Abstract
Purpose
To develop a mouse model of cardiac injury following partial-heart irradiation (PHI) and test whether DE-microCT and 4D-microCT can be used to assess cardiac injury after PHI to complement myocardial perfusion imaging using microSPECT.
Methods and Materials
To study cardiac injury from tangent field irradiation in mice, we used a small-field biological irradiator to deliver a single dose of 12 Gy x-rays to approximately one-third of the left ventricle (LV) of Tie2Cre; p53FL/+ and Tie2Cre; p53FL/− mice, where one or both alleles of p53 are deleted in endothelial cells. Four and 8 weeks after irradiation, mice were injected with gold and iodinated nanoparticle-based contrast agents, and imaged with DE-microCT and 4D-microCT to evaluate myocardial vascular permeability and cardiac function, respectively. Additionally, the same mice were imaged with microSPECT to assess myocardial perfusion.
Results
After PHI with tangent fields, DE-microCT scans showed a time-dependent increase in accumulation of gold nanoparticles (AuNp) in the myocardium of Tie2Cre; p53FL/− mice. In Tie2Cre; p53FL/− mice, extravasation of AuNp was observed within the irradiated LV, whereas in the myocardium of Tie2Cre; p53FL/+ mice, AuNp were restricted to blood vessels. In addition, data from DE-microCT and microSPECT showed a linear correlation (R2=0.97) between the fraction of the LV that accumulated AuNp and the fraction of LV with a perfusion defect. Furthermore, 4D-microCT scans demonstrated that PHI caused Tie2Cre; p53FL/− mice to develop a markedly decreased ejection fraction, and higher end-diastolic and end-systolic volumes, which were associated with compensatory cardiac hypertrophy of the heart that was not irradiated.
Conclusions
Our results show that DE-microCT and 4D-microCT with nanoparticle-based contrast agents are novel imaging approaches complementary to microSPECT to non-invasively assess the change in myocardial vascular permeability and cardiac function of mice that develop myocardial injury after PHI.
INTRODUCTION
Radiation therapy (RT) in the post-lumpectomy or post-mastectomy setting for breast cancer patients has been shown to improve rates of local control, and for some subgroups of patients, rates of survival (1, 2). However, RT to the chest wall can expose the heart to incidental radiation, which can lead to radiation-related heart disease (RRHD) and death (1, 3–6). The overall dose of radiation to the heart and the proportion of the heart irradiated have been reported to correlate with the risk of RRHD (6, 7).
It remains challenging to identify the subset of patients who will develop RRHD and establish reproducible markers to diagnose RRHD. To develop an early surrogate for RRHD, several clinical trials have used SPECT imaging to assess changes in myocardial perfusion after RT (8, 9). A prospective study (9) used SPECT to examine myocardial perfusion of patients with left-sided breast cancer before and after RT, and showed that radiation causes volume-dependent defects in cardiac perfusion, which can persist even 3 to 6 years post-RT (10). Thus, these findings suggest that non-invasive imaging modalities that detect early changes in the irradiated heart may help to identify patients at risk for developing RRHD at an early stage of pathogenesis.
One underlying mechanism of RRHD is damage to vascular endothelial cells (11–17), which contributes to both microvascular and macrovascular injuries (18). Prior studies demonstrated that the tumor suppressor p53 protects cardiac endothelial cells against radiation (16). After a single dose of 12 Gy or 10 fractions of 3 Gy whole-heart irradiation, Tie2Cre; p53FL/− mice, where both alleles of p53 are deleted in endothelial cells, are sensitive to radiation-induced myocardial injury compared to Tie2Cre; p53FL/+ littermates, which retain one allele of p53 in endothelial cells. Histological and physiological examinations indicate that damage to the microvasculature of the heart after irradiation precedes pathological changes in the myocardium. Thus, imaging biomarkers that assess alterations in vascular permeability are promising surrogates to non-invasively identify regions of the myocardium at risk for radiation-induced injury in vivo.
