Abstract
Background: A common obesity-risk variant rs9939609 in the fat mass– and obesity-associated (FTO) gene was recently shown to affect appetite, and the gene is sensitive to the regulation of amino acids.
Objective: We examined the interaction between FTO genotype and protein intake on the long-term changes in appetite in a randomized controlled trial.
Design: We genotyped FTO rs9939609 in 737 overweight adults in the 2-y Preventing Overweight Using Novel Dietary Strategies trial and assessed 4 appetite-related traits including cravings, fullness, hunger, and prospective consumption.
Results: We showed that dietary protein significantly modified genetic effects on changes in food cravings and appetite scores at 6 mo after adjustment for age, sex, ethnicity, baseline body mass index, weight change, and baseline value for respective outcomes (P-interaction = 0.027 and 0.048, respectively). The A allele was associated with a greater decrease in food cravings and appetite scores in participants with high-protein–diet intake (P = 0.027 and 0.047, respectively) but not in subjects in the low-protein–diet group (P = 0.384 and 0.078, respectively). The weight regain from 6 to 24 mo attenuated gene-protein interactions. Protein intakes did not modify FTO genotype effects on other appetite measures.
Conclusion: Our data suggest that individuals with the FTO rs9939609 A allele might obtain more benefits in a reduction of food cravings and appetite by choosing a hypocaloric and higher-protein weight-loss diet. This trial was registered at clinicaltrials.gov as NCT00072995.
INTRODUCTION
A recent study showed that subjects homozygous for the fat mass and obesity associated (FTO) obesity-predisposing rs9939609 A allele have dysregulated circulating concentrations of ghrelin, which is a key appetite-regulating hormone, and attenuated postprandial measurements of hunger and ghrelin concentrations (1). Subjects who carry different FTO genotypes exhibited divergent neural responsiveness to circulating ghrelin within brain regions. These findings offered insight into how FTO obesity-risk alleles induce increased energy intake and obesity in humans (1).
Emerging data have also indicated that FTO may affect adiposity through playing a role in the cellular sensing of nutrients, especially amino acids (2, 3). Therefore, we hypothesized that intakes of dietary protein, which are sources of amino acids, might particularly modify FTO genetic effects on appetite related measures. In the current study, we analyzed associations between FTO rs9939609 and changes in appetite measures (over the past week) in the 2-y diet-intervention study the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST)4 trial (www.clinicaltrials.gov; NCT00072995) and particularly tested the interaction of the FTO genotype and diet interventions that varied in protein intake.
SUBJECTS AND METHODS
The POUNDS LOST trial
The POUNDS LOST trial was a 2-y randomized clinical trial for weight loss conducted at Boston, MA, and Baton Rouge, LA, in 2004–2007. The study design, methods, and main results have been described in detail elsewhere (4). Criteria for exclusion were the presence of diabetes or unstable cardiovascular disease, medication use that affected body weight, and insufficient motivation. Finally, a total of 811 overweight and obese subjects [BMI (in kg/m2) ≥25 to ≤40] aged 30–70 y were randomly assigned to 1 of 4 diets; target percentages of energy derived from fat, protein, and carbohydrate in the 4 diets were as follows 20%, 15% and 65%; 20%, 25%, and 55%; 40%, 15%, and 45%; and 40%, 25%, and 35%. Thus, 2 diets were low fat (20%), 2 diets were high fat (40%), 2 diets were average protein (15%), and 2 diets were high protein (25%), which constituted a 2-by-2 factorial design. All diets were low in saturated fat, high in fiber, and involved a reduction in total energy intake. All participants received a state-of-the-art behavioral counseling program. Each diet was deemed practical and suitable for public health recommendations, and each diet was expected to have a favorable effect on cardiovascular risk factors. Carbohydrates in all diets were intended to have low glycemic indexes. Participants were instructed to take a standard multivitamin with calcium (200–250 mg/d) to ensure the inclusion of sufficient vitamins and minerals in their diets. After 24 mo, 80% of participant (n = 645) completed the trial. All participants provided informed consent, and the study was approved and monitored by the human subjects committee at Harvard School of Public Health, Brigham and Women's Hospital, Pennington Biomedical Research Center, and the National Heart, Lung and Blood Institute.
