Abstract
We evaluated immune reconstitution in 58 adults who received hematopoietic stem cell transplants from allogeneic siblings (allosib), matched unrelated donors (MUD), or cord blood (CB) at 90-day intervals for one year post-transplant. CB recipients had a higher incidence of infections in the first 100 days compared to allosib and MUD recipients. The number of circulating T cells was lower in CB recipients compared to MUD recipients at 90 days and compared to allosib recipients at 180 days. Spectratype analysis of the TCR Vβ complementarity determining region 3 (CDR3) of patient lymphocytes revealed that the TCR repertoire remained poorly diversified even at 360 days in nearly all patients. In contrast, the number of circulating B cells was significantly elevated in CB recipients compared to allosib recipients throughout the first year post-transplant and compared to MUD recipients at 9-12 months. Spectratype analysis of the B cell receptor VH CDR3 showed that the B cell repertoire was diversified in most patients by 90 days. CD5pos B cells from assayed CB recipients expressed intracellular IL-10 early post-transplant. Our data suggest that B cells, in addition to T cells, may play a role in impaired immune responses in CB transplant patients.
Keywords: cord blood, immune reconstitution, hematopoietic stem cell transplant, spectratype analysis
Introduction
Hematopoietic SCT (HSCT) is an option for leukemia patients who cannot achieve long-term remission with chemotherapy. Cord blood (CB) was first used as an alternative to BM as a source of HSCs in pediatric patients due to the low number of HSCs in CB compared to BM.1 Recently, the number of adults receiving CB transplants has been increasing due to advances in the expansion of CB HSCs and an increase in the availability of CB units collected from ethnic minorities. Advantages of using CB as a source of HSCs include a low prevalence of latent viral disease2 and low incidence of GVHD in CB transplant (CBT) recipients compared to BM transplant (BMT) recipients.3-5 However, there are significant disadvantages to CB transplantation including delayed hematopoietic recovery4-9 and a higher incidence of life-threatening infections especially in the first 100 days post-transplant10-12 compared to BM recipients. Taken together, these data suggest that immune responses are impaired in CBT patients especially in the first few months post-transplant.
To identify strategies for improving the success of adult CB transplantation, we compared the incidence of infection and GVHD as well as survival and immune reconstitution in adult transplant patients receiving stem cells from allogeneic siblings (allosib), matched unrelated donors (MUD), or CB. Since T cell numbers are low and the TCR repertoire is not well diversified one year or longer after HSCT in both BM recipients13-15 and CB recipients,16, 17 we hypothesized that immune cells, other than T cells, contribute to impaired immune responses in CBT patients. To address this hypothesis, we quantified multiple T cell subsets, B cells, monocytes, and granulocytes in peripheral blood from CB, allosib, and MUD recipients at 90 day intervals for one year post-transplant. In addition, we performed spectratype analysis to assess the genetic variation in complementarity determining region 3 (CDR3), the third hypervariable region, of the TCR β chain (Vβ) and the BCR heavy chain (VH) in fresh CB and patient peripheral blood lymphocytes.
Materials and Methods
Patients
A total of 22 allosib, 17 MUD, and 19 CBT patients of both sexes and various ethnicities aged 25-67 years were enrolled in this study. Informed consent was obtained from all patients in accordance with the Helsinki Declaration. All procedures were approved by Loyola University Chicago's Institutional Review Board. Preparative regimens were myeloablative in all but two transplant patients with TBI and regimens including one or more of the following: BU; cytarabine; CY; fludarabine; melphalan; pentostatin; BEAM. Single agent tacrolimus (FK-506) was given for 100 days post-transplant and then tapered in all patients. All but one allosib patient received mobilized peripheral blood stem cells (PBSC); the remaining allosib received BM from an HLA-mismatched sibling. Eleven MUD recipients received BM and 6 received PBSC. Keratinocyte growth factor was given to 38 patients to reduce mucositis. Anti-thymocyte globulin was given to 7 patients (2 allosib and 5 CB) as part of their preparative regimen. Peripheral blood was collected from patients undergoing HSCT every 90 days for one year post-transplant. One allosib that received a non-myeloablative regimen was given DLI at 6 months to improve chimerism. Relapsed patients were not evaluated beyond the date of relapse.
Collection and processing of cord blood
CB for control assays was obtained after vaginal or cesarean deliveries at Loyola University Medical Center. CB was collected into sterile pouches containing 35mL of citrate phosphate dextrose anti-coagulant (Fenwal, Lake Zurich, IL) and processed within 36 hours of collection. B cells were enriched from CB using an EasySep kit that does not deplete CD43pos cells (StemCell Technologies, Vancouver, BC, Canada).
Determination of graft-versus-host disease (GVHD) and infection
Acute GVHD (aGVHD) and chronic GVHD (cGVHD) were graded according to Center for International Blood and Marrow Transplant Research guidelines. Infections were confirmed by culture of body fluids, enzyme immunoassay, digene hybrid capture, or polymerase chain reaction (PCR).
