hyperactivity and context-specific sensitization by altering memory consolidation processes, then the immediate, but not delayed, post-session administration of prazosin should disrupt the development of conditioned hyperactivity and context-specific sensitization.
Materials and Methods
1.1. Subjects
Male, adolescent Swiss-Webster mice (N = 160) were obtained from Charles River Laboratories (Raleigh, N.C). Mice were 25-27 days of age at the time of arrival and were 42-44 days of age at the time of testing. Mice were group-housed (four per tub) in a ventilated-caging system (Vent-Air, PA) lined with paper bedding (Care-free Ultra). Food (Purina Fortified Rodent Chow) and water were made available ad libitum. The room was kept at ~ 21 degrees (Celsius) and the lights cycled on a 12:12 light/dark cycle in which the light turned on at 0900 h. All mice were handled for 1 min each day for a week prior to the start of the experiments (acclimation period). The tails of the mice were marked for identification. The average weight of the mice at the start of the 10-day experiments was approximately 30 g. The experiments conform to the guidelines established by the NIH Guide for the Care and Use of Laboratory Animals (2011 Edition) and the APA Ethical Principles of Psychologists and Code of Conduct. The Institutional Animal Care and Use Committee at Dickinson College approved the experiments described.
1.2. Drugs
All drugs were obtained from Sigma (St. Louis, MO). Methamphetamine HCl was dissolved into physiological saline (0.9% vehicle). The methamphetamine was injected subcutaneously (s.c.) at 1.0 mg/kg (body weight) in a volume of 10 ml/kg. Prazosin was dissolved in distilled water. Prazosin was injected intraperitoneally (i.p.) in a volume of 2 ml/kg. All drug doses are expressed as the weight of their salts.
1.3. Apparatus
Eight, open-field chambers (MED-OFA-510; Med-associates, VT) were used for all locomotor activity sessions. Each chamber was placed in a sound-attenuated cubicle (MED-OFA-022, Med-Associates, Burlington, VT) containing a fan and lights. The walls of the activity chambers were made of Plexiglas with the interior dimensions measuring 27.9 cm x 27.9 cm. Locomotor activity, defined as distance traveled (cm), was determined by three, 16-beam I/R arrays (X, Y and Z axes) and photo beam breaks were recorded by a personal computer (Activity Monitor software; Med-associates, VT) located in the same room as the chambers. The chambers were cleaned with a disinfectant solution (Precise QTB/Caltech, Midland, MI) following each 30 min locomotor-activity session. A small, cap-size amount of anise extract (McCormick, Hunt Valley, MD) was used daily to scent each open-field chamber and provide an olfactory cue.
1.4. Procedure
The methamphetamine dose and conditioning procedure chosen were similar to those previously found to produce robust conditioned hyperactivity and context-specific sensitization in mice [3]. Following the acclimation period, the experiments consisted of 2 phases: conditioning and tests. The conditioning phase consisted of 4 alternating chamber and home-cage days. On chamber days (1, 3, 5 and 7), paired mice (n = 14/group) received an injection (i.p.) of vehicle (distilled water) or prazosin (0.5 – 2.0 mg/kg; Experiment 1) 30 min prior to receiving methamphetamine (1.0 mg/kg) and then placed in the locomotor activity chamber for a 30-min period. On chamber days, unpaired mice (n = 14/group) received vehicle or prazosin (0.5 – 2.0 mg/kg) followed 30 min later by an injection of vehicle and placed in the locomotor activity chamber for a 30-minute period. The prazosin doses and injection parameters were based on a previous study [29]. In Experiments 2 and 3, paired and unpaired mice (n = 6/group) received either prazosin (2.0 mg/kg) or vehicle immediately or 24 h after the conditioning sessions, respectively, on chamber days. On the 4 intervening home-cage days (2, 4, 6, and 8), paired mice received an injection of vehicle whereas unpaired mice received an injection of methamphetamine in their home cages. In the case of Experiment 3 in which the injection of either prazosin or vehicle occurred on home-cage days by design, the prazosin or vehicle was administered 30 min after the injections of methamphetamine or saline. The mean plasma half-life of prazosin has been shown to be less than 3 h in rodents [30]. Therefore, prazosin should not have been behaviorally active during the subsequent chamber day. Forty-eight h after the last chamber day, all mice were tested for conditioned hyperactivity (Test 1). At this time, all mice were given an injection of vehicle immediately before placement in the activity chamber for a 30-min session. All mice were tested for context-specific sensitization (methamphetamine challenge) the following day (Test Day 2). On this test day, all mice were given an injection of methamphetamine (1 mg/kg) before a 30-min session in the activity chamber. In all experiments, conditioned hyperactivity and context-specific sensitization were assessed by comparing paired and unpaired mice, an approach taken in a previously-reported study from the laboratory [3]. Mice received their respective treatments Monday through Friday. All mice rested on the weekends.
1.5. Data Analysis
Data for each experiment were analyzed using SPSS (version 21.0). The conditioning data were subjected to three-way analyses of variance (ANOVAs) on the within-subjects factor, Chamber Day (1 and 4), and the between-subjects factors of Conditioning (Paired or Unpaired) and Prazosin Dose (Vehicle, 0.5, 1.0, or 2.0 mg/kg). Two-way ANOVAs were performed on the factors of Conditioning and Prazosin Dose for Test Days 1 and 2, separately. Additional time-course analyses were performed as necessary on particular days of the experiment. These analyses involved three-way ANOVAs on the between-subjects factors, Conditioning and Prazosin Dose, and the within-subjects factor, Session Minute. Significant main effects and/or interactions of interest motivated follow-up post hoc contrasts involving Tukey's Honesty Significant Difference tests. Unless other noted, all statistical decisions were made at α set to 0.05.
3. Results
3.1.Experiment 1
Experiment 1 employed a 4 (Prazosin Dose) x 2 (Conditioning) factor design whereby mice received an injection of vehicle or prazosin (0.5, 1.0 or 2.0 mg/kg) 30 min prior to an injection of vehicle (unpaired mice) or methamphetamine (paired mice) and then placed in the locomotor activity chambers during the Conditioning Phase. Following the Conditioning Phase, all mice were tested for conditioned hyperactivity (Test Day 1) and context-specific sensitization (Test Day 2) 24 h apart.
Nine mice were eliminated from the experiment for various reasons. With the exception of Paired-2.0 Prazosin, one mouse from each group was eliminated due to computer malfunctions. Furthermore, one mouse died from Group Unpaired-0.5 Prazosin and one mouse from Group Paired-2.0 Prazosin received the wrong experimental treatment. These mice were excluded from the data analysis. Thus, the sample sizes per group were as follows: Paired-Vehicle (n = 13), Paired-0.5 Prazosin (n = 13), Paired-1.0 Prazosin (n = 13), Paired-2.0 Prazosin (n = 13), Unpaired-Vehicle (n = 13), Unpaired-0.5 Prazosin (n = 12), Unpaired-1.0 Prazosin (n = 13) and Unpaired-2.0 Prazosin (n = 13).
