Abstract
The dried plant was extracted with dichloromethane and after defatting with hexane, transferred repeatedly on silica columns using dichloromethane-hexane and ethyl acetate-hexane as mobile phases. Finally the fractions were purified by high performance liquid chromatography using a Pack-Sil column and hexane: Ethyl acetate as mobile phase. The structures of the isolated compounds included: cycloart-25-ene-3β, 24-diol (1), cycloart-23(Z)-ene-3β, 25-diol (2), cycloart-23(E)-ene-3β, 25-diol (3), and 24-methylene-cycloart-3β-ol (4) were elucidated by 13C- and 1H-NMR as well as IR and by the aid of mass fragmentation pattern and comparing with the literature. The biological effects of the compounds were done by the MTT assay on two different cancer cell lines including MDA-MB48 and MCF-7. Among these compounds, cycloart-23(E)-ene-3β,25-diol (3) was the most active compound on MDA-MB468 cell line (LD50 = 2.05 μgmL− 1 ) and cycloart-23(Z)-ene-3β, 25-diol (2) was the most active compound on MCF-7 cell line (LD50 = 5.4 μgmL− 1).
Key Words: Euphorboa macrostegia, Cycloartane, Cytotoxicity, MDA-MB468, MCF-7
Introduction
The incidence of cancer in human populations and the increasing need for anti-cancer drugs on the one hand and discovery of effective anti-cancer drugs, such as taxol, vincristine and vinblastin from plants. E.macrostegia as one of the endemic plants to Iran is the subject of this investigation. Euphorbia macrostegia (Persian wood spurge), belongs to the family Euphorbiaceae distributed mostly in central and west parts of Iran. Persian wood spurge is similar to the wood spurge (Euphorbia amygdaloides) and a rare species native of semi-moist woods from south-eastern Europe through Asia Minor. In the Iranian traditional medicine, latex is used to treat warts. Despite their toxicity, the uses of Euphorbia species in traditional medicine in many parts of the world have a long history. They are used to treat inflammations and tumours (1). Previous investigation on the cytotoxicity assessment of E. macrostegia (2), has showed LD50 values of 200, 425, and 390 μgmL− 1 for dichloromethane, ethyl acetate and acetone fractions, respectively while other fractions, remarked as noncytotoxic. Therefore, based on previous studies on cytotoxiciy effects of E. macrostegia and its fractions, the authors decided to investigate phytochemical contents of the dichloromethane extract of this plant as the most active fraction.
Result and Discussion
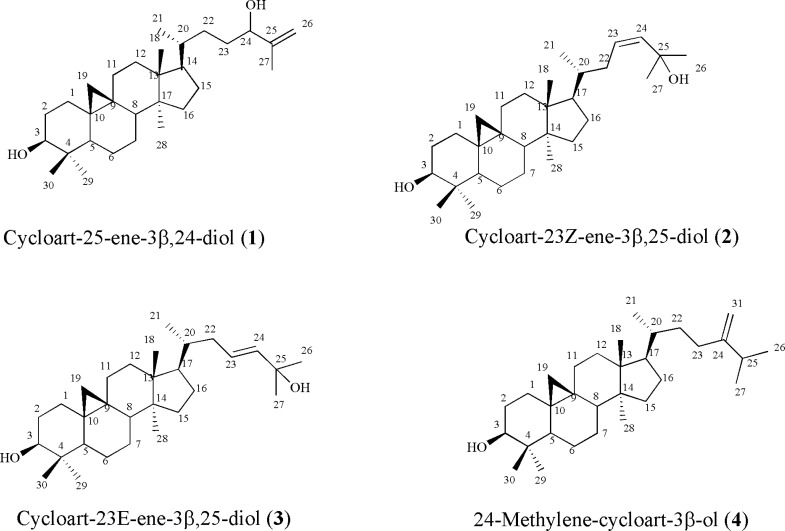
