Abstract
Background: Vitamin D deficiency is associated with obesity; whether repletion supports weight loss and changes obesity-related biomarkers is unknown.
Objective: We compared 12 mo of vitamin D3 supplementation with placebo on weight, body composition, insulin, and C-reactive protein (CRP) in postmenopausal women in a weight-loss intervention.
Design: A total of 218 overweight/obese women (50–75 y of age) with serum 25-hydroxyvitamin D [25(OH)D] ≥10 ng/mL but <32 ng/mL were randomly assigned to weight loss + 2000 IU oral vitamin D3/d or weight loss + daily placebo. The weight-loss intervention included a reduced-calorie diet (10% weight loss goal) and 225 min/wk of moderate-to-vigorous aerobic activity. Mean 12-mo changes in weight, body composition, serum insulin, CRP, and 25(OH)D were compared between groups (intent-to-treat) by using generalized estimating equations.
Results: A total of 86% of participants completed the 12-mo measurements. The mean (95% CI) change in 25(OH)D was 13.6 (11.6, 15.4) ng/mL in the vitamin D3 arm compared with −1.3 (−2.6, −0.3) ng/mL in the placebo arm (P < 0.0001). Changes in weight [−7.1 (−8.7, −5.7) compared with –7.4 (−8.1, −5.4) kg], body mass index (in kg/m2: both −2.8), waist circumference [−4.9 (−6.7, −2.9) compared with −4.5 (−5.6, −2.6) cm], percentage body fat [−4.1 (−4.9, −2.9) compared with −3.5 (−4.5, −2.5)], trunk fat [−4.1 (−4.7, −3.0) compared with −3.7 (−4.3, −2.9) kg], insulin [−2.5 (−3.4, −1.7) compared with −2.4 (−3.3, −1.4) μU/mL], and CRP [−0.9 (−1.2, −0.6) compared with −0.79 (−0.9, −0.4) mg/mL] were similar between groups (all P > 0.05). Compared with women who achieved 25(OH)D <32 ng/mL, women randomly assigned to vitamin D who became replete (ie, 25(OH)D ≥32 ng/mL) lost more weight [−8.8 (−11.1, −6.9) compared with −5.6 (−7.2, −5.0) kg; P = 0.05], waist circumference [−6.6 (−9.3, −4.3) compared with −2.5 (−4.6, −2.0) cm; P = 0.02], and percentage body fat [−4.7 (−6.1, −3.5) compared with −2.6 (−3.9, −2.2); P = 0.04]. Among women with complete pill counts (97% adherence), the mean decrease in CRP was 1.18 mg/mL (46%) in the vitamin D arm compared with 0.46 mg/mL (25%) in the placebo arm (P = 0.03).
Conclusions: Vitamin D3 supplementation during weight loss did not increase weight loss or associated factors compared with placebo; however, women who became replete experienced greater improvements. This trial was registered at clinicaltrials.gov as NCT01240213.
INTRODUCTION
Animal studies support a role for vitamin D in energy regulation (1–3); however, its role in human metabolism remains less clear. In cross-sectional studies, serum 25-hydroxyvitamin D [25(OH)D]4 is inversely associated with obesity (4–7). One recent bidirectional genetic association study suggests that a higher BMI leads to lower 25(OH)D (8), whereas limited human experimental research indicates that vitamin D could potentiate weight loss and improvements in metabolic markers (9–11). However, most previous studies were of short duration and small sample size. One trial tested 12-mo vitamin D supplementation (3320 IU/d compared with placebo) during active weight loss in 200 humans and found no difference in weight change but greater decreases in parathyroid hormone, triglycerides, and the inflammatory marker tumor necrosis factor-α with vitamin D supplementation (10).
Whether weight loss through lifestyle intervention is influenced by vitamin D status remains unknown. A review of randomized clinical trials concluded that there is insufficient evidence to support the contention that vitamin D accelerates weight reduction or fat loss in obesity (12). Because only 2 studies included in the review were designed to test the effects of vitamin D on weight and body-composition changes as primary outcomes, randomized clinical trials to examine the effect of vitamin D on body fat were specifically recommended for future research (12).
Low vitamin D status has been associated with insulin resistance and increased levels of chronic inflammation (13–15)—2 mechanisms through which obesity appears to confer an excess risk of diabetes, cardiovascular disease, and certain cancers (16–18). If vitamin D can modify the metabolic alterations associated with obesity, then changes in circulating concentrations of vitamin D could modify obesity-disease relations.
Because obesity and vitamin D insufficiency are increasingly prevalent worldwide (19, 20), a better understanding of the nature of the relation between them remains an important area of investigation. The purpose of the current study was to investigate the effects of 12 mo of oral vitamin D3 supplementation (2000 IU/d) compared with placebo on changes in weight, body composition, and metabolic markers [insulin and C-reactive protein (CRP)] during a structured behavioral weight-loss program in overweight and obese postmenopausal women.
SUBJECTS AND METHODS
The Vitamin D, Diet and Activity (ViDA) study, conducted from September 2010 to August 2012 in Seattle, WA, was a 12-mo double-blind, placebo-controlled, randomized clinical trial with a parallel-group design that compared the effects of oral vitamin D3 supplementation (cholecalciferol, 2000 IU/d) with those of placebo on weight and related biomarkers in overweight and obese postmenopausal women. A dose of 2000 IU was chosen because, at the time of trial initiation, the Institute of Medicine recommended an upper limit of 2000 IU/d for vitamin D supplements (21). The primary outcome was weight loss. Secondary outcomes included changes in body composition (waist circumference, trunk fat, and percentage body fat) and serum biomarkers (insulin and CRP). All study procedures were reviewed and approved by the Fred Hutchinson Cancer Research Center Institutional Review Board in Seattle, WA, and all participants provided signed informed consent.
Participant recruitment and inclusion and exclusion criteria
The target population for the ViDA study included postmenopausal women from the greater Seattle, WA, area aged 50–75 y who were overweight or obese [BMI (in kg/m2) ≥25, or ≥23 for Asian-American women) and had serum 25(OH)D concentrations ≥10 ng/mL but <32 ng/mL. Specific exclusion criteria included the following: current use of >400 IU vitamin D from supplemental sources; diagnosis of osteoporosis or diabetes; renal disease or history of kidney stones; severe congestive heart failure; history of breast cancer or other invasive cancer, except for nonmelanomatous skin cancer; use of hormone replacement therapy within the past 6 mo; alcohol intake >2 drinks/d; current smoking; contraindication to taking 2000 IU vitamin D/d; current participation in a diet or exercise intervention; history of bariatric surgery; use of weight-loss medications; and additional factors that might interfere with measurement of outcomes or with the success of the intervention (eg, inability to attend facility-based sessions).
