Abstract
Botulinum neurotoxin A (BoNT/A) is used clinically to treat several neurological and metabolic diseases. However, the mechanisms that underlie the clinical use of the toxin remain still to be elusive. BoNT/A inhibits acetylcholine (ACh) release at the motor nerve terminals (MNT) and causes neuroparalysis. The toxic effects of BoNT/A at the MNT occur in sub-pico molar range, and it is invaluable to determine the half-life and the persistence of catalytic activity of the toxin to develop therapeutics against BoNT/A intoxication. However, the use of extremely low concentrations of BoNT/A in cellular, or animal models due to high toxicity makes it difficult to determine new cellular mechanisms and binding or interacting partners of BoNT/A. In order to address this, a catalytically deactivated, non-toxic version of BoNT/A, designated as DrBoNT/A, was characterized. DrBoNT/A lacks endoprotease activity (SNAP-25 cleavage) at concentrations as high as 46,875 – fold, compared to wild-type BoNT/A. Unlike BoNT/A injection (3.2 pg), injection of the recombinant product (150 ng or 3.2 pg) into mouse hind limbs failed to cause neuroparalysis as exhibited by the lack of inhibition of toe spread reflex (ability of the mouse to spread its hindlimb toes), and inhibit ACh release at the MNT. The in vitro experiments also demonstrate that DrBoNT/A uptake (at concentration in 1000–fold excess over BoNT/A), internalization and localization at the MNT remained unaltered. In addition, modeling studies support that DrBoNT/A lacked the zinc binding ability, and the ability to directly participate in the hydrolysis of SNAP-25 substrate. Collectively, we demonstrate that DrBoNT/A is non-toxic to the MNT and can be used as a surrogate tool to understand the mechanism by which BoNT/A modulates signal transduction mechanisms.
Keywords: non-toxic Botulinum neurotoxin A, neuromuscular junction, acetylcholine release, neurotransmission, nerve muscle preparation, deactivated, recombinant botulinum neurotoxin A
1. Introduction
Botulinum neurotoxins (BoNTs) serotypes A through G are extremely potent protein neurotoxins and are lethal to humans with exposure of as little as 1–3 ng/kg (Arnon et al., 2001). The neurotoxins are produced by anaerobic bacteria Clostridium Clostridium and to some extent by other clostridial strains (Simpson, 2004). BoNTs are category A bioterror agent(s) listed among the total six category A select agents by CDC, as they could be consumed through contaminated food unintentionally or from distribution through food supply causing high mortality. Because of its high potency, long duration of action and lethality, any deliberate release of BoNT within a civilian population or large-scale outbreak could cause severe panic, socio- economic impacts (Arnon et al., 2001).
BoNT/A (a 150 kDa protein) consists of a heavy chain (HC) linjked to a light chain (LC) by a disulfide bond. The HC binds to the ectoreceptors at the MNT which sets BoNT/A uptake followed by endosomal pH dependent separation of HC and Lc. Translocation of the LC (Cai et al., 2006; Montal, 2009) into the cytosol results in the proteolysis of SNAP-25 protein, attenuation of ACh release and therefore, flaccid paralysis of the innervating skeletal muscle (Montecucco and Schiavo, 1994). LC of BoNT/A acts as a zinc endopeptidase to cleave SNAP-25 protein that is essential for the fusion of synaptic vesicles at the MNT (Li and Singh, 2000; Montecucco and Schiavo, 1994). The neuromuscular paralysis produced by BoNT/A lasts from weeks to months (Foran et al., 2003). Therapeutic efforts to counteract BoNT intoxication have resulted in the development of serotype-specific antitoxins (Li et al., 2012) which are effective against circulating toxin. This was recently documented with BoNT/F poisoning in humans where neurotoxin was found in circulation as long as 8 days (Sobel et al., 2009).
Generally, the antitoxins are ineffective once BoNT/A enters the neurons. Only recourse for the treatment is artificial ventilation and other supportive therapy (Chertow et al., 2006; Souayah et al., 2006). Therefore, efforts are geared up to develop alternative therapeutics against BoNT/A. Recently, investigators have evaluated various potential therapeutic compounds locally by injecting them into the hindlimb muscles of rats or mice to test their efficacy against BoNT/A-induced paralysis. Small molecule inhibitors to inactivate LC or efforts to enhance degradation of BoNT/A LC (Thyagarajan et al., 2010), use of K+ channel blocker 3, 4-diaminopyridine and Ca2+ channel activator and cycline dependent kinase (CDK) inhibitor, such as roscovitine, as physiological antagonists in rats as post-exposure therapeutic to BoNT/A (Adler et al., 2012) and capsaicin, a transient receptor potential vanilloid receptor 1 (TRPV1) agonist used prior to the injection of neurotoxin in mice (Thyagarajan et al., 2009) have shown some limited success in animals. At the same time, significant progress has been made to in the last 15 to 20 years to harness the therapeutic potential of BoNT/A. The use of neurotoxin (particularly BoNT/A, BOTOX®) for various clinical disorders is expanding. The use of BoNT/A for cosmetic applications for the removal of wrinkles is well documented (Benedetto, 1999; Fabbri et al., 2008). Apart from cosmetic uses, BoNT/A, is used against overactive bladder (Apostolidis, 2012) and to treat a variety of neurologic disorders (Fabbri et al., 2008; Verderio et al., 2006) including diabetic neuropathic pain (Yuan et al., 2009), trigeminal neuralgia (Nurmikko and Cruccu, 2009) and refractory knee pain (Jabbari and Machado, 2011) and for the treatment for spastic disorders such as in cerebral palsy and post-stroke.
