Abstract
Objective
To determine changes in cytokine levels associated with caffeine treatment in a cohort of preterm infants.
Study design
For this observational prospective study, we collected clinical data from 26 preterm infants (≤30 weeks gestational age). In addition to caffeine levels, cytokine profiles in peripheral blood (PB) and tracheal aspirates (TA) were determined with enzyme-linked immunosorbent assay at birth, before and after (at 24 hours and 1 week) initiation of caffeine. Non-parametric statistics were applied.
Results
Included infants were 26.9 1.7 weeks gestational age and weighed 985 ± 202 g. At birth, all cytokine concentrations were significantly greater in TA than PB. Serum caffeine levels were 11.1 μg/mL (interquartile range, 1.85) at approximately 24 hours post-load and 16.4 (8.7) μg/mL at 1 week on treatment. At approximately 24 hours post-load, interleukin (IL)-10 levels decreased by 47.5% (P = .01) in PB and 38.5% (P = .03) in TA, whereas other cytokine levels remained unchanged. At 1 week, caffeine levels were correlated (U-shaped) with changes in proinflammatory tumor necrosis factor-α (R2 = 0.65; P = .0008), interleukin (IL)-1β (R2 = 0.73; P = .0007), and IL-6 (R2 = 0.59; P = .003), whereas inversely correlated (linear) with the anti-inflammatory IL-10 (R2 = 0.64; P = .0008). Altogether, caffeine, at serum levels ≥20 μg/mL, was associated with a proinflammatory profile after 1 week of treatment.
Conclusions
Caffeine treatment for apnea of prematurity correlates with changes in cytokine profile. Caffeine levels ≥20 μg/mL are associated with a proinflammatory profile in our cohort of preterm infants.
Caffeine citrate, a methylxanthine used to treat apnea of prematurity, reduces the risk of bronchopulmonary dysplasia (BPD) and cerebral palsy in extremely premature infants.1,2 Although caffeine, via adenosine receptors (ARs) blockade, stimulates ventilation,3-5 the effect on other processes such as inflammation is still unclear.6-10
Recently, we have reported that in vitro exposure to caffeine at 50 μM (approximately 10 μg/mL) decreases tumor necrosis factor (TNF)-α production with neonatal mononuclear cells from full-term infants via preferential A1R blockade, probably explaining some of the clinical benefits previously reported.1,2,6 However, because caffeine is a non-specific AR antagonist, blockade of other ARs subtypes can produce the opposite effect, potentially increasing inflammation.9 The in vivo translation of these findings in preterm infants is still unknown. Although caffeine citrate has a wide therapeutic index for the treatment of apnea of prematurity and no routine serum levels are recommended,11-13 factors such as the empiric dose adjustment on the basis of clinical response and the variations in renal clearance caused by acute clinical events could lead to serum caffeine levels outside of the recommended therapeutic levels, potentially changing the pattern of binding to ARs.14-17
The understanding of the potential immunomodulatory effects of caffeine exposure in preterm infants is crucial, because this population is at greater risk for morbidities induced by chronic inflammation.3,18 We hypothesized that the non-specific AR blockade produced by caffeine could modulate cytokine production in either direction. To test this hypothesis, we performed in vivo studies in a cohort of preterm infants, with the primary goal of determining the changes in proinflammatory and anti-inflammatory cytokines at 24 hours and 1 week after initiation of caffeine treatment for apnea of prematurity.
Methods
This study complied with the Guidelines for Human Experimentation from the US Department of Health and Human Services and was approved by The Johns Hopkins Medicine institutional review board (NA_00002034). Parents provided informed consent before inclusion in the study.
Included preterm infants were ≤30 weeks gestational age (GA), born in or transferred to the Johns Hopkins Hospital neonatal intensive care unit between February 2007 and January 2009, and required endotracheal intubation and surfactant treatment during the first 24 hours of life. Any infant ≥48 hours old at time of transfer was excluded as were infants who were assessed as being too ill or unlikely to survive by the clinical care team. Infants with major genetic disorders or malformations or intrauterine growth restriction, infants small for GA, and infants with suspected viral infection were also excluded.
GA was calculated with first-trimester ultrasound scanning or assessment using the New Ballard Score.19 Race assignment was determined with maternal race. Prenatal steroid treatment was defined as receiving at least one dose of any steroid 6 hours before delivery. Premature prolonged rupture of membranes was defined as rupture for ≥18 hours at <37 weeks GA. Clinical chorioamnionitis was defined as maternal fever (≥38° C) with uterine tenderness, maternal or fetal tachycardia, or malodorous amniotic fluid;20 whereas pathological chorioamnionitis required inflammatory infiltrate regardless of clinical findings.21 Fetal distress was defined as having decreased fetal movement, late fetal decelerations, or biophysical profile <8/10,22 fetal acidosis (scalp pH <7.20) or was defined by the attending obstetrician. The Score for Neonatal Acute Physiology (SNAP) was used to assess severity of illness at 24 hours of life.23,24 Duration of invasive mechanical ventilation (MV) was determined by counting the number of days that the infant had an endotracheal tube (ETT) without interruptions >30 days. Duration of inotropes was determined from first day of therapy to last day of continuous infusion with no reinstitution of therapy for a period ≥5 days. Infection was confirmed with positive results of any blood, cerebral spinal fluid, or urine culture and the initiation or extension of antibiotic coverage for ≥72 hours. BPD was defined as need for supplemental oxygen at 36 weeks corrected postmenstrual age or at discharge from the neonatal intensive care unit, whichever came first.25 Physiological room air challenge was not included for the diagnosis of BPD.25 Medical treatment for patent ductus arteriosus included indomethacin (Indocin, Merck & Co, Inc, Whitehouse Station, New Jersey), ibuprofen (NeoProfen, Ovation Pharmaceuticals, Deerfield, Illinois), or both in repeated doses.
