Abstract
Background: Previous studies that reported an association of dietary Na+ intake with metabolic syndrome were limited by the use of imprecise measures of obesity, Na+ intake, or exclusion of multiethnic populations. The effect of dietary K+ intake on obesity is less well described.
Objective: We hypothesized that high dietary Na+ and low K+, based on the ratio of urinary Na+ to K+ (U[Na+]/[K+]) in a first-void morning urinary sample, is independently associated with total body fat.
Design: In a prospective population-based cohort, 2782 participants in the community-dwelling, probability-sampled, multiethnic Dallas Heart Study were analyzed. The primary outcome established a priori was total-body percentage fat (TBPF) measured by dual-energy X-ray absorptiometry. The main predictor was U[Na+]/[K+]. Robust linear regression was used to explore an independent association between U[Na+]/[K+] and TBPF. The analyses were stratified by sex and race after their effect modifications were analyzed.
Results: Of the cohort, 55.4% were female, 49.8% African American, 30.8% white, 17.2% Hispanic, and 2.2% other races. The mean (±SD) age was 44 ± 10 y, BMI (in kg/m2) was 30 ± 7, TBPF was 32 ± 10%, and U[Na+]/[K+] was 4.2 ± 2.6; 12% had diabetes. In the unadjusted and adjusted models, TBPF increased by 0.75 (95% CI: 0.25, 1.25) and 0.43 (0.15, 0.72), respectively (P = 0.003 for both), for every 3-unit increase in U[Na+]/[K+]. A statistically significant interaction was found between race and U[Na+] /[K+], so that the non–African American races had a higher TBPF than did the African Americans per unit increase in U[Na+]/[K+] (P-interaction < 0.0001 for both). No interaction was found between sex and U[Na+]/[K+].
Conclusions: The ratio of dietary Na+ to K+ intake may be independently associated with TBPF, and this association may be more pronounced in non–African Americans. Future studies should explore whether easily measured spot U[Na+]/[K+] can be used to monitor dietary patterns and guide strategies for obesity management.
INTRODUCTION
Excess ingestion of Na+ in the diet leads to hypertension, and a decreased Na+ intake leads to a reduction in blood pressure (1). In the United States, there has been a significant increase in Na+ intake of ∼55% from 1983 to 1998 with some stabilization thereafter (2). An increased intake in dietary Na+ is associated with increased thirst and ingestion of water and/or sweetened, carbonated soft drinks (3, 4) that may be linked to the increased prevalence of overweight and obesity (5). A question arises about whether excess ingestion of dietary Na+ contributes directly to obesity simply because of the physiologic renal response to maintain extracellular and whole-body salt constant by excreting excess dietary sodium intake or whether other mechanisms are involved. For example, in the case of hypertension, classic studies and observational data suggest that there is an interaction between the roles of dietary Na+ excess and low K+ intake in the pathogenesis of hypertension, such that the combined effect of diets high in Na+ and low in K+ on blood pressure seem greater than either alone, especially in those of the African race.
The combined role of dietary Na+ and K+ in the pathogenesis of obesity is less clear. Although observational studies have reported a high Na+ intake measured by 24-h urinary Na+ excretion to be associated with mortality and cardiovascular outcomes independent of blood pressure (6–8), a similar consistent association between dietary K+ and poor outcomes is less well delineated (9–11). Few epidemiologic studies reported a positive correlation between high dietary Na+ and low K+ and BMI (12), the metabolic syndrome (13), and diabetes mellitus (5, 14). However, studies were limited by using BMI (in kg/m2) as a measure of body fat content and by exclusion of various ethnic groups or use of dietary diaries that are at risk of recall bias (15).
Given the limitations of previous studies, we evaluated the association between the ratio of dietary Na+ to K+ intake, based on the ratio of urinary Na+ to K+ (U[Na+]/[K+])5 in a first-void morning urine sample, and obesity in a multiethnic cohort consisting of 50% females, 50% African Americans, and 20% Hispanics and of other racial groups. In addition, we used total-body percentage fat (TBPF) measured by dual-energy X-ray absorptiometry (DXA) (16) as a measure of obesity, because BMI does not clearly distinguish between fat and muscle mass, especially in African Americans (17). We hypothesized that U[Na+]/[K+] would be independently associated with TBPF, even after adjustment for confounding variables such as blood pressure, diabetes, blood glucose, serum triglyceride concentrations, and other clinical factors. We also explored whether this association would vary based on racial and sex differences.
