Abstract
The post-translational modification of serine and threonine residues of proteins by O-linked β-N-acetylglucosamine (O-GlcNAc) is highly ubiquitous, dynamic and inducible. Protein O-GlcNAcylation serves as a key regulator of critical biological processes including transcription, translation, proteasomal degradation, signal transduction and apoptosis. Increased O-GlcNAcylation is directly linked to insulin resistance and to hyperglycemia-induced glucose toxicity, two hallmarks of diabetes and diabetic complications. In this review, we briefly summarize what is known about protein O-GlcNAcylation and nutrient metabolism, as well as discuss the commonly used tools to probe changes of O-GlcNAcylation in cultured cells and in animal models. We then focus on some key proteins modified by O-GlcNAc, which play crucial roles in the etiology and progression of diabetes and diabetic complications. Proteomic approaches are also highlighted to provide a system view of protein O-GlcNAcylation. Finally, we discuss how aberrant O-GlcNAcylation on certain proteins may be exploited to develop methods for the early diagnosis of pre-diabetes and/or diabetes.
Keywords: diabetes, diabetic complications, hyperglycemia, insulin resistance, O-GlcNAc, O-GlcNAcomics, proteomics
Diabetes mellitus has reached epidemic proportions worldwide. Estimates from the International Diabetes Federation show that there were 371 million people with diabetes in 2012 [1] and the number of diagnosed cases is expected to rise to 552 million by 2030 [2]. Even more alarmingly, 187 million remain undiagnosed [1]. The high number of undiagnosed diabetes cases means that millions of people are at risk for costly and debilitating diabetic complications.
Diabetes mellitus is a complex metabolic disorder associated with the dysregulation of glucose homeostasis. According to the absolute or relative lack of insulin signaling, diabetes is classified into two major forms: Type 1 diabetes mellitus (T1DM) and Type 2 diabetes mellitus (T2DM). T1DM, an inflammatory autoimmune disease, results from the destruction of the insulin secreting pancreatic β cells leading to a complete absence of insulin in the body [3]. On the other hand, T2DM is characterized by relative insulin deficiency due to progressively decreased insulin secretion and/or the decreased effect of insulin in target tissues (i.e., liver, skeletal muscle and adipose tissue), also known as insulin resistance [4]. Hyperglycemia is a hallmark of both types of diabetes. As hyperglycemia becomes chronic, instead of serving as a substrate and a fuel, glucose takes on a darker role as a toxin that may cause irreversible cellular dysfunction over time, termed glucose toxicity. Chronic hyperglycemia negatively affects not only pancreatic β cells [5–7] and peripheral insulin target tissues [8,9], but also micro- and macro-vascular cells [10,11], leading to complications including diabetic cardiomyopathy, nephropathy, retinopathy, neuropathy and atherosclerosis.
Hyperglycemia, hexosamine biosynthesis pathway & O-GlcNAcylation
Since its discovery in the early 1980s [12,13], O-linked β-D-N-acetylglucosamine (O-GlcNAc) addition (O-GlcNAcylation) to serine/threonine residues has been found to be a key post-translational modification of proteins in the nucleus, cytosol and mitochondria. As a highly dynamic process, O-GlcNAc rapidly cycles onto serine/threonine residues of target proteins in a fashion analogous to phosphorylation. But unlike phosphorylation, only two enzymes are responsible for such an event: O-GlcNAc transferase (OGT) catalyzes the addition of O-GlcNAc to serine/threonine residues, whereas β-D-N-acetylglucosaminidase (O-GlcNAcase) catalyzes O-GlcNAc removal. A huge body of evidence reveals that O-GlcNAcylation plays critical roles in many cellular processes including signal transduction, transcriptional control, cell cycle regulation, protein degradation and stress response, among others [14,15]. And thousands of proteins can be O-GlcNAcylated during their lifecycle.
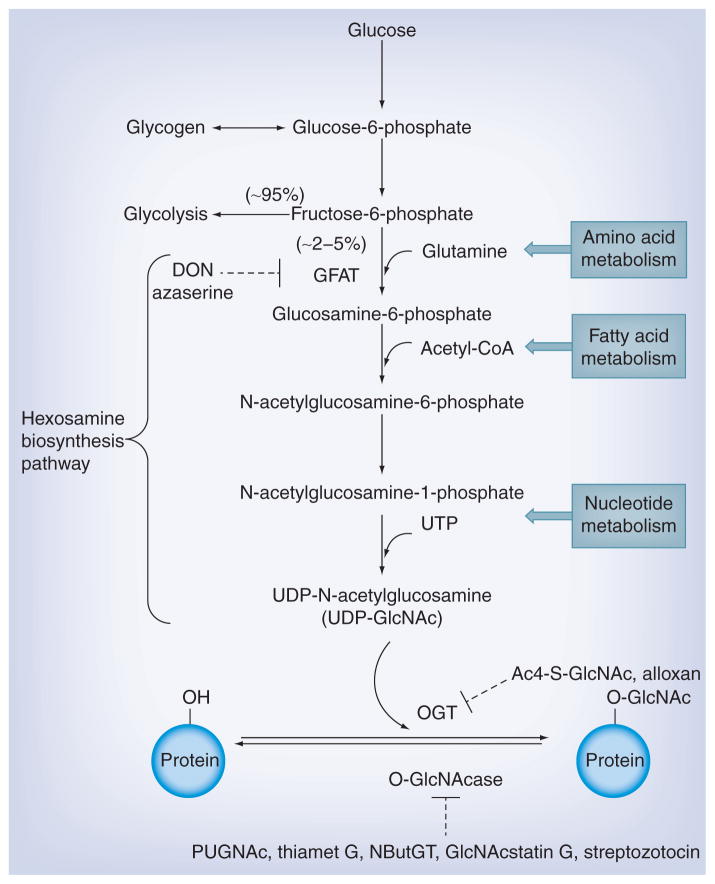
Uridine diphospho-N-acetylglucosamine (UDP-GlcNAc), the end-product of the hexosamine biosynthesis pathway (HBP) [16], serves as the high energy donor substrate for OGT. Due to its high dependency on nearly every major metabolic pathway in the cell (e.g., glucose metabolism, amino acid metabolism, fatty acid metabolism and nucleotide metabolism) (Figure 1), HBP is regarded as a nutrient-sensing pathway. Thus, O-GlcNAc functions as a nutrient sensor since the intracellular UDP-GlcNAc level rapidly responds to these metabolic pathways and OGT activity and specificity are highly dependent upon the concentration of UDP-GlcNAc [17]. Elevated glucose increases flux through the HBP, which often leads to increased UDP-GlcNAc concentrations and increased protein O-GlcNAcylation. In fact, abnormal O-GlcNAcylation has been directly linked to many metabolic diseases including diabetes [18–21].
Figure 1. The hexosamine biosynthesis pathway and protein O-GlcNAcylation (see Table 1 for more information).
To elucidate functional roles of protein O-GlcNAcylation, several different experimental manipulations can be performed, 1) exposure to hyperglycemic conditions (e.g., 4.5 g/l [25 mM] vs 1 g/l [5.5 mM] glucose media); 2) use small molecule inhibitor-based pharmaceutical intervention to induce O-GlcNAc changes, including inhibitors to glutamine:fructose-6-phosphate amidotransferase (GFAT), OGT [22–25] and O-linked N-acetylglucosaminidase (O-GlcNAcase) [26–34] (Figure 1 & Table 1), while cautions should be taken appropriately considering their selectivity and potential off-target effects (e.g., toward lysosomal hexosaminidases) 3) use genetic intervention to induce O-GlcNAc changes, for example, adenovirus-mediated overexpression of OGT/O-GlcNAcase, siRNA-OGT/O-GlcNAcase, inducible OGT knockout [35,36]. Of note is that besides using inhibitors to initiate O-GlcNAcylation changes, several model organisms (e.g., Caenorhabditis elegans [37,38] and Drosophila melanogaster [39,40]) and genetic animal models (e.g., Zucker fatty rats, Goto-Kakizaki rats and ob/ob mice) are widely exploited to investigate potential roles of O-GlcNAcylated proteins in diabetes-mimicking conditions. The increased availability of tools has greatly advanced our understanding of protein O-GlcNAcylation in the development of diabetes and diabetic complications.
Table 1.
Commonly used pharmacological tools for inducing O-GlcNAc changes on proteins in cultured cells and animal models.
| Inhibitors | Name | Structure | Cell culture | Animal model | Remarks | Ref. |
|---|---|---|---|---|---|---|
| GFAT | DON |
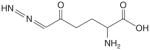
|
+ | + | ||
| Azaserine |
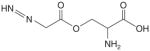
|
+ | ||||
|
| ||||||
| OGT | Alloxan |
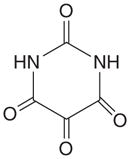
|
+ | + | Low specificity, short half-life, EC50† = 100 μM | [22,23] |
| Ac4-S-GlcNAc |
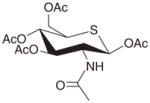
|
+ | EC50= 5 μM; the intracellular active form is UDP-S-GlcNAc | [24,25] | ||
|
| ||||||
| O-GlcNAcase | PUGNAc |
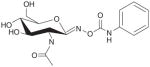
|
+ | + | Low selectivity, EC50= 3 μM, Ki‡ = 0.046 μM | [26] |
| TMG |
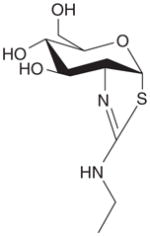
|
+ | + | EC50 = ~30 nM, Ki = 21 nM, orally fed, intraperitoneal or intravenous injection | [27–29] | |
|
| ||||||
| O-GlcNAcase (cont.) | NButGT |
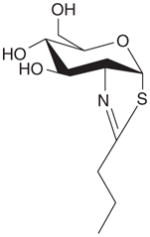
|
+ | + | EC50= 8 μM, Ki = 0.23 μM | [26,30,31] |
| GlcNAc-statin G |
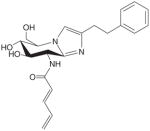
|
+ | EC50 = 3 μM, Ki = 4.1 nM | [32] | ||
| Streptozotocin |
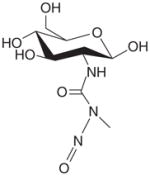
|
+ | Mainly toxic to β cells, leading to increased glucose level; minor inhibition to O-GlcNAcase | [32–34] | ||
EC50: Half maximal effective concentration.
Ki: Inhibition constant.
DON: 6-diazo-5-oxo-L-norleucine; GFAT: Glutamine:fructose-6-phosphate amidotransferase; OGT: O-linked N-acetylglucosamine transferase;
O-GlcNAcase: β-D-N-acetylglucosaminidase; TMG: Thiamet G.
Regulation of several key enzymes: GFAT, OGT & O-GlcNAcase
Regulation of GFAT
As mentioned previously, flux through the HBP and thus the synthesis of UDP-GlcNAc is regulated in large part by the metabolism of glucose; this synthesis is regulated by glutamine:fructose-6-phosphate amidotransferase (GFAT), which converts fructose-6-phosphate to glucosamine-6-phosphate with glutamine as the amine donor. As the first and rate-limiting enzyme in the HBP, GFAT is of crucial importance since it governs the availability of the end product UDP-GlcNAc.
