Abstract
Background:
Stereotactic biopsy of brainstem lesions have been performed with varying indications, with most of the literature reporting on children.
Materials and Methods:
The present study retrospectively analyzed all cases that underwent stereotactic biopsy for brainstem lesion in both adult and pediatric population between 1994 and 2009 in a single tertiary neurosurgical center. The clinical and radiological features, technique of the procedure, morbidity, diagnostic accuracy, spectrum of diagnosis, and variations in adult and pediatric population were analyzed.
Results:
Eighty-two patients were included in the study. Computed tomography (CT) was used as guidance in 73 (38 children and 35 adults) patients and magnetic resonance imaging (MRI) in 9 (3 children and 6 adults). The biopsy was performed in a procedure room under local anesthesia in most adults, while children required sedation. Glioblastoma comprised 29.3% of all pathologies in children, compared with only 4.9% of the pathologies in adult population (P = 0.007). Tuberculosis was the next major diagnosis (9.8%). In 12 patients, initial biopsy was inconclusive. Following a repeat biopsy in 5 of these patients, a diagnosis was possible for 75/82 (91.5%) patients by STB. The location of the target, the choice of entry, the radiological characteristic of the lesion, enhancement pattern, and age group did not significantly correlate with the occurrence of inconclusive biopsy. Permanent complications occurred in two patients (2.4%). There was no mortality in this series.
Conclusion:
Stereotactic biopsy has an important role in brainstem lesions, more significantly in adults, due to wider pathological spectrum. It can be performed safely under local anesthesia through a twist drill craniostomy in most of the adults.
Keywords: Age group difference, brainstem lesion, pathology, pediatric, stereotactic biopsy
Introduction
Brainstem lesions comprise 15% of intracranial space occupying lesions in children and 2% in adults.[1] In pediatric population, most of these lesions are brainstem gliomas while there is a wider diversity in adults.[2] In addition to glioma, the differential diagnosis of a brainstem lesion in adults includes other tumors, vasculitis, AVM, hematoma, infarction, infections, gliosis, and demyelinating disease.[3,4]
Brainstem lesions are sometimes treated on clinical and radiological grounds and it had even been argued that biopsy is not warranted in many of these lesions.[4] This is based on the fact that surgery for brainstem lesions was historically associated with an unacceptably high mortality and morbidity.[5,6,7] Except in the presence of an exophytic component, open biopsy is associated with high morbidity. In case of diffuse lesions, difficulty in identifying the lesion intraoperatively compounds these problems. In general, vast majority of brainstem lesions are not amenable to surgical resection.[8]
Magnetic resonance imaging (MRI) has emerged as the primary diagnostic modality of brainstem lesions.[9] With the introduction of MRI in the 1980s, interest in the histological diagnosis in brainstem lesions declined. But MRI-based diagnosis is reported to be erroneous in 10-20% of the cases.[10] Further, in regard to tumor grade classification, it was noted that the accuracy of MRI brain assessment was correct in 35% of low grade glioma and 27% of high grade glioma.[11]
Different entry points and trajectories are described for reaching the brainstem. The technique used differs based on location of the lesion and surgeon's preference. Similar outcomes are reported with different approaches.[12]
The present study aims to analyze the spectrum of cases that underwent stereotactic biopsy for a brainstem lesion in both adult and pediatric population over a long period in a single tertiary neurosurgical centre. The technique of the procedure, morbidity, diagnostic accuracy, spectrum of diagnosis in Indian population, and variations in adult and pediatric population are analyzed.
Materials and Methods
This study retrospectively analyzed all the stereotactic biopsies performed for lesions involving brainstem in a tertiary neurosurgical institute over a period of 16 years from 1994 to 2009. All cases of radiologically demonstrated lesions localized to brainstem (mid brain, pons, and medulla) were included in the study. In patients with larger lesions involving other regions of brain, in addition to brainstem, or multiple lesions, only the cases where the target of biopsy was brainstem were included. All cases with a target outside the brainstem were excluded even if the bulk of the lesion was in the brainstem. The clinical details, details of the procedure, radiological findings, histopathological diagnosis, and follow up details, wherever available, were reviewed from the case records.
Clinical presentation, location and radiological features of the lesion, stereotactic biopsy technique used, and complications of the procedure were analyzed. The cohort was grouped in to children and adults and subgroup analyses of all these parameters were performed. SPSS version 15.0 software was used for statistical analysis and appropriate tests were applied to determine significance.
Method of stereotactic biopsy
Biopsy was performed in a procedure room under local anesthesia or general anesthesia. After fixing a Leksell stereotactic frame to the head, a contrast enhanced computed tomography (CT) head (Siemens, Pennsylvania, USA)/MRI brain (Siemens Magnetom 1.5 T MRI, Pennsylvania, USA) was done. The lesion was identified and the target point for biopsy was chosen. Coordinates of the target were calculated by the surgeon based on a Cartesian system [Figure 1]. In supine position, through a twist drill craniostomy, biopsy was taken with a side cutting biopsy forceps. Usually three samples were taken during the procedure.
