Abstract
Introduction:
The purpose of this study was to find out the effect of the secondary cognitive and motor task on cued gait in people with Idiopathic Parkinson's disease (PD).
Design and Setting:
A repeated measure same subject design carried out at All India Institute of Medical Sciences, Neurology Department, New Delhi.
Materials and Methods:
The subjects were made to walk in random order on a paper walkway under three conditions: Free walking with cues at preferred walking speed, coin transference while walking with cues at preferred walking speed and digit subtraction while walking with cues at preferred walking speed.
Outcome:
The stride length, cadence, walking speed and stops were recorded.
Results:
There was a significant reduction in their walking speed and stride length, but increase in the cadence and the number of stops was seen, when they had to perform dual tasks along with the cued gait, but the changes were more pronounced when secondary cognitive task was added to the cued gait in people with idiopathic PD.
Conclusion:
The results of this study demonstrated that there is a significant difference in the effect of secondary motor task when compared with secondary cognitive task on cued gait parameters in people with Idiopathic PD.
Keywords: Cueing, dual task, gait, idiopathic Parkinson's disease
Introduction
In Parkinson's disease (PD), alterations in walking, includes reduced speed, shortened stride, reduced swing time, decreased arm swing and increased stride-to-stride variability. Markers of arrhythmicity and reduced automaticity related to gait unsteadiness and fall risk, is also typically observed in patients with PD. To achieve a more normal gait, PD patients may recruit attentional resources to compensate for the impairment in automicity.[1] Cognitive and attentional processes are particularly important in PD to compensate for basal ganglia dysfunction and loss of gait automicity and are integral to cue based rehabilitation strategies.[2]
The decrement in gait performance observed when individuals with PD execute multiple tasks simultaneously and the implied threat to stability has led to the development of multitask training protocols. These training protocols aim to increase functionality through improving the individual's capacity to perform additional tasks concurrent to walking.[3] A recent study showed that 12% of the variance in interference of a dual task on walking speed was explained by motor deficit and reduced executive ysfunction, suggesting that automaticity of performance under complex walking conditions is multi-dimensional. The inability to switch between different functions is recognized as a problem of attentional control in PD that compromises the safe and effective performance of functional tasks such as walking in “the real world” environments, which are unpredictable and require co-ordinated, flexible and immediate cognitive and motor responses. The attentional shifting appears to be mediated by dopaminergic networks. Under dual task conditions, people with PD adopt a posture second strategy, in that they preferentially optimize cognitive responses over stability of gait, thus compromising balance and safety.[2]
Cueing strategies have shown to improve gait in people with PD and are argued to bypass the defective basal ganglia by using alternative pathways unaffected by PD to improve motor performance. External cues provide temporal or spatial stimuli associated with the initiation and facilitation of a motor activity and can be delivered using different modalities (auditory, visual and somatosensory).[2]
The external cues can help in reducing the interference and maintain gait during functional activities. The combination of a rhythmic auditory cue and an attentional strategy has reported to improve gait speed and step amplitude during single as well as in dual task.[2,4] It's also being reported that people with PD do not gain additional benefit combination of attentional and auditory cues.[5]
Since there is conflicting reports on the combination of cueing and attentional strategy this study was undertaken and there is a dearth of studies, which have investigated the effect of secondary task on cued gait in people with PD. The study hypothesized that there is an effect of secondary task on cued gait and the study also tried to find out whether the type of secondary task had any effect on the cued gait in PD.
Materials and Methods
Subjects
A convenient sample of 25 subjects (18 males and 7 females) with idiopathic PD diagnosed by a neurologist, were recruited from All India Institute of Medical Sciences, Neurology Department, New Delhi.
