Abstract
Elevated expression of inhibitory receptors on virus-specific T cells has been implicated as a mechanism by which viruses evade host immune surveillance. Blockade of these pathways during chronic infection leads to increased T cell function and improved immune control of viral replication. To explore the association between costimulatory receptors and HIV replication, we examined the expression of programmed death 1 (PD-1), CTLA-4, T cell Ig domain and mucin domain 3 (TIM-3), and CD28 on HIV-specific CD4+ T cells from HIV-infected subjects. Greater than 30% of HIV-specific CD4+ T cells from untreated subjects coexpressed PD-1, CTLA-4, and TIM-3, whereas <2% of CMV- or varicella-zoster virus-specific CD4+ T cells expressed all three receptors. Coexpression of all three inhibitory receptors on HIV-specific CD4+ T cells was more strongly correlated with viral load compared with the expression of each receptor individually. Suppression of HIV replication with antiretroviral therapy was associated with decreased expression of all three inhibitory receptors on HIV-specific CD4+ T cells. Surprisingly, a high percentage of HIV-specific CD4+ T cells that expressed inhibitory receptors also coexpressed CD28. In vitro blockade of PD-1 binding concurrent with stimulation through CD28 synergistically increased HIV-specific CD4+ T cell proliferation to a greater extent than did either alone. These findings indicate that HIV-specific CD4+ T cell responses during chronic infection are regulated by complex patterns of coexpressed inhibitory receptors and that the synergistic effect of inhibitory receptor blockade and stimulation of costimulatory receptors could be used for therapeutic augmentation of HIV-specific CD4+ T cell function.
Virus-specific memory CD4+ T cells are crucial for effective control of viral replication (1, 2) and are regulated by a delicate balance between costimulatory signals that activate T cells and inhibitory signals that attenuate harmful inflammatory responses (3–5). Simultaneous recognition of the cognate MHC-peptide complex by the TCR (signal 1) and B7 costimulatory family members (CD80/CD86) by CD28 (signal 2) results in T cell activation, proliferation, and differentiation. Conversely, coligation of the TCR with inhibitory receptors results in cell cycle arrest, decreased function, and cell death (3, 4). During chronic viral infection, the balance between positive and negative signals is skewed toward negative regulatory pathways that attenuate virus-specific T cell function, contributing to ongoing viral replication and viral persistence (4, 6–9).
During HIV infection, virus-specific CD4+ T cells undergo exhaustion, which is characterized by progressive loss of effector functions and leads to ineffective T cell responses (4, 6, 9, 10). Although the relationship between HIV-specific CD4+ T cell dysfunction and HIV disease progression is incompletely understood, recent studies suggest that signaling through inhibitory receptors, such as programmed death 1 (PD-1), CTLA-4, and T cell Ig domain and mucin domain 3 (TIM-3), plays a role (7–9, 11–16). PD-1 and CTLA-4 are members of the B7-CD28 family of immunoregulatory molecules (17). PD-1, a negative regulator of T cells (17), was originally identified as a surface receptor involved in apoptosis (18). Engagement of PD-1 with its ligands PD-L1 and PD-L2 inhibits T cell proliferation and cytokine production (17). An increasing body of evidence revealed that PD-1 has a critical role in the regulation of HIV-specific (11, 12, 14–16, 19), SIV-specific (20, 21), and lymphocytic choriomeningitis virus (LCMV)-specific (22–24) T cell responses. CTLA-4 binds to the same ligands as CD28, antagonizes the positive secondary signal mediated by CD28, and inhibits T cell activation by reducing the production of IL-2 and arresting cell-cycle progression (17). Its expression is also increased on total and virus-specific T cells during chronic HIV and hepatitis C virus (HCV) infection (14, 25–27). TIM-3, an Ig superfamily member, was identified as a specific cell surface marker of mouse Th1 CD4+T cells (28). Interaction of mouse TIM-3 with its ligand galectin-9 regulates Th1 responses by promoting the death of IFN-γ– producing Th1 cells (29). TIM-3 was recently shown to be upregulated on T cells from HIV-infected (13) and HCV-infected (30) subjects. Importantly, transient blockade of these inhibitory receptors enhances the function of HIV-specific T cells in humans (11–14), LCMV-specific T cells in mice (22), and SIV-specific T cells in rhesus macaques (20, 21, 31).
In addition to the increased expression of inhibitory receptors, stimulatory receptor expression is also affected by HIVinfection (4). We recently demonstrated that 4-1BB, an inducible costimulatory receptor (5), is downregulated on HIV-specific CD4+ T cells (32). Furthermore, decreased CD28 expression on HIV-specific CD8+T cells has long been implicated as a cause for reduced HIV-specific CTL function (33, 34). Stimulation through CD28 and 4-1BB increased proliferation and cytokine production by HIV-specific CD8+ T cells (35–37). Taken together, these findings suggest that simultaneous blockade of inhibitory receptors and signaling through stimulatory receptors may enhance HIV-specific CD4+ T cell function and may represent a novel therapeutic strategy to control HIV replication.
A recent study using the LCMV model of chronic infection demonstrated that exhausted T cells simultaneously expressed many inhibitory receptors (38). Therefore, we hypothesize that examination of multiple inhibitory receptors on HIV-specific CD4+ T cells will provide a stronger correlate of CD4+ T cell dysfunction than analysis of each receptor alone and that simultaneous blockade of inhibitory receptor ligation and stimulation through a costimulatory receptor will enhance HIV-specific CD4+ T cell function more than each alone. Using intracellular cytokine staining and multiparametric flow cytometry, we show that HIV-specific CD4+ T cells express high levels of CTLA-4, PD-1, and TIM-3 and that the simultaneous expression of these markers on HIV-specific CD4+ T cells correlates more strongly with plasma HIV viral load than with their individual expression. In addition, in vitro blockade of PD-1/PD-L1 pathway and stimulation via CD28 synergistically enhanced HIV-specific CD4+ T cell proliferation. These findings describe the expression of a complex network of costimulatory receptors that can be exploited to increase HIV-specific CD4+ T cell function to potentially better control HIV replication.
Materials and Methods
Study population
Sixty four HIV-1–infected subjects with progressive disease were enrolled into two clinical cohorts based on their treatment status: on antiretroviral therapy (ART) with virological suppression (suppressed) or untreated (viremic). Inclusion criteria for the suppressed cohort (n = 18) included receiving a combination of antiretroviral agents with suppression of plasma viral load to <48 copies of HIV-1 RNA/ml plasma for ≥6 mo (median CD4+ T cell count: 574 cells/μl; range, 396–2,444 cells/μl). Untreated subjects (n = 46) were treatment naive or off treatment for ≥6 mo with a median viral load of 22,000 copies HIV-1 RNA/ml plasma (range, 223–179,000) and a median CD4+ T cell count of 476 cells/μl (range, 306–1,053 cells/μl). HIV-1-seronegative subjects (n = 7) were normal healthy adult volunteers. All study subjects participated voluntarily and gave informed consent. The study was approved by the University of Colorado Denver Institutional Review Board.
T cell stimulations for Ag-specific cytokine production
Blood was collected in BD Vacutainer tubes containing sodium heparin. Within 4 h of venipuncture, PBMCs were isolated from whole blood by density gradient centrifugation on Ficoll (GE Healthcare, Piscataway, NJ). PBMCs (2.5–5 × 106) were resuspended in RPMI 1640 plus 10% human Ab serum and placed in 12 × 75-mm culture tubes. anti-CD28 and -CD49d mAbs (1 μg/ml; BD Biosciences, San Jose, CA) were added, and the cells were stimulated under the following conditions: pooled HIV-1 Gag 15mers (1 μg/ml final concentration of each peptide; clade B HXB2 strain HIV-1; National Institutes of Health AIDS Research and Reference Reagent Program), CMV lysate (1/20 dilution, derived from a G-lung cell line infected with CMV strain AD169, virus titer 2 × 107 PFU/ml; provided by A. Weinberg, University of Colorado Denver), varicella-zoster virus (VZV) lysate (1/10 dilution, derived from a H-lung cell line infected with VZV strain Oka, virus titer 1 × 106 PFU/ml, provided by A. Weinberg), staphylococcal enterotoxin B (SEB, 1 μg/ml; Toxin Technologies), or medium alone. Cells were incubated at a 5° slant for a total of 6 h at 37°C in a humidified 5% CO2 atmosphere with 1 μg/ml brefeldin A (BD Biosciences, San Jose, CA) added after 2 h of stimulation.
Immunofluorescent staining of stimulated T cells
Stimulated PBMCs were washed with PBS containing 1% BSA and stained with biotinylated anti-human TIM-3 Ab (R&D Systems, Minneapolis, MN) for 30 min at 4°C. Cells were then washed and surfaced stained with anti-CD4 (PE-Cy5-5; Invitrogen, Carlsbad, CA), anti-CD3 (Qdot 605; Invitrogen), anti-CD8 (Alexa Fluor 405; Invitrogen), anti-PD-1 (FITC; BD Biosciences), and streptavidin (allophycocyanin; BD Biosciences) for 30 min at 4°C. Cells were washed, fixed, permeabilized (Invitrogen), and intracellularly stained with anti–IFN-γ (PE-Cy7; BD Biosciences), anti–IL-2 (Alexa Fluor 700; BioLegend), and anti–CTLA-4 (PE; BD Biosciences) mAbs for 30 min at 4°C, washed, and fixed with 1% formaldehyde. Fluorescence – 1 (FMO) controls were used in all staining.
