Abstract
A growing body of research has revealed that social evaluative stressors trigger biological and psychological responses that in chronic forms have been linked to aging and disease. Recent research suggests that self-compassion may protect the self from typical defensive responses to evaluation. We investigated whether brief training in self-compassion moderated biopsychological responses to the Trier Social Stress Test (TSST) in women. Compared to attention (placebo) and no-training control conditions, brief self-compassion training diminished sympathetic (salivary alpha-amylase), cardiac parasympathetic, and subjective anxiety responses, though not HPA-axis (salivary cortisol) responses to the TSST. Self-compassion training also led to greater self-compassion under threat relative to the control groups. In that social stress pervades modern life, self-compassion represents a promising approach to diminishing its potentially negative psychological and biological effects.
Keywords: alpha-amylase, cortisol, heart rate variability, stress, social threat, self-compassion
According to social self-preservation theory (Dickerson et al., 2004), humans are motivated to preserve social value, esteem, and status. Social evaluation by others represents a primary source of threat to the social self. Social evaluation threats – job or academic performance reviews, negative judgments by peers, social slights – also represent a regular feature of modern life. Social evaluative stress can elicit marked subjective and biological responses (Dickerson and Kemeny, 2004), and in chronic forms, can lead to mental and physical health problems (Dickerson et al., 2009; Miller et al., 2011; Repetti et al., 2002), and speed biological aging (McEwen and Stellar, 1993; Miller et al., 2011). Given such implications, psychologists have become interested in interventions to buffer social evaluative stress and promote more adaptive threat responses.
Social evaluation directly threatens self-esteem, and evidence indicates that dispositional self-esteem and related self-resources can ameliorate social evaluative threat responses (e.g., Taylor et al., 2003). Yet self-esteem can have psychosocial costs, including self-defensive anger, inaccurate self-perceptions, narcissism, self-worth contingency, and relationship conflict (Emmons, 1984; Leary et al., 2007; Neff and Vonk, 2009). Costs may also be physical; for example, narcissism has been associated with higher cortisol at rest (Reinhard et al., 2012) and in response to social evaluation (Edelstein et al., 2010) among men. Thus, researchers recently have asked whether other forms of self-perception may offer psychosocial benefits in the face of social threat without such associated costs.
Self-compassion appears to represent one such quality. According to Neff (2003a), self compassion involves “being open to and moved by one's own suffering, experiencing feelings of caring and kindness toward oneself, taking an understanding, nonjudgmental attitude toward one's inadequacies and failures, and recognizing that one's experience is part of the common human experience” (p. 224). A self-compassionate person sees his or her weaknesses and shortcomings accurately, yet reacts with self-kindness rather than with self-judgment (Leary, Tate, Adams, Batts Allen, & Hancock, 2007). Relative to trait self-esteem, self-compassion has predicted more stable feelings of self-worth that depend less on external outcomes such as successful performance or social approval (Neff and Vonk, 2009). Self-compassion appears to facilitate adaptive responding to social stressors without resorting to self-esteem enhancement (including narcissism) or a devaluing of either the threat source (Neff and Vonk, 2009) or the self (Leary et al., 2007). Thus, self-compassion appears to have psychological benefits, including resilience to social threats.
To date, most self-compassion research has been correlational or based on brief experimental inductions. However, researchers have begun to examine the effects of short-term training in Buddhist meditative practices aimed at cultivating compassion for the self and others (Hofmann et al., 2011) on social threat responding. In a pioneering study, Pace and colleagues (2009) examined the impact of a 6-week training in compassion meditation on subjective, neuroendocrine (cortisol), and immune (IL-6) responses to an acute social evaluative stressor, the Trier Social Stress Test (TSST; Kirschbaum et al., 2008). The compassion and control groups did not differ in subjective, cortisol, or IL-6 responses to the TSST. However, home practice time in the compassion-trained group correlated with lower IL-6 and distress responses. The compassion group undertook relatively intensive training (totaling 11-18 hours) in compassion meditation for self and others as well as mindfulness and concentration practices. Thus, the training drew from multiple contemplative practices taught over a 6-week period. Also, the training was not specifically focused on managing social stressors. We asked whether briefer and more focused self-compassion training might more strongly impact stressor responses. Focusing on teaching self-compassion, a single dimension of the training undertaken by the Pace et al (2009) participants, also clarifies whether this specific training yields benefits. People preparing for known social stressors, including interviews, performances, or difficult conversations, could more readily undertake such training. We also assessed a different array of psychobiological processes that may track the effects of self-compassion training on acute stress responses.
The present study thus investigated whether brief self-compassion training modulates a range of psychobiological responses to an acute social stressor (using the TSST). Our work builds on the nascent work in this area (Fredrickson et al., 2008; Kok et al., 2013; Pace et al., 2009) in important ways. First, we used a brief (45 minutes total), self-administered training in self-compassion delivered in brief intervals over five days leading up to the stressor. We employed metta (loving-kindness) meditation, which involves the simple repetition of statements of kindness towards the self or others, and thus was well-suited to guided self-practice. Second, we assessed a range of stress-relevant psychobiological outcomes, including subjectively experienced anxiety and measures of two major physiological systems activated during acute social stress: the hypothalamic-pituitary-adrenal (HPA) axis and the autonomic nervous system, implicating both neuroendocrine and cardiovascular systems. Third, we included two rigorous control conditions – an attention control/placebo intervention group and a no-intervention group – to enhance our ability to assess the role of self-compassion training relative to both attention and engagement per se. In summary, we tested the value of a low-demand, stressor-focused training against rigorous control conditions to ameliorate a range of psychobiological responses to social evaluative threat.
We hypothesized that brief self-compassion training would reduce acute social evaluative threat responding (in anticipation of and during the TSST) and speed response recovery. Specifically, we predicted that relative to placebo training and no-training control conditions, self-compassion training would dampen biological stress responses to the TSST. We thus examined salivary cortisol responses, reflecting HPA axis activity (see Sapolsky et al., 1986), salivary alpha amylase (sAA), purported to reflect sympathetic nervous system activity (Rohleder et al., 2004; van Stegeren et al., 2006), and respiratory sinus arrhythmia (RSA; or high frequency heart rate variability), a stress-reactive marker of cardiovascular parasympathetic functioning (Thayer and Lane, 2000). In the TSST context, lower sAA responses are interpreted as dampened sympathetic system responding, which has been linked to lower defensiveness (see Bauer et al., 2002). Higher RSA has been linked to flexible attention deployment and adaptive emotion regulation in threat contexts (Thayer et al., 2012; Thayer et al., 2009; Thayer and Lane, 2000). All three indices have been shown to be reactive to social evaluative threat (Nater et al., 2006) and all are implicated in physical health (Glaser and Kiecolt-Glaser, 2005; McEwen and Stellar, 1993; Thayer and Sternberg, 2006). We also predicted that relative to the control conditions, self-compassion training would reduce TSST-related anxiety and increase self-compassion, both in the TSST environment and in general. We focused on women in this study given that they report lower self-compassion than men (Neff, 2003a), recommending them to an intervention designed to boost this purported stress resilience factor.