3.1.1. Conditioning
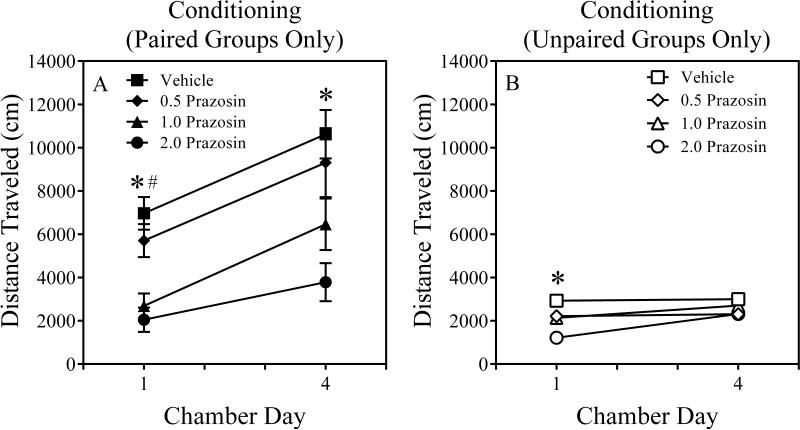
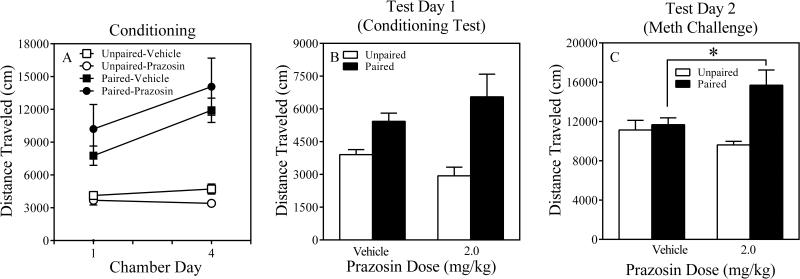
The three-way ANOVA revealed significant main effects of Chamber Day, F (1, 95) = 55.3, P = 0.000, Conditioning, F (1, 95) = 61.0, P = 0.000, and Prazosin Dose, F (3, 95) = 11.6, P = 0.000. The Chamber Day x Conditioning, F (1, 95) = 35. 6, P = 0.000, as well as Conditioning x Prazosin Dose, F (3, 95) = 6.7, P = 0.000, interactions were significant. The Chamber Day x Conditioning x Prazosin Dose interaction approached statistical significance, F (3, 95) = 2.4, P = 0.073. Post hoc contrasts conducted on Chamber Day 1 revealed that paired mice that received the moderate (1.0 mg/kg) and high (2.0 mg/kg) prazosin doses showed less locomotor activity compared to Paired-Vehicle mice (Ps = 0.000), suggesting that these prazosin doses blunted the acute locomotor-activating effects of methamphetamine. However, post hoc contrasts also revealed that unpaired mice that received the high prazosin dose (2.0 mg/kg) were less active compared to Unpaired-Vehicle mice (P = 0.000), suggesting that the high prazosin produced nonspecific locomotor depression (Figure 1A). Post hoc contrasts conducted on Chamber Day 4 revealed that paired mice that received the high (2.0 mg/kg) prazosin dose showed less locomotor activity compared to Paired-Vehicle mice (P = 0.001), suggesting that the high prazosin dose blunted the locomotor-activating effects of methamphetamine following repeated administration. None of the unpaired mice that received prazosin were less active than the Unpaired-Vehicle mice on Chamber Day 4 (Ps > 0.11), suggesting that mice developed tolerance to the initial locomotor-depressing effects of the high prazosin dose (2.0 mg/kg; Figure 1B).
Figure 1.
Results of Chamber Days 1 and 4 of the Conditioning Phase for Paired (Panel A) and Unpaired (Panel B) mice of Experiment 1. The error bars represent ± 1 S.E.M. The pound and asterisk symbols denote a significant difference between 1.0 and 2.0 mg/kg prazosin mice, respectively, and their appropriate vehicle control mice, ps ≤ 0.001.
3.1.2. Test Day 1
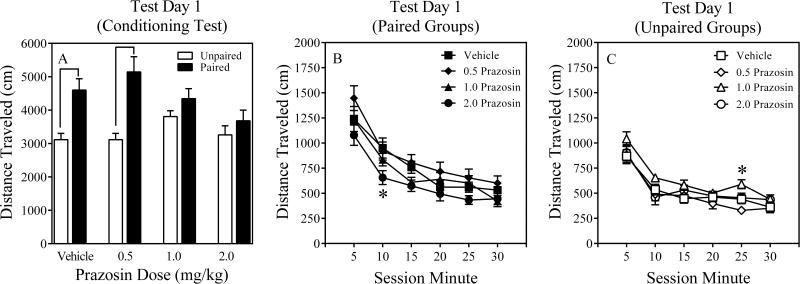
The two-way ANOVA conducted on Test Day 1 revealed a significant main effect of Conditioning, F (1, 95) = 34.6, P = 0.000, as well as a significant Conditioning x Prazosin Dose interaction, F (3, 95) = 3.9, P = 0.01. Post hoc contrasts revealed that none of the active prazosin doses differed from the vehicle control dose in paired mice. However, when paired mice were compared to their respective unpaired counterparts, it was found that Paired-Vehicle and Paired-0.5 Prazosin differed from their unpaired counterparts (Ps ≤ 0.02), suggesting these paired groups displayed conditioned hyperactivity. Furthermore, when Paired-1.0 Prazosin and Paired-2.0 Prazosin were compared to their respective unpaired counterparts, then they did not differ (Ps > 0.9), suggesting that these prazosin doses blocked conditioned hyperactivity (Figure 2A). Moreover, time-course analyses, involving a three-way ANOVA (Conditioning x Prazosin Dose x Session Minute) revealed significant main effects of Conditioning and Session Minute (Fs > 30, Ps = 0.000) as well as significant Conditioning x Session Minute and Conditioning x Prazosin Dose interactions (Fs > 3.9, Ps ≤ 0.01). No other interactions were statistically significant. Post hoc contrasts revealed that Paired-2.0 Prazosin mice were less active than Paired-Vehicle mice during Session Minute 10 (P = 0.066; Figure 2B), suggesting that this prazosin dose attenuated conditioned hyperactivity. Unpaired-1.0 Prazosin mice were more active than Unpaired-Vehicle mice during Session Minute 25 (P = 0.068; Figure 2C). However, no other group differences were detected in unpaired mice on Test Day 1, suggesting that the prazosin-induced attenuation of conditioned hyperactivity in paired mice was not due to motor problems induced by the high prazosin dose (2.0 mg/kg).