CFSE proliferation assay with blockade of PD-1/PD-L1 pathway and stimulation of CD28
To track cell division, PBMCs from untreated subjects with chronic HIV infection (n = 17; median viral load: 28,100 copies HIV-1 RNA/ml plasma [range, 1930–70,000] and median CD4+ T cell count: 518 cells/μl [range, 360–1053]) were isolated as described above. PBMCs were labeled with 1 μM CFSE (Molecular Probes, Eugene, OR) in PBS for 15 min in a 37°C water bath, followed by two washes with PBS. Labeled PBMCs were resuspended in RPMI 1640 supplemented with 10% human Ab serum and incubated for 30 min at 37°C in a humidified 5% CO2 atmosphere. After incubation, PBMCs were plated at 1 × 106 cells/ml in a 48-well plate and stimulated with 2 μg/ml HIV-1 Gag peptides, 2 μg/ml CMV peptides (HCMV pp65 peptide pool), 1 μg/ml SEB, or medium alone (no Ag). Each stimulation condition was incubated with anti–PD-L1 (5 μg/ml; eBioscience, San Diego, CA), anti-CD28 (2 μg/ml; BD Biosciences), anti–PD-L1 and anti-CD28, or nothing at all. The cells were cultured for 6 d in RPMI 1640 plus 10% human serum at 37°C in a humidified 5% CO2 atmosphere. On day 7, cells were washed with PBS containing 1% BSA and surfaced stained with anti-CD4 (PE-Cy5-5; BD Biosciences), anti-CD3 (Qdot 605; Invitrogen), or anti-CD8 (Alexa Fluor 405; Caltag Laboratories) for 30 min at 4°C, washed, and fixed with 1% formaldehyde.
Flow cytometry
Cells were analyzed using an LSR-II flow cytometer (BD Immunocytometry Systems, San Jose, CA); between 1 and 3 million events were collected. Electronic compensation was performed with Ab capture beads (BD Biosciences) stained separately with individual mAbs used in the test samples. To ensure the accuracy and precision of the measurements taken from day to day, quality control was performed on the LSR-II daily using the Cytometer Setup & Tracking (CS&T) feature within BD FACSDiva software. The program uses standardized CS&T beads (BD Biosciences) to determine voltage, laser delays, and area scaling and to track these settings over time. A manual quality control using rainbow beads was also performed daily to verify the laser delay and area scaling determined by CS&T. The data files were analyzed using Diva software (BD Biosciences) and FlowJo Software (Tree Star, Ashland, OR). Lymphocytes were gated by their forward and side scatter profile. CD3+ cells were selected, and expression of CD4 was analyzed in a bivariate dot plot with CD8 to exclude CD4/CD8 double-positive T cells. Biexponential scaling was used in all dot plots. The expression of TIM-3, PD-1, and CTLA-4 was examined on cytokine-producing cells with frequencies ≥0.04% above the background (media control tube) to ensure an adequate number of events for analysis, as previously validated by our laboratory (12, 32, 39, 40). The cutoff value was calculated by stimulating seronegative controls with HIV Gag to determine the background response of the assay and by calculating the frequency needed to obtain a minimum of 100 cytokine-positive events when 2.5 × 106 cells were analyzed on the flow cytometer. FMO controls were used to set the gates for determining the percentage of TIM-3, PD-1 and CTLA-4–positive T cells. The Boolean gating function of FlowJo, in which cells are divided into all possible receptor combinations measured by using the Boolean operations `and' and `not' on analysis gates applied to those measurements, was used to assess each possible inhibitory receptor-expression pattern. Coexpression patterns were analyzed using Simplified Presentation of Incredibly Complex Evaluations (Mario Roederer, National Institutes of Health, Bethesda, MD). The proliferation data are presented as net values after subtracting the percentage of CD3+CD4+ CFSElow T cells observed in unstimulated culture from the corresponding Ag-stimulated culture.
Statistical analysis
Statistical analysis was performed using GraphPad Prism (GraphPad, San Diego, CA). One-way ANOVAwith the Tukey posttest was used to examine the variation of TIM-3, PD-1, and CTLA-4 expression on total and Agspecific CD4+ T cells. The Wilcoxon signed-rank test was used to determine significance of differences between groups. Correlations were calculated using the nonparametric Spearman test. The Friedman test with the Dunn posttest was used to examine the variation of Ag-specific CD4+ T cell proliferation. A p value < 0.05 was considered statistically significant.
Results
HIV-specific IFN-γ–producing CD4+ T cells express high levels of inhibitory receptors
We previously demonstrated that PD-1 expression is elevated on HIV-specific CD4+ T cells (12). In this study we examined the simultaneous expression of PD-1, CTLA-4, and TIM-3 on HIV-, CMV-, and VZV-specific CD4+ T cells from chronically HIV-infected subjects. Because of the heterogeneity in the phenotype and function of virus-specific T cell responses previously described (15, 41, 42), we chose to examine responses directed against two contained (immune controlled) infections (CMV and VZV) as a control for HIV-specific responses. Virus-specific CD4+ T cells were identified by their ability to produce IFN-γ, a T cell function that was shown to persist into the late stages of exhaustion (43). We and other investigators previously demonstrated that in untreated HIV-infected subjects, IFN-γ–producing HIV-specific CD4+ T cells are less functional, exhibiting decreased proliferative capacity compared with CMV-specific IFN-γ–producing CD4+ T cells (40, 44, 45).
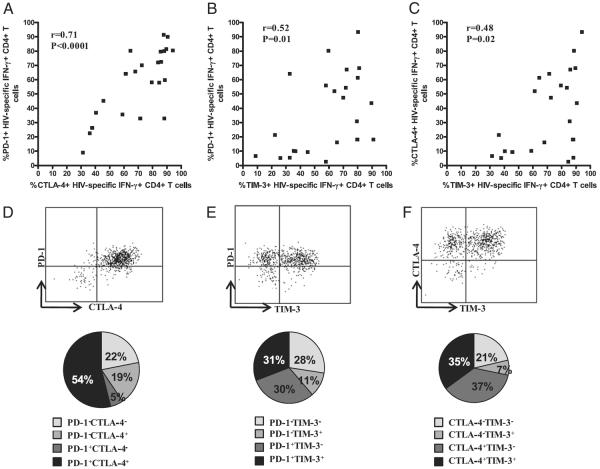
Fig. 1A illustrates representative plots of CTLA-4, PD-1, and TIM-3 expression on HIV-, CMV-, and VZV-specific IFN-γ–producing CD4+ T cells in an untreated HIV-infected subject. As shown in Fig. 1B, the percentage of CTLA-4–, PD-1–, and TIM-3–expressing CD4+ T cells was significantly greater on HIV-specific IFN-γ–producing CD4+ T cells (median: 85%, range, 36–94%; median: 71%, range, 22–91%; median: 50%, range, 5.4–93%, respectively) compared with CMV-specific (median: 46%, range, 16–82%; median: 11%, range, 3.0–55%; median: 18%, range, 2.2–74%, respectively) or VZV-specific IFN-γ–producing CD4+ T cells (median: 35%, range, 23–66%; median: 8.1%, range, 1.3–22%; median: 22%, range, 4.8–86%, respectively). The median expression of these inhibitory receptors on HIV-specific IFN-γ–producing CD4+ T cells was greatest for CTLA-4, followed by PD-1 and TIM-3. HIV-specific IL-2-producing CD4+ T cells also expressed significantly greater levels of CTLA-4 and PD-1 than did CMV- and VZV-specific CD4+ T cells (data not shown). However, unlike on IFN-γ–producing CD4+ T cells, TIM-3 expression was not significantly different on the virus-specific CD4+ T cells that produced IL-2 (data not shown).
FIGURE 1.
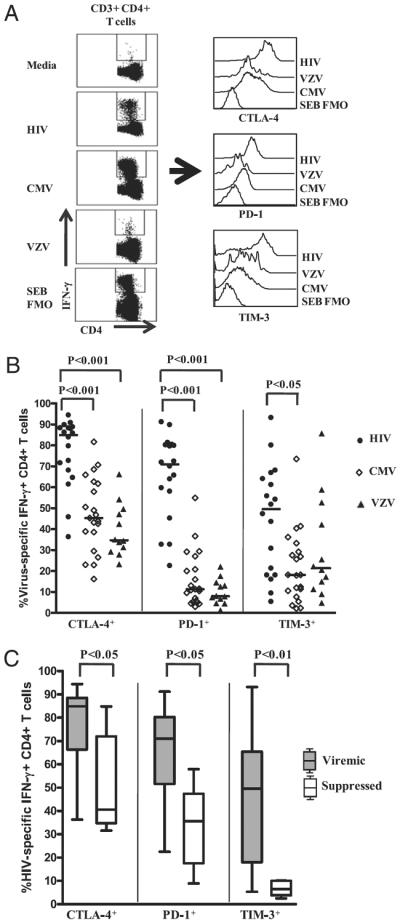
PD-1, CTLA-4, and TIM-3 expression is upregulated on HIV-specific CD4+ T cells in viremic HIV-infected subjects. PBMCs isolated from viremic and suppressed subjects with chronic HIV infection were stimulated with HIV, CMV, VZV, and SEB Ags for 6 h, with brefeldin A added after 2 h of stimulation. Stimulated cells were washed and stained with biotinylated anti-human TIM-3 Ab, followed by anti-CD4 PE-Cy5-5, anti-CD3 Qdot 605, anti-CD8 Alexa Fluor 405 mAbs and streptavidin allophycocyanin for 30 min at 4°C. Cells were washed, fixed, permeabilized, and intracellularly stained with anti–IFN-γ PE-Cy7 and anti–IL-2 Alexa Fluor 700 and anti–CTLA-4 PE mAbs for 30 min at 4°C, washed, fixed, and analyzed using an LSR-II flow cytometer. A, Representative flow-cytometry plots of PD-1, CTLA-4, and TIM-3 staining on HIV-, CMV-, and VZV-specific IFN-γ–producing CD4+ T cells and FMO control from a chronically HIV-infected viremic subject. B, Expression of PD-1, CTLA-4, and TIM-3 on HIV-, CMV-, and VZV-specific IFN-γ–producing CD4+ T cells from chronically HIV-infected subjects (n = 25). Dots represent individual values, with the solid lines indicating medians. Statistical significance was determined using one-way ANOVAwith the Tukey posttest. C, Expression of PD-1, CTLA-4, and TIM-3 on HIV-specific IFN-γ–producing CD4+ T cells from viremic and suppressed chronically HIV-infected subjects. Statistical significance was determined using the Wilcoxon signed-rank test.