METHODS
Participants
Participants were undergraduate women who, to control for known influences on cortisol response to acute stress (Granger et al., 2009; Kirschbaum et al., 1999), reported no use of oral contraception or other prescription medication. All were fluent in English, non-smokers, non-drug users, and physically healthy (no reported fever or illnesses, no diseases/ conditions that may impact stress responses such as endocrine disorders, autoimmune disorders, cardiovascular or respiratory conditions, etc.). Participants were forbidden from drinking caffeinated or alcoholic beverages, or exercising in the 3 hours prior to the TSST session, and consuming anything other than water for the hour prior, or using any drugs or medications for 24 hours prior. One hundred five women completed the TSST (M age = 19.53, SD = 1.88; 76% white, 12.5% Asian-American; 6.7% biracial; and 4.9% African-American, Latina, Native American, or another race/ethnicity); they represent the focus of this paper1. Three were not included in salivary biomarker analyses because of technical problems; they were included in the RSA and self-report-based analyses. Women received course credit or payment for study participation. Human subjects IRB approval was obtained prior to data collection and all participants provided informed consent.
Design and Procedures
Eligible women were randomly assigned to one of three intervention conditions: self-compassion, attention (placebo) control, or no intervention. The study consisted of two sessions scheduled 4 days apart; both sessions took place in the same laboratory room. The experimenters running the study were female; the two TSST judges were always 1 male and 1 female. Judges and experimenters were blind to condition, the latter until participants were asked if they had questions about the condition-specific recordings (see below); thus, experimenters needed to know which recording they referred to. Further, they needed to give non-recording based instructions to the no intervention control group. Different female experimenters ran each session (1 and 2) for the same participant; thus, the session 2 experimenter remained blind to condition at the start of the session. Further, independent evaluators blind to condition viewed randomly selected live sessions in the second half to check for consistency in experimenter behavior.
In session 1 (s1), women randomized to self-compassion and attention control listened to a 10-minute condition-specific recording, were given an opportunity to ask questions, and were instructed to listen to a “similar recording” once per day for the following 3 days (‘self-compassion’ or ‘meditation’ were not mentioned). Women were told that attending to the recordings was “extremely important”, “may help you prepare for your second session” and should be listened to at home “without any distractions.” Recordings were accessed via a secure website.
Session 2 (s2; TSST session) took place between 1-6pm to account for diurnal rhythms in salivary biomarkers. Women first completed questionnaires and baseline psychophysiological recordings, which took approximately 30 minutes and allowed them to rehabituate to the laboratory environment. Immediately prior to the TSST instructions, women in the self-compassion and attention control conditions listened to a final 5 minute recording specific to their condition with instructions that: “The rest of the study will be challenging. To help you prepare for the challenge, we invite you to listen to a recording similar to the ones you listened to at home...”. Women in the no-intervention control condition were simply told, “the rest of the study is challenging” and were invited to wait quietly during the 5 minute period (during which women in the other conditions listened to the recording). TSST followed canonical procedures (Kirschbaum et al., 2008), excepting a 5 min (rather than 10 min) speech anticipation period, and a 35 minute recovery period. Intervention Conditions
Self-compassion
The self-compassion recordings consisted of meditations focused on cultivating kindness and acceptance towards the self, and to a lesser extent, towards others. Meditations consisted of phrases (“may I be happy...may I be at ease...”) that women were asked to repeat silently with intention and self-kindness (Salzberg, 1995). The phrases included traditional and study-specific content that drew from Neff's (2003a, b) conceptualization of self-compassion (“may I know that my joys and struggles are shared by others...”).
Attention (placebo) control
The control recordings consisted of excerpts from a psychology textbook chapter on cognition, with content plausibly relevant to TSST preparation, including discussions of problem solving, judgment, and thinking. One female voice was used for instructions for both active conditions.
No intervention (NI) control
In the NI condition, women did not listen to recordings during or between sessions. They were invited to sit quietly or read (provided) neutral-content magazines during the s2 period that women in the other conditions heard the recording. Because this condition was added into the randomization sequence after the other conditions, we assessed whether salivary biomarker responses to the TSST differed for the SC and Attention Control groups before versus after the addition of the NI control group; no cohort effects were evident, ps > .28.
Measures
Subjective measures
Trait and state self-compassion
To measure training-related changes in self compassion, we administered the 26-item Self Compassion Scale (SCS; Neff, 2003a) at the beginning of s1 and s2; for the latter, a modified version assessed self-compassion “over the past several days” to capture training-related changes. Internal consistency (Cronbach's α) was .94 for each. To assess state self-compassion following the TSST we modified all original SCS items to refer to current experience2 (e.g., “In response to my performance, I am being tough on myself”; α = .88).
State anxiety
At the same time points as saliva collection and after the TSST speech preparation, state anxiety was measured using the widely-used, single-item Subjective Units of Distress (SUDS) scale (Wolpe, 1990). In addition, the Spielberger State-Trait Anxiety Inventory (STAI; Spielberger et al., 1983) assessed how participants were feeling “right now” at: baseline, pre-TSST (immediately after the speech preparation), 10min post TSST, and 20min post TSST (the 3rd and 4th saliva collections). Sample α's were .92-.96.
Trait social anxiety
. To assess baseline group differences in social anxiety, which have been linked to cortisol responses within similar stressors (Condren et al., 2002), we administered the well-validated Social Phobia Scale and Social Interaction Anxiety Scale (Mattick and Clarke, 1998) at s1 baseline. Sample α's were .90 and .93.
Salivary biomarkers
Saliva was collected at five points during s2: baseline, immediately post-TSST, 10 minutes post TSST, 20 minutes post TSST, 35 minutes post TSST. Samples were stored in a –15°C freezer until all samples from the same subject could be processed in the same assay. Salivary cortisol was determined using a commercial expanded range high sensitivity EIA kit (Salimetrics; State College, PA) that detects cortisol levels in the range of 0.083- 82.77 nmol/L (.003 – 3.0 mg/dl). Samples with duplicate CVs greater than 10% were rerun in triplicate and the median value of the rerun was reported. Inter- and intra-assay coefficients of variability were 2.6 and 9.6%, respectively. Salivary alpha-amylase was determined by kinetic assay (Salimetrics; State College, PA). Salivary alpha-amylase samples were run in singlet, diluted 1:200. Inter- and intra-assay coefficients of variability for alpha amylase were 4.7 and 11.3%, respectively.
