Abstract
DEET (N,N-diethyl-m-toluamide) is the most common active ingredient in the insect repellents commonly detected in European groundwater. The aim of this study was to investigate the effect of subchronic DEET exposure on biochemical and haematological parameters, antioxidant enzymes, including catalase, glutathione peroxidase, glutathione reductase, and glutathione S-transferase, and the amount of thiobarbituric acid reactive substances (TBARS) in common carp (Cyprinus carpio L.). Two specific proinflammatory and anti-inflammatory cytokine genes were selected to assess an immunological status of the fish. Fish were exposed for 28 days to three concentrations of DEET (1.0 µg/L, 0.1 mg/L, and 1.0 mg/L) where 1 µg/L is corresponding to the concentration found in the environment. DEET had a significant (P < 0.05) effect on increased RBC, decreased mean corpuscular volume (MCV), and mean corpuscular haemoglobin value (MCH) compared to control groups in the concentration of 1 mg/L. A significant decline (P < 0.05) in triacylglycerols (TAG) in plasma was found in the concentration of 1 mg/L compared to the control groups. The parameters of oxidative stress in tissues of common carp were weekly affected and immunological parameters were not affected.
1. Introduction
DEET (N,N-diethyl-m-toluamide) is the most common active ingredient in insect repellents used around the world due to its high efficacy against insects and arthropods bites [1–3]. DEET was produced and patented for usage in American military by the US government and registered for the general population in the 1950s [4, 5].
WHO and subsequently the US Environmental Protection Agency decided that an application of DEET-containing repellents in compliance with the instruction guidelines does not pose a health risk [6].
Behavioral and electrophysiological studies have demonstrated DEET interactions with antennal olfactory as well as gustatory receptors in insect [7–9]. Ditzen et al. [10] described DEET-dependent blockade of electrophysiological responses of olfactory sensory neurons to attractive odors in Anopheles gambiae and Drosophila melanogaster.
In addition, DEET inhibits insect acetylcholinesterase (AChE) [11] resulting in the accumulation of AChE in the synaptic cleft, which leads to a continuous stimulation of the postsynaptic neuron, finally causing the disruption of the transmission of the nerve impulse [12]. Moreover, it is unknown if the inhibition of the AChE is related to the repellency potential of DEET [11, 13, 14].
DEET is a mobile and persistent chemical which is commonly detected in aquatic environment around the world. Presence of DEET has been studied and monitored in various aquatic environments, such as drinking water, streams, open seawater, effluents from sewage plant, groundwater, treated effluent, and even drinking water treated with conventional water-treatment systems [5, 15–18]. Costanzo et al. [5] state that the concentrations of DEET in aqueous samples are ranging from 40 to 3000 ng/L worldwide, while the acute toxic concentrations for aquatic species vary between 4 and 388 mg/L [19].
The aim of this study was to assess the subchronic influence of DEET-containing formulation on common carp (C. carpio) through biometric, biochemical, and haematological parameters, oxidative stress markers, and selected immunological indices. The lowest tested concentrations of DEET responded to the environmental concentration.
2. Material and Methods
2.1. Experimental Design
The test was performed using two-year-old common carps (C. carpio) with average weight 277.1 ± 42.6 g. After one month of acclimatization to experimental conditions (light/dark: 12/12 h, a flow-through system), the fish were randomly distributed into ten tanks (volume 200 L). Three concentrations of DEET (1.0 μg/L, 0.1 mg/L, and 1.0 mg/L) and two control groups were tested: one control with dilution dechlorinated water only and the second control with dilution dechlorinated water and solvent dimethyl sulfoxide (DMSO) in concentration 5 μL/L). Ten fish in each group were divided into two replicates of five in each.
Concentrations of DEET were prepared from formulation Expedition 100+ (Lifemarque Ltd., UK). This formulation contains 95% of N,N-diethyl-m-toluamide and 5% of inert components. DMSO solvent was added to the formulation in the amount of 5 μL/L of final solution. The duration of this subchronic toxicity test was 28 days. During the test, the condition of fish was checked twice daily and the temperature, pH, and the oxygen saturation of water were daily recorded. Water temperature in the test was 21–22°C. The dissolved oxygen concentrations were above 80–90% and pH ranged from 7.74 to 8.22. Other water quality parameters were as follows: CODMn (chemical oxygen demand) 1.4–1.9 mg/L; total ammonia 0.25–0.6 mg/L; NO3 − 40 mg/L; NO2 − 0.75–1.25 mg/L; Cl− 30 mg/L; Cl−/N–NO2 − 78.9–130,4.
The experiment was conducted in a flow-through system, and the test solutions were changed twice a day. The concentrations of DEET did not decrease 80% of original concentrations during the experiment. The fish were fed commercial pellets at total rate of 1.5% body weight twice a day.
At the end of the experiment, individual blood samples were taken by cardiac puncture and heparinized (50 IU per mL of blood). The carps were euthanized and their body weight and length (with/without tail) were recorded. Samples of tissues, such as kidney, gills, brain, and liver (hepatopancreas), were removed and stored at −85°C until analyses.
2.2. Biometric Parameters
Two biometric parameters were calculated: the condition factor (CF) and the hepatosomatic index (HSI). The condition factor of each fish was calculated as CF = (body weight (g)/standard length (cm)3) × 100. The hepatosomatic index was calculated as HSI = liver weight (g)/body weight (g) × 100.
