Abstract
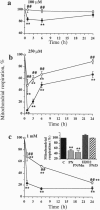
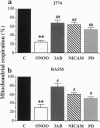
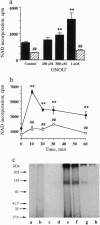
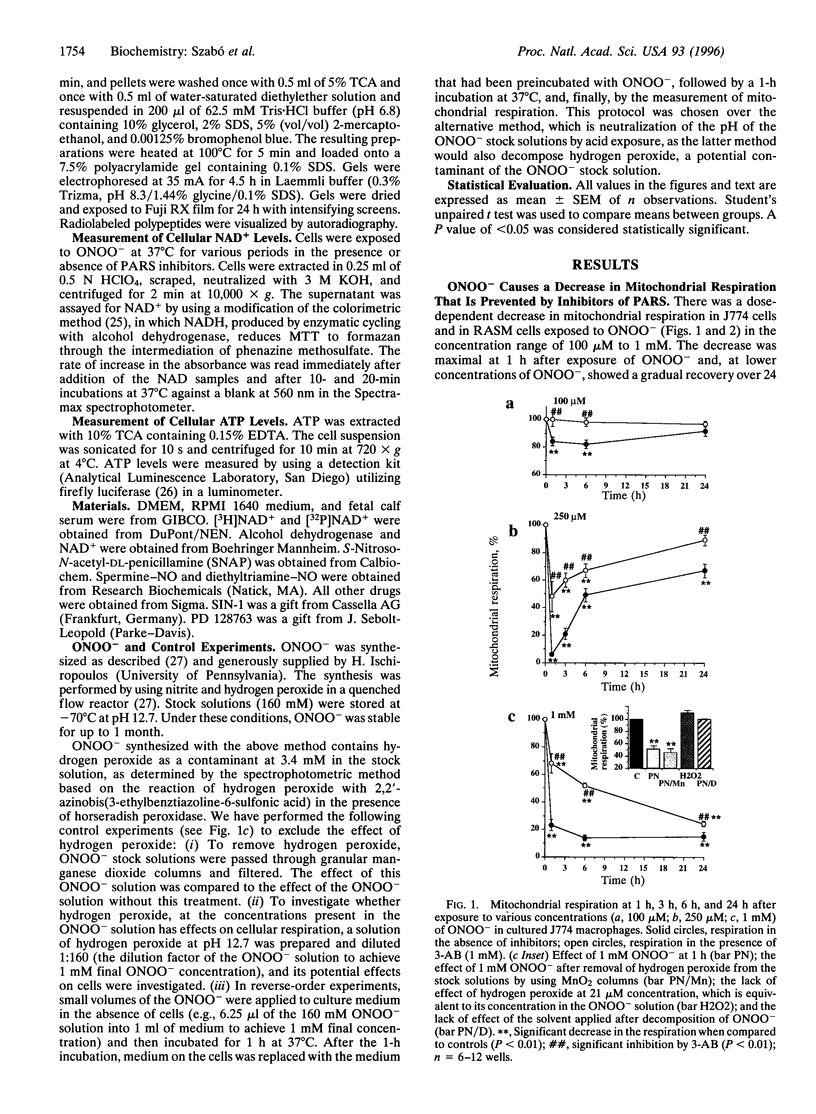
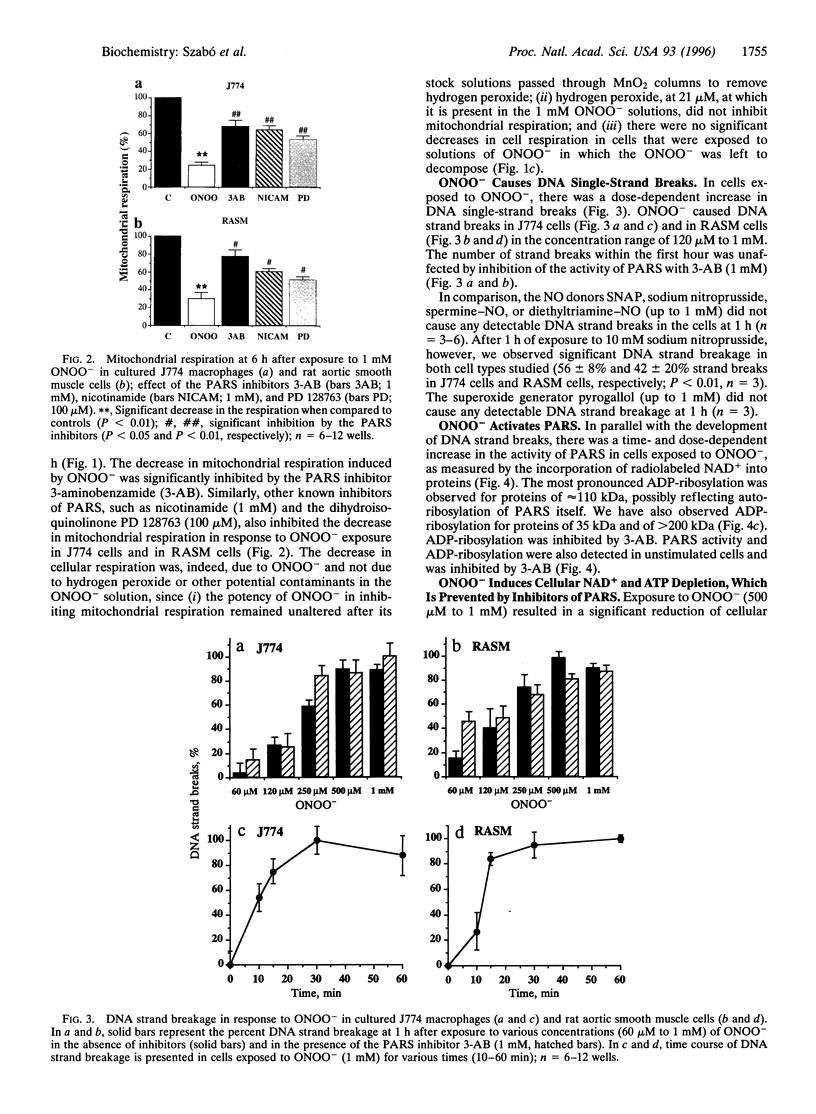
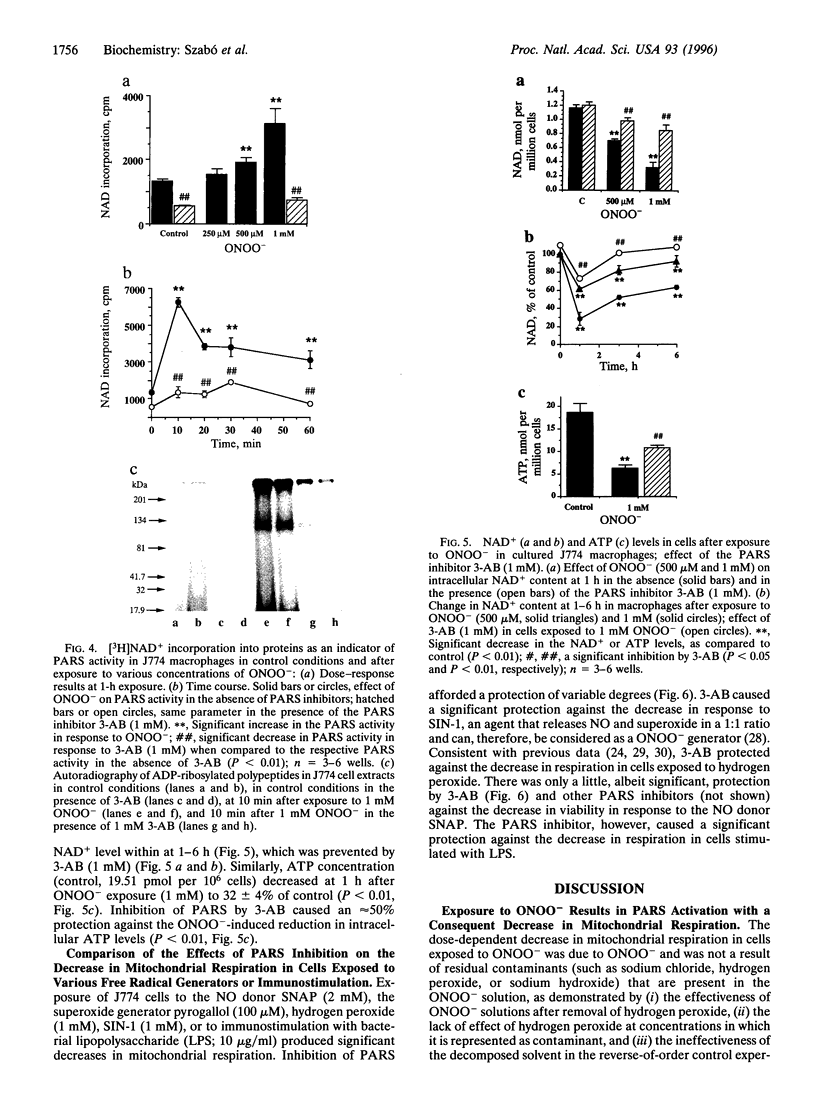
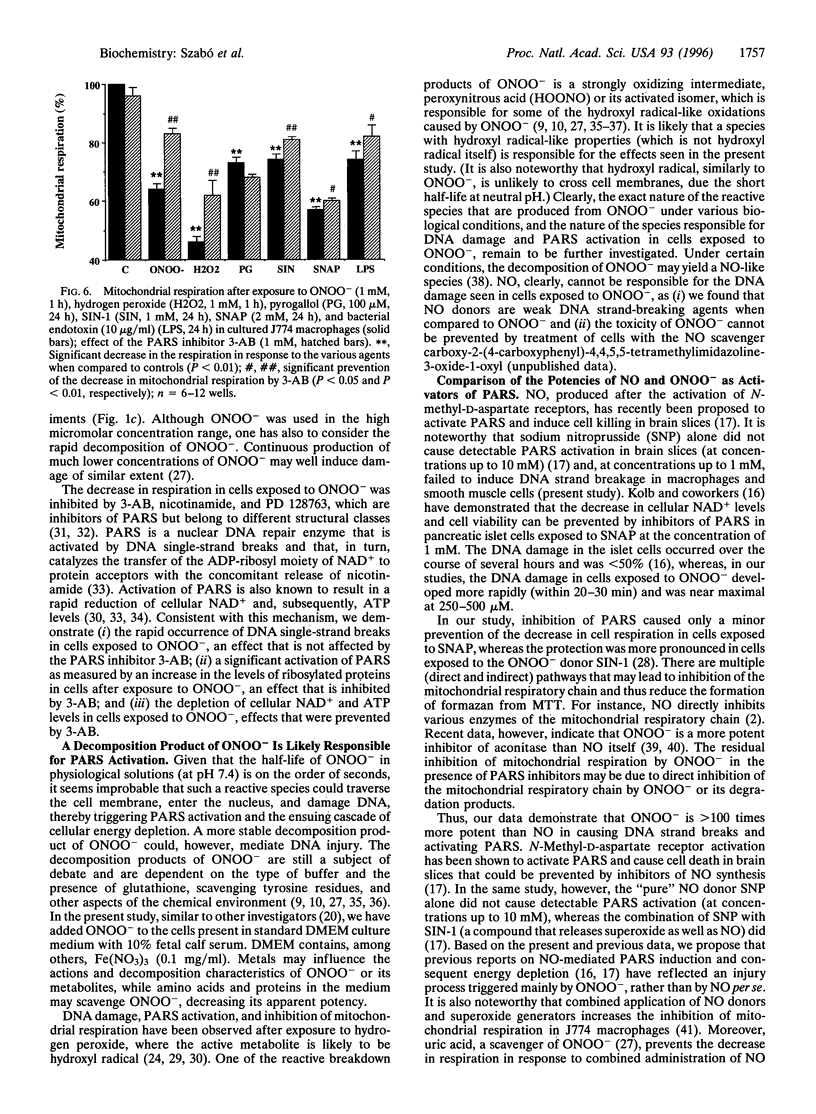
The free radicals nitric oxide and superoxide anion react to form peroxynitrite (ONOO-), a highly toxic oxidant species. In vivo formation of ONOO- has been demonstrated in shock and inflammation. Herein we provide evidence that cytotoxicity in cells exposed to ONOO- is mediated by DNA strand breakage and the subsequent activation of the DNA repair enzyme poly(ADP ribose) synthetase (PARS). Exposure to ONOO- (100 microM to 1 mM) inhibited mitochondrial respiration in cultured J774 macrophages and in rat aortic smooth muscle cells. The loss of cellular respiration was rapid, peaking 1-3 h after ONOO- exposure, and reversible, with recovery after a period of 6-24 h. The inhibition of mitochondrial respiration was paralleled by a dose-dependent increase in DNA strand breakage, reaching its maximum at 20-30 min after exposure to ONOO-. We observed a dose-dependent increase in the activity of PARS in cells exposed to ONOO-. Inhibitors of PARS such as 3-aminobenzamide (1 mM) prevented the inhibition of cellular respiration in cells exposed to ONOO-. Activation of PARS by ONOO--mediated DNA strand breakage resulted in a significant decrease in intracellular energy stores, as reflected by a decline of intracellular NAD+ and ATP content. 3-Aminobenzamide prevented the loss of NAD+ and ATP in cells exposed to ONOO-. In contrast, impairment of cellular respiration by the addition of the nitric oxide donors S-nitroso-N-acetyl-DL-penicillamine or diethyltriamine nitric oxide complex, was not associated with the development of DNA strand breaks, in concentrations up to 1 mM, and was largely refractory to PARS inhibition. Our results suggest that DNA damage and activation of PARS, an energy-consuming futile repair cycle, play a central role in ONOO--mediated cellular injury.