Recently, DE-microCT has been demonstrated as an effective tool for non-invasive assessment of vascular changes in primary tumors after a single therapeutic dose of RT (19). To develop microCT imaging methods to study cardiac injury from tangent field irradiation in mice, we used a small-field biological irradiator to deliver a single dose of 12 Gy x-rays to approximately one third of the LV of Tie2Cre; p53FL/− and Tie2Cre; p53FL/+ mice. To achieve non-invasive and sensitive measurements at both anatomical and functional levels for the murine heart, we performed DE-microCT and 4D-microCT with two nanoparticle-based contrast agents – a blood-pool liposomal Iodine (Lip-I) (20) and a PEGylated gold nanoparticle (AuNp). Theses contrast agents were chosen based on their physical properties and because of their potential for translation into the clinic (21).
While DE-CT and cardiac 4D-CT are both gaining momentum in the clinic, they are still novel in the pre-clinical domain to study cardiac injury after RT. Here, we show that DE-microCT and 4D-microCT are effective pre-clinical imaging approaches that provide not only anatomic, but also functional information to non-invasively measure radiation-induced cardiac injury in mice. For comparison and validation we also assessed cardiac perfusion in the same cohort of mice by microSPECT.
METHODS AND MATERIALS
Mouse strains
All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) in our institute. Tie2Cre; p53FL/− mice and Tie2Cre; p53FL/+ littermates (8-to-10-weeks old), which were characterized previously (16), were used for experiments.
Radiation
Partial-heart irradiation was performed with a small-field biological irradiator, the X-RAD 225 Cx (Precision X-ray). The isocenter was placed in the heart (source-to-subject distance: ~30.75 cm) using cone beam computed tomography. Placement of the isocenter was based on the location of the following landmarks: spinal cord, sternum, and heart using the axial, coronal, sagittal images. Treatment was delivered with two opposing tangent fields using gantry angles of 45° and 225° (see Fig. 1A). Irradiation was performed with a rectangle collimator to produce a radiation field of 10 mm × 10 mm at the treatment isocenter. We delivered 225-kVp x-rays with an average dose rate of 300 cGy/min at target depth using a current of 13 mA and a 0.3-mm Cu filter. The dose rate was measured with an ion chamber by members of the Radiation Safety Division in our institute.
Figure 1.
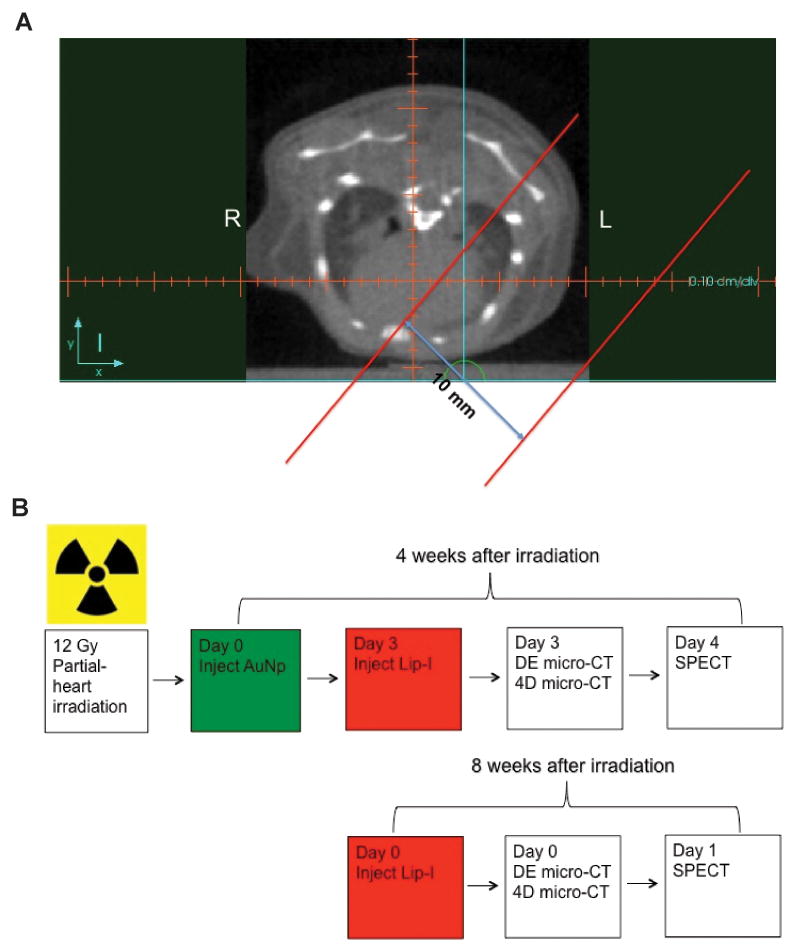
Multimodality imaging of the mouse heart after partial heart irradiation. (A) Representative axial slice of the pre-treatment microCT of a mouse heart. The isocenter is placed in a reproducible position in each mouse using the axial, coronal, and sagittal images based on bony landmarks (sternum and thoracic vertebral bodies) and the heart. Then a 10 mm-wide rectangular collimator is used to deliver RT with a left posterior oblique (gantry angle = 45°) and a right anterior oblique (gantry angle = 225°) field. The tangent radiation fields are shown within the parallel red lines. (B) Schematic of the protocol for DE-microCT, 4D-microCT, and microSPECT imaging. Four weeks after irradiation mice were administered with the gold nanoparticles (AuNp), and 3 days later the liposomal iodine (Lip-I) contrast agent. The mice were then imaged with DE-microCT and 4D-microCT. One day after CT imaging the mice were injected with 99mTc-tetrofosmin and imaged by microSPECT. Eight weeks after irradiation, mice were administered with another bolus of Lip-I and 99mTc-tetrofosmin prior to microCT and microSPECT imaging, respectively.