Peripheral blood (PB) and tracheal aspirates (TA) from enrolled preterm infants were collected within 8 hours of birth and 4 hours before caffeine loading dose (20 mg/kg). TA was also collected at approximately 24 hours (6-24 hours) after caffeine loading dose during routine endotracheal suction using a closed system (Kimberly-Clark, Roswell, Georgia) attached to a sterile mucus trap at the time of extubation. PB specimen at approximately 24 hours (14-24 hours) after caffeine load was collected no later than 8 hours after the TA. Additional PB was collected at 1week (≤ 8 hours before the seventh maintenance dose of 5 μg/kg/day). After centrifugation of PB (500 mL, EDTA microtainer) and TA specimens at 2000 × g for 10 minutes, the TA pellet was used for cellular differential, whereas the TA supernatant and PB plasma were used for cytokine measurements. Serum caffeine levels (additive-free microtainer, Quest Diagnostics, Baltimore, Maryland) and complete blood count (CBC) and differential (EDTA microtainer, JHMI Laboratories, Baltimore, Maryland) were also determined.
Proinflammatory cytokine (interleukin [IL]-1β, IL-2, IL-5, IL-6, IL-7, IL-8, IL-12p70, interferon [INF]-γ, granulocyte macrophage-colony stimulating factor [GM-CSF], tumor necrosis factor [TNF]-α), and anti-inflammatory cytokine (IL-4, IL-10, IL-13) concentrations were measured with enzyme-linked immunosorbent assay, using LINCOplex Multiplex kits (Millipore, Billerica, Massachusetts), according to the manufacturer's protocol, and concentrations were calculated with the Luminex detection system (Milli-pore). The lowest dilution on the standard curve (0.64 pg/mL) was considered to be the lower limit of detection (LLD). Values below the LLD were calculated with the maximum-likelihood estimation only when they represented <50% of all measurements. In contrast, when >50% of the measurements were below LLD, those cytokines were considered non-detectable.26
TA cytokine concentrations were corrected with the calculated dilution factor originated from the blood urea nitrogen (BUN) ratio between TA and PB. Urea concentrations in TA were determined by using the QuantiChrom Urea Assay kit (BioAssay Systems, Hayward, California). Microplate reader (Biorad) detected the chromogenic reagent, urea complex, at 430 nm and the standard curve provided a linear detection range between 0.08 and 100 mg/dL. BUN concentrations were calculated by dividing the measured urea concentration (mg/dL) by 2.14.
Statistical Analysis
A sample size of 26 infants was calculated by using TNF-α plasma concentrations, measured at “birth” from the first 10 enrolled subjects (mean ± SD, 27.1 ± 8 pg/mL), to detect a 20% difference in groups6 with a power of 0.80 and a two-sided α error of 0.05. Four of those initial 10 infants finished the protocol and became part of the final cohort for analysis. Cytokine concentrations at birth were used as a baseline descriptor, whereas 4-hour pre-caffeine levels were used as points of comparison to calculate changes after caffeine treatment initiation.
Categorical variables were compared with χ2 test. Continuous variables, which were non-normally distributed, were reported as medians with interquartile ranges (IQR, 25th-75th percentile), represented as box-and-whisker plots (boxes symbolize IQR) and analyzed with the Wilcoxon signed rank and Mann-Whitney U tests. Descriptive variables, which were normally distributed, were reported as means ± SD. Univariate regression models were applied to determine whether serum caffeine levels correlated with changes in cytokine concentrations observed at approximately 24 hours after caffeine load and at 1 week of caffeine maintenance (versus pre-caffeine load). Significant models were represented as scatter plots of change in cytokine concentrations (y) and serum caffeine levels (x) with the best-fitted curve. Significance was assigned with a P value <.05. SPSS software version 14.0 (SPSS Inc, Chicago, Illinois) was used for analysis and graphics.
Results
The 26 preterm infants included for final analysis were (mean ± SD) 26-6/7 ± 1.7 wk GA, weighed 985 ± 202 g, and 42.4% were male (Table I). At birth, IL-1β, IL-2, IL-7, INF-γ, and GM-CSF were not detected in plasma, whereas all 13 measured cytokines were detected in TA with levels that were significantly higher than cytokine levels measured in PB (P < .001; Table II).
Table I.
Characteristics of included preterm infants
| Demographic and perinatal variables | |
| Gestational age, weeks (mean ± SD) | 26 6/7 ± 1.7 |
| Birth weight, g (mean ± SD) | 985 ± 202 |
| Sex, % male | 42.4% |
| Race, % African-American | 65.4% |
| 1-minute Apgar score, median (IQR) | 5 (3-7) |
| 5 minutes Apgar score, median (IQR) | 7 (6-8) |
| Delivery method, % cesarean delivery | 61.5% |
| Antenatal steroids | 84.9% |
| PPROM | 42.3% |
| Clinical choriamnionitis | 23.1% |
| Pathological chorioamnionitis | 53.8% |
| Fetal distress at delivery | 56.3% |
| Clinical variables | |
| SNAP score, mean ± SD | 16 ± 5 |
| Surfactant doses per infant, median (IQR) | 2 (2-3) |
| Days on mechanical ventilation, mean ± SD | 19.5 ± 17 |
| Days on inotropes, mean ± SD | 2 ± 2 |
| Confirmed infection | 11.5% |
| BPD | 42.3% |
| PDA medically treated | 38.5% |
| PDA surgically treated | 7.7% |
| Hematological parameters | |
| Hematocrit, mean ± SD | 40.5 ± 6.3% |
| White blood cell count (per mm3), mean ± SD | 12401 ± 8377 |
| Neutrophils, mean ± SD | 42.8 ± 17.7% |
| Lymphocytes, mean ± SD | 39.6 ± 18.3% |
| Monocytes, mean ± SD | 11.6 ± 6.1% |
| Platelet count (× 1000 per mm3), mean ± SD | 172 ± 55 |
| Caffeine level at birth (μg/mL), mean ± SD | 0.27 ± 0.44 |
PPROM, Premature prolonged rupture of membranes.
Table II.