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano F., Akamatsu Y. A lipopolysaccharide (LPS)-resistant mutant isolated from a macrophagelike cell line, J774.1, exhibits an altered activated-macrophage phenotype in response to LPS. Infect Immun. 1991 Jun;59(6):2166–2174. doi: 10.1128/iai.59.6.2166-2174.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasik M., Komura H., Shimoyama M., Ueda K. Specific inhibitors of poly(ADP-ribose) synthetase and mono(ADP-ribosyl)transferase. J Biol Chem. 1992 Jan 25;267(3):1569–1575. [PubMed] [Google Scholar]
- Beckman J. S., Chen J., Ischiropoulos H., Crow J. P. Oxidative chemistry of peroxynitrite. Methods Enzymol. 1994;233:229–240. doi: 10.1016/s0076-6879(94)33026-3. [DOI] [PubMed] [Google Scholar]
- Beckman J. S., Crow J. P. Pathological implications of nitric oxide, superoxide and peroxynitrite formation. Biochem Soc Trans. 1993 May;21(2):330–334. doi: 10.1042/bst0210330. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Jevcak J. J. Fluorometric method for rapid detection of DNA strand breaks in human white blood cells produced by low doses of radiation. Cancer Res. 1981 May;41(5):1889–1892. [PubMed] [Google Scholar]
- Castro L., Rodriguez M., Radi R. Aconitase is readily inactivated by peroxynitrite, but not by its precursor, nitric oxide. J Biol Chem. 1994 Nov 25;269(47):29409–29415. [PubMed] [Google Scholar]
- Cochrane C. G. Mechanisms of oxidant injury of cells. Mol Aspects Med. 1991;12(2):137–147. doi: 10.1016/0098-2997(91)90009-b. [DOI] [PubMed] [Google Scholar]