Figure 1.
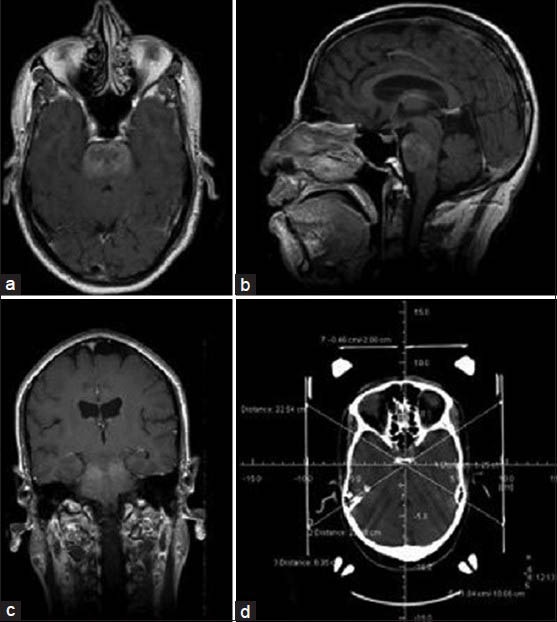
Postcontrast axial (a), sagittal (b) and coronal (c) MRI brain and CT brain with stereotactic frame showing the coordinates (d), of a 12-year-old boy with multiple cranial nerve involvement. The images reveal an ill defined lesion in the pons, which demonstrates heterogenous enhancement with contrast. Initial stereotactic biopsy was inconclusive and subsequent STB yielded a diagnosis of anaplastic astrocytoma
Results
Eighty-two patients underwent stereotactic biopsy for a brainstem lesion during the study period. There were 41 children (≤18 years) and 41 adults (>18 years). The age of the patients ranged from 3 to 60 years (mean 22.11 years, median 18.5 years). When grouped separately, median age of the children was 9 years and that for adults was 34 years. There was a male preponderance in both groups with 26 males (63.4%) among the children and 29 males (70.7%) among the adults. The duration of symptoms ranged from 0.3 to 36 months (in children: range 0.3-24 months, mean 3 months; and in adults: range 0.3-36 months, mean 5.7 months). There was no significant difference in duration of symptoms between pediatric and adult population (P = 0.058). Nine out of 41 children (22%) and 20 out of 41 adults (48.8%) had symptoms of cranial nerve dysfunction as the first feature. Headache was noted in 35.4% patients, while limb weakness and ataxia were noted in 42.7% and 47.6%, respectively. Seizure was documented in one patient (1.2%). The clinical features in pediatric and adult population were comparable, except for the involvement of trigeminal nerve, which was significantly higher in adults as compared with children (43.9% vs. 22%; P = 0.030).
Imaging data was available for review in 72 patients [Table 1]. The location of the biopsied lesions between pediatric and adult population was similar (P = 0.841). Eight out of 13 lower pontine lesions were extending to medulla. Other extensions were to thalamus, middle cerebellar peduncle, and cerebellum [Table 1]. All these cases underwent biopsy from pons or midbrain.
Table 1.
Radiological characteristics of the brainstem lesions

All the lesions were predominantly intrinsic. Lesions, which have a well defined margin in contrast enhanced MRI or CT scan, were considered as “well defined”, while those without definite margins are considered diffuse. Nearly 59.7% were ill defined or diffuse lesions and 40.3% were well defined [Table 1]; 22.2% of lesions did not demonstrate any contrast enhancement. There were three uniformly enhancing lesions. Two of these uniformly enhancing lesions were histologically proven later to be nonHodgkin's lymphoma and one was pilocytic astrocytoma. The remaining lesions were almost equally distributed among heterogeneous (22%), patchy (20.7%), and ring (22%) enhancing type. Of the 72 patients, in whom imaging data was available, contrast enhanced MRI was available in 61 patients. The remaining patients had only imaging findings from the CT scan. MRI was available in 34 children and 27 adults. In children, 22/34 lesions were diffuse, while in adults 15/27 were diffuse lesions as noted in MRI. Of all the diffuse lesions, 26.2% did not show contrast enhancement.
Four patients had other lesions separate from the biopsied lesion. Of these four patients, two had multiple brainstem lesions. One adult had a small deep frontal lesion and one child had a cerebellar hemispheric lesion, both of which were considered nonsignificant at the time of biopsy. In the adult, the biopsy from brainstem was inconclusive. But the deep frontal lesion enlarged on follow up and biopsy at a later date showed metastasis. In the child, biopsy was reported as inflammatory lesion, and he was started on antituberculosis treatment and steroids empirically. But the patient was lost to further follow up.