Modification of binding domain of BoNT/A to produce engineered toxin as a delivery vehicle for LC or other non-native proteins to the cytosol of non-neuronal cells has been shown to help treating non-neuronal secretory diseases (Pickett, 2011). Chimera of BoNT/A and /E utilizing BoNT/A HC, LC of BoNT/A and N-terminal HC of BoNT/E produce rapid uptake and neurotransmission block by BoNT/E (Wang et al., 2012). Such approach could be used in pain mediation (Dolly et al., 2011). A recent report (Wang et al., 2012) characterized and tested physiologically, the chimeras utilizing HC of native tetanus toxin and non-toxic version of tetanus toxin mutant with the LC of BoNT/A and E/ and found that LC of BoNT/E was delivered to the spinal cord by retrograde axonal transport and caused spastic paralysis of the limb on injected side (Wang et al., 2012). For therapeutic drug delivery, the LC of BoNT/A needs to be inactivated (Singh et al., 2010). Recently a catalytically inactive full-length version of recombinant BoNT/A (deactivated recombinant BoNT/A; DrBoNT/A) has been developed by mutating two glutamic acid residues at the active site (Kukreja et al., 2007; Weiping Yang, 2008), which also showed significant loss of systemic toxicity in a mouse bioassay (Singh et al., 2010). Although DrBoNT has been biochemically characterized, it has not been subjected to testing for its physiological action at the MNT. Here, we have examined the effect of DrBoNT/A on the EDL nerve muscle preparations (NMPs) by using in vivo and in vitro techniques to assess the effects of DrBoNT/A on the extent of muscle paralysis, and the effects on spontaneous and stimulus evoked ACh release at the NMT. The effects of DrBoNT/A were compared with those produced by BoNT/A.
2. Methods
Adult C57BL6 mice were used for this study. All animal procedures were approved by the Institutional Animal Care and Use Committee.
2.1 In vivo experiments to test effects of DrBONT/A on EDL muscle after local injection
2.1.1 Surgical procedure and injections
Surgical procedures for in vivo experiments were conducted using aseptic techniques in mice anesthetized with intraperitoneal injection of a mixture of ketamine (100 mg/kg) and xylazine (9 mg/kg). A small incision in the skin was made in the right leg where the peroneal nerve enters into the EDL muscle (Thyagarajan et al., 2009). Three microliters of either BoNT/A (1.066 pg/µl) in HEPES Ringer Solution (Thyagarajan et al., 2009) or the same volume of DrBONT/A (50 ng/µl) was injected into the space surrounding the innervation site of the EDL with a 26-gauge needle attached to a Hamilton syringe. Since the molecular weight of BoNT/A and DrBoNT/A is 150 kDa, the amount of DrBoNT/A injected in the muscle was 46,875 – fold in excess of BoNT/A. The skin incision was closed with silk suture. Saline (3 µl) injected-contralateral EDL muscles in the left limb served as sham-operated controls. Alternative to 150 ng of DrBoNT/A, 3.2 pg of DrBoNT/A (equivalent concentration to wild type BoNT/A) was injected into EDL regions (n=3). In experiments where the effect of prior injection of DrBoNT/A on its ability to protect EDLs from subsequent BoNT/A injection-induced muscle paralysis was to be tested, DrBoNT/A was injected 12 hr., before BoNT/A injection, in the same leg. Toe spread reflex (TSR; ability of the mouse to spread its hindimb toes) was measured 24 to 48 hr. after second injection.
2.1.2 Assessment of TSR
Typically, when the mouse is lifted by tail peroneal muscles spread the 2nd, the 3rd and the 4th toes, and this constitutes the peroneal nerve function index [PFI (Gutmann E, 1942)]. It serves as a sign of functional recovery. The effect of BoNT/A and DrBoNT/A on the TSR was evaluated according to the procedure described in an earlier report (Thyagarajan et al., 2009; Thyagarajan et al., 2010). Since the evaluation of TSR is subjective, the investigators conducting the test were unaware (blinded) of the treatment groups. The TSR was scored from 1 to 5 depending on the number of toes that the mouse could extend from the midline of the foot, when the animal was lifted by the tail. The mice were observed every day for a period of 6 days after injection of the toxins.
2.2 In vitro experiments with EDL nerve-muscle preparation (NMP)
2.2.1 Recording of endplate currents (EPCs) evoked by peroneal nerve stimulation
Right and left EDL muscles with intact nerve were removed from injected or uninjected control mice anesthetized with intraperitoneal injection of a mixture of ketamine (100 mg/kg) and xylazine (9 mg/kg). The muscles were pinned to a Sylgard-lined Plexiglas chamber, and bathed in HEPES Ringer solution (HRS; 22° C) containing (in mM) NaCl 135; KCl 5; CaCl2; MgCl2 1; dextrose 5.5 and HEPES 5. In uninjected control preparations 0.75 µM µ-conotoxin GIIIB (Alamone Labs, Israel) was added to the bath to inhibit muscle contractions in response to the nerve stimulation. In experiments where EDL muscles from uninjected control mice were removed for studying the effects of BoNT/A (10 pM) or DrBoNT/A (10 nM), after in vitro exposure (bath application), the procedure as described elsewhere (Thyagarajan et al., 2009) was followed. Briefly, the muscles were exposed to 10 pM of BoNT/A or 10 nM of DrBoNT/A in the presence HRS containing 40 mM KCl (osmolarity adjusted by decreasing NaCl to 100 mM) for 60–90 min when almost 90% block of twitch at mouse neuromuscular junction had occurred. In experiments where nerve stimulation-evoked loading was performed, the nerve was stimulated at 1 Hz for 60–90 min when maximum stimulus-evoked ACh release occurred with no observable muscle contractions. The EPCs in control muscles or those exposed to BoNT/A or DrBoNT/A were recorded by blocking the contractions with µ-conotoxin as described above. Miniature endplate currents (mEPCs) and endplate currents (EPCs; −75 mV holding potential) were recorded from the endplate region by using two electrode voltage clamp technique as described previously (Thyagarajan et al., 2009; Thyagarajan et al., 2010). Briefly, two electrodes were inserted into the endplate membrane (interelectrode distance of approximately 50 µm). One electrode filled with 4 M potassium acetate served to deliver current while the other electrode filled with 3 M KCI recorded membrane voltage. EPCs were elicited (1 Hz stimulation) by peroneal nerve stump sucked into a suction electrode. EPC and mEPC signals were amplified by Axoclamp 900A amplifier (Molecular Devices Inc., USA USA), acquired and digitized (Digidata 1344, Axon Instruments) and analyzed with PCLAMP software (version 10, Axon Instruments). All currents were recorded at a holding potential of −75 mV. All experiments were performed at 22 °C 24 to 48 hr. following BoNT/A or DrBoNT/A treatment.
2.2.2 Immunoblotting and Confocal imaging
Uptake of 667 pM BoNT/A or DrBoNT/A at the MNT was studied at room temperature by either KCI stimulation mediated loading or nerve stimulation- evoked uptake of the toxin as described above (see section 2.2.1).
