Abstract
Excessive levels of B Cell Activating Factor (BAFF) are found in patients with active chronic graft versus host disease (cGVHD). In mice, BAFF has been shown to be essential for B cell recovery after myeloablation. To assess how BAFF levels relate to transplant factors and subsequent cGVHD development, we prospectively monitored 412 patients in the first year after allogeneic peripheral blood or bone marrow (PB/BM) hematopoietic stem cell transplantation (HSCT) and censoreddata at time ofcGVHD onset. In patients who did not develop cGVHD, we affirmeda temporal pattern of gradual BAFF level decreaseas theB cell numbers increase after myeloablative conditioning (MAC). By contrast, after reduced intensity conditioning (RIC), BAFF levels remained high throughoutthe first post-HSCT year, suggesting that the degree of myeloablation resulted in delayedB cell recovery associated with persistence of higher BAFF levels. Since high BAFF/B ratios have been associated with active cGVHD, weexamined differences in early BAFF/B ratios and found significantly different BAFF/B ratios at 3 months post-HSCT only afterMAC in patients who subsequently developed cGVHD. In addition toHSCT conditioning type, use of sirolimus was significantly associated with higher BAFF levels after HSCT and this wasalso potentially related tolower B cell numbers. Together, our results are important for interpretation of BAFF measurements in cGVHD biomarker studies.
Introduction
Bcell activating factor (BAFF) is a key regulator of B-cell homeostasis. BAFF support is required for normal B-cell proliferation and survival and the absence of BAFF or BAFF-R results in profound B lymphopenia.(1, 2) In contrast, excess BAFF has been associated with persistence of autoreactive B cells and a variety of autoimmune diseases.(3) These homeostatic functions of BAFF are exemplified in astudy of patients with common variable immunodeficiency (CVID).(4) In CVID patients soluble BAFF levels wereinversely correlated with peripheralB-cell numbers and the expression of BAFF receptors. The clinical significance of this cytokine pathway is also highlighted by the recent FDA approval of belimumab, an anti-BAFF monoclonal antibody for the treatment of systemic lupus erythematosus (SLE). In SLE, neutralization of high BAFF levels leads to a reduction of pathogenic autoantibodies and clinical improvement of disease.(5, 6)
BAFF alsohas an important role in the reconstitution of B cells and normal B-cell function after stem cell transplantation. In murine models, BAFF is required for reconstitution of the B-cell compartment after myeloablation.(1) Soluble BAFF levels are characteristically high when patients are B-lymphopenic and BAFF levels gradually decrease as the number of circulating B cells return to normal levels.(7-9) After transplantation, increased numbers of bone marrow B-cell precursors and early recovery of circulating B cells have been observed in patients who do not develop cGVHD.(10-13) In addition to supporting recovery of normal B cells after stem cell transplantation, BAFF has also been proposed to contribute to the development of chronic graft versus host disease (cGVHD).(14, 15) Despite having significantly higher BAFF levels, chronic GVHD patients often have low total numbers of circulating B cells compared to those without cGVHD, resulting in relatively high BAFF/B ratios and the presence of circulating activated B cells. While the mechanisms whereby BAFF contributes to the development of cGVHD are not well established, many studies strongly support a role for B cells in this disease. While the cellular sources of BAFF are unknown in cGVHD, inducible expression from neutrophils, monocytes/macrophages, dendritic cell subsets, T-cells and activated B-cells occurs in other inflammatory states.(16, 17)
Previous studies have suggestedthat regulation of B-cell recovery by BAFF might influence the subsequent development of cGVHD.(18) To examine this issue, we prospectively monitored a large cohort of 412patients with serial measurements of BAFF levels and B-cell numbers in the first year after allogeneic HSCTbefore cGVHD development. This study identifies several key variables such as the intensity of transplant conditioning therapy and sirolimus important for interpretation of differences in BAFF levels and B cell numbers in the first HSCT year.
Methods
Patient Characteristics
Serial blood samples for analysis of BAFF levels and B-cell reconstitution were obtained from 412 patients who underwent allogeneic HSCT and survived at least 100 days after HSCT at the Dana-Farber Cancer Institute and Brigham and Women’s Hospital from 2000-2008. Written informed consent was obtained in accordance with the Declaration of Helsinki and approved by the Human Subjects Protection Committee of the Dana-Farber/Harvard Cancer Center.
Table 1 summarizes clinical characteristicsof the patient cohort. Umbilical cord blood transplantation(UCBT) patients were excluded from this study since B cell recovery and BAFF levels have previously been shown to be very different in these patients.(19, 20) Donors were HLA-matched in 86% of transplants, including 42% with related donors. Acute GVHD developed in 24% of patients and chronic GVHD developed in 67% following HSCT. Chronic GVHD was categorized according to documented clinical examination and laboratory studies using both Seattle criteria(21) and National Institutes of Health cGVHD consensus criteria.(22) The median time from HSCT to the development of cGVHD was 222 days (range 71-994 days). Median follow-up for all surviving patients was 72 months (range 30–133 months) after HSCT. Samples obtained after cGVHD development or after disease relapse were excluded from analysis.
Table 1.
