Abstract
The purpose of this study was to determine the association between measures of disease severity, impairment, and ambulation ability in persons with polyostotic fibrous dysplasia (PFD). A cross-sectional sample of 81 patients (ages 5–57) with polyostotic fibrous dysplasia was evaluated as part of an ongoing study. Subjects were scored on the Skeletal Disease Burden Score (SDBS), completed a 9-minute walk test (9MW), manual muscle testing (MMT), and measurements of range of motion (ROM). Correlations between continuous variables were calculated using the Pearson correlation coefficient and ordinal variables by Spearman correlation coefficient. It was found that subjects with more severe disease walked slower than those with less skeletal disease, with the exception of the youngest subjects. Walking velocity was faster in subjects with better hip strength and range of motion and slower in those with bilateral coxa vara. Those subjects with more severe disease had less range of motion, were weaker at the hips, and more likely to have leg length discrepancy. Skeletal disease severity was associated with hip weakness, leg length discrepancy, and loss of range of motion. Inmost cases, findings did not differ in the presence or absence of associated endocrinopathies. Skeletal disease severity, MMT and ROM each has an impact on walking efficiency in persons with PFD. These findings suggest that treatment focused on strategies to improve or, at least, maintain hip strength and range of motion, correct leg length discrepancies and hip malalignment may help preserve ambulation ability in persons with PFD and that treatment should begin at a young age.
Keywords: Polyostotic fibrous dysplasia, McCune–Albright Syndrome, Ambulation, Functional measures
Introduction
Polyostotic fibrous dysplasia (PFD) is a disorder that derives from a mutated skeletal stem cell resulting in the replacement of normal bone by a benign fibrous connective tissue formed as a consequence of the proliferation of undifferentiated cells of osteogenic lineage [1–5]. It is due to post-zygotic, missense, activating somatic mutations occurring in the GNAS gene, which codes for the α subunit of the stimulatory G protein (Gsα)[6,7]. These mutations result in the upregulation of cAMP, causing defects in osteoblast differentiation and production of an abnormal bone [4]. Additionally, an increased production of IL-6 is found in bones with PFD, which may contribute to osteoclastogenesis and resorption of adjacent normal bone [8]. PFD presents as a mosaic disorder in some but not all bones in a given person and can exist as an isolated skeletal finding or may be coupled with endocrine abnormalities as the McCune–Albright Syndrome (MAS)[9]. MAS is characterized by café-au-lait pigmentation on the skin, hyperfunctioning endocrinopathies such as precocious puberty, hyperthyroidism, and fibrous dysplasia of the bone (FD)[ 10]. The spectrum of involved bones is broad, from a single asymptomatic site detected incidentally, to total skeletal involvement associated with marked morbidity [9,11]. PFD/MAS is a rare disease. While the precise prevalence is unknown, estimates of the prevalence of MAS range between 1/100,000 and 1/1,000,000 [10].
The diagnosis of PFD is usually made on clinical grounds, by some combination of medical history, radiographic evaluation, and radionucleotide bone scan [12]. Occasionally, histopathological and/or molecular confirmation are needed [11,13,14]. Clinical manifestations of FD of the proximal femur, one of the most commonly affected sites, lead to the pathognomonic finding of the “shepherd's crook” deformity [15]. Common findings in the craniofacial and axial skeleton include facial asymmetry and progressive scoliosis, respectively [16–19]. FD is often associated with pain [20]. In MAS, endocrine abnormalities can exacerbate the disease [17,21–24]. Even children who initially present with a great amount of disease on imaging are quite functional in mobility and daily life skills. However, these abilities frequently show significant decline in the progression to adulthood [25].
Treatment with bisphosphonates has provided pain relief for some patients but has no overall or long-term effect on disease progression or function [26–30]. Surgical management of PFD is challenging, particularly because of growth and development through childhood into adolescence. Little has been published with respect to everyday functional capabilities of persons with FD [31–34]. Understanding of the spectrum of impairment and disability in persons with PFD is important for treatment planning and counseling of families. Information about the relationships between disease severity and function may further assist with rehabilitation and surgical treatment planning to optimize the quality of life for persons with PFD.
The goals of this study were to (a) determine the relationship between extent of PFD and the functional skill of ambulation, (b) determine the relationship between extent of PFD and measures of musculoskeletal impairment, and (c) determine the relationship between measures of impairment and ambulation ability.
Methods and materials
Subjects were chosen from a group of ninety-seven patients enrolled in an NIDCR IRB-approved natural history study of PFD/MAS. All subjects or their parents gave informed consent to participate in this study. All subjects were assessed for the presence of café au lait spots (CAL) and endocrinopathies which are associated with MAS. Functional measures obtained as part of the protocol included nine-minute walk test (9MW), joint range of motion measurements (ROM), and manual muscle testing (MMT). Imaging included 99Tc-MDP bone scan. Data for analysis were chosen from the first visit during which every one of these measures was obtained for each subject.
The 9MW test is a standardized, validated measure of ambulation endurance and efficiency [35]. Patients were instructed to walk and/or run at the fastest comfortable pace that they would be able to sustain for a full nine minutes. Walking velocity was compared to age and gender adjusted norms for children under 17. Since there are no specific adult norms for the 9MW, reference values for 17 year olds were used for adult patients. Distance covered in the nine minutes was assigned a percentile according to normalized values. Percentiles were used in statistical analyses.
ROM of lower limb joints was measured by one of two examiners with a goniometer using standard technique. Bilateral lower limb ROM was measured at the hip (flexion, extension, internal rotation, external rotation, and abduction), knee (flexion and extension) and ankle (plantar flexion and dorsiflexion). In order to compare and combine different joint movements, Z-scores for each movement were calculated using published norms and standard errors [36]. Movements were then grouped by joint and mean Z-scores were calculated for each joint.