Procedure
The most common target of biopsy was midbrain in more than half of the patients, followed by pons [Table 1]. Medulla was not targeted in any of the patients. All the biopsies in adults were done under local anesthesia only, except for one patient with altered sensorium, who required general anesthesia. In contrast, most of the biopsies in children were done under general anesthesia or local anesthesia with sedation [Table 2].
Table 2.
Details of STB procedure and complications
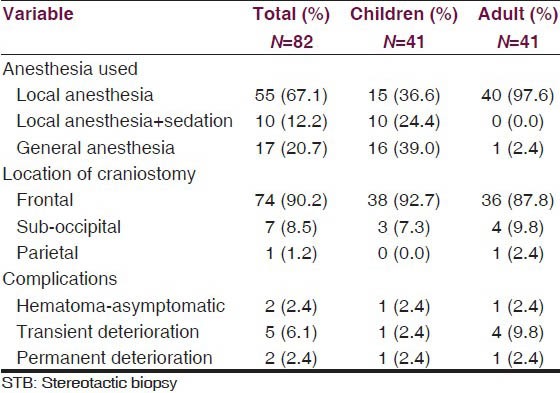
CT was used as guidance in 73 (38 children and 35 adults) patients and MRI in 9 (3 children and 6 adults). In 90.2% of the lesions, a precoronal frontal entry point was selected [Table 2]. An anteriorly located midbrain lesion in one patient was approached from a right parietal entry point. A sub occipital entry point with prone position and sub occipital burr hole was used in seven patients in the early part of the series, in the operating room with lesions limited to below mid pons. General anesthesia was used in two of these patients and local anesthesia in the other five patients [Table 2].
Histological diagnosis
The histological diagnosis of the biopsied lesions, with their distribution among children and adults, are tabulated in Table 3. Malignant gliomas were the most common histological diagnosis in the whole cohort. Glioblastoma was significantly more common in children. It comprised of 29.3% of all pathologies in children, compared to only 4.9% of the pathologies in adult population, the difference being statistically significant (P = 0.007). Tuberculosis was the next major diagnosis (9.8%), with equal distribution in both adults and children [Figure 2a]. Three patients were diagnosed with other primary malignancies. Two adults with well defined and uniformly enhancing mass lesions in the midbrain, one showing extension to thalamus were diagnosed as nonHodgkin's lymphoma [Figure 2b]. The third patient was a 16-year-old child, with a radiological diagnosis of midbrain lesion based on CT, whose histopathology was reported as germinoma [Figure 2c].
Table 3.
Histopathological diagnosis: Distribution between children and adults
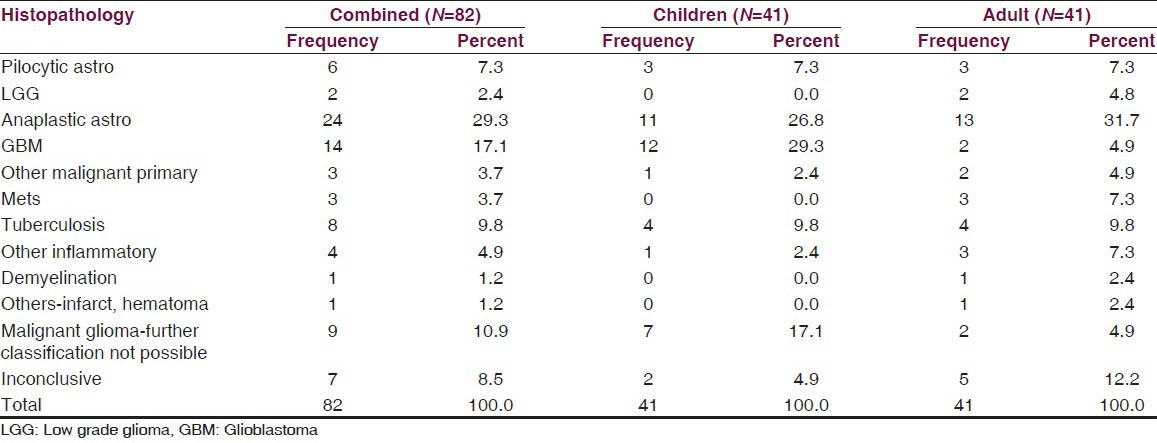
Figure 2.