- Dinerman J. L., Lowenstein C. J., Snyder S. H. Molecular mechanisms of nitric oxide regulation. Potential relevance to cardiovascular disease. Circ Res. 1993 Aug;73(2):217–222. doi: 10.1161/01.res.73.2.217. [DOI] [PubMed] [Google Scholar]
- Gross S. S., Levi R. Tetrahydrobiopterin synthesis. An absolute requirement for cytokine-induced nitric oxide generation by vascular smooth muscle. J Biol Chem. 1992 Dec 25;267(36):25722–25729. [PubMed] [Google Scholar]
- Haddad I. Y., Crow J. P., Hu P., Ye Y., Beckman J., Matalon S. Concurrent generation of nitric oxide and superoxide damages surfactant protein A. Am J Physiol. 1994 Sep;267(3 Pt 1):L242–L249. doi: 10.1152/ajplung.1994.267.3.L242. [DOI] [PubMed] [Google Scholar]
- Hausladen A., Fridovich I. Superoxide and peroxynitrite inactivate aconitases, but nitric oxide does not. J Biol Chem. 1994 Nov 25;269(47):29405–29408. [PubMed] [Google Scholar]
- Hinz M., Katsilambros N., Maier V., Schatz H., Pfeiffer E. F. Significance of streptozotocin induced nicotinamide-adenine-dinucleotide (NAD) degradation in mouse pancreatic islets. FEBS Lett. 1973 Mar 1;30(2):225–228. doi: 10.1016/0014-5793(73)80656-8. [DOI] [PubMed] [Google Scholar]
- Hu P., Ischiropoulos H., Beckman J. S., Matalon S. Peroxynitrite inhibition of oxygen consumption and sodium transport in alveolar type II cells. Am J Physiol. 1994 Jun;266(6 Pt 1):L628–L634. doi: 10.1152/ajplung.1994.266.6.L628. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H., Zhu L., Beckman J. S. Peroxynitrite formation from macrophage-derived nitric oxide. Arch Biochem Biophys. 1992 Nov 1;298(2):446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- Kaur H., Halliwell B. Evidence for nitric oxide-mediated oxidative damage in chronic inflammation. Nitrotyrosine in serum and synovial fluid from rheumatoid patients. FEBS Lett. 1994 Aug 15;350(1):9–12. doi: 10.1016/0014-5793(94)00722-5. [DOI] [PubMed] [Google Scholar]