Imaging Protocol
Both DE-microCT and 4D-microCT acquisitions were performed with a dual source microCT system developed at our center explicitly for dynamic and spectral applications. Imaging was performed 4 and 8 weeks post-irradiation (see Fig. 1B). Mice were intravenously injected with AuNp contrast agent (AuroVist, www.nanoprobes.com; 0.004 mL/g) only once on day 0. Three days later mice were re-injected with Lip-I (0.012 mL/g). The use of both types of nanoparticles was necessary to measure vascular permeability in the damaged myocardium (via AuNp which extravasates after a few days and accumulates in the injured tissue), and to image cardiac function with 4D-microCT (via Lip-I which remains intravascular). DE-microCT was performed immediately after Lip-I injection followed by a 4D-microCT acquisition. One day later (i.e. day 4) mice were injected with 99mTc-tetrofosmin (300 ± 79 MBq) and imaged with microSPECT. At 8 weeks, mice received another intravenous injection of Lip-I (0.012 mL/g) and were scanned again with DE- and 4D-microCT. The following day, mice were injected with 99mTc-tetrofosmin (300 ± 79 MBq) and imaged by microSPECT. The free-breathing animals were scanned while under anesthesia induced with 1% isoflurane delivered by nose cone.
The estimated cumulative radiation dose associated with imaging for the entire study was: 0.26 Gy for DE-microCT, 0.58 Gy for 4D-microCT and 1.34 Gy for microSPECT.
Dual energy-microCT
Images from DE-microCT were used to determine the fraction of damaged LV, which was estimated by semi-automatic segmentation using the Segment Assistant and VoxelCount plugins for ImageJ (http://rsbweb.nih.gov/ij/). The fraction of damaged LV was computed as the ratio between the volume of damaged LV and the total volume of the myocardium in the LV.
Comprehensive methods for DE-microCT imaging and dual energy decomposition are described in Supplementary Materials (www.redjoural.org).
4D-microCT
Measurements of global LV function for 4D-microCT were performed using a commercially available software package that semi-automatically segments the LV over 10 phases of the cardiac cycle and provides volumetric measurements at each phase (Vitrea v5.2 LV Functional Analysis, Vital Images Inc., Minnetonka, MN). The LV end-diastolic (EDV) and end-systolic (ESV) volumes were converted to absolute volumes by multiplying with voxel volumes, and then used to compute stroke volume (SV), ejection fraction (EF), and cardiac output (CO), as in:
Comprehensive methods for 4D-microCT imaging are described in Supplementary Materials (www.redjoural.org).
MicroSPECT
MicroSPECT acquisitions were performed with a U-SPECTII/CT system fitted with a 0.35 mm multi-pinhole collimator (MILabs, Utrecht, The Netherlands). The fraction of the damaged LV was computed on microSPECT using the same method for DE-microCT.
Comprehensive methods for microSPECT imaging are described in Supplementary Materials (www.redjoural.org).
Histological analyses
Methods for sample preparation, hematoxylin and eosin (H&E) staining, Masson’s trichrome staining, and quantification of the average cardiomyocyte cross-sectional area were described previously (16).