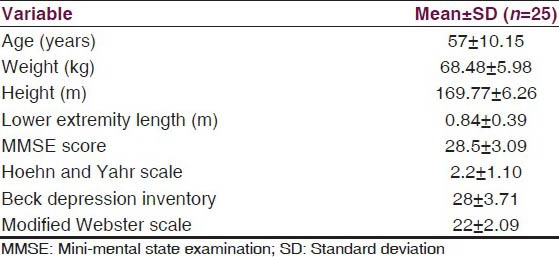
The subjects participated in the study were, between 41 and 80 years of age, mini-mental state examination score of 24 or higher,[6] able to walk independently indoors without any aid,[2] Hoehn and Yahr scale score of 1-4,[7] no long off periods (unified Parkinson's disease rating scale ≥ 1 on item no. 39),[8] score of ≤20 on modified Webster scale,[9] score <30 on Beck's depression inventory,[10] and minimum primary level of education. Adequate vision and hearing, achieved using corrective lenses and/or hearing aids if required, determined informally by ensuring that the subject could read the study information and hear the cueing device.[11] The demographic characteristics of the subjects is given in Table 1.
Table 1.
The characteristics of the subjects
Design and setting
An experimental repeated measure same subject design was used in which each subjects was observed for gait parameters under three different walking conditions and the main factors studied were the effect of concurrent task in cued gait. The study was approved by research and Ethical Committee of ISIC Institute of Rehabilitation Sciences, New Delhi. The subjects duly signed the consent form for participation in the study. Demographic details such as age, sex, weight, height and the lower extremity length were collected from the subjects. After a complete physical examination, subjects were made to follow the testing procedure. The testing was always performed in the morning to avoid the problems of fatigue and they were tested in self-reported “on” phase of their medication cycle.[12] The whole procedure was completed in a single session, which lasted for approximately 1 h for one patient, starting from applying the ink in the foot with socks.
Then subjects were given one practice trial for each of the task conditions namely the cued gait only and cued gait with secondary motor and cognitive task at preferred walking speed.[13] The rhythmic auditory cues were delivered by a metronome, which was worn on a belt around the neck of the researcher while researcher was walking alongside each subject. Frequency of cueing was determined by measuring the time taken to walk 10 steps at the subjects preferred walking speed.[12] The tempo of the metronome was set to this baseline cadence as frequency of cueing during the intervention by matching the number of beats per minute to the number of steps per minute.[14] The subjects walked over an absorbent paper of 10 m length wearing cotton socks applied with ink and foot prints thus formed were used calculating the parameters of gait.[12] The parameters studied were cadence, walking speed and stride length. The stops while walking were also recorded for all the three conditions.
The walking speed was calculated by dividing the total walking distance (6 m) by the time taken to traverse that distance (in seconds) as timed by the stop watch. It was recorded in meters per second. The stride length was calculated by measuring the perpendicular distance from the heel strike of one foot to the next heel strike of the same foot. It was recorded in meters. The normalized stride length was calculated by dividing the stride length by the lower extremity length.[4,15] The cadence was calculated as the number of steps taken by a person per unit of time.[16] The steps per minute were calculated using the conversion formula.[15]
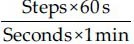
The verbal instruction of “begin walking” was used to prompt each participant to walk across the 10 m paper walkway placed on the floor.[17] The instructions given to the patients were “As you walk try to step your feet in time to the beat.”[2] Their ambulation time was recorded using the stop watch and readings were taken only in the middle 6 m of the walkway to record the most stable phase walking and reduce the effect of acceleration and deceleration.
Experimental conditions
Subjects walked 1 time under three conditions and the order of task conditions was randomly allocated to reduce any learning effect. The three conditions were (1) free walking (“free”) with cues at preferred walking speed, (2) coin transference while walking (“coin”) with cues at preferred walking speed (concurrent motor task) and (3) digit subtraction while walking (“digit”) with cues at preferred walking speed (concurrent cognitive task).
Tasks performed while walking
Subjects were instructed to use their dominant hand to transfer as many coins as they could, one at a time, from the starting pocket to the pocket on the opposite side. During the coin transfer condition, the belt with the pockets was fitted to the subject's waist and the twelve one rupee coins were placed in the pocket on the side of their dominant hand. Subjects reported their dominant hand by indicating, which hand they would use to catch a small ball. For the arithmetic task, the subjects were made to count backward in threes from a number between 20 and 100 randomly selected by the examiner.[18]
The dual task cost (DTC) was calculated using the formula: DTC [%] =100 × (single task – score – dual task score)/single task score.[19]
Data analysis
The data was analyzed using statistical software SPSS version 15.0 (IBM, USA). Descriptive statistics were calculated for all the parameters studied one factor repeated measure analysis of variance and post hoc comparisons was used for multiple comparisons across the different task conditions after the necessary Bonferroni adjustment. One sample t-test was used to find the difference of DTC for different walking conditions (percentage changes of DTC, DTC [%]). A P ≤ 0.05 was considered to be significant.