GFAT has two main isoforms, GFAT1 and GFAT2, which are transcribed from separate genes located in human chromosome 2p13-p14 and 5q23-q35 respectively [41]. Although GFAT2 is mainly expressed in brain and heart, GFAT1 has high expression in other tissues including liver and fat. Of note, GFAT1L, a splice variant of GFAT1, is mainly in skeletal muscle [42]. Besides being transcriptionally regulated, GFAT also undergoes phosphorylation by cAMP-dependent protein kinase A [43,44] and AMP-activated protein kinase [45]. Moreover, glucosamine-6-phosphate [46] and UDP-GlcNAc [47] are potent feedback inhibitors of GFAT, providing important negative feedback loops to regulate HBP. However, even a moderately elevated HBP flux might increase O-GlcNAcylation on proteins [20].
Exhaustive early studies have demonstrated that 1) hyperglycemia in diabetic humans [48,49] and diabetic rats [50] induces or worsens insulin resistance, 2) adipocytes and muscle cells exposed to chronic high glucose levels develop insulin resistance and this phenotype is prevented when adipocytes are incubated with DON, a potent inhibitor of GFAT [51], 3) glucosamine infusion increases O-GlcNAcylation in skeletal muscle proteins in vivo [52] and 4) overexpression of GFAT in transgenic mice results in insulin resistance [53]. Collectively, these results strongly suggest that there is a direct connection between hyperglycemia, HBP flux, intracellular UDP-GlcNAc level, protein O-GlcNAcylation, the occurrence of insulin resistance and diabetes.
In addition to GFAT, perturbing the regulation of the O-GlcNAc cycling enzymes (i.e., OGT and O-GlcNAcase) provides another alternative to pinpoint the roles of protein O-GlcNAcylation in diabetes and diabetic complications.
Regulation of OGT
By transferring the O-GlcNAc moiety to its target proteins, OGT couples metabolic status to the regulation of a wide variety of cellular signaling pathways. However, unlike the presence of 518 human protein kinases [54], protein O-GlcNAcylation is specifically modified by a single enzyme OGT, the gene of which maps to a region on chromosome Xq13 in human [55]. OGT is highly conserved in a number of organisms ranging from Caenorhabditis elegans and Arabidopsis to humans [56,57]. Although OGT is expressed in all cells (predominantly localized in nucleus), it seems to be more abundant in several issues such as brain. Notably, alternative spicing of the single gene produces three isoforms of OGT: nucleocytoplasmic OGT (ncOGT, 110 kDa); a mitochondrial form, mOGT (103 kDa) [58] and a short form OGT (sOGT, 78 kDa). The crystal structure reveals that OGT has two distinct regions: an N-terminal region consisting of a series of tetratricopeptide repeat (TPR) units [56,57] and a multi-domain catalytic region. The three native OGT isoforms differ only in the number of TPRs, (ncOGT, mOGT and sOGT have 13.5, 9, 3 TPRs, respectively) [59]. The TPR domain is proposed to scaffold interactions with other proteins, which may play a role in determining substrate selectivity [60]. Moreover, the TPR domain is the location where OGT homotrimer/heterotrimer forms. OGT mainly exists as a homotrimer of three 110 kDa subunits in most tissues while a heterotrimer consisting of two 110 kDa subunits and one 78 kDa subunit in several tissues including liver, muscle and kidney [61,62].
Whereas the detailed catalytic mechanisms are still under debate [63,64], OGT seems to bind with UDP-GlcNAc and then the polypeptide acceptors, transferring the GlcNAc moiety onto serine/threonine residues of target proteins. Although UDP-GlcNAc can be transported into endoplasmic reticulum and Golgi for the synthesis of diverse forms of glycoconjugates, OGT has a high affinity toward UDP-GlcNAc, providing the nucleocytoplasmic proteins a competitive advantage for O-GlcNAc modification [61]. OGT has three separate Km values (ranging from 6 μM to over 200 μM) for UDP-GlcNAc, suggesting that UDP-GlcNAc level directly affects OGT activity and thus modulates the extent of O-GlcNAcylation of proteins.
Poor protein substrates at low levels of UDP-GlcNAc can become better acceptors at higher UDP-GlcNAc concentrations, indicating a changed substrate spectrum of OGT with an increasing UDP-GlcNAc concentration. Moreover, OGT is also regulated by other complex mechanisms, involving transcriptional regulation, mRNA splicing, proteolytic processing, post-translational modification and multi-merization with itself and other proteins [65]. For example, OGT is itself O-GlcNAcylated and also tyrosine phosphorylated in its catalytic domain [66,67]. Tyrosine or serine/threonine phosphorylation at certain sites appears to activate the enzyme, but the role of O-GlcNAc on OGT is not yet clear.
The regulation of OGT is directly involved in diabetes. OGT and O-GlcNAc-modified protein levels are increased in the pancreatic islets of diabetic rats [68]. Overexpression of OGT in liver, muscle and fat tissues causes insulin resistance [51,69]. Furthermore, OGT overexpression in cardiomyocytes induces impaired myocardium performance [70]. Alloxan, a cyclic uracil analog, has been used as a pharmaceutical inhibitor of OGT [22]. However, it suffers from several drawbacks: it is a fairly non-specific inhibitor of OGT; it is chemically unstable with a half-life time of 1.5 min at physiological pH [71]; and it inhibits OGT with an IC50 of 100 μM in extracts, but requires millimolar concentrations to decrease cellular levels of O-GlcNAcylation [22,23]. In contrast, Ac4-S-GlcNAc, which can penetrate into cells and be converted to its active form UDP-S-GlcNAc via the GlcNAc salvage pathway [24,25], offers a promising tool to selectively inhibit OGT. Besides inhibition of OGT at the enzymatic activity level, siRNA-mediated OGT suppression has been performed as well. Although increased O-GlcNAcylation leads to insulin resistance, it seems that an approximately 90% knockdown of OGT expression (with siRNA) and consequent global decrease in O-GlcNAcylation levels (to a similar degree) do not prevent the development of insulin resistance in 3T3-L1 adipocytes [72]. In addition, since earlier observations have shown that OGT knockout is lethal for the embryo [73], the inducible knockout OGT cell lines might be a very useful approach to elucidate the roles of OGT in the development of insulin resistance and other signaling pathways related to diabetes and diabetic complications [35,74].
Regulation of O-GlcNAcase
Similar to OGT, modulating O-GlcNAcase affords another way to probe the functional roles of O-GlcNAc cycling in diabetes. As with OGT, O-GlcNAcase is encoded by a single gene in human chromosome 10q24 [75], highly conserved across species, and also expressed in all tissues with a similar tissue distribution. The N-terminal domain is the O-GlcNAcase catalytic region and the C-terminal region has a histone acetyl-transferase like domain. The N- and C-terminal domains can be separated by caspase 3 cleavage during apoptosis [76]. O-GlcNAcase has two alternative spliced variants: the full-length/long isoform O-GlcNAcase-L (~130 kDa, localized mainly in the cytosol) and the short isoform O-GlcNAcase-S which lacks the C-terminal domain (~70 kDa, predominantly localized in the nucleus) [77].
Overexpression of O-GlcNAcase in pancreatic β cells of transgenic mice leads to decreased insulin secretion and impaired glucose tolerance [78]. In diabetic (db/db) mice, improvement of glucose tolerance has also been demonstrated with O-GlcNAcase overexpression in liver [79]. Moreover, overexpression of O-GlcNAcase in the liver of non-diabetic mice reduces protein O-GlcNAc modification in the tissue, resulting in a parallel decrease in the transcription of gluconeogenic enzymes [80]. In addition, adenoviral O-GlcNAcase overexpression in diabetic hearts improves calcium cycling and thus enhanced contractile function, which was reversed with OGT overexpression [81]. Of note is that Caenorhabditis elegans with an OGA−/− null allele exhibits a phenotype metabolically similar to that of human T2DM [82].
On the other hand, the discovery of O-GlcNAcase inhibitors, which decrease the activity of O-GlcNAcase instead of affecting its expression, provides a convenient way to aid in our understanding of the roles of O-GlcNAcase. Treating β cells with PUGNAc acutely increases the levels of O-GlcNAcylation, resulting in decreased glucose stimulated insulin secretion [68]. Increased levels of O-GlcNAcylation in 3T3-L1 adipocytes treated with PUGNAc lead to insulin resistance [83]. Moreover, a similar phenomenon has been observed in rat skeletal muscle [84]. However, the treatment with a more specific inhibitor, NButGT, induces elevated global O-GlcNAcylation in cultured 3T3-L1 adipocytes [27] and all tissues tested in rats and mice [30,31], without causing obvious insulin resistance on its own. This discrepancy might be resulted from the off-target effects (e.g., on lysosomal hexosaminidases) of the less-specific PUGNAc, suggesting the necessity of choosing more specific and selective inhibitors to explore the roles of O-GlcNAcase. Caution should also be made in terms of inhibitor usage, since effects of the inhibitors are, generally, in a dose- and time-dependent manner, which may cause significant differences in protein O-GlcNAcylation levels in a tissue-specific manner and thus the related diabetic phenotypes.
O-GlcNAcylated proteins in diabetes & diabetic complications
As aforementioned, O-GlcNAcylation is abundant and occurs on myriad nucleocytoplasmic and mitochondrial proteins, exerting diverse important functions, including gene expression, protein translation, degradation and modulation of multiple signal transduction pathways. Here, we do not intend to summarize all O-GlcNAcylated proteins related to every aspect of diabetes and diabetic complications (interested readers can refer to several excellent reviews [14,15,18–21,85–87]); instead, we are providing a general perspective by focusing on some key players as well as their roles in the progression of diabetes and diabetic complications from a tissue-specific view.
Protein O-GlcNAcylation in pancreas/β-cells
The β-cell is a highly specialized cell in the pancreatic islets whose function is to produce, store and release insulin upon elevation of blood glucose. The dysfunction of β-cells is regarded as a major etiology of diabetes and diabetic complications. Hyperglycemia leads to hyper-GlcNAcylation of several proteins [68,88], including neurogenic differentiation 1 (NeuroD1) [89] and pancreatic/duodenal homeobox-1 (PDX-1) [68,90,91]. NeuroD1, a transcription factor regulating the expression of the insulin gene, interacts with OGT under high glucose conditions, but interacts with O-GlcNAcase under low glucose conditions. Moreover, O-GlcNAcylated NeuroD1a has increased nuclear localization, leading to an increase in DNA binding and glucose-dependent insulin synthesis [89]. O-GlcNAcylation of PDX-1 enhances its DNA binding to the A-box in the HR2 region of the promoter of protein-coupled free fatty acid receptor-1 (FFA1/GPR40), thus stimulating GPR40 gene transcription and insulin secretion [91]. However, in the long term, the increased OGT expression and O-GlcNAcylation level upon hyperglycemia are associated with the impaired insulin secretion [68] and pancreas apoptosis [23,88]. These data suggests that O-GlcNAcylation promotes insulin secretion under physiological conditions while this proliferative response ultimately might be offset by an increase in pancreatic apoptosis via excessive O-GlcNAcylation upon the prolonged exposure of hyperglycemia. Given the crucial function of the pancreas, roles of protein O-GlcNAcylation in the development of pancreatic dysfunction still need to be further investigated.