Endplate-rich regions from EDL NMPs were stained for AChE with the method described previously (Koelle and Horn, 1968) as modified by Gautron (Gautron, 1982). ACh was the substrate for the staining reaction. AChE labeling was performed at room temperature. After a 30-min reaction period, preparations were washed with physiological saline. End plate regions were cut under a stereozoom microscope and flash frozen in liquid nitrogen for storage at −80°C. The procedure for detecting SNAP-25 cleavage from the endplate-rich tissue from the EDL NMPs exposed to BoNT/A or nontoxic DrBoNT/A was according to that described previously (Thyagarajan et al., 2009). Briefly, tissue was homogenized in phosphate-buffered saline (PBS) containing 1% Nonidet 40 and complete protease inhibitor cocktail (GE Healthcare, Little Chalfont, Buckinghamshire, UK). After centrifuging the homogenate at 14,000 rpm for 10 min in the cold, the crude lysate (40 µg) was resolved via SDS-polyacrylamide gel electrophoresis after boiling in 1 X Laemmli buffer. Immunoblotting was performed with anti-rabbit SNAP-25 antibody (Abcam, USA). GAPDH was used as a loading control.
Evaluation of neurotoxin uptake was studied in EDL preparations pinned to a Sylgard-lined Plexiglas chamber and bathed in HRS. BoNT/A or DrBoNT/A uptake in muscles was examined by exposing in 667 pM Alexa Fluor 647-labeled wild type BoNT/A or DrBoNT/A (Alexa Fluor-488 DrBoNT/A). To ensure that the endocytosis indeed occurs at the MNT, equivalent concentrations (667 pM) of wild type BoNT/A or DrBoNT/A were used. The uptake of native or engineered toxin (667 pM Alexa Fluor 647 or 488-labeled product) was initiated in response to nerve stimulation (1 Hz) or exposure to HRS 40 mM KCl (osmolarity adjusted by decreasing NaCl to 100 mM). Postsynaptic ACh receptors were labeled by exposing the fixed tissue to 1 ng/ml of Alexa Fluor 647-labeled or FITC α-bungarotoxin (α-BnTX) at 4 °C for 6 h. The muscle end-plate region was cut out and mounted with Vectashield on a slide and kept frozen at −20°C before imaging in confocal microscope. Ima ges were saved and represented as TIFF files.
2.3 Data Analyses
Data for all figures were expressed as mean ± S.D. The statistical significance between the population means was determined using Student’s t-test and p values ≤ 0.05 were considered significant.
2.4 Chemicals and Drugs
Sources of toxins: BoNT/A was obtained from Metabiologics Inc., Madison, WI or UMASS Dartmouth. Fluorescently labeled BoNT/A was purchased from (BBTech, Dartmouth, MA). DrBoNT/A was obtained from UMASS Dartmouth, MA, USA. DrBoNT/A was nicked in vitro using Immobilized TPCK Trypsin (Catalog # 20230, Thermo Scientific) to produce di-chain format linked through di-sulfide bridge and other non-covalent interactions similar to Clostridial BoNT/A holotoxin (Thirunavukkarasu and Singh, 2010). Sources for other reagents were as follows: SNAP-25 antibody (Abcam, USA); µ-Conotoxin GIIIB (Alamone Labs, Jerusalem, Israel); α-BnTX (Invitrogen, USA), Alexa fluor 647-labeled BoNT/A and Alexa-488 DrBoNT/A (Alexa Fluor and 647 dyes; Invitrogen, Carlsbad, CA, USA). All other chemicals and drugs were obtained from Sigma-Aldrich (St. Louis, MO, USA).
3.0 Results
3.1 Effect of local injection of BoNT/A and DrBoNT/A in the mouse EDL on TSR
An injection of 3.2 pg of BoNT/A in the right EDL of mouse showed localized paralysis of the hindlimb toes within 12 to 24 hr. as illustrated in the inset photograph in Figure 1A. The TSR was totally abolished in the right hindlimb whereas, the saline-injected left hindlimb demonstrated a complete spread of all the toes (n = 9). The lack of TSR was maintained throughout the 6-day observation period. In EDLs (n = 9) of mice receiving 150 ng DrBoNT/A, TSR was not affected and the mice were able to spread all toes to the full extent (Figure 1A). These results clearly indicated that DrBoNT/A failed to paralyze the EDL muscle.
Figure 1. DrBoNT/A does not inhibit TSR or stimulus evoked ACh release.
A. TSR evaluated at 12 to 24 hr. following vehicle, BoNT/A (3.2 pg) or DrBoNT/A (150 ng or 3.2 pg) injection into hindlimbs of mice. Inset photograph shows the inability of mouse to spread toes following wild type BoNT/A. B. Representative traces of EPCs of EDL NMP obtained from vehicle (control), BoNT/A (3.2 pg) or DrBoNT/A (150 ng or 3.2 pg) injected mice. C. Summary of effects of vehicle, BoNT/A (3.2 pg) or DrBoNT/A (150 ng or 3.3 pg) injections on stimulus evoked-ACh release measured by two-electrode voltage clamp.
3.2 Effect of BoNT/A and DrBoNT/A injections on the evoked (EPCs) and spontaneous (mEPCs) transmitter release at the mouse MNT
Typical traces of EPCs recorded from the endplate area from the control, BoNT/A and DrBoNT/A injected EDL muscles of mice are shown in Figure 1B. The muscles were injected with BoNT/A (3.2 pg) or 150 ng DrBoNT/A and the nerve was stimulated at 1 Hz. For control muscles and those injected with DrBoNT/A, muscle contractions in response to nerve stimulations were blocked by µ-conotoxin GIIIB. Toxins were injected in the muscles and recording from these muscles were done 12 to 24 hr. post exposure. As expected no response to nerve stimulation could be observed from BoNT/A injected EDL while EPCs of amplitude ranging between 120 – 140 nA could be recorded from the control and DrBoNT/A injected muscles (Figure 1B and 1C). In the endplates tested in the control muscles (n = 8) EPCs having mean amplitude of 135 ± 29 nA (Figure 1C) were recorded. In control recording from EDL endplates in mice receiving wild type, BoNT/A (n=8), EPCs showed mean amplitude of 0.66 ± 0.08 nA indicating full inhibitory effect of the toxin in abolishing nerve stimulus-evoked ACh release from MNT. Similar injections of DrBoNT/A however, showed full complement of the EPC amplitude (138 ± 39 nA) having normal time-course of the EPCs (n = 8). These results clearly showed that DrBoNT/A had no adverse effect on the nerve stimulus-evoked transmitter release at the MNT.