Results
The mean ± standard deviation (m + S.D) of walking speed, stride length, cadence and number of stops under different conditions is given in Table 2. One factor repeated measure analysis of variance showed that walking speed changed from 0.82 ± 0.06 m/s in cued gait only (C) to 0.80 ± 0.05 m/s in cued gait with secondary motor task (CM), to 0.78 ± 0.048 m/s in gait with secondary cognitive task (CC) condition. The variation seen for the values of normalized stride length were from 1.36 ± 0.27 m in cued gait (C) to 1.22 ± 0.27 m in cued gait with secondary motor task (CM), to 1.08 ± 0.29 m in cued gait with secondary cognitive task (CC). The cadence varied from 88.44 ± 26.39 steps/m in cued gait only (C) to 96.59 ± 25.27 steps/m in cued gait with secondary motor task (CM) to 108.00 ± 35.69 steps/m in cued gait with secondary cognitive task condition (CC). The number of stops changed from 0.12 ± 0.60 in cued gait only (C), 1.00 ± 0.76 in cued gait with secondary motor task (CM), 1.48 ± 0.77 in cued gait with secondary cognitive task (CC).
Table 2.
Comparison of normalized stride length, cadence, walking speed and no. of stops under three different conditions using one factor repeated ANOVA
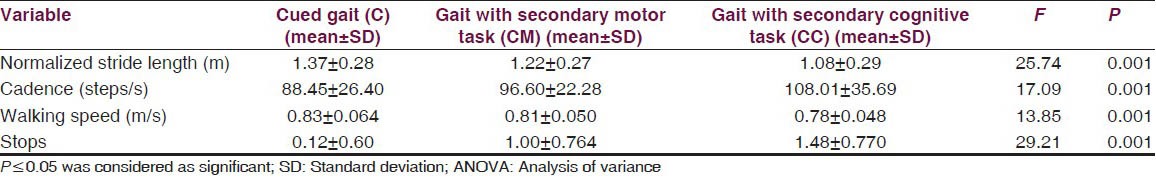
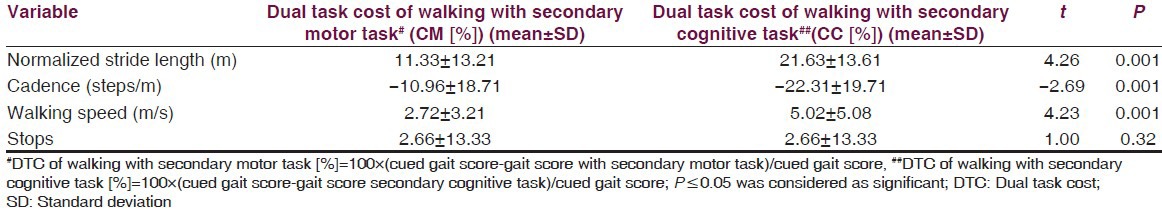
The results showed a significant difference on walking speed, normalized stride length, cadence and number of stops among the three walking conditions [Table 2]. Post-hoc pair wise comparisons showed that there was a significant difference in walking speed between C and CM, C and CC, CM and CC. For the number of stops, there was a significant difference, between C and CM, C and CC, CM and CC [Table 3]. The DTC is tabulated in Table 4 and the difference between the DTC for different task (percentage changes of DTC, DTC [%]) while walking was significant for all the parameters of gait studied except stops [Table 4].
Table 3.
Post hoc pair wise comparison of normalized stride length, cadence, walking speed and no. of stops under three different conditions
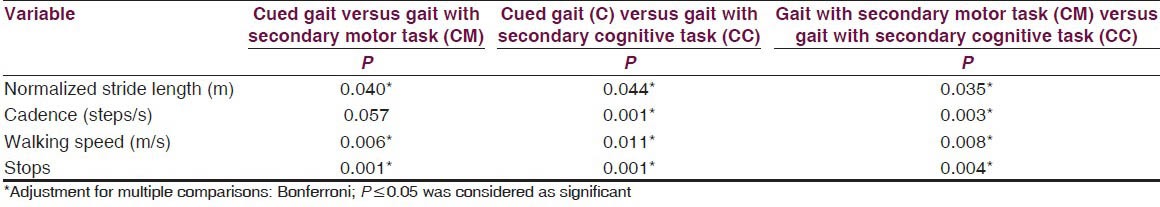
Table 4.