RSA (high-frequency heart rate variability)
Using Mindware (Gahanna, OH) hardware and software, we collected ECG data in 60s epochs, including a 5 min sitting baseline, and derived RSA in accord with recommendations (Berntson et al., 1997). Briefly, the ECG signal was digitized (1000 Hz) and an interbeat interval (IBI) series was derived by a peak-identification algorithm that identifies the peak of the R wave as the fiducial point. Artifacts were flagged by statistical algorithms and then checked visually and edited as necessary according to published guidelines (Berntson et al., 1997). The IBI series was then converted to a time series by resampling at fixed time intervals with interpolation (Berntson, 1995). The time series was linearly detrended to minimize nonstationaries in the data (Litvack et al., 1995). The residual series was then tapered with a Hamming1 window and submitted to the DFT/FFT module of LabVIEW2 (National Instruments, Austin, TX) to derive the spectral distribution. RSA was quantified as the natural log of the integral power within the respiratory frequency band (0.15 to 0.40 Hz), which is equal to the statistical variance of the time series within that band. Respiration rate was derived from the dz/dt signal of the ECG data series.
For RSA analyses, we divided the TSST session into four phases: baseline (5 min), speech preparation (5 min), TSST (10 min), and recovery (first 10 min following TSST). To simplify the modeling and interpretation, we analyzed the first, middle, and last two minutes for the latter two phases. Groups did not differ in mean respiration rate for any phase, ps > .14. Nonetheless, for RSA analyses, we simultaneously covaried respiration rate for each phase.
Statistical Approach
We assessed TSST-related changes in cortisol and sAA by computing the area under the curve with respect to increase (AUCi) using Pruessner et al's (2003) formula. To normalize the data prior to computing AUCi, we used a square root transformation of U/mL for sAA and a log transformation of nmol/L for cortisol. To compare groups on hypothesized outcomes, we used a priori contrast coding within regression equations that compared: a) each control group (to confirm a lack of difference between the two control groups) and b) the control groups to the self-compassion group. We covaried the TSST session start time to account for diurnal variations in cortisol and sAA (Nater et al., 2007; van Cauter, 1990).
We assessed changes in RSA and in the state anxiety measures (SUDS, STAI) using hierarchical linear modeling in HLM 6.08 with linear, quadratic, and cubic time terms at level 2 to capture the curvilinear nature of the outcomes across the TSST session. Prior to analyses, we centered time at different points (for example, at the beginning, middle, and end of TSST recovery), which allowed us to test the mean differences and slopes between groups; we report theoretically relevant results below. For RSA, we covaried epoch-by-epoch respiration rate for the phase of interest at level 1. Mean baseline RSA and respiration rate (see Figure 3a) were covaried at level 2, and retained in the models when significant. Intercepts were random effects; slopes were fixed or random effects dependent on model fit, resulting in differing dfs. For the HLM models, we computed d effect sizes using Feingold's (2009) approach, which accounts for the number of repeated measurement periods.
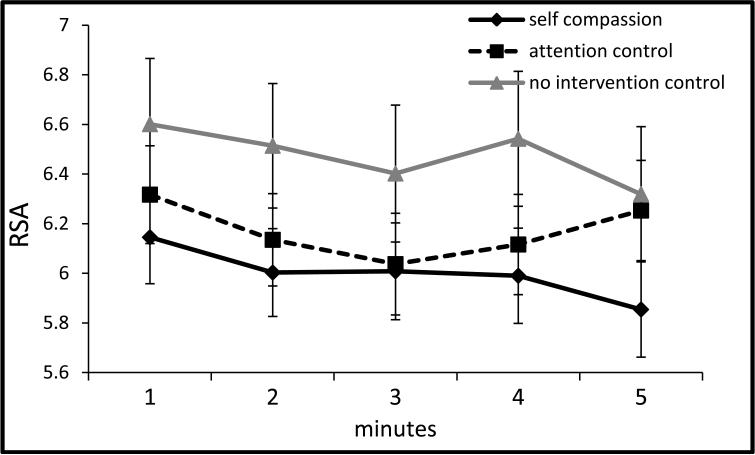
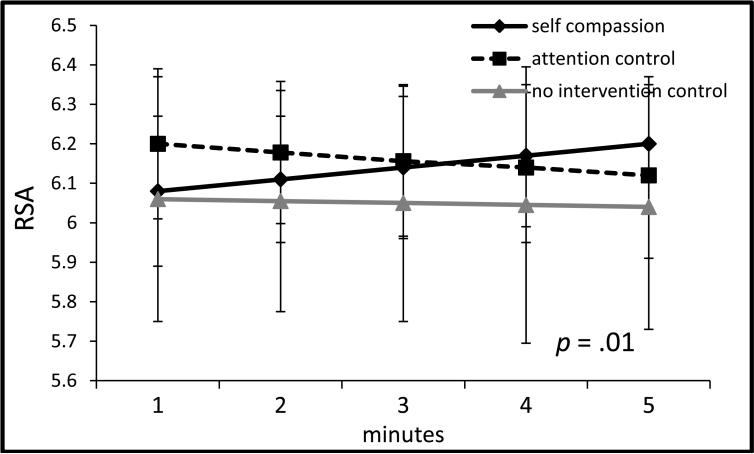
Figure 3a.
RSA during TSST baseline (±1SE).
Note: x-axis denotes minutes of the 5 minute baseline.
Note: RSA = respiratory sinus arrhythmia was quantified as the natural log of the integral power within the respiratory frequency band (0.15 to 0.40 Hz), covarying respiration rate for the period of interest and baseline RSA (for non-baseline analyses); TSST = Trier social stress test.
RESULTS
Manipulation and compliance checks
The self-compassion and attention control conditions showed no differences in frequency of audio-recordings use between s1 and s2 (M = 3.00, SD = .57 and M = 2.80, SD = .69, respectively, t(76.11) = -1.44, p = .15, d = .33. They also showed no differences on a 5-item study-specific measure (α = .80) concerning attention to the recordings (e.g., ‘I tried my best to stay focused on the recordings’), using a 0 to 4 scale, with higher scores = greater attention. Both groups indicated relatively high compliance: self-compassion M = 3.29, SD = .67; attention control M = 3.06, SD = .64; t(83) = -1.59, p = .12, d = .35.
Baseline condition differences
The three groups showed no s1 baseline differences in menstrual cycle phase3 (p = .13), trait self-compassion (p = .17), social phobia symptoms (p = .28), social interaction anxiety (p = .57), or state anxiety (p = .50). In s2, baseline cortisol (p = .76) and sAA (p = .54) did not differ by condition, nor did baseline RSA, with or without covarying respiration rate, ps > .21.