We also examined the effects of viral suppression on inhibitory receptor expression by examining virus-specific CD4+ T cells from subjects receiving successful ART. The percentage of CTLA-4, PD-1, and TIM-3–expressing HIV-specific IFN-γ–producing CD4+ T cells was significantly lower in ART-treated subjects compared with untreated viremic subjects (Fig. 1C). The percentage of CTLA-4, PD-1, and TIM-3–expressing CMV- and VZV-specific IFN-γ–producing CD4+ T cells was not different between viremic and suppressed HIV-infected subjects or HIV seronegative controls (data not shown). Taken together, these findings suggest an association between HIV viremia and increased expression of inhibitory receptors on HIV-specific CD4+ T cells (12, 14).
Expression of inhibitory receptors on HIV-specific IFN-γ–producing CD4+ T cells correlate with one another
To determine whether an association exists between CTLA-4, PD-1, and TIM-3 expression, we evaluated the relationship between their expressions on HIV-specific CD4+ T cells. There was significant positive correlation between the expression of PD-1 and CTLA-4 (r = +0.71, p < 0.0001; Fig. 2A), PD-1 and TIM-3 (r = +0.52, p = 0.01; Fig. 2B), and TIM-3 and CTLA-4 (r = +0.48, p = 0.02; Fig. 2C) on HIV-specific IFN-γ–producing CD4+ T cells. There was also a significant positive correlation between the expression of PD-1 and CTLA-4 on HIV-specific IL-2–producing CD4+ T cells (r = +0.71, p = 0.0001; data not shown). No correlation was observed between the expression of TIM-3 and PD-1 (r = +0.09, p = 0.7) or TIM-3 and CTLA-4 (r = +0.3, p = 0.1) on HIV-specific IL-2–producing CD4+ T cells (data not shown). Because we observed a correlation in the expression of the receptors, we next examined whether the expression of PD-1, CTLA-4, and TIM-3 identifies the same or a distinct population of HIV-specific CD4+ T cells. A larger percentage of IFN-γ–producing HIV-specific CD4+ T cells coexpressed PD-1 and CTLA-4 than either alone (Fig. 2D). However, coexpression of PD-1 and TIM-3 (Fig. 2E) and of CTLA-4 and TIM-3 (Fig. 2F) on IFN-γ–producing HIV-specific CD4+ T cells was more heterogeneous. Taken together, these data indicate covariant expression of PD-1 and CTLA-4 on HIV-specific cytokine-producing CD4+ T cells that is distinct from TIM-3, indicating differential expression pattern of inhibitory receptors.
FIGURE 2.
Relationship between the expression of PD-1, CTLA-4, and TIM-3 on HIV-specific CD4+ T cells. Significant positive correlation between PD-1 and CTLA-4 (A), PD-1 and TIM-3 (B), CTLA4 and PD-1 (C) on HIV-specific IFN-γ+ CD4+ T cells from viremic chronically HIV-infected subjects. The Spearman correlation test was used to determine statistical significance. Representative flow-cytometry plots of the expression of PD-1 and CTLA-4 (D), PD-1 and TIM-3 (E), and CTLA-4 and TIM-3 (F) on HIV-specific IFN-γ+ CD4+ T cells from a viremic chronically HIV-infected subject. The pie charts show expression patterns of PD-1 and CTLA-4 (D), PD-1 and TIM-3 (E), and CTLA-4 and TIM-3 (F) on HIV-specific IFN-γ–producing CD4+ T cells from viremic chronically HIV-infected subjects.
Concurrent expression of CTLA-4, PD-1, and TIM3 on HIV-specific CD4+ T cells
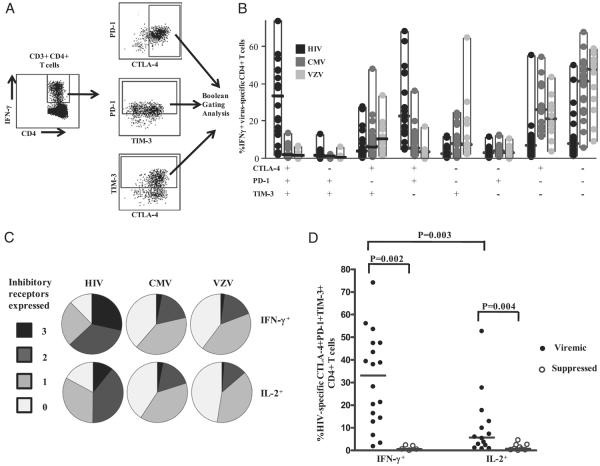
We next examined the simultaneous expression of CTLA-4, PD-1, and TIM-3 on virus-specific CD4+ T cells in subjects with chronic HIV infection. Fig. 3A illustrates the Boolean gating scheme used to analyze the simultaneous expression of the three inhibitory receptors on HIV-specific CD4+ T cells. Eight subpopulations of virus-specific CD4+ T cells were identified based on the different combinations of inhibitory receptors expressed, ranging from cells that did not express any inhibitory receptors to those that expressed all three (Fig. 3B). The pie chart (Fig. 3C) shows the proportion of virus-specific CD4+ T cells expressing three, two, one, or no inhibitory receptors. HIV-specific IFN-γ–producing CD4+ T cells coexpressed significantly greater levels of all three inhibitory receptors (median: 31%; range, 1.8–74%) compared with CMV-specific (median: 1.6%; range, 0.09–14%; p < 0.001) and VZV-specific (median: 1.4%; range, 0.0–6.7%; p < 0.001) CD4+ T cells (Fig. 3B, 3C). About 70% of HIV-specific CD4+ T cells expressed more than one inhibitory receptor compared with 21% of CMV-specific and 18% of VZV-specific CD4+ T cells (Fig. 3C). In addition, only 14% of the HIV-specific CD4+ T cells did not express any inhibitory receptor, whereas 38% and 41% of the CMV- and VZV-specific CD4+ T cells, respectively, did not express these receptors (Fig. 3B, 3C).
FIGURE 3.
Simultaneous expression of PD-1, CTLA-4, and TIM-3 on HIV-specific CD4+ T cells is greater than on CMV- or VZV-specific CD4+ T cells. A, Representative flow-cytometry plots of the Boolean analysis used to examine overlapping expression of CTLA-4, PD-1, and TIM-3 on HIV-specific IFN-γ–producing CD4+ T cells. B, Boolean analysis of the simultaneous expression of PD-1, CTLA-4, and TIM-3 on HIV-, CMV-, and VZV-specific IFN-γ–producing CD4+ T cells from viremic chronically HIV-infected subjects (n = 23). All of the possible combinations of the different markers are shown on the x-axis, whereas their percentages within virus-specific CD4+ T cell populations are shown on the y-axis. Dots represent individual values, and the solid horizontal lines indicate medians. C, Boolean gating analysis of the simultaneous expression of PD-1, CTLA-4, and TIM-3 on virus-specific IFN-γ–producing (upper panels) and IL-2–producing (lower panels) CD4+ T cells from viremic HIV-infected subjects. Individual populations are grouped according to total number of inhibitory receptors expressed. D, Simultaneous expression of CTLA-4, PD-1, and TIM-3 on HIV-specific IFN-γ+ and IL-2+ CD4+ T cells in viremic and suppressed chronically HIV-infected subjects. Dots represent individual values, and the solid horizontal lines indicate medians. Statistical significance was determined using the Mann–Whitney U test.
In a similar approach, we used the Boolean gating analysis to determine the simultaneous expression of CTLA-4, PD-1, and TIM-3 on virus-specific IL-2-producing CD4+ T cells in subjects with chronic HIV infection. We observed significantly increased coexpression of CTLA-4, PD-1, and TIM-3 on HIV-specific (median: 5.70%; range, 0.83–52.70%) versus CMV-specific (median: 0.9%; range, 0.1–26%; p < 0.05) and VZV-specific (median: 0.8%; range, 0.0–5.9%; p < 0.01) IL-2–producing CD4+ T cells (Fig. 3C). More than 50% of HIV-specific IL-2–producing CD4 T cells expressed more than one inhibitory receptor compared with 20% of CMV-specific and 13.6% of VZV-specific similar cells (Fig. 3C). In addition, only 17% of HIV-specific IL-2–producing CD4+ T cells did not express the three inhibitory receptors compared with 41% of CMV-specific and 48% of VZV-specific similar cells (Fig. 3C). Taken together, these data indicate a substantial coexpression of multiple inhibitory receptors on HIV-specific CD4+ T cells during chronic HIV infection.