Comparison of dual task cost (DTC [%]) of walking under different conditions using one sample t test
Discussion
The results of this study showed marked differences in the gait parameters when the subjects were required to perform either the cued gait with secondary motor or the cued gait with secondary cognitive tasks. However, the differences in the parameters were more pronounced when a cognitive task was added to cued gait instead of a motor one and also the DTC was more with the concurrent cognitive task. Thus, interference in walking performance was inferred, when secondary tasks were undertaken with cued gait. The results are supported by the inferences of the study conducted by O’Shea et al.,[18] on dual task interference in people with PD. The changes seen in gait parameters can be explained by capacity-sharing model. The result in decline in performance on either of the task or both will happen with overuse of the resources available according to the above said model.[20]
The mean normalized stride length reduced by 0.15 cm when the secondary motor task (CM) was added to cued gait and it showed a marked reduction, 0.29 cm with the secondary cognitive task (CC). The difference in value of the same was only 0.14 cm on comparison between CM and CC. The maximum drop in mean normalized stride length was visible during the performance of the cognitive task to cued gait, which is in agreement with the study done by Morris et al.[21] The mean value of cadence increased by 8.15 steps per minute when the secondary motor task (CM) was added to cued gait and with a cognitive (CC) there was an increase of 19.59 steps per minute. The value of increment in mean cadence on comparison between CM and CC was 11.41 steps per minute. The mean walking speed reduced by 0.02m/s and by 0.05 m/s with secondary motor task and cognitive task respectively. The walking speed was less than 0.03 m/s with secondary cognitive task as compared with secondary motor task. Furthermore, an upward trend was observed in the mean value of the number of stops with secondary motor task by 0.88 and the mean number of stops increased by 1.36 with secondary cognitive task. Furthermore, a 0.48 increase in the mean number of stops was seen with secondary cognitive task when compared with secondary motor task. The differences highlight different natures of secondary tasks on cued gait in people with PD.
The increase in cadence in this study had occurred as reported in the results on a study on pathogenesis of gait hypokinesia in people with PD.[21] These finding are in contrast to those of Galletly and Brauer and O’Shea et al.,[12,18] In contrast to the study conducted by Galletly and Brauer[12] on the effect of type of secondary task on the visually cued gait in PD patients, this study demonstrated significant changes on the cadence and marked differences in the stride lengths and walking speed while performing the secondary tasks with cued gait and did show a trend of shorter steps and slower gait when they engaged in the dual task conditions when compared with when they were performing cued gait only task, which is in accordance with the results of the study on the effect of dust task on the PD subjects.[18]
The results of this study are well-supported by a study of Morris et al.,[21] on the pathogenesis of gait hypokinesia. The relative increase in cadence exhibited by PD subjects is a compensatory mechanism for the difficulty in regulating stride length. These findings are important in the context of the hypothesized role of the basal ganglia in generating internal cues for the maintenance of the gait sequence. This reduced gait speed is consistent with previous findings and the general description of bradykinesis in PD. A possible explanation for increased gait variability, in PD subjects, is that it is a byproduct of lower gait speed. Indeed, many of the gait changes associated with PD are related to diminished ability to, generate normal stride length and velocity. The increased variability may be the result of disjointedness in the gait so that walking becomes a sequence of disconnected strides rather than a single continuous motion. This may be the result of impairment in anticipatory reflexes, the disruption in the normal internal cueing required to string together sub movements, or the diminished capacity to perform automatic sequential movement.[22]
An explanation to the findings of reduced mean walking speed on secondary cognitive task addition to cued gait is that the subjects prioritized the cognitive task to performance on cued gait where in presumably fewer resources are allocated to cued gait as supported by a study on how does explicit prioritization alter walking during dual task performance. The reduction in the values of mean walking speed when secondary tasks were added to cued gait could possibly be explained by motor deficit and reduced executive function suggesting that the automiticity of performance under complex walking conditions is multi-dimensional as supported by the study on executive dysfunction and attention contributory factors in gait interference. The support to the finding of this study with respect to the increase in the mean number of stops when secondary tasks are added to cued gait comes from evidence to prove that under dual task conditions people with PD adopt a “posture second strategy” in that they preferentially optimize cognitive responses over stability of gait, thus compromising the balance and safety.[11]
Thus, knowing which of the type of the secondary task, requires more attention, we can educate the patient and train him/her using dual task training about the likely outcomes and risks associated with performing them while walking. The results of this study highlight the importance of clinically assessing gait with a variety of concurrent tasks, particularly cognitive ones, in the patient with PD. The effect of different tasks on the gait could be a helpful predictor for the risk of falls. Patients may benefit from treatment specifically selected to improve the levels of dual task performance as the everyday activities involve concurrent cognitive and motor task components. A motor task performed under dual task condition may provide a better index of everyday functional ability than a motor task performed under unitask conditions in typical neurological assessment. Understanding prevalence and prognosis of dual task decrements could therefore, form an important part of assessment and rehabilitation.