MMT of the lower limbs was performed using a standard technique scored on the Medical Research Council (MRC) ordinal scale of 0 to 5 [37]. The median for each muscle (gluteus maximus, gluteus medius, iliopsoas, quadriceps, hamstrings, and ankle plantar and dorsiflexors) was then calculated for purpose of analysis.
The presence or absence of leg length discrepancy at the time of the subject's first visit was determined by review of the physiatry clinical note associated with that visit.
The bone scan was used to calculate the Skeletal Disease Burden Score (SDBS), a validated tool developed to assess the overall disease burden of PFD [38]. This weighted measure was derived from the determination of isotopic activity in the various body segments on Tc99-MDP bone scans. In addition, skeletal location of fibrous dysplasia was recorded. Hip x-rays were reviewed for neck to shaft angle and subjects were classified as normal, unilateral or bilateral coxa valgus or varus or mixed valgus/varus.
Calculations
Correlations between continuous data (SDBS, ROM) were determined using the Pearson correlation coefficient; correlations with ordinal data (9MW percentile, MMT) were found using the Spearman correlation coefficient. Correlations between SDBS and ROM were used to determine the relationship between the extent of PFD and joint mobility. To determine the relationship between the extent of PFD and strength impairment, correlations were calculated between SDBS and MMT. Correlations between MMT and ROM were calculated to determine the relationship between strength impairment and joint mobility. T-tests were also performed to examine for differences in SDBS, 9MW,MMT, and ROM between groups with or without CAL, precocious puberty, abnormal level of growth hormone, abnormal thyroid function, hypercortisolemia, presence of craniofacial, axial or appendicular fibrous dysplasia, femoral FB, or leg length discrepancy (LLD). Chi square or Fisher's exact tests were performed to look for possible associations between leg length discrepancy and either endocrinopathies or distribution of skeletal disease. Analysis of Variance with post-hoc Tukey tests were performed to examine for differences in 9MWin relation to categories of femoral alignment.
Results
Eighty-one subjects (32 males, 49 females) had data sets that met the criteria for evaluation of their records. Patients ranged in age from 5 to 57 years (mean = 25). Seventy-seven (95%) of the participants presented with both PFD and MAS. Further demographic data are summarized in Table 1. Data were extracted from the subjects' first visits at which all tests were recorded. All data were collected prospectively as part of ongoing studies. No significant differences were detected between male and female subjects for range of motion or on manual muscle testing.
Table 1.
Patient demographics.
| Gender | 41% male |
| Age | 25.03 ± 15.7 years |
| CAL | 65.4% |
| PFD | 97.5% |
| Precocious puberty | 45.7% |
| Abnormal GH | 14.8% |
| Thyroid | 39.5% |
| Hypercortisolism | 6.17% |
| Phosphate wasting | 32.1% |
| Craniofacial FD | 86.4% |
| Axial FD | 80.2% |
| Appendicular FD | 88.9% |
| Femoral involvement | 81.5% |
| SDBS score | 28.4 ± 20.0 |
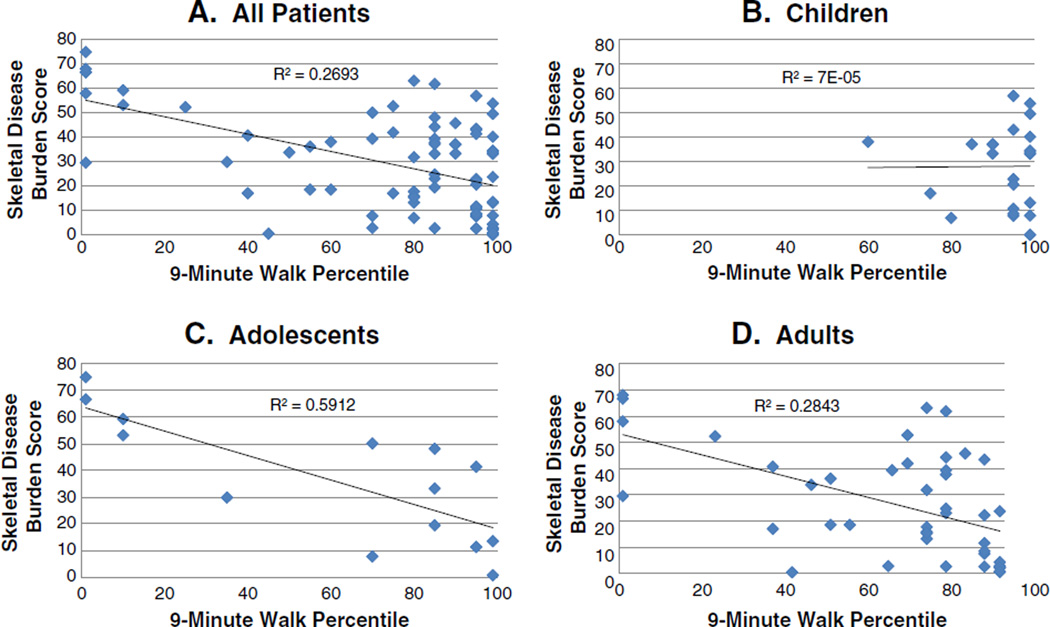
Correlation between SDBS and 9MW was found to be strongly negative (R = −0.52, p < 0.0001, Fig. 1).When separated into children (age < 12), adolescents (12 ≤ age < 18), and adults (age ≥ 18), as shown in Figs. 1B–D, respectively, correlations between SDBS and 9MW were strongly negative and significant in the adolescent and adult age groups (R = −0.78, p = 0.001; R = −0.53, p < 0.001, respectively). However, correlation became insignificant for the subgroup of children less than 12 years of age.
Fig. 1.