SUBJECTS AND METHODS
Study sample
The Dallas Heart Study is a single-site, multiethnic, population-based probability sample of community-dwelling Dallas County residents aged 18–65 y that was designed to study the differences in social and biologic variables among various ethnic groups at the community level to understand potential mechanisms for cardiovascular disease (18). Enrollment began in July 2000. Data collection was performed in 3 separate visits. An extensive household computer-assisted 60-min interview by trained field personnel was conducted in 6101 participants (visit 1) to collect health-related data, vital signs, and weight measurements. A probability-based subset of 3557 persons aged 30–65 y was derived from visit 1, which provided in-home collection of fasting venous blood and early-morning first-void urine samples (visit 2). A third visit (visit 3) consisted of extensive clinic examinations and advanced imaging conducted by trained personnel in the clinical facilities of the University of Texas Southwestern Medical Center, completed in 2971 participants. Our analysis included 2782 participants who completed visits 2 and 3, with data available for urinary electrolytes, and who underwent DXA to assess total-body fat composition (18). The weighted estimates of measured variables derived from visits 1–3 in the Dallas Heart Study cohort are consistent and congruent with the population estimates of the general US population (18).
Clinical variables
Race and ethnic categories were self-assigned by using a similar structured list of categories as used in the third NHANES (19). For the purposes of this analysis, race was classified into 3 categories: African American, white, and Hispanic and other. Five blood pressure measurements were taken at each visit in the seated position by using an automatic oscillometric device (series 52,000; Welch Allyn Inc), previously validated against direct catheter-based intra-arterial pressure (18). Personnel were trained to use the device and to select an appropriately sized cuff. The mean of the third, fourth, and fifth measurements was used for analysis. BMI was expressed as weight in kilograms divided by height squared in meters. Diabetes mellitus was defined on the basis of self-report combined with insulin or oral hypoglycemic agent use, or fasting glucose ≥6.99 mmol/L, or nonfasting glucose ≥11.10 mmol/L (18). An early morning first-void urine sample was collected at visit 2 and maintained at 4°C for ≤4 h before processing in the central laboratory. The ratio of urinary Na+ to K+ (U[Na+]/[K+]), the main independent variable, was calculated by dividing urinary sodium by potassium concentrations, each expressed as mEq/L. Fasting blood was drawn by venipuncture at visit 2 and analyzed for a variety of laboratory variables.
Outcome measures
The primary outcome measure established a priori for this analysis was TBPF measured by DXA (Delphi W scanner; Hologic) with Discovery software version 12.2 in kilograms, normalized for total body weight, and expressed as a percentage (16). The DXA scan is a modern validated method for estimating total-body fat composition. It generates accurate body fat measurements comparable with the standard 4-compartment model, which although comprehensive, requires anthropometric measurements, body density by hydrodensitometry, total body water by radioisotope labeling, and total-body bone mineral mass. The DXA scan is also comparable with multislice computed tomographic imaging for estimating body fat (20). In addition, the DXA method is superior to BMI because previous studies have shown that the diagnostic accuracy of BMI for obesity is limited when compared with the 4-compartment and bioelectrical impedance methods, particularly for individuals in the intermediate BMI ranges (16–21).
Statistical analyses
Baseline characteristics were stratified based on the 3 categories of race (African American, whites, Hispanics and others) and 2 sex categories. Categorical variables were compared by using the chi-square test and continuous variables by using Student's t test or 1-factor ANOVA, with Bonferroni adjustment to P values. Robust linear regression was used to assess the association of U[Na+]/[K+] as the main independent variable with TBPF as the primary outcome. Robust regression, instead of ordinary linear regression, was chosen to mitigate the potential effects of the right-skewed distribution of U[Na+]/[K+] on the regression fit (22). This method generates regression parameter estimates that are less sensitive to the effect of extreme values of predictors than ordinary linear regression. Potential outliers (influence points) were analyzed by using regression diagnostics and were considered acceptable for the model.
Changes in the outcome variable were reported as a parameter estimate for each 3-unit increase in U[Na+]/[K+]. Clinically relevant and/or statistically significant covariates in univariate analyses were included in the multivariable analyses. Participants with missing data for covariates were excluded from the specific multivariable models that included those covariates. The interaction of sex and U[Na]+/[K]+ was analyzed. The interaction of race and U[Na]+/[K]+ was also tested in a separate model, with African American as the referent race. Analyses were then stratified by race and sex. To test whether a potential clinically significant change in BMI in individuals between the visits did not affect the results, sensitivity analyses were performed by excluding participants who had a ≥5% change in BMI between visits 1 and 3.
All statistical analyses used a 2-sided P value <0.05 for significance. For interaction terms, a P value <0.1 was established a priori for statistical significance to promote discovery of potential interactions between variables of interest. All analyses were performed by using SAS Enterprise Guide version 3.0 and SAS version 9.1.3 software (SAS Institute).