- Kilbourn R. G., Griffith O. W. Overproduction of nitric oxide in cytokine-mediated and septic shock. J Natl Cancer Inst. 1992 Jun 3;84(11):827–831. doi: 10.1093/jnci/84.11.827. [DOI] [PubMed] [Google Scholar]
- Kooy N. W., Royall J. A. Agonist-induced peroxynitrite production from endothelial cells. Arch Biochem Biophys. 1994 May 1;310(2):352–359. doi: 10.1006/abbi.1994.1178. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Moro M. A., Darley-Usmar V. M., Goodwin D. A., Read N. G., Zamora-Pino R., Feelisch M., Radomski M. W., Moncada S. Paradoxical fate and biological action of peroxynitrite on human platelets. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6702–6706. doi: 10.1073/pnas.91.14.6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Pryor W. A., Jin X., Squadrito G. L. One- and two-electron oxidations of methionine by peroxynitrite. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):11173–11177. doi: 10.1073/pnas.91.23.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radi R., Rodriguez M., Castro L., Telleri R. Inhibition of mitochondrial electron transport by peroxynitrite. Arch Biochem Biophys. 1994 Jan;308(1):89–95. doi: 10.1006/abbi.1994.1013. [DOI] [PubMed] [Google Scholar]
- Radons J., Heller B., Bürkle A., Hartmann B., Rodriguez M. L., Kröncke K. D., Burkart V., Kolb H. Nitric oxide toxicity in islet cells involves poly(ADP-ribose) polymerase activation and concomitant NAD+ depletion. Biochem Biophys Res Commun. 1994 Mar 30;199(3):1270–1277. doi: 10.1006/bbrc.1994.1368. [DOI] [PubMed] [Google Scholar]
- Rubbo H., Denicola A., Radi R. Peroxynitrite inactivates thiol-containing enzymes of Trypanosoma cruzi energetic metabolism and inhibits cell respiration. Arch Biochem Biophys. 1994 Jan;308(1):96–102. doi: 10.1006/abbi.1994.1014. [DOI] [PubMed] [Google Scholar]