Compound 1, white crystals, showed the molecular formula of C30H50O2 based on EI-MS m/z 442 and number and multiplicity of 13C-NMR spectra. The six-degree of unsaturation and the 13C-NMR data (Table 1), suggested the presence of one double bond and, therefore, a pentacyclic skeleton. EI-MS fragmentation pattern, supported m/z 355 and 302, typical ions of 4,4' dimethyl 9:19 cycloesterols (4). 1H-NMR revealed a pair of doublets in the up-field area 0.57, 0.36 (each 1H, d, J = 4.0 Hz, H-19a, b), characteristic of cycloartane cyclopropane ring (4), one secondary methyl group at 0.90 (3H, d, J = 6.4 Hz, H-21), and five singlet methyls at δH 0.83 (3H, s, H-29), 0.91 (3H, s, H-28), 0.98 (3H, s, H-18), 0.99 (3H, s, H-30), and at 1.73 (3H, s, Me-27). Two double doublet protons at δH 3.30 (1H, dd, J = 4.4, 10.8 Hz, H-3), and δH 4.03 (1H, t, J = 5.8 Hz, H-24) revealed presence of two carbinolic protons and a pair of olefinic protons at δH 4.95 and 4.86 (each 1H, brs, H-26) suggested a terminal methylene. Downfield chemical shift of one singlet methyl proton at δH 1.73 (H-27) of the side chain atoms was in accordance with the quaternary olefinic group on C-25 at δC 128.8. As Ayatollahi and coworkers described EI-MS fragmentation pattern of cycloartanes (4), presence of monounsaturated side chain was also confirmed by the m/z 315 and 297 in EI-MS. In addition, m/z 381 together with 355 [M-H2O-C5H9]+ fragments due to the elimination of parts of side chain during a Mc Lafferty process, inferred presence of one hydroxyl in side-chain. Regarding to these findings, and literature data (4), compound 1 identified as cycloart-25-en-3 β, 24-diol. It is also found in other Euphorbia species like E. aellenii (4), E. heteradena (5) and E. sessiliflora (6).
Table 1.
13C-NMR chemical shifts of the triterpenoids from Euphorbia macrostegia.
| C | 1 | 2 | 3 | 4 | C | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 31.9 | 32.0 | 32.0 | 32.0 | 16 | 26.5 | 26.4 | 26.4 | 26.5 |
| 2 | 30.4 | 30.4 | 30.4 | 30.4 | 17 | 52.2 | 52.0 | 52.1 | 52.3 |
| 3 | 78.9 | 78.8 | 78.8 | 78.9 | 18 | 18.0 | 19.3 | 18.1 | 18.1 |
| 4 | 40.5 | 40.5 | 40.5 | 40.5 | 19 | 29.9 | 30.1 | 29.9 | 29.9 |
| 5 | 47.1 | 47.7 | 47.1 | 47.1 | 20 | 35.9 | 36.4 | 36.3 | 36.4 |
| 6 | 21.1 | 21.1 | 21.1 | 21.1 | 21 | 18.3 | 18.3 | 18.4 | 18.3 |
| 7 | 28.1 | 28.1 | 28.1 | 28.2 | 22 | 32.0 | 39.1 | 39.4 | 35.0 |
| 8 | 48.0 | 48.0 | 48.0 | 48.0 | 23 | 31.5 | 125.6 | 130.8 | 31.3 |
| 9 | 20.1 | 20.0 | 20.0 | 20.0 | 24 | 76.7 | 139.3 | 134.4 | 156.9 |
| 10 | 26.1 | 26.1 | 26.0 | 25.8 | 25 | 128.8 | 70.8 | 68.2 | 33.8 |
| 11 | 26.0 | 26.0 | 26.0 | 26.0 | 26 | 11.4 | 29.9 | 24.4 | 22.0 |
| 12 | 32.9 | 32.8 | 32.8 | 32.9 | 27 | 17.2 | 29.9 | 24.3 | 21.9 |
| 13 | 45.3 | 45.3 | 45.3 | 45.3 | 28 | 19.3 | 19.3 | 19.3 | 19.3 |
| 14 | 48.7 | 48.8 | 48.8 | 48.8 | 29 | 14.0 | 14.0 | 14.0 | 14.0 |
| 15 | 35.6 | 35.6 | 35.6 | 35.9 | 30 | 25.4 | 25.5 | 25.4 | 25.5 |