Stress responses across conditions
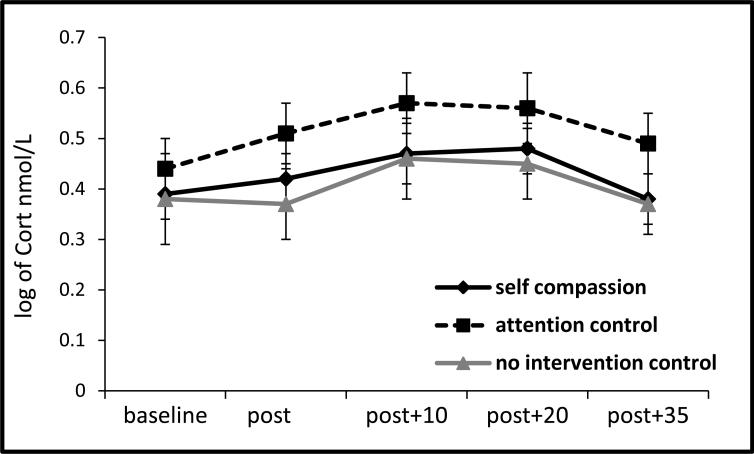
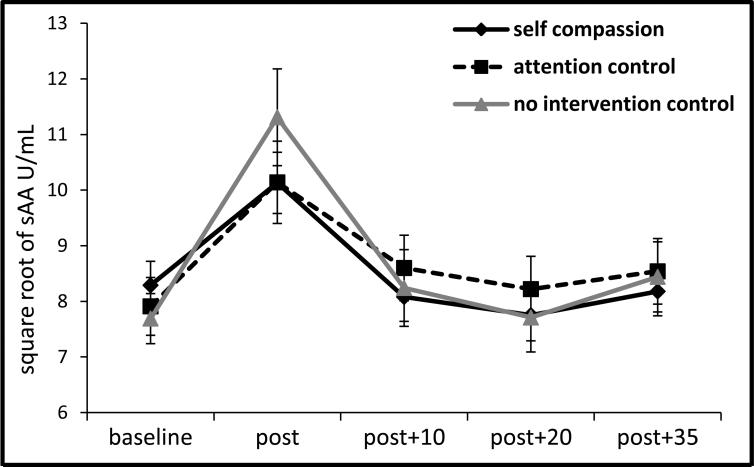
Across the entire sample, the TSST resulted in a significant increase in cortisol: F (4, 404) = 4.82, p = .001, ηp2 = .05, see Figure 1; in sAA: F (4, 404) = 36.56, p < .001, ηp2 = .27, see Figure 2a; in STAI state anxiety: F (3, 312) = 107.05, p < .001, ηp2 = .51, and in SUDS anxiety: F (5, 520) = 214.93, p < .001, ηp2 = .67. From the speech preparation period (phase 2) to the first two minutes of the TSST (start of phase 3), RSA diminished, F (2, 202) = 11.33, p < .001, ηp2 = .10, and then recovered from the middle of the TSST (mid-phase 3) through recovery (phase 4), F (5, 500) = 18.18, p < .001, ηp2 = .15.
Figure 1.
Salivary cortisol response to TSST by condition (±1SE).
Figure 2a.
Salivary alpha-amylase response to TSST by condition (±1SE).
Intervention (condition) effects
Self-compassion responses
As predicted, the self-compassion group reported greater increases in trait self-compassion from s1 to s2 than the controls, b = .06, t(101) = 2.04, p = .04, ΔR2 = .02, whereas the control groups did not differ from one another, b = .00, t(101) = -.01, p = .99, ΔR2 = .00. Further, among women trained in self-compassion, lower s1 self-compassion correlated negatively with s1 to s2 increases (r = -.41, p = .01) such that those lower in baseline self-compassion reported greater increases following the intervention.
Covarying s2 baseline self-compassion, we investigated group differences in state self-compassion following the TSST. As predicted, self-compassion training led to greater state self-compassion during TSST recovery (assessed 35 min post-TSST) than the control conditions, b = .08, t(100) = 2.65, p = .009, ΔR2 = .04, whereas the control groups did not differ, b = -.05, t(100) = -.77, p = .44, ΔR2 = .00, reflecting the following mean levels of state self-compassion: self-compassion group M = 3.79, SD = .53, attention control group M = 3.43, SD = .58, NI control group M = 3.45, SD = .53.
Cortisol and sAA responses
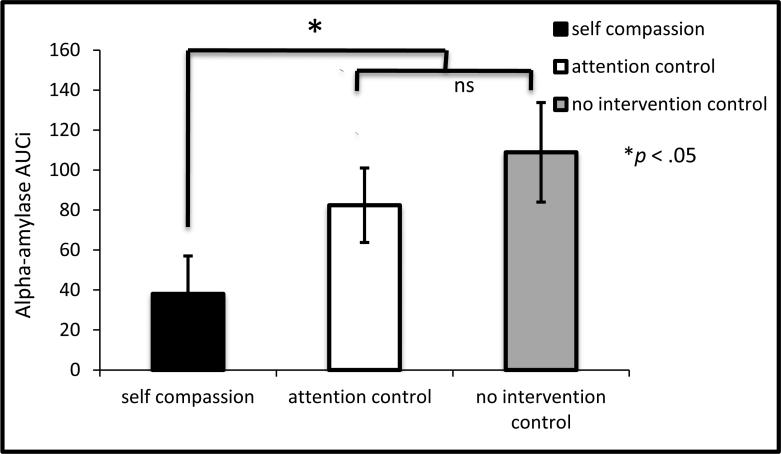
The self-compassion and control groups did not differ in cortisol AUCi to the TSST, b = -.36, t(98) = -.30, p = .77, ΔR2 = .00, nor did the control groups differ from one another, b = -.89, t(98) = -.36, p = .72, ΔR2 = .00 (see Figure 1). However as predicted, the self-compassion group demonstrated lower sAA AUCi than the control groups, b = - 18.73, t(98) = -2.30, p = .02, ΔR2 = .05, and the control groups did not differ from one another, b = 8.64, t(98) = .51, p = .61, ΔR2 = .00 (see Figure 2a and 2b) 4.
Figure 2b.
Salivary alpha-amylase responses to the TSST by condition (AUCi ±1SE).
Note: AUCi was computed from the square-root transform of alpha-amylase in U/mL using Pruessner et al.'s (2003) formula; *p = .02 for self-compassion vs control conditions difference, ns = non-significant (p = .61) difference between the two control conditions.