- Schraufstatter I. U., Hinshaw D. B., Hyslop P. A., Spragg R. G., Cochrane C. G. Oxidant injury of cells. DNA strand-breaks activate polyadenosine diphosphate-ribose polymerase and lead to depletion of nicotinamide adenine dinucleotide. J Clin Invest. 1986 Apr;77(4):1312–1320. doi: 10.1172/JCI112436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraufstatter I. U., Hyslop P. A., Hinshaw D. B., Spragg R. G., Sklar L. A., Cochrane C. G. Hydrogen peroxide-induced injury of cells and its prevention by inhibitors of poly(ADP-ribose) polymerase. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4908–4912. doi: 10.1073/pnas.83.13.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraufstätter I., Hyslop P. A., Jackson J. H., Cochrane C. G. Oxidant-induced DNA damage of target cells. J Clin Invest. 1988 Sep;82(3):1040–1050. doi: 10.1172/JCI113660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X., Lenhart A., Mao Y. ESR spin trapping investigation on peroxynitrite decomposition: no evidence for hydroxyl radical production. Biochem Biophys Res Commun. 1994 Sep 30;203(3):1515–1521. doi: 10.1006/bbrc.1994.2357. [DOI] [PubMed] [Google Scholar]
- Stefanovic-Racic M., Stadler J., Evans C. H. Nitric oxide and arthritis. Arthritis Rheum. 1993 Aug;36(8):1036–1044. doi: 10.1002/art.1780360803. [DOI] [PubMed] [Google Scholar]
- Suto M. J., Turner W. R., Arundel-Suto C. M., Werbel L. M., Sebolt-Leopold J. S. Dihydroisoquinolinones: the design and synthesis of a new series of potent inhibitors of poly(ADP-ribose) polymerase. Anticancer Drug Des. 1991 May;6(2):107–117. [PubMed] [Google Scholar]
- Szabó C., Mitchell J. A., Gross S. S., Thiemermann C., Vane J. R. Nifedipine inhibits the induction of nitric oxide synthase by bacterial lipopolysaccharide. J Pharmacol Exp Ther. 1993 May;265(2):674–680. [PubMed] [Google Scholar]