Regression analyses of the relationship between skeletal disease burden and walking function by age group. Skeletal disease burden was calculated using a standardized, validated tool the measures skeletal disease burden (SDBS, Ref. [38]) and function was assessed by performance on a standardized nine minute walk (9MW, Ref. [35]). The relationship between SDBS and 9MWwas determined by calculating the Pearson correlation coefficient. The R and p values are indicated. The relationship for all patients (A), children younger than 12 (B), adolescents aged 12–17 (C), and for adults (D) are shown. For all groups but the children, there was a significant inverse relationship between skeletal burden and function.
Correlations between SDBS and MMT are shown in Table 2(a). Moderate negative correlation was found between SDBS and hip strength, including both the gluteus medius (GM) and maximus (GX). This relationship existed bilaterally (right GM: R = −0.39, p < 0.001; left GM: R = −0.32, p = 0.004; right GX: R = −0.43, p < 0.001; left GX: R = −0.35, p = 0.001). Weak to moderate negative correlation existed with knee strength; there was weak to insignificant correlation between SDBS and ankle groups. Correlations between SDBS and ROM are shown in Table 2(b). Moderate to strong negative correlations were found with hip extension bilaterally (right: R = −0.51, p < 0.0001; left: R = −0.41, p = 0.00016). Weak to moderate negative correlations existed with hip internal rotation (right: R = −0.34, p = 0.0017; left: R = −0.24, p = 0.029). Weak to insignificant positive correlation existed with hip flexion; weak to insignificant negative correlations existed with knee flexion and extension.
Table 2.
Correlations between Skeletal Disease Burden Score and functional limitation measures.
| (a) Manual muscle testing | ||
|---|---|---|
| Muscle | Right | Left |
| Gluteus medius | −0.39 (p = 0.00032) | −0.32 (p = 0.0037) |
| Gluteus maximus | −0.43 (p = 0.000062) | −0.35 (p = 0.0014) |
| Hamstrings | −0.22 (p = 0.044) | −0.32 (p = 0.0038) |
| Iliopsoas | −0.37 (p = 0.00063) | −0.38 (p = 0.00037) |
| Quadriceps | −0.42 (p = 0.00010) | −0.28 (p = 0.013) |
| Dorsiflexors | −0.22 (p = 0.048) | −0.18 (p = 0.099) |
| Plantarflexors | −0.23 (p = 0.046) | −0.18 (p = 0.14) |
| Ext Hal Long | −0.35 (p = 0.00015) | −0.071 (p = 0.52) |
| (b) Range of motion | ||
|---|---|---|
| Joint movement | Right | Left |
| Hip flexion | 0.12 (p = 0.28) | 0.25 (p = 0.024) |
| Hip extension | −0.51 (p < 0.0001) | −0.41 (p = 0.00016) |
| Hip external rotation | −0.20 (p = 0.080) | −0.097 (p = 0.39) |
| Hip internal rotation | −0.34 (p = 0.0017) | −0.24 (p = 0.029) |
| Hip abduction | −0.19 (p = 0.090) | −0.31 (p = 0.0050) |
| Knee flexion | −0.26 (p = 0.017) | −0.11 (p = 0.33) |
| Knee extension | −0.28 (p = 0.010) | −0.056 (p = 0.62) |
| Plantarflexion | −0.18 (p = 0.11) | −0.29 (p = 0.0082) |
| Dorsiflexion | 0.044 (p = 0.70) | 0.13 (p = 0.24) |
Correlations between 9MW and MMT can be found in Table 3(a). Moderate positive correlations of 9MWvelocity percentile were found with hip muscle and quadriceps strength (right GM: R = 0.31, p = 0.0046; left GM: R = 0.25, p = 0.026; right GX: R = 0.40, p = 0.00020; left GX: R = 0.35, p = 0.0013; right quadriceps: R = 0.29, p = 0.0090; left quadriceps: R = 0.31, p = 0.0058). Correlations between 9MW and ROM can be found in Table 3(b). Moderate to strong correlations existed with hip extension (right: R = 0.56, P < 0.0001; left: R = 0.45, P < 0.0001) and knee flexion (right: R = 0.63, P < 0.0001; left: R = 0.47, P < 0.0001).Weak to insignificant correlations with hip flexion and external rotation were noted.
Table 3.
Correlations between 9-minute walk and functional limitation measures.
| (a) Manual muscle testing | ||
|---|---|---|
| Muscle | Right | Left |
| Gluteus medius | 0.31 (p = 0.0046) | 0.25 (p = 0.026) |
| Gluteus maximus | 0.40 (p = 0.00020) | 0.35 (p = 0.0013) |
| Hamstrings | 0.11 (p = 0.33) | 0.16 (p = 0.16) |
| Iliopsoas | 0.32 (p = 0.0034) | 0.30 (p = 0.0059) |
| Quadriceps | 0.29 (p = 0.0090) | 0.31 (p = 0.0058) |
| Dorsiflexors | 0.15 (p = 0.17) | 0.043 (p = 0.70) |
| Plantarflexors | 0.27 (p = 0.020) | 0.23 (p = 0.049) |
| Ext Hal Long | 0.14 (p = 0.21) | 0.12 (p = 0.26) |
| (b) Range of motion | ||
|---|---|---|
| Joint movement | Right | Left |
| Hip flexion | 0.26 (p = 0.019) | 0.17 (p = 0.12) |
| Hip extension | 0.56 (p < 0.0001) | 0.45 (p < 0.0001) |
| Hip external rotation | 0.26 (p = 0.016) | 0.18 (p = 0.12) |
| Hip internal rotation | 0.48 (p < 0.0001) | 0.33 (p = 0.0028) |
| Hip abduction | 0.41 (p = 0.00023) | 0.34 (p = 0.0022) |
| Knee flexion | 0.63 (p < 0.0001) | 0.47 (p < 0.0001) |
| Knee extension | 0.29 (p = 0.0087) | 0.040 (p = 0.73) |
| Plantarflexion | −0.14 (p = 0.21) | −0.090 (p = 0.42) |
| Dorsiflexion | 0.16 (p = 0.16) | −0.044 (p = 0.70) |
There was no significant difference in 9MWwhen subjects were divided by the presence or absence of CAL or by the presence of FD in specific skeletal locations. With the exception of growth hormone excess (higher 9MW %ile, p = 0.045), there was no significant difference when subjects were divided by the presence or absence of endocrinopathies. The most notable significant differences in ROM were associated with phosphate wasting: increased ROM Z-scores for right total hip (p = 0.026), left total hip (p = 0.004), average of right and left hip (p = 0.007), and right (but not left) knee flexion and extension (p = 0.025). There were higher ROM Z-scores in precocious puberty only with left (p = 0.003) and right (p = 0.008) ankle ROM. There was only a significantly greater left knee ROM (p = 0.003) to growth hormone excess. There were no significant differences in any ROM parameters for thyroid function or hypercortisolemia. Median MMT was greater in patients with café au lait spots (p = 0.024), phosphate wasting (p = 0.032), and in subjects with axial skeletal involvement (p = 0.041). No other endocrinopathies or skeletal disease distributions showed significant differences in strength.
