Abstract
Background: Studies in rodents and older humans have shown that the hippocampus—a brain structure critical to relational/associative memory—has remarkable plasticity as a result of lifestyle factors (eg, exercise). However, the effect of dietary intake on hippocampal-dependent memory during childhood has remained unexamined.
Objective: We investigated the cross-sectional relation of dietary components characteristic of the Western diet, including saturated fatty acids (SFAs), omega-3 (n−3) fatty acids, and refined sugar, with hippocampal-dependent relational memory in prepubescent children.
Design: Participants aged 7–9 y (n = 52) reported their dietary intake by using the Youth-Adolescent Food-Frequency Questionnaire and completed memory tasks designed to assess relational (hippocampal-dependent) and item (hippocampal-independent) memory. Performance on the memory tasks was assessed with both direct (accuracy) and indirect (eye movement) measures.
Results: Partial correlations adjusted for body mass index showed a positive relation between relational memory accuracy and intake of omega-3 fatty acids and a negative relation of both relational and item memory accuracy with intake of SFAs. Potential confounding factors of age, sex, intelligence quotient, socioeconomic status, pubertal timing, and aerobic fitness (maximal oxygen volume) were not significantly related to any of the dietary intake measures. Eye movement measures of relational memory (preferential viewing to the target stimulus) showed a negative relation with intake of added sugar.
Conclusions: SFA intake was negatively associated with both forms of memory, whereas omega-3 fatty acid intake was selectively positively associated with hippocampal-dependent relational memory. These findings are among the first to show a link between habitual dietary intake and cognitive health as pertaining to hippocampal function in childhood. The Fitness Improves Thinking Kids (FITKids) and FITKids2 trials were registered at www.clinicaltrials.gov as NCT01334359 and NCT01619826, respectively.
See corresponding editorial on page 971.
INTRODUCTION
Physical inactivity and a lack of fitness, key factors contributing to obesity, have been associated with poorer cognitive outcomes across species (1–4). However, in school-aged children without nutritional deficiency or learning disabilities, the literature regarding the influence of habitual dietary intake on specific aspects of cognitive function remains equivocal. Rodent models and studies in older adults suggest that the hippocampus is a brain structure that is particularly susceptible to structural and functional modulation by diet, possibly because of its high metabolic demand and capability for neurogenesis beyond the gestational period (5–7).
The hippocampus is essential for relational memory, which refers to the ability to bind together into memory the relations among constituent elements of experience. This includes information about the co-occurrences of people, places, and/or objects and their spatial and temporal context (8, 9)—information that provides a critical foundation for learning and scholastic achievement. A diet high in SFAs and refined sugars increases neural oxidative stress in rats, which results in a decrease in hippocampal brain-derived neurotrophic factor (BDNF)5 (7). Low concentrations of BDNF impair synaptic function, resistance of neurons to the effects of stress and disease (10–12), and hippocampal memory performance (13–16). On the other hand, omega-3 (n−3) fatty acids, particularly DHA, support synaptic plasticity, cell membrane fluidity, and neuronal metabolism (7, 17–20). In middle childhood, plasma DHA concentrations have been shown to relate to neuroelectric measures of continuous recognition performance (21). However, omega-3 supplementation studies in children have provided mixed results (22).
One reason for the mixed findings is that studies have used variable cognitive assessment techniques, overlooking the potential neural specificity of nutrient-brain interactions. Therefore, a critical next step is to identify cross-sectional associations between nutrients and cognitive processes known to be subserved by well-defined neural substrates. Thus, the current study examined cross-sectional relations in prepubescent children between diet and hippocampal-dependent relational memory and hippocampal-independent item memory by using both direct behavioral and indirect eye movement measures. We hypothesized that a higher intake of SFAs and refined sugars would be negatively associated with relational memory performance and that intake of omega-3 fatty acids would be positively associated with relational memory performance.
SUBJECTS AND METHODS
Subjects
Prepubescent children (n = 52) between the ages of 7 and 9 y from the East-Central Illinois community (Table 1) participated in the study during the summers of 2011 and 2012 as part of the larger Fitness Improves Thinking Kids trial. The number of participants was determined based on recruitment and funding. All participants provided written assent, and legal guardians provided written informed consent in accordance with the regulations of the University of Illinois Institutional Review Board. Exclusion criteria included neurological or attentional disorders, physical disabilities, and psychoactive medication status. All participants had normal or corrected-to-normal vision.
TABLE 1.