RSA responses
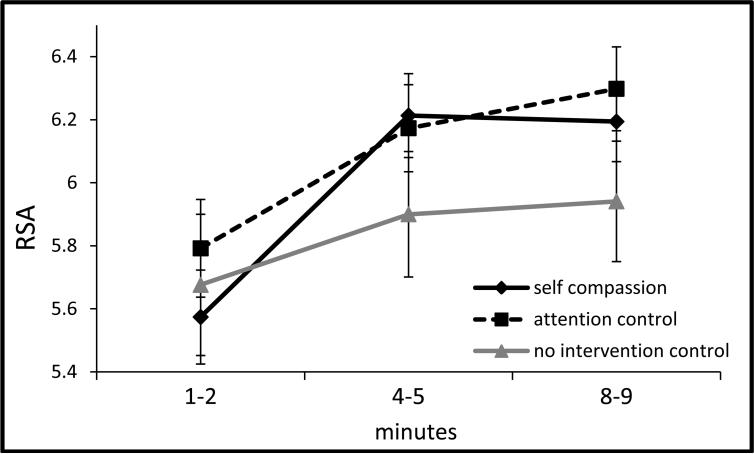
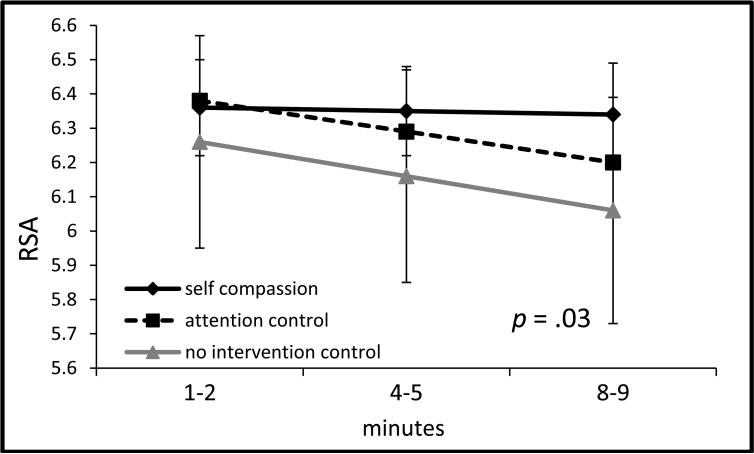
During the speech preparation phase, group by (linear) time differences emerged between the self-compassion and control conditions, b = .03, SE = .01, t(494) = 2.59, p = .01, d = .10, such that the former showed stable RSA levels, b = .03, SE = .02, t(497) = 1.47, p = .14, whereas the control groups showed significant decreases in RSA, b = -.05, SE = .02, t(497) = - 2.55, p = .01 (see Figure 3b). During the TSST phase, RSA showed significant quadratic change across groups, p = .01, but no group differences emerged between the self-compassion and control groups, ps > .15, or between the two control groups, ps > .63 (see Figure 3c). During the recovery phase, group by (linear) time differences were found, b = .05, SE = .02, t(302) = 2.12, p = .03, d = .09, such that the self-compassion group showed stable RSA levels, b = -.01, SE = .05, t(306) = -.20, p = .84, whereas the control groups significantly decreased in RSA, b = -.16, SE = .05, t(306) = -3.44, p = .001 (see Figure 3d).
Figure 3b.
RSA during TSST anticipation (speech preparation period, ±1SE).
Note: x-axis denotes minutes of 5 min speech preparation period; p = .01 for self-compassion vs control groups by time (slope) difference.
Note: RSA = respiratory sinus arrhythmia was quantified as the natural log of the integral power within the respiratory frequency band (0.15 to 0.40 Hz), covarying respiration rate for the period of interest and baseline RSA (for non-baseline analyses); TSST = Trier social stress test.
Figure 3c.
RSA during the TSST (±1SE).
Note: x-axis denotes minutes of the 9-10 min TSST period.
Note: RSA = respiratory sinus arrhythmia was quantified as the natural log of the integral power within the respiratory frequency band (0.15 to 0.40 Hz), covarying respiration rate for the period of interest and baseline RSA (for non-baseline analyses); TSST = Trier social stress test.
Figure 3d.
RSA during TSST recovery (±1SE).
Note: x-axis denotes minutes of the TSST recovery period; p = .03 for self-compassion vs control groups by time (slope) difference.
Note: RSA = respiratory sinus arrhythmia was quantified as the natural log of the integral power within the respiratory frequency band (0.15 to 0.40 Hz), covarying respiration rate for the period of interest and baseline RSA (for non-baseline analyses); TSST = Trier social stress test.
Subjective anxiety responses
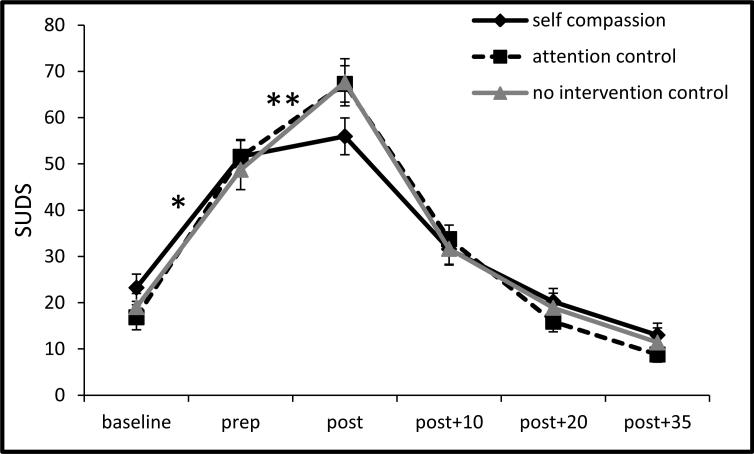
Across groups, SUDS and STAI showed curvilinear changes during the TSST (see Figures 4-5), indicated by significant cubic time terms in the HLM models, ps < .001. As presented in Figure 4, from baseline through speech preparation and speech preparation through the TSST, all groups increased in SUDS ratings, ps < .001. The self-compassion group, however, showed milder SUDS increases than the control groups from baseline through speech preparation, b = -5.25, SE = 2.16, t(102) = -2.43, p = .02, d = .30, and from the speech preparation period through the TSST, b = -1.87, SE = .68, t(102) = -2.76, p = .007, d = .11. The two control groups did not differ at any point, ps > .40.
Figure 4.
SUDS responses to the TSST by condition (±1 SE).
Note: prep = speech preparation period; post = immediately after the TSST; SUDS = subjective units of distress; TSST = Trier social stress test, *p =.02, **p = .01, for self-compassion vs control condition slope difference.