Statistics
Results are presented as mean ± SEM. The Student’s t-test and the Mann-Whitney test (two-tailed) were performed to compare the means and medians of two groups, respectively. R squared values were calculated by Pearson’s r correlation. Significance was assumed at P<0.05 and calculated using GraphPad Prism 5 (GraphPad Software, Inc).
RESULTS
Assessing vascular permeability in the left ventricle by dual energy microCT
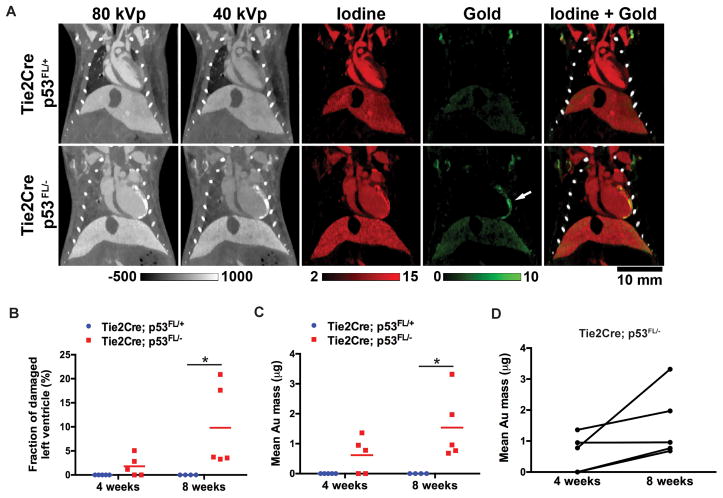
DE-microCT images and their decompositions are shown in Fig. 2A. Four and eight weeks after irradiation, accumulation of AuNp was detected in the portion of the LV that was within the radiation field in Tie2Cre; p53FL/− mice (Fig. 2A). The fraction of damaged LV (Fig. 2B) and the mean mass of accumulated AuNp in the LV (Fig. 2C) were significantly increased in Tie2Cre; p53FL/− mice compared to Tie2Cre; p53FL/+ mice 8 weeks after irradiation. In addition, in 4 out of 5 Tie2Cre; p53FL/− mice, a time-dependent increase in the mean mass of AuNp in the LV were observed (Fig. 2D). Collectively, these results suggest that the AuNp contrast agent and delayed DE-microCT can be utilized to non-invasively assess the change in permeability of myocardial vessels after partial-heart irradiation.
Figure 2.
Dual energy-microCT to assess accumulation of Au nanoparticles in the left ventricle (LV). (A) Iodine maps (red) were used to contour the total volume of the LV, whereas Au maps (green) from the same animal were used to quantify volume of the myocardium that had increased AuNp accumulation (arrow). (B) Quantification of the fraction of damaged LV and (C) of mean Au mass in the LV 4 and 8 weeks after irradiation. (D) Comparison of the mean Au mass in the LV of Tie2Cre; p53FL/− mice 4 and 8 weeks after irradiation. P values were calculated by Mann-Whitney test. *P<0.05
Alterations in tissue architecture of the left ventricle after partial-heart irradiation
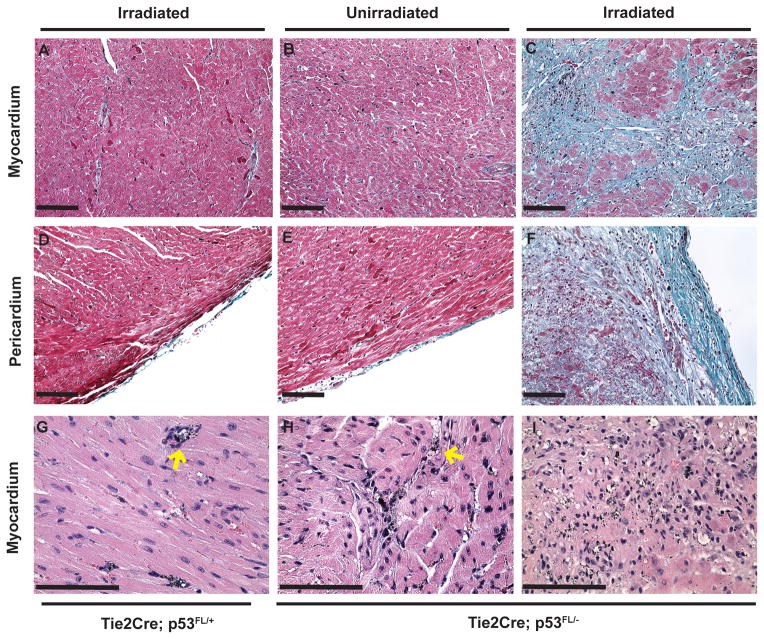
We examined the histology of the heart in Tie2Cre; p53FL/+ and Tie2Cre; p53FL/− mice after the second round of imaging (approximately 10 weeks after irradiation). Masson’s trichrome-stained sections showed normal tissue architecture of the myocardium and pericardium in the irradiated LV of Tie2Cre; p53FL/+ mice (Fig. 3, A and D). In contrast, in Tie2Cre; p53FL/− mice, while the part of the heart that was outside the field of radiation appeared normal (Fig 3, B and E), the portion within the radiation field demonstrated multifocal to extensive myocardial necrosis and fibrosis in the same mice (Fig 3, C and F). Additionally, the pericardium in the irradiated LV was thickened by collagen fibrils and infiltration of lymphocytes (Fig 3, F).