Clinical characteristics of study population
| All | no cGVHD | cGVHD | p-value | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Total | 412 | 100 | 137 | 33.3 | 275 | 66.7 | |
| Age, median (range) | 49 (18-72) | 48 (18,72) | 50 (18-72) | 0.88 | |||
| Female Gender | 184 | 44.7 | 70 | 51.1 | 114 | 41.5 | 0.07 |
| Patient & Donor Sex Mismatch | <0.001 | ||||||
| (Male patient with Female donor) | 98 | 23.8 | 16 | 11.7 | 82 | 29.8 | |
| HLA type | <0.001 | ||||||
| Matched related | 183 | 41.5 | 80 | 51 | 103 | 36.3 | |
| Matched unrelated | 194 | 44 | 48 | 30.6 | 146 | 51.4 | |
| Mismatched | 35 | 8.5 | 9 | 6.6 | 26 | 9.5 | |
| Conditioning regimen | 0.6 | ||||||
| Myeloablative | 187 | 45.4 | 65 | 47.4 | 122 | 44.4 | |
| RIC | 225 | 54.6 | 72 | 52.6 | 153 | 55.6 | |
| Stem Cell Source | 0.008 | ||||||
| Bone Marrow | 37 | 9 | 21 | 15.3 | 16 | 5.8 | |
| PBSC | 375 | 91 | 116 | 84.7 | 259 | 94.2 | |
| Diagnosis | 0.004 | ||||||
| AML | 134 | 32.5 | 52 | 38 | 82 | 29.8 | |
| CLL/SLL/PLL | 46 | 11.2 | 12 | 8.8 | 34 | 12.4 | |
| CML | 45 | 10.9 | 15 | 10.9 | 30 | 10.9 | |
| HD | 24 | 5.8 | 4 | 2.9 | 20 | 7.3 | |
| MM/PCD | 8 | 1.9 | 7 | 5.1 | 1 | 0.4 | |
| Anemia/RCD | 11 | 2.7 | 6 | 4.4 | 5 | 1.8 | |
| ALL | 39 | 9.5 | 15 | 10.9 | 24 | 8.7 | |
| MDS | 33 | 8 | 6 | 4.4 | 27 | 9.8 | |
| MPD | 7 | 1.7 | 1 | 0.7 | 6 | 2.2 | |
| NHL | 65 | 15.8 | 19 | 13.9 | 46 | 16.7 | |
| GVHD prophylaxis | 0.18 | ||||||
| Tac/Sir/MTX | 161 | 39.1 | 50 | 36.5 | 111 | 40.4 | |
| Tac/Sir no MTX | 93 | 22.6 | 24 | 17.5 | 69 | 25.1 | |
| Tac/MTX | 115 | 27.9 | 47 | 34.3 | 68 | 24.7 | |
| Tac no MTX | 21 | 5.1 | 7 | 5.1 | 14 | 5.1 | |
| Cyclosporine contained | 13 | 3.2 | 4 | 2.9 | 9 | 3.3 | |
| Sir/MMF | 5 | 1.2 | 2 | 1.5 | 3 | 1.1 | |
| Other | 4 | 1 | 3 | 2.2 | 1 | 0.4 | |
| grade II-IVaGVHD | 100 | 24.3 | 22 | 16.1 | 78 | 28.4 | 0.007 |
HLA = human leukocyte antigen; GVHD = graft-versus-host disease; NHL = non-Hodgkin lymphoma; CLL = chronic lymphocytic leukemia; HD = Hodgkin disease; AML = acute myelogenous leukemia; CML = chronic myelogenous leukemia; MDS/MPD = myelodysplastic syndrome/myeloproliferative disorder; ALL = acute lymphoblastic leukemia; MM = multiple myeloma; aGVHD = acute GVHD; cGVHD = chronic GVHD
Details of conditioning regimens and GVHD prophylaxis:
Myeloablative Conditioning (MAC): TBI d-4 to -2; cyclophosphamide 1800mg/m2 IV QD d-6,-5 GVHD: MTX 15mg/m2 IV QD d1 then 10mg/m2 IV QD d3,6,11; Tacrolimus 0.02mg/kg IVCI starting day -3 and titrated to level
Reduced Intensity Conditioning (RIC): Fludarabine 30mg/m2 IV QD d-5 to -2; Busulfan 0.8mg/kg IV QD d-5 to -2
GVHD Prophylaxis: MTX 5mg/m2 IV QD d1,3,6 (day 11 is optional); Tacrolimus 0.05mg/kg BID starting day -3 and titrated to level; Sirolimus 12mg po QD d-3, then 4mg PO QD from day-2 on and titrated to level
Processing of patient plasma
Blood was drawn into standard EDTA-containing collection tubes. Plasma was separated from whole blood cells by centrifugation at 600xg, stored in aliquots at -80°C, and used after first or second thaw for BAFF measurements.
BAFF enzyme-linked immunosorbent assay
Soluble BAFF in patient plasma samples was measured using a commercially available enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s recommended procedures (R&D Systems, Minneapolis, MN).
Flow cytometric analysis of peripheral blood cells
Fluorochrome-conjugated monoclonal antibodies specific for: CD3, CD4, CD19, CD20, CD25, CD27, (BD Biosciences); CD8 (Beckman Coulter, Fullerton, CA) were used to stain fresh, whole blood. After staining, red blood cellswere lysed with BD Pharm Lyse or an automated TQ Prep workstation (Beckman Coulter). Flow cytometry was performed on a FACSCanto II (BD Bioscience) and analyzed using BD FACSDiva software, or on a Beckman Coulter FC500, with Beckman Coulter CXP analysis software. We found no difference betweenB cell numbers using CD19 or CD20 cell surface staining. B-cell percentage was based on enumeration of cells expressing CD20.
Statistical Analysis
Patient baseline and transplant characteristics were reported descriptively, and compared using Fisher’s exact test or Wilcoxon rank sum test. BAFF, B cell, BAFF/B-cell ratio data were analyzed descriptively at each time point and compared using the Wilcoxon rank sum test. All p-values are 2-sided at significance level of .05. Multiple comparisons were not considered. The Kaplan-Meier method was used to estimate overall survival (OS) and progression-free survival (PFS). OS was calculated from date of transplantation to date of death. PFS was calculated from date of transplantation to time of relapse/disease progression or death, whichever occurred first. Multivariable linear regression analysis was performed to assess factors that potentially affect elevated BAFF levels. All calculations were performed using SAS 9.2 (SAS Institute, Cary, NC) and R 2.10.1.