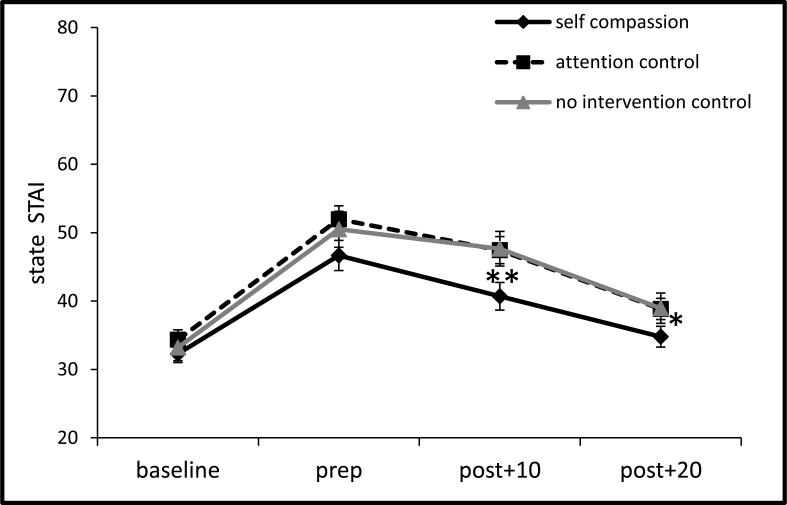
Figure 5.
State STAI responses to the TSST by condition (±1 SE).
Note: prep = speech preparation period; post = post TSST and recovery periods; STAI = state trait anxiety inventory; TSST = Trier social stress test; *p = .04, **p = .01, for self-compassion vs control groups differences in mean SUDS during recovery. Also note p < .05 in group slope difference from prep thru early recovery (post+10)
As presented in Figure 5, all groups showed linear increases in STAI from baseline to speech preparation, ps < .001, and then decreased from speech preparation through the recovery phases, ps < .001. However, from speech preparation through early recovery phases, the self-compassion group showed steeper linear decreases in STAI slope (e.g., faster recovery) than the control groups, b = -1.13, SE = .50, t(102) = -2.27, p = .03, d = .13 . During recovery, the self-compassion group reported lower mean state anxiety than the controls at early recovery (post+10 min): b = -2.28, SE = .85, t(102) = -2.69, p = .009, d = .26, and at later recovery (post+20 min): b = -1.37, SE = .67, t(102) = -2.05, p = .04, d = .16. STAI scores between the control groups did not differ at any point, ps > .52.
DISCUSSION
Past research has shown that self-esteem and self-affirmation can buffer the adverse consequences of social evaluative threat (e.g., Creswell et al., 2005; Taylor et al., 2003). This experiment showed that a brief training in self-compassion produced psychobiological responses to social evaluative threat in young women that were similarly consistent with lower stress – in particular dampened sympathetic nervous system (sAA) reactivity and more adaptive parasympathetic cardiac (RSA) and subjective (anxiety) responses. These findings suggest that social self-preservation can adaptively take the form of self-kindness, including recognizing one's vulnerabilities, without the potential costs of self-esteem enhancement.
Salivary AA responses showed the predicted pattern of group differences whereas salivary cortisol responses did not, suggesting that the brief self-compassion training impacted the sympathetic nervous system to a greater extent than the HPA axis system. The fast-acting sympathetic system has been implicated in defensive reactions whereas the slower-acting HPA system has been implicated in response to uncontrollable situations (see Bauer et al., 2002; Dickerson and Kemeny, 2004). Thus, self-compassion training appears to have reduced defensiveness in the face of social threat to a greater extent than perceptions of uncontrollability. This finding is consistent with Leary et al.'s (2007) study showing that self-compassion (both trait and induced) led to lower reported defensiveness in response to negative life events. Strong sympathetic responses also have been linked to “Type A” personalities or more specifically, a propensity towards hostility that involves effortful or aggressive responses to challenge (Bauer et al., 2002; Brydon et al., 2010; Matthews, 1982). Thus, lower sAA (combined with lower reported anxiety) suggests a stressor response profile of lower defensiveness, active acceptance, and greater easefulness, rather than efforts to exert control. Such an approach may have been adaptive in that the TSST was designed to elicit a reliable cortisol response partly by virtue of its uncontrollability (Dickerson and Kemeny, 2004; Kirschbaum et al., 2008). Thus, while the sample as a whole showed the expected elevated cortisol response, it may not be surprising that brief self-compassion training failed to alter cortisol responses in this context. Self-compassion training appears to have altered women's approach to an uncontrollable situation rather than their perceptions of controllability. RSA, or heart rate variability, responses diminished less during TSST anticipation and recovery in the self-compassion than control groups, suggesting that self-compassion training led to greater attentiveness and more adaptive emotion regulation in response to social threat (see Thayer et al., 2012; Thayer and Lane, 2000).
With regard to subjective responses, the TSST dramatically increased anxiety across all groups, in keeping with previous findings (e.g., Brown et al., 2012), but did so to a lesser degree following self-compassion training. This training was also associated with higher reported trait self-compassion as well as higher state self-compassion during TSST recovery than the control groups. Lower trait self-compassion at baseline (first session) was associated with greater increases in reported self-compassion following training. Future studies should examine whether this effect stems from regression to the mean or reflects the possibility that individuals lower in self-compassion benefit more from self-compassion training.
In addition to the possibility that self-compassion reduces defensiveness, as noted above, it also may activate a biological “caregiving system” implicated in both bonding and stress regulation (Preston, 2013; Swain et al., 2012). Researchers have argued that such a caregiving system is activated by compassion for others. However, self-compassion may also activate this caregiving system, which would help to explain why self-compassion training produced psychobiologically beneficial effects in social stress contexts. Future studies could compare self- and other-directed compassion training to examine whether compassion, regardless of target, has such beneficial effects.
To our knowledge, only one previous study has examined the impact of self-compassion training on social evaluative threat responses (Pace et al., 2009), where experimental condition differences in psychobiological responses were not found. The positive findings of the present study are likely due to three factors. First, although our training was far briefer, it was designed to foster adaptive responses to social stress. For example, participants in all conditions were told that “the rest of the study is challenging”. Both active conditions (self compassion and active control) were then told that “to help you prepare for this challenge, we're having you listen to a recording” which we used to justify that they “focus and concentrate fully on the recording”. This, along with other features, may have helped women to take seriously and draw upon their self-compassion training during the TSST stressor. Thus, self-compassion training may be particularly helpful when people are encouraged to draw upon it before or during stressful situations, rather than merely during neutral times when training-related practice is often done. Of course, the more intensive training undertaken by the Pace et al (2009) participants may have offered more enduring benefits than our briefer training; future studies will need to examine this possibility. Second, we drew upon a different and possibly simpler type of self-compassion training (metta) than used in the Pace et al. study (2009). It is possible that among beginners, some forms of self-compassion training are more effective at modulating social stress responses than others. Finally, our training focused largely on self-compassion, and only briefly touched on compassion for others – in a brief training context, this highly directed training may have promoted greater resiliency during the self-threat that the TSST represents. By focusing on training in self-compassion rather than the range of self- and other-compassion, concentration, and mindfulness practices taught in the Pace et al (2009) study, our study more clearly links the self-compassion dimension of compassion training to adaptive social threat responding.