2.3. Haematological and Biochemical Profile
Haematological values, red blood cells count (RBC), white blood cells count (WBC), packed cell volume (PCV), haemoglobin (Hb), mean corpuscular volume (MCV), mean corpuscular haemoglobin value (MCH), and mean corpuscular haemoglobin concentration (MCHC), were determined according to Svobodová et al. [20]. Biochemical indices in plasma glucose, albumin, total protein, ammonium, lactate dehydrogenase (LDH), triacylglycerols (TAG), cholesterol, total calcium, inorganic phosphorus, lactate, alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), and butyrylcholinesterase (ButChE) activities were determined using the biochemical analyzer Konelab 20i and commercial test kits (BioVendor, Czech Republic). To assess the ferric reducing ability of plasma samples (FRAP), the biochemical analyzer Konelab 20i was also used, according to Benzie and Strain [21] supplemented with slight modifications [22].
2.4. Immunological Profile
Samples of head kidney and spleen from 5 fish from 3 groups (control with DMSO and DEET in 1 μg/L and 1 mg/L) were immediately stabilized with RNAlater (Qiagen) and stored at −80°C. Tissue samples free of RNAlater were then lysed in 1 mL of TRI Reagent RT (Molecular Research Center) and homogenized on MagNA Lyser (Roche) with 2.3 mm zirconia/silica beads (BioSpec Products). Total RNA was obtained using combination of 4-bromoanisole and the RNeasy Kit (Qiagen) according to the manufacturer's instruction. Extracted RNA was reversely transcribed with M-MLV reverse transcriptase (200 U) (Invitrogen) and oligo-dT primers at 37°C for 1.5 h and then stored at −20°C. cDNA diluted 5 times (0.5 μL) was used in triplicate reactions in a final volume of 3 μL using the QuantiTect SYBR Green PCR Kit (Qiagen). Primers (10 pmol per reaction) [23] specific for proinflammatory (TNF-α and IL-1β) and anti-inflammatory cytokine genes (TGF-β and IL-10) and for two candidate reference genes (40S and β-actin) used are shown in Table 1. Each run included a control free of template to test the assay reagents for contamination. PCR was performed on the LightCycler 480 (Roche). To test the variation of mRNA expression in samples, RefFinder tool (http://www.leonxie.com/referencegene.php) was used and β-actin candidate reference gene was selected for normalization of expression data in our experiment. The relative expression of a gene of interest (GOI) was calculated according to formula (1/2^Ct (GOI))/(1/2Ct (reference gene)) [24].
Table 1.
Primers used for gene expression by immunological examination of common carp.
| Gene | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| TNF-α | GCTGTCGCTTCACGCTCAA | CCTTGGAAGTGACATTTGCTTTT |
| IL-1β | AAGGAGGCCAGTGGCTCTGT | CCTGAAGAGGAGGCTGTCA |
| TGF-β | ACGCTTTATTCCCAACCAAA | GAAATCCTTGCTCTGCCTCA |
| IL-10 | AAGGAGGCCAGTGGCTCTGT | CCTGAAGAAGAGGCTGTCA |
| β-actin | GCTATGTGGCTCTTGACTTCGA | CCGTCAGGCAGCTCATAGCT |
2.5. An Activity of Detoxifying Enzymes and Values of Oxidative Stress
An activity of detoxifying enzyme (glutathione S-transferase GST) and indices of oxidative stress (glutathione reductase GR, glutathione peroxidase GPx, catalase CAT, and the amount of thiobarbituric acid reactive substances TBARS) were measured in different fish tissues (liver, kidney, gill, and brain). Tissue samples were weighed and homogenized using phosphate buffer (pH = 7.4). The homogenized samples were divided into two portions: the first one was for measuring of TBARS and the second one was centrifuged (11.000 g, 4°C, 20 min) to obtain supernatant fraction for measurement of GST, GR, GPx, and CAT activities and protein content. The enzyme activities were normalized and expressed per mg of protein content. Protein level was quantified by a spectrophotometric method using bicinchoninic acid [25]. All measurements were determined spectrophotometrically using Varioskan Flash Spectral Scanning Multimode Reader (Thermo Scientific). The GST activity was determined by measuring the conjugation of 1-chloro-2,4-dinitrobenzene with reduced glutathione at 340 nm and the activity was expressed as the nmol of the formed product per min per mg of protein [26]. The GR activity was determined by measuring of NADPH oxidation at 340 nm and expressed as the nmol of NADPH consumption per min per mg of protein [27]. The GPx activity was calculated from the rate of NADPH oxidation by the reaction with GR at 340 nm and expressed as the nmol of NADPH consumption per min per mg of protein [28]. The CAT activity was determined by measuring of H2O2 breakdown at 240 nm and it was expressed as the μmol of decomposed H2O2 per min per mg of protein [29]. To evaluate the level of lipid peroxidation, the amount of malondialdehyde was measured using the TBARS method at 535 nm and the concentration was expressed as nmol of TBARS per gram of tissue wet weight [30].
2.6. DEET Concentration in Water
The level of DEET in water was determined by gas chromatography with ion trap mass spectrometry. A sample was extracted in cyclohexane (4 mL samples: 4 mL cyclohexane). The separation, identification, and quantification of DEET were carried out using a Varian 450-GC gas chromatograph with 220-MS ion trap mass spectrometer and VF-5 ms (30 m × 0.25 mm) column (Varian, Inc., USA). A 1 μL of sample aliquot extract was injected in splitless mode. The injector temperature was 250°C. The initial oven temperature was set at 50°C for 1 min, increased in a rate of 30°C min−1 to 130°C for 1 min, increased in a rate of 16°C min−1 to 230°C, held for 1 min, increased in a rate of 60°C min−1 to 280°C, and held for 1 min. Total run time was 13.75 min. Certified standard DEET was purchased from (Sigma Aldrich, Co.). All solvents were GC/MS-grade purity (Chromservis s.r.o., Czech Republic).