Figure 2.
Results of Test Day 1 (Conditioning Test) of Experiment 1 (Panel A). Time course results of Test Day 1 for paired (Panel B) and unpaired (Panel C) mice of Experiment 1. The error bars represent +/ ± 1 S.E.M. The symbol denotes a significant difference between paired mice and their respective unpaired control mice, ps ≤ 0.02. The asterisks denote a significant difference between paired or unpaired mice and their respective vehicle control mice at different time points, ps < 0.07.
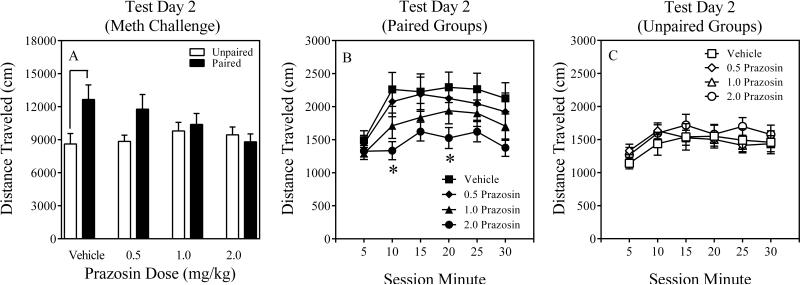
3.1.3. Test Day 2
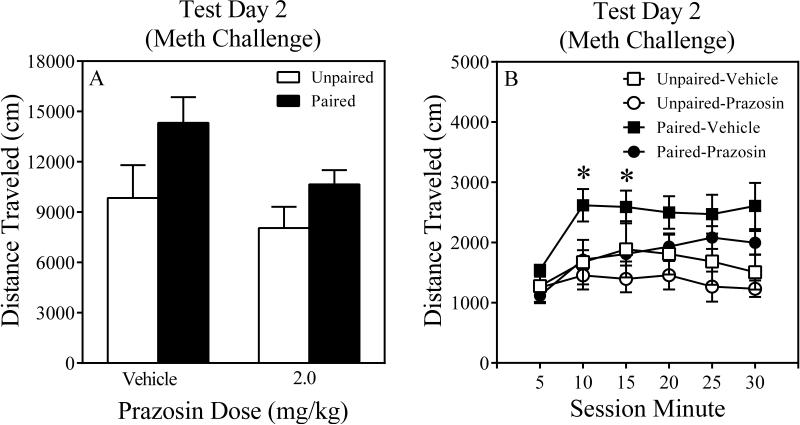
The two-way ANOVA conducted on Test Day 2 revealed a significant main effect of Conditioning, F (1, 95) = 6.4, P = 0.01, as well as a Conditioning x Prazosin Dose interaction that approached statistical significance, F (3, 95) = 2.45, P = 0.068. Despite a non-significant interaction, several exploratory contrasts were conducted comparing paired mice to their respective unpaired controls. It was found that only Paired-Vehicle differed from its unpaired counterpart (P < 0.07), suggesting this paired group displayed context-specific sensitization (Figure 3A). Paired-0.5, Paired-1.0 and Paired-2.0 Prazosin mice did not reliably differ from their respective unpaired counterparts (Ps > 0.41), suggesting that these prazosin doses attenuated context-specific sensitization. Moreover, time-course analyses, involving a three-way ANOVA (Conditioning x Prazosin Dose x Session Minute) revealed significant main effects of Conditioning and Session Minute (Fs > 6.4, Ps ≤ 0.01) as well as two interactions, Conditioning x Session Minute and Conditioning x Prazosin Dose, that approached statistical significance (Fs > 2.4, Ps ≤ 0.067). No other interactions were statistically significant. Post hoc contrasts revealed that Paired-2.0 Prazosin mice were less active than Paired-Vehicle mice during Session Minutes 10 and 20 (Ps ≤ 0.05 Figure 3B), suggesting that this prazosin dose attenuated context-specific sensitization. Unpaired mice receiving active prazosin doses did not differ from Unpaired-Vehicle control mice on Test Day 2 (Ps > 0.87; Figure 3C), suggesting that the prazosin-induced attenuation of context-specific sensitization in paired mice was not due to motor problems induced by the high prazosin dose (2.0 mg/kg).
Figure 3.
Results of Test Day 2 (Meth Challenge) of Experiment 1 (Panel A). Time course results of Test Day 2 for paired (Panel B) and unpaired (Panel C) mice of Experiment 1. The error bars represent +/ ± 1 S.E.M. The symbol denotes a significant difference between paired mice and their respective unpaired control mice, p < 0.07. The asterisk denotes a significant difference between Paired-2.0 Prazosin mice and their respective vehicle control mice at different time points, p ≤ 0.05.
3.2. Experiment 2
Experiment 2 employed a 2 (Conditioning) x 2 (Prazosin Dose) factor design whereby mice received an injection of vehicle (unpaired mice) or methamphetamine (paired mice) prior to placement in the locomotor activity chambers during the Conditioning Phase similar to Experiment 1. However, in Experiment 2, paired and unpaired mice received an injection of either vehicle or prazosin (2.0 mg/kg) immediately following each conditioning session and then tested for conditioned hyperactivity (Test Day 1) and context-specific sensitization (Test Day 2) after the completion of the Conditioning Phase.
In total, 24 mice were used in the experiment; however one mouse left the testing area during Test Day 1 of the experiment. Thus, this mouse's data were not available on this day and not included in the Test Day 1 analyses; however, this mouse's data were included in all other analyses.
3.2.1. Conditioning
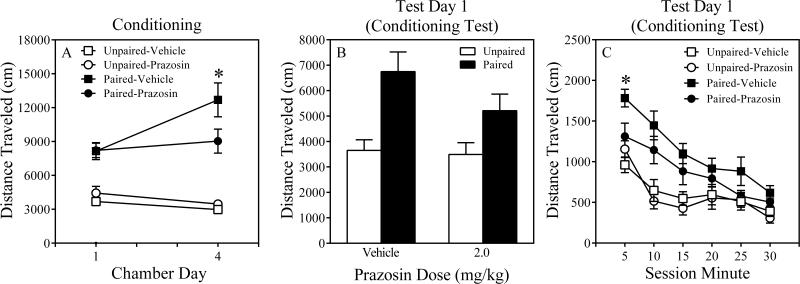
The three-way ANOVA revealed significant main effects of Chamber Day, F (1, 20) = 5.9, P = 0.025 and Conditioning, F (1, 20) = 73.0, P = 0.000. The Chamber Day x Conditioning, F (1, 20) = 21.5, P = 0.000, as well as the Chamber Day x Prazosin Dose, interactions were significant, F (1, 20) = 6.9, P = 0.016. The former significant interaction indicates that paired mice were more active than unpaired mice, reflecting a pharmacological effect of the methamphetamine. The Conditioning x Prazosin Dose approached statistical significance, F (1, 20) = 3.1, P = 0.095. Importantly, a significant Chamber Day x Conditioning x Prazosin Dose interaction was detected, F (1, 20) = 5.3, P = 0.033. This significant three-way interaction motivated a post hoc contrast that found that Paired-Prazosin mice were less active than Paired-Vehicle mice, P = 0.056, on the last conditioning day (i.e., Chamber Day 4; Figure 4A).