Spectratyping
RNA from PBMC was extracted using Trizol reagent (Invitrogen, Carlsbad, CA) and was used for cDNA synthesis. Reverse transcriptase PCR was performed to detect different CDR3 lengths from 11 TCR Vβ regions. A reverse primer, Cβr, with the sequence AGA TCT CTG CTT CTG ATG C that hybridizes within the constant region was paired with each of the following forward primers: Vβ2 GCT TCT ACA TCT GCA GTG C; Vβ3 GCT GAG TCC GCC AGC ACC; Vβ4 GCA ACA TGA GCC CTG AAG; Vβ5.2 GCT GAA TGT GAA CGC CTT GTT G; Vβ6.1 GAT CCA GCG CAC ACA GC; Vβ6.2 GAT CCA GCG CAC AGA GC; Vβ7 CCT GAA TGC CCC AAC AGC; Vβ9 CCC TGG AGC TTG GTG ACT CTG or TTC TGT GCC AGC AGT CCG; Vβ13 GCT CAG GCT GCT GTC GGC TGC; Vβ17 GGA TCC AGC AGG TAG TGC G; or Vβ19 CAC TGT GAC ATC GGC CCA AAA G. Products from the first round of PCR after 30 cycles were used in a second round of PCR containing the 6-carboxyfluorescein (FAM) Cβr primer AGA TCT CTG CTT CTG ATC GCT C for 12 cycles using the following conditions: 94°C 1’, 55°C 1’, 72°C 1’. Reverse transcriptase PCR was also used to detect different CDR3 lengths from 6 BCR variable regions. A reverse JH primer with the sequence TGA GGA GAC GGT GAC CAK GGT that hybridizes within the J region, was paired with each of the following forward primers: VH1 AGC ACA GCC TAC ATG GAG CTG AGC; VH2 STC ACC ATC WCC AAR GAC A; VH3 CTG TAT CTG CAA ATG AAC AGC CTG; VH4 CTC CCT GAA GCT GAG CTC TGT G; VH5 CTA CCT GCA GTG GAG CAG CCT G; VH6 CCA AGA ACC AGT TCT CCC TGC. Products from the first round of PCR after 30 cycles were used in a second round of PCR containing the FAM JH primer TGA CCA KGG TBC CHT GGC CCC for 12 cycles using the following conditions: 94°C 1’, 55°C 1’, 72°C 1’. All primers were ordered from Integrated DNA Technologies (Coralville, IA). PCR products were loaded on to an ABI PRISM 3130 genetic analyzer (Applied Biosystems, Carlsbad, CA) and histograms of CDR3 lengths for each of the Vβ and VH families were generated using GeneMapper 4.1 software. A diversity score was assigned to each family as follows: 0, no peak; 1, 1 peak; 2, 2-7 peaks; 3, 8 or more peaks with non-Gaussian distribution; 4, 8 or more peaks, Gaussian distribution. Representative profiles are shown in Figure 3A. We defined a Gaussian distribution as 8 or more consecutive peaks that were no more than three nucleotides apart.
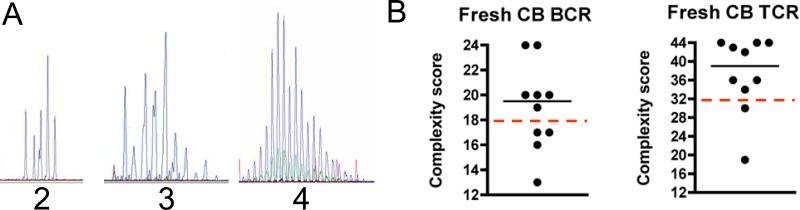
Figure 3. CDR3 spectratype analysis of cord blood (CB).
(A) Representative spectratype profiles for lymphocytes with clones of distinctive CDR3 length shown as peaks. VH (BCR) and Vβ (TCR) families with fewer than 8 peaks were scored as a 2 (left panel). Families with 8 or more peaks were scored as a 3 if they did not display a Gaussian distribution (center panel) or as a 4 if they did display a Gaussian distribution (right panel). (B) CDR3 spectratype analysis of the BCR (left panel) and TCR (right panel) repertoires in 10 CB samples. A complexity score was calculated by summing the individual diversity scores for each BCR or TCR family. The BCR repertoire was considered well-diversified if the complexity score was >18 (score of at least 3 for all 6 VH families), and the TCR repertoire was considered well-diversified if the complexity score was >33 (score of at least 3 for all 11 Vβ families); the dashed lines indicate the minimum score of a well diversified repertoire. The horizontal lines indicate the median.
Enumeration of leukocyte populations
PBMC were stained with the following antibodies from eBioscience (San Diego, CA), Invitrogen, Biolegend (San Diego, CA), or Becton Dickinson (BD) Pharmingen (Franklin Lakes, NJ): CD19 (AF750 or FITC), CD38 (PECy5), IgM (APC), IgD (PE), CD3 (FITC or APC), CD4 (APC-Cy7 or PE), CD8 (PECy7), CD56 (PE), TCRαβ (FITC), TCRγδ (PE), CD14 (FITC), CD25 (PE), CD127 (PECy5), and CD5 (FITC). Cells were analyzed on a FACs Canto or FACs Canto II (BD) and flow cytometry data was analyzed using FlowJo software (Treestar, Ashland, OR).