2.7. Histopathological Examination
Samples of liver, gills, cranial, and caudal kidney were removed from 5 fish in each group. They were fixed in 10% neutral formalin solution and subsequently stained with haematoxylin and eosin. Histological changes in samples were examined by light microscopy.
2.8. Statistical Analysis
Statistical analysis was performed using Unistat 5.6 software. A Shapiro-Wilk test was done for the normal distribution. The differences among test groups were assessed with the Tukey-HSD test. Immunological parameters were evaluated by the unpaired nonparametric Mann-Whitney test.
3. Results
During the experiment, the mortality of fish was not recorded in both control groups as well as in the tested concentrations.
3.1. Biometric Parameters
There were no changes in HSI and CF in fish exposed to all DEET concentrations compared to both control groups after 28 days of exposure (Table 2).
Table 2.
Biometric parameters in C. carpio for each tested group (n = 10).
| Parameter | Control | Control with DMSO | DEET 1 µg/L | DEET 0.1 mg/L | DEET 1 mg/L |
|---|---|---|---|---|---|
| Body weight (g) | 295.06 ± 40.76 | 267.33 ± 42.99 | 267.10 ± 44.88 | 273.17 ± 39.86 | 282.67 ± 46.26 |
| CF | 2.59 ± 0.19 | 2.59 ± 0.17 | 2.51 ± 0.21 | 2.59 ± 0.26 | 2.51 ± 0.14 |
| HSI | 1.87 ± 0.39 | 1.90 ± 0.26 | 1.79 ± 0.26 | 1.74 ± 0.27 | 1.81 ± 0.35 |
3.2. Haematological Profile
The DEET exposure did not affect WBC, MCHC, values of Hb, and PCV of experimental fish. A significant increase (P < 0.05) in RBC was observed in the concentration of 1 mg/L compared to both control groups (Table 3). Further, a significant (P < 0.05) decrease in MCV and a decrease (P < 0.05) in MCH were found in the 1 mg/L concentration compared to both control groups (Table 3).
Table 3.
Haematological values in C. carpio from control groups and groups exposed to DEET (mean ± SD, n = 10).
| Indices | Control | Control with DMSO | DEET 1 µg/L | DEET 0.1 mg/L | DEET 1 mg/L |
|---|---|---|---|---|---|
| RBC (1012/L) | 1.69 ± 0.35a | 1.78 ± 0.41a | 2.21 ± 0.74a,b | 2.20 ± 0.62a,b | 2.49 ± 0.42b |
| Hb (g/L) | 74.05 ± 10.81 | 69.26 ± 13.93 | 73.56 ± 13.95 | 67.14 ± 8.31 | 71.70 ± 17.59 |
| PCV (L/L) | 0.26 ± 0.04 | 0.27 ± 0.03 | 0.26 ± 0.03 | 0.26 ± 0.02 | 0.26 ± 0.02 |
| MCV (1015/L) | 162.11 ± 34.65a | 159.54 ± 34.23a | 134.76 ± 52.87a,b | 128.68 ± 41.65a,b | 107.27 ± 18.43b |
| MCH (1012/L) | 44.12 ± 11.73a | 39.75 ± 8.36a,b | 36.35 ± 11.51a,b | 32.66 ± 10.30a,b | 29.88 ± 9.31b |
| MCHC (g/L) | 0.28 ± 0.04 | 0.25 ± 0.05 | 0.29 ± 0.11 | 0.25 ± 0.04 | 0.27 ± 0.07 |
Significant differences (P < 0.05) between groups are marked by different alphabetic superscripts.
WBC and differential white blood cells count were not affected by treatment (data not shown).
3.3. Biochemical Profile
The only change in biochemical profile of the experimental fish was in the decrease (P < 0.05) of TAG in the DEET concentration of 1 mg/L compared to the control groups. The other parameters including activity of butyrylcholinesterase were not affected (Table 4).
Table 4.
Biochemical indices in plasma of C. carpio from control groups andgroups exposed to DEET (mean ± SD, n = 10).
| Indices | Control | Control with DMSO | DEET 1 µg/L | DEET 0.1 mg/L | DEET 1 mg/L |
|---|---|---|---|---|---|
| ALT (µkat/L) | 0.70 ± 0.22 | 0.65 ± 0.11 | 0.88 ± 0.35 | 0.97 ± 0.36 | 0.67 ± 0.33 |
| AST (µkat/L) | 1.88 ± 0.69 | 1.69 ± 0.47 | 2.11 ± 0.39 | 1.95 ± 0.51 | 1.80 ± 0.52 |
| ALP (µkat/L) | 0.40 ± 0.21 | 0.54 ± 0.19 | 0.55 ± 0.22 | 0.52 ± 0.17 | 0.42 ± 0.17 |
| Albumin (g/L) | 10.94 ± 1.77 | 11.12 ± 1.57 | 10.89 ± 1.30 | 10.86 ± 1.22 | 10.92 ± 2.03 |
| Total protein (g/L) | 29.23 ± 2.12 | 27.67 ± 2.30 | 26.33 ± 3.02 | 27.26 ± 3.15 | 26.36 ± 2.52 |
| Glucose (mmol/L) | 3.46 ± 1.09 | 3.67 ± 1.18 | 3.24 ± 1.12 | 4.13 ± 1.39 | 2.93 ± 0.87 |
| LDH (µkat/L) | 5.67 ± 2.33 | 5.07 ± 0.73 | 4.45 ± 0.99 | 4.78 ± 1.61 | 4.15 ± 1.30 |
| TAG (mmol/L) | 2.37 ± 2.34a | 2.12 ± 0.73a,b | 2.21 ± 0.99a,b | 2.15 ± 1.60a,b | 1.88 ± 1.30b |
| Ammonium (mmol/L) | 196.12 ± 66.35 | 186.6 ± 104.77 | 164.39 ± 37.74 | 156.70 ± 50.76 | 172.33 ± 73.20 |
| Calcium (mmol/L) | 2.14 ± 0.09 | 2.06 ± 0.20 | 2.06 ± 0.06 | 2.03 ± 0.31 | 2.06 ± 0.12 |
| Phosphorus (mmol/L) | 1.85 ± 0.35 | 1.84 ± 0.33 | 1.88 ± 0.22 | 2.01 ± 0.58 | 1.80 ± 0.26 |
| Lactate (mmol/L) | 2.91 ± 1.29 | 2.64 ± 2.48 | 2.11 ± 0.95 | 3.29 ± 2.17 | 2.85 ± 1.77 |
| Cholesterol (mmol /L) | 3.33 ± 0.47 | 3.12 ± 0.54 | 2.97 ± 0.41 | 3.07 ± 0.52 | 3.06 ± 0.63 |
| FRAP (Fe2+ equivalent µmol/L) | 559.76 ± 91.74 | 448.57 ± 104.53 | 506.90 ± 60.87 | 483.81 ± 52.25 | 465.98 ± 116.96 |
| ButChE (µkat/L) | 1.55 ± 0.67 | 1.22 ± 0.85 | 1.54 ± 0.75 | 1.66 ± 0.59 | 1.69 ± 0.53 |
Significant differences (P < 0.05) between groups are marked by different alphabetic superscripts.