SDBS in persons with leg length discrepancy was significantly (p < 0.0001) higher (35 ± 5) than those without leg length discrepancy (15 ± 6). 9MW percentile was significantly higher (p < 0.0001) in persons without leg length discrepancy (92%ile ± 4 vs. 67%ile ± 9). Analyses were also conducted to examine the differences in functional limitation measures (MMT and ROM) in patients with and without LLD; correlations can be found in Table 4. Strength was significantly less in bilateral hip and knee muscle groups in persons with leg length discrepancy, but no significant difference was noted in ankle muscle groups. Significantly less range of motion was noted in bilateral hip extension and abduction when leg length discrepancy was present. Similar difference was noted in right, but not left, hip flexion range of motion. Right external rotation range of motion approached significance (p = 0.07) and just reached significance on the left (p = 0.05). There was no significant difference in hip internal rotation range of motion when accounting for leg length discrepancy. There was a significant difference noted in both right and left plantar flexion range of motion (p = 0.028, p = 0.0014 respectively). Differences in range of motion about the knee were not significant. There were no significant differences in the presence or absence of LLD for subjects with or without various endocrinopathies. The absence of LLD was found to be significantly more frequent with appendicular skeletal involvement (Fisher's exact test, p = 0.07). The presence of LLD was more frequent with femoral disease (Chi2, p < 0.0001).
Table 4.
Correlations between individuals with and without leg length discrepancies in functional limitation measures.
| (a) Manual muscle testing | ||
|---|---|---|
| Muscle | Right | Left |
| Hip, combined (gluteus maximus, gluteus medius, abductors) | p = 0.00023 | p = 0.0046 |
| Knee, combined (flexion, extension) | p = 0.12 | p = 0.051 |
| Ankle, combined (plantarflexion, dorsiflexion) | p = 0.60 | p = 0.37 |
| Lower extremity, average | p = 0.0090 | |
| Lower extremity, median | p = 0.012 | |
| (b) Range of motion | ||
|---|---|---|
| Joint movement | Right | Left |
| Hip flexion | p = 0.035 | p= 0.81 |
| Hip extension | p = 0.0068 | p = 0.049 |
| Hip external rotation | p = 0.071 | p = 0.046 |
| Hip internal rotation | p = 0.19 | p = 0.21 |
| Hip abduction | p = 0.040 | p = 0.013 |
| Knee flexion | p = 0.14 | p = 0.21 |
| Knee extension | p = 0.30 | p = 0.61 |
| Plantar flexion | p = 0.028 | p = 0.0014 |
| Dorsiflexion | p = 0.71 | p = 0.49 |
Bold values indicate significance at p≤0.05.
Analysis of variance revealed significant differences in 9MWpercentiles when subjects were categorized by hip alignment (p < 0.0001). Post-hoc testing revealed that 9MW%iles were significantly less in subjects with bilateral coxa vara in comparison to subjects with hips that were normally aligned bilaterally, had bilateral coxa valga, or unilateral coxa valgus. No significant differences were revealed between subjects with bilateral coxa vara and those with unilateral varus or mixed varus and valgum. No other significant differences were detected between the groups.
Discussion
There have been no previous studies examining the functional status of patients with PFD and its association with disease severity. Without such information, treatment to date has been based solely on clinical experience and anecdote. The significant negative correlation between SDBS and 9MW age-normalized percentiles indicates that the ability to ambulate is adversely affected by skeletal disease severity. This finding is even more evident in the adolescent and adult age groups. There are several reasonable possibilities that can help explain why this correlation was not found to be significant in pre-adolescent age group. For one, it may be a function of how the 9MW is normalized. The 9MWfactors in age and gender but not variation in height within a given age. Gait velocity is dependent on stature and many children with MAS have increased height compared to age peers due to their precocious puberty. However, if the lack of correlation between SDBS and 9MW in preadolescents is independent of this stature issue, it would imply that, even in severely involved children, there may still be a chance to improve or maintain ambulation status through treatment interventions, if started early enough in life.
The results of this study demonstrated that the hip was the lower limb joint whose function was most significantly impacted by the extent of PFD. This was true for both mobility and strength. Analyses of the relationship between measures of impairment in joint mobility, hip alignment, and strength and ambulation ability, as represented by the 9MW, suggest that the degree of impairment at the hip significantly influences ambulation performance.