Several study limitations are notable. First, the study sample was limited to a convenience sample of young undergraduate women, and replication is needed across both sexes and in more diverse samples before firm conclusions can be made about the efficacy of self-compassion training for reducing psychobiological responses to social threat. Second, the TSST represents a standardized stressor in a controlled environment; future research would do well to test the value of self-compassion training for reducing social evaluative stress in naturalistic environments. Third, the training was relayed to novices without ongoing guidance or feedback from instructors. This was deliberate because we were interested in self-directed training that could be replicated in diverse contexts; however, it may have limited participants’ capacity to engage more deeply with the meditations. Relatedly, women listened to a brief (5 minute) condition-specific “reminder” exercise immediately prior to the TSST. Future studies will need to examine the relative contribution to stress modulation of this brief onsite training versus the four days of practice leading up to the TSST session. Finally, we did not assess women's previous experience with contemplative practices such as formal meditation or yoga, which may have influenced their experience of the self-compassion training. However, we assessed trait self-compassion, state anxiety, and social anxiety, as well as the physiological variables, and showed that the conditions did not differ at baseline.
Conclusions
This experiment represents the first to show that self-compassion training can diminish psychobiological responses to social evaluative threat, relative to rigorous control conditions, and offers a fresh perspective on social self-preservation. The findings are noteworthy given the intensity of the TSST stressor relative to the brevity of the self-compassion training (45 minutes over 5 days). In that social stress is a ubiquitous feature of modern life, our findings suggest that self-compassion is a promising approach to diminishing its potentially negative psychological and biological effects.
Acknowledgements
We would like to acknowledge our excellent project coordinators Kelsee Henningsen and Kimberly Brown and research assistants Patrick Autruong, Marc Azoulay, Alaina Carr, Victoria Grunberg, Kristy Gustavson, Ryan Healy, Olivia Johnson, Chandni Merchant, Himgauri Nikrad, Laura Niss, Scott Schulman, and Thomas Parker Twibell for making this project possible. We also thank Patrick Benitez for his expert assistance in processing samples for biomarker analysis and Dr. Charles Judd for his helpful statistical consultation.
Funding
JJA was supported with startup funds from the University of Colorado Boulder. MLL was partially supported by NIH Grant CA126971.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors J. J. Arch developed the study concept. All authors contributed to the study design effort led by J. J. Arch and K. W. Brown. Data collection was performed by J. J. Arch, L. N. Landy, K. Brown, and D. J. Dean at the University of Colorado Boulder. Physiological data cleaning and organization was performed by D. J. Dean. M. L. Laudenslager contributed the salivary assays and assisted in their interpretation. J. J. Arch completed the data analysis with input from K. W. Brown. J. J. Arch drafted the paper and K. W. Brown provided critical revisions. All authors approved the final version of the paper for submission.
Participants who refused to do the TSST (n = 1) or were withdrawn due to obvious high distress at baseline (n = 1) (e.g., pale, shaking, appearing physically unwell - symptoms that appeared to be unrelated to the study) were not included in the analyses.
Please contact J.J.A. to obtain a copy of the state SCS.
Menstrual cycle phase was not covaried in salivary analyses because a substantial minority of women (n = 18) could not remember the start date of their last cycle. However, covarying this with the majority who remembered showed that group differences in AUCi alpha-amylase responses to the TSST were similar in direction and magnitude but became non-significant, p = .13, ΔR2 = .04, no doubt due to the reduced statistical power of this considerably smaller sample. Moreover, menstrual cycle phase did not predict alpha-amylase outcomes, p = .43, ΔR2 = .01. Covarying menstrual cycle phase did not change the lack of group differences in the AUCi salivary cortisol outcomes, nor was it a significant predictor of cortisol responses, p = .18, ΔR2 = .03.
The correlation between AUCi for log-transformed sAA and cortisol was: r = .22, p = .03, in the overall sample; r = .34, p = .02, in the SC group; r = .04, p = .83, in the attention control group; and r = .27, p = .24, in the NI group. The AUCi results were consistent with repeated measures ANOVA results in which the group by time cubic effect for sAA was significant, F(2, 97) = 3.33, p = .04, ηp2 = .06, whereas the group by time effect for cortisol was non-significant, p = .46, ηp2 = .02.
Conflicts of Interest
All authors declare that they have no conflicts of interest.
References
- Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children's behavior: Advantages of a multisystem approach. Developmental and behavioral pediatrics. 2002;23:102–113. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, Van der Molen MW. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. The metrics of cardiac Chronotropism: Biometric perspectives. Psychophysiology. 1995;32:162–171. doi: 10.1111/j.1469-8986.1995.tb03308.x. [DOI] [PubMed] [Google Scholar]
- Brown KW, Weinstein N, Creswell JD. Trait mindfulness modulates neuroendocrine and affective responses to social evaluative theat. Psychoneuroendocrinology. 2012;37:2037–2041. doi: 10.1016/j.psyneuen.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydon L, Strike PC, Bhattacharyya MR, Whitehead DL, McEwan J, Zachary I, Steptoe A. Hostility and physiological responses to laboratory stress in acute coronary syndrome patients. Journal of Psychosomatic Research. 2010;68:109–116. doi: 10.1016/j.jpsychores.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condren RM, O'Neill A, Ryan MCM, Barrett P, Thakore JH. HPA axis response to a psychological stressor in generalised social phobia. Psychoneuroendocrinology. 2002;27:693–703. doi: 10.1016/s0306-4530(01)00070-1. [DOI] [PubMed] [Google Scholar]
- Creswell JD, Welch WT, Taylor SE, Sherman DK, Gruenewald TL, Mann T. Affirmation of personal values buffers neuroendocrine and psychological stress responses. Psychology Science. 2005;16:846–851. doi: 10.1111/j.1467-9280.2005.01624.x. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Gruenewald TL, Kemeny ME. When the social self is threatened: Shame, physiology, and health. Journal of Personality. 2004;72:1191–1216. doi: 10.1111/j.1467-6494.2004.00295.x. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Gruenewald TL, Kemeny ME. Psychobiological responses to social self threat: Functional or detrimental? Self and Identity. 2009;8:270–285. [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Edelstein RS, Yim IS, Quas JA. Narcissism predicts heightened cortisol reactivity to a psychosocial stressor in men. Journal of Research in Personality. 2010;44:565–572. doi: 10.1016/j.jrp.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons RA. Factor analysis and construct validity of the narcissistic personality inventory. Journal of personality assessment. 1984;48:291–300. doi: 10.1207/s15327752jpa4803_11. [DOI] [PubMed] [Google Scholar]