Figure 3.
Histological examination of the myocardium and pericardium 10 weeks after partial-heart irradiation. Masson’s trichrome staining demonstrated relatively normal appearing myocardium and pericardium (red) in the LV of Tie2Cre; p53FL/+ mice that were irradiated (A and D) and of Tie2Cre; p53FL/− mice outside the field of irradiation (B and E). In contrast, in irradiated LV of Tie2Cre; p53 FL/− mice, collagen fibers (blue) were present in the myocardium (C) and pericardium (F). H&E staining demonstrated gold nanoparticles (AuNp; small black particles) were restricted within blood vessels (yellow arrow) in the LV of Tie2Cre; p53FL/+ mice that were irradiated (G) and of Tie2Cre; p53FL/− mice that was outside the field of irradiation (H). Of note, in the irradiated LV of Tie2Cre; p53 FL/− mice, extravasation of AuNp was observed in necrotic and fibrotic areas in the myocardium (I). Scale bar, 100 μm.
Moreover, H&E-stained sections showed that in the myocardium of Tie2Cre; p53FL/+ mice, AuNp was exclusively within blood vessels (Fig 3, G). In contrast, in Tie2Cre; p53FL/− mice, while AuNp was also exclusively within blood vessels in the unirradiated LV fraction (Fig 3, H), extravasation of AuNp into the necrotic myocardium was observed in irradiated portions of the LV (Fig 3, I). Together, our results show that 12 Gy partial-heart irradiation caused necrosis and fibrosis in the myocardium and pericardium of Tie2Cre; p53FL/− mice, in which extravasation of AuNp was present. These data indicate that AuNp accumulation is a specific imaging surrogate for vascular injury induced by irradiation.
Assessing myocardial perfusion by microSPECT
We also assessed myocardial perfusion in the same cohort of Tie2Cre; p53FL/+ and Tie2Cre; p53FL/− mice using microSPECT. MicroSPECT imaging of Tie2Cre; p53FL/− mice demonstrated a decrease in myocardial perfusion in the irradiated LV, which occurred in the same region as the accumulation of AuNp (Fig. 4A). Linear regression demonstrated a significant correlation for the fraction of damaged LV measured by microSPECT and DE-microCT 8 weeks after partial-heart irradiation (R2=0.97) (Fig. 4B).
Figure 4.
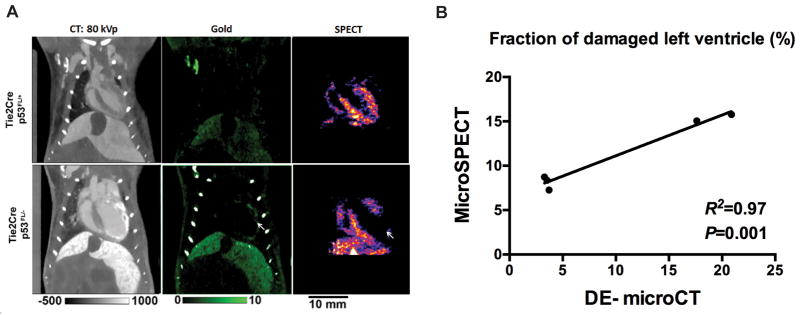
MicroSPECT scans to assess changes in myocardial perfusion. (A) Representative DE-microCT (green) and SPECT images of the heart 8 weeks after 12 Gy partial-heart irradiation. Note the area of decreased perfusion on SPECT (white arrow) matches the region of AuNp accumulation on DE-microCT. (B) Correlation analysis of the fraction of damaged LV that is assessed by SPECT and DE microCT. R squared and P values were calculated with Pearson’s r correlation.