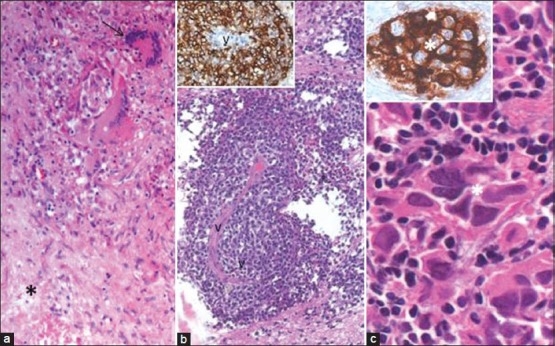
(a) STB from a ring enhancing lesion reveals tuberculoma showing foci of caseous necrosis (asterix), epitheloid cells, and Langhans giant cells (arrow). (b) STB of an uniformly enhancing mid brain lesion reveals NonHodgkin's lymphoma with neoplastic lymphoid cells around a vessel (V), which are strongly labeled by B cell marker, CD20 (B, inset). (c) STB from a midbrain lesion revealed characteristic features of germinoma with large epithelial appearing cells, strongly expressing PLAP (inset), admixed with mature lymphocytes. [a: H and E, ×20; b: H and E, ×10; b, inset: CD20 IHC ×20; c: H and E, ×40; c, inset: PLAP IHC ×40]
There were 11 diffuse nonenhancing lesions, which consisted of 6 children and 5 adults. The histopathology of the lesions in children were pilocytic astrocytoma (n = 1), anaplastic astrocytoma (n = 2), GBM or high grade glioma (n = 1), malignant glioma with further classification not possible (n = 2). In adults, all the five lesions were histologically diagnosed as anaplastic astrocytoma.
Inconclusive biopsy
In 12 (14.6%) patients, initial biopsy was inconclusive. A review of radiological data, available on 11 of these patients revealed that 9 lesions were located in upper pons and midbrain and two in lower pons. Seven lesions were well defined with ring (4/7) or heterogeneous (3/7) enhancement. Four patients with initial inconsistent biopsy underwent a repeat STB from brainstem, which yielded a histological diagnosis of anaplastic astrocytoma (n = 2), tuberculoma (n = 1), and pineocytoma (n = 1). One adult patient had a small frontal lesion at initial presentation, which was considered insignificant, enlarged in size during follow up imaging after inconclusive brainstem biopsy. An STB from the frontal lesion yielded a diagnosis of metastasis. Overall, a diagnosis was possible for 75/82 (91.5%) patients by STB. For the remaining patients with inconclusive biopsy, option of repeat biopsy was offered, but they declined and were lost to follow up. The histology in inconclusive biopsies had shown normal brain tissue, white matter tracts, astrocytes, and occasional neurons in most of the cases. In two cases, reactive astrocytes or focal aggregates of microglial nodules were noted. In two cases, a suspicion of neoplasm was raised, but a definitive diagnosis could not be made. On analyzing the factors contributing to the inconclusive biopsy, the location of the target lesion (P = 0.545), the choice of entry (P = 0.513), the radiological characteristic of the lesion, namely well defined or ill defined (P = 0.14), enhancement pattern (P = 0.842), age group pediatric vs adult; P = 0.349) and imaging modality used for target localization (CT vs MRI; P = 0.620) did not significantly correlate with the occurrence of inconclusive biopsy.
Complications
Complications were relatively rare in the present series. Permanent deterioration in sensorium and fresh cranial nerve deficits occurred in two patients (2.4%). Both patients had anaplastic astrocytoma involving midbrain and pons. One of them had a large intralesional hematoma causing mass effect, which was evacuated through a sub occipital craniectomy. But the patient did not improve in sensorium and persisted to have neurological deficits. The other patient developed small hematoma without any mass effect, which was treated conservatively. Five patients had deterioration in sensorium transiently. The sensorium improved with conservative management. One patient with transient deterioration of sensorium had small amount of intraventricular hemorrhage, which cleared with conservative management. Two patients had small hematoma in post-STB scan without any symptoms. One of these patients had mild hydrocephalus before biopsy, which was managed with insertion of a ventriculo-peritoneal shunt system. There was no significant difference in morbidity between pediatric and adult population. There was no significant influence of location of target and choice of entry in the occurrence of complications. There was no procedure-related mortality in the present series.
Discussion
Image-guided stereotactic biopsy for histopathological diagnosis of cerebral lesions has become a standard component of the neurosurgical armamentarium.[13,14] Image directed stereotactic brainstem biopsy was first reported by Gleason et al. in 1978.[15] CT and MRI directed stereotactic targeting are both used in brainstem biopsies. Most of the literature published regarding brain stem masses has been based on studies in children, where the diversity of pathology is not as broad as in adults.[2] In brain-stem lesions in adults, MRI is limited in its capability for differentiating tumor vs. nontumor, particularly in cases of infection or inflammation.[2,16]
The present study is a retrospective analysis of brainstem lesions, which underwent stereotactic biopsy over a long period, with 50% of the cohort consisting of adults. This is in contrast to studies of brainstem lesions, which reported brainstem lesions occurring in children only.[17,18] But in studies dealing with stereotactic biopsy of brainstem lesions, adults outnumber children indicating that an adult with a brainstem lesion is more likely to undergo biopsy than a child with brainstem lesion.[2,19,20]
Most of the lesions in the present study were located in the midbrain or upper pons in contrast to some previous studies in which the majority of lesions were located in pons followed by medulla.[17,21,22] However, Kratimenos et al. reported that midbrain and upper pontine lesions predominated in their series.[23] The disparity may be due to the various practice guidelines and indications for stereota ctic biopsy in various institutes.