3.4. Immunological Parameters
The exposure to DEET did not influence proinflammatory (TNF-α and IL-1β) and anti-inflammatory cytokine genes (TGF-β and IL-10) in any tested concentration of DEET (Figure 1).
Figure 1.
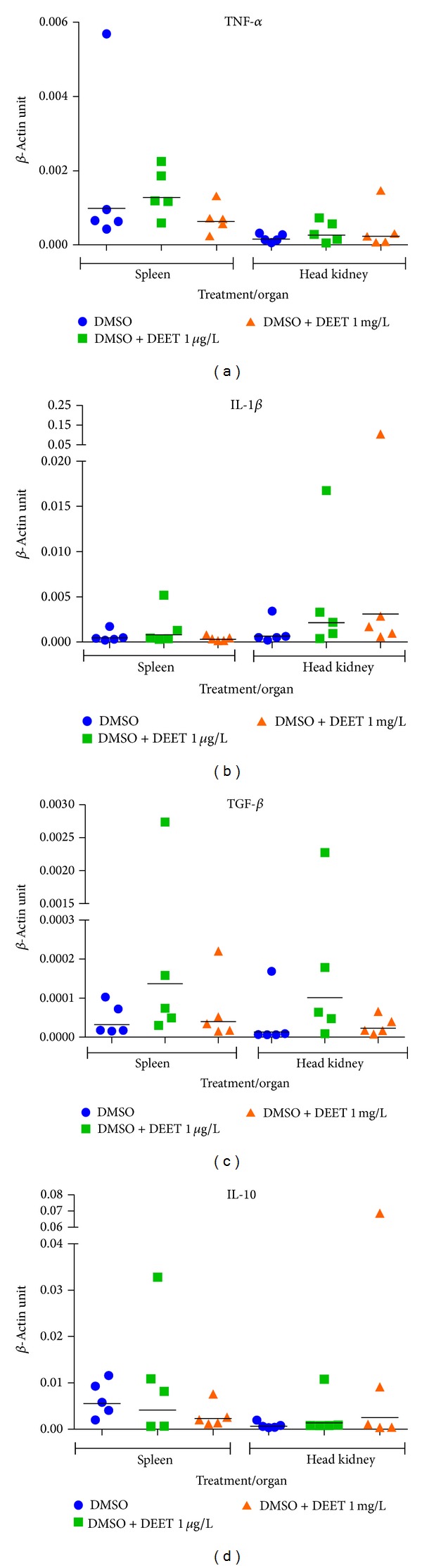
Graphs show individual values of the expression of cytokines versus housekeeping gene β-actin. Bars represent geometric mean values.
3.5. Parameters of Oxidative Stress
Values of antioxidant enzymes activities (GR, GPx, GST, and CAT) and amount of TBARS are presented in tables for individual tissue (Tables 5, 6, 7, and 8). A significant (P < 0.01) increase in GPx was found in kidney in the exposure concentration of 1 mg/L compared to 1 μg/L and a significant (P < 0.05) decrease in GPx was found in gills in the exposure concentration of 1 mg/L compared to the control group with DMSO. The catalase activity could be not determined in brain due to very low activity of this enzyme.
Table 5.
Antioxidant enzymes activities and amount of TBARS in liver of C. carpio in each group (mean ± SD, n = 10).
| Parameter | Units | Control | Control with DMSO | DEET 1 µg/L | DEET 0.1 mg/L | DEET 1 mg/L |
|---|---|---|---|---|---|---|
| GR | (nmol NADPH/min/mg protein) | 5.69 ± 0.98 | 5.03 ± 1.98 | 5.43 ± 1.51 | 4.79 ± 1.14 | 5.86 ± 1.06 |
| GPx | (nmol NADPH/min/mg protein) | 182.3 ± 61.5 | 203.9 ± 70.7 | 169.1 ± 50.3 | 203.8 ± 47.2 | 194.7 ± 39.3 |
| GST | (nmol /min/mg protein) | 260.6 ± 82.9 | 344.9 ± 110.3 | 310.0 ± 91.4 | 284.1 ± 97.3 | 319.6 ± 100.3 |
| CAT | (µmol H2O2/min/mg protein) | 365.7 ± 70.4 | 351.3 ± 67.9 | 332.2 ± 62.7 | 344.7 ± 56.3 | 332.1 ± 73.0 |
| TBARS | (nmol/g sample) | 36.4 ± 10.4 | 30.6 ± 6.6 | 35.8 ± 11.2 | 36.1 ± 12.1 | 32.7 ± 14.4 |
Table 6.