Measuring cardiac function by 4D-microCT
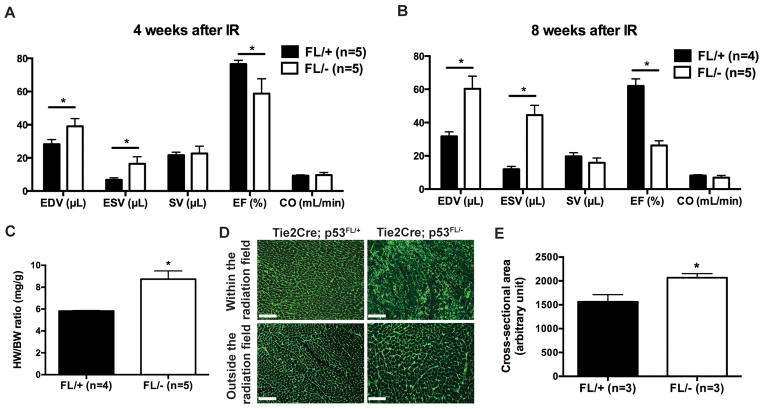
We also utilized 4D-microCT to measure a number of clinically important endpoints of cardiac physiology, including the LV end-diastolic volume (EDV), end-systolic (ESV) volume, stroke volume (SV), ejection fraction (EF), and cardiac output (CO) (22). Our results show that 4 and 8 weeks after partial-heart irradiation, Tie2Cre; p53FL/− mice had a significant increase in both EDV and ESV, and a significant decrease in EF compared to Tie2Cre; p53FL/+ controls (Fig. 5, A and B).
Figure 5.
4D-microCT scans to examine changes in cardiac functions after partial-heart irradiation. (A and B) 4D-micoCT was performed 4 and 8 weeks after irradiation to assess end-diastolic volume (EDV), end-systolic volume (ESV), stroke volume (SV), ejection fraction (EF) and cardiac output (CO) of the LV. (C) Ratio of heart-weight to body-weight (HW/BW) of Tie2Cre; p53FL/+ and Tie2Cre; p53FL/− mice 10 weeks after irradiation. (D) Representative images of wheat germ agglutinin (WGA)-stained myocardium that was within or outside of the radiation field. Scale bar, 100 μm. (E) Quantification of cardiomyocyte cross-sectional area in WGA-stained myocardium that was outside of the radiation field. Data are presented as mean ± SEM (n=3 mice per group). *P<0.05 by Student’s t test.
At necropsy 10 weeks after irradiation, we observed a markedly increased heart-weight to body-weight ratio (Fig. 5C). In addition, the average cardiomyocyte cross-sectional area in the myocardium outside the field of radiation in Tie2Cre; p53FL/− mice were increased compared to Tie2Cre; p53FL/+ littermates (Fig. 5, D and E). Together, the results from 4D-microCT and histology studies indicate that partial-heart irradiation with 12-Gy x-rays not only caused necrosis and fibrosis within the irradiated LV of Tie2Cre; p53FL/− mice, but also caused compensatory enlargement of surviving cardiomyocytes in the myocardium outside the field of radiation, which led to hypertrophy, dilatation and decreased EF in the LV.
DISCUSSION
Although the clinical manifestations of RRHD are diverse, one of the early effects of partial-heart irradiation is decreased myocardial perfusion, which results from damage to the microvasculature within the irradiated heart (18). In this study, DE-microCT was utilized to non-invasively assess radiation-induced changes in myocardial vascular permeability by measuring the accumulation of AuNp (Fig. 2). By histology, we demonstrated that the extravasation of AuNp occurs specifically in the irradiated myocardium and pericardium that became necrotic and fibrotic 10 weeks after irradiation (Fig. 3). Additionally, the accumulation of AuNp in the irradiated LV detected by DE-microCT significantly correlates with defects in myocardial perfusion measured by microSPECT (Fig. 4). Therefore, our results indicate that DE-microCT with AuNp can non-invasively assess radiation-induced damage to the myocardial vasculature in mice.