Our findings relating to the role of the hip are consistent with findings in musculoskeletal disabilities such as osteoarthritis and neurological disabilities such as cerebral palsy (CP). In an article published in 2011 by Thompson et al., researchers studied the relationship between muscle strength and ability to ambulate in patients with CP [39]. Between those who walked independent of gait aids and those who could not, the greatest differences in strength were found in the hip abductors and knee extensors. These movements are both important in maintaining stability about the sagittal and coronal planes throughout the walking cycle. Similarly, in a study of gait biomechanics in adults with osteoarthritis, the strength of muscles supporting and guiding the hip through motion, particularly those involved in hip extension, was found to be directly correlated with the energy cost of walking [40]. The severity of the reduction of hip extension was proportional to the magnitude of the energy cost of walking; this severity was inversely proportional to gait velocity. It is suggested that this is likely due to the role that hip extensors play in step-to-step transitions and lateral swing during the gait cycle and the greater energy expenditure that would occur as a result of decreased muscle strength. Like osteoarthritis, MAS often causes deformation and pain at the hip.
The conclusions of these previous studies and the strong relationships between ambulation ability (as measured by 9MW) and the outcome measures at the hip in PFD revealed in this study strongly suggest that strength and range of motion of the hip in PFD greatly affect one's ability to ambulate. The associations between disease burden (as measured by SDBS) and ambulation ability and between disease burden and impairments in strength and range of motion suggest that dysfunction at the hip is at least part of the mechanism by which disease severity has an impact on the ability to ambulate efficiently. This is significant in that both strength and range of motion can be improved with targeted rehabilitation interventions. Thus, it is possible that targeting and improving strength and range of motion could increase functionality and decrease morbidity. In fact, as specific program of hip range of motion exercises has been shown to improve gait parameters in frail elderly, a population that likely shares similar challenges in strength and joint mobility with more seriously impaired persons with MAS [41].
The findings of an association between leg length discrepancy and disease severity are not surprising. The presence of more fibrous dysplasia makes it more likely that there will be extensive involvement of the lower limbs. The more extensive involvement of the lower limbs can result in leg length discrepancy due to mechanisms including hip deformity, asymmetric presence of fibrous dysplasia in the two lower limbs, or previous fractures. Although, on first glance, it might appear counterintuitive that LLD was more frequent in those with less appendicular skeletal involvement, it makes sense when considering that there is greater appendicular involvement in bilateral disease, where LLD is less likely. Conversely, given the contribution that hip deformity contributes to LLD, the association between LLD and femoral involvement is not surprising. Similarly, the association of leg length discrepancy with hip weakness and decreased range of motion is quite logical. The increased amount of fibrous dysplasia and associated hip deformities is likely to result in decreased strength and range of motion as well as lower limb asymmetry. The association between leg length discrepancy and 9MW performance may be directly due to dynamic gait asymmetry or could simply be due to the association of leg length discrepancy with more severe disease, weakness, hip malalignment (which is supported by the association between bilateral coxa vara and decreased 9MWperformance) and/or diminished range of motion. This may be able to be resolved in future prospective studies. However, there is related research which has demonstrated that gait efficiency is improved when leg length discrepancy is corrected, so the use of shoe lifts should be considered in the presence of asymmetry [43]. Up to 3/8 inch can usually be placed under the insole within the shoe, higher amounts should be built into the midsole of the shoe. The goal should be to achieve a level pelvis.
Similarly, there are reasonable explanations for the associations observed between the presence of leg length discrepancy and both muscle strength and joint range of motion. The significantly increased hip weakness and decreased hip range of motion, most notable in hip extension and abduction, seen in subjects with leg length discrepancy may also be due to the fact that all are associated with more severe bony disease. In the absence of any significant difference in knee joint range of motion, the presence of a significant difference in plantar flexion range of motion is unlikely to be explained only on the basis of disease severity. However, it is common for persons with leg length discrepancy to attempt to achieve amore level pelvis during ambulation by placing the foot of the short leg in an equinus position [44]. This will eventually lead to a plantar flexion contracture on the short side. This is a likely explanation for our finding. Unfortunately, the data in this retrospective sample is insufficient to test the side to side association between LLD and plantar flexion contracture. Prospectively, it would be useful to not only track this association but to determine whether the effect is ameliorated by the use of shoe lifts.
One limitation of the current study is in the analyses of 9MW; it is important to acknowledge that the norms used to determine percentiles are corrected for age and gender but not for stature, which is often above average in the children with precocious puberty in our population. Additionally, the 9MWnormative data is available only for ages 5 through 17, so percentiles for adults 18 and over were calculated from the data available for 17 year olds. A 6-minute walk/run test (6MW), which is now normalized by age and gender across the human lifespan with additional adjustments for height and weight, was developed subsequent to the initiation of this study [40,42]. We would recommend that new research into ambulation and function across all ages use the 6MW instead of the 9MW so that results for all ages and heights may be corrected accordingly. An additional potential limitation of our study relates to the analysis of ROM. Since the normal range for various movements is not the same, it was necessary to calculate Z-scores to combine different joint movements in the analysis. Unfortunately, available means and standard deviations (necessary for Z-score calculations) are neither age nor gender normed.
There are several unexplained patterns in the results that should be acknowledged. In correlations between MMT and SDBS, EHL muscle showed moderate correlation on the right and insignificant correlation on the left. Unexplained unilateral correlation existed between SDBS and ROM of hip abduction; there was moderate, negative correlation in the left and insignificant correlation in the right hip abductors. It is also difficult to explain the unilateral association of leg length discrepancy with hip flexion and hip external rotation. These findings may be the result of the relatively small sample size, which is inevitable in a rare disease. For example, with respect to hip abductor range of motion, it is reasonable to assume that some PFD-related hip deformities will impact on hip abduction, so the inability to detect this on the right may be due to the small sample size. In the case of toe strength, the “asymmetry” of side to side correlations may be due to the small sample size but could also be a result of random error yielding a stronger than anticipated finding in the muscle in one foot but not the other.