Compound 2, and 3 showed the molecular formula of C30H50O2 based on positive EI- MS m/z 442 and in accordance with their number and the multiplicity of 13C-NMR spectra (BB and DEPT). Their 1H-NMR revealed six tertiary singlet methyls, one secondary methyl group, and a pair of doublets in the up-field area characteristic of cycloartane cyclopropane ring and one carbinolic proton related to 3(β)-OH group. In compound 2, in olefinic pair protons, δH 4.94 (1H, brs, H-24) showed low coupling constants with at δH 4.96 (1H, m, H-23) due to their cis orientation while in compound 3, olefinic pair protons at δH 5.72 (1H, ddd, J = 15.6, 8.4, 6.0 Hz, H-23) and 5.54 (1H, d, J = 15.6 Hz, H-24) with large coupling constant (J = 15.6 Hz) allowed assignment of trans geometry to the Δ23(24). In both compounds, downfield chemical shifts of two singlet methyl protons (Me-26, and Me-27) of the side chain atoms were in accordance with the second hydroxyl group on C-25 at δC 70.8 and 68.2, respectively. Therefore, based on aforementioned data and complete agreements of 13C- and 1H-NMR with other reported data in literature (7; 8), compound 2 and 3 were identified as cycloart-23Z-ene-3β, 25-diol and cycloart-23E-ene-3β, 25-diol (Figure 1). They are also reported in Euphorbia spinidens (9), E. rigida (10), and E. humifusa (11).
Figure 1.
Triterpenoids from Euphorbia macrostegia
Compound 4, showed the molecular formula of C31H52O based on EI-MS m/z 440 and number and multiplicity of 13C-NMR spectra. The six-degree of unsaturation and the 13C-NMR data (Table 1), suggested the presence of one double bond and consequently five rings in the molecule. The 13C-NMR data (BB and DEPT), encompassed thirty-one carbons.1H-NMR revealed a pair of doublets in the up-field area at δH 0.30 and 0.53 (J = 4.25 Hz) characteristic of cycloartanes, four singlet methyls at δH 0.83 (3H, s, H-29), 0.91 (3H, s, H-28), and 0.99 (2× 3H, s, H-18, H-30) together with three secondary methyls. A doublet of doublet proton at δH 3.31, indicative of a carbinolic group, and one pair of olefinic protons δH 4.74, and 4.69 (each 1H, bs, H-31a, b) related to exocyclic terminal methylene. According to the literarture and these data, compound 4 was determined as 24-methylene-cycloartan-3β-ol (4). It was found in other spurge species like E. rigida (10), and E. aellenii (4).
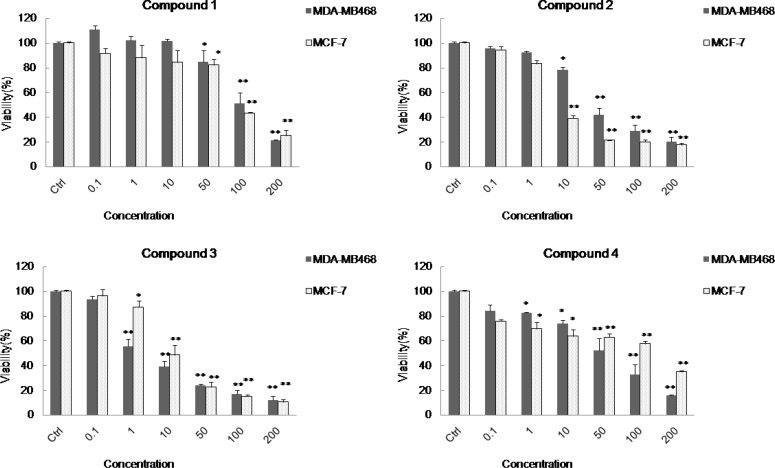
Using MTT assay on two different cancer cell lines (3,12-13), the biological effects of the compounds (1-4) on two different cancer cell lines including MDA-MB48 and MCF-7 showed LD50 values of 102.3, 34.0, 2.05, and 53.8 μgmL−1 on MDA-MB468 cell line, and LD50 values of 88.3, 5.4, 8.9, and 127.3 μgmL− 1 on MCF-7 cell line, respectively. Among these compounds, cycloart-23(E)-ene-3β,25-diol (3) was the most active compound on MDA-MB468 cell line (LD50 = 2.05 μgmL− 1 ) and cycloart-23(Z)-ene-3β,25-diol (2) was the most active compound on MCF-7 cell line (LD50 = 5.4 μgmL− 1 ).