Protein O-GlcNAcylation in adipose tissue/adipocytes
As one insulin-sensing tissue, adipose (especially white adipose tissue) plays an important role in energy balance and glucose homeostasis via storage and turnover of triglycerides. After insulin stimulation, phosphatidylinositol 3,4,5-trisphosphate (PI(3,4,5) P3) recruits OGT from the nucleus to the plasma membrane, leading to the O-GlcNAcylation of several molecules in the insulin signaling pathway and the desensitization to insulin [69]. Upon insulin treatment, OGT can also be recruited to the insulin receptor (IR), tyrosine-phosphorylated and catalytically activated [67], resulting in O-GlcNAcylation of PDK1 and increased GLUT4-mediated glucose uptake [92]. However, with PUGNAc treatment, O-GlcNAcylation of the insulin receptor substrate 1 (IRS-1) decreases its interaction with PI3K p85, reducing phosphorylation of Thr308 of AKT and thus reduced glucose uptake [92]. Notably, hyperglycemia induced O-GlcNAcylation of glycogen synthase decreases its enzymatic activity, leading to maintained high glucose level and further insulin resistance [93]. In-depth site-specific functional assays of these O-GlcNAcylated proteins, together with the discovery of other potentially O-GlcNAc modified members in the insulin signaling pathway [94], should advance our insight of how protein O-GlcNAcylation attenuates insulin sensitivity.
Protein O-GlcNAcylation in liver/hepatic cells
Liver plays a central role in maintaining glucose homeostasis via, 1) storage of glucose as glycogen (glycogenesis) or conversion of glucose to lipid under high glucose conditions (e.g., after feeding), and 2) breakdown of glycogen (glycogenolysis), synthesis of glucose from non-carbohydrate sources such as amino acids (gluconeogenesis), and ketogenesis under low glucose conditions (e.g., during fasting). Insulin and other hormones modulate these events by insulin signaling and gene expression, leading to inhibition or stimulation in glucose production.
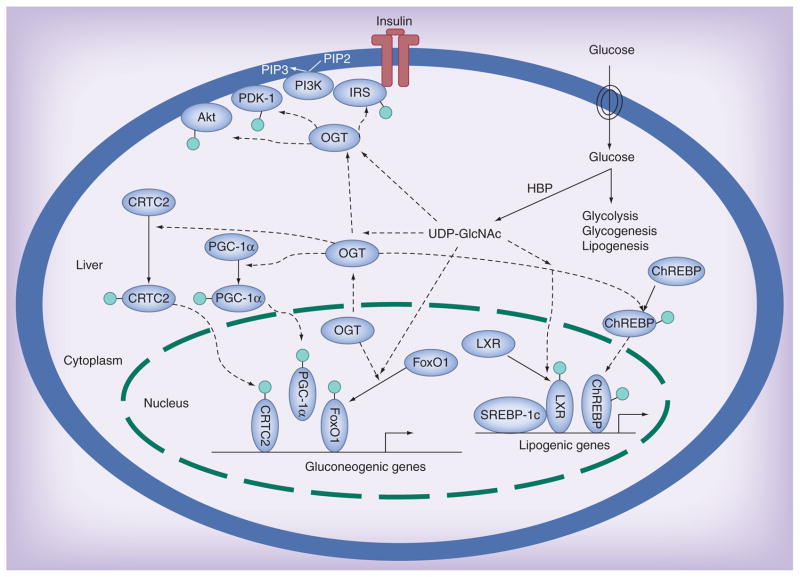
Besides the O-GlcNAcylation of proteins in the insulin signaling [69], quite a few transcriptional factors and co-activators are found to be O-GlcNAcylated, FoxO1, PGC-1α, CRTC2, LXR and ChREBP. And more intriguingly, the O-GlcNAcylation of these proteins is closely linked to high glucose-induced expression of gluconeogenic/lipogenic genes and thus may contribute to glucose toxicity (Figure 2). FoxO1 responds to hyperglycemia through elevated O-GlcNAcylation in the liver. Diabetes-induced O-GlcNAcylation of hepatic FoxO1 elevates expression of phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase), two rate-limiting enzymes in gluconeogenesis [95]. Elevated glucose also increases expression of these two enzymes through increased O-GlcNAcylation of CRTC2, which promotes its nuclear localization and enhanced promoter binding at gluconeogenic genes [79]. As a master regulator of gluconeogenesis, PGC-1α is found to be O-GlcNAcylated as well [96]. PGC-1α binds to OGT and targets OGT to FoxO1, leading to increased O-GlcNAcylation and increased transcriptional activity [78]. Interestingly, host cell factor (HCF-1) seems to recruit OGT to O-GlcNAcylate PGC-1α, facilitating the binding of the deubiquitinase BAP1, thus protecting PGC-1α from degradation and promoting gluconeogenesis [97].
Figure 2. Protein O-GlcNAcylation attenuates insulin sensitivity.
Insulin binding to its tyrosine kinase receptor activates intracellular substrates (e.g., IRS1/2), initiating PI3K-Akt pathways and thus increased glucose uptake and metabolism in cells. PI3K activation also gives rise to PIP3, recruiting OGT to the plasma membrane, where the O-GlcNAcylation of several proximal elements leads to insulin signaling attenuation. Upon chronic hyperglycemia (insulin resistance), a rich UDP-GlcNAc pool produced from the HBP flux results in abnormally high O-GlcNAcylation of proteins (including some key transcription factors and coactivators), stimulating gluconeogenic/lipogenic gene transcription, which further diminishes insulin sensitivity (light green circle denotes the O-GlcNAc moiety).
ChREBP, a central regulator of lipid synthesis in the liver, undergoes O-GlcNAcylation [98]. Elevated O-GlcNAcylation of ChREBP allows its nuclear localization and enhanced ability to bind the carbohydrate responsive element, initiating the transcription of lipogenic genes [98]. Moreover, the O-GlcNAcylation of a nuclear receptor liver X receptor α (LXR-α) increases in response to glucose, concomitant with elevated promoter activity and expression of SREBP-1c, contributing to de novo lipogenesis [99].
Taken together, hyperglycemia induces elevated O-GlcNAcylation of some key transcription factors and cofactors, promoting gluconeogenesis and lipogenesis. This, in turn, further increases glucose level, forming a vicious cycle that worsens the glucose toxicity effects and thus aggravates the progression of diabetes and diabetic complications.
Protein O-GlcNAcylation in skeletal muscle
Given the high energy demand for muscle contraction, skeletal muscle is actually the largest insulin-sensitive tissue and is the source for more than 80% of insulin-stimulated glucose uptake in humans. Raising O-GlcNAc levels in skeletal muscle has been demonstrated to induce insulin resistance [100]. High glucose and/or insulin enhance(s) O-GlcNAcylation of several proteins including a multifaceted transcription factor specificity protein 1 (Sp1), membrane-associated HSP70 and α-tubulin [101]. Moreover, several key contractile proteins (i.e., actin and myosin heavy and light chains) involved in the skeletal muscle metabolism and in the contractile process are also O-GlcNAcylated [102,103]. O-GlcNAcylation of these proteins decreases calcium sensitivity, probably via a modulation of protein-protein interaction dependent on O-GlcNAc moieties [102]. However, detailed functional assays are still required to clarify their roles in the muscular dysfunction in the development of diabetes.
Protein O-GlcNAcylation in heart/cardiomyocytes/cardiac muscles
Diabetic cardiomyopathy, a well-recognized complication of diabetes, leads to an increased risk of heart failure and death and has garnered great attention in recent years [104–107]. Hyperglycemia treatment of cardiomyocytes increases the O-GlcNAcylation of nuclear proteins and induces prolonged calcium transients, corresponding to delayed myocardial relaxation [70]. However, adenoviral overexpression of O-GlcNAcase in diabetic hearts reduces overall O-GlcNAcylation and improves calcium cycling as well as contractility [81]. Inhibition of OGT reduces O-GlcNAcylation levels on voltage dependent anion channel (VDAC) in mitochondria and sensitizes it to calcium-induced mitochondrial permeability transition pore (mPTP) formation [108,109]. More interestingly, high glucose treatment (30 mM glucose) elevates O-GlcNAcylation of several mitochondrial proteins (including NDUFA9 of complex I, core 1 and core 2 of complex III, and the mitochondrial DNA-encoded subunit I of complex IV), leading to lowered cellular ATP content and decreased activity of complex I, III and IV [110]. These results suggest that elevated mitochondrial protein O-GlcNAcylation contributes to impaired mitochondrial function. Moreover, compared with rats artificially selected for high running capacity (HCR), low running capacity (LCR) ones show increased cardiac O-GlcNAcylation on several mitochondrial proteins (including NDUFA9 of complex I and subunit I of complex IV), implying that O-GlcNAcylation-induced mitochondrial dysfunctions might be closely connected with the pathogenesis of insulin resistance observed in the LCR phenotype [111]. In addition, several proteins in cardiac myofilaments (including actin, myosin heavy chain, myosin light chain, and troponin I) are also modified by O-GlcNAc [112]. Exposure to GlcNAc significantly decreased calcium sensitivity [112], indicating that enhanced O-GlcNAcylation contributes to muscle contractile dysfunction.
Taken together, chronic hyperglycemia increases the overall O-GlcNAcylation level of proteins, contributing to cardiac dysfunctions (e.g., aberrant oxidative stress, calcium handling, mitochondrial function and cardiac contractile efficiency). The crucial roles O-GlcNAc plays in the progression of diabetic cardiomyopathy are largely to be explored.
Protein O-GlcNAcylation in kidney/mesangial cells
Diabetic nephropathy is one of the main causes of morbidity and mortality in diabetic patients. p38 MAPK is intimately linked to the development of diabetic nephropathy. Upon high glucose treatment, O-GlcNAcylation promotes activation of p38 MAPK by suppressing the inhibitory actions of Akt. The activated p38 MAPK initiates the expression of plasminogen activator inhibitor-1 (PAI-1), fibronectin, and TGF-β, all of which are important factors in matrix accumulation in diabetic nephropathy [113]. The O-GlcNAcylation of Sp3 diminishes binding to the glucose-responsive GC-box in the promoter of angiopoietin-2 (Ang-2), leading to increased Ang-2 expression and thus increased expression of intracellular adhesion molecule 1 (IAM-1) and vascular cell adhesion molecule 1 (VCAM-1) [114]. Moreover, high glucose induces O-GlcNAc modification of NF-κB, which disrupts its interaction with IκB and causes nuclear translocation of NF-κB, activating the expression of NF-κB-dependent genes, such as VCAM-1 and TNF-α [115]. As a critical glucose-responsive transcriptional factor in almost all cell types, NF-κB undergoes O-GlcNAcylation in other cells [116,117], which might play multiple roles in response to hyperglycemia. Taken together, elevated protein O-GlcNAcylation by hyperglycemia invokes increased expression of proinflammatory cytokines/proteins, accelerating the progression of diabetic nephropathy.
Protein O-GlcNAcylation in macro-/micro-vascular endothelial cells
Because of their location at the interface between the circulating fluid in the lumen and the surrounding tissue, endothelial cells are highly active and closely involved in numerous physiological processes, therefore, providing another good model to investigate the role of hyperglycemia in vascular diseases. Increased glycolysis induced by hyperglycemia results in the overproduction of mitochondrial superoxide, which in turn inhibits the activity of GAPDH activity, a key enzyme in glycolysis [118]. The accumulation of glycolytic intermediates triggers the HBP, leading to increased O-GlcNAcylation of Sp1, Sp1 transactivation and Sp1-dependent gene expression (e.g., PAI-1 and TGF-β). Interestingly, hyperglycemia also increases the O-GlcNAcylation of endothelial nitric oxide synthase (eNOS) [119,120], preventing phosphorylation at its primary positive regulatory site and resulting in reduced production of nitric oxide (NO) and thus diabetes-associated erectile dysfunction [120]. Notably, increased expression of PAI-1 (an inhibitor to fibrinolysis) and TGF-β (a profibrotic factor) resulted from Sp1 O-GlcNAcylation contributes to the development of diabetic atherosclerosis. Moreover, since NO production plays pivotal roles including vasodilation and inhibition of platelet aggregation, the reduced level of NO due to the O-GlcNAcylation of eNOS promotes endothelial cell dysfunction and thus the later-stage atherosclerosis-related complications in diabetes.