RESULTS
Characteristics of study participants
The exclusionary cascade used to derive the sample is shown in Figure 1. For the 2782 participants, the mean (±SD) age was 44.4 ± 9.9 y, 55.4% were female, 49.8% were African Americans, 30.8% were white, 17.2% were Hispanic, and 2.2% were of other races. Twelve percent had diabetes mellitus, and 36% had hypertension. The mean (±SD) BMI was 30.2 ± 6.8, and systolic and diastolic blood pressures were 125.0 ± 18.9 and 78.4 ± 10.2 mm Hg, respectively. TBPF by DXA was 32.2% ± 9.9%, and U[Na+]/[K+] was 4.2 ± 2.6 (Table 1).
FIGURE 1.
Derivation of study sample. DXA, dual-energy X-ray absorptiometry.
TABLE 1.
Baseline characteristics of the cohort1
| Variables | Entire cohort (n = 2782) | African Americans(n = 1386) | Whites(n = 857) | Hispanics and others(n = 539) | P value | Men(n = 1242) | Women(n = 1540) | P value |
| Age (y) | 44.4 ± 9.92 | 45.3 ± 10.0a | 45.2 ± 9.8a | 40.8 ± 9.1b | <0.0001 | 44.3 ± 9.7 | 44.5 ± 10.1 | 0.78 |
| Female [n (%)] | 1,540 (55.4) | 796 (57.4)a | 442 (51.6)b | 302 (56.0)a | 0.02 | |||
| DM [n (%)] | 325 (11.7) | 200 (14.4)a | 58 (6.8)b | 67 (12.4)a | <0.0001 | 144 (11.6) | 181 (11.8) | 0.90 |
| HTN [n (%)] | 1000 (36.0) | 665 (48.0)a | 232 (27.1)b | 103 (19.1)b | <0.0001 | 433 (34.9) | 567 (36.8) | 0.29 |
| BMI (kg/m2) | 30.2 ± 6.8 | 31.2 ± 7.1a | 28.8 ± 6.1b | 30.1 ± 6.2c | <0.0001 | 28.8 ± 5.2 | 31.3 ± 7.6 | <0.0001 |
| TBPF (%) | 32.2 ± 9.9 | 31.9 ± 10.6a | 32.0 ± 9.3 a | 33.0 ± 8.9a | 0.08 | 24.1 ± 6.6 | 38.6 ± 6.9 | <0.0001 |
| Systolic BP (mm Hg) | 125.0 ± 18.9 | 130.5 ± 19.9a | 120.0 ± 15.0b | 118.9 ± 17.4b | <0.0001 | 127.7 ± 17.4 | 122.8 ± 19.7 | <0.0001 |
| Diastolic BP (mm Hg) | 78.4 ± 10.2 | 81.1 ± 10.6a | 76.0 ± 8.7b | 75.1 ± 9.6b | <0.0001 | 79.3 ± 10.0 | 77.6 ± 10.4 | <0.0001 |
| Urinary Na+ (mEq/L) | 121.4 ± 55.9 | 125.9 ± 58.4a | 111.1 ± 50.5b | 126.4 ± 55.3a | <0.0001 | 123.8 ± 54.8 | 119.5 ± 56.7 | 0.04 |
| Urinary K+ (mEq/L) | 37.5 ± 24.8 | 38.6 ± 25.5a | 34.0 ± 22.7b | 40.6 ± 25.7a | <0.0001 | 39.3 ± 25.8 | 36.1 ± 23.9 | <0.001 |
| Ratio of urinary Na+ to K+ | 4.2 ± 2.6 | 4.3 ± 2.8a | 4.2 ± 2.5a | 4.0 ± 2.3a | 0.07 | 4.1 ± 2.7 | 4.2 ± 2.6 | 0.39 |
| Serum glucose (mmol/L) | 5.7 ± 2.4 | 5.9 ± 2.8a | 5.4 ± 1.7b | 5.9 ± 2.4a | <0.0001 | 5.8 ± 2.4 | 5.7 ± 2.5 | 0.38 |
| Serum triglycerides (mmol/L) | 1.4 ± 1.2 | 1.2 ± 1.1a | 1.6 ± 1.2b | 1.7 ± 1.4b | <0.0001 | 1.6 ± 1.4 | 1.3 ± 1.0 | <0.0001 |
Categorical variables were compared by using the chi-square test and continuous variables by using Student's t test or one-factor ANOVA. Means with different superscript letters are significantly different, P < 0.05 (Bonferroni-corrected t tests). BP, blood pressure; DM, diabetes mellitus; HTN, hypertension; TBPF, total-body percentage fat.
Mean ± SD (all such values).
Those of Hispanic origin or other races were about 4 y younger than white and African American participants (P < 0.0001). There was a significantly lower percentage of women and patients with diabetes in the white group than in the other race groups. The prevalence of hypertension was highest among African Americans, and this racial group also had the highest systolic and diastolic blood pressures (P < 0.0001). BMI was highest among African Americans, followed by Hispanics and others (P value significant for all 2-way comparisons). Of the cardiometabolic risk factors, the serum glucose concentration was significantly lower in whites than in the other 2 racial groups, and serum triglyceride concentration was lower in African Americans than in other races. However, no statistically significant difference in U[Na+]/[K+] or TBPF was found (Table 1).