Diagnostic yield of stereotactic biopsy for intracranial lesions show wide variations among different studies. An inconclusive biopsy is reported in 2-30% of the cases in various studies.[24,25] But most of the modern studies report a diagnostic yield of more than 90%. Studies specifically on stereotactic biopsy of brainstem lesions give diagnostic rates comparable to more frequently biopsied supra tentorial lesions.[2,11,17,21,25,26,27,28] A recent meta analysis of 38 studies reported a diagnostic yield of 96.2% by weighted average proportions analysis.[16] In the present study, diagnostic yield of first stereotactic biopsy was noted to be 85.4%. This is slightly less compared with most of the studies, but well within the reported range. The reasons proposed in literature for diagnostic failure in stereotactic brain biopsy include small sample size, inaccurate tissue targeting resulting in sampling error, target choice in areas of high signal on T2-weighted MRI, small target size, necrotic lesion, immunocompromised patient, nonneoplastic lesions, and nonenhancing lesions.[24] The diagnostic yield was directly associated with the number of samples taken during the procedure, in a previous study. Diagnostic accuracy was 84-88% for 2-3 bits and increased with higher numbers. This demonstrates that the diagnostic yield can be improved by taking more tissue samples during a procedure.[29] Most of the inconclusive biopsies in the present series were for lesions with heterogeneous or ring enhancement pattern, suggesting a possibility of biopsy from necrotic area. Five patients who had an initial inconclusive biopsy in the present series underwent a repeat STB, which yielded a total diagnostic accuracy of 91.5%.
Predominant pathology in both children and adults were high grade glioma in the present series, which was in concordance with other studies.[2,20,25] Rajshekhar et al. reported low grade glioma as the most common brainstem pathology in children.[30] In the present series, the lesions were almost exclusively gliomas in children, with exception of tuberculomas in four cases, whereas in adults there was a wider spectrum of diagnosis. Another important finding is that tuberculomas constituted 9.8% of all the pathologies in this series. A similar pattern was observed in other Indian studies also, in contrary to Western studies.[17,30,31]
We noted that all the biopsies in diffuse nonenhancing lesions were gliomas. The histopathology of the lesions in children were pilocytic astrocytoma (n = 1), anaplastic astrocytoma (n = 2), GBM or high grade glioma (n = 1), malignant glioma with further classification not possible (n = 2). In adults, all the five lesions were histologically diagnosed as anaplastic astrocytoma. The present series had six children with nonenhancing diffuse lesions who underwent biopsy in the early part of the series. This probably would indicate the changing practice over the past 15 years. In children, our present practice is to consider a biopsy in an enhancing brainstem mass where the diagnosis is not certain by radiological imaging. Patients with classical MR features of tuberculosis will undergo empirical anti-tubercular therapy and biopsy is considered if there is no response. We consider that diffuse nonenhancing brainstem tumors in children do not require biopsy confirmation before consideration of radiotherapy.
Various approaches and techniques have been used to perform STB in brainstem lesions. While some authors have used a transfrontal access to the brainstem lesions,[17,23] Patel et al.[31] and Abernathey et al.[3] exclusively used transcerebellar approach in all of their patients. Both the approaches have advantages and limitations. A transfrontal approach is performed in supine position, which makes it easier to perform. However, some lesions, especially those placed laterally in the pons cannot be targeted by this route. Also, this route has to traverse a longer distance and has potential risk of penetration of ventral pia/ventricular entry. We use preoperative neuronavigation planning software recently for decision of target and entry point to avoid these potential complications. A transcerebellar approach has the advantage of a shorter trajectory. However, prone or semi sitting position needed for the procedure makes it difficult for both the surgeon and the patient. A study by Dellaretti et al. comparing the transfrontal and transcerebellar approaches for STB in 142 patients concluded that the diagnostic accuracy and the complication rates are similar in both approaches. They reported from a series of 142 patients biopsied by either transfrontal (n = 123) or transcerebellar (n = 19) route, the complication rate in transfrontal route was 17.9%, while the definitive morbidity rate was 9.8%. In transcerebellar group, the complication rate was 21.1%, while the definitive morbidity was 5.3% and mortality was 5.3%.[32] The location of the lesion may partly dictate the approach chosen. Transfrontal approach is the preferred route in most of the cases, which reflects the predominance of upper brainstem lesions.[19,23,33] In the present series, most of the patients underwent STB through a transfrontal access, which reflects the institutional practice. A small number of patients (seven) underwent transcerebellar approach by a suboccipital entry, which was performed in the early part of the series. Some authors reported the transcerebellar approach for brainstem lesions, which reflect the different institutional practice.[3,31]
In the present series, a twist drill craniostomy has been utilized in most of the cases for performing STB. Many authors utilize a burr hole for performing a STB for a brainstem lesion,[31,34] while some series describe the use of twist drill cranisotomy for STB.[8,35] Sanai et al. have reported that the majority of their patients underwent STB using a large twist drill craniostomy in their series.[36] Burr hole may provide placement of a pial incision under vision and may help in avoiding a sulcal entry. However, since the target is fixed by the calculation of the coordinates, a choice of twist drill craniostomy has not hampered any STB procedure in the present series. Furthermore, it obviates the need for any general anesthesia or sedation and the procedure can be easily performed under local anesthesia. The performance of STB under local anesthesia could be appropriate also in multimorbid patients in whom general anesthesia is a high risk factor, for example, due to cardiac disease. No complications related to twist drill craniostomy have been noted in the present series.