Cytokine concentrations at birth
| Peripheral blood (plasma) |
Tracheal aspirates* (supernatant) |
|||
|---|---|---|---|---|
| Cytokines levels | Median | IQR | Median | IQR |
| Pro-inflammatory | ||||
| IL-1β | ND | – | 54 | 18.3-111.6 |
| IL-2 | ND | – | 98 | 82.7-256.4 |
| IL-5 | 1.015 | (0.60-1.05) | 16.1 | 7.3-65.1 |
| IL-6 | 48.1 | (12.26-147.34) | 6434.6 | 2600.8-16228.7 |
| IL-7 | ND | – | 346.9 | 141.5-519.2 |
| IL-8 | 51.24 | (23.69-109.89) | 14655 | 4490.6-33690.9 |
| IL-12p70 | 0.54 | (0.01-3.00) | 153 | 70.4-347.4 |
| INF-γ | ND | – | 123.1 | 79.2-415.4 |
| GM-CSF | ND | – | 67.3 | 41.2-115 |
| TNF-α | 22.52 | (9.52-31.53) | 78.5 | 35.8-190.4 |
| Anti-inflammatory | ||||
| IL-4 | ND | – | 324.2 | 55.9-1275.9 |
| IL-10 | 146.53 | (68.3 – 341.51) | 590.3 | 310.1-1362.3 |
| IL-13 | ND | – | 69.4 | 30.7-273.6 |
ND, Non-detected.
Adjusted for dilution with BUN method.
Plasma IL-10 levels decreased during the first few days of life from 146.53 pg/mL (IQR, 68.30-341.51) at birth to 44.76 pg/mL (41.20-75.91) 4 hours before caffeine treatment (P = .013); however, no significant linear correlation between day of life and changes in plasma IL-10 levels (P = .11, R2 = 0.06) was observed. IL-10 levels in supernatants from tracheal aspirates did not follow the change in plasma (Table III; available at www.jpeds.com).
Table III.
Cytokine and caffeine concentrations in peripheral blood and tracheal aspirates
| Cytokine levels |
4 hours before caffeine (load) |
24 hours after caffeine (load) |
1 week on caffeine* |
||
|---|---|---|---|---|---|
| Median pg/mL (IQR) n=26 | PB | TA† | PB | TA† | PB |
| Pro-inflammatory | |||||
| IL-1β | 0.01 (0.01-0.21) | 81.45 (28.15-146.40) | 0.01(0.01-0.12) | 124.96 (11.88-329.01) | 0.60 (0.11-1.2) |
| IL-6 | 8.34 (4.27-32.15) | 3940.39 (1706.4-8092.41) | 8.89 (3.54-33.49) | 3945.45 (2236.11-6414.15) | 21.6 (12.23-42.12) |
| IL-8 | 33.41 (12.89-60.61) | 17828.97 (6103.4-34398.42) | 37.26 (16.87-73.31) | 22368.59 (7257.66-33360.14) | 31.58 (19.47-54.39) |
| IL-12p70 | 0.94 (0.1-2.32) | 161.20 (53.76-372.45) | 1.17 (0.65-3.27) | 78.41 (38.52-200.34) | 3.83 (1.74-11.07) |
| TNF-α | 18.15 (9.88-26.42) | 63.37 (38.16-200.04) | 20.64 (12.55-30.29) | 85.64 (22.74-188.82) | 27.31 (16.36-32.47) |
| Anti-inflammatory | |||||
| IL-10 | 44.76 (41.20-75.91) | 483.17 (156.53-1082.59) | 40.33 (34.11-60.05) | 156.36 (92.60-547.97) | 66.21 (52.0-116.57) |
| Caffeine level (μg/mL) | 0.1 (0-0.28) | – | 11.1 (10.35-12.2) | – | 16.4 (10.5-19.2) |
Tracheal aspirate not available at this study point.
Adjusted for dilution with BUN method.
Caffeine treatment was started at a median of 3 days of life (IQR, 1-6). Median (IQR) serum caffeine level at approximately 24 hours after load was 11.1 μg/mL (10.35-12.20 μg/mL). Most measured cytokines in plasma and TA did not change by 24 hours after load (versus 4 hours precaffeine; Table III) and did not correlate with caffeine levels (data not shown). Only IL-10 concentrations decreased by 47.5% (–55.8% to –13.9%; P = .01) in plasma and 38.5% (–58.5% to –1.7%; P = .03) in TA (versus 4-hour pre-caffeine load concentrations); however, the change in IL-10 did not show correlation with caffeine levels (data not shown).
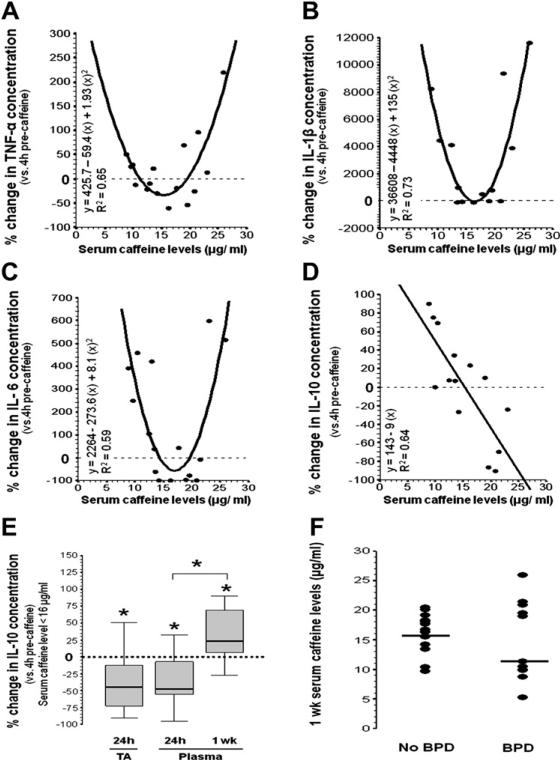
The median serum caffeine level at 1 week of treatment was 16.4 μg/mL (IQR, 10.5-19.2 μg/mL). Median cytokine concentrations are shown in Table III. Changes in plasma levels for the pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 plotted against their respective caffeine levels demonstrated a U-shaped correlation with a R2 of 0.65 (P = .0008), 0.73 (P = .0007), and 0.59 (P = .0029), respectively (Figure, A, B, and C). TNF-α and IL-6 plasma concentrations decreased when caffeine levels were between 11.4 and 19.4 μg/mL and 14.5 and 19.3 μg/mL, respectively. Although IL-1β plasma concentration did not decrease, it was unchanged when serum caffeine levels were approximately 16 μg/mL. The change in the anti-inflammatory cytokine IL-10 in plasma inversely and linearly correlated with caffeine levels (R2 = 0.64, P = .0008) with an inflexion point at a serum caffeine level of 15.9 μg/mL (Figure, D).