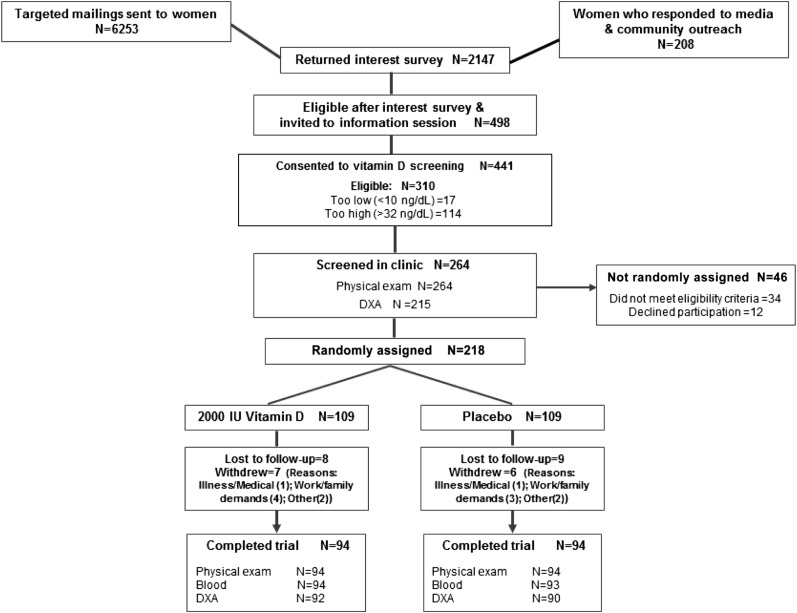
The women were recruited primarily through media publicity and mass mailings between September 2010 and August 2011. Invitation letters (n = 6253) were mailed to potentially eligible women identified from a database of previous study participants and women who had expressed an interest in our studies. In total, 2147 women returned an interest survey, 498 of whom met the initial eligibility criteria and were invited for vitamin D screening. Of these, 310 were eligible based on study 25(OH)D criteria (≥10.0 but <32.0 ng/mL). A total of 264 women underwent a clinic screening interview, and 218 women were randomly assigned into the study (Figure 1).
FIGURE 1.
Flow of participants through the Vitamin D, Diet and Activity study. DXA, dual-energy X-ray absorptiometry.
Study design and randomization
The study design for the ViDA trial is shown in Figure 1. After the baseline data were collected, eligible women were randomly assigned in a 1:1 ratio by permuted blocks into either a lifestyle-based weight-loss program + 2000 IU/d oral vitamin D3 or a lifestyle-based weight-loss program + daily placebo. The random assignment was generated by a computerized program, stratified according to BMI (<30 or ≥30) and consent for optional subcutaneous fat biopsies. Women were randomly assigned into the study by a blinded study coordinator. The number of women randomly assigned to each study arm did not differ by season (chi-square test: P > 0.999). All other study staff, except the study statisticians, was blind to the randomization status.
Power calculations were based on data from 235 overweight/obese women who participated in a previous weight-loss trial conducted by our group (22). On the basis of an assumed 10% dropout rate, 5% drop-in (control adopting equivalent of the intervention) rate (23), and 10% loss to follow-up, we estimated that a total sample size of 228 subjects (n = 114 for each arm) would provide 80% power to detect a 2.87-kg weight-loss difference between the placebo and vitamin D arms.
Vitamin D preparation and dose
The vitamin D preparation consisted of 2000 IU cholecalciferol (vitamin D3). Both the vitamin D and matching placebo (sunflower oil) gel capsules were created and bottled in unmarked containers by J.R. Carlson Laboratories Inc. Two matching bottles, each containing a 6-mo supply of capsules, were prepared for each participant. We randomly selected 10% of the study medication bottles for quality assurance, and the contents of the study capsules were verified at the Horst Laboratory (Heartland Assays). One hundred percent of the tested capsules matched the assigned content provided by the laboratory.
Weight-loss intervention
The ViDA lifestyle program included a diet and an exercise component, adapted from a successful intervention that we used in a similar population of overweight and obese postmenopausal women (22), and was based on the Diabetes Prevention Program and Look Ahead lifestyle change weight-loss programs (24, 25). The goals of the diet program were as follows: total daily energy intake of 1200 to 2000 kcal/d based on baseline weight, <30% daily energy intake from fat, and a 10% reduction in body weight by 6 mo with maintenance thereafter to 12 mo. The diets were not supplemented with calcium, but the women were advised on how to obtain sufficient calcium in their diets. The diet intervention was led by registered dietitians with extensive training in behavior modification. Participants met individually with a study dietitian for personalized goal setting at the start of the program, followed by weekly meetings in groups of ∼5 to 25 women for 6 mo. Thereafter (months 7–12), women attended monthly support groups facilitated by a study dietitian. This combination of individual and group-based sessions was used to maximize the benefits of targeted personalized recommendations along with the social support and greater cost-effectiveness of a group setting. The diet counseling sessions instructed participants on how to achieve the target calorie reduction, including setting calorie and fat goals, food intake and calorie counts of foods, reducing fat and improving fiber intake, self-monitoring, and coping with challenges to eating behavior changes. In addition, the women were asked to record all food eaten daily for ≥6 mo or until they reached their individual weight loss goal (10%). Thereafter, the women were encouraged to keep a food journal for ≥1 wk/mo. Food journals were collected weekly. Journaling, weekly weigh-ins, and session attendance were tracked to promote adherence to the diet intervention.
The goal of the exercise program was ≥45 min of moderate-to-vigorous intensity exercise 5 d/wk week (225 min/wk) for 12 mo. The women attended 2 sessions/wk at our study facility, where they were supervised by an exercise physiologist, and performed their remaining sessions at home. Facility-based exercise consisted of treadmill walking or jogging, stationary bicycling, and use of other aerobic machines; a variety of home exercises were encouraged, including walking/hiking, aerobics, and bicycling. The exercise training program began with 15-min sessions at 60–70% of the age-predicted maximal heart rate and progressed to the target 70–85% maximal heart rate for 45 min by the seventh week after enrollment, when it was maintained for the remainder of the study. The women wore heart rate monitors (Polar Electro) during the exercise sessions at the facility to assist with attaining their target heart rate. They recorded the mode and duration of all exercise sessions and the peak heart rate achieved for each facility sessions and their relative perceived exertion by using a Borg CR10 scale (26) for home sessions. The women were also offered the use of a pedometer throughout the exercise intervention and were encouraged to track their steps daily. Activities of ≥4 metabolic equivalents according to the Compendium of Physical Activities (27) were counted toward the prescribed exercise target.
Study measures and data collection
At baseline (before randomization) and 12 mo, the participants completed a series of questionnaires to assess demographic information, medical history, health habits, sun exposure, reproductive and body weight history, psychosocial attributes, quality of life, dietary intake (with a 120-item self-administered food-frequency questionnaire) (28) and supplement use, and physical activity patterns via the International Physical Activity Questionnaire (29). The participants also wore pedometers (H215-S; Bestek Electronics) while awake for 7 consecutive days at baseline and 12 mo to determine an average daily step count.
Anthropometric measures were taken at baseline and 12 mo by using standard methods previously described (22). BMI was calculated. Waist circumference was measured at the end of normal expiration in the horizontal plane at the iliac crest, by using a fiberglass tape, and rounded to the nearest 0.5 cm; this measure was taken in duplicate and averaged. Total and percentage body fat and trunk fat mass were measured at baseline and 12 mo with a dual-energy X-ray absorptiometry whole-body scanner (GE Lunar) while the participants were in a supine position.
Blood measures and assays
Venous blood samples (50 mL) were collected during clinic visits before randomization and at 12 mo after a 12-h fast, after 24 h without exercise, and after 48 h of no alcohol consumption. Because baseline serum 25(OH)D was used to determine study eligibility, this analyte was measured in a nonfasting blood sample. However, fasting status does not appreciably affect this analyte (30). Blood samples were processed within 1 h and stored at −70°C until the assays were performed.
Blood samples were analyzed in batches such that each participant's samples were assayed simultaneously, the number of samples from each randomization arm were approximately equal, participant randomization dates were similar, and sample order was random.
Vitamin D was measured at Heartland Assays, Inc with a direct, competitive chemiluminescence immunoassay by using the DiaSorin LIAISON 25-OH Vitamin D Total Assay, which is co-specific for 25-hydroxyvitamin D and 25(OH)D2 (31, 32). The inter- and intraassay CVs for this assay were 11.2% and 8.1%, respectively.