- Feingold A. Effect sizes for growth-modeling analysis for controlled clinical trials in the same metric as for classical analysis. Psychological Methods. 2009;14:43–53. doi: 10.1037/a0014699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Cohn MA, Coffey KA, Pek J, Finkel SM. Open hearts build lives: Positive emotions, induced through loving-kindness meditation, build consequential personal resources. Journal of Personality and Social Psychology. 2008;95:1045–1062. doi: 10.1037/a0013262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nature Reviews Immunology. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Granger DA, Hibel LC, Fortunato CK, Kapelewski CH. Medication effects on salivary cortisol: tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology. 2009;34:1437–1448. doi: 10.1016/j.psyneuen.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Grossman P, Hinton DE. Loving-kindness and compassion meditation: Potential for psychological intervention. Clinical Psychology Review. 2011;31:1126–1132. doi: 10.1016/j.cpr.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Medicine. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 2008;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kok BE, Coffey KA, Cohn MA, Catalino LI, Vacharkulksemsuk T, Algoe SB, Brantley M, Fredrickson BL. How positive emotions build physical health perceived positive social connections account for the upward spiral between positive emotions and vagal tone. Psychological Science. 2013;24:1123–1132. doi: 10.1177/0956797612470827. [DOI] [PubMed] [Google Scholar]
- Leary MR, Tate EB, Adams CE, Batts Allen A, Hancock J. Self-compassion and reactions to unpleasant self-relevant events: The implications of treating oneself kindly. Journal of Personality and Social Psychology. 2007;92:887–904. doi: 10.1037/0022-3514.92.5.887. [DOI] [PubMed] [Google Scholar]
- Litvack DA, Oberlander TF, Carney LH, Saul JP. Time and frequency domain methods for heart rate variability analysis: a methodological comparison. Psychophysiology. 1995;32:492–504. doi: 10.1111/j.1469-8986.1995.tb02101.x. [DOI] [PubMed] [Google Scholar]
- Matthews KA. Psychological perspectives on the Type A behavior pattern. Psychological Bulletin. 1982;91:293–323. [PubMed] [Google Scholar]
- Mattick RP, Clarke JC. Development and validation of measures of social phobia scruitiny fear and social interaction anxiety. Behaviour Research and Therapy. 1998;36:455–470. doi: 10.1016/s0005-7967(97)10031-6. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual: Mechanisms leading to disease. Archives of Internal Medicine. 1993;153:2093–2101. [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychological Bulletin. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nater UM, La Marca R, Florin L, Moses A, Langhans W, Koller MM, Ehlert U. Stress-induced changes in human salivary alpha-amylase activity—associations with adrenergic activity. 2006;31:49–58. doi: 10.1016/j.psyneuen.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Nater UM, Rohleder N, Schlotz W, Ehlert U, Kirschbaum C. Determinants of the diurnal course of salivary alpha-amylase. Psychoneuroendocrinology. 2007;32:392–401. doi: 10.1016/j.psyneuen.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Neff KD. The development and validation of a scale to measure self-compassion. Self and Identity. 2003a;2:223–250. [Google Scholar]
- Neff KD. Self-Compassion: An alternative conceptualization of a healthy attitude toward oneself. Self and Identity. 2003b;2:85–101. [Google Scholar]
- Neff KD, Vonk R. Self-compassion versus global self-esteem: Two different ways of relating to oneself. Journal of Personality. 2009;77:23–50. doi: 10.1111/j.1467-6494.2008.00537.x. [DOI] [PubMed] [Google Scholar]
- Pace TWW, Negi LT, Adame DD, Cole SP, Sivilli TI, Brown TD, Issa MJ, Raison CL. Effect of compassion meditation on neuroendocrine, innate immune and behavioral responses to psychosocial stress. Psychoneuroendocrinology. 2009;34:87–98. doi: 10.1016/j.psyneuen.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston SD. The originals of altruism in offspring care. Psychological Bulletin. 2013 doi: 10.1037/a0031755. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Reinhard DA, Konrath SH, Lopez WD, Cameron HG. Expensive egos: narcissistic males have higher cortisol. PLoS ONE. 2012;7:e30858. doi: 10.1371/journal.pone.0030858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: family social environments and the mental and physical health of offspring. Psychological bulletin. 2002;2 [PubMed] [Google Scholar]
- Rohleder N, Nater UM, Wolf JM, Ehlert U, Kirschbaum C. Psychosocial stres-induced activation of salivary alph-amylase: An indicator of sympathetic activity? Annals of the New York Academy of Sciences. 2004;1032:258–263. doi: 10.1196/annals.1314.033. [DOI] [PubMed] [Google Scholar]
- Salzberg S. Lovingkindness: The Revolutionary Art of Happiness. Shambhala; Boston: 1995. [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endorcrine Review. 1986;7 doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory, STAI (Form Y) Mind Garden; Palo Alto, CA.: 1983. [Google Scholar]
- Swain JE, Konrath S, Brown SL, Finegood ED, Akce LB, Dayton CJ, Ho SS. Parenting and beyond: Common neurocircuits underlying parental and altruistic caregiving. Parenting, Science and Practice. 2012;12:115–123. doi: 10.1080/15295192.2012.680409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Lerner JS, Sherman DK, Sage RM, McDowell NK. Portrait of the self-enhancer: well adjusted and well liked or maladjusted and friendless? Journal of Personality and Social Psychology. 2003;84:165–176. [PubMed] [Google Scholar]
- Thayer JF, Ahs F, Fredrikson M, Sollers JJ, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neuroscience & Biobehavioral Reviews. 2012;36:747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Annals of Behavioral Medicine. 2009;37:141–153. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Sternberg EM. Beyond heart rate variability: vagal regulation of allostatic systems. Annals of the New York Academy of Sciences. 2006;1088:361–372. doi: 10.1196/annals.1366.014. [DOI] [PubMed] [Google Scholar]
- van Cauter E. Diurnal and ultradian rhythms in human endorcrine function: A minireview. Hormone Research. 1990;34:45–53. doi: 10.1159/000181794. [DOI] [PubMed] [Google Scholar]
- van Stegeren A, Rohleder N, Everaerd W, Wolf OT. Salivary alpha amylase as marker for adrenergic activity during stress: Effect of betablockade. Psychoneuroendocrinology. 2006;31:137–141. doi: 10.1016/j.psyneuen.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Wolpe J. The practice of behavior therapy. Rev. ed. Pergamon Press; New York: 1990. [Google Scholar]