Smaller sample size is one of the major limitations, where in a larger sample size would increase the statistical power of the study. Furthermore, the blinding of the investigator and the subject is not done. This could in turn may had lead to bias. Furthermore, there was no control group and convenient sampling technique was being used to select the participants. All the patients were tested at peak dose in the levodopa medication cycle and it is not clear whether the results could be generalized to “off” phase performance. The age range of the subjects was too broad that might have caused some effects on the results. The methods used to analyze the gait parameters was a crude one even though its validity and ratability was established.[3]
The use of the stride analyzer could be done to further specifically document the heel toe contact patterns while studying the gait in patients with PD. In addition, the training protocols could be used to enable to the researcher to dwell deeply into the effects of the dual task training regime on cued gait in Parkinson's patients. Testing environment in the real world will broaden the scope of research work. The retention or the carryover effect of the results over weeks and months could be studied. Investigation of the effects of functional tasks during gait in more real-world setting during activities of daily living is needed.
Conclusion
The results of this study, add weight to the growing body of literature demonstrating that people with PD have great difficulty in multi-tasking. The results of this study highlight the importance of clinically assessing gait with a variety of concurrent tasks, particularly cognitive ones, in the patient with PD.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Yogev G, Giladi N, Peretz C, Springer S, Simon ES, Hausdorff JM. Dual tasking, gait rhythmicity and Parkinson's disease: Which aspects of gait are attention demanding? Eur J Neurosci. 2005;22:1248–56. doi: 10.1111/j.1460-9568.2005.04298.x. [DOI] [PubMed] [Google Scholar]
- 2.Baker K, Rochester L, Nieuwboer A. The immediate effect of attentional, auditory and a combined cue strategy on gait during single and dual tasks in Parkinson's disease. Arch Phys Med Rehabil. 2007;88:1593–600. doi: 10.1016/j.apmr.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 3.Brown LA, de Bruin N, Doan J, Suchowersky O, Hu B. Obstacle crossing among people with Parkinson disease is influenced by concurrent music. J Rehabil Res Dev. 2010;47:225–31. doi: 10.1682/jrrd.2009.10.0171. [DOI] [PubMed] [Google Scholar]
- 4.Rochester L, Hetherington V, Jones D, Nieuwboer A, Willems AM, Kwakkel G, et al. The effect of external rhythmic cues (auditory and visual) on walking during a functional task in homes of people with Parkinson's disease. Arch Phys Med Rehabil. 2005;86:999–1006. doi: 10.1016/j.apmr.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 5.Lohnes CA, Earhart GM. The impact of attentional, auditory and combined cues on walking during single and cognitive dual tasks in Parkinson disease. Gait Posture. 2011;33:478–83. doi: 10.1016/j.gaitpost.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 6.Heeren TJ, Lagaay AM, von Beek WC, Rooymans HG, Hijmans W. Reference values for the mini-mental state examination (MMSE) in octo- and nonagenarians. J Am Geriatr Soc. 1990;38:1093–6. doi: 10.1111/j.1532-5415.1990.tb01371.x. [DOI] [PubMed] [Google Scholar]