Figure 4.
Results of Chamber Days 1 and 4 of the Conditioning Phase (Panel A) and Test Day 1 (Conditioning Test; Panel B) for paired and unpaired mice of Experiment 2. Time course results of Test Day 1 for paired and unpaired mice of Experiment 2 (Panel C). The error bars represent +/ ± 1 S.E.M. The asterisks denote a significant difference between Paired-Prazosin and Paired-Vehicle mice, ps < 0.06.
3.2.2. Test Day 1
The two-way ANOVA revealed a significant main effect of Conditioning, F (1, 19) = 14.9, P = 0.001, but no significant main effect of Prazosin Dose, F (1, 19) = 1.8, P = 0.19. The former result suggests that paired mice were more active than unpaired mice (i.e., conditioned hyperactivity; Figure 4B). The Conditioning x Prazosin Dose interaction was not significant, F (1, 19) = 1.2, P = 0.28. Furthermore, a post hoc contrast, comparing Paired-Prazosin and Paired-Vehicle mice, revealed no significant differences, P = 0.31, suggesting that immediate post-session administration of prazosin did not alter conditioned hyperactivity. However, as can be seen in Figure 4B, Paired-Prazosin mice were less active than Paired-Vehicle mice. Thus, to further explore group differences not detected during the analysis of the overall 30-min session, a time-course analysis was conducted. The three-way ANOVA, conducted on the time course data, found a significant main effect of Session Minute, F (5, 95) = 68.6, P = 0 .000, Session Minute x Conditioning interaction, F (5, 95) = 8.1, P = 0.000 and Conditioning x Prazosin Dose x Session Minute interaction, F (5, 95) = 2.8, P = 0.022. No other interactions were statistically significant. Post hoc contrasts found that Paired-Prazosin mice were less active than Paired-Vehicle mice during Session Minute 5, P = 0.058, suggesting that the post-session administration of prazosin attenuated the development of the conditioned hyperactive response. Unpaired-Prazosin and Unpaired-Vehicle mice did not differ during any time point, Ps > 0.71 (Figure 4C).
3.2.3. Test Day 2
The two-way ANOVA revealed a significant main effect of Conditioning, F (1, 20) = 5.9, P = .025, and a marginally non-significant main effect of Prazosin Dose, F (1, 20) = 3.5, P = 0.076. The former result suggests that the methamphetamine challenge produced an increase in activity in paired mice more so than unpaired mice. The Conditioning x Prazosin Dose interaction was not significant, F (1, 20) =.42, P = 0.52, indicating that the prazosin dose did not significantly alter the locomotor activity in the paired mice compared to the unpaired mice. Paired-Prazosin mice tended to be less active than Paired-Vehicle mice; however, this difference was not statistically significant, P = 0.31, (Figure 5A). To further explore group differences not detected during the analysis of the overall 30-min session, a time- course analysis was conducted. The three-way ANOVA, conducted on the time course data, indicates a significant main effects of Session Minute, F (5, 100) = 18.0, P = 0.000, and Conditioning, F (1, 20) = 5.9, P = 0.025. The main effect of Prazosin Dose approached statistical significance, F (1, 20) = 3.5, P = 0.076. The Session Minute x Conditioning interaction was significant, F (5, 100) = 6.7, P = 0.000. No other statistically significant interactions were noted. Paired-Prazosin mice were less active compared to Paired-Vehicle mice during Session Minutes 10 (P = 0.03) and 15 (P = 0.055). Unpaired-Prazosin and Unpaired-Vehicle mice did not differ during any time point, Ps > 0.93 (Figure 5B).
Figure 5.
Results of Test Day 2 (Methamphetamine Challenge) for paired and unpaired mice of Experiment 2 (Panel A). Time course results of Test Day 2 for paired and unpaired mice of Experiment 2 (Panel B). The error bars represent +/ ± 1 S.E.M. The asterisks denote a significant difference between Paired-Prazosin and Paired-Vehicle mice, ps < 0.06.
3.3. Experiment 3
Experiment 3 was similar to Experiment 2 and employed a 2 (Conditioning) x 2 (Prazosin Dose) factor design; however, in Experiment 3 paired and unpaired mice received an injection of either vehicle or prazosin (2.0 mg/kg) 24 h after each conditioning session and then tested for conditioned hyperactivity (Test Day 1) and context-specific sensitization (Test Day 2) at the completion of the Conditioning Phase.
3.3.1. Conditioning
The three-way ANOVA revealed significant main effects of Chamber Day, F (1, 20) = 16.0, P = 0.001, and Conditioning, F (1, 20) = 32.1, P = 0.000, as well as a significant Chamber Day x Conditioning interaction, F (1, 20) =13.7, P = 0.001. The latter result indicates that paired mice were more active than unpaired mice, reflecting a pharmacological effect of the methamphetamine. The Chamber Day x Prazosin Dose, F (1, 20) = .312, P = 0.58, Conditioning x Prazosin Dose, F (1, 20) =1.6, P = 0.21 and Chamber Day x Conditioning x Prazosin dose, F (1, 20) = .080, P = 0.78, interactions were not significant. The lack of a significant three-way interaction suggests that the prazosin did not alter the pharmacological effects of methamphetamine in paired mice (Figure 6A).
Figure 6.
Results of Chamber Days 1 and 4 of the Conditioning Phase (Panel A), Test Day 1 (Conditioning Test; Panel B) and Test Day 2 (Methamphetamine Challenge; Panel C) for paired and unpaired mice of Experiment 3. The asterisks denote a significant difference between Paired-Prazosin and Paired-Vehicle mice, p < 0.05
3.3.2. Test Day 1
The two-way ANOVA revealed a significant main effect of Conditioning, F (1, 20) = 18.5, P = 0.000, but no significant main effect of Prazosin Dose, F (1, 20) = .016, P = 0.90. These results suggest that paired mice were more active than unpaired mice (i.e., conditioned hyperactivity; Figure 6B). A marginally non-significant Conditioning x Prazosin Dose interaction was detected, F (1, 20) =3.1, P = 0.095. Furthermore, a post hoc contrast, comparing Paired-Prazosin and Paired-Vehicle mice, revealed no reliable differences, P = 0.56. Collectively, these results suggest that the administration of prazosin 24 h after the conditioning sessions did not alter methamphetamine conditioned hyperactivity.