Men and women had similar ages, and the proportions of patients with diabetes and hypertension were similar. However, both systolic and diastolic blood pressures were higher in men than in women (Table 1). Although women had a higher BMI and TBPF (38.6 ± 6.9% compared with 24.1 ± 6.6% in men; P < 0.0001), the triglyceride concentration was higher in men. No statistically significant sex differences in the serum glucose concentration or in U[Na+]/[K+] were found.
Association between obesity and U[Na+]/[K+]
U[Na+]/[K+] was independently associated with TBFP. In the unadjusted model (model 1), TBPF increased by 0.75 (95% CI: 0.25, 1.25; P = 0.003) for each 3-unit increase in U[Na+]/[K+]. This association remained significant even after adjustment for age, race, sex, presence of diabetes mellitus, systolic and diastolic blood pressures, and serum glucose and triglycerides. In the adjusted model (model 2), TBPF increased by 0.43 (95% CI: 0.15, 0.72; P = 0.003) for each 3-unit increase in U[Na+]/[K+] (Table 2). A sensitivity analysis excluding participants who had a change in BMI of >5% between visits 1 and 3 (model 3) did not change the significance (Table 2). The variances in TBPF explained by U[Na+]/[K+] for models 1, 2, and 3 were 0.32%, 0.01%, and 0.02%, respectively.
TABLE 2.
Robust linear regression to show the association of total-body percentage fat (dependent variable) with the ratio of urinary Na+ to K+ (independent variable)
| Independent variable1 | n | Change (95% CI)2 | P value |
| Model 1 | 2782 | 0.75 (0.25, 1.25) | 0.003 |
| Model 2 | 2779 | 0.43 (0.15, 0.72) | 0.003 |
| Model 3 | 1741 | 0.42 (0.08, 0.77) | 0.02 |
Model 1: univariate model. Model 2: multivariate model adjusted for age, sex, race, diabetes mellitus, systolic blood pressure, diastolic blood pressure, and serum glucose and triglyceride concentrations. Model 3: sensitivity analysis excluding participants who had a change in BMI ≥5% between visits 1 and 3. Covariates in model 3 are the same as in model 2.
Unit for change in total-body percentage fat is expressed as the percentage per 3-unit change in the ratio of urinary Na+ to K+.
Race interactions
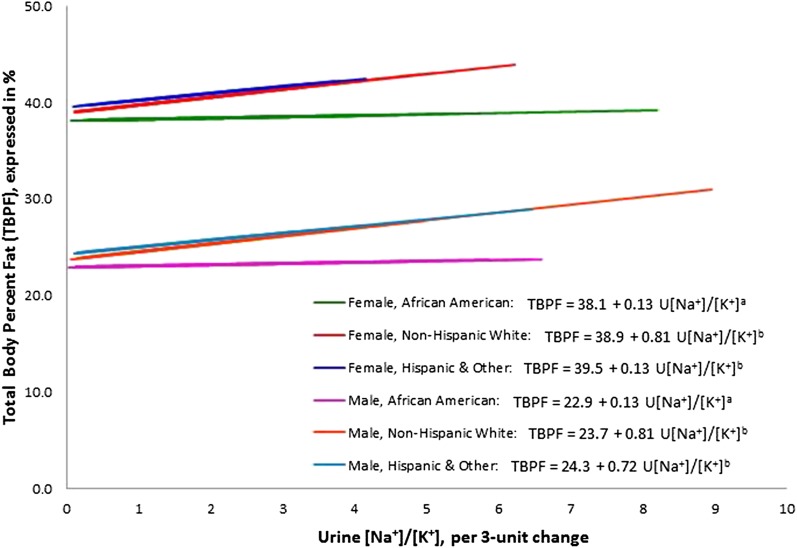
A statistically significant interaction between race and U[Na+]/[K+] (P-overall interaction = 0.08) was found after adjustment for age, race, sex, presence of diabetes mellitus, systolic and diastolic blood pressures, and serum glucose and triglycerides. Specifically, for any given mean value of U[Na+]/[K+], TBPF increased by a greater magnitude for whites and Hispanics and others as compared with African Americans as the referent (P < 0.0001 for both bivariate interaction terms). As seen in multivariable-stratified analyses (Table 3), TBPF increased significantly by 0.65 and 0.73 for each 3-unit increase in U[Na+]/[K+] in whites and Hispanics and others, respectively, which was not observed in African Americans. In the sensitivity analysis, the same pattern of association was observed.
TABLE 3.