Insulin was analyzed at the University of Washington Northwest Lipid Research Laboratories, and quantified by a 48-h, polyethylene glycol-accelerated, double-antibody radioimmunoassay. The intra- and interassay CVs were 6.5% and 9.3%, respectively. CRP was assayed on a Roche Mira Plus Chemistry Analyzer by using a Genzyme CRP Ultra Wide Range Reagent at the University of Washington Immunology Laboratory. Inter- and intraassay CVs were 2.84% and 3.97%, respectively. Extremely high CRP values (≥20 mg/L) at either baseline or 12 mo were excluded (n = 8) as outliers because these values may be a result of transitory events such as acute infections (33).
Study medication adherence
At randomization, participants received a 6-mo supply of study medications. Medication bottles were returned at 6 mo, and the remaining capsules were counted before a second 6-mo supply was provided. Likewise, at 12 mo, the second bottle and any remaining capsules were returned and counted. Medication counts and the 12-mo change in serum vitamin D were used as indicators of study medication adherence.
Safety and adverse effects
For the safety of participants, all women were interviewed after 1, 3, 6, 9, and 12 mo of study participation for any signs or symptoms of vitamin D toxicity or other adverse events, including serious illness or hospitalizations. Reports were reviewed by a physician's assistant with appropriate follow-up as necessary. Summary data were recorded and reviewed according to study group by an independent Data and Safety Monitoring Committee at 6-mo intervals.
Statistical analysis
Because of their nonnormal distribution, blood measures were log transformed. These data are presented as geometric means (and 95% CIs). Pearson correlation coefficients were calculated between baseline anthropometric measures, body-composition measures, blood measures, vitamin D and calcium intakes, average sun exposure, and serum 25(OH)D concentrations.
Mean changes in weight, anthropometric variables, body composition, insulin, and CRP from baseline to 12 mo, stratified by study arm, were computed. The intervention effect on these variables was examined based on the assigned treatment at randomization, regardless of adherence or study retention (ie, intent-to-treat). Mean 12-mo changes in the vitamin D3 group were compared with placebo by using the generalized estimating equation modification of linear regression to account for intraindividual correlation over time. The models were initially unadjusted but then subsequently adjusted for age, race-ethnicity (white or other), baseline BMI, baseline serum 25(OH)D, vitamin D and calcium intakes (diet + supplements), average sun exposure, and percentage weight loss (insulin and CRP only). The generalized estimating equations approach for mixed-model regression using the available data was applied to address missing data. Additional analyses were based on post hoc analyses of specific subgroups.
Among women randomly assigned to receive daily vitamin D3, the 12-mo change in serum 25(OH)D was used to predict the observed change in outcome variables by linear regression. These analyses also included potential confounders, as listed above. Changes in outcome measures were also compared across tertiles of change in serum 25(OH)D in women with and without complete pill counts and separately in overweight compared with obese women. Analyses were performed by using SAS software version 9.2.
RESULTS
Participants
The baseline characteristics of randomized women are shown in Table 1. Participants had a mean ± SD age of 59.6 ± 5.1 y. On average, they were obese (BMI: 32.4 ± 5.8) and had a high percentage body fat (47.4 ± 4.9). Most of the participants were non-Hispanic white (86%) and college graduates (76%). At 12 mo, 188 (86%) of 218 women completed physical examinations, 187 (86%) provided a blood sample, and 182 (83%) underwent a dual-energy X-ray absorptiometry scan; 30 did not complete the study (Figure 1).
TABLE 1.
Selected baseline characteristics of study participants
| Variable | All (n = 218) | Vitamin D (n = 109) | Placebo (n = 109) |
| Age (y) | 59.6 ± 5.11 | 60.3 ± 5.3 | 59.0 ± 4.7 |
| Weight (kg) | 87.7 ± 16.3 | 87.4 ± 15.5 | 88.1 ± 17.1 |
| BMI (kg/m2) | 32.4 ± 5.8 | 32.3 ± 5.5 | 32.5 ± 6.1 |
| Waist circumference (cm) | 100.1 ± 12.3 | 100.0 ± 11.0 | 100.3 ± 13.5 |
| Body fat (%) | 47.4 ± 4.9 | 47.3 ± 5.2 | 47.5 ± 4.5 |
| Race-ethnicity [n (%)] | |||
| Non-Hispanic white | 188 (86.2) | 94 (86.2) | 94 (86.2) |
| Non-Hispanic black | 13 (6.0) | 7 (6.4) | 6 (5.5) |
| Hispanic | 5 (2.3) | 1 (0.9) | 4 (3.7) |
| Other2 | 12 (5.5) | 7 (6.4) | 5 (4.6) |
| College graduate [n (%)] | 161 (73.9) | 82 (75.2) | 79 (72.5) |
| Moderate to vigorous physical activity (min/wk) | 142.2 ± 143.2 | 137.9 ± 146.5 | 146.6 ± 140.4 |
| Energy intake (kcal/d)3 | 2004 ± 699.3 | 2025 ± 722 | 1982 ± 678 |
| Relative energy from fat (%) | 33.0 ± 6.2 | 33.4 ± 6.7 | 32.6 ± 5.7 |
| Relative energy from protein (%) | 17.6 ± 3.2 | 17.2 ± 2.9 | 17.9 ± 3.5 |
| Relative energy from carbohydrate (%) | 48.3 ± 7.4 | 48.5 ± 7.8 | 48.1 ± 7.1 |
| Dietary vitamin D intake (μg) | 6.6 ± 4.6 | 6.3 ± 4.0 | 6.9 ± 5.2 |
| Vitamin D supplement intake (IU) | 332.4 ± 106.2 | 262.7 ± 140.5 | 303.6 ± 125.2 |
| Total calcium intake, diet + supplement (mg) | 1120 ± 600 | 1170 ± 633 | 1071 ± 564 |
| Sun exposure (h/wk)4 | 2.4 ± 1.3 | 2.2 ± 1.3 | 2.5 ± 1.3 |
| Serum 25(OH)D5 (ng/mL) | 21.4 ± 6.1 | 21.4 ± 6.1 | 21.4 ± 6.2 |
Mean ± SD (all such values).
Includes American Indian, Asian, or unknown.
Values derived from a food-frequency questionnaire were truncated to <600 and >4000 kcal. Independent t tests showed no significant differences in baseline measures between groups.
Calculated based on average exposure between 1000 and 1600; reported separately for weekdays and weekends.
25(OH)D, 25-hydroxyvitamin D.
Habitual sun exposure was assessed by a series of questions for which categorical response options were provided (34). No significant difference was found between study arms in the change in average hours/d of sun exposure over 12 mo (P = 0.11). At baseline, the vitamin D arm reported a mean 2.2 h/d of sun exposure; the placebo group reported a mean of 2.5 h/d. At 12 mo, both groups reported a mean sun exposure of 2.0 h/d. Approximately 50% of the women in both arms reported wearing sunscreen “often” or “always” (placebo arm: 54%; vitamin D3 arm: 47%) (see Supplemental Table 1 under “Supplemental data” in the online issue).
Adherence to interventions
Women in the vitamin D3 arm attended a mean 56.1% of all diet counseling sessions and returned 24% of weekly diet journals, whereas women in the placebo arm attended a mean 59.5% of diet sessions and returned 25% of their weekly diet journals.
The 12-mo change in vitamin D intake from dietary sources and supplements did not differ between study arms (P = 0.60) (see Supplemental Table 1 under “Supplemental data” in the online issue), nor did any other major component of dietary intake, with the exceptions of dietary protein (1.5 compared with 0.3 g/d; P = 0.006) and omega-3 (n−3) fatty acids (−0.4 compared with −0.1 g/d; P = 0.02) (see Supplemental Table 2 under “Supplemental data” in the online issue).