- 7.Hoehn MM, Yahr MD. Parkinsonism: Onset, progression and mortality. Neurology. 1967;17:427–42. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 8.Martínez-Martín P, Gil-Nagel A, Gracia LM, Gómez JB, Martínez-Sarriés J, Bermejo F. Unified Parkinson's disease rating scale characteristics and structure. The cooperative multicentric group. Mov Disord. 1994;9:76–83. doi: 10.1002/mds.870090112. [DOI] [PubMed] [Google Scholar]
- 9.Kempster PA, Frankel JP, Bovingdon M, Webster R, Lees AJ, Stern GM. Levodopa peripheral pharmacokinetics and duration of motor response in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1989;52:718–23. doi: 10.1136/jnnp.52.6.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharp LK, Lipsky MS. Screening for depression across the lifespan: A review of measures for use in primary care settings. Am Fam Physician. 2002;66:1001–8. [PubMed] [Google Scholar]
- 11.Nieuwboer A, Kwakkel G, Rochester L, Jones D, van Wegen E, Willems AM, et al. Cueing training in the home improves gait-related mobility in Parkinson's disease: The RESCUE trial. J Neurol Neurosurg Psychiatry. 2007;78:134–40. doi: 10.1136/jnnp.200X.097923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galletly R, Brauer SG. Does the type of concurrent task affect preferred and cued gait in people with Parkinson's disease? Aust J Physiother. 2005;51:175–80. doi: 10.1016/s0004-9514(05)70024-6. [DOI] [PubMed] [Google Scholar]
- 13.Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: A review of an emerging area of research. Gait Posture. 2002;16:1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- 14.Shores M. Footprint analysis in gait documentation. An instructional sheet format. Phys Ther. 1980;60:1163–7. doi: 10.1093/ptj/60.9.1163. [DOI] [PubMed] [Google Scholar]
- 15.Holden MK, Gill KM, Magliozzi MR, Nathan J, Piehl-Baker L. Clinical gait assessment in the neurologically impaired. Reliability and meaningfulness. Phys Ther. 1984;64:35–40. doi: 10.1093/ptj/64.1.35. [DOI] [PubMed] [Google Scholar]
- 16.Olney SJ. Gait. In: Levangie PK, Norkin CC, editors. Joint Structure and Function: A Comprehensive Analysis. India: Jaypee Publications; 2006. pp. 517–64. [Google Scholar]
- 17.Nelson AJ, Zwick D, Brody S, Doran C, Pulver L, Rooz G, et al. The validity of the GaitRite and the functional ambulation performance scoring system in the analysis of Parkinson gait. NeuroRehabilitation. 2002;17:255–62. [PubMed] [Google Scholar]
- 18.O’Shea S, Morris ME, Iansek R. Dual task interference during gait in people with Parkinson disease: Effects of motor versus cognitive secondary tasks. Phys Ther. 2002;82:888–97. [PubMed] [Google Scholar]
- 19.Hausdorff JM, Edelberg HK, Mitchell SL, Goldberger AL, Wei JY. Increased gait unsteadiness in community-dwelling elderly fallers. Arch Phys Med Rehabil. 1997;78:278–83. doi: 10.1016/s0003-9993(97)90034-4. [DOI] [PubMed] [Google Scholar]
- 20.Pashler H. Dual-task interference in simple tasks: Data and theory. Psychol Bull. 1994;116:220–44. doi: 10.1037/0033-2909.116.2.220. [DOI] [PubMed] [Google Scholar]
- 21.Morris ME, Iansek R, Matyas TA, Summers JJ. The pathogenesis of gait hypokinesia in Parkinson's disease. Brain. 1994;117:1169–81. doi: 10.1093/brain/117.5.1169. [DOI] [PubMed] [Google Scholar]
- 22.Cubo E, Leurgans S, Goetz CG. Short-term and practice effects of metronome pacing in Parkinson's disease patients with gait freezing while in the ‘on’ state: Randomized single blind evaluation. Parkinsonism Relat Disord. 2004;10:507–10. doi: 10.1016/j.parkreldis.2004.05.001. [DOI] [PubMed] [Google Scholar]