Statistics
Statistical analyses were performed using GraphPad Prism software version 4 (GraphPad software, La Jolla, CA).
Results
Patient Characteristics
The main patient characteristics are summarized in Table 1. Most allosib and MUD recipients were matched at both alleles for the HLA A, B, C, and DR. In addition, MUD patients were matched at both alleles for HLA-DQ. Most CB recipients were antigen mismatched for HLA-A and/or HLA-B but were all matched for both alleles of HLA-DR. BMT recipients received more total nucleated cells and CD34pos cells compared to CBT recipients (Table 2). Three CBT patients were enrolled in a clinical trial sponsored by Gamida Cell Ltd (Jerusalem, Israel); one day after stem cell infusion, these patients received cells that had been thawed from a fraction of their CB unit and expanded in vitro for 21 days. As their immune recovery and outcomes were similar to CB recipients that did not receive expanded cells, they were included in the analyses.
Table 1.
Clinical characteristics of patients that received hematopoietic stem cells from an allogeneic sibling (allosib), a matched unrelated donor (MUD), or cord blood (CB).
| Allosib≠ (n = 22) | MUD‡ (n = 17) | CB (n = 19) | |
|---|---|---|---|
| Number of female/male | 11/11 | 7/10 | 10/9 |
| Median age at transplant (years) | 53 | 47 | 46 |
| Median weight (kg) | 85 | 91 | 83 |
| weight range | 50-124 | 57-103 | 48-101 |
| HLA matching | |||
| matched | 21 | 14 | 3 |
| mismatched* | 1 | 3 | 16 |
| Disease, status at transplantation | |||
| Acute leukemia | |||
| CR1 | 9 | 3 | 6 |
| >CR1 | 2 | 7 | 2 |
| REF | 0 | 0 | 1 |
| Chronic leukemia | |||
| Blast phase | 1 | 0 | 0 |
| >CR1 | 0 | 0 | 1 |
| REF | 0 | 1 | 0 |
| Lymphoma | |||
| CR1 | 0 | 0 | 1 |
| >CR1 | 1 | 1 | 2 |
| PR1 | 1 | 0 | 0 |
| >PR1/REF | 4 | 1 | 3 |
| Multiple Myeloma | 0 | 1 | 0 |
| Myelofibrosis/Myelodysplastic syndrome | 4 | 3 | 3 |
| Prior autologous or CB transplant** | 1 | 3 | 4 |
| Conditioning Regimen | |||
| TBI† | 16 | 10 | 12 |
| No TBI | 6 | 7 | 7 |
>CR1, complete remission subsequent to CR1; REF, refractory; >PR1, partial remission subsequent to PR1.
One patient received BM cells from an HLA-mismatched sibling, all other patients received mobilized PBSC.
Nine of the MUD recipients received BM cells; the remaining 6 patients received PBSC.
Allosib and 2 MUD mismatched for 1 allele, one MUD mismatched for 2 alleles, 7 CB mismatched for 1 antigen, 9 CB mismatched for 2 antigens.
Allosib had autologous transplant 12 months prior, MUD had autologous transplant 9, 23, or 77 months prior, CB had autologous transplant 13, 5, or 89 months prior. One patient had a CB transplant 40 months prior to receiving a second CB transplant.
Two allosib patients received low dose TBI.
Table 2.
Infused cell dose and engraftment in transplant patients receiving BM, PBSC from allogeneic siblings (allosib) or matched unrelated donors (MUD), or cord blood (CB).
| TNC≠ (108/kg) (range) | CD34pos cells† (106/kg) (range) | Median days to neutrophil engraftment (range) | Median days to platelet engraftment (range) | |
|---|---|---|---|---|
| BM‡ (n=12) | 2.6 (1.4-3.7) | NA | 13 (12-18)* | 15 (12-31)* |
| PBSC (n=27) | NA | 6.6 (1.3-25.1) | 12 (10-15)* | 13 (0-36)* |
| Allosib (n=21) | 6.3 (1.3-11.9) | 12 (10-15) | 13 (0-36) | |
| MUD (n=6) | 7.68 (5.2-25.1) | 13 (10-14) | 11 (9-17) | |
| CB (n=19) | 0.19 (0.1-0.4) | 0.09 (0.03-0.9) | 16 (11-32)* | 38 (14-50)* |
TNC, total nucleated cell count; NA, not available.
TNC is not reported for 21 allosib and 6 MUD recipients that received mobilized PBSC.
CD34 count is not routinely performed on BM biopsies and was not available for one CB unit (n=18).
One allosib received BM from an HLA-mismatched sibling; 11 patients received BM from MUD.
For neutrophil engraftment, BM vs PBSC and PBSC vs CB p <0.01; For platelet engraftment, BM vs CB and PBSC vs CB p <0.01, Mann-Whitney test.