The potent cytotoxicity observed by compound 2 and 3 with double bound on C-23 suggested that the cytotoxicity activities of these compounds are related to the position of the olefinic or the hydroxyl group on side chain.
Figure 2.
Cytotoxicity effects of the cycloartanes (1-4) in Euphorbia macrostegia on two cancer cell lines MDA-MB48 and MCF-7 . In this panel the cytotoxicity tests were presented on two different cancer cell lines including MDA-MB48 and MCF-7 in the presence of different concentrations (0.1, 1, 10, 50, 100 and 200 μg/mL) of cycloart-25-ene-3β,24-diol (1), cycloart-23(Z)-ene-3β,25-diol (2), cycloart-23(E)-ene-3β, 25-diol (3), and 24-methylene-cycloart-3β-ol (4), and control cells which were not treated (set to 100%). For statistical significance one-way ANOVA was used to analyze the differences between each sample and control (*P < 0.05, **P < 0.01).
By the literature, cycloartanes isolated from Euphorbia species showed also apoptosis induction on mouse lymphoma cells (14). Cycloart-25-en-3(β), 24-diol and 24-methylene-cycloartan-3(β)-ol (compound 1 and 4) presented antiproliferated activity on human peripheral blood lymphocytes (4). Cycloartanes were also reported for other biological activities like immunomodulatory effects like positive effect on Th1 cytokine release (IL-2 and IFN-γ), and suppression on Th2 cytokine production (IL-4) (15), inhibition of 11β-hydroxysteroid dehydrogenases (11β-HSD1 and 11β-HSD2) as a strategy for reducing glucocorticoid action on insulin resistance in type 2 diabetes mellitus and metabolic syndrome (16,17), or stimulating GLP-1 amide secretion in streptozotocin-nicotinamide induced diabetic Sprague Dawley rats (18). Therefore, interesting properties of cycloartanes, especially their antiproliferative effects, candidate them as investigational lead compounds in cancer research.
Acknowledgment
This paper is part of theses of Somayeh Baniadam submitted in partial fulfillment of the requirements for the degree of. Masters of Science. She is also grateful to the Isfahan Pharmaceutical Sciences Research Center, Isfahan University of Medical Sciences, Isfahan, I.R. and Shahid Beheshti University of Medical Sciences, Tehran, I.R. Iran for their support.
Exprerimental
General experimental procedures
The NMR spectra were recorded on a Bruker Avance AV 400, using CDCl3 as solvent. HPLC was carried out on a waters 515 using a YMC-Pack-Sil column (250 × 20 mm i.d.) and hexane: EtOAc as mobile phase. Chromatographic materials were silica gel (Merck Co., Germany). Thin layer chromatography detection was achieved by spraying the silica gel plates with cerium sulfate in 10% aq.H2SO4, followed by heating.
Plant material
Plant material was collected from Margoon water fall with elevation of 2130 m A.S.L. located in Yasooj, a city of Kohkilouyeh Va Boyer Ahmad province at Iran. It was identified by Department of Biology, Faculty of Science at University of Isfahan and a voucher specimen (#3340) was deposited in the herbarium of the Isfahan University (Iran).