Measurements of appetite-related variables
Subjects completed baseline visual analog scale (VAS) questionnaires that measured their motivation to eat. The motivation-to-eat VAS questionnaire, which was used to assess appetite (5, 6), was composed of 4 standardized questionnaires or scales as follows: 1) How often did you experience cravings over the past week? (“never” to “always”), 2) How hungry have you felt over the past week? (“not hungry at all” to “as hungry as I've ever felt”); 3) How full have you felt over the past week? (“not full at all” to “very full”); and 4) How much do you think you could have eaten over the past week? (“nothing at all” to “a large amount”). Each VAS consisted of a 100-mm line anchored at the beginning and end by opposing statements. Subjects marked an X on the line to indicate their feelings at that given moment. Scores were determined by measuring the distance (in mm) from the left starting point of the line to the intersection of the X. Subjects remained seated throughout the experimental session. An average appetite score was calculated at each time of measurement for each treatment as
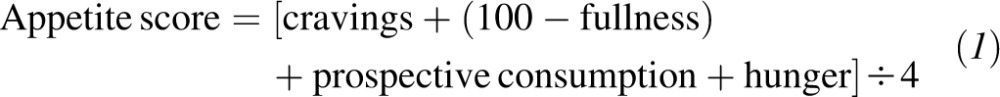 |
The formula reflected the 4 questions on the motivation-to-eat questionnaire (5, 6). Finally, the appetite-related measurements at 6 mo of 609 participants were available.
Measurements of other variables
Body weight and waist circumference were measured in the morning before breakfast at baseline and 6 and 24 mo. Height was measured at baseline. BMI was calculated as weight divided by the square of height. Dietary intake was assessed in a random sample of 50% of participants by a review of the 5-d diet record at baseline and a 24-h recall during a telephone interview on 3 nonconsecutive days at 6 and 24 mo. Biomarkers of nutrient intake were used to validate the self-reported adherence to macronutrient targets as follows: HDL cholesterol for carbohydrate, urinary nitrogen excretion for protein, and respiratory quotient for fat (4).
Genotyping
DNA was extracted from the buffy coat fraction of centrifuged blood by using the QIAmp Blood Kit (Qiagen). Single nucleotide polymorphism (SNP) rs9939609 was selected because it had emerged as the top variant of the FTO locus for BMI (7), food intake, and food choice (8). The SNP was genotyped successfully in 737 of 811 total participants by using the OpenArray SNP Genotyping System (BioTrove). The genotype success rate was 99% in available DNA samples. Replicated quality-control samples (10%) were included in every genotyping plate with >99% concordance (9). The allele frequency in 2 major ethnic groups (white and black) was consistent with Hardy-Weinberg equilibrium (P > 0.05 for both ethnic groups).
Statistical analysis
Primary endpoints for this study were changes in appetite over the intervention. General linear models (PROC GLM, SAS version 9.1; SAS Institute Inc) for continuous variables and chi-square test (PROC FREQ, SAS version 9.1; SAS Institute Inc) for categorical variables were applied for the comparison according to genotype groups at baseline. We compared changes in primary endpoints, biomarkers of adherence, and nutrient intakes across genotype groups at 6 and 24 mo by using generalized linear models. For gene-diet interactions, data were pooled for the following 2 factorial comparisons: low fat compared with high fat or low protein compared with high protein. To test for interactions, we examined the genotype and genotype-by-intervention interactions as independent predictors of changes in primary endpoints adjusted for age, sex, ethnicity, baseline BMI, weight change, and the baseline value for the respective outcome trait in generalized linear models. All reported P values were nominal and 2-side, and P = 0.05 was considered statistically significant. We used the Quanto 1.2.4 program (University of Southern California) to estimate detectable effect sizes of genotype-by-diet interactions. The study had 80% power to detect significant (P < 0.05) gene-diet–interaction effect sizes of 2.6 kg for weight loss, 2.5 mm for changes in appetite scores, 2.3 mm for changes in food cravings, 4.1 mm for changes in fullness, 5.5 mm for changes in prospective consumption, and 6.7 mm for changes in hunger at 6 mo, respectively, under an additive model. Statistical analyses were performed with SAS version 9.1 software (SAS Institute Inc).