Engraftment and chimerism
Neutrophil engraftment was defined as the first of 3 consecutive days after HSCT when the ANC was at least 500 cells/μl. Neutrophil engraftment was more rapid in PBSC recipients compared to BM and CB recipients (Table 2). Platelet engraftment was defined as the time to reach a sustained platelet count of at least 20,000/μl without the use of transfusions. Platelet engraftment was significantly delayed in CB recipients compared to BM or PBSC recipients (Table 2).
Donor-recipient chimerism was determined by PCR analysis on whole blood for short tandem repeat sequences and results were expressed as percent donor-derived DNA. By 3 months, all but one CBT recipient achieved 98% donor chimerism; this patient had 92% donor chimerism at 90 days but relapsed and was excluded from further study. Two allosib patients that did not achieve 98% chimerism until 5 months or 7 months post-transplant expired within the first year; one died following a myocardial infarction that was not treatment-related and one died due to sepsis and GVHD.
Post-transplant complications
Several patients relapsed or succumbed to infection in the first year post-transplant. The percent of patients alive at one year was similar for patients receiving stem cells from different donor sources (allosib (92%), MUD (95%), CB (63%); Kaplan-Meier survival analysis, p>0.05, data not shown). The incidence of aGVHD and cGVHD was similar between CBT patients and allosib and MUD patients (X2 = 0.5451, p >0.05, d.f. = 1, chi-square test; p >0.05, Fisher's exact test). However, the severity of cGVHD differed between patient groups. The disease was severe in only 3 of 13 CBT patients whereas the disease was severe in more than half of the 29 allosib and MUD patients (Table 3). Of note, all 5 of the MUD PBSC recipients that could be evaluated for cGVHD developed severe cGVHD.
Table 3.
Post-transplant outcome for patients receiving hematopoietic stem cells from an allogeneic sibling (allosib), a matched unrelated donor (MUD), or cord blood (CB).
| Allosib and MUD | CB | |
|---|---|---|
| Incidence of acute GVHD≠ | 7/39 | 5/19 |
| Grade I | 5 | 2 |
| Grade II | 0 | 1 |
| Grade III-IV | 2 | 2 |
| Incidence of chronic GVHD† | 29/32 | 13/16 |
| Limited/Mild | 13 | 10 |
| Extensive/Severe | 16 | 3 |
| Incidence of infection before day 100 | 10/39* | 11/19* |
| Number of patients with bacterial infection | 4 | 5 |
| Number of patients with viral infection | 2 | 3 |
| Number of patients with fungal infection | 1 | 1 |
| Number of patients with multiple infections | 3 | 2 |
Four allosib and one MUD BM recipient developed Grade I aGVHD. One MUD PBSC and one MUD BM recipient developed Grade III-IV.
Eight allosib and 5 MUD BM recipients developed mild cGVHD. Nine allosib, 5 MUD PBSC, and 2 MUD BM recipients developed severe cGVHD. Seven allosib or MUD and 3 CB recipients that expired or relapsed before the development of chronic GVHD could be assessed were excluded from the analysis.
X2 = 5.754, p <0.02, d.f. = 1, chi-square test.
Since it has been reported that CBT recipients have a higher incidence of infections compared to BMT recipients during the first 100 days post-transplant,4, 12, 18 we determined the number of bacterial, viral, and fungal infections that our patient population acquired during this time period (Table 3). Only infections that were confirmed by laboratory tests were included except for one incident of varicella zoster infection which was based on the symptoms presented by the patient. HSCT patients acquired infections that were primarily caused by bacteria or by viruses that were reactivated in the lymphopenic hosts. Two CBT patients, one allosib patient, and 2 MUD transplant patients acquired multiple infections. CBT patients had a higher risk of developing opportunistic infections in the early post-transplant period compared to allosib and MUD recipients (p=0.02, Fisher's exact test). All patients survived 100 days post-transplant however, several succumbed to infections within the first year. One MUD recipient became infected with Stenotrophomonas maltophilia and had oral candidiasis within the first 100 days post-transplant and expired at 5 months from sepsis. Another MUD recipient and 2 allosib recipients died of pneumonia caused by an unidentified organism within the first year. One CBT patient died of fungal pneumonia subsequent to infection with Klebsiella pneumoniae in the first 100 days. Three other CBT patients, one with sepsis, one with viral pneumonia, and one with pneumonia caused by Pseudomonas aeruginosa expired in the first year. We hypothesized that CBT patients are more susceptible to infection and experience less severe GVHD because immune reconstitution in these patients differs from reconstitution in patients receiving HSCs from other sources.
Influence of graft source and donor source on lymphocyte reconstitution
Previous studies have shown differences in immune reconstitution between patients receiving BM cells and patients receiving PBSC.19, 20 To explore the influence of graft source on immune reconstitution, we enumerated multiple T cell subsets and B cells in the peripheral blood of 6 MUD BM and 11 MUD PBSC recipients. In our study, we were unable to compare graft source in allosib recipients since all but one of 22 allosib patients received PBSC. MUD PBSC recipients had significantly more CD4 T cells at 90 days than MUD BM recipients (p=0.04, Mann-Whitney test, data not shown). The number of other T cell subsets including CD8 T cells, natural killer T (NKT) cells, CD3posCD4posCD25posCD127neg regulatory T cells, and CD4posCD25posCD127pos activated T cells and the number of B cells was not different throughout the first year post-transplant (data not shown).