Antioxidant enzymes activity and amount of TBARS in kidney of C. carpio in each tested group (mean ± SD, n = 10).
| Parameter | Units | Control | Control with DMSO | DEET 1 µg/L | DEET 0.1 mg/L | DEET 1 mg/L |
|---|---|---|---|---|---|---|
| GR | (nmol NADPH/min/mg protein) | 5.02 ± 2.23 | 4.41 ± 1.62 | 5.67 ± 2.41 | 4.98 ± 1.68 | 4.76 ± 2.26 |
| GPx | (nmol NADPH/min/mg protein) | 193.4 ± 33.1a,b | 183.7 ± 45.7b | 164.9 ± 39.4a,b | 201.7 ± 29.8a,b | 221.8 ± 31.4a |
| GST | (nmol/min/mg protein) | 252.3 ± 43.7 | 275.4 ± 45.7 | 256.5 ± 55.9 | 265.2 ± 65.9 | 301.9 ± 65.1 |
| CAT | (µmol H2O2/min/mg protein) | 52.63 ± 8.60 | 42.90 ± 12.49 | 49.21 ± 15.98 | 42.06 ± 9.87 | 44.36 ± 17.40 |
| TBARS | (nmol/g sample) | 24.12 ± 4.53 | 25.06 ± 5.11 | 24.99 ± 4.66 | 26.27 ± 6.26 | 27.00 ± 8.85 |
Significant differences (P < 0.05) between groups are marked by different alphabetic superscripts.
Table 7.
Antioxidant enzymes activity and amount of TBARS in brain of C. carpio in each tested group (mean ± SD, n = 10).
| Parameter | Units | Control | Control with DMSO | DEET 1 µg/L | DEET 0.1 mg/L | DEET 1 mg/L |
|---|---|---|---|---|---|---|
| GR | (nmol NADPH/min/mg protein) | 3.64 ± 0.49 | 3.94 ± 0.35 | 3.88 ± 0.59 | 4.33 ± 0.81 | 4.01 ± 0.62 |
| GPx | (nmol NADPH/min/mg protein) | 56.20 ± 6.76 | 60.96 ± 6.87 | 56.86 ± 7.30 | 58.33 ± 6.16 | 56.93 ± 3.97 |
| GST | (nmol/min/mg protein) | 212.30 ± 50.11 | 220.28 ± 53.98 | 263.46 ± 62.05 | 222.51 ± 40.33 | 231.73 ± 78,67 |
| TBARS | (nmol/g sample) | 9.09 ± 3.59 | 8.3 ± 2.23 | 8.92 ± 3.15 | 10.37 ± 4.50 | 10.02 ± 4.32 |
Table 8.
Antioxidant enzymes activity and amount of TBARS in gills of C. carpio in each tested group (mean ± SD, n = 10).
| Parameter | Units | Control | Control with DMSO | DEET 1 µg/L | DEET 0.1 mg/L | DEET 1 mg/L |
|---|---|---|---|---|---|---|
| GR | (nmol NADPH/min/mg protein) | 6.38 ± 1.04 | 7.29 ± 1.37 | 6.93 ± 2.16 | 7.96 ± 0.96 | 6.35 ± 1.05 |
| GPx | (nmol NADPH/min/mg protein) | 43.67 ± 13.94a,b | 54.87 ± 18.11a | 43.09 ± 21.65a,b | 37.31 ± 6.94a,b | 34.28 ± 11.39b |
| GST | (nmol /min/mg protein) | 116.9 ± 24.2 | 112.6 ± 36.1 | 104.7 ± 30.3 | 116.4 ± 33.2 | 110.1 ± 22.7 |
| CAT | (µmol H2O2/min/mg protein) | 10.12 ± 3.55 | 9.20 ± 2.61 | 10.40 ± 2.73 | 11.07 ± 2.44 | 10.88 ± 2.27 |
| TBARS | (nmol/g sample) | 39.76 ± 17.62 | 29.23 ± 8.87 | 31.01 ± 9.66 | 36.66 ± 14.03 | 31.86 ± 8.61 |
Significant differences (P < 0.05) between groups are marked by different alphabetic superscripts.
3.6. Histological Examination
A subchronic exposure to DEET did not cause marked specific histopathological changes in the DEET-treated fish.
4. Discussion
The amount of data on mechanism of action and chronic toxicity for DEET to aquatic environment is still limited. Acute toxic studies have found DEET to be slightly toxic for fish: 96 h LC50 for tilapia mossambica (Oreochromis mossambicus) and rainbow trout (Oncorhynchus mykiss) is 120–150 mg/L and 71.3 mg/L, respectively [31, 32]. Nevertheless our study has shown that even low concentration of DEET can influence red blood parameters of fish after 28 days of exposure. The increase in red blood cells in DEET concentration 1 mg/L indicates rise of erythropoiesis. Although the total amount of haemoglobin and haematocrit in blood was not changed, erythrocytes (MCV and MCH) decreased. Two-third decrease in mean corpuscular volume (MCV) of erythrocyte indicates a breakdown of erythropoiesis and a development of nonadequate erythrocytes. Higher occurrence of erythroblast was not recorded. In the study of dogs, a weak reduction of haemoglobin and haematocrit was noticed after 6 and 12 months of oral intake of DEET in concentration 400 mg/kg/day [33], but other red blood parameters were not affected. In adult fish, a spleen, the head kidney (pronephros), and mesonephros have been found to be sites of erythropoiesis [34]; specific histopathological changes of these organs in the DEET-treated fish were not noticed in our study.