Protein O-GlcNAcylation in blood cells
Body fluids (e.g., blood, urea, saliva), which are often excreted or secreted from the body, directly reflect the physiological/pathological status, representing an appealing target for prediagnosis/diagnosis (which will be discussed later). Amongst the body fluids, blood cells are of particular interest due to 1) they are directly exposed to blood glucose; 2) the degree of protein O-GlcNAcylation is a sensitive indicator of the level of glucose. By proteomics screening, dozens of erythrocytes proteins are differentially O-GlcNAcylated between control and diabetes [121]. Therefore, it is possible that the changes of O-GlcNAcylation on proteins in erythrocytes could serve as a potential ‘marker’ for the early detection of diabetic progression.
O-GlcNAcylation interaction with other modifications
It should be pointed out that although many proteins (especially some transcription factors and co-activators, e.g., FoxO1, PGC-1α, Sp1 and NF-κB) reside preferentially in certain tissues or cells, they may also be expressed in other tissues and O-GlcNAcylated differently, exerting distinct roles in a given biological context. Considering the hydroxyl group on serine/threonine can undergo phosphorylation by protein kinases as well, an extensive interplay between O-GlcNAcylation and serine/threonine phosphorylation occurs on many proteins (see recent reviews [122–124]). Intereactions between O-GlcNAcylation and other post-translational modifications (e.g., tyrosine phosphorylation [125], methylation [126], acetylation [117,126,127], ubiquitination [97,128] and methylglyoxal modification [114]) have also been revealed recently. Undoubtedly, it is just these diverse modifications and their intimate crosstalks that contributes together to the regulation of gene expression and/or protein functions, leading to intricate molecular networks evolving in the pathology of diabetes and diabetic complications.
Proteomic approaches to profile O-GlcNAcylated proteins
Profiling all proteins in a given organism, cell, or organelle provides a whole picture of biological events, advancing our understanding of physiological and pathological processes in a time and space manner. Although great progress has been made in O-GlcNAc research, as aforementioned, identification and quantification of O-GlcNAcylated proteins are still required to facilitate site-specific functional assays.
Generally, the detection of O-GlcNAcylation can be performed in three major ways: tritiated UDP-galactose labeling followed by autoradiography [12], immunoblots with pan specific antibodies (e.g., CTD 110.6 [129] and RL2 [130]) and mass spectrometry (MS). The first two traditional approaches are still commonly used to probe O-GlcNAcylation in proteins. Notably, the newly emerging bioinformatic resources (e.g., dbOGAP [131]) have provided a helpful tool for the prediction of potential O-GlcNAcylation sites, which may be used as a reference when accurate site mapping by MS is not available. As a powerful approach, MS can be applied to the unambiguous assignment of the O-GlcNAc modification sites as well as their stoichiometry in a high-throughput manner.
The MS-based detection of O-GlcNAc modification has been slow, largely because O-GlcNAc is very labile in the gas phase. The glycosidic linkage between the peptide chain and the O-GlcNAc moiety is readily cleaved in the traditional collision induced dissociation tandem mass spectrometry (CID-MS/MS), losing the modification site information. The newly developed ion-trap electron transfer dissociation tandem mass spectrometry (ETD-MS/MS), which can well preserve O-GlcNAc on peptide chains, has advanced the detection of O-GlcNAc tremendously [132]. Undoubtedly, ETD-MS/MS is becoming a popular approach for direct O-GlcNAc site mapping. On the other hand, to make use of the currently prevalent CID mass spectrometers in most laboratories, one way is to convert the CID-labile O-GlcNAc moiety to CID-stable groups. Beta-elimination/Michael Addition with dithiothreitol (BEMAD) is such an approach, in which the O-GlcNAc sugar undergoes a β-elimination reaction and dithiothreitol is reacted with the resulting carbonyl in a Michael addition reaction, producing a sulphide adduct. Since the resulting adduct is stable enough upon CID, the original modification sites (replaced with dithiothreitol) can be easily determined [133–135]. Notably, besides O-GlcNAc, other groups (e.g., phosphate) may also undergo BEMAD treatment under certain conditions. Therefore, optimization should be performed and appropriate controls included to avoid possible false positive identifications.
Some success has been achieved by direct detection with ETD-MS/MS [95,96] or even CID-MS/MS [136], however, selective O-GlcNAc enrichment is still required to render the detection of low abundant modified peptides possible especially when complex samples are analyzed. To this end, according to the properties of O-GlcNAc proteins/peptides, several methods have been developed to enrich O-GlcNAc followed by MS detection.
Affinity based O-GlcNAc enrichment
Although antibody-based enrichment works well for other modified proteins/peptides (e.g., phosphorylated and acetylated peptides), immunoaffinity purification of O-GlcNAc proteins/peptides with pan-specific antibodies (e.g., CTD 110.6 and RL2) has been tentatively applied due to the low binding avidity. In a recent study, three newly generated monoclonal antibodies [137] were used to enrich O-GlcNAc proteins in HEK293 cell lysates, with the digests subjected to a combination of high-energy C-trap dissociation (HCD) and ETD fragmentation, leading to the assignment of 83 O-GlcNAc sites [138]. The cocktail usage of multiple antibodies, and more appealingly, the discovery of more specific antibodies toward O-GlcNAc, should enable antibody-based O-GlcNAc enrichment more promising.
Lectin-carbohydrate interactions represent another approach for the enrichment of O-GlcNAc-containing peptides. Wheat germ agglutinin (WGA) is an old [139] but still useful tool to enrich O-GlcNAc proteins/peptides [140,141]. In a very recent report, an amazingly high number of 1750 O-GlcNAc sites were identified from mouse brain synaptosomes by a combination of WGA enrichment, offline separation via high pH reversed phase high-performance liquid chromatography (RPLC), and RPLC-ETD-MS/MS [141]. Lectins with higher selectivity and specificity are still worthy to be exploited to improve the O-GlcNAc enrichment efficiency.
Chemical/chemoenzymatic derivatization based O-GlcNAc enrichment
Chemical/chemoenzymatic derivatization is another tool for the enrichment of O-GlcNAc proteins/peptides. This technique usually consists of several steps: ‘activate’ the O-GlcNAc moiety by transferring UDP-galactose analogs (e.g., azido/ketone-containing ones) with a mutant galactosyltransferase (GalT1 labeling), use biotin-bearing regents to react with the labeled peptides (e.g., via click chemistry/hydrazine chemistry), capture the tagged O-GlcNAc proteins/peptides with avidin beads and release the peptides (enzymatic digestion is required if proteins are captured) and inject into MS. Due to the presence of diagnostic ions provided by the unique tag, O-GlcNAc peptides and their exact modification sites can be assigned.
In one study, O-GlcNAc proteins were labeled with ketone-containing UDP-galactose analog followed by hydrazine chemistry, digestion, and the isotopic dimethyl labeling [142]. Avidin beads were then used to capture the biotin–tagged O-GlcNAc peptides, with the released peptides subjected to ETD-MS/MS. About ten sites in several O-GlcNAc peptides in cortical neurons were found to be significantly increased in O-GlcNAcylation upon PUGNAc treatment [142]. Since the biotinavidin interaction is pretty stable, the elution efficiency of peptides from avidin beads should be addressed for its routine use as a robust method of O-GlcNAc site mapping.
In several other studies [143–145], a different strategy was adopted, in which an azide-substituted UDP-galactose (UDP-GalNAz) was used by GalT1 to transfer GalNAz onto O-GlcNAc peptides, the resulting peptides were reacted with a multi-functional reagent (which bears an alkyne terminus, a photo-cleavable linker, and a biotin handle) via click chemistry and then captured onto an avidin column, tagged peptides were released by UV cleavage and detected with ETD-MS/MS. Totally 458 O-GlcNAc sites in 195 proteins were identified from mouse cerebrocortical brain tissue [144]. By coupling this enrichment method with stable isotope labeling by amino acids in cell culture (SILAC) for O-GlcNAc protein identification and quantification [145], previously unknown O-GlcNAc sites on proteins that function in spindle assembly and cytokinesis in HeLa cells were assigned. Moreover, by combining GalT1 labeling, BEMAD, isobaric tag for relative and absolute quantitation technique (iTRAQ), and CID-MS/MS, 25 O-GlcNAcylated proteins corresponding to 35 O-GlcNAc sites were identified from erythrocytes [121]. The comparison of occupancy levels (relative occupancy ratio [ROR]) between normal and diabetic erythrocytes reveals that O-GlcNAcylation is differentially regulated at individual sites on proteins in response to glycemic status.
Compared with in vitro labeling methods mentioned above, metabolic labeling presents another avenue to identify O-GlcNAc sites. This strategy involves tagging O-GlcNAc-modified proteins through metabolic labeling of living cells with azido-containing N-acetylglucosamine analog (GlcNAz), since the azido sugar can be processed by enzymes in the hexosamine salvage pathways to form UDP-GlcNAz which can then be incorporated into proteins by OGT [146–149]. The bio-orthogonal azide moiety can then be derivatized with a FLAG peptide or a biotin tag using the Staudinger ligation or click chemistry. With a combination of metabolic labeling, click chemistry, BEMAD and CID-MS/MS, 185 O-GlcNAc modification sites on 80 proteins were identified from HEK293 cells [149]. To increase the labeling efficiency of low-abundant endogenous O-GlcNAc proteins with this Trojan horse approach, a higher GlcNAz concentration in the growth medium seems to be helpful [148].
Taken together, great achievements have been made for O-GlcNAc protein profiling (Table 2), the O-GlcNAcome is still largely underrepresented in comparison to proteomes of other post-translational modifications [150,151]. The major bottleneck is the lack of highly efficient enrichment tools. Therefore, one hotspot is to develop more straightforward, simplified, sensitive and robust enrichment methods. With the combination of the most efficient enrichment and quantification technologies as well as new mass spectrometry approaches (e.g., ETD-MS/MS), large-scale O-GlcNAcomic profiling will become routine, which would certainly accelerate the whole O-GlcNAc research process and facilitate the elucidation of the roles of protein O-GlcNAcylation in the pathological progression of diabetes and diabetic complication.
Table 2.