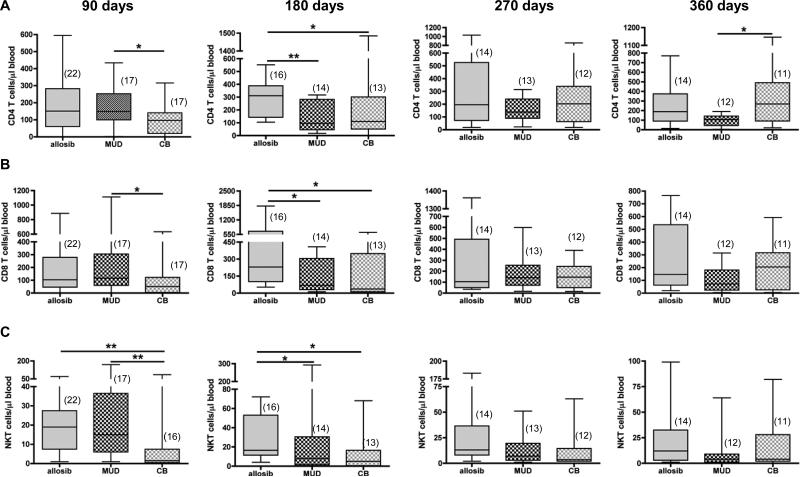
Post-transplant outcomes differ between adult BMT and adult CBT patients; 4, 7, 8, 12 therefore, we examined whether donor source influences immune reconstitution. The number of CD4 T and CD8 T cells was significantly higher in MUD recipients compared to CB recipients at 90 days. However, the median number of CD4 T and CD8 T cells was below normal in all patient groups as long as one year post-transplant except for allosib patients at 180 days when the median CD8 T cell count was at the low end of the normal physiological range (Figure 1A, 1B). CD4 T cell and CD8 T cell counts at day 180 were higher in patients receiving allosib cells compared to patients receiving MUD or CB cells (Figure 1A, 1B). A comparison of CD4 T and CD8 T cell count in 21 allosib PBSC recipients and 11 MUD PBSC recipients revealed that allosib PBSC recipients had more circulating CD4 T and CD8 T cells than MUD PBSC recipients (p<0.05, Mann-Whitney test, data not shown). By 360 days post-transplant, the number of CD4 T cells was similar between allosib and CB recipients however CB recipients had significantly more CD4 T cells than MUD recipients (Figure 1A). The number of NKT cells was significantly higher in allosib and MUD recipients compared to CB recipients at 90 days (Figure 1C). At 180 days, allosib recipients had significantly more NKT cells than either MUD or CB recipients (Figure 1C), and allosib PBSC recipients had more circulating NKT cells than MUD PBSC recipients (p=0.04, Mann-Whitney test, data not shown). The number of TCRγδ T cells and regulatory T cells was within the range observed in healthy adults and was similar in all patients (data not shown).
Figure 1. The number of CD4 T, CD8 T, and natural killer T (NKT) cells in the peripheral blood of transplant patients receiving hematopoietic stem cells from an allogeneic sibling (allosib), a matched unrelated donor (MUD), or from cord blood (CB).
The percentage of CD3posCD4pos T cells, CD3posCD8pos T cells, or CD3posCD56pos NKT cells was determined by flow cytometry. This percentage was multiplied by the number of lymphocytes, which was quantified by a complete blood cell count to determine the number of each T cell subset. The number of CD4 T cells (A) CD8 T cells (B) and NKT cells (C) is shown for 90-day intervals up to one year post-transplant. The number of patients analyzed at each time point is indicated in parentheses. The horizontal bars indicate the median and range of each patient group. *p<0.05, **p<0.01, Mann-Whitney test.
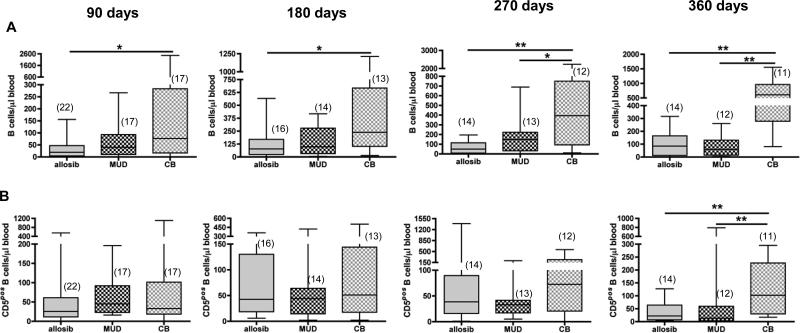
To evaluate if B cell reconstitution differs in transplant patients that receive cells from different donor sources, we enumerated IgMposIgDpos B cells and CD5pos B cells in the peripheral blood of HSCT recipients. The number of IgMposIgDpos B cells was higher in CB recipients compared to allosib recipients at 90 days and at 180 days (Figure 2A). In some CBT patients, the number of B cells was as much as four times the number found in healthy individuals. At 270 and 360 days post-transplant, CB recipients had significantly more circulating IgMposIgDpos B cells than either MUD or allosib recipients (Figure 2A). CB recipients also had significantly more CD5pos B cells in circulation compared to allosib or MUD recipients at 360 days (Figure 2B).