Behavioral data and participant demographics, weight status, and fitness1
| Characteristic | Girls (n = 25) | Boys (n = 27) | All children (n = 52) |
| Age (y) | 8.61 ± 0.132 | 8.70 ± 0.11 | 8.66 ± 0.08 |
| IQ | 114.92 ± 2.34 | 111.33 ± 2.85 | 113.06 ± 1.86 |
| SES [n (%)] | |||
| Low | 5 (20) | 9 (33) | 14 (27) |
| Middle | 7 (28) | 8 (30) | 15 (29) |
| High | 13 (52) | 10 (37) | 23 (44) |
| BMI (kg/m2) | 17.59 ± 0.58 | 19.35 ± 0.80 | 18.50 ± 0.51 |
| BMI-for-age percentile | 61.00 ± 5.16 | 73.75 ± 5.32 | 67.62 ± 3.79 |
| Underweight, BMI percentile <5 [n (%)] | 1 (4) | 1 (4) | 2 (4) |
| Normal weight, BMI percentile ≤5 and <84.9 [n (%)] | 19 (76) | 12 (44) | 31 (60) |
| Overweight, BMI percentile ≤85 and <94.9 [n (%)] | 2 (8) | 6 (22) | 8 (15) |
| Obese, BMI percentile >95 [n (%)] | 3 (12) | 8 (30) | 11 (21) |
| BMI z score | 0.38 ± 0.19 | 0.91 ± 0.21 | 0.66 ± 0.14 |
 O2max (mL ⋅ kg−1 ⋅ min−1) O2max (mL ⋅ kg−1 ⋅ min−1) |
40.26 ± 1.15 | 41.83 ± 1.22 | 41.08 ± 0.84 |
| Relational memory accuracy (%) | 62.09 ± 2.65 | 64.84 ± 2.22 | 63.51 ± 1.71 |
| Item memory accuracy (%) | 85.56 ± 1.97 | 87.55 ± 1.57 | 86.59 ± 1.25 |
| Relational memory response time, correct trials only (ms) | 2304.41 ± 100.50 | 2689.17 ± 77.683 | 2504.19 ± 67.88 |
| Item memory response time, correct trials only (ms) | 2472.28 ± 94.75 | 2937.40 ± 80.443 | 2713.78 ± 69.30 |
Weight status was based on the Centers for Disease Control and Prevention BMI-for-age growth charts. IQ, intelligence quotient; SES, socioeconomic status; 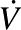 O2max, maximal oxygen volume.
O2max, maximal oxygen volume.
Mean ± SEM (all such values).
Significantly different from girls, P < 0.05 (t tests).
Measures
Memory task
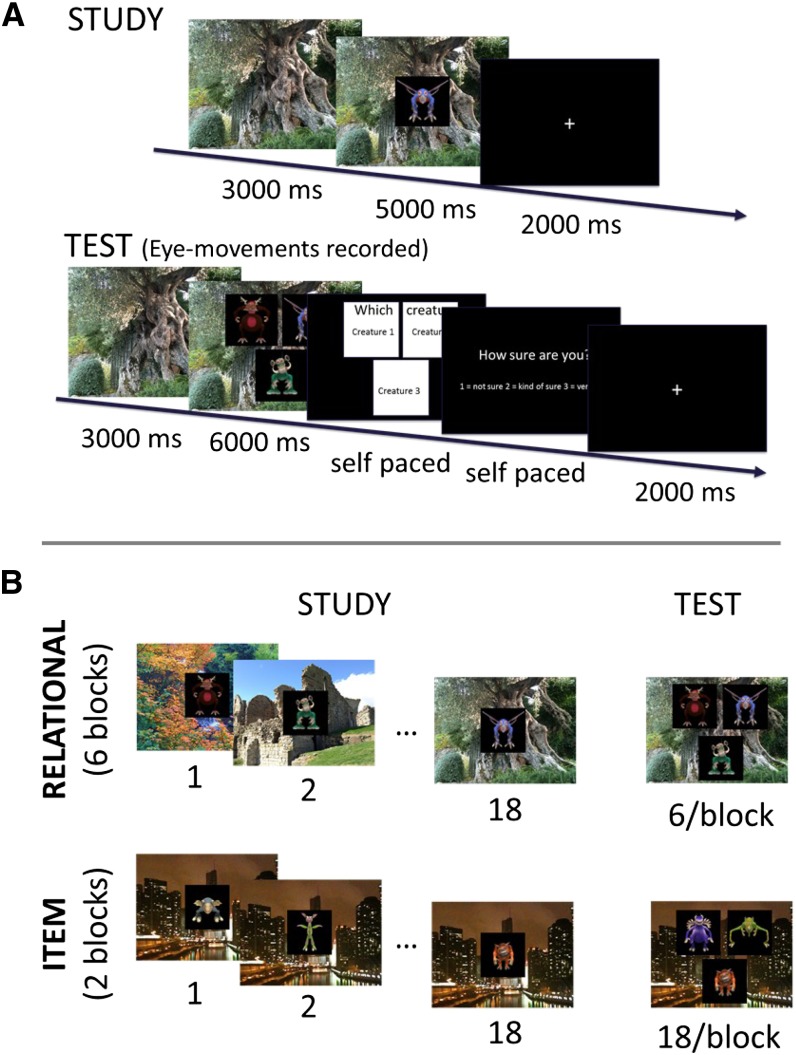
Children completed a memory task designed to assess both hippocampal-dependent relational memory and hippocampal-independent item memory (Figure 1). The task, adapted from Monti et al (23), was divided into 8 study-test blocks, each designed to test either relational or item memory. Briefly, in the Relational condition (6 blocks of 6 test trials), participants studied 18 unique creature-scene pairings. The background scene was trial-unique, which results in nonoverlapping creature-scene pairings. After each study block, participants were tested on 6 probe trials in which they were instructed to find the creature originally studied with that scene. Each test trial consisted of 1 of the 18 studied backgrounds superimposed with 3 of the creatures studied in that block. One of the creatures had been studied with that scene and 2 had been studied with other scenes; familiarity across the 3 creatures was thus matched, necessitating the use of relational memory (23). Participants were instructed to press a single response button when they felt that they knew which creature had been studied together with that scene. In the Item memory condition (2 blocks of 18 test trials), participants studied 18 creature-scene pairings. In contrast with the Relational memory condition, the background scene was the same for all 18 study trials during each Item block. After the study period, a test phase was presented in which 3 creatures, 1 studied and 2 novel, were simultaneously presented atop the scene. Participants were instructed to press a single response button when they felt they knew which creature had been studied. Given that 2 creatures were novel and 1 studied, the Item condition could be solved by using familiarity alone—an ability that has been shown to be independent of the hippocampus (8, 24). Before starting the task, participants were instructed to minimize head movement and give their best performance. During the test, children were cued to remain still or make button press responses if necessary and were given regular positive verbal reinforcement for following directions, regardless of accuracy.