Results
BAFF levels and B cell recovery associated with intensity of conditioning regimen and stem cell source
We examined changes in plasma BAFF levelsand reconstitution of B cells in the first year after HSCT in412adult patientswith hematologic malignancies who underwent allogeneic HSCT. This was the first allogeneic HSCT for 96.4% of patients. Patients received granulocyte colony stimulating factor (G-CSF)-mobilized peripheral blood (91%) or bone marrow (9%) stem cells. The 5-year overall and progression-free survival for the entire cohort was 56% and 47%, respectively. Since subsequent treatment would affect both circulating B cells and BAFF levels, patients were censored at time of cGVHD onset or relapse. None of the patients developed graft failure. Of the 412 patients, 275 (67%) developed cGVHD at a median of 7.4 months after transplant (cGVHD cohort) and 137 patients (33%) did not develop cGVHD during the follow-up period (No-cGVHD cohort). The cGVHD cohort differed from the No-cGVHD cohort in several important variables(Table 1); there were significantly more male patients with female donors, patients with matched unrelated donors (MUD), mobilized PB stem cell grafts and previous history of grade II-IV acute GVHD. All of these variables have previously been associated with increased risk of developing cGVHD.
We first examined whether intensity of the conditioning regimen influences B-cell reconstitution following transplantation.(23-27) We found that the patterns of BAFF and B cell recoverydiffered in the MAC and RIC groups. BAFF levels in RIC versus MAC HSCT were significantly different at 3 month and 12 month time points. Specifically, BAFF levels 3 months after MAC HSCT were significantly higher vs RIC at 10.62ng/ml, (range 1.65-40.47) versus 7.93 ng/ml (range 0.79-61-.03), p=0.02; and BAFF levels were significantly higher in RIC at 12 months with MAC at 5.48 (range 2.37-25.3) and RIC at 8.0 (range 2.14-20.69), p=0.02. These increased BAFF levels did not appear to associate with total B cell numbers, since we found no significant difference in B cell numbers or BAFF/B cell ratios at any time point after MAC versus RIC HSCT conditioning (data not shown). To determine whether B cell recovery was related to the level of engraftment with donor cells, we examined available total peripheral blood cell chimerism. No significant correlation between BAFF/B cell and chimerism at Day 30 orDay 100 post transplantation was found (Supplementary Table 1). Since this potential effect of HSCT conditioning on BAFF and B cell values would impact how these values are interpreted in cGVHD studies, we further examined data by stratifying according to subsequent cGVHD development.
BAFF levels and B cell recovery before chronic GVHD development
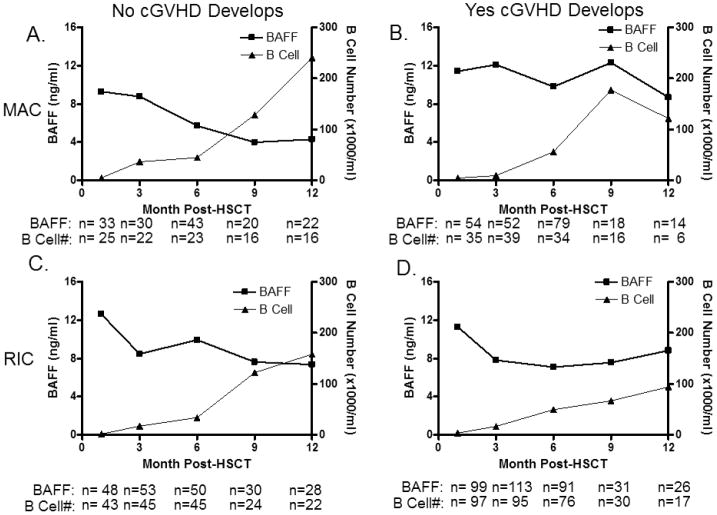
To identify differences in B cell recovery prior to development of cGVHD, patients were censored after onset of cGVHD, but stratified according to subsequent cGVHD development. First, to further examine the relationship between B cell number and BAFF levels, we compared BAFF and B-cell recovery separately in patients who received either MAC or RIC transplants and either later developed or who never developed cGVHD. Of these412patients,275(67%) developed cGVHD at a median of 7.4 months after transplant (cGVHD cohort) and 137 patients (33%) did not develop cGVHD during the follow-up period (No-cGVHD cohort). BAFF levels and B-cell numbers were measured at 1, 3, 6, 9, and 12 months after HSCT in all available samples from both MAC and RIC cohorts. Figure 1 shows the comparison of patients who received RIC with patients who received MAC before cGVHD development. The general pattern of BAFF and B-cell recovery was similar in patients who do not develop cGVHD in both groups, but these patterns were temporally distinct and appeared to reflect the level and rate of B-cell recovery. Early after transplant, BAFF levels were high in both MAC and RIC groups. B-cell recovery after RIC with support was slightly slower than after MAC transplants. In each group, the decrease in BAFF levels reflected the tempo of recovery of peripheral B cells. This coordinated pattern of BAFF and B-cell number increaseis consistent with previous reports demonstrating a low incidence of cGVHD in patients who have rapid recovery of normal B cells. Taken together and consistent with previous findings, this data suggests a relationship between BAFF levels and degree of B lymphopenia.
Figure 1. BAFF levels and B cells recovery after allogeneicstem cell transplantation.
Patients who received either myelablative (MAC) or reduced intensity conditioning (RIC) are shown separately according to subsequent cGVHD development. A) BAFF levels (squares) and B cell numbers (triangles) after MAC in patients who never developed cGVHD. B) BAFF levels (squares) and B cell numbers (triangles) after MAC in patients who developed cGVHD. C) BAFF levels (squares) and B cell numbers (triangles) after RIC in patients who never developed cGVHD. D) BAFF levels (squares) and B cell numbers (triangles) after RIC in patients who subsequently cGVHD.