3.3.3. Test Day 2
The two-way ANOVA revealed a significant main effect of Conditioning, F (1, 20) = 11.1, P = 0.003, but no significant main effect of Prazosin Dose, F (1, 20) = 1.6, P = 0.22. This result suggests that paired mice were more active than unpaired mice (i.e. context-specific sensitization; Figure 6C). However, a significant Conditioning x Prazosin Dose interaction was observed, F (1, 20) = 7.8, P = 0.011. A post hoc contrast, comparing Unpaired-Prazosin and Unpaired-Vehicle mice, found that groups were not statistically different, P = 0.71. However, a similarly-conducted post hoc contrast revealed that Paired-Prazosin mice were more active than Paired-Vehicle mice, P = 0.044, suggesting that the Paired-Prazosin mice showed a greater pharmacological response to the methamphetamine compared to the Paired-Vehicle mice.
4. Discussion
While adolescent compared to adult mice have been shown to be less active following either acute [31] or repeated [32] methamphetamine administration, methamphetamine (1.0 mg/kg) enhanced locomotor activity following either acute (Chamber Day 1) or repeated (Chamber Day 4) administration in adolescent Swiss-Webster mice in the present set of experiments. Furthermore, methamphetamine (1.0 mg/kg) produced a robust conditioned hyperactive response in all experiments, replicating previous work in the laboratory [3].
When prazosin was administered 30 min prior to the methamphetamine, then prazosin dose-dependently blocked the locomotor-activating effects of methamphetamine, following acute and repeated administration (Experiment 1). These results are similar to other studies that have shown that antagonism of the α1-adrenergic receptor blocks the unconditioned, pharmacological effects of amphetamine in rodents [23, 25]. In Experiment 1, we also found that prazosin dose-dependently blocked the development of conditioned hyperactivity and context-specific sensitization in mice. To our knowledge, these latter findings are the first to show that α1-adrenergic receptor antagonism blocks the conditioned component of behavioral sensitization. Unfortunately, all prazosin doses initially decreased locomotor activity in unpaired mice, suggesting that these prazosin doses produced nonspecific motor impairments, complicating the interpretation of the disrupting effects of prazosin in paired mice. Furthermore, because prazosin was administered prior to the methamphetamine during the conditioning phase, the results did not indicate if prazosin blocked the development of methamphetamine conditioned hyperactivity and context-specific sensitization by disrupting acquisition (i.e., at the time of training) or post-acquisition (i.e., memory consolidation) processes. Thus, in order to address these limitations, two additional experiments (Experiments 2 and 3) were conducted in which prazosin was administered immediately or 24 h after the conditioning sessions.
The results of Experiment 2 showed that immediate post-session administration of prazosin attenuated the pharmacological effects of methamphetamine (Chamber Day 4, Figure 4A) and conditioned hyperactivity (Figure 4C). In contrast, the results of Experiment 3 illustrated that when prazosin was administered 24 h after conditioning it had no effects on the pharmacological effects of methamphetamine (Figure 6A) or conditioned hyperactivity (Figure 6B). Collectively, these results suggest that the post-session administration of prazosin disrupted memory consolidation, and not acquisition, processes. Moreover, in Experiments 2 and 3, when prazosin was administered after the conditioning session, a decrease in locomotor activity was not detected in unpaired mice, suggesting that the memory-disrupting effects of prazosin in paired mice was not due to nonspecific motor impairments. These results are consistent with other recent pharmacological studies examining the role of α1-adrenergic receptors in mediating memory consolidation mechanisms and employing different behavioral conditioning paradigms [33]. For example, Bernardi and Lattel (2010) examined if post-session prazosin treatment altered memory consolidation of extinction, as assessed by contextual fear conditioning and cocaine-conditioned place preference paradigms. These authors found that post-session prazosin treatment retarded the rate of contextual fear extinction and reduced the persistence of extinction following reconditioning of cocaine-produced conditioned place preference, suggesting a role for α1-adrenergic receptors in mediating memory consolidation.
The results of Experiment 2 and 3, furthermore, showed that immediate, but not delayed, post-session administration of prazosin attenuated context-specific sensitization, consistent with the idea that post-session prazosin administration blunted context-specific sensitization by altering memory consolidation processes. This finding and interpretation is consistent with another recent behavioral study that found that altering memory consolidation processes (specifically memory reconsolidation) attenuates context-specific sensitization [34]. Given that Experiments 2 and 3 of the present report involved post-session pharmacological manipulations done in the context of a multiple-session conditioning paradigm (i.e., 4 chamber + methamphetamine pairings), it is perhaps most accurate to suggest that immediate, not delayed, post-session administration of prazosin disrupted memory consolidation and/or reconsolidation processes, resulting in an attenuation of conditioned hyperactivity and context-specific sensitization. The two memory processes have been shown to involve different neuronal mechanisms [35]; however, the multiple-session conditioning paradigm used in the present report does not permit us to specify the precise process (consolidation vs. reconsolidation) targeted by the current pharmacological manipulation.
Two limitations of the context-specific sensitization data were noted in the present report. First, the sensitization procedure used in the present experiments lacked a control group that never received methamphetamine prior to the methamphetamine challenge (Test Day 2). While evidence of sensitization was shown by an increase in locomotor activity following repeated administration of methamphetamine in Paired mice during the Conditioning Phase in the present experiments (see Figures 1A, 4A and 6A), a stronger demonstration of sensitization would have compared mice repeatedly-administered methamphetamine to methamphetamine-naïve mice on the methamphetamine challenge day, Test Day 2. However, the present experiments specifically wanted to examine the effects of prazosin on context-specific sensitization and compare those effects to context nonspecific sensitization; therefore, a protocol was adopted to compare paired and unpaired mice, respectively. A similar approach was adopted in a previous study from the laboratory [3]. Second, paradoxically, Paired-Prazosin mice showed a greater sensitized response following methamphetamine administration compared to Paired-Vehicle mice on Test Day 2 of Experiment 3 (see Figure 6C). Moreover, Unpaired-Vehicle mice displayed an uncharacteristically heightened sensitized response to methamphetamine (M = ~ 12,000 cm) on Test Day 2 of Experiment 3 compared to the Unpaired-Vehicle mice's sensitized response to methamphetamine on Test Day 2 in previous experiments (e.g., Experiment 2 = M = ~ 9,000 cm; see Figure 5A). At present, it is not known the reason for the enhanced sensitized response to methamphetamine in Paired-Prazosin mice compared to Paired-Vehicle mice. An informal inspection of the data suggests that Paired-Prazosin mice sensitized to methamphetamine more so, though not statistically speaking, than Paired-Vehicle mice during the Conditioning Phase (see Figure 6A), perhaps leading to an enhanced sensitized response to methamphetamine on Test Day 2. Indeed, Paired-Prazosin mice also tended to show, though not statistically speaking, a heightened CR compared to Paired-Vehicle mice on Test Day 1 of Experiment 3 (see Figure 6B). Thus, the methamphetamine challenge on Test Day 2 may have unmasked pre-existing group differences.