FIGURE 1.
Test. A: Single-trial progression in the study (top) and test (bottom) phases. Durations of each trial component are specified in milliseconds. B: Example study and test trials from the Relational condition (top) and Item condition (bottom).
Stimuli included 216 novel creatures created in Spore Creature Creator (Electronic Arts Inc; see Figure 1 for examples) and were presented by using Presentation software (Neurobehavioral Systems; http://nbs.neuro-bs.com) on a 21-inch color monitor. Creatures were presented on black backgrounds and resized to 480 × 480 pixels; 110 color images of real-world scenes measuring 1280 × 1280 pixels and taken by Brand X photography were used as scene backgrounds.
An Eyelink 1000 eye-tracker (SR Research) was used to record eye movements. The eye tracker was calibrated immediately before each test block of the memory task. A desk-mounted gel-padded chin rest was used to minimize head movement during eye-tracking data collection. Lists of stimuli presented in the Item or Relational conditions and target location on test trials were counterbalanced across participants. In addition, order of study-test blocks was counterbalanced across participants in the following manner: “Item, Rel, Rel, Rel, Item, Rel, Rel, Rel” or “Rel, Rel, Rel, Item, Rel, Rel, Rel, Item.”
Nutritional intake assessment
The Youth-Adolescent Food-Frequency Questionnaire (YAQ) was used to assess dietary intake. The YAQ has been previously validated for use in 9–18-y-olds (25). The YAQ contains 152 questions pertaining to various foods and dietary habits over the preceding 1 y. Instructions were for the child and parent to complete the YAQ together. Questionnaires were analyzed by the Harvard School of Public Health. Although a comprehensive diet analysis was performed, only the primary variables of interest (ie, total energy, dietary lipids and carbohydrates) were included in the statistical analyses to test the proposed hypotheses. Dietary intake data are summarized in Table 2.
TABLE 2.
Dietary intake based on the Youth-Adolescent Food-Frequency Questionnaire1
| Characteristic | Girls (n = 25) | Boys (n = 27) | All children (n = 52) |
| Energy (kcal) | 2020.75 ± 115.97 | 1898.46 ± 103.23 | 1957.25 ± 77.05 |
| Fat (g) | 70.30 ± 4.41 | 65.31 ± 3.86 | 67.71 ± 2.91 |
| Protein (g) | 83.35 ± 5.08 | 78.26 ± 5.39 | 80.71 ± 3.70 |
| Carbohydrates (g) | 270.65 ± 16.79 | 255.05 ± 13.97 | 262.55 ± 10.80 |
| SFAs (g) | 25.52 ± 1.75 | 23.29 ± 1.36 | 24.36 ± 1.10 |
| Omega-3 fatty acids (g) | 1.31 ± 0.09 | 1.29 ± 0.09 | 1.30 ± 0.06 |
| MUFAs (g) | 24.07 ± 1.58 | 22.25 ± 1.39 | 23.12 ± 1.05 |
| trans Fatty acids (g) | 2.58 ± 0.18 | 2.40 ± 0.15 | 2.48 ± 0.12 |
| Total sugars (g) | 129.98 ± 9.10 | 111.52 ± 5.99 | 120.40 ± 5.50 |
| Added sugars (g) | 57.70 ± 4.34 | 54.52 ± 3.63 | 56.05 ± 2.80 |
All values are means ± SEMs. No statistically significant differences between boys and girls were observed.
Control variables
In addition to the memory measures that were the primary target of this investigation, data were also collected on other cognitive functions [intelligence quotient (IQ), inhibition abilities], and demographic variables [socioeconomic status (SES), BMI, pubertal status, and aerobic fitness] to test for the selectivity of the dietary effects. BMI values were converted to z scores as recommended by the US CDC (26). Fluid and crystallized intelligence were assessed by using 1 of 2 standardized tests: the Kaufman Brief Intelligence Test (27) or the Woodcock-Johnson Tests of Cognitive Abilities (28). Inhibition was measured by using a modified flanker task (29). SES was estimated based on household income, participation in a school meal-assistance program, maternal and paternal education levels, and how many parents work full time (30). Pubertal status was assessed by using the Tanner Staging Scales (31). Finally, a maximal oxygen consumption test (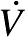 O2max) was used to assess aerobic fitness (32).
O2max) was used to assess aerobic fitness (32).
Statistics and data analysis
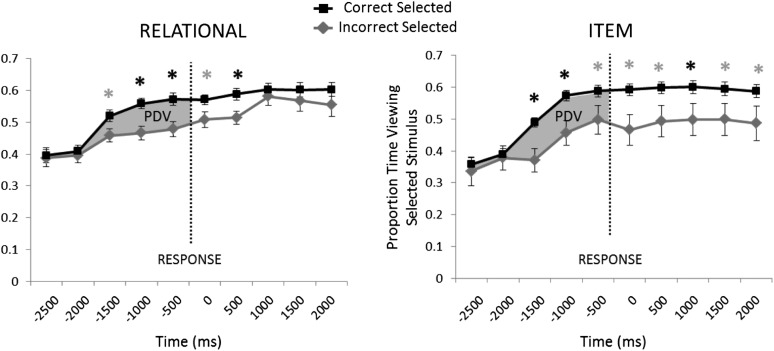
Eye-movement data were divided into trials in which participants correctly selected the matching target and trials in which participants incorrectly selected a competitor and were analyzed by using response-locked time courses. Response-locked time courses of eye-movement data were analyzed by using Accuracy × Time Window (500-ms windows from 2500 ms before and 2500 ms after behavioral response) repeated-measures ANOVAs using Greenhouse-Geisser correction where appropriate (33). Time courses were quantified by using preferential disproportionate viewing (PDV), which was defined as the difference in the proportion of time spent viewing correctly selected creatures relative to the proportion of time spent viewing incorrectly selected creatures before behavioral response (Figure 2). A priori t tests comparing viewing with correctly selected creatures relative to incorrectly selected creatures were corrected for multiple comparisons by using Bonferroni correction.