Robust linear regression, stratified by race, to show the association of total-body percentage fat (dependent variable) with the ratio of urinary Na+ to K+ (independent variable)
| African Americans |
Whites |
Hispanics and others |
|||||||
| Independent variable1 | n | Change (95% CI)2 | P | n | Change (95% CI)2 | P | n | Change (95% CI)2 | P |
| Model 1 | 1386 | 0.65 (−0.06, 1.37) | 0.07 | 857 | 1.12 (0.27, 1.97) | 0.01 | 539 | 0.30 (−0.87, 1.46) | 0.62 |
| Model 2 | 1386 | 0.17 (−0.24, 0.57) | 0.41 | 855 | 0.65 (0.14, 1.17) | 0.01 | 538 | 0.73 (0.10, 1.35) | 0.02 |
| Model 3 | 825 | 0.03 (−0.48, 0.55) | 0.90 | 619 | 0.66 (0.09, 1.24) | 0.02 | 297 | 1.14 (0.35, 1.94) | 0.005 |
Model 1: univariate model. Model 2: multivariate model adjusted for age, sex, diabetes mellitus, systolic blood pressure, diastolic blood pressure, and serum glucose and triglyceride concentrations. Model 3: sensitivity analysis excluding participants who had a change in BMI ≥5% between visits 1 and 3. Covariates in model 3 are the same as in model 2. Adjusted for age, race, sex, presence of diabetes mellitus, systolic and diastolic blood pressures, and serum glucose and triglyceride concentrations, there was a statistically significant interaction between race and the ratio of urinary Na+ to K+ (P-overall interaction = 0.08). The P values for bivariate interactions between the ratio of urinary Na+ to K+ and whites and between the ratio of urinary Na+ to K+ and Hispanics and others, with African American race as the referent category, were both <0.0001.
Unit for change in total-body percentage fat is expressed as the percentage per 3-unit change in the ratio of urinary Na+ to K+.
Sex interactions
No statistically significant interaction between sex and U[Na+]/[K+] was found on the association with TBPF (P-interaction > 0.1). In the unadjusted analyses (Table 4), TBPF increased by 0.59 and 0.62 for each 3-unit increase in U[Na+]/[K+] in women and men, respectively. This association remained significant for women, even after adjustment for covariates. However, statistical significance was no longer observed in men in the adjusted model. Women showed a significant association, even in the sensitivity analysis, when those with a change in BMI of ≥5% were excluded.
TABLE 4.
Robust linear regression, stratified by sex, to show the association of total-body percentage fat (dependent variable) with the ratio of urinary Na+ to K+ (independent variable)
| Men |
Women |
|||||
| Independent variable1 | n | Change (95% CI)2 | P | n | Change (95% CI)2 | P |
| Model 1 | 1242 | 0.62 (0.19, 1.05) | 0.004 | 1540 | 0.59 (0.18, 1.00) | 0.005 |
| Model 2 | 1241 | 0.38 (−0.03, 0.78) | 0.07 | 1538 | 0.43 (0.04, 0.83) | 0.03 |
| Model 3 | 811 | 0.29 (−0.20, 0.78) | 0.24 | 930 | 0.51 (0.02, 0.99) | 0.04 |
Model 1: univariate model. Model 2: multivariate model adjusted for age, race, diabetes mellitus, systolic blood pressure, diastolic blood pressure, and serum glucose and triglyceride concentrations. Model 3: sensitivity analysis excluding subjects who had a change in BMI ≥5% between visits 1 and 3. Covariates in model 3 are the same as in model 2. There was no statistically significant interaction between sex and the ratio of urinary Na+ to K+ on the association with total-body percentage fat (P-interaction > 0.1).
Unit for change in total-body percentage fat is expressed as the percentage per 3-unit change in the ratio of urinary Na+ to K+.
The regression of U[Na+]/[K+] on TBPF and race interactions, adjusted for age, sex, diabetes mellitus, systolic blood pressure, diastolic blood pressure, and serum glucose and triglyceride concentrations, is shown in Figure 2. The slopes of the regression lines are different between African Americans (the referent) and whites and Hispanics and others.
FIGURE 2.
Linear regression of U[Na+]/[K+] on TBPF and race interactions, adjusted for age, sex, diabetes mellitus, systolic blood pressure, diastolic blood pressure, and serum glucose and triglyceride concentrations (covariates from model 2). Values are plotted at the mean levels of the covariates. Lines denoted by different letters have a significantly different slope (P-interaction < 0.10) from that of the referent category of African Americans. TBPF, total-body percentage fat; U[Na+]/[K+], ratio of urinary Na+ to K+.
DISCUSSION
This study reports an independent direct association between U[Na+]/[K+] and total body fat, measured by DXA, even after adjustment for blood pressure, diabetes, and serum glucose and triglyceride concentrations. Moreover, this study was conducted in a multiethnic, population-based cohort in which we found racial differences in the association of U[Na+]/[K+] with total body fat. The rise in TBPF was greater in whites and Hispanic and others than in African Americans for each unit increase in U[Na+]/[K+].