Suppression of HIV replication is associated with reduced simultaneous expression of inhibitory receptors
Because we observed a significant reduction in the expression of CTLA-4, PD-1, and TIM-3 on HIV-specific CD4+ T cells in subjects on ART with suppressed plasma viral load compared with viremic subjects (Fig. 1C), we next investigated the level of simultaneous expression on virus-specific CD4+ T cells from the same subjects. IFN-γ–producing HIV-specific CD4+ T cells from viremic subjects expressed significantly greater levels of CTLA-4, PD-1, and TIM-3 (median: 31.3%; range, 1.8–74.10%) than did suppressed subjects (median: 0.54; range, 0.0–2.4%; p = 0.002) or IL-2–producing HIV-specific CD4+ T cells in viremic subjects (median: 5.7%; range: 0.8–53%; p = 0.003; Fig. 3D). In addition, the percentage of CTLA-4, PD-1, and TIM-3–expressing HIV-specific IL-2–producing CD4+ T cells was significantly greater in untreated viremic subjects (median: 5.7%; range, 0.8–53%) compared with its level in subjects on ART with suppressed plasma viral load (median: 0.7%; range, 0.0–4.6%; p = 0.004; Fig. 3D). The percentage of CTLA-4, PD-1, and TIM-3–coexpressing CMV- and VZV-specific IFN-γ–producing CD4+ T cells was not different between viremic and suppressed subjects (data not shown). However, their coexpression was significantly greater on CMV-specific IFN-γ–producing CD4+ T cells in HIV-infected subjects compared with similar cells in healthy HIV-seronegative controls (data not shown). The findings suggest that there is an association between HIV viremia and elevated coexpression of inhibitory receptors on HIV-specific CD4+ T cells.
CTLA-4, PD-1, and TIM-3 expression on HIV-specific CD4+ T cells correlates with plasma HIV viral load
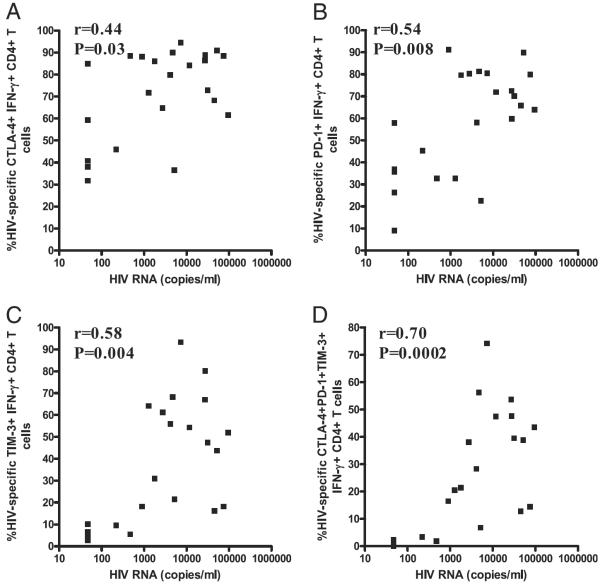
To determine whether an association exists between expression of CTLA-4, PD-1, and TIM-3 and markers of HIV disease progression, we evaluated the relationship between their expression on HIV-specific CD4+ T cells, HIV plasma viral load, and CD4 count. Significant positive correlation was observed between HIV plasma viral load and CTLA-4 (r = +0.44, p = 0.03; Fig. 4A), PD-1 (r = +0.54, p = 0.008; Fig. 4B), and TIM-3 (r = +0.58, p = 0.004; Fig. 4C) expression on IFN-γ–producing HIV-specific CD4+ T cells. Except for TIM-3 (r = +0.37, p = 0.07), expression of CTLA-4 (r = +0.54, p = 0.008) and PD-1 (r = +0.54, p = 0.008) on IL-2–producing HIV-specific CD4+ T cells also correlated positively with viral load (data not shown). Interestingly, the simultaneous expression of CTLA-4, PD-1, and TIM-3 on IFN-γ–producing (r = +0.70, p = 0.0002; Fig. 4D) and IL-2–producing (r = +0.62, p = 0.002; data not shown) HIV-specific CD4+ T cells correlated more strongly with plasma viral load than did their individual expression. These correlations were not significant when data from only viremic subjects were used (CTLA-4: r = +0.14, p = 0.6; PD-1: r = +0.21, p = 0.4; TIM-3: r = +0.18, p = 0.5; CTLA-4, PD-1, and TIM-3: r = +0.40, p = 0.09; data not shown). Although we observed a significant negative correlation between CD4 count and PD-1 expression on HIV-specific IFN-γ+ CD4+ T cells (r = −0.45, p = 0.02), no such correlation was observed with CTLA-4 and TIM-3 or their simultaneous expression (data not shown). There was no correlation between expression of the inhibitory receptors on CMV- and VZV-specific CD4+ T cells and HIV viral load or CD4 count (data not shown).
FIGURE 4.
Correlation between the percentage of HIV-specific CD4+ T cells expressing inhibitory marker and plasma HIV viral load. Significant positive correlation between viral load and expression of CTLA-4 (A), PD-1 (B), TIM-3 (C), and CTLA-4, PD-1, and TIM-3 (D) on HIV-specific IFN-γ+ CD4+ T cells from chronically HIV-infected subjects. Statistical analyses were performed using the Spearman correlation test.
CTLA-4, PD-1, and TIM-3 expression is greater on HIV-specific IFN-γ–producing than on IL-2–producing CD4+ T cell subsets
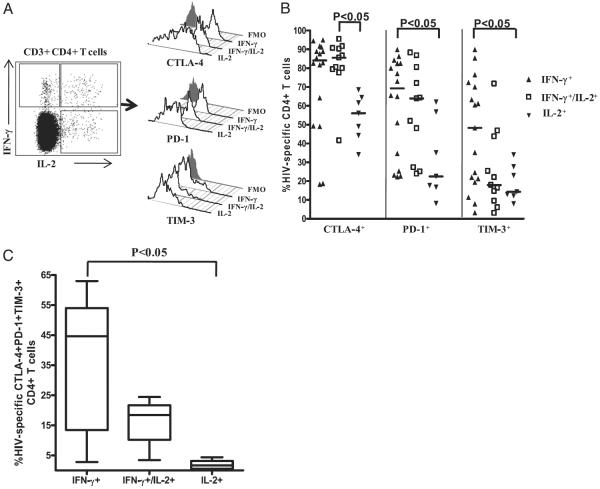
We previously showed that IFN-γ–producing HIV-specific CD4+ T cells are more differentiated than IL-2–producing cells. The production of IFN-γ was also associated with an effector memory phenotype and greater levels of the senescence marker CD57, whereas IL-2 production was associated with a less-differentiated central memory phenotype and a paucity of CD57 (39, 40). To determine whether inhibitory receptor expression differs during the various stages of CD4+ T cell differentiation, we compared the expression of CTLA-4, PD-1, and TIM-3 on HIV-specific CD4+ T cells that produced IFN-γ only (IFN-γ+IL-2−), IL-2 only (IFN-γ−IL-2+), or IFN-γ and IL-2 (IFN-γ+IL-2+) from untreated chronically HIV-infected subjects (Fig. 5A). HIV-specific CD4+ T cells producing IFN-γ alone expressed greater levels of CTLA-4, PD-1 (p < 0.05), and TIM-3 (p < 0.05) than did similar cells producing only IL-2 (Fig. 5B). HIV-specific CD4+ T cells producing IFN-γ and IL-2 expressed significantly greater levels of CTLA-4 than did HIV-specific CD4+ T cells producing IL-2 alone (p < 0.05). However, PD-1 and TIM-3 expression was not significantly different between HIV-specific CD4+ T cells producing both IFN-γ and IL-2 or IL-2 alone, although the median level of PD-1 was greater on dual cytokine-producing cells, unlike that of TIM-3, which was the same as the level on cells producing only IL-2 (Fig. 5B). Taken together, our data show that CTLA-4 and PD-1 are expressed on a large percentage of HIV-specific CD4+ T cells that produce IFN-γ only and dual cytokines (IFN-γ+IL-2+), unlike TIM-3, which was expressed on a very low percentage of dual cytokine-producing HIV-specific CD4+ T cells.
FIGURE 5.
Distribution of CTLA-4, PD-1, and TIM3 expression on HIV-specific cytokine-producing CD4+ T cell subsets. A, Representative flow-cytometry plots showing staining of CTLA-4, PD-1, and TIM-3 on HIV-specific IFN-γ only-producing (IFN-γ+IL-2−), IL-2 only-producing (IFN-γ−IL-2+), or dual IFN-γ and IL-2–producing (IFN-γ+IL-2+) CD4+ T cells from a chronically HIV-infected viremic individual relative to FMO control. B, Expression of CTLA-4, PD-1, and TIM-3 on HIV-specific cytokine-producing CD4+ T cell subsets from chronically HIV-infected viremic subjects. Individual values are presented, with the solid lines indicating medians. Statistical significance was determined using one-way ANOVA with the Tukey posttest. C, Simultaneous expression of CTLA-4, PD-1, and TIM-3 on HIV-specific cytokine-producing CD4+ T cell subsets from chronically HIV-infected viremic subjects. Statistical significance was determined using one-way ANOVA with the Dunn posttest.