The results of mEPCs recorded from EDL muscles injected with BoNT/A or DrBoNT/A are shown in Figure 2. The recordings were made at 22 °C within 48 hr. post injection. Typical record of traces of mEPCs from a control saline injected EDL and one receiving wild type BoNT/A and similar tracings obtained from a mouse injected with DrBoNT/A are shown in Figure 2A and 2D. It is obvious that both control and DrBoNT/A had hardly noticeable influence on either the amplitude or the time-course of an mEPC at 24 hr. In control muscles the mean amplitude and frequency of mEPCS were 2.1 ± 0.8 nA and 1.2 ± 0.42 /s respectively (n = 8) whereas mEPCS recorded from BoNT/A–injected EDLs exhibited spontaneous events having slightly increased mEPC amplitude of 2.6 ± 1.1 nA (p < .05 with respect to control; n = 8) and a significantly lower (p < .01) frequency of occurrence with respect to controls (0.7 ± 0.41 /s in BoNT/A vs. 1.2 ± 0.42 /s in controls; n = 8; Figure 2B and 2C). It is worth noting that mEPCs recorded from the corresponding control and DrBoNT/A injected muscles had no decrement in either the amplitude (2.2 ± 1.1 nA in controls vs. 2.1 ± 0.8 nA DrBoNT/A; Figure 2E and 2F) or the frequency (1.2 ± 0.6 /s in controls vs. 1.2 ± 0.58 /s DrBoNT/A; n=8, figure 2E and 2F).
Figure 2. DrBoNT/A does not alter the properties of mEPC.
A and D represent typical records of mEPCs from EDL muscles injected with wild type BoNT/A (A) and DrBoNT/A (D) respectively. Histograms in B and C denote mEPC amplitude and the frequency for control and BoNT/A injected EDLs respectively. Histograms E and F represent mEPC amplitude and the frequency for control and DrBoNT/A injected EDL NMP respectively.
3.3 The effect of exposure of EDL muscles removed from uninjected control mice and subjected to in vitro exposure of BoNT/A and DrBoNT/A
The results of these experiments are shown in Figure 3. Typical traces of EPCs in response to peroneal nerve stimulation in preparations where EDL contractions from control (uninjected) mice were inhibited with the use of µ-conotoxin GIIIB are shown in Figure 3A. It is obvious that the traces obtained from either control or DrBoNT/A exposed muscles showed hardly any alteration either in the amplitude or the time-course after exposure to DrBoNT/A (Figure 3A and 3C). On the contrary, EDLs exposed to BoNT/A (10 pM) for the same duration failed to exhibit any response to the nerve stimulation indicating BoNT/A – induced block of evoked ACh release from MNT (Figure 3A). The lack of response to even increased stimulus strength was obvious as shown by large stimulus artifact in Figure 3A middle panel. The data gathered for the measurement of amplitude and quantal content of EPCs recorded from EDLs exposed in control condition and those after perfusion with DrBoNT/A for 60–90 min are shown in Figure 3B and 3C. Both, EPC amplitude and quantal content values in DrBoNT/A-exposed muscles were comparable to those recorded from untreated controls (amplitude- 135 ± 44 vs. 132 ± 48 p >.05; quantal content- 64 ± 13 vs. 66 ± 17.6; n=4 muscles, Figure 3B and 3C). These observations reinforced the findings from EDL muscles that had received native BoNT/A or mutant DrBoNT/A by injections and confirmed the lack of toxicity of the recombinant product.
Figure 3. DrBoNT/A treatment, in vitro, does not affect stimulus evoked ACh release.
A. Representative traces of EPCs of vehicle (control), BoNT/A (10 pM) or DrBoNT/A (10 nM) treated EDL NMP. Note- large stimulus artifact for BoNT/A (middle trace in A) indicating lack of EPC response to stimulus strength several- fold higher than that used for control or DrBoNT/A treated EDLs. Histograms in B and C show mean values (± S.D.) for the EPCs and quantal content measured for control, BoNT/A and DrBoNT/A treated EDL NMP respectively.
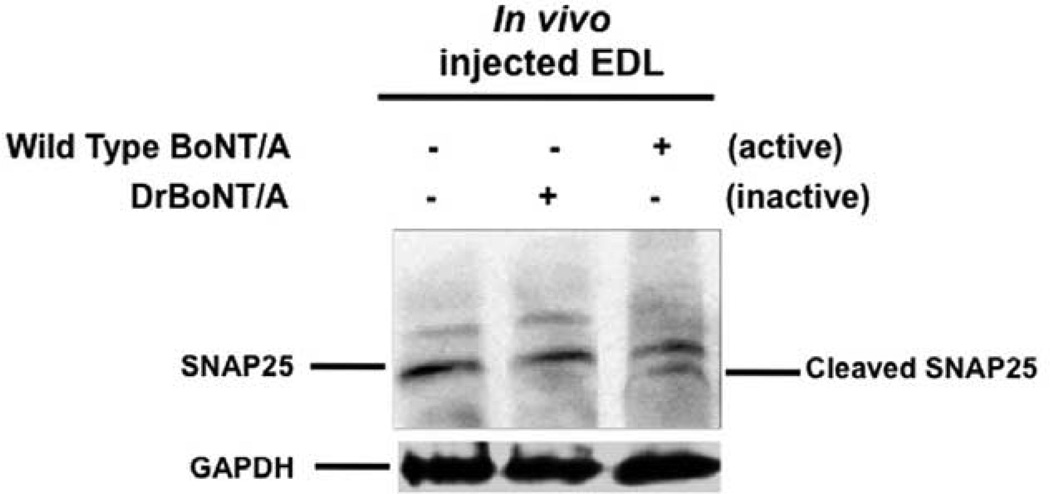
3.4 Ability of DrBoNT/A to cleave SNAP-25 in the EDL
Western blot of EDL muscles exposed to BoNT/A or DrBoNT/A in vivo and subjected to the procedure to test the presence or absence of cleavage of SNAP-25 at indicated concentrations of BoNT/A and DrBoNT/A (see methods) is shown in Figure 4. It is evident that the cleavage of SNAP-25 occurred only in the muscles treated with the wild type BoNT/A. (lane 3, Figure 4). In DrBoNT/A exposed muscles cleavage of SNAP-25 substrate was absent (lane 2, Figure 4) indicating that the engineered mutant protein lacked the ability to cleave its target substrate.