In the same cohort of mice, blood pool Lip-I and 4D-microCT enabled us to measure LV volumes over the cardiac cycle to assess cardiac function. Four and 8 weeks after partial-heart irradiation, Tie2Cre; p53FL/− mice showed a significant decrease in EF compared to Tie2Cre; p53FL/+ littermates (Fig. 5, A and B). The decrease in EF was associated with LV dilation and hypertrophy. Nevertheless, in Tie2Cre; p53FL/− mice there was no significant decrease in SV and CO, likely due to compensatory changes in the heart reflected by the increase in both EDV and ESV and the length of follow-up.
Prior studies showed that after whole-heart irradiation, Tie2Cre; p53FL/− mice developed multifocal myocardial necrosis and systolic dysfunction (16). However, the LVs in those mice were not dilated and showed only limited fibrosis in the myocardium and pericardium. In contrast, in this study we found that after partial-heart irradiation Tie2Cre; p53FL/− mice developed LV dilation (Fig. 5, A and B) and extensive fibrosis in the irradiated myocardium and pericardium (Fig. 3, C and F), which suggest more of a chronic injury. Together, these results suggest that compared to whole-heart irradiation, partial-heart irradiation may cause different changes in cardiac physiology and pathology, which better model pericarditis and myocardial injury that can occur in patients receiving left chest wall irradiation with tangent fields.
In this study, we performed partial-heart irradiation with 12-Gy x-rays to model radiation-induced myocardial injury in Tie2Cre; p53FL/− mice, in which endothelial cells are sensitive to radiation. The histological manifestations of cardiac injury in this model, which include myocardial fibrosis and pericarditis, mimic the pathological changes observed in humans (18) and wild-type mice (15) after heart irradiation. However, in wild-type animals exposed to whole-heart irradiation, cardiac injury varies with the total-dose and time after irradiation (7, 13, 15, 23). Therefore, in future studies we will use the imaging techniques developed in this study to characterize the physiological and cellular events that lead to cardiac injury in wild-type mice after partial-heart irradiation with a more clinically relevant radiation dose, such as 2 Gy per day up to 50 Gy.
It is possible that the phenotype we observed in this study was influenced by the additional radiation exposure from microCT and microSPECT (approximately 2.18 Gy per mouse for the entire study). This additional radiation exposure represents 18% of the partial-heart irradiation dose. This dose was not sufficient to elicit radiation injury to the part of the heart in Tie2Cre; p53FL/− mice outside of the tangent fields (Fig. 3), but may have additive effects on the myocardium within the tangent fields. Therefore, it will be important to limit the number of scans, or to choose either microCT or microSPECT in future studies to minimize the radiation epxosure from imaging.
In conclusion, our results show that DE-microCT and 4D-microCT with nanoparticle-based contrast agents are promising approaches to assess changes in vascular permeability and cardiac function in the pre-clinical setting. These imaging approaches may be useful to identify patients treated with RT who have developed vascular injury in the heart that may lead to radiation-related heart disease.
Supplementary Material
Acknowledgments
We thank Howard Rockman and Ato Wright for critically reading the manuscript. These studies were supported by grants from Susan G. Komen, NIAID R01 AI080488 and K02 AI093866 (D.G.K). All imaging was performed by the Duke Center for In Vivo Microscopy, an NIH/NIBIB Biomedical Technology Resource Center (P41 EB015897).