*Indicates differences that are statistically significant (p<0.05).
Note: Blood samples after the clinical onset of cGVHD were excluded from analysis.
We found that patterns of BAFF and B-cell recovery were different in patients who subsequently developed cGVHD. In MAC and RIC patients who subsequently developed cGVHD (Figure 1), BAFF levels remained elevated and were relatively stable while B-cell numbers slowly increased in the first year after transplant. Toascertain whether high BAFF was related to naïve B lymphopenia, we examined CD27, a marker of antigen-experienced B cells. CD19+CD27+ B cell percentages were available for only a small subset of these patients (64 out of 412) at various time points. In this small patient subset, thepercentage of CD19+CD27+ B cells was not significantly different between groups at any time point, revealing that the majority of cells were likely naive B cells. Interestingly, the frequency of CD27+ B cells was high at 1 month in both cGVHD and no cGVHD groups after HSCT (data not shown), potentially reflecting increased proportions of antigen-experienced B cells or early bone marrow CD27+ B cells (28, 29). Thus, taken together our data show that cGVHD and No-cGVHD cohorts within each PB/BM transplantation conditioning cohort have variations in the kinetics of BAFF level change with B cell number increase following transplant, but with delayed B cell recovery in those with subsequent cGVHD development.
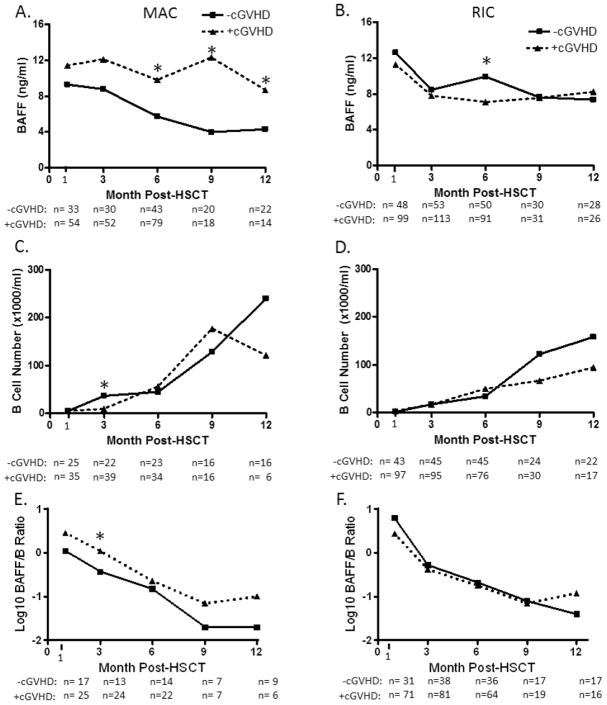
Since high BAFF/B ratios in patients with active cGVHD may be pathobiologically relevant(13, 30), we examined whether a difference in BAFF/B cell ratio occurred prior to disease onset. Thus, a more detailed analysis of the data comparing results in the cGVHD and No-cGVHD cohorts is shown in Figure 2. After MAC, BAFF levels were persistently and significantly higher in the cGVHD cohort compared with the No-cGVHD cohort (Figure 2A) (6 months: 9.79 vs 5.71 ng/mL, p=0.025; 9 months: 12.3 vs. 3.99 ng/mL, p=0.004; 12 months: 8.68 vs. 4.3 ng/mL, p=0.02). After MAC,B-cell numberwas significantly lower 3 months after HSCT in patients who subsequently developed cGVHDcompared to those without cGVHD development (10vs. 37cells/uL, p=0.008, Figure2C). This corresponded to ahigherBAFF/B-cell ratio at this time (1.11 vs. o.37, p=0.01,Figure 2E). The only significant difference following RIC was at 6 months when BAFF levels were significantly higher in the No-cGVHD group (Figure 2B)(9.92 vs 7.09 ng/mL in +cGVHD, p=0.05). This peak in BAFF after RIC was followed by a more rapid, albeit not statistically significant, increase in total B-cell numbers at 9 months and 12 months in the No-cGVHD compared with the cGVHD group (Figure 2D). Taken together, our data suggests that BAFF levels in the first year after transplantation are affected by the intensity of the transplant conditioning. The persistence of high BAFF levels associated with the subsequent development of cGVHD is most evident after MAC.
Figure 2. Patterns of BAFF levels, B-cells and BAFF/B-cell ratios after stem cell transplantation before cGVHD onset.
Serial values during the first year after transplant for patients who received eitherMAC (three left panels, A, C, and E) or RIC (three right panels, B, D, and F) prior to HSCT. Patients who later developed cGVHD (triangles) are compared to patients who did not develop cGVHD (squares).
*Indicates differences that are statistically significant (p<0.05).
Median values and ranges (in parentheses) are listed for those that were significantly different after MAC are as follows: 6 months: yes cGVHD 9.79 (0.62-58.43) vs no cGVHD 5.71 (0.08-34.49) ng/mL, p=0.025; 9 months: yes cGVHD 12.3 (1.75-37.38) vs. no cGVHD 3.99 (0.83-31.82) ng/mL, p=0.004; 12 months: yes cGVHD 8.68 (2.37-22.17) vs. no cGVHD 4.3 (3.48-25.3) ng/mL, p=0.02); or after RIC are as follows: 6 months: yes cGVHD 7.09 (0-26.01] vs no cGVHD 9.92 (0-80.49) ng/mL, p=0.05). Significant differences in total B cell numbers (median values with range in parentheses) are listed as follows: 3 months after MAC: yes cGVHD 10 (0- 631.41) versus no cGVHD 37 (0-434.64) cells/uL, p=0.008; BAFF/B-cell ratios were calculatedas previously described and are depicted on a log scale. Significant difference was determined at the 3 month timepoint after MAC with median values and ranges (in parentheses) as follows: yes cGVHD 1.11 (0.05-20.07)vs No-cGVHD 0.37 (0.03-10.75), p=0.01.