Representative methods for O-GlcNAcomic protein profiling.
| Enrichment method | Quantification | MS | Sample information and modification sites | Ref. | |
|---|---|---|---|---|---|
| Affinity | Antibody-based | HCD/ETD-MS/MS | 83 sites from HEK293 cell lysate | [138] | |
| Lectin-based (WGA) | ETD-MS/MS | 1750 sites from mouse brain synaptosomes | [141] | ||
|
| |||||
| Chemical/ chemoenzymatic derivatization | Chemoenzymatic labeling + hydrazine chemistry | Isotopic dimethyl labeling | ETD-MS/MS | Increased O-GlcNAcylation of ~10 sites in several peptides in cortical neurons induced by PUGNAc | [142] |
| Chemoenzymatic labeling + click chemistry | HCD-CID/ETD-MS/MS | 458 O-GlcNAc sites from mouse cerebrocortical brain | [144] | ||
| Chemoenzymatic labeling + click chemistry | SILAC | ETD-MS/MS | 141 sites from HeLa spindle and midbody proteins | [145] | |
| Chemozymatic labeling + click chemistry + BEMAD | iTRAQ | CID-MS/MS | 35 sites from erythrocyte | [121] | |
| Metabolic labeling + click chemistry + BEMAD | CID-MS/MS | 185 sites from HEK293 cell lysate | [149] | ||
BEMAD: Beta elimination/Michael addition with dithiothreitol; CID: Collision induced dissociation; ETD: Electron transfer dissociation; iTRAQ: Isobaric tag for relative and absolute quantitation; MS: Mass spectrometry; O-GlcNAc: O-linked β-D-N-acetylglucosamine; SILAC: Stable isotope labeling of amino acids in cell culture; WGA: Wheat germ agglutinin.
O-GlcNAc as a biomarker for diagnosis of prediabetes/diabetes
Despite the availability of several diagnostic tests, diabetes remains under diagnosed [1], providing impetus for the development of novel assays for its earlier and more efficient detection, which would permit the changes of lifestyle to delay and/or prevent the onset of diabetes and diabetic complications.
The O-GlcNAcomic profiling method allows for the quantification of site-specific O-GlcNAc ROR of erythrocytes proteins between normal and diabetic samples [121]. Among the 35 O-GlcNAc sites uncovered, the O-GlcNAcylation of catalase at serine-114 shows a significantly high ROR of >3 when comparing diabetic to normal samples. Similar results have also been achieved using an O-GlcNAc site-specific antibody toward serine-114 (Park K and Hart GW, unpublished data). Coincidently, biochemical analysis of erythrocyte proteins (from patients diagnosed as normal, prediabetic or diabetic) reveals that the levels of both protein O-GlcNAcylation and O-GlcNAcase are elevated in diabetic compared to that of the control group [152]. And more importantly, protein O-GlcNAcylation and O-GlcNAcase levels are significantly elevated in prediabetic samples as well. This finding is extremely important since assessment of hemoglobin A1c (HbA1c), a universal marker used for the diagnosis of diabetic status, fails to distinguish between normal and prediabetic samples. Collectively, these results demonstrate that the assessment of the O-GlcNAcase levels in erythrocytes and/or the O-GlcNAc occupancy level of catalase serine-114 could be developed as a diagnostic indicator for monitoring diabetic progression.
Similarly, another recent study shows that prediabetic and diabetic individuals display increased protein O-GlcNAcylation in leukocytes (particularly granulocytes) and differential O-GlcNAcase expression in leukocytes is found in diabetic subjects [153]. Taken together, these results highlight the emerging paradigm that changes in protein O-GlcNAcylation may serve as a potential tool for the early screening of prediabetes and diabetes.
Expert commentary & five-year view
Substantial evidence suggests that hyperglycemia upregulates O-GlcNAcylation in various proteins through the HBP, leading to insulin resistance and glucose toxicity in diabetes and diabetic complications. Promising efforts have been made in our understanding of the underlying molecular mechanisms, however, the information about the important roles of protein O-GlcNAcylation is still somewhat limited and scattered. Therefore, how to explore new underlying mechanisms of glucose toxicity and put the pieces together might be a daunting task that must be fulfilled in the near future. Of note is that, given its role as a nutrient sensor and signal processing integrator, more attention should be paid to the protein O-GlcNAcylation in mitochondria. Last but not least, more sensitive and reliable tools for O-GlcNAc site mapping and quantification should be developed, which would allow site-specific functional assays of proteins as well as a proteomic view of the complex molecular events, while the widespread availability of site-specific O-GlcNAc antibodies would have a huge impact upon many studies. We believe that the elucidation of the roles that O-GlcNAc plays will not only provide us deeper insights to our understanding of molecular mechanisms leading to pathological development, but also will offer novel ways to diagnose and, even more importantly, develop effective therapies to diabetes and diabetic complications.
Key issues.
Numerous evidence suggests that protein O-GlcNAcylation, through the hexosamine biosynthesis pathway, plays key roles in the pathological progression of diabetes and diabetic complications.
Great progress has been made to elucidate the molecular mechanisms linking insulin resistance, chronic hyperglycemia and protein O-GlcNAcylation.
Proteomics approaches have been applied to large-scale identification and quantification of O-GlcNAcylated proteins.
Status of certain proteins involved in O-GlcNAcylation may be exploited as a tool for screening prediabetes and diabetes.
The availability of important tools (e.g., specific inhibitors to OGT/ O-GlcNAcase, more facile and robust O-GlcNAc site mapping methods, and O-GlcNAc site-specific antibodies) are critically needed to further advance our understanding of the roles of protein O-GlcNAcylation in diseases including diabetes and diabetic complications.
Acknowledgments
The authors especially thank Kaoru Sakabe and Ronald Copeland for critical reading and suggestions of this manuscript and the Hart laboratory.
Footnotes
Financial & competing interests disclosure
Original research in the author’s laboratory is supported by NIH R01CA42486, R01DK61671 and P01HL107153. Dr. Hart receives a share of royalty received by the university on sales of the CTD 110.6 antibody, which are managed by JHU. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.IDF Diabetes Atlas. 5. International Diabetes Federation; Brussels, Belgium: 2012. [Google Scholar]
- 2.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Daneman D. Type 1 diabetes. Lancet. 2006;367:847–858. doi: 10.1016/S0140-6736(06)68341-4. [DOI] [PubMed] [Google Scholar]
- 4.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365:1333–1346. doi: 10.1016/S0140-6736(05)61032-X. [DOI] [PubMed] [Google Scholar]
- 5.DeFronzo RA. The triumvirate: (-cell muscle, liver: a collusion) responsible for NIDDM. Diabetes. 1988;37:677–687. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- 6.Rossetti L, Giaccari A, DeFronzo RA. Glucose toxicity. Diabetes Care. 1990;13:610–630. doi: 10.2337/diacare.13.6.610. [DOI] [PubMed] [Google Scholar]
- 7.Robertson RP, Harmon J, Tran PO, et al. Glucose toxicity in beta-cells: Type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes. 2003;52:581–587. doi: 10.2337/diabetes.52.3.581. [DOI] [PubMed] [Google Scholar]
- 8.Rossetti L, Smith D, Shulman GI, et al. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987;79:1510–1515. doi: 10.1172/JCI112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garvey WT, Olefsky JM, Matthaei S, et al. Glucose and insulin co-regulate the glucose transport system in primary cultured adipocytes. J Biol Chem. 1987;262:189–197. [PubMed] [Google Scholar]
- 10.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 11.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 12•.Torres CR, Hart GW. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem. 1984;259:3308–3317. The first paper about protein O-GlcNAcylation. [PubMed] [Google Scholar]
- 13.Holt GD, Hart GW. The subcellular distribution of terminal N-acetylglucosamine moieties. Localization of a novel protein-saccharide linkage, O-linked GlcNAc. J Biol Chem. 1986;261:8049–8057. [PubMed] [Google Scholar]
- 14•.Hart GW, Slawson C, Ramirez Correa GA, et al. Cross talk between GlcNAcylation and phosphorylation: Roles in signaling, transcription, and chronic disease. Annu Rev Biochem. 2011;80:825–858. doi: 10.1146/annurev-biochem-060608-102511. Together with [15], it provides a very comprehensive review on protein O-GlcNAcylation and its important physiological and pathological roles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15•.Bond MB, Hanover JA. O-GlcNAc Cycling: A link between metabolism and chronic disease. Annu Rev Nutr. 2013;33:13.1–13.25. doi: 10.1146/annurev-nutr-071812-161240. Together with [14], it provides a very comprehensive review on protein O-GlcNAcylation and its important physiological and pathological roles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16•.Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem. 1991;266:4706–4712. This paper demonstrates the link between the hexosamine biosynthesis pathway and insulin resistance. [PubMed] [Google Scholar]
- 17.Haltiwanger RS, Holt GD, Hart GW. Enzymatic addition of O-GlcNAc to nuclear and cytoplasmic proteins. Identification of a uridine diphospho-N -acetylglucosamine: peptide beta-N-acetyl-glucosaminyltransferase. J Biol Chem. 1990;265:2563–2568. [PubMed] [Google Scholar]
- 18.Buse MG. Hexosamines, insulin resistance, and the complications of diabetes: current status. Am J Physiol Endocrinol Metab. 2006;290:E1–E8. doi: 10.1152/ajpendo.00329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dias WB, Hart GW. O-GlcNAc modification in diabetes and Alzheimer’s disease. Mol Biosyst. 2007;3:766–772. doi: 10.1039/b704905f. [DOI] [PubMed] [Google Scholar]
- 20.Copeland RJ, Bullen JW, Hart GW. Cross-talk between GlcNAcylation and phosphorylation: roles in insulin resistance and glucose toxicity. Am J Physiol Endocrinol Metab. 2008;295:E17–E28. doi: 10.1152/ajpendo.90281.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slawson C, Copeland RJ, Hart GW. O-GlcNAc signaling: a metabolic link between diabetes and cancer? Trends Biochem Sci. 2010;35:547–555. doi: 10.1016/j.tibs.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konrad RJ, Zhang FX, Hale JE, et al. Alloxan is an inhibitor of the enzyme O-linked N-acetylglucosamine transferase. Biochem Biophys Res Commun. 2002;293:207–212. doi: 10.1016/S0006-291X(02)00200-0. [DOI] [PubMed] [Google Scholar]