It is also somewhat difficult to explain the associations between most associated endocrinopathies and range of motion in isolated joints. More notable was an association between phosphate wasting and greater overall ROM. There is no obvious reason for this association but it does suggest exploring the role of phosphate metabolism in elastic tissues. Similarly, it is difficult to explain an association between phosphate wasting and greater muscle strength. Associations between greater muscle strength and both café au lait spots and axial skeletal involvement are equally difficult to theorize on and may just be statistical aberrations.
The results of our analyses suggest that hip function is critical to ambulation in MAS/PFD, and treatment should focus on strategies to improve or, at least, maintain hip strength and range of motion. The significant negative correlation between 9MWpercentile and SDBS appears to emerge in the adolescent subgroup and persist into the adult subgroup. The lack of any correlation in younger children suggests that there may be a window of opportunity in childhood for the initiation of treatment to aid hip function. Loss of function may also be due to lack of adequate analgesia in older patients leading to disuse and weakness. We have previously shown that pain can emerge and/or worsen in patients with FD as they age, and that pain is often inadequately treated [20]. This possibility emphasizes the need for adequate pain control in PFD.
In light of the risk of fractures in this population, aquatic therapy would be the safest approach to hip strengthening exercises and improving range of motion at the hip. Other low impact approaches include both indoor stationary cycling and outdoor bi- or tricycling in patients where this is deemed safe. Resistive exercise against body weight and/or gravity is probably safe in MAS/PFD, but use of free weights or machines should be approached with caution and close supervision. These recommendations are also supported by studies in the osteoarthritis and osteoporosis literature. In a study of patients with osteoarthritis at the hip, researchers discussed a case report in a patient who focused on hip strengthening and static stretches to improve ROM [45]. After treatment, the patient had improved 6MW scores. Another study randomized elderly patients' post-hip fracture between controls and an exercise regimen using home-based leg strengthening exercises [45]. The study found that patients randomized to leg strengthening exercises outperformed those in the control group in strength and performance tests. These patients improved in isometric force production, gait velocity, and 6MW distance, while those in the control group showed no significant changes. More patients in the leg-strengthening group improved significantly on each functional measure than those randomized to the control. These studies support our prediction that focusing treatment to strengthening and increasing RO Mat the hip can improve functional outcomes.
For the subpopulation of patients with leg length discrepancy, it is advisable to offer non-operative correction through the use of shoe orthoses and lifts. As noted above, this may offer a more efficient gait and reduce secondary complications such as joint contractures. A variety of factors must be carefully considered in the prescription of the shoe system since there may be pelvic obliquity due to pelvic asymmetry and deformity unrelated to leg length discrepancy and femoral length discrepancy that may lead to asymmetric knee centers, which cannot be corrected by shoe systems.
Conclusions
Correlations of range of motion, disease burden (as measured by SDBS), and ambulation strongly suggest that dysfunction at the hip is a significant mechanism by which disease severity has an impact on the ability to ambulate efficiently. Focused rehabilitation interventions with particular consideration of hip range of motion and strength should play a major role in the treatment of persons with PFD. Additional research, including a prospective intervention study, to confirm and further elucidate the findings of this study would be of merit. At present, it is probably prudent to recommend a careful, clinician-directed, slowly progressive program of joint mobility and strengthening exercises to attempt to preserve ambulation function in persons with MAS/PFD with judicious use of shoe orthoses and modifications when indicated.
Acknowledgments
Funding
This research was funded entirely by the National Institutes of Health, Division of Intramural Research; the NIDCR and the Clinical Center Rehabilitation Medicine Department.
The authors would like to dedicate this article in memory of the late Usha Chaudhry, MD who made significant contributions to the study of MAS at the NIH, and who showed unwavering commitment to patient care and kindness to everyone whose life she touched.
Abbreviations
- 9MW
9-minute walk
- CP
cerebral palsy
- EHL
extensor hallucis longus
- GM
gluteus minimus
- GX
gluteus maximus
- IRB
institutional review board
- MMT
manual muscle testing
- MAS
McCune–Albright Syndrome
- MRC
Medical Research Council
- NIDCR
National Institute of Dental and Craniofacial Research
- NIH
National Institutes of Health
- PFD
polyostotic fibrous dysplasia
- ROM
range of motion
- SDBS
Skeletal Disease Burden Score
Footnotes
Conflicts of interest
The authors have no conflicts to report.
Contributor Information
Scott M. Paul, Email: SPaul@cc.nih.gov.
Lisa R. Gabor, Email: lisa.gabor@gmail.com.
Scott Rudzinski, Email: rudzinskism@upmc.edu.
David Giovanni, Email: giovannidavidmd@msn.com.
Alison M. Boyce, Email: boyceam@nidcr.nih.gov.
Marilyn R.N. Kelly, Email: marilynhkelly@gmail.com.
Michael T. Collins, Email: mc247k@nih.gov.