Women randomly assigned to the vitamin D3 arm completed a mean (±SD) of 138 ± 147) min/wk of moderate-to-vigorous physical activity, whereas the placebo group completed a mean (±SD) of 147 ± 140 min/wk of activity. Both groups increased their average pedometer steps/d (+2602 and +2816 steps/d, respectively) compared with baseline. No significant differences in adherence to the weight-loss program were detected between study arms.
Complete (6 and 12 mo) study medication counts were obtained for 120 women [vitamin D3 arm: n = 59 (54%); placebo arm: n = 61 (56%)]. Of those with medication counts available, medication adherence was 97.9% in the vitamin D3 arm and 95.8% in the placebo arm. In all participants, regardless of medication count availability, serum 25(OH)D decreased by a mean of 1.3 ng/mL in the placebo arm and increased by a mean of 13.6 ng/mL in the vitamin D3 arm over 12 mo (P < 0.0001).
Baseline associations
At baseline, the serum 25(OH)D concentration was not significantly correlated with age (−0.07, P = 0.31), BMI (−0.06, P = 0.40), percentage body fat (−0.09, P = 0.20), insulin (−0.07, P = 0.28), or CRP (0.01, P = 0.87). Baseline 25(OH)D was significantly correlated with average hours of sun exposure per day (0.79, P = 0.02) and vitamin D intake from diet and supplemental sources (0.21, P = 0.004).
Intervention effects
At 12 mo, the mean weight change was −8.2% in the vitamin D3 group compared with −8.4% in the placebo arm (P = 0.41). Changes in waist circumference (−4.9 cm compared with −4.5 cm; P = 0.42), percentage body fat (−4.1% compared with −3.5%; P = 0.70), and trunk fat mass (−4.1% compared with −3.7%; P = 0.70) were also similar between the 2 groups (Table 2). Insulin and CRP were reduced in both groups; however, the magnitude of the improvement was not significantly different between the vitamin D and placebo groups (both P > 0.1) (Table 2).
TABLE 2.
Twelve-month changes in serum 25(OH)D, weight, anthropometric measures, body composition, and metabolic markers by study arm1
| Placebo |
Vitamin D3 |
|||||||||||||
| n | Baseline | n | 12 mo | Change | Percentage | n | Baseline | n | 12 mo | Change | Percentage | P2 | P3 | |
| Serum 25(OH)D (ng/mL) | 109 | 21.4 ± 6.14 | 94 | 20.1 ± 6.7 | −1.3 | −6.2 | 109 | 21.4 ± 6.2 | 93 | 35.0 ± 9.4 | 13.6 | 63.4 | <0.0001 | <0.0001 |
| Weight (kg) | 109 | 88.1 ± 17.1 | 94 | 80.7 ± 17.6 | −7.4 | −8.4 | 109 | 87.4 ± 15.5 | 94 | 80.2 ± 15.6 | −7.1 | −8.2 | 0.66 | 0.41 |
| BMI (kg/m2) | 109 | 32.5 ± 6.1 | 94 | 29.7 ± 6.1 | −2.8 | −8.8 | 109 | 32.3 ± 5.5 | 94 | 29.5 ± 5.6 | −2.8 | −8.7 | 0.67 | 0.58 |
| Waist circumference (cm) | 109 | 100.3 ± 13.5 | 94 | 95.8 ± 13.8 | −4.5 | −4.5 | 109 | 100.0 ± 11.0 | 93 | 95.1 ± 12.7 | −4.9 | −4.9 | 0.58 | 0.42 |
| Hip circumference (cm) | 109 | 116.7 ± 12.7 | 94 | 110.8 ± 13.1 | −5.9 | −5.1 | 109 | 116.4 ± 11.7 | 93 | 109.8 ± 14.4 | −6.6 | −5.7 | 0.34 | 0.35 |
| Body fat (%) | 107 | 47.5 ± 4.5 | 92 | 44.0 ± 7.0 | −3.5 | −7.4 | 108 | 47.3 ± 5.2 | 90 | 43.1 ± 7.5 | −4.1 | −8.7 | 0.58 | 0.70 |
| Trunk fat mass (kg) | 107 | 24.2 ± 6.0 | 92 | 20.5 ± 6.9 | −3.7 | −15.3 | 108 | 23.7 ± 6.2 | 90 | 19.6 ± 6.6 | −4.1 | −17.1 | 0.6 | 0.70 |
| Insulin (μU/mL)5 | 109 | 11.06 (10.0, 12.3) | 94 | 8.66 (7.7, 9.7) | −2.40 | −21.7 | 109 | 10.86 (9.9, 11.9) | 93 | 8.38 (7.5, 9.3) | −2.47 | −22.8 | 0.61 | 0.89 |
| CRP (mg/mL)5 | 106 | 2.18 (1.8, 2.7) | 93 | 1.48 (1.2, 1.9) | −0.70 | −32.3 | 106 | 2.29 (1.9, 2.8) | 92 | 1.39 (1.1, 1.8) | −0.90 | −39.3 | 0.19 | 0.16 |
CRP, C-reactive protein; 25(OH)D, 25-hydroxyvitamin D.
Derived from a general estimating equation model that compared the 12-mo change between vitamin D and placebo; all available data, unadjusted.
Adjusted for age, race-ethnicity, baseline serum 25(OH)D, total vitamin D intake (diet + supplement), total calcium intake (diet + supplement), sun exposure (h/d), and percentage weight loss (for insulin and CRP only).
Mean ± SD (all such values).
Log-transformed variables are presented as geometric means (95% CIs).
The between-group differences in weight change, body composition, and blood measures did not differ significantly according to baseline 25(OH)D (<20 or 20–32 ng/mL) (28) or BMI (<30 or ≥30) (data not shown).
However, in the vitamin D3 arm, the tertile of change in serum 25(OH)D over 12 mo was significantly inversely associated with the magnitude of change in weight (P-trend = 0.003), BMI (P-trend = 0.002), percentage body fat (P-trend = 0.01), trunk fat mass (P-trend = 0.006), insulin (P-trend = 0.04), and CRP (P-trend = 0.02), although the strength of the associations were attenuated to nonsignificance, with the exception of trunk fat mass (P-trend = 0.02), after adjustment for age, race-ethnicity, baseline BMI, baseline serum 25(OH)D, vitamin D and calcium intakes (diet + nonstudy supplements), sun exposure, and percentage weight loss (insulin and CRP only) (Table 3).
TABLE 3.