- 23.Kang E-S, Han D, Park J, et al. O-GlcNAc modulation at Akt1 Ser473 correlates with apoptosis of murine pancreatic beta cells. Exp Cell Res. 2008;314:2238–2248. doi: 10.1016/j.yexcr.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Dorfmueller HC, Borodkin VS, Blair DE, et al. Substrate and product analogues as human O-GlcNAc transferase inhibitors. Amino Acids. 2011;40:781–792. doi: 10.1007/s00726-010-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gloster TM, Zandberg WF, Heinonen JE, et al. Hijacking a biosynthetic pathway yields a glycosyltransferase inhibitor within cells. Nat Chem Biol. 2011;7:174–181. doi: 10.1038/nchembio.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haltiwanger RS, Grove K, Philipsberg GA. Modulation of O-linked N-acetylglucosamine levels on nuclear and cytoplasmic proteins in vivo using the peptide O-GlcNAc-beta-N-acetylglucosaminidase inhibitor O-(2-acetamido-2-deoxy-D-gluco-pyranosylidene)amino-N-phenylcarbamate. J Biol Chem. 1998;273:3611–3617. doi: 10.1074/jbc.273.6.3611. [DOI] [PubMed] [Google Scholar]
- 27.Macauley MS, Bubb AK, Martinez-Fleites C, et al. Elevation of global O-GlcNAc levels in 3T3-L1 adipocytes by selective inhibition of O-GlcNAcase does not induce insulin resistance. J Biol Chem. 2008;283:34687–34695. doi: 10.1074/jbc.M804525200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuzwa SA, Macauley MS, Heinonen JE, et al. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat Chem Biol. 2008;4:483–490. doi: 10.1038/nchembio.96. [DOI] [PubMed] [Google Scholar]
- 29.Andres-Bergos J, Tardio L, Larranaga-Vera A, et al. The increase in O-linked N-acetyl-glucosamine protein modification stimulates chondrogenic differentiation both in vitro and in vivo. J Biol Chem. 2012;287:33615–33628. doi: 10.1074/jbc.M112.354241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macauley MS, He Y, Gloster TM, et al. Inhibition of O-GlcNAcase using a potent and cell-permeable inhibitor does not induce insulin resistance in 3T3-L1 adipocytes. Chem Biol. 2010;17:937–948. doi: 10.1016/j.chembiol.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macauley MS, Shan X, Yuzwa SA, et al. Elevation of global O-GlcNAc in rodents using a selective O-GlcNAcase inhibitor does not cause insulin resistance or perturb glucohomeostasis. Chem Biol. 2010;17:949–958. doi: 10.1016/j.chembiol.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dorfmueller HC, Borodkin VS, Schimpl M, et al. Cell-penetrant, nanomolar O-GlcNAcase inhibitors selective against lysosomal hexosaminidases. Chem Biol. 2010;17:1250–1255. doi: 10.1016/j.chembiol.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reed MJ, Meszaros K, Entes LJ, et al. A new rat model of Type 2 diabetes: The fat-fed, streptozotocin-treated rat. Metabolism. 2000;49:1390–1394. doi: 10.1053/meta.2000.17721. [DOI] [PubMed] [Google Scholar]
- 34.Roos MD, Xie W, Su K, et al. Streptozotocin, an analog of N-acetylglucosamine, blocks the removal of O-GlcNAc from intracellular proteins. Proc Assoc Am Physicians. 1998;110:422–432. [PubMed] [Google Scholar]
- 35.O’Donnell N, Zachara NE, Hart GW, et al. Ogt-dependent X-chromosome-linked protein glycosylation is a requisite modification in somatic cell function and embryo viability. Mol Cell Biol. 2004;24:1680–1690. doi: 10.1128/MCB.24.4.1680-1690.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watson LJ, Facundo HT, Ngoh GA, et al. O-linked β-N-acetyl-glucosamine transferase is indispensable in the failing heart. Proc Natl Acad Sci USA. 2010;107:17797–17802. doi: 10.1073/pnas.1001907107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanover JA, Forsythe ME, Hennessey PT, et al. A Caenorhabditis elegans model of insulin resistance: altered macronutrient storage and dauer formation in an OGT-1 knockout. Proc Natl Acad Sci USA. 2005;102:11266–11271. doi: 10.1073/pnas.0408771102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mondoux MA, Love DC, Ghosh SK, et al. O-Linked-N-Acetylglucosamine cycling and insulin signaling are required for the glucose stress response in Caenorhabditis elegans. Genetics. 2011;188:369–382. doi: 10.1534/genetics.111.126490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sinclair DAR, Syrzycka M, Macauley MS, et al. Drosophila O-GlcNAc transferase (OGT) is encoded by the Polycomb group (PcG) gene, super sex combs (sxc) Proc Natl Acad Sci USA. 2009;106:13427–13432. doi: 10.1073/pnas.0904638106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sekine O, Love DC, Rubenstein DS, et al. Blocking O-linked GlcNAc cycling in Drosophila insulin-producing cells perturbs glucose-insulin homeostasis. J Biol Chem. 2010;285:38684–38691. doi: 10.1074/jbc.M110.155192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oki T, Yamazaki K, Kuromitsu J, et al. cDNA cloning and mapping of a novel subtype of glutamine:fructose-6-phosphate amido-transferase (GFAT2) in human and mouse. Genomics. 1999;57:227–234. doi: 10.1006/geno.1999.5785. [DOI] [PubMed] [Google Scholar]
- 42.DeHaven JE, Robinson KA, Nelson BA, et al. A novel variant of glutamine: fructose-6-phosphate amidotransferase-1 (GFAT1) mRNA is selectively expressed in striated muscle. Diabetes. 2001;50:2419–2424. doi: 10.2337/diabetes.50.11.2419. [DOI] [PubMed] [Google Scholar]
- 43.Zhou J, Huynh QK, Hoffman RT, et al. Regulation of glutamine:fructose-6-phosphate amidotransferase by cAMP-dependent protein kinase. Diabetes. 1998;47:1836–1840. doi: 10.2337/diabetes.47.12.1836. [DOI] [PubMed] [Google Scholar]
- 44.Chang Q, Su K, Baker JR, et al. Phosphorylation of human glutamine:fructose-6-phosphate amidotransferase by cAMP-dependent protein kinase at serine 205 blocks the enzyme activity. J Biol Chem. 2000;275:21981–21987. doi: 10.1074/jbc.M001049200. [DOI] [PubMed] [Google Scholar]
- 45.Li Y, Roux C, Lazereg S, et al. Identification of a novel serine phosphorylation site in human glutamine:fructose-6-phosphate amidotransferase isoform 1. Biochemistry. 2007;46:13163–13169. doi: 10.1021/bi700694c. [DOI] [PubMed] [Google Scholar]
- 46.Broschat KO, Gorka C, Page JD, et al. Kinetic characterization of human glutamine-fructose-6-phosphate amidotransferase I: potent feedback inhibition by glucosamine 6-phosphate. J Biol Chem. 2002;277:14764–14770. doi: 10.1074/jbc.M201056200. [DOI] [PubMed] [Google Scholar]
- 47.Kornfeld R. Studies on L-glutamine D-fructose 6-phosphate amidotransferase. I Feedback inhibition by uridine diphosphate-N-acetylglucosamine. J Biol Chem. 1967;242:3135–3141. [PubMed] [Google Scholar]
- 48.Henry RR, Wallace P, Olefsky JM. Effects of weight loss on mechanisms of hyper-glycemia in obese non-insulin dependent diabetes mellitus. Diabetes. 1986;35:990–998. doi: 10.2337/diab.35.9.990. [DOI] [PubMed] [Google Scholar]
- 49.Yki-Jarvinen H, Helve E, Koivisto VA. Hyperglycemia decreases glucose uptake in Type 1 diabetes. Diabetes. 1987;36:892–896. doi: 10.2337/diab.36.8.892. [DOI] [PubMed] [Google Scholar]
- 50.Kahn B, Schulman GI, DeFronzo RA, et al. Normalization of blood glucose in diabetic rats with phlorizin treatment reverses insulin-resistant glucose transport in adipose cells without restoring glucose transporter gene expression. J Clin Invest. 1991;87:561–570. doi: 10.1172/JCI115031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McClain DA, Lubas WA, Cooksey RC, et al. Altered glycan-dependent signaling induces insulin resistance and hyper-leptinemia. Proc Natl Acad Sci USA. 2002;99:10695–10699. doi: 10.1073/pnas.152346899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herbert L, Daniels M, Zhou J, et al. Over-expression of glutamine:fructose-6-phosphate amidotransferase in transgenic mice leads to insulin resistance. J Clin Invest. 1996;98:930–936. doi: 10.1172/JCI118876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yki-Jarvinen H, Virkamaki A, Daniels MD, et al. Insulin and glucosamine infusions increase O-linked N-acetyl-glucosamine in skeletal muscle proteins in vivo. Metabolism. 1998;47:449–455. doi: 10.1016/s0026-0495(98)90058-0. [DOI] [PubMed] [Google Scholar]
- 54.Manning G, Whyte DB, Martinez R, et al. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 55.Muller U, Steinberger D, Nemeth AH. Clinical and molecular genetics of primary dystonias. Neurogenetics. 1998;1:165–177. doi: 10.1007/s100480050025. [DOI] [PubMed] [Google Scholar]
- 56.Lubas WA, Frank DW, Krause M, et al. O-linked GlcNAc transferase is a conserved nucleocytoplasmic protein containing tetratricopeptide repeats. J Biol Chem. 1997;272:9316–9324. doi: 10.1074/jbc.272.14.9316. [DOI] [PubMed] [Google Scholar]
- 57.Jacobsen SE, Binkowski KA, Olszewski NE. SPINDLY, a tetratricopeptide repeat protein involved in gibberellin signal transduction in Arabidopsis. Proc Natl Acad Sci USA. 1996;93:9292–9296. doi: 10.1073/pnas.93.17.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hanover JA, Yu S, Lubas WB, et al. Mitochondrial and nucleocytoplasmic isoforms of O-linked GlcNAc transferase encoded by a single mammalian gene. Arch Biochem Biophys. 2003;409:287–297. doi: 10.1016/s0003-9861(02)00578-7. [DOI] [PubMed] [Google Scholar]
- 59.Lazarus MB, Nam Yunsun, Jiang J, et al. Structure of human O-GlcNAc transferase and its complex with a peptide substrate. Nature. 2011;469:564–568. doi: 10.1038/nature09638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jinek M, Rehwinkel J, Lazarus BD, et al. The superhelical TPR-repeat domain of O-linked GlcNAc transferase exhibits structural similarities to importin alpha. Nat Struct Mol Biol. 2004;11:1001–1007. doi: 10.1038/nsmb833. [DOI] [PubMed] [Google Scholar]
- 61.Kreppel LK, Hart GW. Regulation of a cytosolic and nuclear O-GlcNAc transferase. Role of the tetratricopeptide repeats. J Biol Chem. 1999;274:32015–32022. doi: 10.1074/jbc.274.45.32015. [DOI] [PubMed] [Google Scholar]
- 62.Haltiwanger RS, Blomberg MA, Hart GW. Glycosylation of nuclear and cytoplasmic proteins. Purification and characterization of a uridine diphospho-N-acetylglucosamine:polypeptide beta-N-acetylglucosaminyltransferase. J Biol Chem. 1992;267:9005–9013. [PubMed] [Google Scholar]
- 63.Lazarus MB, Jiang J, Gloster TM, et al. Structural snapshots of the reaction coordinate for O-GlcNAc transferase. Nat Chem Biol. 2012;8:966–968. doi: 10.1038/nchembio.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schimpl M, Zheng X, Borodkin VS, et al. O-GlcNAc transferase invokes nucleotide sugar pyrophosphate participation in catalysis. Nat Chem Biol. 2012;8:969–974. doi: 10.1038/nchembio.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hart GW, Akimoto Y. The O-GlcNAc modification. In: Varki ACR, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. 2. Cold Spring Harbor Laboratory Press; Cold Spring, Harbor NY, USA: 2009. [Google Scholar]
- 66.Kreppel LK, Blomberg MA, Hart GW. Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J Biol Chem. 1997;272:9308–9315. doi: 10.1074/jbc.272.14.9308. [DOI] [PubMed] [Google Scholar]
- 67••.Whelan SA, Lane DM, Hart GW. Regulation of the O-GlcNAc transferase by insulin signaling. J Biol Chem. 2008;283:21411–21417. doi: 10.1074/jbc.M800677200. Together with [69], it shows that the O-GlcNAc transferase translocation and thus increased O-GlcNAcylation of proteins in insulin signaling pathway compromises the insulin sensitivity, providing a molecular link between insulin signaling and protein O-GlcNAcylation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Akimoto Y, Hart GW, Wells L, et al. Elevation of the post-translational modification of proteins by O-linked N-acetylglucosamine leads to deterioration of the glucose-stimulated insulin secretion in the pancreas of diabetic Goto-Kakizaki rats. Glycobiology. 2007;17:127–140. doi: 10.1093/glycob/cwl067. [DOI] [PubMed] [Google Scholar]