References
- 1.Riminucci M, Fisher LW, Shenker A, Spiegel AM, Bianco P, Gehron Robey P. Fibrous dysplasia of bone in the McCune–Albright syndrome: abnormalities in bone formation. Am J Pathol. 1997;151:1587–1600. [PMC free article] [PubMed] [Google Scholar]
- 2.Riminucci M, Saggio I, Robey PG, Bianco P. Fibrous dysplasia as a stem cell disease. J Bone Miner Res. 2006;21(Suppl. 2):P125–P131. doi: 10.1359/jbmr.06s224. [DOI] [PubMed] [Google Scholar]
- 3.Robey PG, Kuznetsov S, Riminucci M, Bianco P. The role of stem cells in fibrous dysplasia of bone and the McCune–Albright syndrome. Pediatr Endocrinol Rev. 2007;4(Suppl 4):386–394. [PubMed] [Google Scholar]
- 4.Riminucci M, Gehron Robey P, Saggio I, Bianco P. Skeletal progenitors and the GNAS gene: fibrous dysplasia of bone read through stem cells. J Mol Endocrinol. 2010;45:355–364. doi: 10.1677/JME-10-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lichtenstein L. Poloyostotic fibrous dysplasia. Arch Surg. 1938;36:874–898. [Google Scholar]
- 6.Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune–Albright syndrome. N Engl J Med. 1991;325:1688–1695. doi: 10.1056/NEJM199112123252403. [DOI] [PubMed] [Google Scholar]
- 7.Schwindinger WF, Francomano CA, Levine MA. Identification of a mutation in the gene encoding the α subunit of the stimulatory G protein of adenylyl cyclase in McCune–Albright syndrome. Proc Natl Acad Sci U S A. 1992;89:5152–5156. doi: 10.1073/pnas.89.11.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riminucci M, Kuznetsov SA, Cherman N, Corsi A, Bianco P, Gehron Robey P. Osteoclastogenesis in fibrous dysplasia of bone: in situ and in vitro analysis of IL-6 expression. Bone. 2003;33:434–442. doi: 10.1016/s8756-3282(03)00064-4. [DOI] [PubMed] [Google Scholar]
- 9.Collins MT. Spectrum and natural history of fibrous dysplasia of bone. J Bone Miner Res. 2006;21(Suppl 2):P99–P104. doi: 10.1359/jbmr.06s219. [DOI] [PubMed] [Google Scholar]
- 10.Dumitrescu CE, Collins MT. McCune–Albright syndrome. Orphanet J Rare Dis. 2008;3:12. doi: 10.1186/1750-1172-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins MT, Bianco P. Fibrous dysplasia. In: Favus MJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Washington, D.C.: American Society for Bone and Mineral Research; 2006. pp. 415–418. [Google Scholar]
- 12.Leet AI, Collins MT. Current approach to fibrous dysplasia of bone and McCune–Albright syndrome. J Child Orthop. 2007;1 doi: 10.1007/s11832-007-0006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karadag A, Riminucci M, Bianco P, Cherman N, Kuznetsov SA, Nguyen N, et al. A novel technique based on a PNA hybridization probe and FRET principle for quantification of mutant genotype in fibrous dysplasia/McCune–Albright syndrome. Nucleic Acids Res. 2004;32:e63. doi: 10.1093/nar/gnh059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riminucci M, Liu B, Corsi A, Shenker A, Spiegel AM, Robey PG, et al. The histopathology of fibrous dysplasia of bone in patients with activating mutations of the Gs alpha gene: site-specific patterns and recurrent histological hallmarks. J Pathol. 1999;187:249–258. doi: 10.1002/(SICI)1096-9896(199901)187:2<249::AID-PATH222>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 15.Harris WH, Dudley HR, Barry RJ. The natural history of fibrous dysplasia. J Bone Joint Surg. 1962;44-A:207–233. [PubMed] [Google Scholar]
- 16.Leet AI, Magur E, Lee JS, Wientroub S, Robey PG, Collins MT. Fibrous dysplasia in the spine: prevalence of lesions and association with scoliosis. J Bone Joint Surg Am. 2004;86-A:531–537. [PubMed] [Google Scholar]
- 17.Cutler CM, Lee JS, Butman JA, FitzGibbon EJ, Kelly MH, Brillante BA, et al. Long-term outcome of optic nerve encasement and optic nerve decompression in patients with fibrous dysplasia: risk factors for blindness and safety of observation. Neurosurgery. 2006;59:1011–1017. doi: 10.1227/01.NEU.0000254440.02736.E3. discussion 1017–1018. [DOI] [PubMed] [Google Scholar]
- 18.Lee JS, FitzGibbon E, Butman JA, Dufresne CR, Kushner H, Wientroub S, et al. Normal vision despite narrowing of the optic canal in fibrous dysplasia. N Engl J Med. 2002;347:1670–1676. doi: 10.1056/NEJMoa020742. [DOI] [PubMed] [Google Scholar]
- 19.Chen YR, Chang CN, Tan YC. Craniofacial fibrous dysplasia: an update. Chang Gung Med J. 2006;29:543–549. [PubMed] [Google Scholar]
- 20.Kelly MH, Brillante B, Collins MT. Pain in fibrous dysplasia of bone: age-related changes and the anatomical distribution of skeletal lesions. Osteoporos Int. 2008;19:57–63. doi: 10.1007/s00198-007-0425-x. [DOI] [PubMed] [Google Scholar]
- 21.Akintoye SO, Chebli C, Booher S, Feuillan P, Kushner H, Leroith D, et al. Characterization of gsp-mediated growth hormone excess in the context of McCune–Albright syndrome. J Clin Endocrinol Metab. 2002;87:5104–5112. doi: 10.1210/jc.2001-012022. [DOI] [PubMed] [Google Scholar]
- 22.Collins MT, Chebli C, Jones J, Kushner H, Consugar M, Rinaldo P, et al. Renal phosphate wasting in fibrous dysplasia of bone is part of a generalized renal tubular dysfunction similar to that seen in tumor-induced osteomalacia. J Bone Miner Res. 2001;16:806–813. doi: 10.1359/jbmr.2001.16.5.806. [DOI] [PubMed] [Google Scholar]
