Abstract
Purpose.
Retinal edema, the accumulation of extracellular fluid in the retina is usually attributed to inner blood retina barrier (BRB) leakage. Vascular endothelial growth factor plays an important role in this process. The effects of VEGF on the outer BRB, the RPE, however, have received limited attention. Here, we present a methodology to assess how VEGF modulates the integrity of the RPE barrier in vivo.
Methods.
Control subretinal blebs (1–5 μL) and blebs containing VEGF (1–100 μg/mL), placental growth factor (PlGF; 100 μg/mL), or albumin (100–1000 μg/mL) were injected into New Zealand White or Dutch Belted rabbits with IOP maintained at 10, 15, or 20 mm Hg. One-hour intravitreal pretreatment with ZM323881 (10 μM/L) was used to inhibit the VEGF response. Fluid resorption was followed by optical coherence tomography for 1 hour. Retinal pigment epithelium leakage was assessed by fluorescein angiography.
Results.
Increasing IOP resulted in an elevated rate of bleb resorption, while increasing albumin concentration in the bleb decreased the rate of resorption. Vascular endothelial growth factor, but not PlGF, caused a significant, concentration-dependent decrease in the rate of fluid resorption, which was reversed by ZM323881. Compared with albumin-filled blebs, VEGF-filled blebs showed accelerated early-phase leakage from the choroid.
Conclusions.
Consistent with a localized modulation of RPE function, VEGF induced a significant reduction in fluid resorption and an increase in hydraulic conductivity. Our results establish VEGF as a major cytokine regulating RPE barrier properties in vivo and indicate that the RPE is a principal factor in the pathogenesis of retinal edema.
Keywords: VEGF, retinal edema, RPE, barrier, fluid transport, resorption
Retinal edema is usually not attributed to a breakdown in RPE function. In this article, VEGF actions on RPE functional integrity were studied using an improved methodology in vivo, providing new evidence that the RPE is likely to be a key factor in the pathogenesis of retinal edema.
Introduction
Retinal edema (RE) is the localized accumulation of extracellular fluid in the neurosensory retina. The occurrence of edema in the macular area is a primary cause for loss of visual acuity in various eye diseases, such as diabetic retinopathy, wet age-related macular degeneration, posterior segment inflammatory disease, and venous occlusive disease.1,2 These conditions share some of the same features, such as general thickening resulting from radially-oriented cystoid spaces filled with optically-clear fluid.1 The development of RE is attributed to the disruption of one or both blood retina barriers (BRB), which leads to an imbalance of the hemodynamic forces (hydrostatic and oncotic) between the blood and the retina.3–5
Vascular endothelial growth factor is a dimeric glycoprotein with potent vasoactive6 and angiogenic potential,7 a cytokine that has been shown to disrupt the inner BRB.8–10 The disruption of the inner BRB allows protein to infiltrate the retina,11 often leading to an accumulation of extracellular fluid. Thus, VEGF is generally accepted to be a major player in the pathogenesis of RE,12–14 and anti-VEGF therapy is traditionally believed to be beneficial by preventing the VEGF-induced effect on inner retinal vasculature.15–19
Although leakage across the endothelium of retina vessels can contribute to the accumulation of extracellular fluid in the retina, it is the RPE that is ultimately responsible for the maintenance of the retina in a relatively dehydrated state.20 The tight junctional complexes between the cells of the RPE, which form the outer BRB, together with the active regulation of ion transport across the RPE, are conducive for the removal of retinal extracellular fluid into the choroidal circulation. Therefore, a tight RPE barrier is a prerequisite for both maintaining fluid balance within the retina and resolving edematous fluid once it accumulates.4 We have recently shown that the receptors for VEGF are expressed by the RPE,21,22 and we provided evidence that VEGF can modulate RPE barrier properties through apically-oriented VEGF type 2 receptors (VEGF-R2) in vitro.23 Therefore, VEGF action on the RPE was postulated to influence retinal fluid dynamics; however, the effect of VEGF on RPE function in vivo has not been investigated.
The experimental assessment of RPE barrier integrity is a particularly challenging task in vivo because hydrodynamic processes through the RPE versus inner retinal vasculature are usually hard to separate in most species. However, the merangiotic rabbit retina allows observations limited exclusively to the RPE, as only a small streak of the retina is vascularized, and most of the retina remains avascular. Thus, experiments in the area devoid of vessels can be utilized to understand RPE function, and these principles have been implemented in the past for in vivo RPE studies.24–28
In this article, we present a methodology to study the RPE barrier function in vivo using rabbits under observation with spectral-domain optical coherence tomography (SD-OCT). These experiments allow a unique opportunity for high-precision measurements to experimentally determine the modulatory effects of VEGF on the RPE in vivo.
Methods
Animals
Eight New Zealand White and 24 Dutch Belted rabbits weighing 1.5 to 2 kg were anesthetized using intramuscular ketamine (20 mg/kg) and xylazine (2 mg/kg) (both from Butler Schein, Columbus, OH, USA). Additional doses were administered as needed during the procedure. The pupils were dilated using 10% phenylephrine hydrochloride (Bausch & Lomb, Tampa, FL, USA) and atropine (Bausch & Lomb) drops administered topically. For local anesthesia, proparacaine (0.5%; Bausch & Lomb) drops were administered topically before and during the procedure. Unless noted otherwise, one bleb per eye was created to evaluate individual treatment in each animal. Animal handling was performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and the study protocol was approved by the Animal Care and Use Committee at the Medical University of South Carolina.