RESULTS
Baseline characteristics of participants according to the FTO rs9939609 genotype are presented in Table 1. The risk allele frequency (A allele) was 0.44 in the total population. Genotype frequencies were not significantly different for sexes and ethnicities. Baseline adiposity measures of body weight, BMI, and waist circumference were not significantly correlated with the FTO genotype after adjustment for age, sex, and ethnicity.
TABLE 1.
Characteristics by FTO rs9939609 at baseline in participants in the POUNDS LOST trial1
| Characteristic | TT (n = 227) | TA (n = 360) | AA (n = 150) | P |
| Age (y) | 50.0 ± 9.92 | 51.5 ± 9.1 | 51.4 ± 8.5 | 0.19 |
| Sex [n (%)] | 0.71 | |||
| F | 141 (31.3) | 214 (47.5) | 96 (21.2) | |
| M | 86 (30.1) | 146 (51.1) | 54 (18.8) | |
| Race or ethnic group [n (%)] | 0.09 | |||
| White | 181 (30.7) | 284 (48.2) | 124 (21.1) | |
| Black | 28 (25.0) | 61 (54.5) | 23 (25.5) | |
| Hispanic | 11 (44.0) | 11 (44.0) | 3 (12.0) | |
| Asian or other | 7 (63.6) | 4 (36.4) | 0 (0) | |
| Weight (kg) | 93.5 ± 15.6 | 92.9 ± 15.8 | 93.9 ± 14.9 | 0.37 |
| BMI (kg/m2) | 32.7 ± 3.8 | 32.5 ± 3.9 | 33.1 ± 3.8 | 0.28 |
| Waist circumference (cm) | 103.3 ± 13.2 | 103.5 ± 13.2 | 104.2 ± 12.2 | 0.31 |
| Dietary intake per day3 | ||||
| Energy (kcal) | 1993 ± 563 | 1960 ± 555 | 1933 ± 575 | 0.05 |
| Carbohydrate (%) | 44 ± 7 | 44 ± 8 | 43 ± 7 | 0.07 |
| Fat (%) | 36 ± 5 | 36 ± 6 | 38 ± 5 | 0.09 |
| Protein (%) | 18 ± 3 | 18 ± 3 | 18 ± 3 | 0.84 |
| Biomarkers of adherence | ||||
| Respiratory quotient | 0.84 ± 0.01 | 0.84 ± 0.02 | 0.83 ± 0.01 | 0.88 |
| Urinary nitrogen (g) | 12.1 ± 4.1 | 12.6 ± 4.8 | 11.5 ± 3.7 | 0.61 |
| Appetite variables4 | ||||
| Appetite score | 48.8 ± 8.5 | 48.5 ± 9.5 | 49.8 ± 8.4 | 0.38 |
| Cravings | 50.3 ± 24.6 | 51.8 ± 26.6 | 49.2 ± 25.4 | 0.88 |
| Fullness | 62.5 ± 19.6 | 65.2 ± 19.6 | 60.5 ± 19.6 | 0.96 |
| Prospective consumption | 64.4 ± 16.2 | 65.5 ± 17.7 | 66.1 ± 16.9 | 0.21 |
| Hunger | 42.9 ± 19.7 | 42.0 ± 19.7 | 44.4 ± 19.9 | 0.89 |
| Weight loss at 6 mo (kg) | −3.7 ± 7.8 | −4.1 ± 7.5 | −4.5 ± 7.1 | 0.12 |
| Weight loss at 2 y (kg) | −5.4 ± 7.6 | −5.9 ± 7.9 | −5.9 ± 7.9 | 0.32 |
P values were calculated by using the chi-square test for categorical variables and F tests after adjustment for age, sex, and ethnicity for continuous variables. FTO, fat mass and obesity associated gene; POUNDS LOST, Preventing Overweight Using Novel Dietary Strategies.