Figure 4. DrBoNT/A does not cleave SNAP-25.
Western blot of EDL muscles injected with BoNT/A (3.2 pg) or DrBoNT/A (150 ng) and processed for detection of SNAP-25 cleavage (see methods). Note the absence of cleaved product with DrBoNT/A (lane 2) and a cleaved band of the product with BoNT/A treated EDL (lane 3)
3.5 In vitro exposure of EDL muscles to BoNT/A and DrBoNT/A and the uptake of neurotoxin in MNT
Studies with 40 mM KCl in HRS stimulated uptake update of alexa 647 labeled BoNT/A (Figure 5 b) or alexa 488 labeled DrBoNT/A (Figure 5 d) demonstrate that the native as well as mutant toxins were endocytosed in the MNT. Equivalent concentration of 667 pM of the wild type BoNT/A or DrBoNT/A was used for these experiments. The same figure also shows confocal images of postsynaptic ACh receptors labeled with alexa- Fluor 488 (Figure 5 a) or alexa-647 and FITC conjugated α bungarotoxin (Figure 5 a and c). The confocal micrographs clearly show that uptake of both BoNT/A (Figure 5 b) and DrBoNT/A (Figure 5 d) had occurred into the MNT at equivalent concentrations.
Figure 5. DrBoNT/A is endocytosed into MNT.
The images show the 40 mM KCl in HRS-stimulated uptake of both BoNT/A and DrBoNT/A into MNT. Confocal images of MNT of EDL NMPs exposed to fluorescence labeled toxins. a and c represent postsynaptic αBnTX labeled with alexa 488 or alexa 647. b and d represent the corresponding presynaptic nerve terminal labeled with BoNT/A (alexa 647; b) and DrBoNT/A (alexa 488; d). Equivalent concentration (667 pM) of BoNT/A or DrBoNT/A for the experiments.
3.6 Effect of prior injection of DrBoNT/A on BoNT/A –induced paralysis in EDL
Local injection of DrBoNT/A (150 ng / 3µl) was made at the EDL region in the left hind limbs 12 hours prior to the injection of wild type BoNT/A (3.2 pg/3µl). As shown in Figure 6, BoNT/A alone, as expected, displayed mean TSR of 0 (n= 12 muscles) while muscles receiving DrBoNT/A only showed full TSR value of 5 (n= 8 muscles). DrBoNT/A injection 12 hr. prior to wild type BoNT/A injection failed to protect from neuroparalytic effect of BoNT/A (n=8 muscles). The TSR was totally abolished indicating inadequate nerve stimulus-evoked ACh release needed for normal function of TSR.
Figure 6. DrBoNT/A preinjection does not protect mice from the neuroparalytic effects of wild type BoNT/A.
Effect of injection of DrBoNT/A, 12 hr. before the injection of BoNT/A, into the EDL muscles on the TSR. Note the lack of protection by DrBoNT/A in preventing BoNT/A –induced paralysis.
3.7 Effects of the mutation at the molecular level determined through structural calculations
Figures 7A and B show the vicinity of the Zn2+ coordination sites for BoNT/A (Lacy et al., 1998) and DrBoNT/A, respectively. The ligands to the metal centers and amino acids R362, E350, and Y365 are represented in ball and stick. Figure 7C depicts the overlap of the structures displayed in 7 A and B. This figure shows only the amino acids that are important for Zn2+ binding and catalytic activity of BoNT/A (Binz et al., 2010), as well as the mutated amino acids in DrBoNT/A (A261 and A223). As can be seen from Figure 7C, the mutation of E residues to A causes a big disturbance of the metal binding site, increasing the ligand-metal distances in DrBoNT/A (Figure 7D). Amino acids Y365, R362 and E350, proposed earlier to play important roles in the hydrolytic mechanism of BoNT/A (Binz et al., 2010; Brunger et al., 2009) suffer only slight displacement from their original position in BoNT/A upon mutation.
Figure 7. Mutations in DrBoNT/A modulate the Zn2+ coordination sites.
A and B represent the vicinity of the Zn coordination sites for BoNT/A and DrBoNT/A, respectively. The ligands to the metal centers and amino acids R362, E350, and Y365 are represented in ball and stick. C. Overlap of the structures displayed in A and B. Only the amino acids that are important for Zn binding and catalytic activity of BoNT/A are displayed to show their relative locations before and after mutation. D. Metal binding sites for the Zn ion in BoNT/A and DrBoNT/A. Ligand-to-metal distances are in Å.
4.0 Discussion
This study is designed to investigate whether DrBoNT/A is toxic, at higher concentrations, at the mouse MNT. DrBoNT/A, is a double mutant in the light chain at the amino acid residues E224A and E262A (Yang et al., 2008) and previous research work has evaluated the endopeptidase activity of DrBoNT/A in cell free systems and its systemic toxicity (Singh et al., 2010). In the present investigation we have systematically tested the local effects of DrBoNT/A by injecting it into the hind limbs of mice and evaluated the ability of DrBoNT/A to inhibit TSR and ACh release. The effects were compared with those shown by native BoNT/A. In addition, we have also examined the effects of in vitro exposure of EDLs to DrBoNT/A to see if effects seen after local EDL injections could be mimicked after bath application.
As described in Figure 1A, injection of wild type BoNT/A (3.2 pg / 3 µl) at the EDL region in the hindlimbs inhibited the TSR (n = 8) within 24 hours following injection while DrBoNT/A (150 ng / 3µl or 3.2 pg / 3µl) injection did not cause TSR inhibition. This was consistent with the finding that the EPCs of EDL NMPs isolated from wild type BoNT/A injected mice hindlimbs were inhibited while no inhibition was observed in NMPs isolated from DrBoNT/A injected animals (Figures 1B and C).