Next, we determined the proportion of the cytokine-producing HIV-specific CD4+ T cell subsets (IFN-γ only, IFN-γ/IL-2, and IL-2 only) that coexpress CTLA-4, PD-1, and TIM-3. This analysis was performed on a subset of untreated chronically HIV-infected subjects who had significant responses in all three cytokine-producing populations. A greater percentage of HIV-specific CD4+ T cells that produced only IFN-γ coexpressed all three inhibitory receptors (median: 44.6%, range, 2.8–63.0%) than did dual cytokine-producing cells (median: 18.4%; range, 3.4–24.4%) or cells producing only IL-2 (median: 1.6%; range, 0–4.3%) (Fig. 5C), indicating an association between increased coexpression of multiple inhibitory receptors and IFN-γ production.
We previously showed an association between a costimulatory receptor (4-1BB) expression and the ability of virus-specific CD4+ T cells to produce cytokine on a per-cell basis (32). In the current study, we also examined whether HIV-specific CD4+ T cells expressing inhibitory receptors produced less cytokine on a per-cell basis than did cells that did not express inhibitory receptors. We found no significant difference in the amount, as measured by the mean fluorescence intensity, of IFN-γ made by PD-1, CTLA-4, and TIM-3–positive and -negative subsets (data not shown). We also examined the frequency of IFN-γ–producing CD4+ T cells that expressed the inhibitory markers compared with those that did not express the markers by segregating total CD4+ T cells into PD-1, CTLA-4, or TIM-3–positive and -negative populations. The majority of HIV-specific IFN-γ–producing CD4+ T cells fall into the PD-1– and CTLA-4–expressing fractions (p = 0.03 and 0.001, respectively; data not shown). However, when the same analysis was performed on the TIM-3–positive and -negative populations, the percentage of IFN-γ–producing CD4+ T cells was similar in both populations (p = 0.7; data not shown). Analysis of the data using the three-tiered gating system described by Jones et al. (13) (Fig. 6A) showed that HIV-specific IFN-γ–producing CD4+ T cells were found in the TIM-3+ and TIM-3− subsets (Fig. 6B). In line with Jones et al. (13), we revealed a similar trend, with significantly lower frequencies of IFN-γ–producing CMV-specific, VZV-specific, and SEB-reactive CD4+ T cells in the TIM-3+ fractions (Fig. 6B).
FIGURE 6.
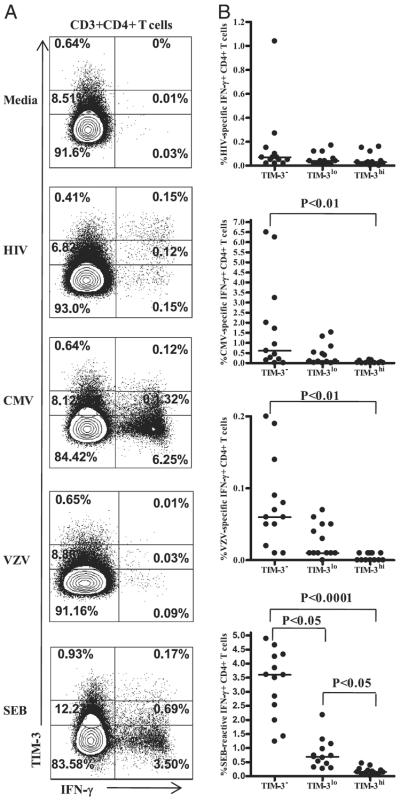
TIM-3 expression on Ag-specific IFN-γ–producing CD4+ T cells A, Representative flow-cytometry plots showing a three-tiered gating system (13) for analyzing IFN-γ responses to HIV, CMV, VZV, and SEB in TIM-3neg, TIM-3low, and TIM-3high CD4+ T cells from untreated subjects with chronic HIV infection. B, Pooled data showing IFN-γ response in TIM-3neg, TIM-3low, and TIM-3high CD4+ T cells.
Substantial coexpression of inhibitory receptors and CD28 on HIV-specific IFN-γ+ CD4+ T cells
We previously showed that 4-1BB, an inducible costimulatory molecule receptor, is downregulated on HIV-specific CD4+ T cells (32). However, unlike HIV-specific CD8+ T cells, CD4+ T cells were reported to maintain the expression of CD28 (46). To confirm this and to determine the relationship between CD28 and the expression of inhibitory receptors, we examined the level of coexpression of CD28 and inhibitory receptors on HIV- and CMV-specific CD4+ T cells from subjects with untreated chronic HIV infection. Surprisingly, a large percentage of CD28+ T cells also expressed inhibitory receptors (Fig. 7A). As shown in Fig. 7B, a large proportion of HIV-specific CD4+ T cells coexpressed CD28 and PD-1, CD28 and CTLA-4, and CD28 and TIM-3. This indicates the expression of costimulatory and inhibitory core-ceptors on the same HIV-specific CD4+ T cell, suggesting that these cells may be dysfunctional, despite the expression of a costimulatory molecule. Unlike on HIV-specific CD4+ T cells, the coexpression of CD28 with PD-1, CTLA-4, or TIM-3 on CMV-specific CD4+ T cells was not remarkable (Fig. 7B). Consistent with a previous report (46), the percentage of CD28-expressing CD4+ T cells was significantly greater on HIV-specific IFN-γ+ CD4+ T cells than on CMV-specific IFN-γ+ CD4+ T cells (p < 0.01; Fig. 7C). CD28 expression on total CD4+ T cells (median: 84%; range, 56–94%) was slightly greater than on HIV-specific CD4+ T cells (median: 75%; range, 41–97%), but it was significantly greater than on CMV-specific CD4+ T cells (median: 34%; range, 8–67%; p < 0.001; Fig. 7C). The expression of CD28 on total CD4+ T cells was reported to decline with HIV disease progression (47).
FIGURE 7.
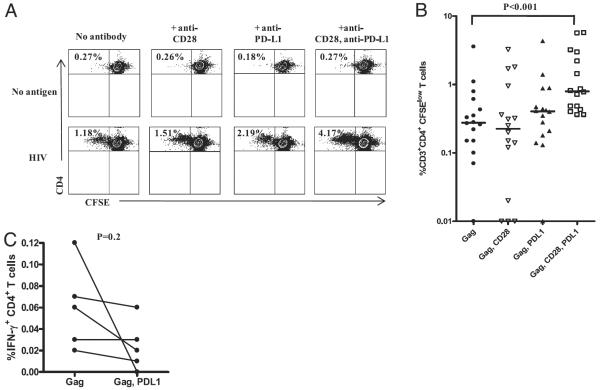
Coexpression of CD28 and inhibitory receptors on HIV-specific IFN-γ+ CD4+ T cells and the effect of blockade of inhibitory receptor pathway and/or engagement of stimulatory pathway on HIV-specific CD4+ T cell function. A, Representative flow-cytometry profile of CD28 and PD-1, CD28 and CTLA-4, and CD28 and TIM-3 expression on HIV-specific IFN-γ–producing CD4+ T cells from a viremic subject with chronic HIV infection. B, Bar graphs showing the percentage of CD28 and PD-1–, CD28 and CTLA-4–, and CD28 and TIM-3–expressing HIV- and CMV-specific IFN-γ–producing CD4+ T cells from chronically HIV-infected viremic subjects. Data are presented as median and range. C, Pooled data showing the percentage of CD28-expressing total, HIV-specific, and CMV-specific IFN-γ–producing CD4+ T cells from viremic patients with chronic HIV infection (n = 9). Statistical significance was determined using the Wilcoxon signed-rank test. D, Media-adjusted data (mean ± SEM) showing the percentage of CFSElow HIV-specific CD4+ T cells in the presence and absence of the blocking (PD-L1, CTLA-4, TIM-3) and/or stimulating (CD28) Abs (n = 3).
Because cooperation between costimulatory and inhibitory receptors is required for driving T cell responses toward immunity or tolerance (3–5), we hypothesized that blockade of inhibitory receptor binding and activation of a costimulatory molecule might synergistically improve HIV-specific CD4+ T cell responses. To examine whether simultaneous blockade of inhibitory receptor binding and stimulation through CD28 improved HIV-specific CD4+ T cell proliferation, PBMCs from untreated subjects were labeled with CFSE and incubated for 6 d with anti–PD-L1, anti–CTLA-4, anti–TIM-3, and anti–CD28 mAbs or each anti-inhibitory receptor mAb with anti-CD28 mAb in the presence of HIV-1 Gag peptide pool or media. Proliferation was measured by calculating the percentage of CFSElow (divided) CD4+ T cells using multiparametric flow cytometry. These preliminary experiments revealed that the combination of anti–PD-L1 and CD28 mAbs induced the greatest increase in HIV-specific CD4+ T cell proliferation (Fig. 7D).
Blockade of PD-1/PD-L1 pathway with concurrent stimulation though CD28 enhances HIV-specific CD4+ T cell proliferation
Because we observed the greatest increase in HIV-specific CD4+ T cell proliferation using anti–PD-L1 and anti-CD28 mAbs (Fig. 7D), we focused the proliferation studies on blockade of the PD1/PD-L1 pathway and stimulation of CD28. Fig. 8A shows representative flow-cytometry dot plots depicting the CFSE staining and proliferation of HIV-specific CD4+T cells. Background-adjusted pooled data showing the percentage of CFSElow HIV-specific CD4+ T cells in the presence and absence of the blocking and/or stimulating Abs are shown in Fig. 8B. Stimulation with HIV-1 Gag peptide and CD28 alone or blockade of PD-L1 alone resulted in a 1.14- and a 1.43-fold increase, respectively, in the expansion of Gag-specific CD4+ T cells compared with expansion following stimulation with peptide alone. Interestingly, stimulation with Gag peptide in the presence of anti–PD-L1 and anti-CD28 Ab further enhanced proliferation, resulting in a 3.51-fold increase in HIV-specific CD4+ T cells compared with each separately (p < 0.001; Fig. 8B), indicating that there was a synergistic effect of targeting the inhibitory and costimulatory receptors simultaneously. In contrast, there was no significant effect on CMV-specific CD4+ T cell proliferation in the same subjects (data not shown). In a subset of subjects, we also assessed the effect of PD-1 blockade on IFN-γ production by HIV-specific CD4+ T cells using a 6-h intracellular cytokine-secretion assay. In line with a previous report on HIV-specific CD8+ T cells (15), we saw no significant difference in the amount of IFN-γ/cell (mean fluorescence intensity) or an increase in the frequency of HIV-specific CD4+ T cells producing IFN-γ with or without PD-1 blockade (Fig. 8C). These data suggest that PD-1 blockade did not directly affect cytokine production by HIV-specific CD4+ T cells in a short-term assay.