Effects of acute GVHD and corticosteroid therapy on BAFF levels
To examine the role of acute GVHD we compared patients with cGVHD who also had a history of aGVHD with patients with de novo cGVHD with respect to BAFF levels, B-cell numbers, and BAFF/B-cell ratios. There were no significant differences in any measure at any time point between the two groups (data not shown). Because high dose corticosteroid therapy is known to be associated with lower BAFF levels (31), each patient was further categorized for use of prednisone, either at any dose or only at doses >30mg/day prednisone (or equivalent) at the time that samples were obtained for BAFF measurement. 216 patients received prednisone at any dose in the first year after HSCT; 100 patients received >30mg/day prednisone. When samples obtained from patients receiving any dose or only >30mg/day of prednisone at the time of sample collection were excluded from analysis, we observed a similar temporal pattern of BAFF levels between the two groups as had been observed for all patients: 1 month after HSCT, BAFF levels remained significantly higher in the No-cGVHD cohort (15.21 versus 11.44 ng/ml, p=0.004), but decreased with time such that they were significantly lower in the No-cGVHD cohort 12 months after HSCT (5.57 versus 9.57, p=0.009) (data not shown). This analysis suggests that steroid treatment did not affect the relative differences in BAFF levels found in patients who subsequently developed cGVHD versus those who did not develop disease.
Influence of clinical factors and sirolimus on BAFF and B-cell recovery
We attempted to uncover additional factors contributing to high BAFF levels by examining factors that potentially affect BAFF and B-cell levels. Importantly, prior treatment with anti-B cell or lymphocyte antibody therapy was notassociated with cGVHD development in our cohort. None of the patients in this study received ATG in their transplant conditioning regimen. Of the 114 patients with B-cell non-Hodgkin lymphoma or chronic lymphocytic leukemia (CLL), 60 received rituximab or ibritumomab tiuxetan and 6 received alemtuzumab within 6 months prior to transplantation. Importantly, when we excluded the 60 patients who received B-cell specific depletion therapy, the pattern of BAFF levels was the same but B-cell recovery was more robust for the cGVHD cohort and less robust for the “no cGVHD” cohort resulting in no difference between the two groups for B cells or BAFF/B cell ratios (data not shown). Various clinical factors are known to increase the risk of developing cGVHD, but multivariable linear regression analysis did not detect a significant association between age, donor and patient sex mismatch, related or unrelated HSCT, stem cell source and BAFF levels at any time in the first year post transplant (Table 2). B cell recovery was faster in younger patients than in older patients (median 143.7 vs 27.7, respectively, p=0.049 at 9 month; median 229.4 vs 46, respectively, p=0.004 at 12 month). In multivariable analysis,older recipient age was associated with a significantly lower total B cell number, but only at the 12 monthtime point (p=0.002). In multivariable linear regression analysis, we found that conditioning regimen and donor cell source were also significantly associated with BAFF levels at 3 months after HSCT (p=0.02, Table 2).
Table 2.
Multivariable linear regression models on Log (BAFF) and Log10 (B cell) at 1,3,6,9, and 12 months after HSCT.
| Transplant Factor | p-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Log (BAFF) | Log 10 (B cell) | |||||||||
|
| ||||||||||
| 1 | 3 | 6 | 9 | 12 | 1 | 3 | 6 | 9 | 12 | |
| Older vs Younger Age a | 0.69 | 0.62 | 0.69 | 0.25 | 0.43 | 0.55 | 0.50 | 0.90 | 0.13 | 0.002 |
| Male Patient with Female | ||||||||||
| Donor vs Other | 0.41 | 0.36 | 0.08 | 0.10 | 0.23 | 0.64 | 0.54 | 0.99 | 0.85 | 0.54 |
| PBSC vs BMSC | 0.62 | 0.02 | 0.91 | 0.47 | 0.32 | 0.54 | 0.24 | 0.61 | 0.60 | 0.76 |
| MRD vs MUD/Mismatched | 0.73 | 0.12 | 0.70 | 0.49 | 0.11 | 0.54 | 0.89 | 0.75 | 0.66 | 0.69 |
| Sirolimus Use vs Other | 0.97 | 0.054 | 0.0006 | 0.07 | 0.02 | 0.11 | 0.0005 | 0.14 | 0.37 | 0.32 |
| Conditioning: MAC vs RIC | 0.20 | 0.02 | 0.15 | 0.36 | 0.87 | 0.41 | 0.40 | 0.26 | 0.10 | 0.51 |
: ’older’ is defined as age >=50 in MAC and age>=60 in RIC
Multivariable analysis also revealed that sirolimuswasmost significantly associated with higher BAFF levelsat 6months after HSCT (Table 2, p=0.0006). Notably, this significant increase in BAFF level was preceded at the 3 month time point by a significant decrease in B cell number (Table 2, p=0.0005). As shown in Figure 3A, BAFF levels remained high at all times in patients receiving sirolimus and decreased after MAC as B cell number increased and notablydid not decrease asB-cell numbers recovered in those who received sirolimus, suggesting that factors other than total B cell number affect BAFF levels. In contrast, B-cell numbers recovered more rapidly in patients not receiving sirolimus and this was associated with gradual lowering of BAFF levels in these patients(Figure 3B). Of note, GVHD prophylaxis was typically discontinued by 6 months after transplant when patients had no signs of cGVHD. Due to the relatively small number of patients who did not receive sirolimusas GVHD prophylaxis in this study, we were not able to furtheranalyze the effect of sirolimus in the cGVHD and No-cGVHD cohorts.
Figure 3. Changes in BAFF levels and B-cell recoveryin patients receiving sirolimus for GVHD prophylaxis after stem cell transplantation.