Figure.
Change in plasma cytokine concentrations and serum caffeine levels after 1 week of treatment. Scatter plots showing percentage change in A, TNF-α, B, IL-1β, C, IL-6, and D, IL-10 concentrations (y-axis) versus caffeine levels (x-axis). Significant regression models are shown with estimated fitted curves and coefficient of determination (R2). Reference line sitting at 0 represents the cytokine concentration at 4-hour pre-caffeine load. E, Comparison between 24-hour and 1-week IL-10 levels in TA and plasma are shown for the subset maintaining caffeine levels <16 μg/mL. F, Scatter plot of caffeine levels at 1 week stratified by BPD diagnosis, with solid line representing median.
Although the highest caffeine level at approximately 24 hours after initiation of caffeine was 16μg/mL, the highest level after 7 days of caffeine maintenance was 26 μg/mL. The analysis of the subset of infants that maintained serum caffeine levels <16μg/mL showed that at approximately 24 hours, IL-10 levels, in both TA and plasma, decreased, whereas after 7 days on maintenance, IL-10 levels in plasma increased (Figure, E).
Analysis of Clinical Variables
In our cohort, those infants with caffeine levels ≥16 μg/mL after 1 week of therapy were older at birth (26.9 weeks [range, 26.4-29.5 weeks] versus 25.5 weeks [range, 25-28.5], P = .03), had better Apgar scores at 1 and 5 minutes (P = .03 and .02, respectively), were more frequently delivered via cesarean delivery (79%, P = .03), and were more likely to be Caucasian (57%, P = .01). The rest of the clinical variables (Table I) were not different.
Renal or liver function variables (creatinine, BUN, albumin, transaminases, direct bilirubin or alkaline phosphatase levels) were not correlated with serum caffeine levels (or cytokine concentrations) after 1 week of treatment (data not shown). We also found no correlation between the day of life of the subjects and the changes in cytokine concentrations at approximately 24 hours and at 1 week after the initiation of caffeine treatment.Antibiotics use forsuspected sepsis(P = .49), mechanical ventilation for respiratory failure (P = .37), or ibuprofen/indomethacin for patent ductus arteriosus treatment (P = .29) did not significantly change caffeine levels. Excluding those infants who had confirmed infection at the time of collection of the specimens (n = 3) from the analysis did not modify the significant correlation between the changes in cytokines levels and corresponding caffeine levels.
Cellular differential of tracheal aspirates were similar at birth, before and approximately 24 hours after caffeine load, with polymorphonuclear cells representing (mean ± SD) 70% ± 26%, 73% ± 28%, and 69% ± 26% of total cell count at each of the 3 evaluation points.
Subgroup Analysis by Bronchopulmonary Dysplasia Diagnosis
In our cohort, those preterm infants in whom BPD developed (n = 11) showed a longer exposure to mechanical ventilation (median, 26 versus 2 days; P = .04) and a higher incidence of confirmed infection (27% versus 0%, P = .02). Although median caffeine levels at 1 week were not statistically different in infants in whom BPD developed than in infants without BPD (11.4 versus 16.5 μg/mL, respectively; P = .43; Table IV), caffeine levels showed a different distribution, with most of the extreme values observed in infants in whom BPD developed (Figure, F). The analysis of plasma cytokine concentrations during caffeine treatment (1 week) demonstrated that infants in whom BPD developed also showed higher plasma IL-1β (P = .008) and IL-6 (P = .01) concentrations as well as their ratios with IL-10 (P < 0.01; Table IV).
Table IV.
Subgroup analysis by bronchopulmonary dysplasia
| Demographic and perinatal factors n = 26 | No BPD n = 15 | BPD n = 11 | P value |
|---|---|---|---|
| Gestational age, weeks (median [IQR]) | 27 0/7 (3) | 26 3/7 (1.6) | .26* |
| Birth weight, g (median [IQR]) | 870 (450) | 880 (240) | .44* |
| Male, % | 46.6 | 36.4 | .60† |
| African-American, % | 66.7 | 63.6 | .87† |
| 1-minute Apgar score, median (IQR) | 5 (5) | 4 (3) | .33* |
| 5-minute Apgar score, median (IQR) | 7 (2) | 7 (2) | .61* |
| Cesarean delivery, % | 53.3 | 72.7 | .31† |
| No antenatal steroids, % | 6.7 | 27.3 | .15† |
| PPROM, % | 40 | 45.5 | .78† |
| Clinical choriamnionitis, % | 26.6 | 18.2 | .61† |
| Pathological chorioamnionitis, % | 66.7 | 36.4 | .12† |
| History of fetal distress, % | 66.6 | 50.0 | .51† |
| Clinical factors | |||
| SNAP score, median (IQR) | 17 (7) | 15 (10) | .26* |
| Surfactant doses, median (IQR) | 2 (2) | 2 (1) | .61* |
| Days on MV, median (IQR) | 4 (26) | 26 (25) | .04*‡ |
| Days on inotropes, median (IQR) | 1 (2) | 2 (2) | .10* |
| Confirmed infection, % | 0 | 27.3 | .02†‡ |
| PDA medically treated, % | 26.7 | 54.5 | .14† |
| PDA surgically treated, % | 6.7 | 9.1 | .81† |
| Caffeine level at 1-week, μg/mL (median [IQR]) | 16.5 | 11.4 | .43* |
| 1-week plasma cytokine concentrations, pg/mL (median [IQR]) | |||
| TNF-α | 29.6 (22.3) | 23.8 (24.3) | .285* |
| IL-1β | 0.005 (0.59) | 1.07 (3.85) | .008*† |
| IL-6 | 10.8 (17.9) | 30.7 (31.5) | .011*† |
| IL-10 | 64.3 (80.0) | 67.8 (58.6) | .936* |
| TNF-α : IL-10 | 0.48 (0.51) | 0.32 (0.23) | .472* |
| IL-1β : IL-10 | 0.0001 (0.01) | 0.028 (0.05) | .009*† |
| IL-6 : IL-10 | 0.14 (0.18) | 0.39 (0.44) | .005*† |
MV, mechanical ventilation.