- 23.Corsi A, Collins MT, Riminucci M, Howell PG, Boyde A, Robey PG, et al. Osteomalacic and hyperparathyroid changes in fibrous dysplasia of bone: core biopsy studies and clinical correlations. J Bone Miner Res. 2003;18:1235–1246. doi: 10.1359/jbmr.2003.18.7.1235. [DOI] [PubMed] [Google Scholar]
- 24.Riminucci M, Collins MT, Fedarko NS, Cherman N, Corsi A, White KE, et al. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest. 2003;112:683–692. doi: 10.1172/JCI18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly MH, Brillante B, Kushner H, Gehron Robey P, Collins MT. Physical function is impaired but quality of life preserved in patients with fibrous dysplasia of bone. Bone. 2005;37:388–394. doi: 10.1016/j.bone.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 26.Plotkin H, Rauch F, Zeitlin L, Munns C, Travers R, Glorieux FH. Effect of pamidronate treatment in children with polyostotic fibrous dysplasia of bone. J Clin Endocrinol Metab. 2003;88:4569–4575. doi: 10.1210/jc.2003-030050. [DOI] [PubMed] [Google Scholar]
- 27.Chapurlat RD, Meunier PJ. Fibrous dysplasia of bone. Baillieres Best Pract Res Clin Rheumatol. 2000;14:385–398. doi: 10.1053/berh.1999.0071. [DOI] [PubMed] [Google Scholar]
- 28.Chapurlat RD, Hugueny P, Delmas PD, Meunier PJ. Treatment of fibrous dysplasia of bone with intravenous pamidronate: long-term effectiveness and evaluation of predictors of response to treatment. Bone. 2004;35:235–242. doi: 10.1016/j.bone.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Lala R, Matarazzo P, Andreo M, Marzari D, Bellone J, Corrias A, et al. Bisphosphonate treatment of bone fibrous dysplasia in McCune–Albright syndrome. J Pediatr Endocrinol Metab. 2006;19(Suppl 2):583–593. doi: 10.1515/jpem.2006.19.s2.583. [DOI] [PubMed] [Google Scholar]
- 30.DiMeglio LA. Bisphosphonate therapy for fibrous dysplasia. Pediatr Endocrinol Rev. 2007;4(Suppl 4):440–445. [PubMed] [Google Scholar]
- 31.Stanton RP. Surgery for fibrous dysplasia. J Bone Miner Res. 2006;21(Suppl 2):P105–P109. doi: 10.1359/jbmr.06s220. [DOI] [PubMed] [Google Scholar]
- 32.Saglik Y, Atalar H, Yildiz Y, Basarir K, Erekul S. Management of fibrous dysplasia. A report on 36 cases. Acta Orthop Belg. 2007;73:96–101. [PubMed] [Google Scholar]
- 33.Stanton RP, Diamond L. Surgical management of fibrous dysplasia in McCune– Albright syndrome. Pediatr Endocrinol Rev. 2007;4(Suppl 4):446–452. [PubMed] [Google Scholar]
- 34.Ippolito E, Bray EW, Corsi A, De Maio F, Exner UG, Robey PG, et al. Natural history and treatment of fibrous dysplasia of bone: a multicenter clinicopathologic study promoted by the European Pediatric Orthopaedic Society. J Pediatr Orthop B. 2003;12:155–177. doi: 10.1097/01.bpb.0000064021.41829.94. [DOI] [PubMed] [Google Scholar]
- 35.American Alliance for Health, P.E. Recreation and Dance. AAHPERD Health Related Physical Fitness Test Manual. Reston, VA: AAHPERD; 1980. [Google Scholar]
- 36.Boone DC, Azen SP. Normal range of motion of joints in male subjects. J Bone Joint Surg Am. 1979;61:756–759. [PubMed] [Google Scholar]
- 37.Wright W. Muscle training in the treatment of infantile paralysis. Boston Med Surg J. 1912;167:567. [Google Scholar]
- 38.Collins MT, Kushner H, Reynolds JC, Chebli C, Kelly MH, Gupta A, et al. An instrument to measure skeletal burden and predict functional outcome in fibrous dysplasia of bone. J Bone Miner Res. 2005;20:219–226. doi: 10.1359/JBMR.041111. [DOI] [PubMed] [Google Scholar]
- 39.Thompson N, Stebbins J, Seniorou M, Newham D. Muscle strength and walking ability in diplegic cerebral palsy: implications for assessment and management. Gait Posture. 2011;33:321–325. doi: 10.1016/j.gaitpost.2010.10.091. [DOI] [PubMed] [Google Scholar]
- 40.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158:1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 41.Watt JR, Jackson K, Franz JR, Dicharry J, Evans J, Kerrigan DC. Effect of a supervised hip flexor stretching program on gait in frail elderly patients. PM&R. 2011;2011(4):330–335. doi: 10.1016/j.pmrj.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 42.Geiger R, Strasak A, Treml B, Gasser K, Kleinsasser A, Fischer V, et al. Six-minute walk test in children and adolescents. J Pediatr. 2007;150:395–399. doi: 10.1016/j.jpeds.2006.12.052. [399 e391–392]. [DOI] [PubMed] [Google Scholar]
- 43.Lai K, Lin C, Jou I, Su F. Gait analysis after total hip arthroplasty with leg-length equalization in women with unilateral congenital complete dislocation of the hip—comparison with untreated patients. J Orthop Res. 2001;19:1147–1152. doi: 10.1016/S0736-0266(01)00032-8. [DOI] [PubMed] [Google Scholar]
- 44.Kaufman KR, Miller LS, Sutherland DH. Gait asymmetry in patients with limb-length inequality. J Pediatr Orthop. 1996;16:144–150. doi: 10.1097/00004694-199603000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Mangione KK, Craik RL, Palombaro KM, Tomlinson SS, Hofmann MT. Home-based leg-strengthening exercise improves function 1 year after hip fracture: a randomized controlled study. J Am Geriatr Soc. 2010;58:1911–1917. doi: 10.1111/j.1532-5415.2010.03076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]