FIGURE 8.
Effect of blockade of PD-1/PD-L1 pathway and stimulation through CD28 on HIV-specific CD4+ T cell function. A, Representative flow-cytometry plots showing the percentage of CFSElow CD4+ T cells after 6 d of stimulation with HIV Gag peptides or media alone in the presence or absence of anti–PD-L1 and/or anti-CD28 Ab. B, Media-adjusted pooled data showing the percentage of CFSElow HIV-specific CD4+ T cells in the presence and absence of the blocking and/or stimulating Abs (n = 17). Individual values are presented, with the solid lines indicating medians. Statistical significance was determined using the Friedman test with the Dunn posttest. C, Percentage of HIV-1 Gag-specific IFN-γ–producing CD4+ T cells by blockade of PD-1/PD-L1 pathway in a 6-h intracellular cytokine staining assay. Statistical significance was determined using the Mann–Whitney U test.
Discussion
An optimal balance between positive and negative signals delivered through costimulatory receptors on the surface of T cells is critical for the generation of an effective cellular immune response (3–5). However, during chronic HIV infection, this balance is skewed toward increased expression of inhibitory receptors (11–13, 15), with a commensurate downregulation of costimulatory receptors (32–34, 48) on HIV-specific T cells. Costimulatory receptor dys-regulation contributes to impaired effector function of HIV-specific T cells in subjects with chronic HIV infection. Understanding the relationship between inhibitory receptor expression on HIV-specific CD4+ T cells and HIV disease is critical, because functional impairment of HIV-specific CD4+ T cells during chronic HIV infection is closely linked to disease progression (7–9, 49, 50). We previously reported that HIV-specific CD4+ T cells in subjects with untreated chronic HIV infection expressed increased levels of an inhibitory receptor PD-1 (12) and decreased levels of a costimulatory receptor 4-1BB (32). A recent study using the LCMV mouse model of chronic infection demonstrated the involvement of multiple inhibitory pathways in regulating T cell function (38). In the current study, we show for the first time that HIV-specific CD4+ T cells from subjects with chronic HIV infection simultaneously express high levels of PD-1, CTLA-4, and TIM-3; coexpression of the inhibitory receptors correlate more strongly with HIV plasma viremia than their individual expression; TIM-3 expression is greater on HIV-specific cells than on CMV-specific CD4+ T cells; TIM-3 expression is greater on HIV-specific CD4+ T cells from viremic subjects than from suppressed subjects with chronic HIV infection; a large percentage of HIV-specific CD4+ T cells that express inhibitory receptors also express CD28; and activation of a costimulatory pathway (CD28) and blockade of an inhibitory pathway (PD-1) synergistically enhance HIV-specific CD4+ T cell proliferation. These novel findings confirm the earlier observations for the individual receptors, as well as extend them by relating the expression of all three receptors and demonstrating the usefulness of targeting positive and negative costimulatory receptor pathways to enhance the function of HIV-specific CD4+ T cells during chronic HIV infection.
Previous studies reported an elevated expression of PD-1 and CTLA-4 on HIV-specific CD4+ T cells from subjects with untreated chronic infection (12, 14). However, unlike the previous studies, we found for the first time that >30% of HIV-specific CD4+ T cells simultaneously express PD-1, CTLA-4, and TIM-3, suggesting that multiple inhibitory receptor pathways cooperate to restrain CD4+ T cell responses during chronic infection with HIV. These findings were in contrast with the low expression of inhibitory receptors on CMV- and VZV-specific CD4+ T cells from the same subjects, providing evidence for differential regulation of virus-specific T cell function during chronic viral infections (41, 42). Furthermore, we report that extrinsic control of HIV replication by ART is associated with reduced expression of all three inhibitory receptors compared with untreated subjects with chronic infection. The link between the expression of inhibitory receptors and viral replication is much stronger when the simultaneous expression of all three, rather than single expression, is compared, suggesting that analysis of multiple receptors provides a better marker of disease progression. However, these correlations are only significant when all subjects (viremic and ART suppressed) are included, although examination of all three trends toward significant correlation with viral load (r = +0.40, p = 0.09), unlike that of each receptor separately. No published study links the expression of PD-1 or TIM-3 on HIV-specific CD4+ T cells from untreated subjects with chronic HIV infection to viral load. We previously reported a correlation between the expression of PD-1 on HIV-specific CD4+ T cells and viral replication (12), although like in this study we only saw a significant correlation when subjects on and off ART were combined. However, a correlation between CTLA-4 expression on HIV-specific CD4+ T cells and viral replication, without including highly active ART-treated subjects, was reported, but the cohort included subjects with acute HIV infection, elite and viremic controllers, as well as those with chronic HIV infection (14). Expression of all three receptors correlates more strongly with viral replication than does each alone, and this correlation is strengthened by the addition of data from suppressed subjects, suggesting that expression of these inhibitory receptors is driven, in part, by HIV replication. Alternatively, it is possible that the expression of these inhibitory receptors is upregulated, with low levels of replicating virus, indicative of a threshold effect. This is supported by recent findings that demonstrate PD-1 is upregulated during SIV and HIV infection on virus-specific T cells even with low viral load, indicating that PD-1 is a sensitive indicator of low-level viral replication (51). In addition, decreased expression of CTLA-4 in HIV-specific CD4+ T cells in elite controllers compared with viremic controllers or subjects with ART-suppressed viral load was shown, suggesting that the presence of even very small amounts of persistent Ag is sufficient to upregulate CTLA-4 expression on HIV-specific CD4+ T cells (14).
TIM-3 expression was also increased on HIV-specific IFN-γ- producing CD4+ T cells compared with CMV- and VZV-specific CD4+ T cells from subjects with untreated chronic HIV infection. In addition, subjects on ART with suppressed plasma viral load had significantly lower levels of TIM-3–expressing HIV-specific IFN-γ–producing CD4+ T cells than did viremic subjects. These findings are in line with a recent study by Jones et al. (13) that demonstrated TIM-3 expression was greater on HIV-specific CD8+ T cells than on CMV-specific CD8+ T cells from chronically infected HIV subjects, and TIM-3 expression on total CD4+ and CD8+ T cells declined with the initiation of ART. Unlike CTLA-4 and PD-1, which were expressed on a substantial proportion of HIV-specific CD4+ T cells that produced IFN-γ and IL-2, TIM-3 was expressed on a very low percentage of dual cytokine-producing HIV-specific CD4+ T cells, indicating differential expression of the inhibitory receptors on cytokine-producing HIV-specific CD4+ T cells. Furthermore, although the expressions of PD-1 and CTLA-4, which have been implicated in the control of proliferation (52–54), are highly correlated, the correlations between these two receptors and TIM-3, implicated in the control of IFN-γ production (55), are less robust. It is likely that PD-1 and CTLA-4 expression marks populations exhibiting features of relatively early HIV-specific CD4+ T cell exhaustion, where cell survival and proliferation are impaired but cytokine production remains intact (9, 43, 56), whereas TIM-3 expression denotes advanced stages of T cell exhaustion where cytokine production is impaired. PD-1 and CTLA-4 inhibit T cell activation by using distinct (53) but partially overlapping signaling pathways that converge on inhibition of phosphorylation of the kinase Akt (17, 57). Although the intracellular domain of TIM-3 contains six tyrosine phosphorylation motifs, its downstream signaling targets leading to the death of Th1 cells remain unknown (58). These findings suggest that inhibitory receptors PD-1 and CTLA-4, which belong to the B7/CD28 family and are involved in decreased proliferation, act together, whereas TIM-3, a T cell Ig and mucin domain-containing molecule involved in regulating Th1 cytokine responses, is not linked as closely.
TIM-3 was shown to negatively regulate Th1 responses in mice (29). Recent studies suggest that this may also be true in humans (13, 30). However, in our study, we detected TIM-3+ cells that produced IFN-γ. Populations of TIM-3–expressing HCV-specific CD4+ T cells that produce IFN-γ have also been described (30). When total CD4+ T cells were segregated into PD-1 and CTLA-4–positive and -negative populations, the majority of HIV-specific IFN-γ–producing CD4+ T cells fell into the PD-1– and CTLA-4–expressing fractions. However, when the same analysis was performed on the TIM-3–positive and -negative populations, the percentage of IFN-γ–producing CD4+ T cells was similar in both populations. This suggests that although there clearly are TIM-3+ CD4+ T cells that produce IFN-γ, they are significantly more rare compared with cells that express PD-1 or CTLA-4. We also found no significant difference in the amount of IFN-γ produced by PD-1, CTLA-4, and TIM-3–positive and -negative subsets of HIV-specific CD4+ T cells. Although we were not surprised to see a difference in PD-1 and CTLA-4–positive and -negative populations, we assumed that cells that expressed TIM-3 would produce less IFN-γ, which was not the case. These inhibitory receptors have all been shown to modulate the proliferative capacity of HIV-specific CD4+ T cells; however, the influence on the ability to produce cytokines is still in question (12, 14, 15). Although MHC class II tetramer analysis has been used by a limited number of groups to examine HIV-specific CD4+ T cells (59, 60), and it would have been highly informative for the study of dysfunctional T cells, technological limitations render it difficult to use in combination with Ag-specific intracellular cytokine staining (61–65).