Footnotes
CONFLICT OF INTEREST
None
References
- 1.EBCTCG. Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Lancet. 2000;355:1757–1770. [PubMed] [Google Scholar]
- 2.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. The New England Journal of Medicine. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 3.Darby SC, McGale P, Taylor CW, et al. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6:557–565. doi: 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 4.Cuzick J, Stewart H, Rutqvist L, et al. Cause-specific mortality in long-term survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol. 1994;12:447–453. doi: 10.1200/JCO.1994.12.3.447. [DOI] [PubMed] [Google Scholar]
- 5.EBCTCG. Effects of radiotherapy and surgery in early breast cancer. An overview of the randomized trials. The New England Journal of Medicine. 1995;333:1444–1455. doi: 10.1056/NEJM199511303332202. [DOI] [PubMed] [Google Scholar]
- 6.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. The New England Journal of Medicine. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 7.Schultz-Hector S, Trott KR. Radiation-induced cardiovascular diseases: is the epidemiologic evidence compatible with the radiobiologic data? Int J Radiat Oncol Biol Phys. 2007;67:10–18. doi: 10.1016/j.ijrobp.2006.08.071. [DOI] [PubMed] [Google Scholar]
- 8.Seddon B, Cook A, Gothard L, et al. Detection of defects in myocardial perfusion imaging in patients with early breast cancer treated with radiotherapy. Radiotherapy and Oncology. 2002;64:53–63. doi: 10.1016/s0167-8140(02)00133-0. [DOI] [PubMed] [Google Scholar]
- 9.Marks LB, Yu X, Prosnitz RG, et al. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys. 2005;63:214–223. doi: 10.1016/j.ijrobp.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 10.Prosnitz RG, Hubbs JL, Evans ES, et al. Prospective assessment of radiotherapy-associated cardiac toxicity in breast cancer patients: analysis of data 3 to 6 years after treatment. Cancer. 2007;110:1840–1850. doi: 10.1002/cncr.22965. [DOI] [PubMed] [Google Scholar]
- 11.Fajardo LF, Stewart JR. Pathogenesis of radiation-induced myocardial fibrosis. Lab Invest. 1973;29:244–257. [PubMed] [Google Scholar]
- 12.Fajardo LF, Stewart JR. Experimental radiation-induced heart disease. I. Light microscopic studies. Am J Pathol. 1970;59:299–316. [PMC free article] [PubMed] [Google Scholar]
- 13.Lauk S, Kiszel Z, Buschmann J, et al. Radiation-induced heart disease in rats. Int J Radiat Oncol Biol Phys. 1985;11:801–808. doi: 10.1016/0360-3016(85)90314-1. [DOI] [PubMed] [Google Scholar]
- 14.Yeung TK, Lauk S, Simmonds RH, et al. Morphological and functional changes in the rat heart after X irradiation: strain differences. Radiat Res. 1989;119:489–499. [PubMed] [Google Scholar]
- 15.Seemann I, Gabriels K, Visser NL, et al. Irradiation induced modest changes in murine cardiac function despite progressive structural damage to the myocardium and microvasculature. Radiotherapy and Oncology. 2012;103:143–150. doi: 10.1016/j.radonc.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Lee CL, Moding EJ, Cuneo KC, et al. p53 functions in endothelial cells to prevent radiation-induced myocardial injury in mice. Sci Signal. 2012;5:ra52. doi: 10.1126/scisignal.2002918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart FA, Hoving S, Russell NS. Vascular damage as an underlying mechanism of cardiac and cerebral toxicity in irradiated cancer patients. Radiat Res. 2010;174:865–869. doi: 10.1667/RR1862.1. [DOI] [PubMed] [Google Scholar]
- 18.Darby SC, Cutter DJ, Boerma M, et al. Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys. 2010;76:656–665. doi: 10.1016/j.ijrobp.2009.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moding EJ, Clark DP, Qi Y, et al. Dual-energy micro-computed tomography imaging of radiation-induced vascular changes in primary mouse sarcomas. Int J Radiat Oncol Biol Phys. 2013;85:1353–1359. doi: 10.1016/j.ijrobp.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukundan S, Ghaghada K, Badea C, et al. A Nanoscale, Liposomal Contrast Agent for Preclincal MicroCT Imaging of the Mouse. AJR. 2006;186:300–307. doi: 10.2214/AJR.05.0523. [DOI] [PubMed] [Google Scholar]
- 21.Libutti SK, Paciotti GF, Byrnes AA, et al. Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clin Cancer Res. 2010;16:6139–6149. doi: 10.1158/1078-0432.CCR-10-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Busch S, Johnson TR, Wintersperger BJ, et al. Quantitative assessment of left ventricular function with dual-source CT in comparison to cardiac magnetic resonance imaging: initial findings. European Radiology. 2008;18:570–575. doi: 10.1007/s00330-007-0767-y. [DOI] [PubMed] [Google Scholar]
- 23.Yeung TK, Hopewell JW. Effects of single doses of radiation on cardiac function in the rat. Radiotherapy and Oncology. 1985;3:339–345. doi: 10.1016/s0167-8140(85)80047-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.