Subretinal Bleb Formation
A 26-gauge needle was inserted 2 to 3 mm posterior to the limbus and a 33-gauge blunt needle was advanced through the 26-gauge needle, under infrared guidance with a Spectralis OCT (Heidelberg Engineering, Heidelberg, Germany), across the vitreous toward the subretinal space. Once reaching the subretinal space, sterilized fluid (1–5 μL) was slowly injected to produce a subretinal bleb. To control IOP at 10, 15, and 20 mm Hg, the anterior chamber was cannulated using a 25-gauge needle connected to a heparinized saline reservoir positioned at the corresponding height. For most experiments (except for those with IOP as a variable), pressure was maintained at 10 mm Hg. Intraocular pressure was confirmed using the Model 30 Pneumatonometer (Reichert Technologies, Depew, NY, USA). Infrared fundus images and OCT tomograms (both in transverse and sagittal directions) as well as full-volume scans of the injected fluid (bleb) were taken at every 10 minutes over the course of 1 hour using the Spectralis instrument.
Compounds for Subretinal Injections
Various compounds were injected subretinally (at a concentration of 1–100 μg/mL) following the procedures described above in the Subretinal Bleb Formation section. These compounds included human recombinant VEGF (VEGF-A165), human recombinant placental growth factor (PlGF), bovine serum albumin (albumin), PBS, and Na-fluorescein (1 mg/mL diluted in sterile PBS; hereafter termed fluorescein). All these compounds were purchased from Sigma (St. Louis, MO, USA). In selected experiments, 10 μL ZM323881 (10 μM; Tocris Bioscience, Ellisville, MO, USA), a relatively selective VEGF-R2 antagonist, was injected into the vitreous 1 hour prior to creating VEGF- or albumin-filled blebs. Before injection, all compounds were sterilized by filtration through a syringe filter.
Fluorescein Angiography
To evaluate the integrity of the RPE barrier, fluorescein was injected intravenously into the main ear vein. For these studies, two blebs of similar size were created in one eye. One bleb contained VEGF (100 μg/mL) and the other contained an equal concentration of albumin. In selected animals, eyes were pretreated with 10 μL ZM323881 (10 μM) 1 hour prior to the creation of both blebs. Blebs were created using the same procedures as described above. Early-phase angiographies were recorded as both infrared and fluorescence (1exc = 488) signals using the Spectralis for 1 minute immediately post fluorescein injections. Images were extracted from these videos using the instrument software.
Data Analysis
The linear dimensions of blebs were determined using instrument software (Heidelberg Eye Explorer 1.7, Heidelberg Engineering). The data were then tabulated, and linear regressions were determined in Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA). Tabulated data are shown as SEM of 3 to 6 independent experiments (different animals). Total fluorescence intensity in the blebs was calculated by comparing total pixel intensities in extracted images within the same size circle for each bleb using ImageJ software (National Institutes of Health [NIH], Bethesda, MD, USA). Calculation of error propagation followed basic rules outlined in the literature.29 Statistical significance was determined at P < 0.05 using the Student's t-test.
Results
Resolution of Subretinal Fluid Measured in the OCT
Injection of fluid under the retina resulted in the appearance of dome-shaped blebs as observed under the OCT instrument (Fig. 1A). For purposes of volume calculations, the blebs were considered to be half ellipsoids; thus each volume was calculated using the following equation:
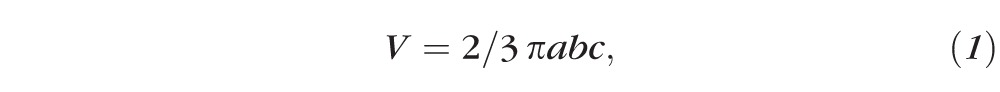 , ,
|
where a and b are the largest radii from sagittal and transverse directions, respectively, and c is the largest height of the blebs.
Figure 1.
The process of subretinal bleb resorption. (A) A typical bleb visualized by a transverse OCT scan through its largest width. The corresponding retina structures are indicated. (B) The blebs showed a linear decrease in volume with time. The rate of fluid resorption is calculated from the linear regression of the change in bleb volume over the course of 1 hour. Values are expressed as SEM; n = 4.
As shown in Figure 1B, the volume of the blebs exhibited a constant decrease over a period of 1 hour. As the volume changed linearly with time, the rate of volume change (fluid current) was equal to the slope of the linear regression fitted to the bleb volume over the course of the experiment (Fig. 1B):
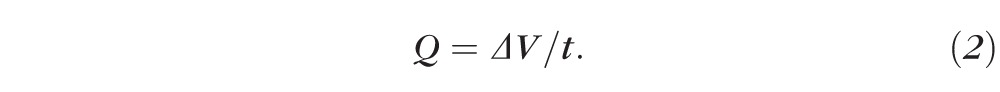 . .
|
From this, the rate of resorption (the volume flux) is calculated as the current passing through the changing bleb surface, A(t), of the RPE:
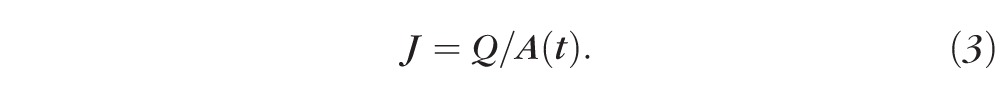 . .
|
Injections of blebs filled with fluorescein-containing saline provided evidence of no lateral leakage and no leakage into the vitreous during bleb resorption (data not shown). These experiments supported the idea that the entire volume of the subretinal blebs was resorbed by fluid transport through the RPE directly under the bleb.