In some published studies, a high complication rate of more than 10% was reported for stereotactic biopsy of brainstem lesions.[20,21] Based on this observation, some authors have questioned the use of STB for these lesions.[5,37] STB of brainstem lesions was found to be a safe procedure in the present study, with 2.4% permanent deterioration and 6% transient deterioration, which are comparable to other similar studies.[20,25,30,38] This is also comparable to the complication rates reported for supratentorial biopsy.[2,27] The recent meta analysis of 1480 cases reported 7.8% overall morbidity, 1.7% permanent morbidity, and 0.9% mortality.[16]
Kratimenos et al.,[23] Patel et al.,[31] and Abernathy et al.[3] used general anesthesia in their cases and the procedure was done in the operating room. In younger children, STB is usually done under general anesthesia with local anesthesia used for older children and adults.[12] Two series with 13 patients each, have reported performance of STB under local anesthesia in operating room with good patient compliance.[8,36] While most of the present day stereotactic procedures have become complex procedures, which are performed under general anesthesia, using surgical neuronavigation systems or intraoperative MRI in a fully equipped operating room, our series demonstrates that these procedures can be done with patients awake most of the time, under local anesthesia in a procedure room using a twist drill craniostomy, with a comparable diagnostic and complication rate to other brainstem biopsy series. This avoids the need for operating room and anesthesia time, which is a very important factor in busy neurosurgical institutes in developing as well as developed countries. In children, the biopsies were performed under local anesthesia with sedation or under general anesthesia in the procedure room.
In conclusion, it can be reiterated that stereotactic biopsy has an important role, the treatment of brainstem lesions, more significantly in adults, due to the larger variety of mass lesions affecting the brainstem. It can be performed safely under local anesthesia through a twist drill craniostomy in most of the adults. Children may require sedation for the procedure. The procedure yields a high diagnostic rate and the complication rates are minimal.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Pereira EA, Jegan T, Green AL, Aziz TZ. Awake stereotactic brainstem biopsy via a contralateral, transfrontal, transventricular approach. Br J Neurosurg. 2008;22:599–601. doi: 10.1080/02688690802220387. [DOI] [PubMed] [Google Scholar]
- 2.Samadani U, Stein S, Moonis G, Sonnad SS, Bonura P, Judy KD. Stereotactic biopsy of brain stem masses: Decision analysis and literature review. Surg Neurol. 2006;66:484–90. doi: 10.1016/j.surneu.2006.05.056. [DOI] [PubMed] [Google Scholar]
- 3.Abernathey CD, Camacho A, Kelly PJ. Stereotaxic suboccipital transcerebellar biopsy of pontine mass lesions. J Neurosurg. 1989;70:195–200. doi: 10.3171/jns.1989.70.2.0195. [DOI] [PubMed] [Google Scholar]
- 4.Epstein F, McCleary EL. Intrinsic brain-stem tumors of childhood: Surgical indications. J Neurosurg. 1986;64:11–5. doi: 10.3171/jns.1986.64.1.0011. [DOI] [PubMed] [Google Scholar]
- 5.Albright AL. Diffuse brainstem tumors: When is a biopsy necessary? Pediatr Neurosurg. 1996;24:252–5. doi: 10.1159/000121047. [DOI] [PubMed] [Google Scholar]
- 6.Albright AL, Packer RJ, Zimmerman R, Rorke LB, Boyett J, Hammond GD. Magnetic resonance scans should replace biopsies for the diagnosis of diffuse brain stem gliomas: A report from the Children's Cancer Group. Neurosurgery. 1993;33:1026–9. doi: 10.1227/00006123-199312000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Epstein F, Wisoff JH. Surgical management of brain stem tumors of childhood and adolescence. Neurosurg Clin N Am. 1990;1:111–21. [PubMed] [Google Scholar]