Next, we evaluated the effects of mEPC (spontaneous release) of NMPs isolated from the wild type BoNT/A or DrBoNT/A injected animals. As shown in Figure 2, wild type BoNT/A inhibited the mEPC frequency significantly in comparison to the controls and slightly increased mEPC amplitude while these parameters remained unaffected by DrBoNT/A. The lack of any effect on the nerve stimulus-evoked or the spontaneous ACh release at the MNT of DrBoNT/A injected EDL suggests that the engineered mutant is unable to block the release of ACh at the MNT.
In vivo injection effects of BoNT/A and DrBoNT/A could be mimicked by in vitro exposure of isolated EDL NMPs for 60 to 90 min with BoNT/A or DrBoNT/A (Figure 3). Neither EPC amplitude nor quantal content were altered by the recombinant non-toxic product.
If DrBoNT/A did not inhibit ACh release or TSR, then it should not cause SNAP-25 proteolysis. Therefore, we determined SNAP-25 cleavage in EDL MNTs isolated from wild type BoNT/A or DrBoNT/A injected animals. As shown in Figure 4, SNAP-25 cleavage was observed for wild type BoNT/A but not DrBoNT/A injected EDL.
It could be argued that the lack of toxicity of DrBoNT/A seen with electrophysiological evidence could be due to the possibility that DrBoNT/A failed to enter the MNTs. To verify this, we performed in vitro experiments to study the uptake of fluorescent DrBoNT/A in isolated NMPs. As shown in figure 5, equivalent concentration (667 pM) of either Alexa 647 labeled wild type BoNT/A or Alexa 488 labeled DrBoNT/A was effectively taken up into the MNTs after stimulation with 40 mM KCL containing HRS. Alexa 488 or Alexa 647 labeled α-bungarotoxin (α-BnTX) was used to label postsynaptic ACh receptors. These results together with consistent electrophysiological evidence from acute treatment of DrBoNT/A showing that it did not modify the ACh release while wild type BoNT/A inhibited EPC (Figure 4), conclusively demonstrate that the recombinant DrBoNT/A is not toxic to MNTs. One can also conclude that DrBoNT/A endocytosed into MNT but did not cleave SNAP-25 or inhibit ACh release unlike wild type BoNT/A.
One interesting question that arises based on these data is whether DrBoNT/A pretreatment will saturate its receptors at the MNT and as a result will decrease the inhibitory effects of wild type BoNT/A. To answer this question, we injected DrBoNT/A (150 ng / 3µl) at the EDL region in hind limbs 12 hr. before the injection of wild type BoNT/A. As shown in Figure 6, EDL muscles injected with DrBoNT/A before wild type BoNT/A exposure did not prevent the local paralysis and exhibited a total absence of TSR. The failure of DrBoNT/A to prolong the time to paralysis produced by subsequent injection of BoNT/A could be due to either lack of DrBoNT/A’s effect on synaptic vesicle recycling or perhaps DrBoNT/A might be using other pathways for endocytosis. It appears that DrBoNT/A although binds to its receptors at the presynaptic nerve terminals and is endocytosed normally, it does not affect synaptic vesicle fusion and moreover, the recombinant toxin does not decrease the binding of wild type BoNT/A to its receptors at the MNT. Future research work should address this as this is out of scope of this manuscript. Also, our data on molecular modeling (Figure 7) demonstrate that the mutation at E224A alters the zinc binding of light chain in DrBoNT/A. Notably, besides the involvement of Zn2+ center, the carboxyl group of Glu-224 also directly participates in the hydrolysis of SNAP-25 peptide bond (Li and Singh, 2000). Therefore, lack of such characteristics due to mutation at E224A leading to catalytic deactivation may underlie the mechanisms behind its non-toxic effects. The model given in Figure 8 summarizes the effects of wild type BoNT/A and DrBoNT/A at the MNT.
Figure 8. Model showing BoNT/A and DrBoNT/A undergo binding, internalization and translocation at the MNT.
Only native BoNT/A is able to cleave SNAP-25 and cause inhibition of ACh release.
The disturbance of the metal-binding site of BoNT/A caused by the E261A and E223A can be explained by considering that glutamic acids are negatively charged amino acids that will be attracted to the positive charge on the Zn2+ ion, their replacement with alanine abolishes this attraction. Both alanine residues shift away from the Zn2+-binding site as shown in Figures 7A, 7B and 7C. The shift of A223 in turn shifts H222 away from the Zn ion due to the direct connection between these two amino acids. Figure 7D shows only the coordination sites for Zn2+ in BoNT/A and DrBoNT/A with metal-atom distances in Å. Examination of this figure indicates that for the mutated protein the coordinating nitrogen in H222 has shifted to a distance of 8.4 Å from the Zn ion. This shift would prevent the H222 form being a ligand to the metal atom. Although H226 is not shifted dramatically in the mutated protein, the atom-metal distance for its coordinating nitrogen becomes 3.7 Å after mutation. This distance is long enough to make coordination of this residue very unlikely. In summary, the mutations E261A and E223A remove one of the ligands to the metal center (E261), and leads to the rearrangement of the other ligands that would preclude their ligation to the Zn ion. Mutation of E223 has been shown to deactivate of BoNT/A (Li et al., 2000). This deactivation has been attributed to a role that E223 plays in the proposed catalytic mechanism of BoNT/A (Binz et al., 2010; Brunger and Rummel, 2009). In the present study, we have shown that Zn2+ binding would be very difficult upon mutations of E261A and E223A. The absence of a Zn ion in any BoNT would preclude the polarization of the Zn2+-bound H2O molecule required for the hydrolytic mechanism proposed for these bacterial protein toxins. Fu and co-workers reported the structure of the light chain of a recombinant BoNT/A treated with EDTA in 2006 (Fu et al., 2006). This structure was almost identical to that of the native light chain regarding the Zn2+-coordination site, and exhibited no catalytic activity. Therefore, even if all the amino acids required for Zn2+ binding, water polarization, and oxyanion stabilization are present in the protein, no catalytic activity would be observed. In light of these facts, we propose that the mutated protein is inactive because it does not bind the metal center required for its toxic activity, and not because of the loss ofits catalytic activity due to the mutation of E223A.