Recently, authors have suggested that state-dependent learning mechanisms may contribute to drug conditioning and context-specific sensitization [36]. Braga, Dias, Carey and Carrera (2009) suggested that drug USs, particularly dopamine agonists, produce conditioned hyperactive responses that reflect the presence of both internal (drug effects) and external (e.g., smell of the chamber) cues at the time of testing. Moreover, internal drug cues serve as retrieval cues by way of dopaminergic activation. When animals are tested for conditioned hyperactivity in a non-drug state, the weakened CR stems from the absence of internal drug cues, and lack of dopaminergic activation, relative to those present during conditioning. Conversely, when animals are tested for context-specific sensitization, the drug US is present, resulting in dopaminergic activation and aiding as retrieval cues. Such retrieval cues result in a more robust CR relative to that seen during the test for conditioned hyperactivity. By this account, any mismatch between internal and external cues (differences in dopaminergic states) from the Conditioning-to-Testing Phases would produce a weakened CR, as measured either during tests for conditioned hyperactivity or context-specific sensitization. Thus, it could be argued that the results of Experiment 1, results that found that prazosin dose-dependently attenuated conditioned hyperactivity and context-specific sensitization reflect state-dependent learning. That is, prazosin dose-dependently blunted methamphetamine's dopaminergic activity during conditioning. At the time of testing for conditioned hyperactivity, when there was more dopaminergic activity relative to that present during the Conditioning Phase, the internal cue was weakened and the CR was attenuated subsequently. Likewise, the attenuated response observed in prazosin-pretreated mice during the test for context-specific sensitization, resulted from a mismatch between internal and external cues from the Conditioning-to-Testing Phases (differences in dopaminergic states). While this retrieval hypothesis may explain the results of Experiment 1, the results of Experiment 2 seem incapable with this account. Namely, because prazosin was administered immediately after the conditioning session, there would not be a mismatch between internal and external cues from the Conditioning-to-Testing Phases and should not weaken conditioned hyperactivity or context-specific sensitization according to the retrieval hypothesis [36]. However, such a weakening was observed in mice treated with prazosin immediately after the conditioning session. Thus, a memory consolidation account seems to be the most parsimonious explanation of the data.
The ability of prazosin to disrupt memory consolidation/reconsolidation may involve an interaction between the dopaminergic and noradrenergic systems. The dopaminergic system's influence on memory consolidation processes has been localized to several brain regions including the striatum and associated structures. The striatum receives excitatory innervation from the amygdala, hippocampus, ventral tegmental area and the substantia nigra pars compacta, with evidence showing that the ventral tegmental area and substantia nigra pars compacta contribute to the consolidation of context-dependent memories [37]. Additionally, the ventral striatum subsumes the nucleus accumbens region, which has been heavily implicated in several addiction-related and memory paradigms [14]. At a cellular level, the D1-dopaminergic receptor is coupled to G-proteins which, when activated, promote adenylate cyclase cyclic-adenosine monophosphate (c-AMP) activity. Moreover, c-AMP increases protein kinase A and transcription factors (e.g. cAMP response element-binding protein) in an important signal transduction pathway [37], a pathway suggested as necessary for memory consolidation leading to long-term memory formation [38]. Given the role of the striatum and associated structures in memory consolidation, it is interesting to note that both the ventral tegmental area and the nucleus accumbens receive excitatory inputs that are mediated through α1-adrenergic receptors. Antagonism of the α1-adrenergic receptors inhibits burst firing of ventral tegmental area neurons [18] whereas stimulation of α1-adrenergic receptors, typically from the locus coeruleus, directly increases the likelihood of action potentials in both the ventral tegmental area and nucleus accumbens [17]. These results collectively suggest that the increased activity of the α1-adrenergic receptor, through excitatory innervations of both the ventral tegmental area and nucleus accumbens, has an upstream role in increasing dopamine levels in several brain areas including those directed linked to conditioned place preference, amphetamine-conditioned hyperactivity and memory consolidation. Collectively, our results, when viewed in tandem with the previous literature, provide a likely mechanism by which memory consolidation and/or memory reconsolidation can be mediated through a synergistic relationship between noradrenergic and dopaminergic systems. Most likely, this interaction involves the activation of the D1-dopaminergic receptor and its associated signaling cascade by upstream α1-adrenergic receptors; however, other authors have suggested different mechanisms (e.g., α1-β and/or α1-α2 adrenergic receptor interactions) to account for the role of α1-adrenergic receptors in memory consolidation [33].
Drug addiction has been conceptualized as a disorder of memory [39]. That is, Hyman (2005) has theorized that “addiction represents a pathological hijacking of memory systems related to reward” (p. 1418). Viewed within this conceptual framework, drug addiction bears some similarities to another psychiatric condition, Post-traumatic Stress Disorder (PTSD), in which memory problems feature prominent. Indeed, authors recently have drawn a link between drug addiction and PTSD, suggesting that both disorders stem from prefrontal cortical pathology, resulting in impaired extinction processes [40]. Furthermore, prazosin has been shown to reduce certain memory-based symptoms (e.g., trauma-related nightmares) associated with PTSD in combat veterans [41]. The results of Experiments 1 and 2 bolster the idea that prazosin has a role in attenuating consolidation and/or reconsolidation of context-drug memories. Therefore, given that prazosin attenuates some of the memory-based symptoms of PTSD (e.g., nightmares), and it also disrupts the conditioned effects of methamphetamine by altering memory consolidation and/or reconsolidation processes, then a novel therapeutic approach for treating drug addiction may involve pharmacological treatments (i.e. prazosin) that target consolidation or reconsolidation processes of drug memories, as suggested by other authors [42-44]. Finally, it has been suggested that memory processes, related to associative learning, contribute to drug sensitization [45, 46] and drug addiction [47, 48] in people. In their seminal theoretical paper [46], Robinson and Berridge (1993) suggested that the “behavioral expression of sensitization-related neuroadaptations should be strongly influenced by associative factors” (p. 259). Moreover, Robinson and Berridge (1993) speculated that the “Incentive Sensitization Theory of Addiction predicts that an especially effective pharmacotherapeutic agent would reverse sensitization-related neuroadaptations” (p. 271). The results of the present report suggest that pharmacologically targeting memory consolidation and/or reconsolidation processes may be a novel approach to alter expression of sensitization-related neuroadaptations, thereby blunting the incentive value of drugs and drug-associated stimuli and reducing the likelihood of relapse in addicts.