FIGURE 2.
Response locked eye-movement time courses depicting viewing to selected creatures during the test phase of the memory task (n = 52). The x-axis indicates onset of each 500-ms time bin. The vertical dotted line indicates the timing of behavioral response. Error bars indicate SEMs. Black asterisks indicate a significant pairwise difference in t tests at P < 0.05 (Bonferroni corrected). Gray asterisks indicate a significant pairwise difference at P < 0.05 (uncorrected). Gray shading indicates PDV—an eye-movement outcome measure assessed before behavioral response. PDV, preferential disproportionate viewing.
Nutrient intake was normalized by average total daily kilocalorie consumption within participants (34). Bivariate correlations (Pearson's r) between the primary variables of interest were followed by partial correlations that adjusted for the control variables. All analyses were performed by using SPSS Statistical version 21 (IBM).
RESULTS
Memory performance
Memory accuracy and response time by sex and in aggregate are summarized in Table 1. In addition, significant differences in accuracy (t51 = 15.17, P < 0.001) and response time (t51 = 3.21, P < 0.002) were found between the Relational and Item conditions.
Eye movement
Response-locked time courses in the Relational memory condition showed significant main effects of accuracy (F1,51 = 22.57, P < 0.001) and of time window (F4.56,232.97 = 19.34, P < 0.001), but failed to yield an accuracy × time window interaction (F5.59,284.98 = 1.35, P < 0.24; Figure 2). The Relational condition showed significant PDV emerging before response, beginning during the 1000–500-ms time bin, which was sustained until response. Response-locked time courses in the Item condition showed significant main effects of accuracy (F1,41 = 24.95, P < 0.001) and of time window (F5.29,216.92 = 10.03, P < 0.001) but no accuracy × time window interaction (F6.05,247.94 = 0.98, P < 0.44). Comparisons of individual time points showed significant PDV in the Item condition emerging 1500–1000 ms before response, continuing through the 1000–500-ms time bin before response, and then becoming nonsignificant in the 500 ms leading up to response when Bonferroni corrected for multiple comparisons.
Correlations of nutritional intake with relational and item memory
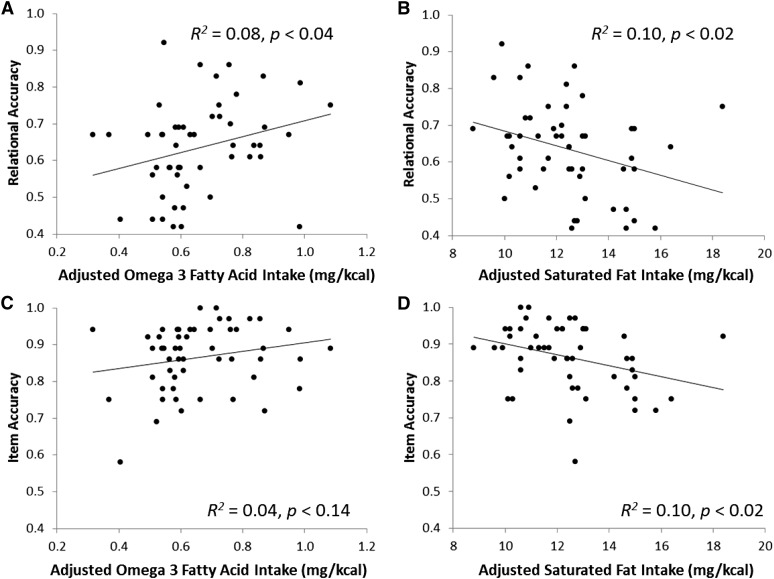
Bivariate correlations showed a significant positive correlation between accuracy on the Relational condition and omega-3 fatty acid intake (r = 0.28, P = 0.04; Figure 3; Table 3). Behavioral accuracy on both the Relational and Item conditions was negatively correlated with SFA intake [r = −0.32 (P < 0.02) and r = −0.32 (P < 0.02), respectively]. Furthermore, PDV in the Item condition was marginally correlated with SFA intake (r = −0.26, P < 0.07). Measures of total sugar, added sugar, trans fatty acids, and MUFAs were not significantly related to performance (Table 3). A separate analysis of boys and girls showed that behavioral correlations were statistically carried by boys (|r| > 0.44, P < 0.03), with nonsignificant numerical trends in the same directions for girls (|r| < 0.23, P > 0.27). In contrast, the eye-movement effect was present in girls (r = −0.47, P < 0.02) but not in boys (r = 0.14, P < 0.95).
FIGURE 3.
Scatterplots depicting relations between intake of omega-3 (n−3) fatty acids (A and C) and SFAs (B and D) with Relational and Item memory accuracy (n = 52). Statistical relations were determined by using bivariate correlations (Pearson's r) with a significance threshold of P < 0.05. Each dietary intake measure is expressed as milligrams per kilocalorie consumed per day.
TABLE 3.