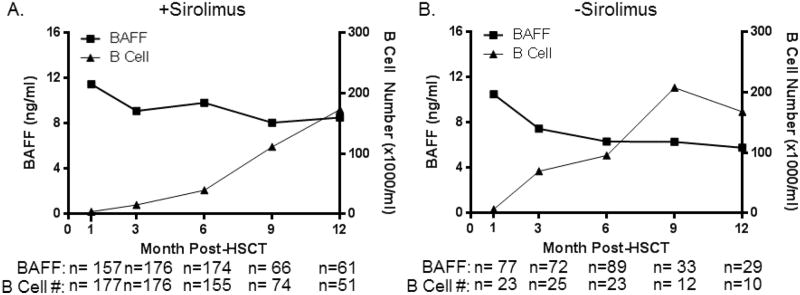
Serial median BAFF levels (left y-axis, squares) are compared to B cell numbers (right y-axis, triangles) in patients at 1, 3, 6, 9, and 12 months post-transplantation. A) Patients who received sirolimus after transplant. B) Patients who did not receive sirolimus after transplant. Note: All patients studied after HSCT, before cGVHDdevelopment, were combined in this analysis.
Discussion
Chronic GVHD results from a complex series of immune interactionsthat occur as the donor immune system develops in an antigenically disparate host/recipient. Patients with chronic GVHD frequently produce auto-antibodies as well as allo-antibodies, suggesting that the pathogenesis of this disease reflects a critical breakdown in peripheral B-cell tolerance. After allogeneic HSCT, the re-establishment of B-cell homeostasis withoutalloimmunity or autoimmunity may reflectan improved capacity for B-cell recovery early after HSCT.(10) This is similar to what has been demonstrated in mouse models of autoimmunity, wherethe reconstitution of peripheral naïve B cells is critical for the maintenance of B-cell tolerance. Thus, the amount of available soluble BAFF is potentially a pivotal determinant of B cell homeostasis after HSCT(32, 33). Thus, while pre-emptive treatment of cGVHD would likely alter patient outcomes, abrogation of BAFF in the early post transplantation period may not be beneficial. While BAFF has been implicated in cGVHD, whether factors in the early post-transplant period affected patterns of BAFF levels required further examination, since this information impacts interpretation of BAFF levels in ongoing GVHD biomarker studies.
The current prospective study in 412 patients reveals the dynamic interaction between BAFF and B-cell reconstitution and their relationship to cGVHD. Patients were analyzed serially for up to 1 year prior to the onset of cGVHD to examine the relationship between BAFF and BAFF/B-cell ratios and subsequent development of cGVHD. While induction of lymphopenia after transplant was generally associated withincreased BAFF levels, higher BAFF levels tended to precedemore rapid B-cell reconstitution, and this was particularly evident in patients who subsequently never developed cGVHDafter RIC (Figure 2B and 2D). This finding is consistent with previous studies demonstrating increased numbers of precursor B cells in patients early after HSCT who do not develop cGVHD.(10, 34)Following a more rapid B-cell recovery and concurrent decline in BAFF levels, patients who did not develop cGVHD had a significantly lower BAFF/B-cell ratio 12 months following HSCT. This observation suggests thatonce B cells recover and normal B-cell homeostasis is established, BAFF levels become limiting. This decrease in the amount of BAFF available per B cell (the BAFF/B-cell ratio) potentially promotesB-cell tolerance with preferential survival of non-alloreactive B cells that are less likely to contribute to cGVHD.(32, 33, 35) Taken together, these results suggest that pre-emptive treatment of cGVHD directed at BAFF in the early post-transplantation period may not be beneficial. In contrast, prophylactic rituximab therapy would also delay B-cell reconstitution, but this approach would result in high BAFF levels and allow for a “reset” of BAFF and B-cell kinetics that leads to cGVHD prevention. While, in the current analysis, no patients received prophylactive rituximab, the increased B cell number and decreased BAFF/B ratio found in patients after prophylactic rituximab who did not develop cGVHD affirms this notion.(40)
Our study of a large number of transplant patients allowed for an investigation of the impact of various HSCT-related factors on BAFF levels and B cell recovery and the subsequent development of cGVHD. We found that transplant conditioning (MAC versus RIC) significantly impacted the kinetics of BAFF and B-cell numbers following transplant. Older age of HSCT recipients was significantly associated with decreased B cell number at the 12 month time point. An unexpected finding in our study was the highly significant effect of sirolimus on BAFF levels and B-cell reconstitution. Overall, 64% of patients in this study received sirolimus as part of their GVHD prophylaxis and this was associated with persistence of high BAFF levels as well as delayed B-cell reconstitution. Although not statistically significant, and not found in previously published reports at our center [rev. in(36)],these patients also experienced a higher incidence of cGVHD than patients who did not receive a sirolimus-containing GVHD prophylaxis regimen (67% vs 59% respectively, p=0.1) in the recently presented Clinical Trials Network study.(37) Our analysis did not allow us to decipher the mechanisms responsible for elevated BAFF associated withsirolimus administration. The higher BAFF levels that were associated with cGVHD development in the sirolimus cohort may have been independent of sirolimus use. Since sirolimus was associated with significantly lower B cell numbers prior to the significant increase in BAFF levels, it is tempting to speculate that sirolimus itself may result in higher BAFF levels because of B lymphopenia induction. This is plausible because sirolimus in mice is known to hinder B cell development.(38) Previous studies havealso suggested that mTor inhibition may contribute to increased BAFF production(39) or elimination of activated B cells.(40, 41) Taken together,our data reveal the effects of HSCT conditioning, age and sirolimus onB cell recovery kinetics and BAFF levels after HSCT.
Understanding a potential role for early high BAFF levels in relation to B-cell reconstitution and attainment of B-cell tolerance versus whether high BAFF relates to the promotion of autoreactive B cells will be critical if we are to understand cGVHD pathophysiology.