Baseline and 12-mo outcome measures in the placebo group and stratified by tertile of change in serum 25(OH)D in women receiving 2000 IU vitamin D3/d (n = 109)1
| Baseline | 12 mo | Change | P2 | P2 | P3 | |
| % | ||||||
| Weight (kg) | ||||||
| Placebo | 88.1 ± 17.14 | 80.7 ± 17.6 | −8.4 | Ref | ||
| Tertile 1 | 89.7 ± 17.4 | 85.2 ± 17.8 | −5.1 | 0.04 | Ref | Ref |
| Tertile 2 | 86.0 ± 15.1 | 78.4 ± 13.5 | −8.8 | 0.61 | 0.06 | 0.16 |
| Tertile 3 | 86.5 ± 13.4 | 76.9 ± 14.5 | −11.1 | 0.08 | 0.003 | 0.05 |
| P-trend | 0.10 | 0.003 | 0.06 | |||
| BMI (kg/m2) | ||||||
| Placebo | 32.5 ± 6.1 | 29.7 ± 6.1 | −8.8 | Ref | ||
| Tertile 1 | 32.8 ± 5.5 | 31.1 ± 5.7 | −5.1 | 0.03 | Ref | Ref |
| Tertile 2 | 31.4 ± 6.0 | 28.6 ± 5.3 | −8.9 | 0.63 | 0.06 | 0.21 |
| Tertile 3 | 32.4 ± 5.0 | 28.9 ± 5.7 | −11.0 | 0.08 | 0.002 | 0.07 |
| P-trend | 0.10 | 0.002 | 0.07 | |||
| Waist circumference (cm) | ||||||
| Placebo | 100.3 ± 13.5 | 95.8 913.8) | −4.5 | Ref | ||
| Tertile 1 | 100.1 ± 12.1 | 97.0 ± 16.2 | −3.2 | 0.69 | Ref | Ref |
| Tertile 2 | 97.8 ± 10.6 | 93.0 ± 10.4 | −4.9 | 0.65 | 0.54 | 0.87 |
| Tertile 3 | 101.6 ± 10.10 | 95.2 ± 10.6 | −6.3 | 0.11 | 0.21 | 0.6 |
| P-trend | 0.15 | 0.21 | 0.58 | |||
| Hip circumference (cm) | ||||||
| Placebo | 116.7 ± 12.7 | 110.8 ± 13.1 | −51 | Ref | ||
| Tertile 1 | 117.3 ± 12.6 | 112.3 ± 18.5 | −4.2 | 0.8 | Ref | Ref |
| Tertile 2 | 115.2 ± 11.7 | 108.0 ± 11.8 | −6.3 | 0.33 | 0.41 | 0.76 |
| Tertile 3 | 117.0 ± 11.3 | 109.2 ± 11.9 | −6.7 | 0.07 | 0.25 | 0.63 |
| P-trend | 0.06 | 0.25 | 0.62 | |||
| Body fat (%) | ||||||
| Placebo | 47.5 ± 4.5 | 44.0 ± 7.0 | −7.4 | Ref | ||
| Tertile 1 | 47.4 ± 5.2 | 45.3 ± 6.7 | −4.4 | 0.09 | Ref | Ref |
| Tertile 2 | 45.4 ± 5.7 | 40.9 ± 8.5 | −9.9 | 0.46 | 0.06 | 0.12 |
| Tertile 3 | 48.0 ± 4.6 | 42.9 ± 6.8 | −10.5 | 0.14 | 0.009 | 0.04 |
| P-trend | 0.13 | 0.01 | 0.05 | |||
| Trunk fat mass (kg) | ||||||
| Placebo | 24.2 ± 6.0 | 20.5 ± 6.9 | −15.3 | Ref | ||
| Tertile 1 | 23.8 ± 6.5 | 21.4 ± 6.4 | −10.0 | 0.04 | Ref | Ref |
| Tertile 2 | 22.3 ± 6.4 | 17.9 ± 6.6 | −19.6 | 0.40 | 0.02 | 0.05 |
| Tertile 3 | 24.5 ± 5.5 | 19.5 ± 6.6 | −20.5 | 0.12 | 0.005 | 0.02 |
| P-trend | 0.10 | 0.006 | 0.02 | |||
| Insulin (μU/mL) | ||||||
| Placebo | 11.06 (10.1, 12.3)5 | 8.66 (7.7, 9.7) | −21.7 | Ref | ||
| Tertile 1 | 11.29 (9.3, 13.8) | 10.06 (8.4, 12.1) | −10.9 | 0.21 | Ref | Ref |
| Tertile 2 | 10.47 (8.8, 12.4) | 7.15 (6.0, 8.5) | −31.7 | 0.11 | 0.02 | 0.04 |
| Tertile 3 | 11.49 (10.1, 13.1) | 8.19 (6.8, 9.9) | −28.7 | 0.25 | 0.04 | 0.48 |
| P-trend | 0.12 | 0.04 | 0.56 | |||
| CRP (mg/mL) | ||||||
| Placebo | 2.33 (1.9, 2.9) | 1.54 (1.2, 2.0) | −33.9 | Ref | ||
| Tertile 1 | 1.86 (1.2, 2.8) | 1.31 (0.9, 2.0) | −29.5 | 0.96 | Ref | Ref |
| Tertile 2 | 1.92 (1.3, 2.8) | 1.38 (0.9, 2.1) | −28.0 | 0.85 | 1.00 | 0.07 |
| Tertile 3 | 3.82 (2.5, 5.9) | 1.65 (1.1, 2.6) | −56.8 | 0.004 | 0.02 | 0.91 |
| P-trend | 0.02 | 0.02 | 0.76 |
A general estimating equation model was used to compare the 12-mo change between groups. Tertile 1: n = 31, 25(OH)D <9.43 ng/mL; tertile 2: n = 31, 25(OH)D 9.43–16.65 ng/mL; tertile 3: n = 31, 25(OH)D ≥16.65 ng/mL. CRP, C-reactive protein; Ref, reference; 25(OH)D, 25-hydroxyvitamin D.
Unadjusted.
Adjusted for age, race-ethnicity, baseline BMI, baseline serum 25(OH)D, vitamin D intake (diet + nonstudy supplements), total calcium intake (diet + supplements), sun exposure (h/d), and percentage weight loss (insulin and CRP only).
Mean ± SD (all such values).
Geometric mean; 95% CI in parentheses (all such values).
Compared with women who did not become replete despite vitamin D3 supplementation, women who became replete [ie, 25(OH)D ≥32 ng/mL] by 12 mo lost more weight (−9.9% compared with −6.2%; P = 0.05), had a greater waist circumference (−6.6 cm compared with −2.5 cm; P = 0.02), and had a greater percentage body fat (−10.1% compared with −5.5%; P = 0.04). These findings were not substantially altered after adjustment of the model for relevant covariates; however, only changes in waist circumference remained statistically significant, whereas changes in BMI became statistically significant (Table 4).
TABLE 4.
Change in weight, anthropometric measures, body composition, and metabolic markers in women randomly assigned to vitamin D treatment who did or did not become 25(OH)D replete (≥32 ngm/L) by 12 mo1
| 25(OH)D <32 ng/mL |
25(OH)D ≥32 ng/mL |
|||||||||||
| n | Baseline | n | 12 mo | Percentage | n | Baseline | n | 12 mo | Percentage | 2 | 3 | |
| Serum 25(OH)D (ng/mL) | 40 | 19.2 ± 5.54 | 40 | 26.4 ± 4.6 | 37.2 | 53 | 23.5 ± 5.7 | 53 | 41.5 ± 6.5 | 76.8 | <0.001 | <0.001 |
| Weight (kg) | 40 | 89.7 ± 16.6 | 40 | 84.1 ± 16.7 | −6.2 | 53 | 85.7 ± 14.2 | 53 | 77.2 ± 14.3 | −9.9 | 0.05 | 0.07 |
| BMI (kg/m2) | 40 | 33.1 ± 5.8 | 40 | 31.0 ± 5.7 | −6.3 | 53 | 31.5 ± 5.1 | 53 | 28.4 ± 5.3 | −9.9 | 0.06 | 0.02 |
| Waist circumference (cm) | 40 | 100.9 ± 12.2 | 40 | 98.4 ± 11.6 | −2.5 | 53 | 99.0 ± 10.0 | 53 | 92.5 ± 12.9 | −6.6 | 0.03 | 0.03 |
| Hip circumference (cm) | 40 | 117.4 ± 11.9 | 40 | 113.2 ± 13.0 | −3.6 | 53 | 115.8 ± 11.8 | 53 | 107.3 ± 15.0 | −7.4 | 0.02 | 0.03 |
| Body fat (%) | 39 | 47.7 ± 5.4 | 36 | 45.1 ± 7.9 | −5.5 | 53 | 46.3 ± 5.1 | 53 | 41.7 ± 7.0 | −10.1 | 0.04 | 0.16 |
| Trunk fat mass (kg) | 39 | 24.1 ± 6.4 | 36 | 20.9 ± 6.6 | −13.1 | 53 | 23.1 ± 6.0 | 53 | 18.7 ± 6.5 | −19.1 | 0.10 | 0.20 |
| Insulin (μU/mL)5 | 40 | 11.43 (9.8, 13.4) | 40 | 8.95 (7.6, 10.6) | −21.7 | 53 | 10.81 (9.6, 12.2) | 53 | 7.98 (7.0, 9.2) | −26.2 | 0.53 | 0.88 |
| CRP (mg/mL)5 | 40 | 1.75 (1.3, 2.4) | 40 | 1.20 (0.8, 1.7) | −31.5 | 51 | 2.69 (2.0, 3.6) | 52 | 1.56 (1.1, 2.1) | −42.1 | 0.20 | 0.62 |
CRP, C-reactive protein; 25(OH)D, 25-hydroxyvitamin D.