Bivariate correlations between nutrient intake and memory performance1
| Relational accuracy |
Item accuracy |
|||
| Characteristic | r | P | r | P |
| Energy (kcal/d) | −0.16 | 0.26 | 0.02 | 0.88 |
| Fat (g/d) | −0.19 | 0.18 | −0.01 | 0.93 |
| Protein (g/d) | −0.16 | 0.27 | 0.04 | 0.78 |
| Carbohydrates (g/d) | −0.12 | 0.39 | 0.03 | 0.84 |
| SFAs (mg ⋅ kcal −1 ⋅ d −1) | −0.32 | 0.02 | −0.32 | 0.02 |
| Omega-3 fatty acids (mg ⋅ kcal−1 ⋅ d−1) | 0.28 | 0.04 | 0.21 | 0.14 |
| MUFAs (mg ⋅ kcal−1 ⋅ d−1) | −0.15 | 0.29 | −0.09 | 0.55 |
| trans Fatty acids (mg ⋅ kcal−1 ⋅ d−1) | −0.19 | 0.19 | −0.04 | 0.76 |
| Total sugars (mg ⋅ kcal−1 ⋅ d−1) | −0.12 | 0.38 | −0.05 | 0.72 |
| Added sugars (mg ⋅ kcal−1 ⋅ d−1) | 0.03 | 0.84 | 0.13 | 0.38 |
Dietary intake was based on self-reported measures on the Youth-Adolescent Food-Frequency Questionnaire.
Next, to determine whether measures of age, sex, inhibition abilities, SES, IQ, 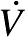 O2max, and BMI z score explained these correlations, we performed bivariate correlations, 1-factor ANOVAs, or t tests, where appropriate, comparing each of these measures relative to the dietary intake values of interest. Results showed that the BMI z score was positively related to intake of trans fatty acids (r = 0.28, P < 0.04), but no other relations were significant (all |r| < 0.20, P > 0.15), which indicated that they did not account for a significant portion of the variance observed in dietary intake. Partial correlations thus included BMI z score as a factor, but not age, sex, inhibition abilities, SES, IQ, or
O2max, and BMI z score explained these correlations, we performed bivariate correlations, 1-factor ANOVAs, or t tests, where appropriate, comparing each of these measures relative to the dietary intake values of interest. Results showed that the BMI z score was positively related to intake of trans fatty acids (r = 0.28, P < 0.04), but no other relations were significant (all |r| < 0.20, P > 0.15), which indicated that they did not account for a significant portion of the variance observed in dietary intake. Partial correlations thus included BMI z score as a factor, but not age, sex, inhibition abilities, SES, IQ, or 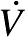 O2max.
O2max.
Partial correlations of the dietary factors with behavioral memory accuracy measures, adjusted for BMI z score, showed the same pattern of results as the uncorrected correlations: omega-3 intake was positively correlated with Relational accuracy (r = 0.29, P < 0.04), and SFA intake was negatively correlated with both Relational (r = −0.31, P < 0.03) and Item (r = −0.30, P < 0.03) accuracy. Interestingly, adjustment for BMI z score changed the eye-movement results such that PDV in the Relational condition was negatively correlated with intake of added sugar (r = −0.34, P < 0.02). No other relations were significant (P > 0.05 for all).
Secondary exploratory analyses examining individual omega-3 fatty acids showed a positive correlation between intake of DHA and Relational accuracy (r = 0.32, P < 0.03) and marginal correlations between intake of EPA and α-linolenic acid with Relational accuracy (r = 0.27, P < 0.06 and r = 0.27, P < 0.07, respectively) after adjustment for BMI z score and exclusion of one outlier in DHA intake.
DISCUSSION
The current study was among the first to examine the effects of diet on specific aspects of memory in healthy prepubescent children. The major findings were that dietary intake of omega-3 fatty acids was positively correlated with behavioral accuracy on a hippocampal-dependent relational memory task and that intake of SFAs was negatively correlated with behavioral accuracy on both the relational memory and item memory tasks. Individuals who had a lower intake of SFAs had marginally greater preferential viewing of previously viewed stimuli (item memory), but this effect was associated with differences in BMI.
Observed relations between dietary intake and memory performance cannot be attributed simply to greater body weight, because the behavioral correlations with diet remained even after adjustment for BMI. A similar effect has been seen in studies in rodents, where dietary intake of fats or sugars affected cognition even before weight gain or accumulation of adipose tissue was evident (35, 36), and the intake of particular nutrients was associated with memory impairment among experimental animals who were matched in weight (37).
The current findings add clarity to previous research on the role of omega-3 fatty acids in neurodevelopment, because the extant literature is contradictory. On one hand, several studies have found a positive relation between intake of omega-3 fatty acids and verbal learning between 7 and 9 y (38), working memory between 6 and 16 y (39), and intelligence at 18 y (40). Conversely, other studies have failed to observe a significant relation between omega-3 fatty acid intake and global intelligence at 4 y (41) or 6–10 y (42, 43). One possibility for the discrepancy in the literature is that intake of omega-3 fatty acids may preferentially affect specific cognitive domains and specific brain systems, such as relational memory, which depends on the hippocampus, and broader measures such as global intelligence tests may dilute positive relations.
Although little developmental work in this area has been published in animal models (17), the current results further converge with the adult animal literature in that omega-3 and saturated fats have been shown to facilitate or impair behavioral measures of hippocampal memory, respectively (7, 16, 17). For example, Wu et al (16) showed that adult rats fed feed pellets supplemented with omega-3 fatty acids showed enhanced spatial (relational) memory performance relative to animals fed standard feed pellets. In contrast, rodents consuming diets high in saturated fat and refined sugar have been shown to be impaired in spatial (relational) memory relative to rats fed control diets (10, 35, 36, 44–47). Similarly, in human adults, a high intake of saturated fat has been associated with impairments in verbal and prospective memory (48, 49).