The Effect of Changing Parameters of Fluid Dynamics on the Rate of Resorption
Fluid dynamics across the RPE is governed by Starling's equation, which states that for equilibrium,
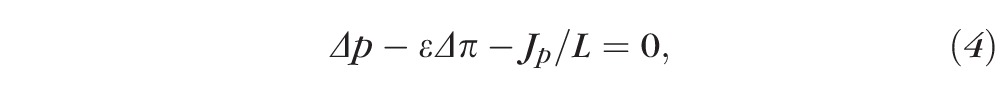 , ,
|
where Δp is the difference of the choroidal and intraocular pressures, ε is a coefficient, Δπ is the difference between choroidal and intraocular osmotic forces, Jp represents the flux due to active water pumping, and L is the hydraulic conductivity of the RPE. Our measurement system allowed a unique opportunity to observe the validity of these parameters.
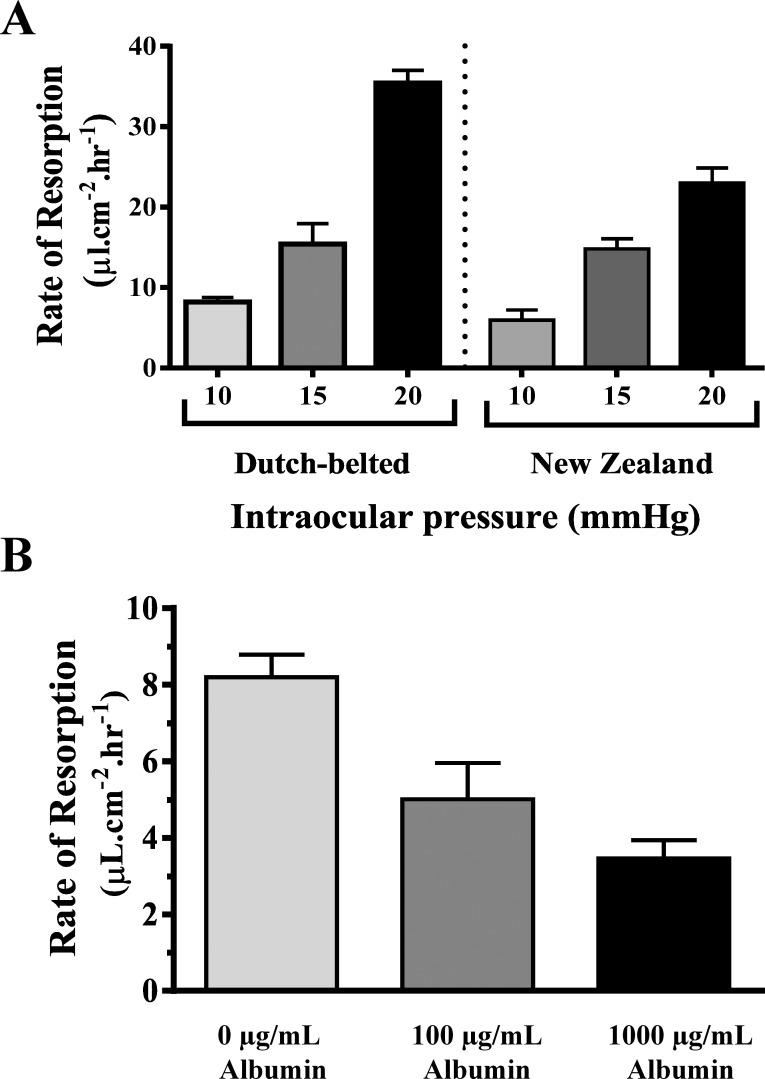
To test the effect of IOP on the rate of resorption, blebs were created at various IOP settings (10, 15, and 20 mm Hg; Fig. 2A). In Dutch Belted rabbits, the rate of resorption of the blebs was 8.2 ± 0.59 μL·cm−2·h−1 at IOP 10 mm Hg, 15.4 ± 2.5 μL·cm−2·h−1 at 15 mm Hg, and 35.4 ± 1.6 μL·cm−2·h−1 at 20 mm Hg (all changes were statistically significant). To test whether this response is strain specific, experiments in New Zealand White rabbits found similar resorption rates of 5.95 ± 1.2 μL·cm−2·h−1 at IOP 10 mm Hg, 14.83 ± 1.2 μL·cm−2·h−1 at 15 mm Hg, and 22.99 ± 1.88 μL·cm−2·h−1 at 20 mm Hg.
Figure 2.
The rate of bleb resorption depends on basic parameters of fluid dynamics. (A) In both Dutch Belted and New Zealand White rabbits, the rate of PBS-filled bleb resorption was proportional to the IOP preset with anterior segment cannulation. (B) In Dutch Belted rabbits with the IOP maintained at 10 mm Hg, the rate of bleb resorption was inversely proportional to the oncotic pressure of the bleb filled with various concentrations of albumin (0 μg/mL albumin blebs were filled with PBS). Values are expressed as SEM; n = 3 to 4.
To assess the effects of the oncotic pressure of the blebs on fluid resorption, increasing concentrations of albumin were injected subretinally with the IOP maintained at 10 mm Hg. Contrary to changing the IOP, Starling's equation predicts that the higher the oncotic pressure in the bleb, the lower the rate of fluid resorption (Fig. 2B). In Dutch Belted rabbits, at 0 μg/mL albumin (PBS-filled blebs), the resorption rate was 8.2 ± 0.59 μL·cm−2·h−1; at a concentration of 100 μg/mL albumin, it was 5.0 ± 0.9 μL·cm−2·h−1; and increasing the albumin concentration to 1000 μg/mL resulted in a resorption rate of 3.46 ± 0.47 μL·cm−2·h−1.