Highlights.
Examined role of α1-adrenergic receptor in conditioned hyperactivity/sensitization
Pre-session prazosin dose-dependently attenuated locomotor activity nonspecifically
Immediate post-session prazosin attenuated conditioned hyperactivity/sensitization
Delayed post-session prazosin did not alter conditioned hyperactivity/sensitization
Disruption of memory consolidation processes is a possible mechanism of action
Acknowledgments
The authors appreciate the assistance of Margaret Della Vecchia, Neil Devoe and Ee-Rah Sung in conducting the experiments. These experiments were supported by the Department of Psychology, Dickinson College, and grant (DA019866) awarded by USPHS to A. S. Rauhut.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- 2.Anagnostaras SG, Robinson TE. Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning. Behav Neurosci. 1996;110:1397–1414. doi: 10.1037//0735-7044.110.6.1397. [DOI] [PubMed] [Google Scholar]
- 3.Rauhut AS, Bialecki V. Development and persistence of methamphetamine-conditioned hyperactivity in Swiss-Webster mice. Behav Pharmacol. 2011;22:228–238. doi: 10.1097/FBP.0b013e328345f741. doi: 10.1097/FBP.0b013e328345f741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tilson HA, Rech RH. Conditioned drug effects and absence of tolerance to d-amphetamine induced motor activity. Pharmacology, biochemistry, and behavior. 1973;1:149–153. doi: 10.1016/0091-3057(73)90068-3. [DOI] [PubMed] [Google Scholar]
- 5.Stewart J, Vezina P. Extinction procedures abolish conditioned stimulus control but spare sensitized responding to amphetamine. Behav Pharmacol. 1991;2:65–71. [PubMed] [Google Scholar]
- 6.Beninger RJ. The role of dopamine in locomotor activity and learning. Brain Res. 1983;287:173–196. doi: 10.1016/0165-0173(83)90038-3. [DOI] [PubMed] [Google Scholar]
- 7.Gold LH, Swerdlow NR, Koob GF. The role of mesolimbic dopamine in conditioned locomotion produced by amphetamine. Behav Neurosci. 1988;102:544–552. doi: 10.1037//0735-7044.102.4.544. [DOI] [PubMed] [Google Scholar]
- 8.Schiff SR. Conditioned dopaminergic activity. Biol Psychiatry. 1982;17:135–154. [PubMed] [Google Scholar]
- 9.Beninger RJ, Hahn BL. Pimozide blocks establishment but not expression of amphetamine-produced environment-specific conditioning. Science. 1983;220:1304–1306. doi: 10.1126/science.6857251. [DOI] [PubMed] [Google Scholar]
- 10.Mazurski EJ, Beninger RJ. Effects of selective drugs for dopaminergic D1 and D2 receptors on conditioned locomotion in rats. Psychopharmacology (Berl) 1991;105:107–112. doi: 10.1007/BF02316871. [DOI] [PubMed] [Google Scholar]
- 11.McDougall SA, Reichel CM, Cyr MC, Karper PE, Nazarian A, Crawford CA. Importance of D(1) receptors for associative components of amphetamine-induced behavioral sensitization and conditioned activity: a study using D(1) receptor knockout mice. Psychopharmacology (Berl) 2005;183:20–30. doi: 10.1007/s00213-005-0146-9. doi: 10.1007/s00213-005-0146-9. [DOI] [PubMed] [Google Scholar]
- 12.Aujla H, Sokoloff P, Beninger RJ. A dopamine D3 receptor partial agonist blocks the expression of conditioned activity. Neuroreport. 2002;13:173–176. doi: 10.1097/00001756-200201210-00039. [DOI] [PubMed] [Google Scholar]
- 13.Aujla H, Beninger RJ. Intra-BLA or intra-NAc infusions of the dopamine D3 receptor partial agonist, BP 897, block intra-NAc amphetamine conditioned activity. Behav Neurosci. 2004;118:1324–1330. doi: 10.1037/0735-7044.118.6.1324. doi: 10.1037/0735-7044.118.6.1324. [DOI] [PubMed] [Google Scholar]
- 14.Weinshenker D, Schroeder JP. There and back again: a tale of norepinephrine and drug addiction. Neuropsychopharmacology. 2007;32:1433–1451. doi: 10.1038/sj.npp.1301263. doi: 10.1038/sj.npp.1301263. [DOI] [PubMed] [Google Scholar]
- 15.Jones BE, Halaris AE, McIlhany M, Moore RY. Ascending projections of the locus coeruleus in the rat. I. Axonal transport in central noradrenaline neurons. Brain Res. 1977;127:1–21. doi: 10.1016/0006-8993(77)90377-8. [DOI] [PubMed] [Google Scholar]
- 16.Tanoue A, Koshimizu TA, Shibata K, Nasa Y, Takeo S, Tsujimoto G. Insights into alpha1 adrenoceptor function in health and disease from transgenic animal studies. Trends Endocrinol Metab. 2003;14:107–113. doi: 10.1016/s1043-2760(03)00026-2. [DOI] [PubMed] [Google Scholar]
- 17.Grenhoff J, North RA, Johnson SW. Alpha 1-adrenergic effects on dopamine neurons recorded intracellularly in the rat midbrain slice. Eur J Neurosci. 1995;7:1707–1713. doi: 10.1111/j.1460-9568.1995.tb00692.x. [DOI] [PubMed] [Google Scholar]
- 18.Grenhoff J, Nisell M, Ferre S, Aston-Jones G, Svensson TH. Noradrenergic modulation of midbrain dopamine cell firing elicited by stimulation of the locus coeruleus in the rat. J Neural Transm Gen Sect. 1993;93:11–25. doi: 10.1007/BF01244934. [DOI] [PubMed] [Google Scholar]
- 19.Swanson LW, Hartman BK. The central adrenergic system. An immunofluorescence study of the location of cell bodies and their efferent connections in the rat utilizing dopamine-beta-hydroxylase as a marker. J Comp Neurol. 1975;163:467–505. doi: 10.1002/cne.901630406. doi: 10.1002/cne.901630406. [DOI] [PubMed] [Google Scholar]
- 20.Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20:3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ventura R, Cabib S, Alcaro A, Orsini C, Puglisi-Allegra S. Norepinephrine in the prefrontal cortex is critical for amphetamine-induced reward and mesoaccumbens dopamine release. J Neurosci. 2003;23:1879–1885. doi: 10.1523/JNEUROSCI.23-05-01879.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blanc G, Trovero F, Vezina P, Herve D, Godeheu AM, Glowinski J, Tassin JP. Blockade of prefronto-cortical alpha 1-adrenergic receptors prevents locomotor hyperactivity induced by subcortical D-amphetamine injection. Eur J Neurosci. 1994;6:293–298. doi: 10.1111/j.1460-9568.1994.tb00272.x. [DOI] [PubMed] [Google Scholar]