Although the evidence in humans remains limited, several studies in rodents provide mechanistic support for the differential association of dietary lipids on hippocampal memory function. These factors include possible dietary effects on neurobiological processes such as neurogenesis, insulin signaling, oxidative stress and neuroinflammation, and maintenance of the blood-brain barrier. Low amounts of omega-3 fatty acids in a typical Western diet may interfere with neurogenesis, because omega-3 fatty acids are important for the creation and maintenance of neuronal plasma membranes (16, 17). Compounding this effect, a high intake of saturated fats can further reduce the formation of new neurons potentially through its role in inhibiting the formation of BDNF (10). Consumption of a high-fat diet may also increase insulin resistance in the brain (50) and interfere with hippocampal insulin signaling (5). Exacerbating these deleterious effects, SFAs have been shown to increase oxidative damage to neurons (14), increase neural expression of inflammation (51), and diminish the integrity of the blood-brain barrier (5). Conversely, intake of omega-3 fatty acids has been shown to reduce oxidative stress and inhibit expression of proinflammatory genes (52). Interestingly these effects may affect the hippocampus and resulting functionality earlier than other cortical memory systems (5).
Although one or more of these mechanisms may mediate the observed relation between dietary intake and memory function, the current study was limited in its ability to discern among these factors by lack of biomedical outcomes of metabolic dysregulation. Future studies should consider including measures of serum BDNF (2), insulin sensitivity (53), neuroinflammation (54), or oxidative stress (55). Social, personality, and genetic factors such as parental feeding practices, reward sensitivity, or BDNF allele status likely also contribute to the current findings and should be evaluated in future research. An additional limitation of the current study was that children were not required to fast before participation, so it remains a possibility that food consumption immediately before participation may have influenced memory performance.
It was surprising that dietary SFA intake was associated with reduced accuracy in the Item condition (in addition to the Relational condition). One explanation for this effect is that some of the deleterious effects of dietary saturated fat, such as neuroinflammation and oxidative stress, may have broader neural effects. This perhaps reflects an influence on brain plasticity more generally. It is worth noting that SFA intake was not correlated with IQ or performance on an executive function task; as such, this effect appeared to be specific to the plasticity involved in the memory domain. This distinction furthermore argues against the alternative explanation that the observed memory effects can be explained by reduced motivation secondary to habitual intake of saturated fat.
Another hallmark of the Western diet is a high intake of refined sugars, which were found in the current study to correlate with reduced PDV in the Relational condition, after adjustment for BMI. Although it was not clear why this effect emerged only after body size was accounted for, a diet high in refined sugars has been shown in animal models to cause impairments in learning and memory independent of body weight (37) and in healthy children to cause impairments in immediate memory after ingestion of sugars (56). When taken together with a diet high in saturated fat, a diet high in refined sugar causes impairments in hippocampal spatial memory (57). Like saturated fat, the deleterious effects of high fructose consumption on memory performance can be counteracted by adequate amounts of omega-3 fatty acids in the diet (18).
In conclusion, the current study found that prepubescent children who consumed more SFAs showed impairments in hippocampal-dependent relational memory and cortical-dependent item memory relative to children who consumed less SFAs. Conversely, children who consumed a greater amount of omega-3 fatty acids showed relational memory that was superior to children who consumed a lower amount of omega-3 fatty acids. Given the broad range of reported dietary intake values and range in outcome of control variables, these results may be generalized across a broad cross section of children; however, the cross-sectional design of the current study cannot provide causal evidence that consuming higher amounts of omega-3 fatty acids will improve hippocampal-dependent relational memory performance. The major implication of these findings is that a chronically low intake of omega-3 fatty acids during development, typical of the Western diet, may not be sufficient to support optimal functioning of the hippocampus—a brain region critical for learning and memory. That this influence can be observed after just a few years of following these dietary patterns raises concerns for brain health and the learning capabilities of children consuming a typical Western diet. Therefore, the public health consequences of early intervention to improve dietary intake patterns in children are of considerable significance. Thus, the current findings underscore the importance of developing healthy eating habits early in life.
Acknowledgments
We thank Bonnie Hemrick for recruiting and scheduling participants and Teresa Borowski, Becky Delgado, Inge Karosevica, Ari Pence, Jackie Rodriguez, Grace Song, and Sebastian Wraight for assisting with the data collection.
The authors’ responsibilities were as follows—CLB, NAK, JMM, AFK, CHH, and NJC: designed the research; CLB, NAK, LBR, ESD, RDM, and MRS: conducted the research; CLB and NAK: analyzed the data; CLB, NAK, CHH, and NJC: wrote the manuscript; and CLB: had primary responsibility for the final content. All authors read and approved the final manuscript. None of the authors declared a conflict of interest.
Footnotes
Abbreviations used: BDNF, brain-derived neurotrophic factor; IQ, intelligence quotient; PDV, preferential disproportionate viewing; SES, socioeconomic status;  O2max, maximal oxygen volume; YAQ, Youth-Adolescent Food-Frequency Questionnaire.
O2max, maximal oxygen volume; YAQ, Youth-Adolescent Food-Frequency Questionnaire.