The Effect of VEGF Filling on Fluid Resorption Across the RPE
Vascular endothelial growth factor has been shown to modulate RPE barrier function in vitro.21,23 To determine if VEGF can also affect RPE function in vivo, VEGF-containing subretinal blebs were created in Dutch Belted rabbits with the IOP maintained at 10 mm Hg (Fig. 3). Increasing concentrations of VEGF (1, 10, 100 μg/mL) in the blebs resulted in a dose-dependent decrease in the rate of fluid resorption (Fig. 3A). Blebs filled with VEGF at a concentration of 1 μg/mL did not cause any significant changes in resorption (7.8 ± 0.9 μL·cm−2·h−1) compared with PBS (8.2 ± 0.59 μL·cm−2·h−1). However, bleb containing VEGF at a concentration of 10 μg/mL showed significant decrease in resorption, with a rate of 2.16 ± 0.5 μL·cm−2·h−1; and increasing VEGF levels to 100 μg/mL reduced the rate to 0.1 ± 0.16 μL·cm−2·h−1. The resorption rate of 100 μg/mL albumin-filled blebs (5.0 ± 0.9 μL·cm−2·h−1) was not significantly different from the PBS controls. Regression analysis of VEGF concentration-response data (Fig. 3B) yielded a half maximal effective concentration (EC50) value of 4.9 μg/mL (log EC50 = −5.3 ± 0.15).
Figure 3.
The resorption of VEGF-filled subretinal blebs. (A) In Dutch Belted rabbits, VEGF filling exhibited a concentration-dependent reduction in the rate of resorption compared with both PBS and albumin. As 10 μg/mL VEGF reduced the resorption rate more than 100 μg/mL albumin, the response to VEGF was beyond the one expected from oncotic forces alone. (B) The concentration-response curve exhibited sigmoid characteristics with a unitary slope. The EC50 concentration for VEGF was equal to 4.9 μg/mL (log EC50 = −5.3 ± 0.15). IOP was set at 10 mm Hg. Values are expressed as SEM; n = 3 to 4; **P < 0.01, ***P < 0.001.
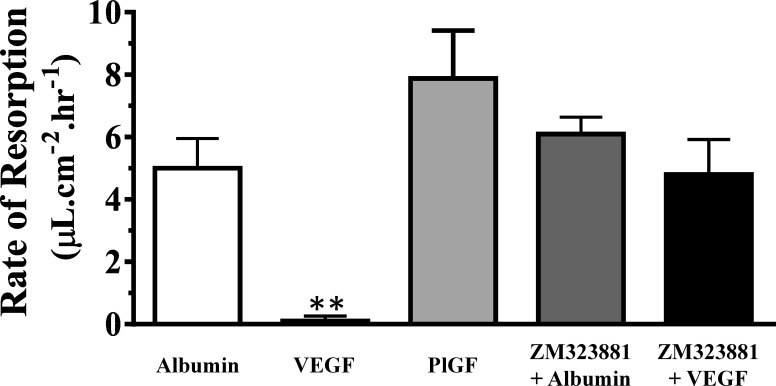
Experiments with a selective agonist and antagonist identified the receptor subtype involved in the VEGF response (Fig. 4). Blebs filled with the VEGF type 1 receptor (VEGF-R1) agonist, PlGF (100 μg/mL), did not produce any significant change in the rate of fluid resorption (7.8 ± 1.5 μL·cm−2·h−1) compared with control (100 μg/mL) albumin-filled blebs (5.0 ± I 0.9 μL·cm−2·h−1). The lack of a response to PlGF supports the idea that the effect of VEGF is mediated by the VEGF-R2 receptor. To confirm the direct involvement of VEGF-R2, we investigated if pretreatment with ZM323881 could reverse the actions of VEGF on bleb reabsorption. Intravitreal treatment with 10 μL ZM323881 (10 μM) 1 hour prior to the subretinal fluid injection suppressed the effect of VEGF on the rate of fluid resorption (4.8 ± 1.1 μL·cm−2·h−1). However, pretreating eyes with ZM323881 1 hour prior to the creation of albumin-filled bleb formation had no significant effect on the rate of resorption (6.1 ± 0.5 μL·cm−2·h−1).
Figure 4.
VEGF receptor specificity. Blebs filled with PlGF (a selective VEGF-R1 agonist) showed no significant difference in rate of resorption when compared with albumin-filled blebs, while VEGF (a nonselective VEGF receptor agonist) significantly reduced the resorption. Moreover, 1-hour intravitreal pretreatment with 10 μL of 10 μM ZM323881 (a specific VEGF-R2 antagonist) resulted in a rate of VEGF-filled bleb resorption that was not significantly different from the rate of albumin-filled bleb resorption. Both the agonist and antagonist experiments indicate a VEGF-R2–specific response. Concentrations were 100 μg/mL; **P < 0.01. Values are expressed as SEM; n = 3 to 4.
To investigate if VEGF induced a significant leakage from the choroid toward the subretinal space, early-phase fluorescein angiography experiments were performed (Fig. 5). Two adjacent blebs, one filled with VEGF (100 μg/mL) and the other with albumin (100 μg/mL), were created in the same retina as shown in the infrared image of the fundus in the presence or absence of ZM323881 in the vitreous (Figs. 5A, 5D). Fluorescent images of the fundus immediately following intravenous angiography (before any significant change in bleb volume) provided evidence that VEGF-filled blebs contained a higher amount of fluorescein filtering through the RPE from the choroidal vasculature compared with albumin-filled blebs (Figs. 5B, 5C). This effect was reversed with administration of ZM323881 into the vitreous 1 hour prior to the creation of the blebs (Figs. 5E, 5F). Plotting the total fluorescence intensity over time (Fig. 5G) showed an increased rate of fluorescein-filling with VEGF-filled blebs (kv = 3.5 ± 0.2 s−1) compared both to albumin-filled blebs (kn = 2.2 ± 0.1 s−1) and VEGF-filled blebs following pretreatment with ZM323881 (kv+i = 1.8 ± 0.1 s−1).