Collectively, our data describe the non-toxic effects of DrBoNT/A at the MNTs. This study assumes its significance in demonstrating the safety of DrBoNT/A. These data support a notion that the non-toxic potential of DrBoNT/A makes it a surrogate tool to identify unknown binding partners or signal transduction mechanisms that are sensitive to wild type BoNT/A. This surrogate tool, DrBoNT/A, will also help in determining the biological half-life and persistence at the MNT and to develop therapeutics against BoNT/A intoxication. Future studies are needed to address this as it will be important to delineate the mechanisms that underlie the growing therapeutic uses of wild type BoNT/A and also will serve to provide the proof of concept for the treatment of diseases by BoNT/A.
HIGHLIGHTS FOR REVIEW.
Deactivated recombinant BoNT/ A (DrBoNT/A) is not toxic to neuromuscular junction
DrBoNT/A is endocytosed into motor nerve terminals similar to that of wild type BoNT/A
DrBoNT/A pre-exposure does not protect the inhibitory effects of wild type BoNT/A
DRBoNT/A is a surrogate tool to understand neuronal signaling mechanisms
Acknowledgement
The work was supported by the Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P30 GM103398, National Institute of General Medical Sciences of the National Institutes of Health under Award Number 8P20GM103432-12 and by the American Association of Colleges of Pharmacy New Investigator Award to BT.
Abbreviations used
- BoNT/A
Botulinum neurotoxin A
- DrBoNT/A
deactivated recombinant BoNT/A
- Ach
acetylcholine
- NMPs
nerve muscle preparations
- MNT
motor nerve terminals
- HC
heavy chain
- LC
light chain
- TSR
toe spread reflex
- EPC
end plate current
- mEPC
miniature EPC
- EDL
extensor digitorum longus
- HRS
HEPES Ringer Solution
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethical Statement
The experimental protocols used in the work entitled “Effects of Enzymatically Inactive Recombinant Botulinum Neurotoxin Type A at the Mouse Neuromuscular Junctions” were approved by the Ethical Committee for the use of Animals of the University of Wyoming, 1000 East University Avenue, Laramie, WY 82071.
REFERENCES
- Adler M, Deshpande SS, Apland JP, Murray B, Borrell A. Reversal of BoNT/A-mediated inhibition of muscle paralysis by 3,4-diaminopyridine and roscovitine in mouse phrenic nerve-hemidiaphragm preparations. Neurochem Int. 2012 doi: 10.1016/j.neuint.2012.07.015. [DOI] [PubMed] [Google Scholar]
- Apostolidis A. Treatment of overactive bladder with botulinum toxin: are there more challenges to deal with? Eur Urol. 2012;62:515–517. doi: 10.1016/j.eururo.2012.05.043. [DOI] [PubMed] [Google Scholar]
- Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Hauer J, Layton M, Lillibridge S, Osterholm MT, O'Toole T, Parker G, Perl TM, Russell PK, Swerdlow DL, Tonat K. Botulinum toxin as a biological weapon: medical and public health management. Jama. 2001;285:1059–1070. doi: 10.1001/jama.285.8.1059. [DOI] [PubMed] [Google Scholar]
- Benedetto AV. The cosmetic uses of Botulinum toxin type A. Int J Dermatol. 1999;38:641–655. doi: 10.1046/j.1365-4362.1999.00722.x. [DOI] [PubMed] [Google Scholar]
- Binz T, Sikorra S, Mahrhold S. Clostridial neurotoxins: mechanism of SNARE cleavage and outlook on potential substrate specificity reengineering. Toxins (Basel) 2010;2:665–682. doi: 10.3390/toxins2040665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunger AT, Rummel A. Receptor and substrate interactions of clostridial neurotoxins. Toxicon. 2009;54:550–560. doi: 10.1016/j.toxicon.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S, Kukreja R, Shoesmith S, Chang TW, Singh BR. Botulinum neurotoxin light chain refolds at endosomal pH for its translocation. Protein J. 2006;25:455–462. doi: 10.1007/s10930-006-9028-1. [DOI] [PubMed] [Google Scholar]
- Chertow DS, Tan ET, Maslanka SE, Schulte J, Bresnitz EA, Weisman RS, Bernstein J, Marcus SM, Kumar S, Malecki J, Sobel J, Braden CR. Botulism in 4 adults following cosmetic injections with an unlicensed, highly concentrated botulinum preparation. Jama. 2006;296:2476–2479. doi: 10.1001/jama.296.20.2476. [DOI] [PubMed] [Google Scholar]
- Dolly JO, Wang J, Zurawski TH, Meng J. Novel therapeutics based on recombinant botulinum neurotoxins to normalize the release of transmitters and pain mediators. Febs J. 2011;278:4454–4466. doi: 10.1111/j.1742-4658.2011.08205.x. [DOI] [PubMed] [Google Scholar]
- Dong M, Liu H, Tepp WH, Johnson EA, Janz R, Chapman ER. Glycosylated SV2A and SV2B mediate the entry of botulinum neurotoxin E into neurons. Mol Biol Cell. 2008;19:5226–5237. doi: 10.1091/mbc.E08-07-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri A, Travaglione S, Falzano L, Fiorentini C. Bacterial protein toxins: current and potential clinical use. Curr Med Chem. 2008;15:1116–1125. doi: 10.2174/092986708784221430. [DOI] [PubMed] [Google Scholar]
- Foran PG, Mohammed N, Lisk GO, Nagwaney S, Lawrence GW, Johnson E, Smith L, Aoki KR, Dolly JO. Evaluation of the therapeutic usefulness of botulinum neurotoxin B, C1, E, and F compared with the long lasting type A. Basis for distinct durations of inhibition of exocytosis in central neurons. J Biol Chem. 2003;278:1363–1371. doi: 10.1074/jbc.M209821200. [DOI] [PubMed] [Google Scholar]
- Fu Z, Chen S, Baldwin MR, Boldt GE, Crawford A, Janda KD, Barbieri JT, Kim JJ. Light chain of botulinum neurotoxin serotype A: structural resolution of a catalytic intermediate. Biochemistry. 2006;45:8903–8911. doi: 10.1021/bi060786z. [DOI] [PubMed] [Google Scholar]