REFERENCES
- 1.Chaddock L, Erickson KI, Prakash RS, Kim JS, Voss MW, VanPatter M, Pontifex MB, Raine LB, Konkel A, Hillman CH. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res 2010;1358:172–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA 2011;108:3017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA 1999;96:13427–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci 2013;17:525–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav 2011;103:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science 1977;197:1092–4. [DOI] [PubMed] [Google Scholar]
- 7.Gómez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci 2008;9:568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davachi L. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol 2006;16:693–700. [DOI] [PubMed] [Google Scholar]
- 9.Eichenbaum H, Cohen NJ.From conditioning to conscious recollection:multiple memory systems in the brain. New York, NY: Oxford University Press, 2001. [Google Scholar]
- 10.Molteni R, Barnard R, Ying Z, Roberts C, Gomez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience 2002;112:803–14. [DOI] [PubMed] [Google Scholar]
- 11.McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci 1999;22:295–318. [DOI] [PubMed] [Google Scholar]
- 12.Radecki DT, Brown LM, Martinez J, Teyler TJ. BDNF protects against stress-induced impairments in spatial learning and memory and LTP. Hippocampus 2005;15:246–53. [DOI] [PubMed] [Google Scholar]
- 13.Kanoski SE, Meisel RL, Mullins AJ, Davidson TL. The effects of energy-rich diets on discrimination reversal learning and on BDNF in the hippocampus and prefrontal cortex of the rat. Behav Brain Res 2007;182:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu A, Ying Z, Gomez-Pinilla F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. Eur J Neurosci 2004;19:1699–707. [DOI] [PubMed] [Google Scholar]
- 15.Wu A, Ying Z, Gomez-Pinilla F. Dietary curcumin counteracts the outcome of traumatic brain injury on oxidative stress, synaptic plasticity, and cognition. Exp Neurol 2006;197:309–17. [DOI] [PubMed] [Google Scholar]
- 16.Wu A, Ying Z, Gomez-Pinilla F. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience 2008;155:751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Innis SM. Dietary (n−3) fatty acids and brain development. J Nutr 2007;137:855–9. [DOI] [PubMed] [Google Scholar]
- 18.Agrawal R, Gomez-Pinilla F. ‘Metabolic syndrome’ in the brain: deficiency in omega-3 fatty acid exacerbates dysfunctions in insulin receptor signalling and cognition. J Physiol 2012;590:2485–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhatia HS, Agrawal R, Sharma S, Huo Y-X, Ying Z, Gomez-Pinilla F. Omega-3 fatty acid deficiency during brain maturation reduces neuronal and behavioral plasticity in adulthood. PLoS ONE 2011;6:e28451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu A, Ying Z, Gomez-Pinilla F. Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J Neurotrauma 2004;21:1457–67. [DOI] [PubMed] [Google Scholar]
- 21.Boucher O, Burden MJ, Muckle G, Saint-Amour D, Ayotte P, Dewailly E, Nelson CA, Jacobson SW, Jacobson JL. Neurophysiologic and neurobehavioral evidence of beneficial effects of prenatal omega-3 fatty acid intake on memory function at school age. Am J Clin Nutr 2011;93:1025–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirby A, Woodward A, Jackson S. Benefits of omega-3 supplementation for schoolchildren: review of the current evidence. Br Educ Res J 2010;36:699–732. [Google Scholar]
- 23.Monti JM, Hillman CH, Cohen NJ. Aerobic fitness enhances relational memory in preadolescent children: the FITKids randomized control trial. Hippocampus 2012;22:1876–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: insights from relational and item-based learning. J Neurophysiol 2002;88:982–90. [DOI] [PubMed] [Google Scholar]
- 25.Rockett HR, Breitenbach M, Frazier AL, Witschi J, Wolf AM, Field AE, Colditz GA. Validation of a youth/adolescent food frequency questionnaire. Prev Med 1997;26:808–16. [DOI] [PubMed] [Google Scholar]
- 26. Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat Ser 11 2002:1–190. [PubMed]
- 27.Kaufman A, Kaufman N, Test KBI.American guidance service. CirclePines, MN:.American Guidance Service, 1990. [Google Scholar]
- 28.McGrew KS. Itasca, IL: Riverside Publishing, 2001. [Google Scholar]
- 29.Voss MW, Chaddock L, Kim JS, VanPatter M, Pontifex MB, Raine LB, Cohen NJ, Hillman CH, Kramer AF. Aerobic fitness is associated with greater efficiency of the network underlying cognitive control in preadolescent children. Neuroscience 2011;199:166–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Birnbaum AS, Lytle LA, Murray DM, Story M, Perry CL, Boutelle KN. Survey development for assessing correlates of young adolescents’ eating. Am J Health Behav 2002;26:284–95. [DOI] [PubMed] [Google Scholar]
- 31.Taylor SJ, Whincup PH, Hindmarsh PC, Lampe F, Odoki K, Cook D. Performance of a new pubertal self-assessment questionnaire: a preliminary study. Paediatr Perinat Epidemiol 2001;15:88–94. [DOI] [PubMed] [Google Scholar]
- 32.Kamijo K, Pontifex MB, O'Leary KC, Scudder MR, Wu CT, Castelli DM, Hillman CH. The effects of an afterschool physical activity program on working memory in preadolescent children. Dev Sci 2011;14:1046–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika 1959;24:95–112. [Google Scholar]