Mann-Whitney U rank test.
χ2 test.
P < .05.
Discussion
Caffeine citrate, is a non-specific AR antagonist used for the treatment of apnea of prematurity.1,2,5,27-29 Because ARs are also broadly expressed on the surface of immune cells, their blockade by caffeine has been found to modulate their function in vitro.6-9 Preterm infants are frequently exposed to caffeine and have co-morbidities that are mediated by inflammation; however, we are unaware of any study that has evaluated the potential change in cytokine profile during caffeine treatment in preterm infants.13,27,28,30-32 In this study, we prospectively determined in a cohort of preterm infants the changes in plasma and TA cytokine profiles after the initiation of caffeine treatment. We report that at 1 week after initiation of treatment, serum caffeine levels were correlated with changes in pro-inflammatory and anti-inflammatory cytokines in PB, which could tip the balance to a proinflammatory profile at caffeine levels outside the therapeutic range (10-20 ug/mL).
Previous in vitro studies have demonstrated that caffeine concentrations of 50 to 100 μM inhibit the production of the proinflammatory cytokines IL-1β,33 IL-2,34,35 IL-5,36 and TNF-α,6,7,34 and early use of caffeine citrate in vivo has been found to decrease the risk of BPD and cerebral palsy in extremely low birth weight infants.1,2 Although the shorter exposure to mechanical ventilation in the infants who received caffeine could be the sole reason for these reported clinical outcomes, the immunomodulatory effects associated with the blockade of ARs, particularly A1R, may also be operative.1,2,6 Our study describes the correlation between therapeutic caffeine levels (range, 10-20 μg/mL) and the decrease in IL-6 and TNF-α (pro-inflammatory cytokines) levels and the increase in IL-10 (anti-inflammatory cytokine) levels, which could be the biological mechanism for the observed clinical findings previously reported in preterm infants treated with caffeine for apnea of prematurity.1,2 However, we also report that caffeine levels outside the therapeutic range (>20 μg/mL) are potentially associated with a proinflammatory cytokine profile of unknown clinical significance.
At standard doses for apnea of prematurity, the therapeutic range (10-20 μg/mL) for caffeine can be exceeded in 30% of the infants because of changes in fluid balance and illnesses that alter the metabolism, distribution, and excretion of the drug.14,16,37,38 Furthermore, because of the wide therapeutic and safety index, caffeine dosing is frequently symptom-based and levels are rarely monitored.32,34 Although, in our cohort, caffeine levels were not modified by sepsis, PDA treatment, or renal/ liver function, we still observed a wide range of serum caffeine levels (7-16 μg/mL at approximately 24 hours and 5.5-26.0 μg/mL at 1 week of caffeine treatment), although similar loading (20 mg/kg) and maintenance (5 mg/kg/day) doses were administered to all subjects. Some of this variability could be related to the collection times, because daily variability is reported to be between 6.1% to 35.0%.30,39 However, because the peak caffeine level is at 30 to 120 minutes, the half-life is approximately 100 hours and the dosing is every 24 hours, the differences between levels within the 12 hours before the next dose are expected to be minimal by 1 week of treatment.15,30 In our cohort, two factors appeared to be positively associated with higher caffeine levels (≥16 μg/mL after 1 week of treatment): older GA and Caucasian race. Older GA could be associated with a relative lower volume of distribution (lower total body water) leading to higher serum levels, and Caucasian infants have higher cytochrome P450 (CYP1A2) polymorphisms, changing the metabolism of the drug.40
Some cytokines, such as IL-10, decrease after birth,41 making cumbersome the interpretation of its changes.41 The decrease in IL-10 levels at approximately 24 hours post-caffeine load observed in our cohort of preterm infants could be argued without further multivariate analysis even more because those changes were not correlated with caffeine levels. However, the changes in plasma IL-10 concentrations correlate with their respective serum caffeine levels after 7 days on maintenance treatment, with an inflexion point of 16μg/mL, as shown in the Figure. These data suggest that decrease in IL-10 concentrations, one of the most important counter-balance cytokines, is associated with higher caffeine levels.
The biological mechanism accounting for the potential changes in the cytokine profile associated with caffeine exposure in premature infants is unclear. However, the order of affinity of ARs for caffeine potentially is the physiological principle behind the parabolic distribution of the proinflammatory cytokines. AR subtype A1 is coupled to protein Gi, and its activation inhibits adenylyl cyclase, decreasing cyclic adenosine monophosphate (cAMP) production. The opposite effect is produced by activation of A2a and A2b (coupled to protein Gs). Once caffeine reaches therapeutic levels, preferential blockade of A1R increases cAMP accumulation, thereby decreasing cytokine production.6 However, because caffeine is a non-specific AR antagonist, higher concentrations also blocks A2Rs, thereby decreasing cAMP and increasing proinflammatory cytokine transcription, reversing the anti-inflammatory effect observed at lower concentrations.9 Additionally, the concomitant decrease in IL-10 production, which down-regulates the release of proinflammatory cytokines, could also be contributing.42 Other mechanisms of action, such as inhibition of phosphodiesterases, activation of calcium channels, and the blockade of certain sites at GABAA receptors,43-46 require concentrations of 10-fold higher than those observed in serum. Thus, the increase in ventilation and any other effect seen in infants receiving caffeine is probably primarily mediated by AR blockade. Although these mechanisms are beyond the scope of this paper, we are currently exploring potential molecular pathways mediating the regulation of cytokine production by caffeine.