A general estimating equation model was used to compare the 12-mo change between women who became replete (≥32 ng/mL) and those who did not; all available data, unadjusted.
Adjusted for age, race-ethnicity, baseline BMI, baseline serum 25(OH)D, total vitamin D intake (diet + supplements), and total calcium intake (diet + supplements).
Mean ± SD (all such values).
Log-transformed variables are presented as geometric means (95% CIs).
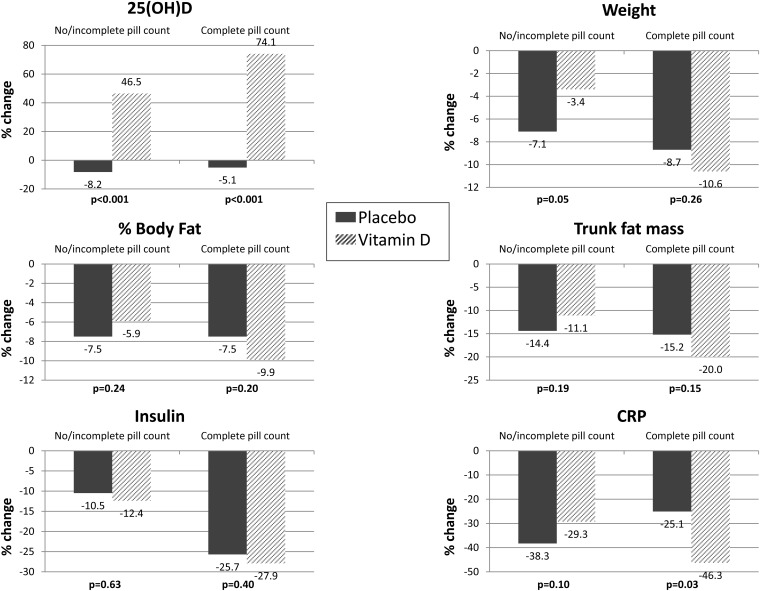
Among women with complete pill counts available, in whom study medication adherence was 97%, CRP decreased by a mean of 1.18 mg/mL (46%) in the vitamin D3 and by a mean of 0.46 mg/mL (25%) in the placebo arm (P = 0.03). No statistically significant differences in insulin or body composition were found between the groups (Figure 2).
FIGURE 2.
Change in serum 25(OH)D, weight, body composition, and metabolic markers among women with and without complete study pill counts. CRP, C-reactive protein; 25(OH)D, 25-hydroxyvitamin D.
Adverse effects
No serious adverse events related to vitamin D3 supplementation or to participation in the weight-loss program were found between groups. However, at 12-mo, 7 women receiving vitamin D3 supplementation had a 25(OH)D concentration >50 ng/mL, which is potentially harmful (30). Nonserious adverse events reported by 1–4 women in both groups included lightheadedness, severe headaches, nausea, rash/hives, weakness/numbness, and constipation.
DISCUSSION
Vitamin D has multiple physiologic functions beyond its classically recognized role in calcium homeostasis and bone metabolism. Vitamin D receptors are found in >30 cell types, including adipocytes (35), and a growing body of evidence has implicated vitamin D status in a range of adverse health conditions (6, 36–39) in addition to premature mortality from all causes, cardiovascular disease, and cancer (40).
Despite previous observations of reduced serum vitamin D with increased adiposity (4–7), vitamin D supplementation of 2000 IU/d in women with insufficient concentrations at baseline had no overall effect on weight or fat loss in postmenopausal women consuming a calorie-restricted diet and following an exercise program. Our findings are consistent with those of Zittermann et al (10), who reported no difference in weight change among 200 overweight participants with a mean 25(OH)D concentration of 12 ng/mL (30 nmol/L) who received 83 μg/d (3320 IU) vitamin D3 or placebo for 12 mo while participating in a weight-loss program.
Our findings are also consistent with those of previous placebo-controlled studies that tested vitamin D3 supplementation, without a weight-loss program, on measures of adiposity. For example, Sneve et al (41) saw no effect of either 20,000 IU vitamin D3 twice per week or 20,000 IU once per week for 12 mo on changes in weight, waist-to-hip ratio, or percentage body fat in 334 overweight and obese men and women (21–70 y of age) with a mean baseline 25(OH)D concentration of 53.1 nmol/L (21.2 ng/mL). More recently, Wamberg et al (42) reported that 26 wk of vitamin D supplementation (7000 IU/d) in 26 obese adults (18–50 y of age) did not change body fat, subcutaneous fat, visceral fat, or intramyocellular lipids compared with placebo, nor did vitamin D treatment affect insulin resistance or several inflammatory markers, including CRP and IL-6. However, the authors did acknowledge the potential for statistical type 2 error given the small sample size.
In contrast, Nagpal et al (11) reported improved insulin sensitivity in 100 men given 3 high doses (120,000 IU) of vitamin D over 6 wk compared with placebo, whereas von Hurst et al (43) reported a significant improvement in insulin resistance among insulin-resistant women, despite no effect on body weight or CRP after 6 mo of daily 4000 IU vitamin D supplementation. Interestingly, insulin resistance was most improved when endpoint serum 25(OH)D reached ≥32 ng/mL. Although we did not measure insulin resistance directly, we did not observe a significant overall or repletion effect [reaching 25(OH)D ≥32 ng/mL] of vitamin D on fasting serum insulin or on CRP. We did observe greater improvements in insulin and CRP with greater increases in serum vitamin D, which suggested that a dose-response relation could exist; however, this effect was no longer significant after adjustment for percentage weight loss.
It remains unknown whether vitamin D–related outcomes are more strongly related to a specific magnitude of change in 25(OH)D (eg, 10 ng/mL) or to a change in status defined by reaching a specific threshold concentration (eg, >32 ng/mL). However, few studies of vitamin D and weight loss have specifically examined these issues. Our observation that women randomly assigned to vitamin D supplementation who became replete [ie, 25(OH)D ≥32 ng/mL] lost more weight and had greater improvements in body composition compared with women who did not become replete suggests a potential threshold effect and highlights the importance of considering changes in nutrient status rather than only the mean magnitude of change (44). Supplementation that does not alter nutrient status may be insufficient to detect an effect; thus, consistent with the argument recently put forward by Heaney (44), the nutrient response curve should be carefully considered when designing future clinical trials to test nutrient effects. Our observations also suggest that greater effects may be detected in future studies with the ability to individualize supplementation such that a higher proportion of the study sample becomes replete.