Figure 5.
Fluorescein angiogram post subretinal bleb injections. Fluorescein angiograms were performed following the creation of approximately equal size side-by-side VEGF- (red arrow) and albumin-filled blebs (white arrow) at equal 100 μg/mL concentrations. Blebs were injected in the absence (A–C) or the presence of 10 μL of 10 μM ZM323881 administered 1 hour before bleb injection (D–F). The infrared fundus images (A, D) show the blebs immediately prior to the intravenous injection of fluorescein. Representative fluorescence (λexc = 488) images taken consecutively at 20 seconds (B, E) and 30 seconds (C, F) post intravenous injection of fluorescein. (G) The total fluorescence (arbitrary unit) of blebs filled with VEGF (▪, kv), albumin (▴, kn), and VEGF with ZM323881 in the vitreous (•, kv+i) after intravenous injection of fluorescein exhibits linear early-phase kinetics. The k values represent the rate of fluorescence accumulation.
The Hydraulic Conductivity of the RPE
The rate of fluid resorption is related to the hydraulic conductivity of the RPE by the Dracy equation:
 , ,
|
where Jp represents active water pumping by the RPE, L is the hydraulic conductivity, and ΔP is the pressure gradient moving the fluid across the RPE. Therefore, the hydraulic conductivity of the normal RPE (Ln) is the slope of the linear regression between IOP and the rate of fluid resorption, while Jp can be calculated from the intersect,
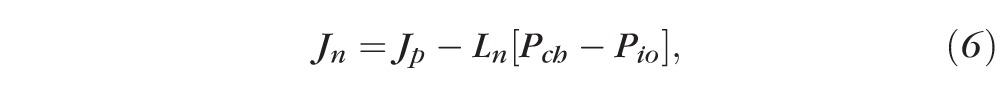 , ,
|
if we use Pch = 25 mm Hg for the choroidal perfusion pressure (from the literature)30 and Pio is the IOP. From regression analysis of the rate of bleb resorption against increasing IOP in Dutch Belted and New Zealand White rabbits (data shown in Fig. 2A), the appropriate equations are
 for Dutch Belted and
for Dutch Belted and
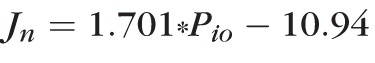 for New Zealand White animals. Therefore, for Dutch Belted rabbits, Ln = 2.7 ± 0.3 μL·cm−2·h−1·mm Hg−1 and Jp = 47.3 ± 4.7 μL·cm−2·h−1. Similarly, for New Zealand White rabbits, Ln = 1.7 ± 0.04 μL·cm−2·h−1·mm Hg−1 and Jp = 31.6 ± 0.7 μL·cm−2·h−1.
for New Zealand White animals. Therefore, for Dutch Belted rabbits, Ln = 2.7 ± 0.3 μL·cm−2·h−1·mm Hg−1 and Jp = 47.3 ± 4.7 μL·cm−2·h−1. Similarly, for New Zealand White rabbits, Ln = 1.7 ± 0.04 μL·cm−2·h−1·mm Hg−1 and Jp = 31.6 ± 0.7 μL·cm−2·h−1.
The same formula can be utilized to describe the fluid resorption post subretinal VEGF administration. Assuming that Jp is independent of the pressure gradient
 3.15 ± 0.4 μL·cm−2·h−1·mm Hg−1, where Jv is measured (0.1 ± 0.16 μL·cm−2·h−1), Jp was determined above, and P'io was 10 mm Hg (the preset IOP). These calculations showed that VEGF caused an increase in the hydraulic conductivity of the RPE and a subsequent increase in fluid movement from the choroid toward the subretinal space.
3.15 ± 0.4 μL·cm−2·h−1·mm Hg−1, where Jv is measured (0.1 ± 0.16 μL·cm−2·h−1), Jp was determined above, and P'io was 10 mm Hg (the preset IOP). These calculations showed that VEGF caused an increase in the hydraulic conductivity of the RPE and a subsequent increase in fluid movement from the choroid toward the subretinal space.
Discussion
The occurrence of retinal edema in the macula is a principal cause for the decrease in visual acuity in various eye conditions. The most promising treatment nowadays is based on targeting VEGF, which is believed to work by primarily preventing retinal vascular changes. Our laboratory has recently shown that the receptors for VEGF are expressed in the RPE,21,22 and we have demonstrated that VEGF can modulate RPE barrier function in vitro.23 In this study, we used SD-OCT in the merangiotic rabbit retina to study the role of the RPE in retinal fluid dynamics, as well as the effect of VEGF on RPE function.