Supplementary Material
Acknowledgments
This study was supported by the NIH grants P01CA142106and K08HL107756, the Dunkin’ Donuts’ Rising Stars Program, the TriadGolfersAgainstCancer, the Jock and Bunny Adams Research and Education Endowmentand the Ted and Eileen Pasquarello Research Fund. We thank the patients and their families for graciously participating in this study. We are grateful to Doreen Hersey and the members of the Pasquarello Tissue bank for their help with identifying and processing patient samples. Dr. Yishay Ofran is thanked for help with patient chart review.
Footnotes
Authorship
Contribution: C.A.J. designed the research, performed research, and wrote the paper; L.S. and H.T.K. analyzed data and helped write the manuscript; S.M.M., C.G.R, M.I.H. and M.S. performed research; J.K., C.S.C., V.T.H., E.P.A., P.A., B.R.B., R.J.S, J.H.A. provided vital patient samples and clinical information and they helped write the manuscript; J.R. provided vital patient samples, designed the research and wrote the paper; S.S. conceived of the study, analyzed data and wrote the paper.
Financial Disclosure Statement. The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gorelik L, Gilbride K, Dobles M, Kalled SL, Zandman D, Scott ML. Normal B cell homeostasis requires B cell activation factor production by radiation-resistant cells. The Journal of experimental medicine. 2003;198:937–945. doi: 10.1084/jem.20030789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rickert RC, Jellusova J, Miletic AV. Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunological reviews. 2011;244:115–133. doi: 10.1111/j.1600-065X.2011.01067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scapini P, Hu Y, Chu CL, et al. Myeloid cells, BAFF, and IFN-gamma establish an inflammatory loop that exacerbates autoimmunity in Lyn-deficient mice. The Journal of experimental medicine. 2010;207:1757–1773. doi: 10.1084/jem.20100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kreuzaler M, Rauch M, Salzer U, et al. Soluble BAFF levels inversely correlate with peripheral B cell numbers and the expression of BAFF receptors. J Immunol. 2012;188:497–503. doi: 10.4049/jimmunol.1102321. [DOI] [PubMed] [Google Scholar]
- 5.Manzi S, Sanchez-Guerrero J, Merrill JT, et al. Effects of belimumab, a B lymphocyte stimulator-specific inhibitor, on disease activity across multiple organ domains in patients with systemic lupus erythematosus: combined results from two phase III trials. Annals of the rheumatic diseases. 2012;71:1833–1838. doi: 10.1136/annrheumdis-2011-200831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hahn BH. Belimumab for systemic lupus erythematosus. The New England journal of medicine. 2013;368:1528–1535. doi: 10.1056/NEJMct1207259. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez B, Valdez H, Freimuth W, Butler T, Asaad R, Lederman MM. Plasma levels of B-lymphocyte stimulator increase with HIV disease progression. Aids. 2003;17:1983–1985. doi: 10.1097/00002030-200309050-00018. [DOI] [PubMed] [Google Scholar]
- 8.Sarantopoulos S, Stevenson KE, Kim HT, et al. Recovery of B-cell homeostasis after rituximab in chronic graft-versus-host disease. Blood. 2011;117:2275–2283. doi: 10.1182/blood-2010-10-307819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan WN. B cell receptor and BAFF receptor signaling regulation of B cell homeostasis. J Immunol. 2009;183:3561–3567. doi: 10.4049/jimmunol.0800933. [DOI] [PubMed] [Google Scholar]
- 10.Fedoriw Y, Samulski TD, Deal AM, et al. Bone marrow B cell precursor number after allogeneic stem cell transplantation and GVHD development. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18:968–973. doi: 10.1016/j.bbmt.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Storek J, Witherspoon RP, Webb D, Storb R. Lack of B cells precursors in marrow transplant recipients with chronic graft-versus-host disease. American journal of hematology. 1996;52:82–89. doi: 10.1002/(SICI)1096-8652(199606)52:2<82::AID-AJH3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 12.Storek J, Ferrara S, Ku N, Giorgi JV, Champlin RE, Saxon A. B cell reconstitution after human bone marrow transplantation: recapitulation of ontogeny? Bone marrow transplantation. 1993;12:387–398. [PubMed] [Google Scholar]
- 13.Sarantopoulos S, Stevenson KE, Kim HT, et al. Altered B-cell homeostasis and excess BAFF in human chronic graft-versus-host disease. Blood. 2009;113:3865–3874. doi: 10.1182/blood-2008-09-177840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujii H, Cuvelier G, She K, et al. Biomarkers in newly diagnosed pediatric-extensive chronic graft-versus-host disease: a report from the Children’s Oncology Group. Blood. 2008;111:3276–3285. doi: 10.1182/blood-2007-08-106286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levine JE, Paczesny S, Sarantopoulos S. Clinical applications for biomarkers of acute and chronic graft-versus-host disease. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18:S116–124. doi: 10.1016/j.bbmt.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nardelli B, Belvedere O, Roschke V, et al. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood. 2001;97:198–204. doi: 10.1182/blood.v97.1.198. [DOI] [PubMed] [Google Scholar]
- 17.Moore PA, Belvedere O, Orr A, et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285:260–263. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- 18.Scapini P, Carletto A, Nardelli B, et al. Proinflammatory mediators elicit secretion of the intracellular B-lymphocyte stimulator pool (BLyS) that is stored in activated neutrophils: implications for inflammatory diseases. Blood. 2005;105:830–837. doi: 10.1182/blood-2004-02-0564. [DOI] [PubMed] [Google Scholar]