Because the balance between anti-inflammatory and proinflammatory cytokines determines the magnitude of local tissue damage contributing to the development of comorbidities, such as BPD, our observation that levels of caffeine outside the therapeutic range are potentially associated with a proinflammatory profile may have significant clinical implications.47,48 Although studies have not definitively linked the increase of TNF-α concentrations with the development of BPD,47,49 the increase of other cytokines such as IL-1β and IL-6 have been more closely linked.41,48,50-52 Impaired early IL-10 production by mononuclear cells has also been associated with BPD;48,53,54 however, recently published data found a higher “early” IL-10 level in infants in who BPD develops or who die.41 Because IL-10 is key in controlling inflammation, low production could represent an impaired counter-balance system, while high production could be a marker of prenatal infection/inflammation, which in either case will lead to lung tissue damage. In our cohort of preterm infants, BPD was associated with higher concentrations of IL-1β and IL-6 and a greater imbalance between these cytokines and IL-10. Also, serum caffeine levels at 1 week demonstrate a different distribution in infants in whom BPD develops, with a suggestive tendency for extreme values.
Although our analysis might suggest an association among caffeine levels, change in cytokine profile, and co-morbidities such as BPD, we recognize that we cannot establish causality because of the limitation imposed by the small sample size of our study. Because of this limitation, we are unable to perform further subgroup analysis of other variables in a multivariate regression model. Larger studies are needed to clarify these suggestive data, and until then, a more careful assessment of adjustment of caffeine dose should be considered.
We report the correlation between serum caffeine levels and prospective changes in cytokine profile in a cohort of preterm infants treated with caffeine citrate for apnea of prematurity. Our results suggest that serum caffeine levels out of the therapeutic range of 10 to 20μg/mL are correlated with a proinflammatory cytokine profile characterized by increased levels of IL-1β, IL-6, and TNF-α. Additionally, high caffeine levels are also correlated with decreased IL-10 concentrations. Further subgroup analysis of our cohort shows a significant link between the imbalance of proinflammatory and anti-inflammatory cytokines and BPD, with more extreme caffeine levels observed in infants with BPD. We speculate that caffeine, at therapeutic levels, is beneficial in preventing the persistent activation of inflammatory cascade and its subsequent clinical consequences, as previously reported;1,2 however, our data also suggest that those benefits could be potentially diminished when caffeine levels are out of the therapeutic range.
Acknowledgments
The authors acknowledge the Johns Hopkins Hospital neonatal intensive care unit and obstetric service staff for their assistance with patient enrollment, and Mrs Debra Flock, Mrs Sandra Sauer, Ms Ariel Mason, Mrs Kelli Price, and Ms Julia Hinojos for their administrative assistance. We are also indebted to our patients and their families for their willingness to participate in this study.
Supported by grants from Johns Hopkins University School of Medicine General Clinical Research Center (M01-RR00052), the National Center for Research Resources (NCRR)/The National Institutes of Health (NIH) (HL-072748 to E.B.G), Johns Hopkins University Institutional Research Grant, and the Thomas Wilson Sanitar ium for Children of Baltimore City.
Glossary
- AR
Adenosine receptor
- BPD
Bronchopulmonary dysplasia
- BUN
Blood urea nitrogen
- cAMP
Cyclic adenosine monophosphate
- CBC
Complete blood count
- ETT
Endotracheal tube
- GA
Gestational age
- GM-CSF
Granulocyte and macrophage-colony stimulating factor
- IL
Interleukin
- INF
Interferon
- IQR
Interquartile range
- LLD
Lower limit of detection
- MV
Mechanical ventilation
- PB
Peripheral blood
- SNAP
Score for neonatal acute physiology
- TA
Tracheal aspirates
- TNF
Tumor necrosis factor
Footnotes
The authors declare no conflicts of interest.
References
- 1.Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354:2112–21. doi: 10.1056/NEJMoa054065. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med. 2007;357:1893–902. doi: 10.1056/NEJMoa073679. [DOI] [PubMed] [Google Scholar]
- 3.Herlenius E, Aden U, Tang LQ, Lagercrantz H. Perinatal respiratory control and its modulation by adenosine and caffeine in the rat. Pediatr Res. 2002;51:4–12. doi: 10.1203/00006450-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Lopes JM, Davis GM, Mullahoo K, Aranda JV. Role of adenosine in the hypoxic ventilatory response of the newborn piglet. Pediatr Pulmonol. 1994;17:50–5. doi: 10.1002/ppul.1950170109. [DOI] [PubMed] [Google Scholar]
- 5.Millar D, Schmidt B. Controversies surrounding xanthine therapy. Semin Neonatol. 2004;9:239–44. doi: 10.1016/j.siny.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Chavez-Valdez R, Wills-Karp M, Ahlawat R, Cristofalo EA, Nathan A, Gauda EB. Caffeine modulates TNF-alpha production by cord blood monocytes: the role of adenosine receptors. Pediatr Res. 2009;65:203–8. doi: 10.1203/PDR.0b013e31818d66b1. [DOI] [PubMed] [Google Scholar]