Figure 2. The number of CD19posIgMposIgDpos B cells and CD5pos B cells in the peripheral blood of transplant patients receiving hematopoietic stem cells from an allogeneic sibling (allosib), a matched unrelated donor (MUD), or from cord blood (CB).
The percentage of CD19posIgMposIgDpos or CD3negCD5pos B cells was determined by flow cytometry. This percentage was multiplied by the number of lymphocytes, which was quantified by a complete blood cell count to determine the number of each B cell subset. The number of CD19posIgMposIgDpos B cells (A) and CD3negCD5pos B cells (B) is shown for 90-day intervals up to one year post-transplant. The number of patients analyzed at each time point is indicated in parentheses. The horizontal bars indicate the median and range of each patient group. *p<0.05, **p<0.01, Mann-Whitney test.
Diversity of the lymphocyte repertoire in HSCT patients
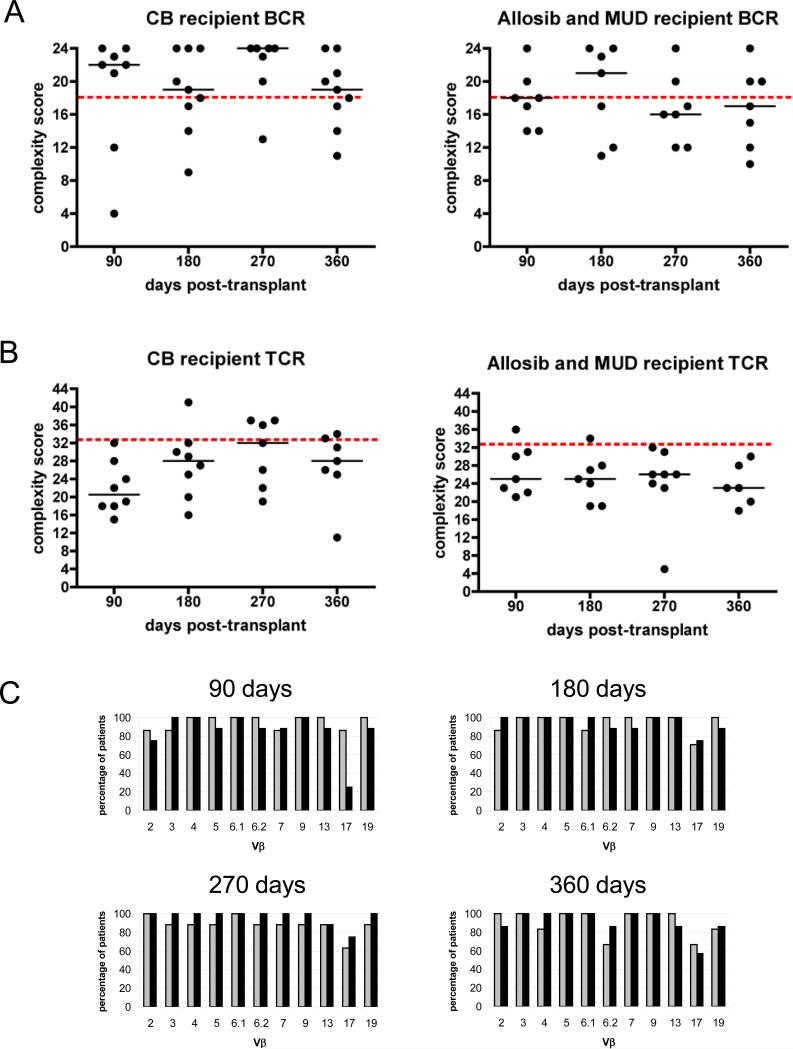
We hypothesized that immune competence in CBT patients may be compromised by a restricted lymphocyte repertoire. To determine whether or not CB-derived cells were capable of giving rise to a diversified T and B cell repertoire, we analyzed the CDR3 region of each of 6 BCR VH and 11 TCR Vβ gene families (representative profiles are shown in Figure 3A) in PBMC from fresh CB samples. Six of the 10 samples analyzed had a well diversified BCR repertoire and 8 of the 10 samples had a well diversified TCR repertoire (Figure 3B). We then analyzed the lymphocyte repertoire of HSCT patients. The majority of B cells from CBT recipients displayed a diversified repertoire as early as 90 days and remained diversified as long as 360 days post-transplant (Figure 4A). B cells from 4 of 7 allosib or MUD recipients displayed a diversified repertoire at 90 days; however, the repertoire became more restricted in some patients by 270 days post-transplant (Figure 4A). In contrast, the TCR repertoire was poorly diversified in most HSCT recipients as long as 360 days post-transplant (Figure 4B). We compared Vβ and VH usage in CBT and allosib or MUD recipients and found that Vβ usage was similar in both patient groups (Figure 4C). A higher percentage of CBT recipients utilized VH2 compared to allosib or MUD recipients but no other differences in VH usage were noted (data not shown).