More than 2 decades ago, the large epidemiologic INTERSALT study reported a bivariate positive correlation between U[Na+]/[K+] and BMI (r = 0.06, P value not reported) (12). However, <10% of participants were of African descent, and the analysis was not adjusted for confounding clinical variables. More recently, Hoffmann and Cubeddu (13) reported that urinary sodium excretion was higher in those with metabolic syndrome (defined by Adult Treatment Panel III guidelines) than in those without it in 766 Venezuelan male and female participants. There was also a weak correlation between 24-h urinary K+ excretion and body weight, BMI, and waist-to-hip ratio only in women (r = 0.14, P = 0.001). Our study extends the observations on the association between U[Na+]/[K+] and percentage body fat in a contemporary population-based cohort consisting of an equal percentage of men and women, of whom ∼50% were of African descent. In fact, the findings remained significant despite adjustments for age, sex, race, blood pressure, diabetes mellitus, and serum glucose and triglyceride concentrations.
Few other studies that reported an association of urinary Na+ and K+ measurements with type 2 diabetes mellitus or BMI were instructive but had varying results. Hu et al (14) reported that urinary Na+ but not K+ excretion was an independent predictor of incident type 2 diabetes mellitus in Finnish participants (HR: 2.24; 95% CI: 1.32, 3.79), even after adjustment for age, sex, blood pressure, obesity, physical inactivity, and antihypertensive drug treatment. When compared with the US population, participants in the Finnish study were more likely to be white and to consume a diet higher in K+ content (68–90 mEq/d), but were less likely to be obese (14). Colditz et al (23) reported that dietary K+ was inversely associated with the risk of developing type 2 diabetes mellitus in 84,941 American women from the Nurses’ Health Study followed for 6 y. This study relied on dietary recall for estimations of K+ intake. Mente et al (24) reviewed a highly selected cohort of 220 kidney stone patients and reported that high 24-h urinary K+ inversely correlated with BMI (r = −0.15, P < 0.05).
We also report a statistically significant interaction between race and U[Na+]/[K+], so that for any given mean value of U[Na+]/[K+], TBPF increased by a greater magnitude in whites and Hispanics and other racial groups than in African Americans, which indicated that a diet high in Na+ and low in K+ may have a greater association with total body fat in non–African American individuals than it does in African Americans. This may also indicate that Hispanics and whites who make unhealthy dietary choices may be more susceptible to obesity than African Americans. Although higher BMIs have been reported in certain ethnic groups, such as Hispanics (25)—similar to our report from a multiethnic cohort—there is still a paucity of information regarding racial differences in the effect of the urinary or dietary ratio of Na+ to K+ on measures of obesity.
The methods used in this study also differed from those used in previous studies. First, we measured body fat by DXA—a method that is increasingly preferred to the use of BMI, principally because the latter fails to measure body fat in various ethnic groups (26, 27). Our study population included >50% African Americans, and others have shown that BMI is inadequate to assess body fat in this ethnic group (28) and that the density of lean body mass is greater in those of African ethnicity than in whites. Second, we used U[Na+]/[K+] as an index of the dietary intake of Na+ and K+. Data derived by using this approach are preferred to data derived from 7-d dietary recalls because it involves a biochemical measure of the nutrients of interest. Spot urine samples can provide low-burden, low-cost close approximations of dietary salt intake at the population level, such as reported in an analysis of the INTERSALT study, which provides better correlation coefficients for group-level analyses involving population means (29). However, measurements in spot urine samples compared with 24-h urine collections may have low levels of reproducibility and reliability at the individual level, especially at very low and high salt intakes (30). Although 24-h urine samples were not available for this cohort to validate spot urine ratios, previous studies reported a correlation between spot U[Na+]/[K+] and 24-h measurements in pairs of urine samples from healthy individuals (30, 31). Despite this limitation, we minimized measurement error by calculating the early-morning spot U[Na+]/[K+]. Importantly, the mean U[Na+]/[K+] in our study was 4.2, which reflects an average American diet of 100 to 300 mEq Na+/d and 30 to 70 mEq K+/d (32, 33), and extends generalizability.
Another limitation of this analysis was that the percentage of variance in body fat explained by U[Na+]/[K+] across models was modest, although there was a statistically significant association between U[Na+]/[K+] and total body fat, even after adjustment for potential confounders. Hence, there may remain other unexplained (and perhaps unmeasured) sources of variability that would lead to a better model fit for predicting total body fat. Finally, although 50% of participants were African American, the lack of an association between U[Na+]/[K+] and total body fat in this subgroup does not necessarily mean that this association does not exist given the observational nature of the study, and these results need to be confirmed in future longitudinal studies.