- 19.Kanda J, Chiou LW, Szabolcs P, et al. Immune recovery in adult patients after myeloablative dual umbilical cord blood, matched sibling, and matched unrelated donor hematopoietic cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18:1664–1676. e1661. doi: 10.1016/j.bbmt.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobson CA, Turki AT, McDonough SM, et al. Immune reconstitution after double umbilical cord blood stem cell transplantation: comparison with unrelated peripheral blood stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18:565–574. doi: 10.1016/j.bbmt.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S, editor. Chronic graft-versus-host disease. New York, NY: Cambridge University Press; 2004. [Google Scholar]
- 22.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Levine JE, Uberti JP, Ayash L, et al. Lowered-intensity preparative regimen for allogeneic stem cell transplantation delays acute graft-versus-host disease but does not improve outcome for advanced hematologic malignancy. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2003;9:189–197. doi: 10.1053/bbmt.2003.50012. [DOI] [PubMed] [Google Scholar]
- 24.Perez-Simon JA, Diez-Campelo M, Martino R, et al. Influence of the intensity of the conditioning regimen on the characteristics of acute and chronic graft-versus-host disease after allogeneic transplantation. British journal of haematology. 2005;130:394–403. doi: 10.1111/j.1365-2141.2005.05614.x. [DOI] [PubMed] [Google Scholar]
- 25.Kim DH, Sohn SK, Baek JH, et al. Retrospective multicenter study of allogeneic peripheral blood stem cell transplantation followed by reduced-intensity conditioning or conventional myeloablative regimen. Acta haematologica. 2005;113:220–227. doi: 10.1159/000084674. [DOI] [PubMed] [Google Scholar]
- 26.Turner BE, Kambouris ME, Sinfield L, et al. Reduced intensity conditioning for allogeneic hematopoietic stem-cell transplant determines the kinetics of acute graft-versus-host disease. Transplantation. 2008;86:968–976. doi: 10.1097/TP.0b013e3181874787. [DOI] [PubMed] [Google Scholar]
- 27.Shi-Xia X, Hai-Qin X, Xian-Hua T, Bo F, Xiang-Feng T. Comparison of reduced intensity and myeloablative conditioning regimens for stem cell transplantation in patients with malignancies: a meta-analysis. Clinical transplantation. 2011;25:E187–198. doi: 10.1111/j.1399-0012.2010.01361.x. [DOI] [PubMed] [Google Scholar]
- 28.Nolte MA, Arens R, van Os R, et al. Immune activation modulates hematopoiesis through interactions between CD27 and CD70. Nat Immunol. 2005;6:412–418. doi: 10.1038/ni1174. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson A, de Milito A, Mowafi F, et al. Expression of CD27-CD70 on early B cell progenitors in the bone marrow: implication for diagnosis and therapy of childhood ALL. Exp Hematol. 2005;33:1500–1507. doi: 10.1016/j.exphem.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Allen JL, Fore MS, Wooten J, et al. B cells from patients with chronic GVHD are activated and primed for survival via BAFF-mediated pathways. Blood. 2012;120:2529–2536. doi: 10.1182/blood-2012-06-438911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarantopoulos S, Stevenson KE, Kim HT, et al. High levels of B-cell activating factor in patients with active chronic graft-versus-host disease. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:6107–6114. doi: 10.1158/1078-0432.CCR-07-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thien M, Phan TG, Gardam S, et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Lesley R, Xu Y, Kalled SL, et al. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004;20:441–453. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- 34.Cho BS, Min CK, Kim HJ, et al. High levels of B cell activating factor during the peritransplantation period are associated with a reduced incidence of acute graft-versus-host disease following myeloablative allogeneic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010;16:629–638. doi: 10.1016/j.bbmt.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 35.Kuzmina Z, Greinix HT, Weigl R, et al. Significant differences in B-cell subpopulations characterize patients with chronic graft-versus-host disease-associated dysgammaglobulinemia. Blood. 2011;117:2265–2274. doi: 10.1182/blood-2010-07-295766. [DOI] [PubMed] [Google Scholar]
- 36.Cutler C, Antin JH. Sirolimus immunosuppression for graft-versus-host disease prophylaxis and therapy: an update. Current opinion in hematology. 2010;17:500–504. doi: 10.1097/MOH.0b013e32833e5b2e. [DOI] [PubMed] [Google Scholar]
- 37.Cutler C, Logan BR, Nakamura R, Johnston L, SChoi SW, Porter DL, Hogan WJ, Pasquini WJ, MacMillan MC, Wingard JR, Waller EK, Grupp SA, McCarthy PL, Wu J, Hu Z, Carter SL, Horowitz MM, Antin JA. Tacrolimus/Sirolimus Vs. Tacrolimus/Methotrexate for Graft-Vs.-Host Disease Prophylaxis After HLA-Matched, Related Donor Hematopoietic Stem Cell Transplantation: Results of Blood and Marrow Transplant Clinical Trials Network Trial 0402. Blood. 2012;120 Abstract 739. [Google Scholar]
- 38.Zhang S, Readinger JA, DuBois W, et al. Constitutive reductions in mTOR alter cell size, immune cell development, and antibody production. Blood. 2011;117:1228–1238. doi: 10.1182/blood-2010-05-287821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weichhart T, Haidinger M, Katholnig K, et al. Inhibition of mTOR blocks the anti-inflammatory effects of glucocorticoids in myeloid immune cells. Blood. 2011;117:4273–4283. doi: 10.1182/blood-2010-09-310888. [DOI] [PubMed] [Google Scholar]
- 40.Limon JJ, Fruman DA. Akt and mTOR in B Cell Activation and Differentiation. Frontiers in immunology. 2012;3:228. doi: 10.3389/fimmu.2012.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heidt S, Roelen DL, Eijsink C, van Kooten C, Claas FH, Mulder A. Effects of immunosuppressive drugs on purified human B cells: evidence supporting the use of MMF and rapamycin. Transplantation. 2008;86:1292–1300. doi: 10.1097/TP.0b013e3181874a36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.