- 34.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65:1220S–8S. [DOI] [PubMed] [Google Scholar]
- 35.Lindqvist A, Mohapel P, Bouter B, Frielingsdorf H, Pizzo D, Brundin P, Erlanson-Albertsson C. High-fat diet impairs hippocampal neurogenesis in male rats. Eur J Neurol 2006;13:1385–8. [DOI] [PubMed] [Google Scholar]
- 36.Kanoski SE, Davidson TL. Different patterns of memory impairments accompany short-and longer-term maintenance on a high-energy diet. J Exp Psychol Anim Behav Process 2010;36:31–9. [DOI] [PubMed] [Google Scholar]
- 37.Jurdak N, Kanarek RB. Sucrose-induced obesity impairs novel object recognition learning in young rats. Physiol Behav 2009;96:1–5. [DOI] [PubMed] [Google Scholar]
- 38.Dalton A, Wolmarans P, Witthuhn RC, van Stuijvenberg ME, Swanevelder SA, Smuts CM. A randomised control trial in schoolchildren showed improvement in cognitive function after consuming a bread spread, containing fish flour from a marine source. Prostaglandins Leukot Essent Fatty Acids 2009;80:143–9. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, Hebert JR, Muldoon MF. Dietary fat intake is associated with psychosocial and cognitive functioning of school-aged children in the United States. J Nutr 2005;135:1967–73. [DOI] [PubMed] [Google Scholar]
- 40.Åberg MA, Åberg N, Brisman J, Sundberg R, Winkvist A, Torén K. Fish intake of Swedish male adolescents is a predictor of cognitive performance. Acta Paediatr 2009;98:555–60. [DOI] [PubMed] [Google Scholar]
- 41.Ghys A, Bakker E, Hornstra G, Van den Hout M. Red blood cell and plasma phospholipid arachidonic and docosahexaenoic acid levels at birth and cognitive development at 4 years of age. Early Hum Dev 2002;69:83–90. [DOI] [PubMed] [Google Scholar]
- 42.Osendarp SJ, Baghurst K, Bryan J, Calvaresi E, Hughes D, Hussaini M, Karyadi S, van Klinken B, Van Der Knaap H, Lukito W. Effect of a 12-mo micronutrient intervention on learning and memory in well-nourished and marginally nourished school-aged children: 2 parallel, randomized, placebo-controlled studies in Australia and Indonesia. Am J Clin Nutr 2007;86:1082–93. [DOI] [PubMed] [Google Scholar]
- 43.Muthayya S, Eilander A, Transler C, Thomas T, van der Knaap HC, Srinivasan K, van Klinken BJW, Osendarp SJ, Kurpad AV. Effect of fortification with multiple micronutrients and n−3 fatty acids on growth and cognitive performance in Indian schoolchildren: the CHAMPION (Children's Health and Mental Performance Influenced by Optimal Nutrition) Study. Am J Clin Nutr 2009;89:1766–75. [DOI] [PubMed] [Google Scholar]
- 44.Molteni R, Wu A, Vaynman S, Ying Z, Barnard R, Gomez-Pinilla F. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience 2004;123:429–40. [DOI] [PubMed] [Google Scholar]
- 45.Boitard C, Etchamendy N, Sauvant J, Aubert A, Tronel S, Marighetto A, Layé S, Ferreira G. Juvenile, but not adult exposure to high-fat diet impairs relational memory and hippocampal neurogenesis in mice. Hippocampus 2012;22:2095–100. [DOI] [PubMed] [Google Scholar]
- 46.Kosari S, Badoer E, Nguyen JC, Killcross AS, Jenkins TA. Effect of western and high fat diets on memory and cholinergic measures in the rat. Behav Brain Res 2012;235:98–103. [DOI] [PubMed] [Google Scholar]
- 47.Park HR, Park M, Choi J, Park K-Y, Chung HY, Lee J. A high-fat diet impairs neurogenesis: involvement of lipid peroxidation and brain-derived neurotrophic factor. Neurosci Lett 2010;482:235–9. [DOI] [PubMed] [Google Scholar]
- 48.Eskelinen MH, Ngandu T, Helkala EL, Tuomilehto J, Nissinen A, Soininen H, Kivipelto M. Fat intake at midlife and cognitive impairment later in life: a population-based CAIDE study. Int J Geriatr Psychiatry 2008;23:741–7. [DOI] [PubMed] [Google Scholar]
- 49.Okereke OI, Rosner BA, Kim DH, Kang JH, Cook NR, Manson JE, Buring JE, Willett WC, Grodstein F. Dietary fat types and 4-year cognitive change in community-dwelling older women. Ann Neurol 2012;72:124–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alsaif MA, Duwaihy M. Influence of dietary fat quantity and composition on glucose tolerance and insulin sensitivity in rats. Nutr Res 2004;24:417–25. [Google Scholar]
- 51.Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK, Bruce-Keller AJ. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol 2010;219:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bazan NG. Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci 2006;29:263–71. [DOI] [PubMed] [Google Scholar]
- 53.Stockhorst U, de Fries D, Steingrueber H-J, Scherbaum WA. Insulin and the CNS: effects on food intake, memory, and endocrine parameters and the role of intranasal insulin administration in humans. Physiol Behav 2004;83:47–54. [DOI] [PubMed] [Google Scholar]
- 54.Yaffe K, Lindquist K, Penninx B, Simonsick E, Pahor M, Kritchevsky S, Launer L, Kuller L, Rubin S, Harris T. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology 2003;61:76–80. [DOI] [PubMed] [Google Scholar]
- 55.Block G, Dietrich M, Norkus EP, Morrow JD, Hudes M, Caan B, Packer L. Factors associated with oxidative stress in human populations. Am J Epidemiol 2002;156:274–85. [DOI] [PubMed] [Google Scholar]
- 56.Benton D, Maconie A, Williams C. The influence of the glycaemic load of breakfast on the behaviour of children in school. Physiol Behav 2007;92:717–24. [DOI] [PubMed] [Google Scholar]
- 57.Jurdak N, Lichtenstein AH, Kanarek RB. Diet-induced obesity and spatial cognition in young male rats. Nutr Neurosci 2008;11:48–54. [DOI] [PubMed] [Google Scholar]