Spectral-domain OCT observation provided evidence that injecting fluid under the retina created dome-shaped blebs best approximated with half ellipsoids (blebs used to be considered spheroids),27,31 which exhibited a linear decrease in volume over time. This linear volume decrease, the lack of lateral diffusion, and the result that fluorescein-filled blebs showed no leakage into the vitreous after 30 minutes of the bleb creation, supported the idea that subretinal fluid resorption is through the RPE. These results are broadly consistent with the previous bleb evaluations through fundus microscope-based observation systems.27
The movement of fluid across the RPE is expected to be governed by Starling's law of fluid dynamics based on the balance of hydrostatic and oncotic forces.4 Under normal conditions, the hydrostatic pressure in the choroid is higher than the IOP (by ∼10 mm Hg); which will result in a net hydrostatic pressure toward the retina.30 On the other hand, oncotic pressure pulls fluid in the opposite direction, from the retina toward the choroidal circulation.4 As oncotic forces arising simply from the difference in protein levels are inadequate to achieve this balance by themselves, the RPE transports ions to remove water out of the retina, keeping it in a dehydrated state. Although there are a high number of independent parameters (six), our experiments provided a way to experimentally verify that Starling's law governs the fluid movements across the RPE in vivo. Increasing the IOP caused an increase in the rate of fluid resorption. Previous studies have shown very limited correlation between IOP and bleb resorption.24 The ability of the current study to demonstrate a strong correlation between IOP and bleb resorption likely reflects a more precise measurement of bleb volume using the OCT instrument compared with previous microscopic observations.27 Our data, showing that IOP can influence fluid movement in the retina, are consistent with clinical observations of edema and treatment in patients with hypotony maculopathy and pseudophakic cystoid macular edema.32–34 Increasing the concentration of subretinal albumin lowered the rate of fluid resorption. Again, clinically, it has been shown that patients with diabetic macular edema have higher intraocular protein concentration compared with normal individuals.4,11 Using the change in the rate of resorption at different IOPs, we were able to calculate the hydraulic conductivity and the active pumping of the RPE in two rabbit strains. Although the values were strain specific, they were in agreement with data published for the hydraulic conductivity (0.756 μL·cm−2·h−1·mm Hg−1)35 and active pumping (∼10−8m·s−1 ≈3.6 μL·cm−2·h−1) of ex vivo RPE tissue for other species.31,35 Our experiments represent the first direct measurement of RPE hydraulic conductivity in vivo.30
Vascular endothelial growth factor is widely accepted as a vascular permeability factor in endothelial cells6,36 including retinal vasculature,37 and human recombinant VEGF has been shown to be active on the blood vessels in rabbit eyes.38–40 Yet, its role in the RPE is still debated. Experiments with cultured ARPE-19 and primary porcine RPE cells in our laboratory have shown that VEGF significantly decreases transepithelial electrical resistance (TEER) and increases permeability to radiolabeled inulin.22 Other laboratories, however, found no significant VEGF response in RPE cells.41,42 Subsequent data from our studies provided evidence that the lack of VEGF responses in earlier studies could be attributed to the high endogenous secretion of the anti-angiogenic molecule, pigment-epithelial derived factor.21
The bleb resorption experiments described in the current manuscript provided evidence that VEGF can regulate RPE function in vivo. The presence of VEGF in subretinal blebs reduced the rate of fluid resorption in a concentration-dependent manner. Although the EC50 for this response (4.9 μg/mL) is higher than concentrations of intravitreal VEGF expected in diseased human eyes (1 ng/mL),43,44 the utilized levels were similar to those used to elicit responses in other ocular rabbit studies (500 ng to 10 μg).38,39 The inconsistency between rabbit and diseased human eyes may reflect differences between the respective VEGF receptors or that these acute experiments required a higher level of VEGF compared with chronic responses to VEGF in disease conditions. Please note that VEGF, even at a concentration of 10 μg/mL, was more potent than albumin at a concentration of 100 μg/mL, arguing that the response to VEGF is beyond oncotic.
In our earlier in vitro studies, we found that the VEGF response in human-derived RPE cultures was mediated by VEGF-R2.23 In the current study, PlGF (a specific VEGF-R1 agonist) had no effect on rate of resorption, and ZM323881 (a selective VEGF-R2 antagonist known to inhibit VEGF-R2 in human,23 porcine,23 rat,45 and frog46 tissues) reversed the VEGF-induced decline in subretinal fluid reabsorption. Taken together, these results supported the idea that responses to VEGF in the rabbit RPE are also mediated by the VEGF-R2 receptor subtype.
To begin to understand the mechanism responsible for the VEGF-induced reduction in subretinal fluid resorption, fluorescein leakage across the RPE was evaluated. As shown in Figure 5, the initial rate of fluorescein accumulation into the VEGF-filled blebs was greater compared with albumin-filled blebs. This VEGF-induced increase was blocked by pretreatment with the VEGF-R2 antagonist, ZM323881, and the ratio of initial rates with and without VEGF was 0.63. This ratio is consistent with both the ratio of hydraulic conductivities with and without VEGF (0.85) as well as the ratio of TEER values from in vitro studies following VEGF treatment compared with control (0.6–0.85 depending on VEGF exposure time and cell type).21,23 Based on these data, we concluded that the activation of VEGF-R2 receptors in the RPE in vivo can increase RPE permeability. The increased permeability likely contributes to the development of retinal edema in disease states where VEGF is elevated in the intraocular environment.
In conclusion, we presented a significantly improved in vivo model system to study the involvement of RPE in the pathogenesis of RE. Our results confirmed that hydrodynamics in the outer retina is governed by Starling's equation across the RPE. Taken together, our data showed for the first time that in addition to affecting the inner BRB, VEGF can also modulate the outer BRB, thereby causing a decrease in the rate of resorption, which leads to an accumulation of fluid. These studies are consistent with the hypothesis that VEGF induces edematous complications in the eye by targeting both the inner and outer BRB, and in the RPE the cellular response to VEGF is mediated by an increase in epithelial permeability. Finally, we propose that pharmacologic intervention to suppress VEGF responses in the RPE is a feasible approach to accelerate the resolution of retinal edema.