Stimulating an antiviral immune response to reduce persisting virus has been suggested as a potentially promising strategy to control chronic HIV infection (66). In fact, in vivo blockade of PD-1 binding in SIV-infected macaques (21) and LCMV-infected mice (22) enhanced T cell responses and improved viral control. We and other investigators previously demonstrated that blocking the PD-1 pathway enhances HIV-specific CD4+ T cell proliferation (11, 12). In the current study, we evaluated whether blockade of an inhibitory receptor with concurrent stimulation of a costimulatory receptor would augment proliferation to a greater extent than blockade alone. We chose to evaluate the effects of stimulation though CD28 because its expression was elevated on HIV-specific CD4+ T cells compared with CMV-specific CD4+ T cells (46), and its expression greatly overlaps with inhibitory receptors on HIV-specific CD4+ T cells. Although there was no significant increase in HIV-specific CD4+ T cell proliferation with the addition of CD28 or PD-L1 mAbs alone, we observed a significant increase in HIV-specific CD4+ T cell proliferation when PBMCs were cultured with both mAbs, indicating a synergistic effect of blockade and stimulation on HIV-specific CD4+ T cell proliferation. PD-1 engagement was shown to alter CD3- and CD28-mediated changes to the T cell transcriptional profile, resulting in decreased activation of the cell (17, 57), suggesting that an inhibitory signal delivered though PD-1 may override the positive signal delivered via CD28.
In summary, our data reveal that the HIV-specific CD4+ T cell response during chronic HIV infection is regulated by complex patterns of coexpressed inhibitory receptors. The increased coexpression of multiple inhibitory receptors may contribute to the loss of HIV-specific CD4+ T cell function in subjects with chronic HIV infection. Expression of all three receptors correlates more strongly with viral replication than does each alone; this correlation is driven by the addition of data from suppressed subjects suggesting that expression of these inhibitory receptors is, in part, a consequence of viral replication. Enhancement of HIV-specific CD4+ T cell function via PD-1 blockade with concurrent stimulation though CD28 is better than PD-1R blockade alone. Taken together, these data suggest that immune dysfunction associated with chronic HIV infection is mediated by multiple inhibitory pathways and that simultaneous targeting of inhibitory and stimulatory receptor pathways synergistically augments virus-specific CD4+ T cell function to a greater extent than inhibitory receptor blockade alone.
Acknowledgments
We thank the physicians, staff, and patients in the Infectious Diseases Group Practice at the University of Colorado Hospital for assistance and participation in this study. We thank the Colorado Center for AIDS Research Laboratory Core for access to flow cytometry and Clinical Investigation Core for recruitment of study participants.
This work was supported by National Institutes of Health Grant 1R21 AI076161-01A2 (to B.E.P.).
Abbreviations used in this paper
- ART
antiretroviral therapy
- CS&T
cytometer setup & tracking
- FMO
fluorescence – 1
- HCV
hepatitis C virus
- LCMV
lymphocytic choriomeningitis virus
- PD-1
programmed death 1
- SEB
staphylococcal enterotoxin B
- TIM-3
T cell Ig domain and mucin domain 3
- VZV
varicella-zoster virus
Footnotes
Disclosures The authors have no financial conflicts of interest.
References
- 1.Pitcher CJ, Quittner C, Peterson DM, Connors M, Koup RA, Maino VC, Picker LJ. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat. Med. 1999;5:518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg ES, Billingsley JM, Caliendo AM, Boswell SL, Sax PE, Kalams SA, Walker BD. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 3.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat. Rev. Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 4.Crawford A, Wherry EJ. The diversity of costimulatory and inhibitory receptor pathways and the regulation of antiviral T cell responses. Curr. Opin. Immunol. 2009;21:179–186. doi: 10.1016/j.coi.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu. Rev. Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 6.Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 7.Kaufmann DE, Walker BD. Programmed death-1 as a factor in immune exhaustion and activation in HIV infection. Curr. Opin. HIV AIDS. 2008;3:362–367. doi: 10.1097/COH.0b013e3282f9ae8b. [DOI] [PubMed] [Google Scholar]
- 8.Kaufmann DE, Walker BD. PD-1 and CTLA-4 inhibitory cosignaling pathways in HIV infection and the potential for therapeutic intervention. J. Immunol. 2009;182:5891–5897. doi: 10.4049/jimmunol.0803771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Far M, Halwani R, Said E, Trautmann L, Doroudchi M, Janbazian L, Fonseca S, van Grevenynghe J, Yassine-Diab B, Sékaly RP, Haddad EK. T-cell exhaustion in HIV infection. Curr. HIV/AIDS Rep. 2008;5:13–19. doi: 10.1007/s11904-008-0003-7. [DOI] [PubMed] [Google Scholar]
- 10.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 12.D'Souza M, Fontenot AP, Mack DG, Lozupone C, Dillon S, Meditz A, Wilson CC, Connick E, Palmer BE. Programmed death 1 expression on HIV-specific CD4+ T cells is driven by viral replication and associated with T cell dysfunction. J. Immunol. 2007;179:1979–1987. doi: 10.4049/jimmunol.179.3.1979. [DOI] [PubMed] [Google Scholar]
- 13.Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM, et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J. Exp. Med. 2008;205:2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufmann DE, Kavanagh DG, Pereyra F, Zaunders JJ, Mackey EW, Miura T, Palmer S, Brockman M, Rathod A, Piechocka-Trocha A, et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat. Immunol. 2007;8:1246–1254. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 15.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 17.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu. Rev. Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 18.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrovas C, Chaon B, Ambrozak DR, Price DA, Melenhorst JJ, Hill BJ, Geldmacher C, Casazza JP, Chattopadhyay PK, Roederer M, et al. Differential association of programmed death-1 and CD57 with ex vivo survival of CD8+ T cells in HIV infection. J. Immunol. 2009;183:1120–1132. doi: 10.4049/jimmunol.0900182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finnefrock AC, Tang A, Li F, Freed DC, Feng M, Cox KS, Sykes KJ, Guare JP, Miller MD, Olsen DB, et al. PD-1 blockade in rhesus macaques: impact on chronic infection and prophylactic vaccination. J. Immunol. 2009;182:980–987. doi: 10.4049/jimmunol.182.2.980. [DOI] [PubMed] [Google Scholar]
- 21.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, Vanderford TH, Chennareddi L, Silvestri G, Freeman GJ, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 23.Ha SJ, Mueller SN, Wherry EJ, Barber DL, Aubert RD, Sharpe AH, Freeman GJ, Ahmed R. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J. Exp. Med. 2008;205:543–555. doi: 10.1084/jem.20071949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lukens JR, Cruise MW, Lassen MG, Hahn YS. Blockade of PD-1/B7-H1 interaction restores effector CD8+ T cell responses in a hepatitis C virus core murine model. J. Immunol. 2008;180:4875–4884. doi: 10.4049/jimmunol.180.7.4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaunders JJ, Ip S, Munier ML, Kaufmann DE, Suzuki K, Brereton C, Sasson SC, Seddiki N, Koelsch K, Landay A, et al. Infection of CD127+ (interleukin-7 receptor+) CD4+ cells and overexpression of CTLA-4 are linked to loss of antigen-specific CD4 T cells during primary human immunodeficiency virus type 1 infection. J. Virol. 2006;80:10162–10172. doi: 10.1128/JVI.00249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamoto N, Cho H, Shaked A, Olthoff K, Valiga ME, Kaminski M, Gostick E, Price DA, Freeman GJ, Wherry EJ, Chang KM. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog. 2009;5:e1000313. doi: 10.1371/journal.ppat.1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leng Q, Bentwich Z, Magen E, Kalinkovich A, Borkow G. CTLA-4 upregulation during HIV infection: association with anergy and possible target for therapeutic intervention. AIDS. 2002;16:519–529. doi: 10.1097/00002030-200203080-00002. [DOI] [PubMed] [Google Scholar]
- 28.Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 29.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 30.Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon BJ, Castelblanco N, Kuchroo V, Gretch DR, Rosen HR. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J. Virol. 2009;83:9122–9130. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hryniewicz A, Boasso A, Edghill-Smith Y, Vaccari M, Fuchs D, Venzon D, Nacsa J, Betts MR, Tsai WP, Heraud JM, et al. CTLA-4 blockade decreases TGF-beta, IDO, and viral RNA expression in tissues of SIVmac251-infected macaques. Blood. 2006;108:3834–3842. doi: 10.1182/blood-2006-04-010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kassu A, D'Souza M, O'Connor BP, Kelly-McKnight E, Akkina R, Fontenot AP, Palmer BE. Decreased 4-1BB expression on HIV-specific CD4+ T cells is associated with sustained viral replication and reduced IL-2 production. Clin. Immunol. 2009;132:234–245. doi: 10.1016/j.clim.2009.03.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gamberg J, Pardoe I, Bowmer MI, Howley C, Grant M. Lack of CD28 expression on HIV-specific cytotoxic T lymphocytes is associated with disease progression. Immunol. Cell Biol. 2004;82:38–46. doi: 10.1111/j.1440-1711.2004.01204.x. [DOI] [PubMed] [Google Scholar]