Figure 4. CDR3 spectratype analysis of peripheral blood lymphocytes from patients that underwent hematopoietic stem cell (HSC) transplantation.
CDR3 spectratype analysis of the BCR repertoire (A) and TCR repertoire (B) from CB transplant recipients (left panels) and from patients receiving stem cells from an allogeneic sibling (allosib) or matched unrelated donor (MUD) (right panels). A complexity score was calculated by summing the individual diversity scores for each BCR or TCR family. The BCR repertoire was considered well-diversified if the complexity score was >18 (score of at least 3 for all 6 VH families), and the TCR repertoire was considered well-diversified if the complexity score was >33 (score of at least 3 for all 11 Vβ families); the dashed lines indicate the minimum score of a well diversified repertoire. The horizontal lines indicate the median. (C) The percentage of allosib and MUD patients (light bars) and the percentage of CBT patients (dark bars) with T cells utilizing each of the 11 Vβ families analyzed is shown at 90 days (allosib and MUD n=7, CB n=8), 180 days (allosib and MUD n=7, CB n=8), 270 days (allosib and MUD n=8, CB n=7), and 360 days (allosib and MUD n=6, CB n=7) post-transplant.
Phenotype of B cells in CBT recipients
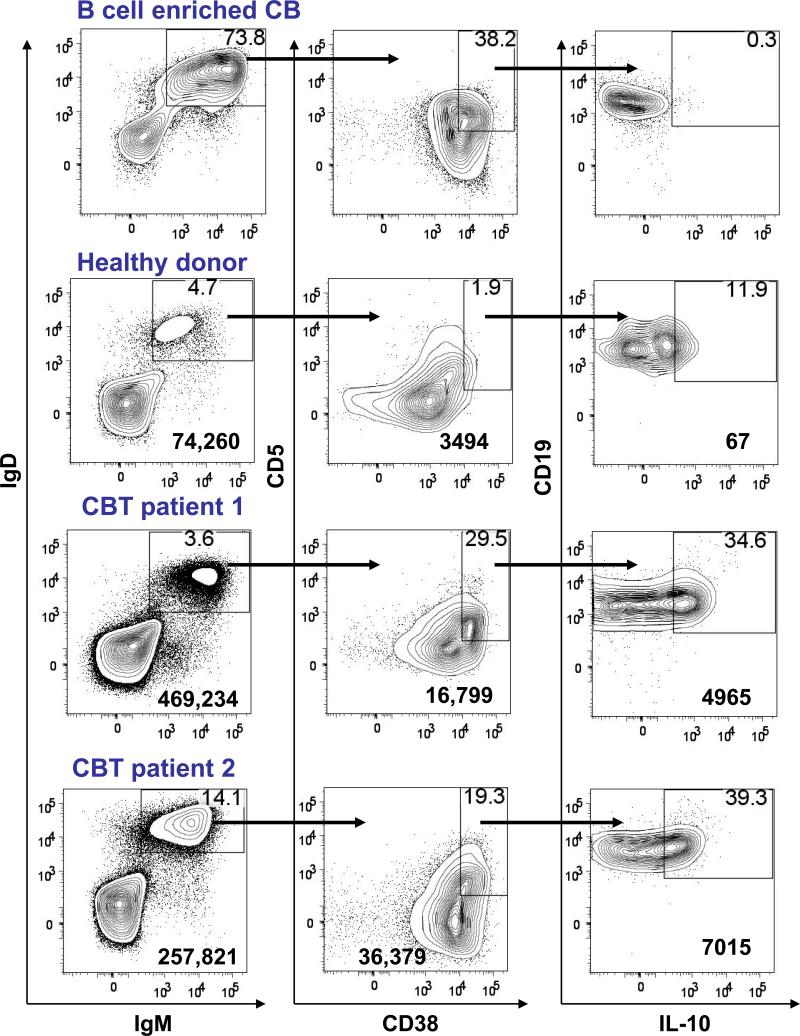
Since the elevated numbers of B cells in CBT recipients was not due to oligoclonal expansion, we investigated the phenotype of B cells in some of these patients. The percentage of IgMposIgDposCD38pos CD5pos B cells was higher in CBT recipients compared to healthy donors (Figure 5). Since the phenotype of these CD5pos B cells is similar to that described for regulatory B cells (Breg) that can suppress T cell function in mice,21, 22 we tested if these cells expressed the immunosuppressive cytokine IL-10. Ex vivo CD38posCD5pos B cells enriched from CB do not express intracellular IL-10 (Figure 5). IL-10 producing CD38posCD5pos B cells were not observed in the circulation of 2 CBT patients that underwent a traditional conditioning regimen at either 270 days or 360 days post-transplant (data not shown). We then examined peripheral blood B cells from an additional 3 patients that underwent a reduced intensity conditioning (RIC) regimen consisting of fludarabine, CY, and TBI prior to CBT. One patient had too few circulating B cells to be analyzed further. The other two RIC patients evaluated at 90 days and at 180 days had a circulating population of IgMposIgDposCD38posCD5pos B cells that expressed intracellular IL-10 as determined by flow cytometry (Figure 5). IL-10 producing B cells were not observed in the circulation of these patients at day 270 or at day 360 (data not shown). These data suggest that IL-10 producing peripheral B cells are present in the first 6 months post-transplant but do not persist.