- 7.Horrigan LA, Kelly JP, Connor TJ. Caffeine suppresses TNF-alpha production via activation of the cyclic AMP/protein kinase A pathway. Int Immunopharmacol. 2004;4:1409–17. doi: 10.1016/j.intimp.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Montesinos MC, Yap JS, Desai A, Posadas I, McCrary CT, Cronstein BN. Reversal of the antiinflammatory effects of methotrexate by the nonselective adenosine receptor antagonists theophylline and caffeine: evidence that the antiinflammatory effects of methotrexate are mediated via multiple adenosine receptors in rat adjuvant arthritis. Arthritis Rheum. 2000;43:656–63. doi: 10.1002/1529-0131(200003)43:3<656::AID-ANR23>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 9.Ohta A, Lukashev D, Jackson EK, Fredholm BB, Sitkovsky M. 1,3,7-trimethylxanthine (caffeine) may exacerbate acute inflammatory liver injury by weakening the physiological immunosuppressive mechanism. J Immunol. 2007;179:7431–8. doi: 10.4049/jimmunol.179.11.7431. [DOI] [PubMed] [Google Scholar]
- 10.Tofovic SP, Salah EM, Jackson EK, Melhem M. Early renal injury induced by caffeine consumption in obese, diabetic ZSF1 rats. Ren Fail. 2007;29:891–902. doi: 10.1080/08860220701569846. [DOI] [PubMed] [Google Scholar]
- 11.Comer AM, Perry CM, Figgitt DP. Caffeine citrate: a review of its use in apnoea of prematurity. Paediatr Drugs. 2001;3:61–79. doi: 10.2165/00128072-200103010-00005. [DOI] [PubMed] [Google Scholar]
- 12.Harrison H., Jr. Apnea of prematurity: theophylline v caffeine. Alaska Med. 1992;34:173–6. [PubMed] [Google Scholar]
- 13.Natarajan G, Botica ML, Thomas R, Aranda JV. Therapeutic drug monitoring for caffeine in preterm neonates: an unnecessary exercise? Pediatrics. 2007;119:936–40. doi: 10.1542/peds.2006-2986. [DOI] [PubMed] [Google Scholar]
- 14.Ginsberg G, Hattis D, Sonawane B. Incorporating pharmacokinetic differences between children and adults in assessing children’s risks to environmental toxicants. Toxicol Appl Pharmacol. 2004;198:164–83. doi: 10.1016/j.taap.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Le Guennec JC, Billon B, Pare C. Maturational changes of caffeine concentrations and disposition in infancy during maintenance therapy for apnea of prematurity: influence of gestational age, hepatic disease, and breast-feeding. Pediatrics. 1985;76:834–40. [PubMed] [Google Scholar]
- 16.Gillot I, Gouyon JB, Guignard JP. Renal effects of caffeine in preterm infants. Biol Neonate. 1990;58:133–6. doi: 10.1159/000243252. [DOI] [PubMed] [Google Scholar]
- 17.Aranda JV, Chemtob S, Laudignon N, Sasyniuk BI. Pharmacologic effects of theophylline in the newborn. J Allergy Clin Immunol. 1986;78:773–80. doi: 10.1016/0091-6749(86)90060-6. [DOI] [PubMed] [Google Scholar]
- 18.Lahra MM, Beeby PJ, Jeffery HE. Intrauterine inflammation, neonatal sepsis, and chronic lung disease: a 13-year hospital cohort study. Pediatrics. 2009;123:1314–9. doi: 10.1542/peds.2008-0656. [DOI] [PubMed] [Google Scholar]
- 19.Ballard JL, Khoury JC, Wedig K, Wang L, Eilers-Walsman BL, Lipp R. New Ballard score, expanded to include extremely premature infants. J Pediatr. 1991;119:417–23. doi: 10.1016/s0022-3476(05)82056-6. [DOI] [PubMed] [Google Scholar]
- 20.Hauth JC, Gilstrap LC, 3rd, Hankins GD, Connor KD. Term maternal and neonatal complications of acute chorioamnionitis. Obstet Gynecol. 1985;66:59–62. [PubMed] [Google Scholar]
- 21.Mueller-Heubach E, Rubinstein DN, Schwarz SS. Histologic chorioamnionitis and preterm delivery in different patient populations. Obstet Gynecol. 1990;75:622–6. [PubMed] [Google Scholar]
- 22.Manning FA, Morrison I, Lange IR, Harman CR, Chamberlain PF. Fetal assessment based on fetal biophysical profile scoring: experience in 12 620 referred high-risk pregnancies. I. Perinatal mortality by frequency and etiology. Am J Obstet Gynecol. 1985;151:343–50. doi: 10.1016/0002-9378(85)90301-1. [DOI] [PubMed] [Google Scholar]
- 23.Richardson DK, Gray JE, McCormick MC, Workman K, Goldmann DA. Score for Neonatal Acute Physiology: a physiologic severity index for neonatal intensive care. Pediatrics. 1993;91:617–23. [PubMed] [Google Scholar]
- 24.Richardson DK, Phibbs CS, Gray JE, McCormick MC, Workman-Daniels K, Goldmann DA. Birth weight and illness severity: independent predictors of neonatal mortality. Pediatrics. 1993;91:969–75. [PubMed] [Google Scholar]
- 25.Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics. 1988;82:527–32. [PubMed] [Google Scholar]
- 26.Wong HL, Pfeiffer RM, Fears TR, Vermeulen R, Ji S, Rabkin CS. Reproducibility and correlations of multiplex cytokine levels in asymptomatic persons. Cancer Epidemiol Biomarkers Prev. 2008;17:3450–6. doi: 10.1158/1055-9965.EPI-08-0311. [DOI] [PubMed] [Google Scholar]
- 27.Henderson-Smart DJ, Davis PG. Prophylactic methylxanthine for extubation in preterm infants. Cochrane Database Syst Rev. 2000:CD000139. doi: 10.1002/14651858.CD000139. [DOI] [PubMed] [Google Scholar]
- 28.Henderson-Smart DJ, Steer PA. Prophylactic methylxanthine for preventing of apnea in preterm infants. Cochrane Database Syst Rev. 2000:CD000432. doi: 10.1002/14651858.CD000432. [DOI] [PubMed] [Google Scholar]
- 29.Du W, Warrier I, Tutag Lehr V, Salari V, Ostrea E, Aranda JV. Changing patterns of drug utilization in a neonatal intensive care population. Am J Perinatol. 2006;23:279–85. doi: 10.1055/s-2006-946719. [DOI] [PubMed] [Google Scholar]
- 30.Charles BG, Townsend SR, Steer PA, Flenady VJ, Gray PH, Shearman A. Caffeine citrate treatment for extremely premature infants with apnea: population pharmacokinetics, absolute bioavailability, and implications for therapeutic drug monitoring. Ther Drug Monit. 2008;30:709–16. doi: 10.1097/FTD.0b013e3181898b6f. [DOI] [PubMed] [Google Scholar]
- 31.Reiter-Reisacher RB, Wald M, Weninger M, Herkner KR. Caffeine monitoring in infants: comparison of automated (VITROS 5, 1 FS) chemistry system versus HPLC analysis. Clin Lab. 2008;54:89–94. [PubMed] [Google Scholar]
- 32.Leon AE, Michienzi K, Ma CX, Hutchison AA. Serum caffeine concentrations in preterm neonates. Am J Perinatol. 2007;24:39–47. doi: 10.1055/s-2006-958163. [DOI] [PubMed] [Google Scholar]