Extraction and isolation
The air-dried plant material (2 Kg) was macerated with chloroform (20 L×3) at room temperature for 5 days. Filtration and in vacuo concentration resulted in a green gum (110 g), which was subjected on silica gel CC (hexane/dichloromethane, 0→100) to several fractions: Fr 1-Fr 5. Inferred from TLC and 1H-NMR, fraction Fr.1 and Fr.2 contained alkanes and fats, Fr.3 containing beta-sitosterol and fraction Fr. 4 and Fr.5 triterpenes. Fr.4 and Fr.5 were chromatographed on another normal column (hexane/acetone, 0→20). Finally triterpenes was further purified on HPLC using YMC-Pak-Sil column (250 × 20 mm) and hexane:ethylacetate (80:20) as mobile phase to yield compounds 1-4.
Cycloart-25-ene-3β,24-diol (1)
White crystals; MW(g/mol): 442; yield: 0.0010%; 1H-NMR (CDCI3, 400 MHz): δH 4.95, 4.86 (each 1H, brs, H-26), 4.03 (1H, t, J = 5.8 Hz, H-24), 3.30 (1H, dd, J = 4.4, 10.8 Hz, H-3), 1.73 (3H, s, H-27), 0.99 (3H, s, H-30), 0.98 (3H, s, H-18), 0.91 (3H, s, H-28), 0.90 (3H, d, J = 6.4 Hz, H-21), 0.83 (3H, s, H-29), 0.57, 0.36 (each 1H, d, J = 4.0 Hz, H-19a, b); 13C-NMR data: see Table 1. EIMS m/z: 442 (5), 427 (5), 424 (12), 409 (17), 381 (8), 355 (2), 315 (7), 302 (21), 297 (8), 203 (28), 175 (59), 43 (100).
Cycloart-23Z-ene-3β,25-diol (2)
White crystals; MW(g/mol): 442; yield: 0.0004%; 1H-NMR (CDCI3, 400 MHz): δH 4.96 (1H, m, H-23), 4.94 (1H, brs, H-24), 3.22 (1H, dd, J = 4.4, 11.2 Hz, H-3), 1.27 (3H, s, H-26), 1.26 (3H, s, H-27), 0.90 (2× 3H, s, H-18, H-29), 0.81 (3H, s, H-30), 0.79 (3H, d, J = 6.4 Hz, H-21), 0.74 (3H, s, H-28), 0.49, 0.25 (each 1H, d, J = 4.4 Hz, H-19a, b) ); 13C-NMR data: see Table 1; EIMS m/z: 442 (3), 427 (6), 425 (15), 409 (5), 383 (3), 363 (5), 357 (3), 326 (16), 315 (6), 302 (13), 300 (30), 297 (9), 269 (7), 175 (52), 43 (100).
Cycloart-23E-ene-3β,25-diol (3)
White crystals; MW(g/mol): 442; yield: 0.0015%; 1H-NMR (CDCI3, 400 MHz): δH 5.72 (1H, ddd, J = 15.6, 8.4, 6.0 Hz, H-23), 5.54 (1H, d, J = 15.6 Hz, H-24), 3.30 (1H, dd, J = 4.4, 10.8 Hz, H-3), 1.37 (2 × 3H, s, H-26, H-27), 1.0 (3H, s, H-29), 0.99 (3H, s, H-18), 0.91 (3H, s, H-30), 0.89 (3H, d, J = 6.4 Hz, H-21), 0.83 (3H, s, H-28), 0.58, 0.36 (each 1H, d, J = 4.0 Hz, H-19a, b) ); 13C-NMR data: see Table 1. EIMS m/z: 442 (3), 424 (10), 409 (14), 315 (6), 302 (9), 297 (10), 255 (16), 203 (36), 187 (45), 175 (60), 145 (67), 43 (100).