When we limited our analysis to women with complete pill counts, among whom adherence to study medications was 97%, we observed a significantly greater decrease in CRP with vitamin D than with placebo, after adjustment for percentage weight loss. Thus, low pill compliance in a subset of study participants may have diminished the strength of the observed outcomes and suggests that future study designs to test vitamin D effects should use methods to optimize study medication adherence, such as once-weekly dosing, medication diaries, reminder phone or electronic messaging, electronic pill bottle counting, and more frequent clinic visits for monitoring and promoting compliance (45, 46).
It remains unclear to what extent low vitamin D status is a consequence of obesity or is some way involved in its etiology. In a similar sample of women, we previously published that 12 mo of dietary weight loss and/or aerobic exercise yielding a weight loss of >15% resulted in significantly increased serum 25(OH)D (47). However, on the basis of our current findings, vitamin D supplementation at 2000 IU/d does not, on average, increase weight loss during calorie restriction and exercise and does not improve insulin or CRP independently of weight loss. Whether higher doses of vitamin D would yield significant effects, or whether significant effects would be evident among women with more severe vitamin D deficiency (eg, <10 ng/mL), will need to be examined in future studies. Because our results indicate that women whose concentrations increased by ≥16.7 ng/mL by 12 mo did experience a significant reduction in insulin and CRP, the effect of individualizing vitamin D therapy to repletion at specific levels also warrants further investigation. Careful consideration not only of the participants’ baseline status but also of changes in vitamin D status resulting from a given intervention will help future studies continue to elucidate the role of vitamin D in weight loss and other outcomes.
This study had several limitations. First, our sample did not include women with a 25(OH)D concentration <10 ng/mL, among whom the potential effects of vitamin D supplementation on weight loss could be the greatest. We did not measure parathyroid hormone or additional biomarkers related to insulin metabolism or inflammation, which could have helped elucidate the potential mechanisms through which vitamin D exerts its physiologic roles. Only 55% of women had complete pill counts for the entire 12-mo study duration. We tested only one dose of vitamin D (2000 IU), and we did not test the independent effects of vitamin D without a weight-loss intervention. The degree to which our findings may be generalized to nonwhite populations, and populations considered truly vitamin D deficient [ie, 25(OH)D <12 ng/mL] (30), is not known. Finally, all significant results are based on post hoc analyses of some subgroups, and thus results are hypothesis-generating for future studies but do not prove significant vitamin D effects.
The strengths of this study included the double-blind, randomized, placebo-controlled design—which allows for causative conclusions—and the large sample size and long duration (≥12 mo) relative to previous studies. Although epidemiologic evidence indicates that higher concentrations of circulating 25(OH) is associated with less weight gain (7) and a lower risk of developing obesity-related metabolic complications (48, 49), 2000 IU daily vitamin D supplementation does not increase weight loss or improve fasting insulin or CRP concentrations in overweight and obese women with suboptimal 25(OH)D concentrations, although there is a suggestion of a potential dose-response effect on some markers.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—CM and AM: full access to the data and responsibility for the integrity of the data and the accuracy of the analysis; CM, II, CD, C-YW, and AM: study concept and design; CM, II, CD, and AM: acquisition of the data; CM, LX, II, C-YW, and AM: analysis and interpretation of the data; CM: drafting of the manuscript; II, CD, C-YW, LK, and AM: critical revision of the manuscript for important intellectual content; LX and CM: statistical analysis; AM: funding and study supervision; and LX: administrative, technical, or material support. None of the authors disclosed any conflicts of interest.
Footnotes
Abbreviations used: CRP, C-reactive protein; ViDA, Vitamin D, Diet and Activity; 25(OH)D, 25-hydroxyvitamin D.
REFERENCES
- 1.Wong KE, Szeto FL, Zhang W, Ye H, King J, Zhang Z, Sun XJ, Li YC. Involvement of the vitamin D receptor in energy metabolism: regulation of uncoupling proteins. Am J Physiol Endocrinol Metab 2009;296:E820–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Narvaez CJ, Matthews D, Broun E, Chan M, Welsh J. Lean phenotype and resistance to diet-induced obesity in vitamin D receptor knockout mice correlates with induction of uncoupling protein-1 in white adipose tissue. Endocrinology 2009;150:651–6124(7):644–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin Y, Yu Z, Xia M, Luo X, Lu X, Ling W. Vitamin D attenuates high fat diet-induced hepatic steatosis in rats by modulating lipid metabolism. Eur J Clin Invest 2012;42:1189–96. [DOI] [PubMed] [Google Scholar]
- 4.Beydoun MA, Boueiz A, Shroff MR, Beydoun HA, Wang Y, Zonderman AB. Associations among 25-hydroxyvitamin D, diet quality, and metabolic disturbance differ by adiposity in adults in the United States. J Clin Endocrinol Metab 2010;95:3814–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brock K, Huang WY, Fraser DR, Ke L, Tseng M, Stolzenberg-Solomon R, Peters U, Ahn J, Purdue M, Mason RS, et al. Low vitamin D status is associated with physical inactivity, obesity and low vitamin D intake in a large US sample of healthy middle-aged men and women. J Steroid Biochem Mol Biol 2010;121:462–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng S, Massaro JM, Fox CS, Larson MG, Keyes MJ, McCabe EL, Robins SJ, O'Donnell CJ, Hoffman U, Jacques PF, et al. Adiposity, cardiometabolic risk, and vitamin D status: the Framingham Heart Study. Diabetes 2010;59:242–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mai XM, Chen Y, Camargo CA, Jr, Langhammer A. Cross-sectional and prospective cohort study of serum 25-hydroxyvitamin D level and obesity in adults: the HUNT study. Am J Epidemiol 2012;175:1029–36. [DOI] [PubMed] [Google Scholar]
- 8.Vimaleswaran KS, Berry DJ, Lu C, Tikkanen E, Pilz S, Hiraki L, Cooper JD, Dastani Z, Li R, Houston DK, et al. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med 2013;10:e1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortega RM, Lopez-Sobaler AM, Aparicio A, Rodriguez-Rodriguez E, Bermejo LM, Perea JM, Lopez-Sobaler AM, Ruiz-Roso B, Andres P. Vitamin D status modification by two slightly hypocaloric diets in young overweight/obese women. Int J Vitam Nutr Res 2009;79:71–8. [DOI] [PubMed]
- 10.Zittermann A, Frisch S, Berthold HK, Gotting C, Kuhn J, Kleesiek K, Stehle P, Koertke H, Koertke R. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr 2009;89:1321–7. [DOI] [PubMed] [Google Scholar]
- 11.Nagpal J, Pande JN, Bhartia A. A double-blind, randomized, placebo-controlled trial of the short-term effect of vitamin D3 supplementation on insulin sensitivity in apparently healthy, middle-aged, centrally obese men. Diabet Med 2009;26:19–27. [DOI] [PubMed] [Google Scholar]
- 12.Soares MJ, Chan She Ping-Delfos W, Ghanbari MH. Calcium and vitamin D for obesity: a review of randomized controlled trials. Eur J Clin Nutr 2011;65:994–1004. [DOI] [PubMed] [Google Scholar]
- 13.Hewison M. Vitamin D and the immune system: new perspectives on an old theme. Endocrinol Metab Clin North Am 2010;39:365–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pham NM, Akter S, Kurotani K, Nanri A, Sato M, Hayabuchi H, Yasuda K, Mizoue T. Serum 25-hydroxyvitamin D and markers of insulin resistance in a Japanese working population. Eur J Clin Nutr 2012;66:1323–8. [DOI] [PubMed] [Google Scholar]
- 15.Sung CC, Liao MT, Lu KC, Wu CC. Role of vitamin D in insulin resistance. J Biomed Biotechnol 2012;2012:634195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Renehan AG, Roberts DL, Dive C. Obesity and cancer: pathophysiological and biological mechanisms. Arch Physiol Biochem 2008;114:71–83. [DOI] [PubMed] [Google Scholar]
- 17.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006;113:898–918. [DOI] [PubMed] [Google Scholar]
- 18.Johnson AR, Milner JJ, Makowski L. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunol Rev 2012;249:218–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prentice A. Vitamin D deficiency: a global perspective. Nutr Rev 2008;66(suppl 2):S153–64. [DOI] [PubMed] [Google Scholar]
- 20.Organization for Economic Co-operation and Development. Obesity and the economics of prevention: fit not fat. 2010. Available from: http://www.oecd.org/health/healthpoliciesanddata/obesityandtheeconomicsofpreventionfitnotfat.htm (cited 1 November 2012).