Mean ±SD (all such values).
Targeted percentages of energy derived from fat, protein, and carbohydrate from groups 1 to 4 were as follows: 20%, 15%, and 65%; 20%, 25%, and 55%; 40%, 15%, and 45%; and 40%, 25%, and 35%, respectively.
Cravings: how often did you experience cravings over the past week? Fullness: how full have you felt over the past week? How much (prospective consumption): how much do you think you could have eaten over the past week? Hunger: how hungry have you felt over the past week? Appetite score = [cravings score+ (100-full score) + how much score + hungry score] ÷ 4.
Dietary intake was assessed to evaluate the dietary adherence across the intervention in a random sample of 50% of participants. There were no significant differences in mean values of nutrient intakes and biomarkers of adherence over the course of the 2-y intervention (P > 0.05; Table 2). In all participants, no significant associations of FTO rs9939609 with baseline appetite-related phenotypes were observed.
TABLE 2.
Nutrient intake and biomarkers of adherence according to FTO rs9939609 at 6 and 24 mo1
| At 6 mo |
At 24 mo |
|||||
| TT | TA | AA | TT | TA | AA | |
| Dietary intake per day2 | ||||||
| Energy (kcal) | 1641 ± 499 | 1624 ± 563 | 1582 ± 451 | 1515 ± 558 | 1493 ± 436 | 1596 ± 468 |
| Carbohydrate (%) | 51 ± 10 | 52 ± 11 | 49 ± 10 | 49 ± 10 | 49 ± 10 | 50 ± 12 |
| Fat (%) | 30 ± 8 | 30 ± 8 | 31 ± 9 | 30 ± 8 | 31 ± 8 | 31 ± 10 |
| Protein (%) | 20 ± 4 | 20 ± 5 | 20 ± 5 | 21 ± 5 | 20 ± 4 | 20 ± 5 |
| Biomarkers of adherence | ||||||
| Urinary nitrogen (g)3 | 11.7 ± 4.7 | 11.7 ± 4.7 | 10.9 ± 4.0 | 11.8 ± 4.3 | 12.0 ± 5.0 | 12.2 ± 3.7 |
| Respiratory quotient4 | 0.85 ± 0.04 | 0.84 ± 0.04 | 0.84 ± 0.04 | 0.83 ± 0.04 | 0.83 ± 0.04 | 0.83 ± 0.04 |
All values are means ± SDs. Generalized linear models were used to compare biomarkers of adherence and nutrient intakes across genotype groups at 6 and 24 mo. There were no significant differences in mean values of nutrient intakes and biomarkers of adherence across genotypes over the course of the 2-y intervention (P > 0.05).
Data were included for 68–159 participants per diet group at 6 mo and 36–78 participants per diet group at 2 y.
Data were included for 109–271 participants per diet group at 6 mo and 75–189 participants per diet group at 2 y.
Data were included for 121–271 participants per diet group at 6 mo and 90–239 participants per diet group at 2 y.
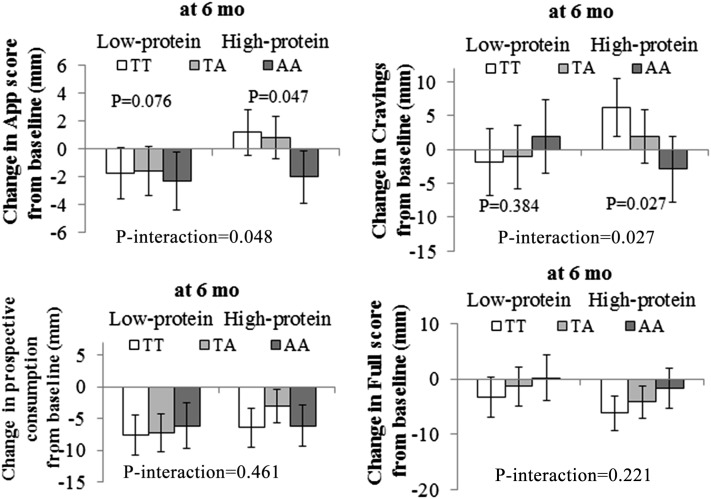
We showed significant interactions between the FTO rs9939609 genotype and dietary protein intake on changes in food cravings during 6 mo intervention after adjustment for age, sex, ethnicity, baseline BMI, weight change, and baseline values for food cravings (P-interaction = 0.027). The A allele was associated with a greater decrease in food-craving responses to a high- protein diet intake (P = 0.027) but not associated with change in food-craving responses to low-protein diet intake (P = 0.384) (Figure 1).