Cycloart-24-en-3β-ol (4)
White crystals; MW(g/mol): 440; yield: 0.0005%; 1H-NMR (CDCI3, 400 MHz): δH 4.73, 4.71 (each 1H, bs, H-31a,b), 3.31 (1H, dd, J = 4.4, 11.2 Hz, H-3), 1.05 (3H, d, J = 6.4, H-27), 1.04 (3H, d, J = 6.8, H-26), 0.99 (2× 3H, s, H-18, H-30), 0.91 (3H, s, H-28), 0.90 (3H, d, J = 9.2 Hz, H-21), 0.83 (3H, s, H-29), 0.57, 0.36 (each 1H, d, J = 4.0 Hz, H-19a, b) ); 13C-NMR data: see Table 1; EIMS m/z: 440 (7), 425 (12), 407 (21), 315 (8), 300 (19), 297 (11), 286 (28), 203 (55), 175 (72), 69 (100).
Cell culture
MCF-7 and MDA-MB468 human breast cancer cell lines were obtained from Pasteur Institute of Iran. The cell lines were grown adherently in RPMI-1640 media supplemented with10% fetal calf serum, 100 U/mL penicillin and 100 µg/mL streptomycin at 37 °C in 5% CO2/ 95% air.
MTT viability assay
Cell viability was determined by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. The MCF-7 and MDA-MB468 cells were seeded at 5 × 103 cells/well in 5% CO2 at 37 °C in RPMI medium (containing 10% FBS, 100 units⁄mL penicillin and 100 μg⁄mL streptomycin) in 96-well plates. After incubation overnight to allow for cell attachment, the RPMI medium in each well was replaced with by media containing various concentrations of compounds and incubated for 48 h. Afterwards, 20 μL of MTT (5 mg/mL in PBS) was added to each well and the cells were incubated for another 4 h at 37 °C. The supernatants were then aspirated carefully and 200 μL of dimethyl sulfoxide (DMSO) was added to each well. The plates were shaken for an additional 10 min and the absorbance values were read by the microplate reader (Bio-Rad, Hercules, CA, USA) at 570 nm. Cell viability was calculated as a percentage using the formula: (mean OD of treated cells /mean OD of control cells) ×100. The results expressed as percent of control cells which were not treated (3).
Statistical analysis
All samples were presented as mean ± SD for three measurements. Significance was attributed to p-values (P < 0.05) and the probability values obtained by the student t-test between sample and control data.
References
- 1.Jassbi AR. Chemistry and biological activity of secondary metabolites in Euphorbia from Iran. Phytochem. 2006;67:1977–1984. doi: 10.1016/j.phytochem.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 2.Toghiani MH. Cytotoxicity of different extracts of Euphorbia macrostegia and E. boissierana worn. Iran: Isfahan faculty of Pharmacy; 2004. Prokh. Pharm D thesis. [Google Scholar]
- 3.Zarei SM, Ayatollahi AM, Ghanadian M, Aghaei M, Choudhary MI, Fallahian F. Unusual ingenoids from Euphorbia erythradenia Bioss with pro-apoptotic effects. Fitoterapia. 2013;91:87–94. doi: 10.1016/j.fitote.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 4.Ayatollahi AM, Ghanadian M, Afsharypuor S, Mesaik MA, Abdella MO, Shahlaei M, Farzandi G, Mostafavi H. Cycloartanes from Euphorbia aellenii Rech. f. and their antiproliferative activity. Iran. J. Pharm. Res. 2011;10:105–112. [PMC free article] [PubMed] [Google Scholar]
- 5.Öksüz S, Ulubelen A, Barla A, Voelter W. Terpenoids and aromatic compounds from Euphorbia heteradena. Turk J. Chem. 2002;26:457–463. [Google Scholar]