- 33.van Furth AM, Seijmonsbergen EM, Langermans JA, van der Meide PH, van Furth R. Effect of xanthine derivates and dexamethasone on Streptococcus pneumoniae-stimulated production of tumor necrosis factor alpha, interleukin-1 beta (IL-1 beta), and IL-10 by human leukocytes. Clin Diagn Lab Immunol. 1995;2:689–92. doi: 10.1128/cdli.2.6.689-692.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ritter M, Hohenberger K, Alter P, Herzum M, Tebbe J, Maisch M. Caffeine inhibits cytokine expression in lymphocytes. Cytokine. 2005;30:177–81. doi: 10.1016/j.cyto.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 35.Rosenthal LA, Taub DD, Moors MA, Blank KJ. Methylxanthine-induced inhibition of the antigen- and superantigen-specific activation of T and B lymphocytes. Immunopharmacology. 1992;24:203–17. doi: 10.1016/0162-3109(92)90076-o. [DOI] [PubMed] [Google Scholar]
- 36.Horrigan LA, Kelly JP, Connor TJ. Immunomodulatory effects of caffeine: friend or foe? Pharmacol Ther. 2006;111:877–92. doi: 10.1016/j.pharmthera.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Thomson AH, Kerr S, Wright S. Population pharmacokinetics of caffeine in neonates and young infants. Ther Drug Monit. 1996;18:245–53. doi: 10.1097/00007691-199606000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Skopnik H, Bergt U, Heimann G. Neonatal theophylline intoxication: pharmacokinetics and clinical evaluation. Eur J Pediatr. 1992;151:221–4. doi: 10.1007/BF01954390. [DOI] [PubMed] [Google Scholar]
- 39.al-Alaiyan S, al-Rawithi S, Raines D, Yusuf A, Legayada E, Shoukri MM, et al. Caffeine metabolism in premature infants. J Clin Pharmacol. 2001;41:620–7. doi: 10.1177/00912700122010500. [DOI] [PubMed] [Google Scholar]
- 40.Grosso LM, Triche EW, Belanger K, Benowitz NL, Holford TR, Bracken MB. Caffeine metabolites in umbilical cord blood, cytochrome P-450 1A2 activity, and intrauterine growth restriction. Am J Epidemiol. 2006;163:1035–41. doi: 10.1093/aje/kwj125. [DOI] [PubMed] [Google Scholar]
- 41.Ambalavanan N, Carlo WA, D'Angio CT, McDonald SA, Das A, Schendel D, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Cytokines associated with bronchopulmonary dysplasia or death in extremely low birth weight infants. Pediatrics. 2009;123:1132–41. doi: 10.1542/peds.2008-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shmarina GV, Pukhalsky AL, Kokarovtseva SN, Pukhalskaya DA, Shabalova LA, Kapranov NI, et al. Tumor necrosis factor-alpha/interleukin-10 balance in normal and cystic fibrosis children. Mediators Inflamm. 2001;10:191–7. doi: 10.1080/09629350123387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butcher RW, Sutherland EW. Adenosine 3’,5’-phosphate in biological materials. I. Purification and properties of cyclic 3’,5’-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3’,5’-phosphate in human urine. J Biol Chem. 1962;237:1244–50. [PubMed] [Google Scholar]
- 44.Chasin M, Harris DN. Inhibitory and activators of cyclic nucleotide phosphodiesterase. Adv Cyclic Nucleotide Res. 1976;7:225–64. [PubMed] [Google Scholar]
- 45.Shi D, Padgett WL, Daly JW. Caffeine analogs: effects on ryanodine-sensitive calcium-release channels and GABAA receptors. Cell Mol Neurobiol. 2003;23:331–47. doi: 10.1023/A:1023688604792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daly JW. Caffeine analogs: biomedical impact. Cell Mol Life Sci. 2007;64:2153–69. doi: 10.1007/s00018-007-7051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Copland IB, Post M. Understanding the mechanisms of infant respiratory distress and chronic lung disease. Am J Respir Cell Mol Biol. 2002;26:261–5. doi: 10.1165/ajrcmb.26.3.f231. [DOI] [PubMed] [Google Scholar]
- 48.De Dooy JJ, Mahieu LM, Van Bever HP. The role of inflammation in the development of chronic lung disease in neonates. Eur J Pediatr. 2001;160:457–63. doi: 10.1007/s004310100785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bolt RJ, van Weissenbruch MM, Lafeber HN, Delemarre-van de Waal HA. Glucocorticoids and lung development in the fetus and pre-term infant. Pediatr Pulmonol. 2001;32:76–91. doi: 10.1002/ppul.1092. [DOI] [PubMed] [Google Scholar]
- 50.Kotecha S, Silverman M, Shaw RJ, Klein N. Soluble L-selectin concentration in bronchoalveolar lavage fluid obtained from infants who develop chronic lung disease of prematurity. Arch Dis Child Fetal Neonatal Ed. 1998;78:F143–7. doi: 10.1136/fn.78.2.f143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kotecha S, Wilson L, Wangoo A, Silverman M, Shaw RJ. Increase in interleukin (IL)-1 beta and IL-6 in bronchoalveolar lavage fluid obtained from infants with chronic lung disease of prematurity. Pediatr Res. 1996;40:250–6. doi: 10.1203/00006450-199608000-00010. [DOI] [PubMed] [Google Scholar]
- 52.Schultz C, Rott C, Temming P, Schlenke P, Moller JC, Bucsky P. Enhanced interleukin-6 and interleukin-8 synthesis in term and preterm infants. Pediatr Res. 2002;51:317–22. doi: 10.1203/00006450-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 53.Oei J, Lui K, Wang H, Henry R. Decreased interleukin-10 in tracheal aspirates from preterm infants developing chronic lung disease. Acta Paediatr. 2002;91:1194–9. doi: 10.1111/j.1651-2227.2002.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 54.Jones CA, Cayabyab RG, Kwong KY, Stotts C, Wong B, Hamdan H, et al. Undetectable interleukin (IL)-10 and persistent IL-8 expression early in hyaline membrane disease: a possible developmental basis for the predisposition to chronic lung inflammation in preterm newborns. Pediatr Res. 1996;39:966–75. doi: 10.1203/00006450-199606000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]