The decrease in triacylglycerides in DEET concentration 1 mg/L was recorded. TAG are the most important energy-storing lipids and belong to major energy sources for the fish [35]. In this study, TAG decrease can indicate exhaustion of energy sources due to long-term stress.
Because DEET is reported to act as a neurotoxin through inhibition of cholinesterase [11], we concentrated on butyrylcholinesterase activity. However, butyrylcholinesterase was not affected. This finding supports results of studies about elevation of cholinesterase inhibition in insect only after common impact of DEET and cholinesterase-inhibiting insecticides [36, 37].
The immunological toxicity of DEET has not been extensively studied in fish before. Our observation was focused on the expression of proinflammatory (TNF-α and IL-1β) and anti-inflammatory cytokine genes (TGF-β and IL-10). There were not changes of the cytokine expression in head kidney and spleen in tested fish. Cytokines are the key initiator of immune reaction. They arise at the sites of entry of pathogens into organism; they stimulate inflammatory signals and thus the ability of resident and newly recruited phagocytes to eliminate the invading pathogens is regulated [38]. In teleostean fishes, such as carp and rainbow trout, the expression of interleukin 1β (IL-1β) mRNA can be stimulated by lipopolysaccharide alone or in combination with cortisol [39–41]. On the contrary, some toxic compounds as cyanotoxin anatoxin-a, for example, significantly inhibited proinflammatory (IL-1β and TNF-α) cytokines and induced anti-inflammatory (IL-10 and TGF-β) cytokines in common carp [42].
The effect of DEET on formation of oxidative stress was studied especially in insect [43] and rats [44–46]. Antioxidant enzymes, that is, GPx, GR, CAT, and SOD, keep the oxidative status in the cell. They reduce either free or membrane-bound hydroperoxides [47]. Glutathione S-transferase catalyzes the conjugation of the reduced form of glutathione to xenobiotic substrates for the purpose of detoxification [48]. In our study, we observed alterations only in case of GPx activities. The activity of GPx in kidney tissues increased in experimental group exposed to 1 mg/L of DEET compared to the DMSO control group. This tissue-specific GPx increase might indicate the adaptive approach by the fish to defend the oxidative stress [49]. Moreover, we also found decline in GPx activity in gill tissues of experimental group exposed to 1 mg/L of DEET compared to the DMSO control group. This alteration in GPx in gills might be due to the depletion of the enzyme. In fact, the fish gills were the first organ exposed to the toxic effluent [50].
5. Conclusions
Fish are an appropriate model for a further investigation of the biological effect of DEET on vertebrates due to its high frequency of occurrence in aquatic environments around the world. Although acute toxicity levels of DEET are high, low concentration after subchronic exposition can cause adverse effects on haematological parameters. To assess the effect of diethyltoluamide on the fish immune system, more immunological parameters need to be included in the future studies.
Acknowledgments
This study was supported by IGA VFU 17/2013/FVHE and Ministry of Education, Youth and Sports, Czech Republic (CZ 1.05/2.1.00/01.0006; ED 0006/01/01 AdmireVet).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Robbins PJ, Cherniack MG. Review of the biodistribution and toxicity of the insect repellent N,N-diethyl-m-toluamide (DEET) Journal of Toxicology and Environmental Health. 1986;18(4):503–525. doi: 10.1080/15287398609530891. [DOI] [PubMed] [Google Scholar]
- 2.McConnell R, Fidler AT, Chrislip D. 83-085. Washington, DC, USA: U.S. Department of Health and Human Services, NIOSH; 1986. Health Hazard Evaluation Determination. [Google Scholar]
- 3.Fradin MS, Day JF. Comparative efficacy of insect repellents against mosquito bites. New England Journal of Medicine. 2002;347(1):13–18. doi: 10.1056/NEJMoa011699. [DOI] [PubMed] [Google Scholar]
- 4.Osimitz TG, Murphy JV, Fell LA, Page B. Adverse events associated with the use of insect repellents containing N,N-diethyl-m-toluamide (DEET) Regulatory Toxicology and Pharmacology. 2010;56(1):93–99. doi: 10.1016/j.yrtph.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Costanzo SD, Watkinson AJ, Murby EJ, Kolpin DW, Sandstrom MW. Is there a risk associated with the insect repellent DEET (N,N-diethyl-m-toluamide) commonly found in aquatic environments? Science of the Total Environment. 2007;384(1–3):214–220. doi: 10.1016/j.scitotenv.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 6.United States Environmental Protection Agency. Registration Eligibility Decision (RED) EPA738-R-98-010. DEET; 1998. [Google Scholar]
- 7.Lee Y, Kim SH, Montell C. Avoiding DEET through insect gustatory receptors. Neuron. 2010;67(4):555–561. doi: 10.1016/j.neuron.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis EE, Rebert CS. Elements of olfactory receptor coding in the yellowfever mosquito. Journal of Economic Entomology. 1972;65(4):1058–1061. doi: 10.1093/jee/65.4.1058. [DOI] [PubMed] [Google Scholar]