- 6.Sutthivaiyakit S, Thapsut M, Prachayasittikul V. Constituents and bioactivity of the tubers of Euphorbia sessiliflora. Phytochem. 2000;53:947–950. doi: 10.1016/s0031-9422(99)00606-8. [DOI] [PubMed] [Google Scholar]
- 7.Khan MT, Khan SB, Ather A. Tyrosinase inhibitory cycloartane type triterpenoids from the methanol extract of the whole plant of Amberboa ramosa Jafri and their structure-activity relationship. Bioorg Med. Chem. 2006;14:938–943. doi: 10.1016/j.bmc.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi S, Satoh H, Hongo Y, Koshino H. Structural revision of terpenoids with a (3Z)-2-methyl-3-penten-2-ol moiety by the synthesis of (23E)- and (23Z)-cycloart-23-ene-3beta,25-diols. J Org. Chem. . 2007;72:4578–4581. doi: 10.1021/jo070478m. [DOI] [PubMed] [Google Scholar]
- 9.Ghanadian M, Akhavan A, Abdalla OM, Ayatollahi AM, Mohammadi-kamalabadi M, Ghazanfari H. Triterpenes from Euphorbia spinidens with immunomodulatory activity. Res Pharm. Sci. . 2013;8:205–210. [PMC free article] [PubMed] [Google Scholar]
- 10.Gherraf N, Zellagui A, Mohamed NS, Hussien TA, Mohamed TA, Hegazy MF, Rhouati S, Moustafa MF, El-Sayed MA, Mohamed AH. Triterpenes from Euphorbia rigida. Pharmacognosy Res. 2010;2:159–162. doi: 10.4103/0974-8490.65510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pei YG, Wu QX, Shia YP. Triterpenoids and other constituents from Euphorbia humifusa. J Chin. Chem. Soc. . 2007;5:1565–1572. [Google Scholar]
- 12.Tayarani-Najaran Z, Asili J, Aioubi E, Emami SA. Growth Inhibition and Apoptosis Induction of Salvia chloroleuca on MCF-7 Breast Cancer Cell Line. Iran J. Pharm. Res. . 2013;12:789–799. [PMC free article] [PubMed] [Google Scholar]
- 13.Adibi H, Majnooni MB, Mostafaie A, Mansouri K, Mohammadi M. Synthesis and In-vitro Cytotoxicity Studies of a Series of Triazene Derivatives on Human Cancer Cell Lines. Iran J. Pharm. Res. 2013;12:695–703. [PMC free article] [PubMed] [Google Scholar]
- 14.Madureira AM, Spengler G, Molnár A, Varga A, Molnár J, Abreu PM, Ferreira MJ. Effect of cycloartanes on reversal of multidrug resistance and apoptosis induction on mouse lymphoma cells. Anticancer Res. 2004;24:859–864. [PubMed] [Google Scholar]
- 15.Nalbantsoy A, Nesil T, Yılmaz-Dilsiz O, Aksu G, Khan S, Bedir E. Evaluation of the immunomodulatory properties in mice and in-vitro anti-inflammatory activity of cycloartane type saponins from Astragalus species. J Ethnopharmacol. 2012;139:574–581. doi: 10.1016/j.jep.2011.11.053. [DOI] [PubMed] [Google Scholar]
- 16.Mpetga JD, Shen Y, Tane P, Li SF, He HP, Wabo HK, Tene M, Leng Y, Hao XJ. Cycloartane and friedelane triterpenoids from the leaves of Caloncoba glauca and their evaluation for inhibition of 11β- hydroxysteroid dehydrogenases. J Nat. Prod. 2012;75:599–604. doi: 10.1021/np200831c. [DOI] [PubMed] [Google Scholar]
- 17.Lipson VV, Zamigajlo LL, Petrova ON. Development of 11βHSD1 inhibitors for the treatment of metabolic syndrome. Ukrain Bioorgan. Acta . 2011;2:3–13. [Google Scholar]
- 18.Badole SL, Mahamuni SP, Bagul PP, Khose RD, Joshi AC, Ghule AE, Bodhankar SL, Raut CG, Khedkar VM, Coutinho EC, Wagh NK. Cycloart-23-ene-3β, 25-diol stimulates GLP-1 (7-36) amide secretion in streptozotocin-nicotinamide induced diabetic Sprague Dawley rats: A mechanistic approach. Eur. J. Pharmacol. 2013; 698:470–479. doi: 10.1016/j.ejphar.2012.10.002. [DOI] [PubMed] [Google Scholar]