- 8.Shad A, Green A, Bojanic S, Aziz T. Awake stereotactic biopsy of brain stem lesions: Technique and results. Acta Neurochir (Wien) 2005;147:47–9. doi: 10.1007/s00701-004-0420-3. [DOI] [PubMed] [Google Scholar]
- 9.Jallo GI, Biser-Rohrbaugh A, Freed D. Brainstem gliomas. Childs Nerv Syst. 2004;20:143–53. doi: 10.1007/s00381-003-0870-6. [DOI] [PubMed] [Google Scholar]
- 10.Massager N, David P, Goldman S, Pirotte B, Wikler D, Salmon I, et al. Combined magnetic resonance imaging- and positron emission tomography-guided stereotactic biopsy in brainstem mass lesions: Diagnostic yield in a series of 30 patients. J Neurosurg. 2000;93:951–7. doi: 10.3171/jns.2000.93.6.0951. [DOI] [PubMed] [Google Scholar]
- 11.Rachinger W, Grau S, Holtmannspotter M, Herms J, Tonn JC, Kreth FW. Serial stereotactic biopsy of brainstem lesions in adults improves diagnostic accuracy compared with MRI only. J Neurol Neurosurg Psychiatry. 2009;80:1134–9. doi: 10.1136/jnnp.2009.174250. [DOI] [PubMed] [Google Scholar]
- 12.Zrinzo LU, Thomas DG. Stereotactic Approaches to the Brain Stem. In: Lozano AM, Gildenberg PL, Tasker RR, editors. Textbook of Stereotactic and Functional Neurosurgery. Vol. 1. Berlin: Springer-Verlag; 2009. pp. 789–95. [Google Scholar]
- 13.Apuzzo ML, Chandrasoma PT, Cohen D, Zee CS, Zelman V. Computed imaging stereotaxy: Experience and perspective related to 500 procedures applied to brain masses. Neurosurgery. 1987;20:930–7. doi: 10.1227/00006123-198706000-00019. [DOI] [PubMed] [Google Scholar]
- 14.Barnett GH, Miller DW, Weisenberger J. Frameless stereotaxy with scalp-applied fiducial markers for brain biopsy procedures: Experience in 218 cases. J Neurosurg. 1999;91:569–76. doi: 10.3171/jns.1999.91.4.0569. [DOI] [PubMed] [Google Scholar]
- 15.Gleason CA, Wise BL, Feinstein B. Stereotactic localization (with computerized tomographic scanning), biopsy, and radiofrequency treatment of deep brain lesions. Neurosurgery. 1978;2:217–22. doi: 10.1227/00006123-197805000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Kickingereder P, Willeit P, Simon T, Ruge MI. Diagnostic value and safety of stereotactic biopsy for brainstem tumors: A systematic review and meta-analysis of 1480 cases. Neurosurgery. 2013;72:873–81. doi: 10.1227/NEU.0b013e31828bf445. [DOI] [PubMed] [Google Scholar]
- 17.Selvapandian S, Rajshekhar V, Chandy MJ. Brainstem glioma: Comparative study of clinico-radiological presentation, pathology and outcome in children and adults. Acta Neurochir (Wien) 1999;141:721–6. doi: 10.1007/s007010050367. [DOI] [PubMed] [Google Scholar]
- 18.Guzman-De-Villoria JA, Fernandez-Garcia P, Ferreiro-Arguelles C. Differential diagnosis of T2 hyperintense brainstem lesions: Part 1. Focal lesions. Semin Ultrasound CT MR. 2010;31:246–59. doi: 10.1053/j.sult.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Chen SY, Chen CH, Sun MH, Lee HT, Shen CC. Stereotactic biopsy for brainstem lesion: Comparison of approaches and reports of 10 cases. J Chin Med Assoc. 2011;74:110–4. doi: 10.1016/j.jcma.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 20.Steck J, Friedman WA. Stereotactic biopsy of brainstem mass lesions. Surg Neurol. 1995;43:563–7. doi: 10.1016/0090-3019(95)00156-5. [DOI] [PubMed] [Google Scholar]
- 21.Guillamo JS, Monjour A, Taillandier L, Devaux B, Varlet P, Haie-Meder C, et al. Brainstem gliomas in adults: Prognostic factors and classification. Brain. 2001;124:2528–39. doi: 10.1093/brain/124.12.2528. [DOI] [PubMed] [Google Scholar]
- 22.Kwon JW, Kim IO, Cheon JE, Kim WS, Moon SG, Kim TJ, et al. Paediatric brain-stem gliomas: MRI, FDG-PET and histological grading correlation. Pediatr Radiol. 2006;36:959–64. doi: 10.1007/s00247-006-0256-5. [DOI] [PubMed] [Google Scholar]