- 34.Trimble LA, Shankar P, Patterson M, Daily JP, Lieberman J. Human immunodeficiency virus-specific circulating CD8 T lymphocytes have down-modulated CD3zeta and CD28, key signaling molecules for T-cell activation. J. Virol. 2000;74:7320–7330. doi: 10.1128/jvi.74.16.7320-7330.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bukczynski J, Wen T, Wang C, Christie N, Routy JP, Boulassel MR, Kovacs CM, Macdonald KS, Ostrowski M, Sekaly RP, et al. Enhancement of HIV-specific CD8 T cell responses by dual costimulation with CD80 and CD137L. J. Immunol. 2005;175:6378–6389. doi: 10.4049/jimmunol.175.10.6378. [DOI] [PubMed] [Google Scholar]
- 36.Serghides L, Bukczynski J, Wen T, Wang C, Routy JP, Boulassel MR, Sekaly RP, Ostrowski M, Bernard NF, Watts TH. Evaluation of OX40 ligand as a costimulator of human antiviral memory CD8 T cell responses: comparison with B7.1 and 4-1BBL. J. Immunol. 2005;175:6368–6377. doi: 10.4049/jimmunol.175.10.6368. [DOI] [PubMed] [Google Scholar]
- 37.Wang C, Wen T, Routy JP, Bernard NF, Sekaly RP, Watts TH. 4-1BBL induces TNF receptor-associated factor 1-dependent Bim modulation in human T cells and is a critical component in the costimulation-dependent rescue of functionally impaired HIV-specific CD8 T cells. J. Immunol. 2007;179:8252–8263. doi: 10.4049/jimmunol.179.12.8252. [DOI] [PubMed] [Google Scholar]
- 38.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palmer BE, Blyveis N, Fontenot AP, Wilson CC. Functional and phenotypic characterization of CD57+CD4+ T cells and their association with HIV-1-induced T cell dysfunction. J. Immunol. 2005;175:8415–8423. doi: 10.4049/jimmunol.175.12.8415. [DOI] [PubMed] [Google Scholar]
- 40.Palmer BE, Boritz E, Wilson CC. Effects of sustained HIV-1 plasma viremia on HIV-1 Gag-specific CD4+ T cell maturation and function. J. Immunol. 2004;172:3337–3347. doi: 10.4049/jimmunol.172.5.3337. [DOI] [PubMed] [Google Scholar]
- 41.Betts MR, Harari A. Phenotype and function of protective T cell immune responses in HIV. Curr. Opin. HIV AIDS. 2008;3:349–355. doi: 10.1097/COH.0b013e3282fbaa81. [DOI] [PubMed] [Google Scholar]
- 42.Harari A, Dutoit V, Cellerai C, Bart PA, Du Pasquier RA, Pantaleo G. Functional signatures of protective antiviral T-cell immunity in human virus infections. Immunol. Rev. 2006;211:236–254. doi: 10.1111/j.0105-2896.2006.00395.x. [DOI] [PubMed] [Google Scholar]
- 43.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iyasere C, Tilton JC, Johnson AJ, Younes S, Yassine-Diab B, Sekaly RP, Kwok WW, Migueles SA, Laborico AC, Shupert WL, et al. Diminished proliferation of human immunodeficiency virus-specific CD4+ T cells is associated with diminished interleukin-2 (IL-2) production and is recovered by exogenous IL-2. J. Virol. 2003;77:10900–10909. doi: 10.1128/JVI.77.20.10900-10909.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Younes SA, Yassine-Diab B, Dumont AR, Boulassel MR, Grossman Z, Routy JP, Sekaly RP. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J. Exp. Med. 2003;198:1909–1922. doi: 10.1084/jem.20031598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yue FY, Kovacs CM, Dimayuga RC, Parks P, Ostrowski MA. HIV-1-specific memory CD4+ T cells are phenotypically less mature than cytomegalovirus-specific memory CD4+ T cells. J. Immunol. 2004;172:2476–2486. doi: 10.4049/jimmunol.172.4.2476. [DOI] [PubMed] [Google Scholar]
- 47.Ostrowski SR, Gerstoft J, Pedersen BK, Ullum H. A low level of CD4+CD28+ T cells is an independent predictor of high mortality in human immunodeficiency virus type 1-infected patients. J. Infect. Dis. 2003;187:1726–1734. doi: 10.1086/375239. [DOI] [PubMed] [Google Scholar]
- 48.Choremi-Papadopoulou H, Viglis V, Gargalianos P, Kordossis T, Iniotaki-Theodoraki A, Kosmidis J. Downregulation of CD28 surface antigen on CD4+ and CD8+ T lymphocytes during HIV-1 infection. J. Acquir. Immune Defic. Syndr. 1994;7:245–253. [PubMed] [Google Scholar]
- 49.Palmer BE, Boritz E, Blyveis N, Wilson CC. Discordance between frequency of human immunodeficiency virus type 1 (HIV-1)-specific gamma interferon-producing CD4(+) T cells and HIV-1-specific lymphoproliferation in HIV-1-infected subjects with active viral replication. J. Virol. 2002;76:5925–5936. doi: 10.1128/JVI.76.12.5925-5936.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McNeil AC, Shupert WL, Iyasere CA, Hallahan CW, Mican JA, Davey RT, Jr., Connors M. High-level HIV-1 viremia suppresses viral antigen-specific CD4(+) T cell proliferation. Proc. Natl. Acad. Sci. USA. 2001;98:13878–13883. doi: 10.1073/pnas.251539598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salisch NC, Kaufmann DE, Awad AS, Reeves RK, Tighe DP, Li Y, Piatak M, Jr., Lifson JD, Evans DT, Pereyra F, et al. Inhibitory TCR coreceptor PD-1 is a sensitive indicator of low-level replication of SIV and HIV-1. J. Immunol. 2010;184:476–487. doi: 10.4049/jimmunol.0902781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brunner MC, Chambers CA, Chan FK, Hanke J, Winoto A, Allison JP. CTLA-4-Mediated inhibition of early events of T cell proliferation. J. Immunol. 1999;162:5813–5820. [PubMed] [Google Scholar]
- 53.Carter L, Fouser LA, Jussif J, Fitz L, Deng B, Wood CR, Collins M, Honjo T, Freeman GJ, Carreno BM. PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur. J. Immunol. 2002;32:634–643. doi: 10.1002/1521-4141(200203)32:3<634::AID-IMMU634>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 54.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J. Exp. Med. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hastings WD, Anderson DE, Kassam N, Koguchi K, Greenfield EA, Kent SC, Zheng XX, Strom TB, Hafler DA, Kuchroo VK. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur. J. Immunol. 2009;39:2492–2501. doi: 10.1002/eji.200939274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronicviral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 57.Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol. Cell. Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodriguez-Manzanet R, DeKruyff R, Kuchroo VK, Umetsu DT. The costimulatory role of TIM molecules. Immunol. Rev. 2009;229:259–270. doi: 10.1111/j.1600-065X.2009.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scriba TJ, Purbhoo M, Day CL, Robinson N, Fidler S, Fox J, Weber JN, Klenerman P, Sewell AK, Phillips RE. Ultrasensitive detection and phenotyping of CD4+ T cells with optimized HLA class II tetramer staining. J. Immunol. 2005;175:6334–6343. doi: 10.4049/jimmunol.175.10.6334. [DOI] [PubMed] [Google Scholar]
- 60.Scriba TJ, Zhang HT, Brown HL, Oxenius A, Tamm N, Fidler S, Fox J, Weber JN, Klenerman P, Day CL, et al. HIV-1-specific CD4+ T lymphocyte turnover and activation increase upon viral rebound. J. Clin. Invest. 2005;115:443–450. doi: 10.1172/JCI23084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vollers SS, Stern LJ. Class II major histocompatibility complex tetramer staining: progress, problems, and prospects. Immunology. 2008;123:305–313. doi: 10.1111/j.1365-2567.2007.02801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cecconi V, Moro M, Del Mare S, Dellabona P, Casorati G. Use of MHC class II tetramers to investigate CD4+ T cell responses: problems and solutions. Cytometry A. 2008;73:1010–1018. doi: 10.1002/cyto.a.20603. [DOI] [PubMed] [Google Scholar]
- 63.James EA, LaFond R, Durinovic-Bello I, Kwok W. Visualizing antigen specific CD4+ T cells using MHC class II tetramers. J. Vis. Exp. 2009;(25):1167. doi: 10.3791/1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Landais E, Romagnoli PA, Corper AL, Shires J, Altman JD, Wilson IA, Garcia KC, Teyton L. New design of MHC class II tetramers to accommodate fundamental principles of antigen presentation. J. Immunol. 2009;183:7949–7957. doi: 10.4049/jimmunol.0902493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tilton JC, Luskin MR, Johnson AJ, Manion M, Hallahan CW, Metcalf JA, McLaughlin M, Davey RT, Jr., Connors M. Changes in paracrine interleukin-2 requirement, CCR7 expression, frequency, and cytokine secretion of human immunodeficiency virus-specific CD4+ T cells are a consequence of antigen load. J. Virol. 2007;81:2713–2725. doi: 10.1128/JVI.01830-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ha SJ, West EE, Araki K, Smith KA, Ahmed R. Manipulating both the inhibitory and stimulatory immune system towards the success of therapeutic vaccination against chronic viral infections. Immunol. Rev. 2008;223:317–333. doi: 10.1111/j.1600-065X.2008.00638.x. [DOI] [PubMed] [Google Scholar]