Acknowledgments
Supported in part by National Institutes of Health (NIH) Grants EY019065 (ZA) and EY021368 (CEC); the Ola B. Williams Foundation (CEC); an unrestricted grant to the Department of Ophthalmology at the Medical University of South Carolina from Research to Prevent Blindness; the Medical Scientist Training Program (NIH/National Institute of General Medical Sciences Grant T32 GM008716 to MD and OA); and the South Carolina Lions Association.
Disclosure: M. Dahrouj, None; O. Alsarraf, None; J.C. McMillin, None; Y. Liu, None; C.E. Crosson, None; Z. Ablonczy, None
References
- 1. Tranos PG, Wickremasinghe SS, Stangos NT, Topouzis F, Tsinopoulos I, Pavesio CE. Macular edema. Surv Ophthalmol. 2004; 49: 470–490 [DOI] [PubMed] [Google Scholar]
- 2. Johnson MW. Etiology and treatment of macular edema. Am J Ophthalmol. 2009; 147: 11–21 [DOI] [PubMed] [Google Scholar]
- 3. Antcliff RJ, Marshall J. The pathogenesis of edema in diabetic maculopathy. Semin Ophthalmol. 1999; 14: 223–232 [DOI] [PubMed] [Google Scholar]
- 4. Stefansson E. Physiology of vitreous surgery. Graefes Arch Clin Exp Ophthalmol. 2009; 247: 147–163 [DOI] [PubMed] [Google Scholar]
- 5. Aiello LM. Perspectives on diabetic retinopathy. Am J Ophthalmol. 2003; 136: 122–135 [DOI] [PubMed] [Google Scholar]
- 6. Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983; 219: 983–985 [DOI] [PubMed] [Google Scholar]
- 7. Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989; 246: 1306–1309 [DOI] [PubMed] [Google Scholar]
- 8. Kenyon BM, Voest EE, Chen CC, Flynn E, Folkman J, D'Amato RJ. A model of angiogenesis in the mouse cornea. Invest Ophthalmol Vis Sci. 1996; 37: 1625–1632 [PubMed] [Google Scholar]
- 9. Ozaki H, Hayashi H, Vinores SA, Moromizato Y, Campochiaro PA, Oshima K. Intravitreal sustained release of VEGF causes retinal neovascularization in rabbits and breakdown of the blood-retinal barrier in rabbits and primates. Exp Eye Res. 1997; 64: 505–517 [DOI] [PubMed] [Google Scholar]
- 10. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994; 331: 1480–1487 [DOI] [PubMed] [Google Scholar]
- 11. Ouchi M, West K, Crabb JW, Kinoshita S, Kamei M. Proteomic analysis of vitreous from diabetic macular edema. Exp Eye Res. 2005; 81: 176–182 [DOI] [PubMed] [Google Scholar]
- 12. Ehlken C, Rennel ES, Michels D, et al. Levels of VEGF but not VEGF(165b) are increased in the vitreous of patients with retinal vein occlusion. Am J Ophthalmol. 2011; 152: 298–303 [DOI] [PubMed] [Google Scholar]
- 13. Brooks HL, Caballero S, Newell CK, et al. Vitreous levels of vascular endothelial growth factor and stromal-derived factor 1 in patients with diabetic retinopathy and cystoid macular edema before and after intraocular injection of triamcinolone. Arch Ophthalmol. 2004; 122: 1801–1807 [DOI] [PubMed] [Google Scholar]
- 14. Shimada H, Akaza E, Yuzawa M, Kawashima M. Concentration gradient of vascular endothelial growth factor in the vitreous of eyes with diabetic macular edema. Invest Ophthalmol Vis Sci. 2009; 50: 2953–2955 [DOI] [PubMed] [Google Scholar]
- 15. Bandello F, Berchicci L, La Spina C. Battaglia Parodi M, Iacono P. Evidence for anti-VEGF treatment of diabetic macular edema. Ophthalmic Res. 2012; 48 (suppl 1): S16–S20 [DOI] [PubMed] [Google Scholar]
- 16. Boras I, Lazic R, Gabric N, Lukic M, Dekaris I. Anti-VEGF in treatment of diabetic macular edema. Coll Antropol. 2011; 35 (suppl 2): S15–S18 [PubMed] [Google Scholar]
- 17. Rarey K, Friberg TR. Indirect laser treatment and anti-VEGF therapy of a retinal angioma, with resolution of a large serous retinal detachment, macular exudates, and macular edema. Semin Ophthalmol. 2010; 25: 21–26 [DOI] [PubMed] [Google Scholar]
- 18. Mitry D, Bunce C, Charteris D. Anti-vascular endothelial growth factor for macular oedema secondary to branch retinal vein occlusion. Cochrane Database Syst Rev. 2013; 1: CD009510 [DOI] [PubMed] [Google Scholar]
- 19. Avery RL, Pearlman J, Pieramici DJ, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006; 113: 1695–1705 [DOI] [PubMed] [Google Scholar]
- 20. Wimmers S, Karl MO, Strauss O. Ion channels in the RPE. Prog Retin Eye Res. 2007; 26: 263–301 [DOI] [PubMed] [Google Scholar]
- 21. Ablonczy Z, Dahrouj M, Tang PH, et al. Human retinal pigment epithelium cells as functional models for the RPE in vivo. Invest Ophthalmol Vis Sci. 2011; 52: 8614–8620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ablonczy Z, Prakasam A, Fant J, Fauq A, Crosson C, Sambamurti K. Pigment epithelium-derived factor maintains retinal pigment epithelium function by inhibiting vascular endothelial growth factor-R2 signaling through gamma-secretase. J Biol Chem. 2009; 284: 30177–30186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ablonczy Z, Crosson CE. VEGF modulation of retinal pigment epithelium resistance. Exp Eye Res. 2007; 85: 762–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Negi A, Kawano S, Marmor MF. Effects of intraocular pressure and other factors on subretinal fluid resorption. Invest Ophthalmol Vis Sci. 1987; 28: 2099–2102 [PubMed] [Google Scholar]