FIGURE 1.
Mean (±SE) effects of the FTO rs9939609 genotype on 6-mo changes in appetite measurements in response to dietary protein. Data were included for 84 (TT), 146 (TA), and 68 (AA) participants in low-protein diet groups and 100 (TT), 154 (TA), and 57 (AA) participants in high-protein diet groups at 6 mo. Data were calculated by using general linear models after adjustment for age, sex, ethnicity, weight change, and baseline values for respective phenotypes. To test for interactions, generalized linear models were used to examine genotype-by-intervention interactions as independent predictors of changes in primary endpoints after adjustment for age, sex, ethnicity, baseline BMI, weight change, and the baseline value for the respective outcome trait. All reported P values were nominal and 2-sided, and P = 0.05 was considered significant. App, appetite.
Similarly, dietary protein intake significantly modified the effect of the FTO rs9939609 genotype on changes in appetite scores during 6 mo intervention after adjustment for age, sex, ethnicity, baseline BMI, weight change, and baseline values for appetite scores (P-interaction = 0.048). The A allele was associated with a greater decrease in appetite scores in response to high-protein diet intake (P = 0.047) but not associated with changes in appetite scores in response to low-protein diet intake (P = 0.078) (Figure 1).
There were no significant interactions between the FTO genotype and other appetite measures during 24 mo intervention. Furthermore, the gene-diet interaction on weight loss was not significant, although the direction was consistent with changes in appetite measures (Figure 1).
DISCUSSION
In the 2-y randomized, weight-loss intervention trial, we observed significant interactions between the FTO rs9939609 SNP and dietary protein intake in relation to changes in food cravings and appetite. Carriers of the A allele exhibited a greater reduction in food cravings and appetite by choosing a hypocaloric and higher-protein weight-loss diet.
Evidence has suggested that the association between SNPs in FTO and obesity is predominantly driven by increased energy intake (8, 10, 11). However, little is known about the precise pathway that links the genotype and altered energy intake. It was recently shown that the FTO genotype was related to ghrelin, which is a key mediator of appetite (1). Individuals with distinct FTO rs9939609 genotypes exhibited a divergent neural responsiveness to circulating ghrelin within brain regions, probably through effects of the expression and methylation concentration of messenger RNA (1). Similarly, a recent intervention study showed that the FTO rs9939609 genotype modified the metabolic response of weight loss after a 3-mo intervention with a hypocaloric diet (12). These findings supported our current results that showed that the A allele was related to greater decreases in food cravings and appetite in response to the high-protein diet.
The mechanism underlying our findings is currently not clear. However, proteins are known to induce satiety and increase the secretion of gastrointestinal hormones (13). Evidence has supported that a high consumption of protein increases weight loss and prevents weight regain (patients generally regain lost weight) (14). Furthermore, the amino acid composition may be an important factor for protein-stimulated metabolic effects (15). It has been suggested that total amino acids dramatically downregulates the FTO messenger RNA concentration (3). Furthermore, FTO itself may influence the cellular amino acids levels (2). It is plausible that dietary protein (sources of amino acids) modifies the effect of FTO genetic variant on changes in appetite through their effects on FTO gene expression. More experimental studies are needed in the future to further explore how the FTO genetic variant rs9939609 may interact with dietary protein or amino acids in the determination of appetite. In addition, previous results have suggested that the portion size of craved foods is a significant predictor of lifetime maximum BMI (16). Therefore, our results suggested that FTO effects on adiposity may be, at least partly, through an effect on appetite.