- 23.Kratimenos GP, Nouby RM, Bradford R, Pell MF, Thomas DG. Image directed stereotactic surgery for brain stem lesions. Acta Neurochir (Wien) 1992;116:164–70. doi: 10.1007/BF01540871. [DOI] [PubMed] [Google Scholar]
- 24.Hall WA. The safety and efficacy of stereotactic biopsy for intracranial lesions. Cancer. 1998;82:1749–55. doi: 10.1002/(sici)1097-0142(19980501)82:9<1756::aid-cncr23>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 25.Perez-Gomez JL, Rodriguez-Alvarez CA, Marhx-Bracho A, Rueda-Franco F. Stereotactic biopsy for brainstem tumors in pediatric patients. Childs Nerv Syst. 2010;26:29–34. doi: 10.1007/s00381-009-1000-x. [DOI] [PubMed] [Google Scholar]
- 26.Kesari S, Kim RS, Markos V, Drappatz J, Wen PY, Pruitt AA. Prognostic factors in adult brainstem gliomas: A multicenter, retrospective analysis of 101 cases. J Neurooncol. 2008;88:175–83. doi: 10.1007/s11060-008-9545-1. [DOI] [PubMed] [Google Scholar]
- 27.Samadani U, Judy KD. Stereotactic brainstem biopsy is indicated for the diagnosis of a vast array of brainstem pathology. Stereotact Funct Neurosurg. 2003;81:5–9. doi: 10.1159/000075097. [DOI] [PubMed] [Google Scholar]
- 28.Rajshekhar V, Chandy MJ. Computerized tomography-guided stereotactic surgery for brainstem masses: A risk-benefit analysis in 71 patients. J Neurosurg. 1995;82:976–81. doi: 10.3171/jns.1995.82.6.0976. [DOI] [PubMed] [Google Scholar]
- 29.Jain D, Sharma MC, Sarkar C, Deb P, Gupta D, Mahapatra AK. Correlation of diagnostic yield of stereotactic brain biopsy with number of biopsy bits and site of the lesion. Brain Tumor Pathol. 2006;23:71–5. doi: 10.1007/s10014-006-0204-y. [DOI] [PubMed] [Google Scholar]
- 30.Rajshekhar V, Moorthy RK. Status of stereotactic biopsy in children with brain stem masses: Insights from a series of 106 patients. Stereotact Funct Neurosurg. 2010;88:360–6. doi: 10.1159/000319044. [DOI] [PubMed] [Google Scholar]
- 31.Patel P, Balamurugan M. Transcerebellar stereotactic biopsy for brainstem lesions in children. J Pediatr Neurosci. 2009;4:17–9. doi: 10.4103/1817-1745.49101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dellaretti M, Reyns N, Touzet G, Dubois F, Gusmao S, Pereira JL, et al. Stereotactic biopsy for brainstem tumors: Comparison of transcerebellar with transfrontal approach. Stereotact Funct Neurosurg. 2012;90:79–83. doi: 10.1159/000335502. [DOI] [PubMed] [Google Scholar]
- 33.Coffey RJ, Lunsford LD. Stereotactic surgery for mass lesions of the midbrain and pons. Neurosurgery. 1985;17:12–8. doi: 10.1227/00006123-198507000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Heper AO, Erden E, Savas A, Ceyhan K, Erden I, Akyar S, et al. An analysis of stereotactic biopsy of brain tumors and nonneoplastic lesions: A prospective clinicopathologic study. Surg Neurol. 2005;64(Suppl 2):S82–8. doi: 10.1016/j.surneu.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 35.Kondziolka D, Lunsford LD. The role of stereotactic biopsy in the management of gliomas. J Neurooncol. 1999;42:205–13. doi: 10.1023/a:1006105415194. [DOI] [PubMed] [Google Scholar]
- 36.Sanai N, Wachhorst SP, Gupta NM, McDermott MW. Transcerebellar stereotactic biopsy for lesions of the brainstem and peduncles under local anesthesia. Neurosurgery. 2008;63:460–6. doi: 10.1227/01.NEU.0000324731.68843.74. [DOI] [PubMed] [Google Scholar]
- 37.Albright AL, Guthkelch AN, Packer RJ, Price RA, Rourke LB. Prognostic factors in pediatric brain-stem gliomas. J Neurosurg. 1986;65:751–5. doi: 10.3171/jns.1986.65.6.0751. [DOI] [PubMed] [Google Scholar]
- 38.Kondziolka D, Lunsford LD. Results and expectations with image-integrated brainstem stereotactic biopsy. Surg Neurol. 1995;43:558–62. doi: 10.1016/0090-3019(95)00009-7. [DOI] [PubMed] [Google Scholar]