- Szabó C., Salzman A. L. Endogenous peroxynitrite is involved in the inhibition of mitochondrial respiration in immuno-stimulated J774.2 macrophages. Biochem Biophys Res Commun. 1995 Apr 17;209(2):739–743. doi: 10.1006/bbrc.1995.1561. [DOI] [PubMed] [Google Scholar]
- Szabó C., Salzman A. L., Ischiropoulos H. Endotoxin triggers the expression of an inducible isoform of nitric oxide synthase and the formation of peroxynitrite in the rat aorta in vivo. FEBS Lett. 1995 Apr 24;363(3):235–238. doi: 10.1016/0014-5793(95)00322-z. [DOI] [PubMed] [Google Scholar]
- Szabó C., Thiemermann C. Invited opinion: role of nitric oxide in hemorrhagic, traumatic, and anaphylactic shock and thermal injury. Shock. 1994 Aug;2(2):145–155. [PubMed] [Google Scholar]
- Ueda K., Hayaishi O. ADP-ribosylation. Annu Rev Biochem. 1985;54:73–100. doi: 10.1146/annurev.bi.54.070185.000445. [DOI] [PubMed] [Google Scholar]
- Vane J. R. The Croonian Lecture, 1993. The endothelium: maestro of the blood circulation. Philos Trans R Soc Lond B Biol Sci. 1994 Jan 29;343(1304):225–246. doi: 10.1098/rstb.1994.0023. [DOI] [PubMed] [Google Scholar]
- Wizemann T. M., Gardner C. R., Laskin J. D., Quinones S., Durham S. K., Goller N. L., Ohnishi S. T., Laskin D. L. Production of nitric oxide and peroxynitrite in the lung during acute endotoxemia. J Leukoc Biol. 1994 Dec;56(6):759–768. doi: 10.1002/jlb.56.6.759. [DOI] [PubMed] [Google Scholar]
- Zhang J., Dawson V. L., Dawson T. M., Snyder S. H. Nitric oxide activation of poly(ADP-ribose) synthetase in neurotoxicity. Science. 1994 Feb 4;263(5147):687–689. doi: 10.1126/science.8080500. [DOI] [PubMed] [Google Scholar]
- Zingarelli B., O'Connor M., Wong H., Salzman A. L., Szabó C. Peroxynitrite-mediated DNA strand breakage activates poly-adenosine diphosphate ribosyl synthetase and causes cellular energy depletion in macrophages stimulated with bacterial lipopolysaccharide. J Immunol. 1996 Jan 1;156(1):350–358. [PubMed] [Google Scholar]