- 23.Darracq L, Blanc G, Glowinski J, Tassin JP. Importance of the noradrenaline-dopamine coupling in the locomotor activating effects of D-amphetamine. J Neurosci. 1998;18:2729–2739. doi: 10.1523/JNEUROSCI.18-07-02729.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohammed AK, Danysz W, Ogren SO, Archer T. Central noradrenaline depletion attenuates amphetamine-induced locomotor behavior. Neurosci Lett. 1986;64:139–144. doi: 10.1016/0304-3940(86)90089-3. [DOI] [PubMed] [Google Scholar]
- 25.Dickinson SL, Gadie B, Tulloch IF. Alpha 1- and alpha 2-adrenoreceptor antagonists differentially influence locomotor and stereotyped behaviour induced by d-amphetamine and apomorphine in the rat. Psychopharmacology (Berl) 1988;96:521–527. doi: 10.1007/BF02180034. [DOI] [PubMed] [Google Scholar]
- 26.Drouin C, Darracq L, Trovero F, Blanc G, Glowinski J, Cotecchia S, Tassin JP. Alpha1badrenergic receptors control locomotor and rewarding effects of psychostimulants and opiates. J Neurosci. 2002;22:2873–2884. doi: 10.1523/JNEUROSCI.22-07-02873.2002. doi: 20026237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGaugh JL. Time-dependent processes in memory storage. Science. 1966;153:1351–1358. doi: 10.1126/science.153.3742.1351. [DOI] [PubMed] [Google Scholar]
- 28.Parent MB, McGaugh JL. Posttraining infusion of lidocaine into the amygdala basolateral complex impairs retention of inhibitory avoidance training. Brain Res. 1994;661:97–103. doi: 10.1016/0006-8993(94)91186-x. [DOI] [PubMed] [Google Scholar]
- 29.Duteil J, Rambert FA, Pessonnier J, Hermant JF, Gombert R, Assous E. Central alpha 1-adrenergic stimulation in relation to the behaviour stimulating effect of modafinil; studies with experimental animals. Eur J Pharmacol. 1990;180:49–58. doi: 10.1016/0014-2999(90)90591-s. [DOI] [PubMed] [Google Scholar]
- 30.Taylor JA, Twomey TM, von Wittenau MS. The metabolic fate of prazosin. Xenobiotica. 1977;7:357–364. doi: 10.3109/00498257709035794. doi: 10.3109/00498257709035794. [DOI] [PubMed] [Google Scholar]
- 31.Zombeck JA, Gupta T, Rhodes JS. Evaluation of a pharmacokinetic hypothesis for reduced locomotor stimulation from methamphetamine and cocaine in adolescent versus adult male C57BL/6J mice. Psychopharmacology (Berl) 2009;201:589–599. doi: 10.1007/s00213-008-1327-0. doi: 10.1007/s00213-008-1327-0; 10.1007/s00213-008-1327-0. [DOI] [PubMed] [Google Scholar]
- 32.Zakharova E, Leoni G, Kichko I, Izenwasser S. Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent male rats. Behav Brain Res. 2009;198:45–50. doi: 10.1016/j.bbr.2008.10.019. doi: 10.1016/j.bbr.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernardi RE, Lattal KM. A role for alpha-adrenergic receptors in extinction of conditioned fear and cocaine conditioned place preference. Behav Neurosci. 2010;124:204–210. doi: 10.1037/a0018909. doi: 10.1037/a0018909; 10.1037/a0018909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernardi RE, Lattal KM, Berger SP. Anisomycin disrupts a contextual memory following reactivation in a cocaine-induced locomotor activity paradigm. Behav Neurosci. 2007;121:156–163. doi: 10.1037/0735-7044.121.1.156. doi: 10.1037/0735-7044.121.1.156. [DOI] [PubMed] [Google Scholar]
- 35.Lee JL, Everitt BJ, Thomas KL. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304:839–843. doi: 10.1126/science.1095760. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- 36.Braga PQ, Dias FR, Carey RJ, Carrera MP. Low dose apomorphine induces context-specific sensitization of hypolocomotion without conditioning: support for a new state dependent retrieval hypothesis of drug conditioning and sensitization. Pharmacol Biochem Behav. 2009;93:128–133. doi: 10.1016/j.pbb.2009.04.019. doi: 10.1016/j.pbb.2009.04.019; 10.1016/j.pbb.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 37.Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- 38.Korzus E, Rosenfeld MG, Mayford M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004;42:961–972. doi: 10.1016/j.neuron.2004.06.002. doi: 10.1016/j.neuron.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- 40.Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–288. doi: 10.1101/lm.1041309. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raskind MA, Peskind ER, Hoff DJ, Hart KL, Holmes HA, Warren D, Shofer J, O'Connell J, Taylor F, Gross C, Rohde K, McFall ME. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61:928–934. doi: 10.1016/j.biopsych.2006.06.032. doi: 10.1016/j.biopsych.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 42.Diergaarde L, Schoffelmeer AN, De Vries TJ. Pharmacological manipulation of memory reconsolidation: towards a novel treatment of pathogenic memories. Eur J Pharmacol. 2008;585:453–457. doi: 10.1016/j.ejphar.2008.03.010. doi: 10.1016/j.ejphar.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 43.Milton AL, Everitt BJ. The psychological and neurochemical mechanisms of drug memory reconsolidation: implications for the treatment of addiction. Eur J Neurosci. 2010;31:2308–2319. doi: 10.1111/j.1460-9568.2010.07249.x. doi: 10.1111/j.1460-9568.2010.07249.x. [DOI] [PubMed] [Google Scholar]
- 44.Taylor JR, Olausson P, Quinn JJ, Torregrossa MM. Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology. 2009;56(Suppl 1):186–195. doi: 10.1016/j.neuropharm.2008.07.027. doi: 10.1016/j.neuropharm.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bradberry CW. Cocaine sensitization and dopamine mediation of cue effects in rodents, monkeys, and humans: areas of agreement, disagreement, and implications for addiction. Psychopharmacology (Berl) 2007;191:705–717. doi: 10.1007/s00213-006-0561-6. doi: 10.1007/s00213-006-0561-6. [DOI] [PubMed] [Google Scholar]
- 46.Leyton M. Conditioned and sensitized responses to stimulant drugs in humans. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1601–1613. doi: 10.1016/j.pnpbp.2007.08.027. doi: 10.1016/j.pnpbp.2007.08.027. [DOI] [PubMed] [Google Scholar]
- 47.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 48.Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]