- Gautron J. Ultrastructural localization of acetylcholinesterase. A direct method for light and electron microscopy. Histochemistry. 1982;76:469–478. doi: 10.1007/BF00489902. [DOI] [PubMed] [Google Scholar]
- Gutmann EGL, Medawar PB, Young JZ. The rate of regeneration of the nerve. Journal of Experimental Biology. 1942;19:14–44. [Google Scholar]
- Jabbari B, Machado D. Treatment of refractory pain with botulinum toxins—an evidence-based review. Pain Med. 2011;12:1594–1606. doi: 10.1111/j.1526-4637.2011.01245.x. [DOI] [PubMed] [Google Scholar]
- Koelle GB, Horn RS. Acetyl disulfide, (CH3COS)2, a major active component in the thiolacetic acid histochemical method for acetylcholinesterase. J Histochem Cytochem. 1968;16:743–753. doi: 10.1177/16.12.743. [DOI] [PubMed] [Google Scholar]
- Kukreja RV, Sharma S, Cai S, Singh BR. Role of two active site Gluresidues in the molecular action of botulinum neurotoxin endopeptidase. Biochim Biophys Acta. 2007;1774:213–222. doi: 10.1016/j.bbapap.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Li D, Mattoo P, Keller JE. New equine antitoxins to botulinum neurotoxins serotypes A and B. Biologicals. 2012;40:240–246. doi: 10.1016/j.biologicals.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Binz T, Niemann H, Singh BR. Probing the mechanistic role of glutamate residue in the zinc-binding motif of type A botulinum neurotoxin light chain. Biochemistry. 2000;39:2399–2405. doi: 10.1021/bi992321x. [DOI] [PubMed] [Google Scholar]
- Li L, Singh BR. Role of zinc binding in type A botulinum neurotoxin light chain's toxic structure. Biochemistry. 2000;39:10581–10586. doi: 10.1021/bi0007472. [DOI] [PubMed] [Google Scholar]
- Montal M. Translocation of botulinum neurotoxin light chain protease by the heavy chain protein-conducting channel. Toxicon. 2009;54:565–569. doi: 10.1016/j.toxicon.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecucco C, Schiavo G. Mechanism of action of tetanus and botulinum neurotoxins. Mol Microbiol. 1994;13:1–8. doi: 10.1111/j.1365-2958.1994.tb00396.x. [DOI] [PubMed] [Google Scholar]
- Nurmikko T, Cruccu G. Botulinum toxin for trigeminal neuralgia. Eur J Neurol. 2009;16:e104. doi: 10.1111/j.1468-1331.2009.02583.x. [DOI] [PubMed] [Google Scholar]
- Pickett AaPK. Towards New Uses of Botulinum Toxin as a Novel Therapeutic Tool. Toxins. 2011;3:63–81. doi: 10.3390/toxins3010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavo G, Matteoli M, Montecucco C. Neurotoxins affecting neuroexocytosis. Physiol Rev. 2000;80:717–766. doi: 10.1152/physrev.2000.80.2.717. [DOI] [PubMed] [Google Scholar]
- Simpson LL. Identification of the major steps in botulinum toxin action. Annu Rev Pharmacol Toxicol. 2004;44:167–193. doi: 10.1146/annurev.pharmtox.44.101802.121554. [DOI] [PubMed] [Google Scholar]
- Singh BR, Thirunavukkarasu N, Ghosal K, Ravichandran E, Kukreja R, Cai S, Zhang P, Ray R, Ray P. Clostridial neurotoxins as a drug delivery vehicle targeting nervous system. Biochimie. 2010;92:1252–1259. doi: 10.1016/j.biochi.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Sobel J, Dill T, Kirkpatrick CL, Riek L, Luedtke P, Damrow TA. Clinical recovery and circulating botulinum toxin type F in adult patient. Emerg Infect Dis. 2009;15:969–971. doi: 10.3201/eid1506.070571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souayah N, Karim H, Kamin SS, McArdle J, Marcus S. Severe botulism after focal injection of botulinum toxin. Neurology. 2006;67:1855–1856. doi: 10.1212/01.wnl.0000244417.34846.b6. [DOI] [PubMed] [Google Scholar]
- Thirunavukkarasu NaSBR. Structure and Trafficking Potentials of Botulinum Neurotoxin in Drug Delivery. The Botulinum J. 2010;1:349–357. [Google Scholar]
- Thyagarajan B, Krivitskaya N, Potian JG, Hognason K, Garcia CC, McArdle JJ. Capsaicin protects mouse neuromuscular junctions from the neuroparalytic effects of botulinum neurotoxin a. J Pharmacol Exp Ther. 2009;331:361–371. doi: 10.1124/jpet.109.156901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyagarajan B, Potian JG, Garcia CC, Hognason K, Capkova K, Moe ST, Jacobson AR, Janda KD, McArdle JJ. Effects of hydroxamate metalloendoprotease inhibitors on botulinum neurotoxin A poisoned mouse neuromuscular junctions. Neuropharmacology. 2010;58:1189–1198. doi: 10.1016/j.neuropharm.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verderio C, Rossetto O, Grumelli C, Frassoni C, Montecucco C, Matteoli M. Entering neurons: botulinum toxins and synaptic vesicle recycling. EMBO Rep. 2006;7:995–999. doi: 10.1038/sj.embor.7400796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zurawski TH, Meng J, Lawrence GW, Aoki KR, Wheeler L, Dolly JO. Novel chimeras of botulinum and tetanus neurotoxins yield insights into their distinct sites of neuroparalysis. Faseb J. 2012 doi: 10.1096/fj.12-210112. [DOI] [PubMed] [Google Scholar]
- Weiping Yang PL, Stephen Riding, Tzuu-Wang Chang, Shuowei Cai, Thuan Van, Roshan Kukreja, Yu Zhou, Kruti Vasa, Singh Bal Ram. Expression, purification and comparative characterisation of enzymatically deactivated recombinant botulinum neurotoxin type A. The Botulinum J. 2008;1:219. [Google Scholar]
- Yuan RY, Sheu JJ, Yu JM, Chen WT, Tseng IJ, Chang HH, Hu CJ. Botulinum toxin for diabetic neuropathic pain: a randomized double-blind crossover trial. Neurology. 2009;72:1473–1478. doi: 10.1212/01.wnl.0000345968.05959.cf. [DOI] [PubMed] [Google Scholar]