- 21.Institute of Medicine. Dietary Reference Intakes: calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, DC: National Academy Press, 1997. Available from: http://www.nal.usda.gov/fnic/DRI/DRI_Calcium/calcium_full_doc.pdf (cited 2 August 2013).
- 22.Foster-Schubert KE, Alfano CM, Duggan C, Xiao L, Campbell KL, Kong A, Bain C, Wang CY, Blackburn GL, McTiernan A. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity (Silver Spring) 2012;20:1628–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedman LM, Furberg C, DeMets DL. Sample size. In: Fundamentals of clinical trials. 4th ed. New York, NY: Springer, 2010:145–6. [Google Scholar]
- 24.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, Kahn SE, Knowler WC, Yanovski SZ. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials 2003;24:610–28. [DOI] [PubMed] [Google Scholar]
- 26.Noble BJ, Borg GA, Jacobs I, Ceci R, Kaiser P. A category-ratio perceived exertion scale: relationship to blood and muscle lactates and heart rate. Med Sci Sports Exerc 1983;15:523–8. [PubMed] [Google Scholar]
- 27.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR, Schmitz KH, Emplaincourt PO, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000;32(Suppl):S498–504. [DOI] [PubMed] [Google Scholar]
- 28.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol 1999;9:178–87. [DOI] [PubMed] [Google Scholar]
- 29.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Ainsworth BE, Pratt ME, Ekelun U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381–95. [DOI] [PubMed] [Google Scholar]
- 30.Institute of Medicine. Dietary Reference Intakes for calcium and vitamin D. National Academy of Sciences. 2010. Available from: http://www.iom.edu/Reports/2010/Dietary-Reference-Intakes-for-Calcium-and-Vitamin-D.aspx (cited 10 April 2012).
- 31.Wagner D, Hanwell HE, Vieth R. An evaluation of automated methods for measurement of serum 25-hydroxyvitamin D. Clin Biochem 2009;42:1549–56. [DOI] [PubMed] [Google Scholar]
- 32.Ersfeld DL, Rao DS, Body JJ, Sackrison JL, Miller AB, Parikh N, Eskridge TL, Polinske A, Olson GT, MacFarlane GD. Analytical and clinical validation of the 25 OH vitamin D assay for the LIAISON automated analyzer. Clin Biochem 2004;37:867–74. [DOI] [PubMed] [Google Scholar]
- 33.Saremi A, Anderson RJ, Luo P, Moritz TE, Schwenke DC, Allison M, Reaven PD. Association between IL-6 and the extent of coronary atherosclerosis in the veterans affairs diabetes trial (VADT). Atherosclerosis 2009;203:610–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glanz K, Yaroch AL, Dancel M, Saraiya M, Crane LA, Buller DB, Manne S, O'Riordan DL, Heckman CJ, Hay J, et al. Measures of sun exposure and sun protection practices for behavioral and epidemiologic research. Arch Dermatol 2008;144:217–22. [DOI] [PubMed] [Google Scholar]
- 35.Gouni-Berthold I, Krone W, Berthold HK. Vitamin D and cardiovascular disease. Curr Vasc Pharmacol 2009;7:414–22. [DOI] [PubMed] [Google Scholar]
- 36.Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis: longitudinal studies of serum vitamin D and colorectal cancer risk. Aliment Pharmacol Ther 2009;30:113–25. [DOI] [PubMed] [Google Scholar]
- 37.Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis: serum vitamin D and breast cancer risk. Eur J Cancer 2010;46:2196–205. [DOI] [PubMed] [Google Scholar]
- 38.Fiscella K, Franks P. Vitamin D, race, and cardiovascular mortality: findings from a national US sample. Ann Fam Med 2010;8:11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Kinkeldei J, Boehm BO, Weihrauch G, Maerz W. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med 2008;168:1340–9. [DOI] [PubMed] [Google Scholar]
- 40.Schöttker B, Haug U, Schomburg L, Kohrle J, Perna L, Muller H, Holleczek B, Brenner H. Strong associations of 25-hydroxyvitamin D concentrations with all-cause, cardiovascular, cancer, and respiratory disease mortality in a large cohort study. Am J Clin Nutr 2013;97:782-93. [DOI] [PubMed] [Google Scholar]
- 41.Sneve M, Figenschau Y, Jorde R. Supplementation with cholecalciferol does not result in weight reduction in overweight and obese subjects. European journal of endocrinology / European Federation of Endocrine Societies 2008;159:675–84. [DOI] [PubMed] [Google Scholar]
- 42.Wamberg L, Kampmann U, Stodkilde-Jorgensen H, Rejnmark L, Pedersen SB, Richelsen B. Effects of vitamin D supplementation on body fat accumulation, inflammation, and metabolic risk factors in obese adults with low vitamin D levels—results from a randomized trial. Eur J Intern Med 2013;24:644–9. [DOI] [PubMed] [Google Scholar]
- 43.von Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient—a randomised, placebo-controlled trial. Br J Nutr 2010;103:549–55. [DOI] [PubMed] [Google Scholar]
- 44.Heaney RP. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr Rev 2014;72:48–54. [DOI] [PubMed] [Google Scholar]
- 45.Conn VS, Hafdahl AR, Cooper PS, Ruppar TM, Mehr DR, Russell CL. Interventions to improve medication adherence among older adults: meta-analysis of adherence outcomes among randomized controlled trials. Gerontologist 2009;49:447–62. [DOI] [PubMed] [Google Scholar]
- 46.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev 2008; (2):CD000011. [DOI] [PubMed] [Google Scholar]
- 47.Mason C, Xiao L, Imayama I, Duggan C, Bain C, Foster-Schubert KE, Kong A, Campbell KL, Wang CY, Neuhouser ML, et al. Effects of weight loss on serum vitamin D in postmenopausal women. Am J Clin Nutr 2011;94:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mattila C, Knekt P, Mannisto S, Rissanen H, Laaksonen MA, Montonen J, Reunanen A. Serum 25-hydroxyvitamin D concentration and subsequent risk of type 2 diabetes. Diabetes Care 2007;30:2569–70. [DOI] [PubMed] [Google Scholar]
- 49.Gagnon C, Lu ZX, Magliano DJ, Dunstan DW, Shaw JE, Sikaris K, Ebeling PR, Daly RM. Low serum 25-hydroxyvitamin D is associated with increased risk of the development of the metabolic syndrome at five years: results from a national, population-based prospective study (The Australian Diabetes, Obesity and Lifestyle Study: AusDiab). J Clin Endocrinol Metab 2012;97:1953–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.