- 9.Alzogaray RA, Fontan A, Zerba EN. Repellency of deet to nymphs of Triatoma infestans . Medical and Veterinary Entomology. 2000;14(1):6–10. doi: 10.1046/j.1365-2915.2000.00213.x. [DOI] [PubMed] [Google Scholar]
- 10.Ditzen M, Pellegrino M, Vosshall LB. Insect odorant receptors are molecular targets of the insect repellent DEET. Science. 2008;319(5871):1838–1841. doi: 10.1126/science.1153121. [DOI] [PubMed] [Google Scholar]
- 11.Corbel V, Stankiewicz M, Pennetier C, et al. Evidence for inhibition of cholinesterases in insect and mammalian nervous systems by the insect repellent deet. BMC Biology. 2009;7(article 47) doi: 10.1186/1741-7007-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casida JE, Durkin KA. Neuroactive insecticides: targets, selectivity, resistance, and secondary effects. Annual Review of Entomology. 2013;58:99–117. doi: 10.1146/annurev-ento-120811-153645. [DOI] [PubMed] [Google Scholar]
- 13.Robbins PJ, Cherniack MG. Review of the biodistribution and toxicity of the insect repellent N,N-diethyl-m-toluamide (DEET) Journal of Toxicology and Environmental Health. 1986;18(4):503–525. doi: 10.1080/15287398609530891. [DOI] [PubMed] [Google Scholar]
- 14.Sudakin DL, Trevathan WR. DEET: a review and update of safety and risk in the general population. Journal of Toxicology: Clinical Toxicology. 2003;41(6):831–839. doi: 10.1081/clt-120025348. [DOI] [PubMed] [Google Scholar]
- 15.Kolpin DW, Skopec M, Meyer MT, Furlong ET, Zaugg SD. Urban contribution of pharmaceuticals and other organic wastewater contaminants to streams during differing flow conditions. Science of the Total Environment. 2004;328(1–3):119–130. doi: 10.1016/j.scitotenv.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Weigel S, Berger U, Jensen E, Kallenborn R, Thoresen H, Hühnerfuss H. Determination of selected pharmaceuticals and caffeine in sewage and seawater from Tromsø/Norway with emphasis on ibuprofen and its metabolites. Chemosphere. 2004;56(6):583–592. doi: 10.1016/j.chemosphere.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 17.Barnes KK, Christenson SC, Kolpin DW, et al. Pharmaceuticals and other organic waste water contaminants within a leachate plume downgradient of a municipal landfill. Ground Water Monitoring and Remediation. 2004;24(2):119–126. [Google Scholar]
- 18.Stackelberg PE, Furlong ET, Meyer MT, Zaugg SD, Henderson AK, Reissman DB. Persistence of pharmaceutical compounds and other organic wastewater contaminants in a conventional drinking-water-treatment plant. Science of the Total Environment. 2004;329(1–3):99–113. doi: 10.1016/j.scitotenv.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Weeks JA, Guiney PD, Nikiforov AI. Assessment of the environmental fate and ecotoxicity of N,N-diethyl-m-toluamide (DEET) Integrated Environmental Assessment and Management. 2012;8(1):120–134. doi: 10.1002/ieam.1246. [DOI] [PubMed] [Google Scholar]
- 20.Svobodová Z, Pravda D, Modrá H. Methods of Haematological Examination of Fish. Manuals of Institute of Fish Culture and Hydrobiology. Vodňany, Czech Republic: Institute of Fish Culture and Hydrobiology; 2012. [Google Scholar]
- 21.Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of ’antioxidant power’: the FRAP assay. Analytical Biochemistry. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 22.Haluzová I, Modrá H, Blahová J, et al. Effects of subchronic exposure to Spartakus (prochloraz) on common carp Cyprinus carpio . Neuroendocrinology Letters. 2010;31:105–113. [PubMed] [Google Scholar]
- 23.Stolte EH, Nabuurs SB, Bury NR, et al. Stress and innate immunity in carp: corticosteroid receptors and pro-inflammatory cytokines. Molecular Immunology. 2008;46(1):70–79. doi: 10.1016/j.molimm.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 24.Zelníčková P, Matiašovic J, Pavlová B, Kudláčková H, Kovářů F, Faldyna M. Quantitative nitric oxide production by rat, bovine and porcine macrophage. Nitric Oxide. 2008;19(1):36–41. doi: 10.1016/j.niox.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Smith PK, Krohn RI, Hermanson GT. Measurement of protein using bicinchoninic acid. Analytical Biochemistry. 1985;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 26.Habig WH, Pabst MJ, Jakoby WB. Glutathione S transferases. The first enzymatic step in mercapturic acid formation. Journal of Biological Chemistry. 1974;249(22):7130–7139. [PubMed] [Google Scholar]
- 27.Carlberg I, Mannervik B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. Journal of Biological Chemistry. 1975;250(14):5475–5480. [PubMed] [Google Scholar]
- 28.Flohe L, Gunzler WA. Assays of glutathione peroxidase. Methods in Enzymology. 1984;105:114–121. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 29.Aebi H. Catalase in vitro. Methods in Enzymology. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 30.Lushchak VI, Bagnyukova TV, Lushchak OV, Storey JM, Storey KB. Hypoxia and recovery perturb free radical processes and antioxidant potential in common carp (Cyprinus carpio) tissues. International Journal of Biochemistry and Cell Biology. 2005;37(6):1319–1330. doi: 10.1016/j.biocel.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Mathai AT, Pillai KS, Deshmukh PB. Acute toxicity od DEET to a fresh-water fish, Tilapia Mossambica—effect on tissue glutathione levels. Journal of Environmental Biology. 1989;10:87–91. [Google Scholar]