- 25. Negi A, Marmor MF. Effects of subretinal and systemic osmolality on the rate of subretinal fluid resorption. Invest Ophthalmol Vis Sci. 1984; 25: 616–620 [PubMed] [Google Scholar]
- 26. Negi A, Marmor MF. The resorption of subretinal fluid after diffuse damage to the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1983; 24: 1475–1479 [PubMed] [Google Scholar]
- 27. Frambach DA, Marmor MF. The rate and route of fluid resorption from the subretinal space of the rabbit. Invest Ophthalmol Vis Sci. 1982; 22: 292–302 [PubMed] [Google Scholar]
- 28. Yao XY, Hageman GS, Marmor MF. Retinal adhesiveness is weakened by enzymatic modification of the interphotoreceptor matrix in vivo. Invest Ophthalmol Vis Sci. 1990; 31: 2051–2058 [PubMed] [Google Scholar]
- 29. Bevington PR, Robinson DK. Data Reduction and Error Analysis for the Physical Sciences. New York: McGraw-Hill; 2002. [Google Scholar]
- 30. Maepea O. Pressures in the anterior ciliary arteries, choroidal veins and choriocapillaris. Exp Eye Res. 1992; 54: 731–736 [DOI] [PubMed] [Google Scholar]
- 31. Chou T, Siegel M. A mechanical model of retinal detachment. Phys Biol. 2012; 9: 046001 [DOI] [PubMed] [Google Scholar]
- 32. Kokame GT, de Leon MD, Tanji T. Serous retinal detachment and cystoid macular edema in hypotony maculopathy. Am J Ophthalmol. 2001; 131: 384–386 [DOI] [PubMed] [Google Scholar]
- 33. Stefánsson E. Ocular hypotony: what is the mechanism of effusion and oedema? Acta Ophthalmol Scand. 2007; 85: 584–585 [DOI] [PubMed] [Google Scholar]
- 34. Civerchia LL, Balent A. Treatment of pseudophakic cystoid macular edema by elevation of intraocular pressure. Ann Ophthalmol. 1984; 16: 890–894 [PubMed] [Google Scholar]
- 35. Tsuboi S. Measurement of the volume flow and hydraulic conductivity across the isolated dog retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1987; 28: 1776–1782 [PubMed] [Google Scholar]
- 36. Zachary I, Gliki G. Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc Res. 2001; 49: 568–581 [DOI] [PubMed] [Google Scholar]
- 37. Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME. Vascular endothelial growth factor in eye disease. Prog Retin Eye Res. 2008; 27: 331–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tari SR, Youssif M, Samson CM, et al. Polychromatic angiography for the assessment of VEGF-induced BRB dysfunction in the rabbit retina. Invest Ophthalmol Vis Sci. 2013; 54: 5550–5558 [DOI] [PubMed] [Google Scholar]
- 39. Iwase T, Oveson BC, Hashida N, et al. Topical pazopanib blocks VEGF-induced vascular leakage and neovascularization in the mouse retina but is ineffective in the rabbit. Invest Ophthalmol Vis Sci. 2013; 54: 503–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Castro MR, Lutz D, Edelman JL. Effect of COX inhibitors on VEGF-induced retinal vascular leakage and experimental corneal and choroidal neovascularization. Exp Eye Res. 2004; 79: 275–285 [DOI] [PubMed] [Google Scholar]
- 41. Peng S, Adelman RA, Rizzolo LJ. Minimal effects of VEGF and anti-VEGF drugs on the permeability or selectivity of RPE tight junctions. Invest Ophthalmol Vis Sci. 2010; 51: 3216–3225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Campa C. Effect of VEGF and anti-VEGF compounds on retinal pigment epithelium permeability: an in vitro study. Eur J Ophthalmol. 2013; 23: 690–696 [DOI] [PubMed] [Google Scholar]
- 43. Moromizato Y, Hayashi H, Kato H, Ozaki H, Oshima K. Concentration of vascular endothelial growth factor within the subretinal space and vitreous fluid in rhegmatogenous retinal detachment [in Japanese]. Nihon Ganka Gakkai Zasshi. 1997; 101: 498–502 [PubMed] [Google Scholar]
- 44. Su CY, Chen MT, Wu WS, Wu WC. Concentration of vascular endothelial growth factor in the subretinal fluid of retinal detachment. J Ocul Pharmacol Ther. 2000; 16: 463–469 [DOI] [PubMed] [Google Scholar]
- 45. Gyurkovics M, Lohinai Z, Gyorfi A, et al. Venodilatory effect of vascular endothelial growth factor on rat gingiva. J Periodontol. 2009; 80: 1518–1523 [DOI] [PubMed] [Google Scholar]
- 46. Whittles CE, Pocock TM, Wedge SR, et al. ZM323881, a novel inhibitor of vascular endothelial growth factor-receptor-2 tyrosine kinase activity. Microcirculation. 2002; 9: 513–522 [DOI] [PubMed] [Google Scholar]