Why consider U[Na+]/[K+] in relation to obesity? First, salt intake is a major determinant of sugar-sweetened soft drink consumption (4), and soft drink consumption is associated with weight gain and incident diabetes mellitus (3, 5). A diet high in fat is generally high in Na+ and low in K+ (5, 34). Second, salt sensitivity may be related to insulin sensitivity (35) through the interplay of an abnormal renin-angiotensin system. Thus, a diet high in Na+ and low in K+ may contribute to obesity.
In summary, we report an independent association between the ratio of dietary Na+ to K+ intake and percentage body fat in a large multiethnic population-based sample, even after control for other cardiovascular risk factors. Our findings raise the possibility of using easily measured U[Na+]/[K+] as a surrogate of a poor-quality diet associated with obesity. We further report a racial interaction so that the effect of dietary Na+ and K+ on obesity may be more pronounced in non–African American racial groups than in African Americans. Future large, prospective, longitudinal studies should explore this interaction and investigate whether easily measured spot urine U[Na+]/[K+] could be used by clinicians to monitor dietary patterns in individuals and guide strategies for obesity management.
Acknowledgments
The authors’ responsibilities were as follows—NJ and SSH: conception and design or analysis and interpretation of the data, drafting of the manuscript or revising it critically for important intellectual content, and final approval of the manuscript submitted; ATM: analysis and interpretation of the data and final approval of the manuscript submitted; and IJN, EFE, and GLV: revision of the manuscript critically for important intellectual content and final approval of the manuscript submitted. None of the authors declared a conflict of interest.
Footnotes
Abbreviations used: DXA, dual-energy X-ray absorptiometry; TBPF, total-body percentage fat; U[Na+]/[K+], ratio of urinary Na+ to K+.
REFERENCES
- 1.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, III, Simons-Morton DG, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- 2.Briefel RR, Johnson CL. Secular trends in dietary intake in the United States. Annu Rev Nutr 2004;24:401–31. [DOI] [PubMed] [Google Scholar]
- 3.Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, Hu FB. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA 2004;292:927–34. [DOI] [PubMed] [Google Scholar]
- 4.He FJ, Marrero NM, MacGregor GA. Salt intake is related to soft drink consumption in children and adolescents: a link to obesity? Hypertension 2008;51:629–34. [DOI] [PubMed] [Google Scholar]
- 5.Schulze MB, Hoffmann K, Manson JE, Willett WC, Meigs JB, Weikert C, Heidemann C, Colditz GA, Hu FB. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr 2005;82:675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). BMJ 2007;334:885–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stolarz-Skrzypek K, Kuznetsova T, Thijs L, Tikhonoff V, Seidlerová J, Richart T, Jin Y, Olszanecka A, Malyutina S, Casiglia E, et al. Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA 2011;305:1777–85. [DOI] [PubMed] [Google Scholar]
- 8.O'Donnell MJ, Yusuf S, Mente A, Gao P, Mann JF, Teo K, McQueen M, Sleight P, Sharma AM, Dans A, et al. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA 2011;306:2229–38. [DOI] [PubMed] [Google Scholar]
- 9.Cook NR, Obarzanek E, Cutler JA, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK, Trials of Hypertension Prevention Collaborative Research Group. Joint effects of sodium and potassium intake on subsequent cardiovascular disease: the Trials of Hypertension Prevention follow-up study. Arch Intern Med 2009;169:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geleijnse JM, Witteman JC, Stijnen T, Kloos MW, Hofman A, Grobbee DE. Sodium and potassium intake and risk of cardiovascular events and all-cause mortality: the Rotterdam Study. Eur J Epidemiol 2007;22:763–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bazzano LA, He J, Ogden LG, Loria C, Vupputuri S, Myers L, Whelton PK. Dietary potassium intake and risk of stroke in US men and women: National Health and Nutrition Examination Survey I epidemiologic follow-up study. Stroke 2001;32:1473–80. [DOI] [PubMed] [Google Scholar]
- 12.Dyer AR, Elliott P. The INTERSALT study: relations of body mass index to blood pressure. INTERSALT Co-operative Research Group. J Hum Hypertens 1989;3:299–308. [PubMed] [Google Scholar]
- 13.Hoffmann IS, Cubeddu LX. Salt and the metabolic syndrome. Nutr Metab Cardiovasc Dis 2009;19:123–8. [DOI] [PubMed] [Google Scholar]
- 14.Hu G, Jousilahti P, Peltonen M, Lindstrom J, Tuomilehto J. Urinary sodium and potassium excretion and the risk of type 2 diabetes: a prospective study in Finland. Diabetologia 2005;48:1477–83. [DOI] [PubMed] [Google Scholar]
- 15.Rosell MS, Hellenius ML, de Faire UH, Johansson GK. Associations between diet and the metabolic syndrome vary with the validity of dietary intake data. Am J Clin Nutr 2003;78:84–90. [DOI] [PubMed] [Google Scholar]
- 16.Vega GL, Adams-Huet B, Peshock R, Willett D, Shah B, Grundy SM. Influence of body fat content and distribution on variation in metabolic risk. J Clin Endocrinol Metab 2006;91:4459–66. [DOI] [PubMed] [Google Scholar]
- 17.Gallagher D, Visser M, Sepulveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol 1996;143:228–39. [DOI] [PubMed] [Google Scholar]
- 18.Victor RG, Haley RW, Willett DL, Peshock RM, Vaeth PC, Leonard D, Basit M, Cooper RS, Iannacchione VG, Visscher WA, et al. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol 2004;93:1473–80. [DOI] [PubMed] [Google Scholar]
- 19.CDC. National Health and Nutrition Examination survey manual. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/fieldint.pdf (cited 4 December 2013).