- 69••.Yang X, Ongusaha P, Miles P, et al. Phosphoinositide signaling links O-GlcNAc transferase to insulin resistance. Nature. 2008;451:964–970. doi: 10.1038/nature06668. Together with [67], it shows that the O-GlcNAc transferase translocation and thus increased O-GlcNAcylation of proteins in insulin signaling pathway compromises the insulin sensitivity, providing a molecular link between insulin signaling and protein O-GlcNAcylation. [DOI] [PubMed] [Google Scholar]
- 70.Clark RJ, McDonough PM, Swanson E, et al. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear GlcNAcylation. J Biol Chem. 2003;278:44230–44237. doi: 10.1074/jbc.M303810200. [DOI] [PubMed] [Google Scholar]
- 71.Lenzen S, Munday R. Thiol-group reactivity, hydrophilicity and stability of alloxan, its reduction products and its N-methyl derivatives and a comparison with ninhydrin. Biochem Pharmacol. 1991;42:1385–1391. doi: 10.1016/0006-2952(91)90449-f. [DOI] [PubMed] [Google Scholar]
- 72.Robinson KA, Ball LE, Buse MG. Reduction of O-GlcNAc protein modification does not prevent insulin resistance in 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab. 2007;292:E884–E890. doi: 10.1152/ajpendo.00569.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shafi R, Lyer SP, Ellies LG, et al. The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc Natl Acad Sci USA. 2000;97:5735–5739. doi: 10.1073/pnas.100471497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kazemi Z, Chang H, Haserodt S, et al. O-Linked Beta-N-acetylglucosamine (O-GlcNAc) regulates stress-induced heat shock protein expression in a GSK-3β-dependent manner. J Biol Chem. 2010;285:39096–39107. doi: 10.1074/jbc.M110.131102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de la Monte SM, Tong M, Lester-Coll N, et al. Therapeutic rescue of neurodegeneration in experimental Type 3 diabetes: relevance to Alzheimer’s disease. J Alzheimers Dis. 2006;10:89–109. doi: 10.3233/jad-2006-10113. [DOI] [PubMed] [Google Scholar]
- 76.Butkinaree C, Cheung WD, Park S, et al. Characterization of beta-N-acetylglucosaminidase cleavage by caspase-3 during apoptosis. J Biol Chem. 2008;283:23557–23566. doi: 10.1074/jbc.M804116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Comtesse N, Maldener E, Meese E. Identification of a nuclear variant of MGEA5, a cytoplasmic hyaluronidase and a beta-N-acetylglucosaminidase. Biochem Biophys Res Commun. 2001;283:634–640. doi: 10.1006/bbrc.2001.4815. [DOI] [PubMed] [Google Scholar]
- 78.Soesant Y, Luo B, Parker G, et al. Pleiotropic and age-dependent effects of decreased protein modification by O-Linked N-acetylglucosamine on pancreatic beta-cell function and vascularization. J Biol Chem. 2011;286:26118–26126. doi: 10.1074/jbc.M111.249508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79•.Dentin R, Hedrick S, Xie J, et al. Hepatic glucose sensing via the CREB coactivator CRTC2. Science. 2008;319:1402–1405. doi: 10.1126/science.1151363. Together with [95], it serves as an excellent example to show how transcription factors respond to glucose stimulation. [DOI] [PubMed] [Google Scholar]
- 80.Soesanto YA, Luo B, Jones D, et al. Regulation of Akt signaling by O-GlcNAc in euglycemia. Am J Physiol Endocrinol Metab. 2008;295:E974–E980. doi: 10.1152/ajpendo.90366.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hu Y, Belke D, Suarez J, et al. Adenovirus-mediated over-expression of O-GlcNAcase improves contractile function in the diabetic heart. Circ Res. 2005;96:1006–1013. doi: 10.1161/01.RES.0000165478.06813.58. [DOI] [PubMed] [Google Scholar]
- 82.Forsythe ME, Love DC, Lazarus BD, et al. Caenorhabditis elegans ortholog of a diabetes susceptibility locus: oga-1 (O-GlcNAcase) knockout impacts O-GlcNAc cycling, metabolism, and dauer. Proc Natl Acad Sci USA. 2006;103:11952–11957. doi: 10.1073/pnas.0601931103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vosseller K, Wells L, Lane MD, et al. Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proc Natl Acad Sci USA. 2002;99:5313–5318. doi: 10.1073/pnas.072072399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arias EB, Kim J, Cartee GD. Prolonged incubation in PUGNAc results in increased protein O-Linked glycosylation and insulin resistance in rat skeletal muscle. Diabetes. 2004;53:921–930. doi: 10.2337/diabetes.53.4.921. [DOI] [PubMed] [Google Scholar]
- 85.Issad T, Kuo M. O-GlcNAc modification of transcription factors, glucose sensing and glucotoxicity. Trends Endocrinol Metab. 2008;19:380–389. doi: 10.1016/j.tem.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 86.Ozcan S, Andrali SS, Cantrell JE. Modulation of transcription factor function by O-GlcNAc modification. Biochim Biophys Acta. 2010;1799:353–364. doi: 10.1016/j.bbagrm.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Slawson C, Hart GW. O-GlcNAc signalling: implications for cancer cell biology. Nat Rev Cancer. 2011;11:678–684. doi: 10.1038/nrc3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu K, Paterson AJ, Chin E, et al. Glucose stimulates protein modification by O-linked GlcNAc in pancreatic b cells: Linkage of O-linked GlcNAc to beta cell death. Proc Natl Acad Sci USA. 2000;97:2820–2825. doi: 10.1073/pnas.97.6.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Andrali SS, Qian Q, Ozcan S. Glucose mediates the translocation of NeuroD1 by O-linked glycosylation. J Biol Chem. 2007;282:15589–15596. doi: 10.1074/jbc.M701762200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gao Y, Miyazaki J, Hart GW. The transcription factor PDX-1 is posttranslationally modified by O-linked N-acetyl-glucosamine and this modification is correlated with its DNA binding activity and insulin secretion in min6 beta-cells. Arch Biochem Biophys. 2003;415:155–163. doi: 10.1016/s0003-9861(03)00234-0. [DOI] [PubMed] [Google Scholar]
- 91.Kebede M, Ferdaoussi M, Mancini A, et al. Glucose activates free fatty acid receptor 1 gene transcription via phosphatidylinositol-3-kinase-dependent O-GlcNAcylation of pancreas-duodenum homeobox-1. Proc Natl Acad Sci USA. 2012;109:2376–2381. doi: 10.1073/pnas.1114350109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Whelan SA, Dias WB, Lakshmanan T, et al. Regulation of insulin receptor substrate 1 (IRS-1)/AKT kinase-mediated insulin signaling by O-linked beta-N-acetylglucosamine in 3T3-L1 adipocytes. J Biol Chem. 2010;285:5204–5211. doi: 10.1074/jbc.M109.077818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Parker GJ, Lund KP, Taylor RP, et al. Insulin resistance of glycogen synthase mediated by O-linked N-acetylglucosamine. J Biol Chem. 2003;278:10022–10027. doi: 10.1074/jbc.M207787200. [DOI] [PubMed] [Google Scholar]
- 94.Teo CF, Wollaston-Hayden EE, Wells L. Hexosamine flux, the O-GlcNAc modification, and the development of insulin resistance in adipocytes. Mol Cell Endocrinol. 2010;318:44–53. doi: 10.1016/j.mce.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95•.Housley MP, Rodgers JT, Udeshi ND, et al. O-GlcNAc regulates FoxO activation in response to glucose. J Biol Chem. 2008;283:16283–16292. doi: 10.1074/jbc.M802240200. Together with [79], it serves as an excellent example to show how transcription factors respond to glucose stimulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Housley MP, Udeshi ND, Rodgers JT, et al. A PGC-1alpha-O-GlcNAc transferase complex regulates FoxO transcription factor activity in response to glucose. J Biol Chem. 2009;284:5148–5157. doi: 10.1074/jbc.M808890200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ruan H, Han X, Li M, et al. O-GlcNAc Transferase/host cell factor C1 complex regulates gluconeogenesis by modulating PGC-1a stability. Cell Metab. 2012;16:226–237. doi: 10.1016/j.cmet.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guinez C, Filhoulaud G, Rayah-Benhamed F, et al. O-GlcNAcylation increases ChREBP protein content and transcriptional activity in the liver. Diabetes. 2011;60:1399–1413. doi: 10.2337/db10-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Anthonisen EH, Berven L, Holm S, et al. Nuclear receptor liver X receptor is O-GlcNAc-modified in response to glucose. J Biol Chem. 2010;285:1607–1615. doi: 10.1074/jbc.M109.082685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Arias EB, Cartee GD. Relationship between protein O-linked glycosylation and insulin-stimulated glucose transport in rat skeletal muscle following calorie restriction or exposure to O-(2-acetamido-2-deoxy-d-glucopyranosylidene)amino-N-phenylcarbamate. Acta Physiol Scand. 2005;183:281–289. doi: 10.1111/j.1365-201X.2004.01403.x. [DOI] [PubMed] [Google Scholar]
- 101.Walgren JL, Vincent TS, Schey KL, et al. High glucose and insulin promote O-GlcNAc modification of proteins, including alpha-tubulin. Am J Physiol Endocrinol Metab. 2003;284:E424–E434. doi: 10.1152/ajpendo.00382.2002. [DOI] [PubMed] [Google Scholar]
- 102.Hedou J, Cieniewski-Bernard C, Leroy Y, et al. O-linked N-acetylglucosaminylation is involved in the Ca2+ activation properties of rat skeletal muscle. J Biol Chem. 2007;282:10360–10369. doi: 10.1074/jbc.M606787200. [DOI] [PubMed] [Google Scholar]
- 103.Hedou J, Bastide B, Page A, et al. Mapping of O-linked beta-N-acetylglucosamine modification sites in key contractile proteins of rat skeletal muscle. Proteomics. 2009;9:2139–2148. doi: 10.1002/pmic.200800617. [DOI] [PubMed] [Google Scholar]
- 104.Fulop N, Marchase RB, Chatham JC. Role of protein O-linked N-acetylglucosamine in mediating cell function and survival in the cardiovascular system. Cardiovasc Res. 2007;73:288–297. doi: 10.1016/j.cardiores.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Laczy B, Hill BG, Wang K, et al. Protein O-GlcNAcylation: a new signaling paradigm for the cardiovascular system. Am J Physiol Heart Circ Physiol. 2009;296:H13–H28. doi: 10.1152/ajpheart.01056.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ngoh GA, Facundo HT, Zafir A, et al. O-GlcNAc Signaling in the Cardiovascular System. Circ Res. 2010;107:171–185. doi: 10.1161/CIRCRESAHA.110.224675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zachara NE. The roles of O-linked β-N-acetylglucosamine in cardiovascular physiology and disease. Am J Physiol Heart Circ Physiol. 2012;302:H1905–H1918. doi: 10.1152/ajpheart.00445.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jones SP, Zachara NE, Ngoh GA, et al. Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation. 2008;117:1172–1182. doi: 10.1161/CIRCULATIONAHA.107.730515. [DOI] [PubMed] [Google Scholar]