- 32.Office of Pesticide Programs. Pesticide Ecotoxicity Database (Formerly: Environmental Effects Database (EEDB)) Washington, DC, USA: Environmental Fate and Effects Division, US EPA; 2000. [Google Scholar]
- 33.Schoenig GP, Osimitz TG, Gabriel KL, Hartnagel R, Gill MW, Goldenthal EI. Evaluation of the chronic toxicity and oncogenicity of N,N-diethyl-m-toluamide (DEET) Toxicological Sciences. 1999;47(1):99–109. doi: 10.1093/toxsci/47.1.99. [DOI] [PubMed] [Google Scholar]
- 34.Moritz KM, Lim GB, Wintour EM. Developmental regulation of erythropoietin and erythropoiesis. American Journal of Physiology—Regulatory Integrative and Comparative Physiology. 1997;273(6):R1829–R1844. doi: 10.1152/ajpregu.1997.273.6.R1829. [DOI] [PubMed] [Google Scholar]
- 35.Haluzová I, Modrá H, Blahová J, et al. The effects of Click 500 SC (terbuthylazine) on common carp Cyprinus carpio under (sub)chronic conditions. Neuroendocrinology Letters. 2011;32:15–24. [PubMed] [Google Scholar]
- 36.Abou-Donia MB, Wilmarth KR, Abdel-Rahman AA, Jensen KF, Oehme FW, Kurt TL. Increased neurotoxicity following concurrent exposure to pyridostigmine bromide, DEET, and chlorpyrifos. Fundamental and Applied Toxicology. 1996;34(2):201–222. doi: 10.1006/faat.1996.0190. [DOI] [PubMed] [Google Scholar]
- 37.Wille T, Thiermann H, Worek F. In vitro kinetic interactions of DEET, pyridostigmine and organophosphorus pesticides with human cholinesterases. Chemico-Biological Interactions. 2011;190(2-3):79–83. doi: 10.1016/j.cbi.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 38.Wang T, Secombes JCh. The cytokine networks of adaptive immunity in fish. Fish and Shellfish Immunology. 2013;35(6):1703–1718. doi: 10.1016/j.fsi.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 39.Engelsma MY, Stet RJM, Schipper H, Verburg-Van Kemenade BML. Regulation of interleukin 1 beta RNA expression in the common carp, Cyprinus carpio L. Developmental and Comparative Immunology. 2001;25(3):195–203. doi: 10.1016/s0145-305x(00)00059-8. [DOI] [PubMed] [Google Scholar]
- 40.Engelsma MY, Stet RJM, Saeij JP, Verburg-Van Kemenade BML. Differential expression and haplotypic variation of two interleukin-1β genes in the common carp (Cyprinus carpio L.) Cytokine. 2003;22(1-2):21–32. doi: 10.1016/s1043-4666(03)00102-9. [DOI] [PubMed] [Google Scholar]
- 41.Zou J, Holland J, Pleguezuelos O, Cunningham C, Secombes CJ. Factors influencing the expression of interleukin-1β in cultured rainbow trout (Oncorhynchus mykiss) leucocytes. Developmental and Comparative Immunology. 2000;24(6-7):575–582. doi: 10.1016/s0145-305x(99)00085-3. [DOI] [PubMed] [Google Scholar]
- 42.Rymuszka A, Adaszek L. Pro- and anti-inflammatory cytokine expression in carp blood and head kidney leukocytes exposed to cyanotoxin stress—an in vitro study. Fish and Shellfish Immunology. 2012;33(2):382–388. doi: 10.1016/j.fsi.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 43.Hellestad VJ, Witthuhn BA, Fallon AM. The insect repellent DEET (N,N-diethyl-3-methylbenzamide) increases the synthesis of glutathione S-transferase in cultured mosquito cells. Cell Biology and Toxicology. 2011;27(2):149–157. doi: 10.1007/s10565-010-9177-z. [DOI] [PubMed] [Google Scholar]
- 44.Abu-Qare AW, Abou-Donia MB. Combined exposure to DEET (N,N-diethyl-m-toluamide) and permethrin: pharmacokinetics and toxicological effects. Journal of Toxicology and Environmental Health B: Critical Reviews. 2003;6(1):41–53. doi: 10.1080/10937400390155481. [DOI] [PubMed] [Google Scholar]
- 45.Abu-Qare A, Abou-Donia M. Increased 8-hydroxy-2′-deoxyguanosine, a biomarker of oxidative DNA damage in rat urine following a single dermal dose of DEET (N,N-diethyl-m-toluamide), and permethrin, alone and in combination. Toxicology Letters. 2000;117(3):151–160. doi: 10.1016/s0378-4274(00)00257-5. [DOI] [PubMed] [Google Scholar]
- 46.Abu-Qare AW, Suliman HB, Abou-Donia MB. Induction of urinary excretion of 3-nitrotyrosine, a marker of oxidative stress, following administration of pyridostigmine bromide, DEET (N,N-diethyl-m-toluamide) and permethrin, alone and in combination in rats. Toxicology Letters. 2001;121(2):127–134. doi: 10.1016/s0378-4274(01)00330-7. [DOI] [PubMed] [Google Scholar]
- 47.Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 48.Schlenk D, Celander M, Gallagher EP, et al. Biotransformation in Fihes, the Toxicology of Fishes. Boca Raton, Fla, USA: CRC Press, Taylor and Francis Group; 2008. [Google Scholar]
- 49.Srikanth K, Pereira E, Duarte AC, Ahmad I. Glutathione and its dependent enzymes' modulatory responses to toxic metals and metalloids in fish—a review. Environmental Science and Pollution Research. 2013;20:2133–2149. doi: 10.1007/s11356-012-1459-y. [DOI] [PubMed] [Google Scholar]
- 50.San Juan-Reyes N, Gómez-Oliván LM, Galar-Martínez M, et al. Effluent from an NSAID-manufacturing plant in Mexico induces oxidative stress on Cyprinus carpio . Water, Air and Soil Pollution. 2013;224(article 1689) [Google Scholar]