In the current study, the A allele was not associated with changes in appetite and related measurements with the high-protein diet from 6 to 24 mo when the body weight of participants was regained (17). This observation was similar to findings in our previous analyses on other phenotypes such as blood pressure (18) and insulin resistance (9). A lack of association between FTO genetic variant and appetite from 6 to 24 mo may be partly a result of diminished adherence that occurred between 6 and 24 mo in the POUNDS LOST trial (4) as observed in other weight-loss trials (19–21). A relatively large number of dropouts (n = 179) at 24 mo may also have reduced the statistical power. However, we still observed that participants with the FTO rs9939609 A allele showed a greater decrease in food cravings and appetite than did subjects without this allele who were assigned to the high-protein diet at 6 mo.
To the best of our knowledge, this is the first study to investigate interactions between FTO genetic variation and dietary protein on appetite and food cravings in a large and long-term randomized trial. Our findings provide new insights into the role of the FTO genotype in determining food intakes and the regulation of energy balance. However, several limitations need to be considered when interpreting our findings. Because the adherence to various diets declined after 6 mo, the power to detect a long-term genotype effect in response to the real difference in macronutrient intake in diet groups was reduced. Most participants (80%) in our study were white, and additional studies are needed to determine whether our findings are generalizable to other ethnic groups. Even though the randomized clinical trial is thought to be the best model to test gene-environment interactions, we acknowledge that replication in diverse populations is needed to verify our findings. In addition, no measurement of ghrelin was a potential limitation, and thus, FTO genotype-diets interaction on ghrelin could not be tested.
In conclusion, we showed that the FTO rs9939609 modified food cravings in response to weight-loss diets in a 2-y randomized trial. Individuals with the FTO rs9939609 A allele might obtain more benefits in decreases in food cravings and appetite than those without this allele in response to a high-protein diet. These novel findings provide supportive evidence for the notion of a personalized nutrition intervention in preventing obesity.
Acknowledgments
We are particularly grateful to all participants in the trial for their dedication and contribution to the research.
The authors’ responsibilities were as follows—TH, QQ, and LQ: contributed to study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript; YL, FBH, GAB, DAW, and FMS: contributed to the study concept and design and critical revision of the manuscript; TH and QQ: contributed to the statistical analysis; GAB, DAW, and FMS: were involved in the collection and analysis of data and funding of the initial project; FMS, FBH, and LQ: contributed to the administration, material support, and study supervision; and LQ: is the guarantor of this work and, as such, had full access to all the data in the study and took responsibility for the integrity of data and the accuracy of the data analysis. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: POUNDS LOST, Preventing Overweight Using Novel Dietary Strategies; SNP, single nucleotide polymorphism; VAS, visual analog scale.