- 109.Ngoh GA, Watson LJ, Facundo HT, et al. Noncanonical glycosyltransferase modulates post-hypoxic cardiac myocyte death and mitochondrial permeability transition. J Mol Cell Cardiol. 2008;45:313–325. doi: 10.1016/j.yjmcc.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110•.Hu Y, Suarez J, Fricovsky E, et al. Increased enzymatic O-GlcNAcylation of mitochondrial proteins impairs mitochondrial function in cardiac myocytes exposed to high glucose. J Biol Chem. 2009;284:547–555. doi: 10.1074/jbc.M808518200. The first report shows the presence of several O-GlcNAcylated proteins in mitochondria. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Johnsen VL, Belke DD, Hughey CC, et al. Enhanced cardiac protein glycosylation (O-GlcNAc) of selected mitochondrial proteins in rats artificially selected for low running capacity. Physiol Genomics. 2013;45:17–25. doi: 10.1152/physiolgenomics.00111.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ramirez-Correa GA, Jin W, Wang Z, et al. O-linked GlcNAc modification of cardiac myofilament proteins: a novel regulator of myocardial contractile function. Circ Res. 2008;103:1354–1358. doi: 10.1161/CIRCRESAHA.108.184978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Goldberg H, Whiteside C, Fantus I. O-linked beta-N-acetylglucosamine supports p38 MAPK activation by high glucose in glomerular mesangial cells. Am J Physiol Endocrinol Metab. 2011;301:E713–E726. doi: 10.1152/ajpendo.00108.2011. [DOI] [PubMed] [Google Scholar]
- 114.Yao D, Taguchi T, Matsumura T, et al. High glucose increases angiopoietin-2 transcription in microvascular endothelial cells through methylglyoxal modification of mSin3A. J Biol Chem. 2007;282:31038–31045. doi: 10.1074/jbc.M704703200. [DOI] [PubMed] [Google Scholar]
- 115.James LR, Tang D, Ingram A, et al. Flux through the hexosamine pathway is a determinant of nuclear factor kappaB-dependent promoter activation. Diabetes. 2002;51:1146–1156. doi: 10.2337/diabetes.51.4.1146. [DOI] [PubMed] [Google Scholar]
- 116.Yang WH, Park SY, Nam HW, et al. NF-kappaB activation is associated with its O-GlcNAcylation state under hyperglycemic conditions. Proc Natl Acad Sci USA. 2008;105:17345–17350. doi: 10.1073/pnas.0806198105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Allison DF, Wamsley JJ, Kumar M, et al. Modification of RelA by O-linked N-acetylglucosamine links glucose metabolism to NF-κB acetylation and transcription. Proc Natl Acad Sci USA. 2012;94:2927–2932. doi: 10.1073/pnas.1208468109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Du XL, Edelstein D, Rossetti L, et al. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci USA. 2000;97:12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Du XL, Edelstein D, Dimmeler S, et al. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest. 2001;108:1341–1348. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Musicki B, Kramer MF, Becker RE, et al. Inactivation of phosphorylated endothelial nitric oxide synthase (Ser-1177) by O-GlcNAc in diabetes-associated erectile dysfunction. Proc Natl Acad Sci USA. 2005;102:11870–11875. doi: 10.1073/pnas.0502488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121••.Wang Z, Park K, Comer F, et al. Site-specific GlcNAcylation of human erythrocyte proteins: potential biomarker(s) for diabetes. Diabetes. 2009;58:309–317. doi: 10.2337/db08-0994. Combining O-GlcNAc enrichment and iTRAQ quantification for large-scale site mapping and site occupancy assessment of O-GlcNAcylated proteins between normal and diabetic samples. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hu P, Shimoji S, Hart GW. Site-specific interplay between O-GlcNAcylation and phosphorylation in cellular regulation. FEBS Lett. 2010;584:2526–2538. doi: 10.1016/j.febslet.2010.04.044. [DOI] [PubMed] [Google Scholar]
- 123.Butkinaree C, Park K, Hart GW. O-linked N-acetylglucosamine (O-GlcNAc): Extensive crosstalk with phosphorylation to regulate signaling and transcription in response to nutrients and stress. Biochem Biophys Acta. 2010;1800:96–106. doi: 10.1016/j.bbagen.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zeidan Q, Hart GW. The intersections between O-GlcNAcylation and phosphorylation: implications for multiple signaling pathways. J Cell Sci. 2010;123:13–22. doi: 10.1242/jcs.053678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ande SR, Moulik S, Mishra S. Interaction between O-GlcNAc modification and tyrosine phosphorylation of prohibitin: Implication for a novel binary switch. PLoS One. 2009;4:e4586. doi: 10.1371/journal.pone.0004586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sakabe K, Hart GW. O-GlcNAc transferase regulates mitotic chromatin dynamics. J Biol Chem. 2010;285:34460–34468. doi: 10.1074/jbc.M110.158170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sakabe K, Wang Z, Hart GW. β-N-acetylglucosamine (O-GlcNAc) is part of the histone code. Proc Natl Acad Sci USA. 2010;107:19915–19920. doi: 10.1073/pnas.1009023107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fujiki R, Hashiba W, Sekine H, et al. GlcNAcylation of histone H2B facilitates its monoubiquitination. Nature. 2011;480:557–560. doi: 10.1038/nature10656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Comer FI, Vosseller K, Wells L, et al. Characterization of a mouse monoclonal antibody specific for O-linked N-acetyl-glucosamine. Anal Biochem. 2001;293:169–177. doi: 10.1006/abio.2001.5132. [DOI] [PubMed] [Google Scholar]
- 130.Snow CM, Senior A, Gerace L. Monoclonal antibodies identify a group of nuclear pore complex glycoproteins. J Cell Biol. 1987;104:1143–1156. doi: 10.1083/jcb.104.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang J, Torij M, Liu H, et al. dbOGAP-an integrated bio-informatics resource for protein O-GlcNAcylation. BMC Bioinformatics. 2011;12:91. doi: 10.1186/1471-2105-12-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Syka JE, Coon JJ, Schroeder MJ, et al. Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc Natl Acad Sci USA. 2004;101:9528–9533. doi: 10.1073/pnas.0402700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wells L, Vosseller K, Cole RN, et al. Mapping sites of O-GlcNAc modification using affinity tags for serine and threonine post-translational modifications. Mol Cell Proteomics. 2002;1:791–804. doi: 10.1074/mcp.m200048-mcp200. [DOI] [PubMed] [Google Scholar]
- 134.Vosseller K, Hansen KC, Chalkley RJ, et al. Quantitative analysis of both protein expression and serine/threonine post-translational modifications through stable isotope labeling with dithiothreitol. Proteomics. 2005;5:388–398. doi: 10.1002/pmic.200401066. [DOI] [PubMed] [Google Scholar]
- 135.Overath T, Kuckelkorn U, Henklein P, et al. Mapping of O-GlcNAc sites of 20S proteasome subunits and Hsp90 by a novel biotin-cystamine tag. Mol Cell Proteomics. 2012;11:467–477. doi: 10.1074/mcp.M111.015966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hahne H, Moghaddas-Gholami A, Kuster B. Discovery of O-GlcNAc-modified proteins in published large-scale proteome data. Mol Cell Proteomics. 2012;11:843–850. doi: 10.1074/mcp.M112.019463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Teo CF, Ingale S, Wolfert M, et al. Glycopeptide-specific monoclonal antibodies suggest new roles for O-GlcNAc. Nat Chem Biol. 2010;6:338–343. doi: 10.1038/nchembio.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhao P, Viner R, Teo CF, et al. Combining high-energy C-trap dissociation and electron transfer dissociation for protein O-GlcNAc modification site assignment. J Proteome Res. 2011;10:4088–4104. doi: 10.1021/pr2002726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kelly WG, Hart GW. Glycosylation of chromosomal proteins: localization of O-linked N-acetylglucosamine in Drosophila chromatin. Cell. 1989;57:243–251. doi: 10.1016/0092-8674(89)90962-8. [DOI] [PubMed] [Google Scholar]
- 140.Chalkley RJ, Thalhammer A, Schoepfer R, et al. Identification of protein O-GlcNAcylation sites using electron transfer dissociation mass spectrometry on native peptides. Proc Natl Acad Sci USA. 2009;106:8894–8899. doi: 10.1073/pnas.0900288106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141•.Trinidad JC, Barkan DT, Gulledge BF, et al. Global identification and characterization of both O-GlcNAcylation and phosphorylation at the murine synapse. Mol Cell Proteomics. 2012;11:215–229. doi: 10.1074/mcp.O112.018366. The largest dataset of O-GlcNAc site mapping in one study to date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Khidekel N, Ficarro SB, Clark MC, et al. Probing the dynamics of O-GlcNAc glycosylation in the brain using quantitative proteomics. Nat Chem Biol. 2007;3:339–348. doi: 10.1038/nchembio881. [DOI] [PubMed] [Google Scholar]
- 143.Wang Z, Udeshi ND, O’Malley M, et al. Enrichment and sitemapping of O-linked N-acetylglucosamine by a combination of chemical/enzymatic tagging, photo-chemical cleavage, and electron transfer dissociation mass spectrometry. Mol Cell Proteomics. 2010;9:153–160. doi: 10.1074/mcp.M900268-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Alfaro JF, Gong CX, Monroe ME, et al. Tandem mass spectrometry identifies many mouse brain O-GlcNAcylated proteins including EGF domain-specific O-GlcNAc transferase targets. Proc Natl Acad Sci USA. 2012;109:7280–7285. doi: 10.1073/pnas.1200425109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145••.Wang Z, Udeshi ND, Slawson C, et al. Extensive crosstalk between O-GlcN-Acylation and phosphorylation regulates cytokinesis. Sci Signal. 2010;3:ra2. doi: 10.1126/scisignal.2000526. A combination of O-GlcNAc enrichment and SILAC for large-scale identification and quantification of O-GlcNAcylated and phosphorylated proteins, showing the dynamic interplay between the two important post-translational modifications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vocadlo DJ, Hang HC, Kim EJ, et al. A chemical approach for identifying O-GlcNAc-modified proteins in cells. Proc Natl Acad Sci USA. 2003;100:9116–9121. doi: 10.1073/pnas.1632821100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nandi A, Sprung R, Barma DK, et al. Global identification of O-GlcNAc-modified proteins. Anal Chem. 2006;78:452–458. doi: 10.1021/ac051207j. [DOI] [PubMed] [Google Scholar]
- 148.Zaro BW, Yang YY, Hang HC, et al. Chemical reporters for fluorescent detection and identification of O-GlcNAc-modified proteins reveal glycosylation of the ubiquitin ligase NEDD4-1. Proc Natl Acad Sci USA. 2011;108:8146–8151. doi: 10.1073/pnas.1102458108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hahne H, Sobotzki N, Tamara N, et al. Proteome wide purification and identification of O-GlcNAc-modified proteins using click chemistry and mass spectrometry. J Proteome Res. 2013;12(2):927–936. doi: 10.1021/pr300967y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Choudhary C, Mann M. Decoding signaling networks by mass spectrometry-based proteomics. Nat Rev Mol Cell Bio. 2010;11:427–439. doi: 10.1038/nrm2900. [DOI] [PubMed] [Google Scholar]
- 151.Topf F, Schvartz D, Gaudet P, et al. The Human Diabetes Proteome Project (HDPP): from network biology to targets for therapies and prevention. Transl Proteomics. 2013;1:3–11. [Google Scholar]
- 152.Park K, Saudek CD, Hart GW, et al. Increased expression of beta-N-acetyl-glucosaminidase in erythrocytes from individuals with pre-diabetes and diabetes. Diabetes. 2010;59:1845–1850. doi: 10.2337/db09-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Springhorn C, Matsha TE, Erasmus RT, et al. Exploring leukocyte O-GlcNAcylation as a novel diagnostic tool for the earlier detection of Type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012;97:4640–4649. doi: 10.1210/jc.2012-2229. [DOI] [PubMed] [Google Scholar]