- 20.Salamone LM, Fuerst T, Visser M, Kern M, Lang T, Dockrell M, Cauley JA, Nevitt M, Tylavsky F, Lohman TG. Measurement of fat mass using DEXA: a validation study in elderly adults. J Appl Physiol 2000;89:345–52. [DOI] [PubMed] [Google Scholar]
- 21.Deurenberg P, Andreoli A, Borg P, Kukkonen-Harjula K, de Lorenzo A, van Marken Lichtenbelt WD, Testolin G, Vigano R, Vollaard N. The validity of predicted body fat percentage from body mass index and from impedance in samples of five European populations. Eur J Clin Nutr 2001;55:973–9. [DOI] [PubMed] [Google Scholar]
- 22.Hedayati SS, Minhajuddin AT, Ijaz A, Moe OW, Elsayed EF, Reilly RF, Huang CL. Association of urinary sodium/potassium ratio with blood pressure: sex and racial differences. Clin J Am Soc Nephrol 2012;7:315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colditz GA, Manson JE, Stampfer MJ, Rosner B, Willett WC, Speizer FE. Diet and risk of clinical diabetes in women. Am J Clin Nutr 1992;55:1018–23. [DOI] [PubMed] [Google Scholar]
- 24.Mente A, Irvine EJ, Honey RJ, Logan AG. Urinary potassium is a clinically useful test to detect a poor quality diet. J Nutr 2009;139:743–9. [DOI] [PubMed] [Google Scholar]
- 25.Carpenter CL, Yan E, Chen S, Hong K, Arechiga A, Kim WS, Deng M, Li Z, Heber D. Body fat and body-mass index among a multiethnic sample of college-age men and women. J Obes 2013;2013:790654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord 1998;22:1164–71. [DOI] [PubMed] [Google Scholar]
- 27.Garrido-Chamorro RP, Sirvent-Belando JE, Gonzalez-Lorenzo M, Martin-Carratala ML, Roche E. Correlation between body mass index and body composition in elite athletes. J Sports Med Phys Fitness 2009;49:278–84. [PubMed] [Google Scholar]
- 28.Schutte JE, Townsend EJ, Hugg J, Shoup RF, Malina RM, Blomqvist CG. Density of lean body mass is greater in blacks than in whites. J Appl Physiol 1984;56:1647–9. [DOI] [PubMed] [Google Scholar]
- 29.Brown IJ, Dyer AR, Chan Q, Cogswell ME, Ueshima H, Stamler J, Elliott P, INTERSALT Co-Operative Research Group. Estimating 24-hour urinary sodium excretion from casual urinary sodium concentrations in Western populations: the INTERSALT study. Am J Epidemiol 2013;177:1180–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ji C, Miller MA, Venezia A, Strazzullo P, Cappuccio FP. Comparisons of spot vs 24-h urine samples for estimating population salt intake: validation study in two independent samples of adults in Britain and Italy. Nutr Metab Cardiovasc Dis (Epub ahead of print 9 October 2013). [DOI] [PubMed] [Google Scholar]
- 31.Milne FJ, Gear JS, Laidley L, Ritchie M, Schultz E. Spot urinary electrolyte concentrations and 24 hour excretion. Lancet 1980;2:1135. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan NM. Primary hypertension: pathogenesis In:Kaplan NM. ed. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2006:50–121. [Google Scholar]
- 33.National Academies Press. Dietary reference intakes for water potassium, sodium, chloride, and sulfate. Washington, DC: National Academies Press, 2005. [Google Scholar]
- 34.Bowman SA, Vinyard BT. Fast food consumption of U.S. adults: impact on energy and nutrient intakes and overweight status. J Am Coll Nutr 2004;23:163–8. [DOI] [PubMed] [Google Scholar]
- 35.Melander O, Groop L, Hulthen UL. Effect of salt on insulin sensitivity differs according to gender and degree of salt sensitivity. Hypertension 2000;35:827–31. [DOI] [PubMed] [Google Scholar]