REFERENCES
- 1.Karra E, O'Daly OG, Choudhury AI, Yousseif A, Millership S, Neary MT, Scott WR, Chandarana K, Manning S, Hess ME, et al. A link between FTO, ghrelin, and impaired brain food-cue responsivity. J Clin Invest 2013;123:3539–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gulati P, Cheung MK, Antrobus R, Church CD, Harding HP, Tung YC, Rimmington D, Ma M, Ron D, Lehner PJ, et al. Role for the obesity-related FTO gene in the cellular sensing of amino acids. Proc Natl Acad Sci USA 2013;110:2557–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheung MK, Gulati P, O'Rahilly S, Yeo GS. FTO expression is regulated by availability of essential amino acids. Int J Obes (Lond) 2013;37:744–7. [DOI] [PubMed] [Google Scholar]
- 4.Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, McManus K, Champagne CM, Bishop LM, Laranjo N, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med 2009;360:859–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson GH, Catherine NL, Woodend DM, Wolever TM. Inverse association between the effect of carbohydrates on blood glucose and subsequent short-term food intake in young men. Am J Clin Nutr 2002;76:1023–30. [DOI] [PubMed] [Google Scholar]
- 6.Samra RA, Anderson GH. Insoluble cereal fiber reduces appetite and short-term food intake and glycemic response to food consumed 75 min later by healthy men. Am J Clin Nutr 2007;86:972–9. [DOI] [PubMed] [Google Scholar]
- 7.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007;316:889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cecil JE, Tavendale R, Watt P, Hetherington MM, Palmer CN. An obesity-associated FTO gene variant and increased energy intake in children. N Engl J Med 2008;359:2558–66. [DOI] [PubMed] [Google Scholar]
- 9.Qi Q, Bray GA, Smith SR, Hu FB, Sacks FM, Qi L. Insulin receptor substrate 1 gene variation modifies insulin resistance response to weight-loss diets in a 2-year randomized trial: the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial. Circulation 2011;124:563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wardle J, Llewellyn C, Sanderson S, Plomin R. The FTO gene and measured food intake in children. Int J Obes (Lond) 2009;33:42–5. [DOI] [PubMed] [Google Scholar]
- 11.Timpson NJ, Emmett PM, Frayling TM, Rogers I, Hattersley AT, McCarthy MI, Davey Smith G. The fat mass- and obesity-associated locus and dietary intake in children. Am J Clin Nutr 2008;88:971–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Luis DA, Aller R, Conde R, Izaola O, Sagrado MG, Castrodeza Sanz J. The rs9939609 gene variant in FTO modified the metabolic response of weight loss after a 3-month intervention with a hypocaloric diet. J Investig Med 2013;61:22–6. [DOI] [PubMed] [Google Scholar]
- 13.Rebello CJ, Johnson WD, Martin CK, Xie W, O'Shea M, Kurilich A, Bordenave N, Andler S, Klinken BJ, Chu YF, et al. Acute effect of oatmeal on subjective measures of appetite and satiety compared to a ready-to-eat breakfast cereal: a randomized crossover trial. J Am Coll Nutr 2013;32:272–9. [DOI] [PubMed] [Google Scholar]
- 14.Stocks T, Angquist L, Hager J, Charon C, Holst C, Martinez JA, Saris WH, Astrup A, Sorensen TI, Larsen LH. TFAP2B -dietary protein and glycemic index interactions and weight maintenance after weight loss in the DiOGenes trial. Hum Hered 2013;75:213–9. [DOI] [PubMed] [Google Scholar]
- 15.Bendtsen LQ, Lorenzen JK, Bendsen NT, Rasmussen C, Astrup A. Effect of dairy proteins on appetite, energy expenditure, body weight, and composition: a review of the evidence from controlled clinical trials. Adv Nutr 2013;4:418–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilhooly CH, Das SK, Golden JK, McCrory MA, Dallal GE, Saltzman E, Kramer FM, Roberts SB. Food cravings and energy regulation: the characteristics of craved foods and their relationship with eating behaviors and weight change during 6 months of dietary energy restriction. Int J Obes (Lond) 2007;31:1849–58. [DOI] [PubMed] [Google Scholar]
- 17.Xu M, Qi Q, Liang J, Bray GA, Hu FB, Sacks FM, Qi L. Genetic determinant for amino acid metabolites and changes in body weight and insulin resistance in response to weight-loss diets: the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial. Circulation 2013;127:1283–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, Qi Q, Liang J, Hu FB, Sacks FM, Qi L. Neuropeptide Y promoter polymorphism modifies effects of a weight-loss diet on 2-year changes of blood pressure: the preventing overweight using novel dietary strategies trial. Hypertension 2012;60:1169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, Szapary PO, Rader DJ, Edman JS, Klein S. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med 2003;348:2082–90. [DOI] [PubMed] [Google Scholar]
- 20.Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA 2005;293:43–53. [DOI] [PubMed] [Google Scholar]
- 21.Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, Golan R, Fraser D, Bolotin A, Vardi H, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med 2008;359:229–41. [DOI] [PubMed] [Google Scholar]