Figure 5. Flow cytometric analysis of B cells from fresh cord blood (CB), a healthy donor, and two CB transplant (CBT) recipients.
B cells were enriched from fresh CB and PBMC were isolated from a healthy donor or from CBT patients. IgMposIgDpos B cells (left panels) were gated on and analyzed for their expression of CD38 and CD5 (center panels). CD38hiCD5pos cells were gated and analyzed for their expression of CD19 and intracellular IL-10 (right panels). Data are representative of three fresh CB samples and two healthy donors. Profiles shown for CBT patients are at 90 days for patient 1 and at 180 days for patient 2. Percentages are shown in the upper right hand quadrant. The number in the bottom right corner is the total number of cells shown. Axes show log10 fluorescence.
Myeloid reconstitution in HSCT patients
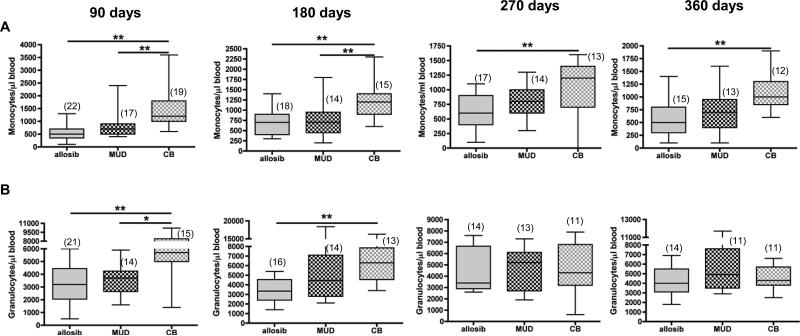
Myeloid recovery as measured by the time to neutrophil engraftment is delayed in CBT recipients compared to BMT recipients.3-5 We determined if the number of monocytes and granulocytes in HSCT recipients was different among patients receiving HSCs from different sources. Our data show that the number of monocytes was significantly higher in CBT patients compared to allosib patients at all time points. CBT patients had more circulating monocytes than MUD recipients at 90 days and at 180 days, but the numbers were similar in both groups at later time points. The number of monocytes was supraphysiological in some patients throughout the first year post-transplant (Figure 6A). Granulocytes were elevated in CBT patients in the first 6 months post-transplant but were comparable to allosib and MUD patients thereafter (Figure 6B).
Figure 6. The number of monocytes and granulocytes in the peripheral blood of transplant patients receiving hematopoietic stem cells (HSC) from an allogeneic sibling (allosib), a matched unrelated donor (MUD), or from cord blood (CB).
The number of monocytes and granulocytes in peripheral blood of HSC transplant patients was determined by a complete blood cell count. The number of patients analyzed at each time point is indicated in parentheses. The horizontal bars indicate the median and range of each patient group. *p<0.05, **p<0.01, Mann-Whitney test.
Discussion
Infection, predominantly caused by bacteria, is the primary cause of death in 15-47% of CBT patients.6, 12, 23 Although many studies have attributed infection in CBT patients to low T cell numbers and a restricted TCR repertoire,16, 24, 25 our data show that T cell numbers are below the normal physiological range and that the TCR repertoire is restricted in nearly all HSCT patients as long as one year post-transplant. These data suggest that immune cells other than T cells may account for impaired immune responses in CBT patients.
There is limited data on B cell reconstitution in HSCT patients. Some studies have reported that the BCR repertoire is fully diversified as early as 3 months post-transplant26, 27 whereas other studies report that diversification does not occur until 9-12 months post-transplant.28, 29 Though B cell numbers were lower in allosib and MUD recipients compared to CB recipients, the BCR repertoire was well diversified in all patient groups as early as 90 days post-transplant. The higher number of B cells observed in CBT patients was not due to oligoclonal expansion since the BCR repertoire was not skewed in these patients. CBT patients in this study show an increase in the number of circulating B cells even after T cell numbers are equivalent to those observed in allosib and MUD patients. These data suggest that elevated B cell numbers in CBT recipients are not the result of compensatory expansion but the result of increased B cell lymphopoiesis or survival. Our data show that monocytes are more abundant in CBT recipients than allosib recipients throughout the first year post-transplant. B cell activating factor produced by activated monocytes has been shown to promote the survival of peripheral B cells30, 31 and to protect leukemic B cells from apoptosis.32, 33 A recent study has shown that patients receiving a double CBT have elevated B cell numbers and elevated plasma levels of B cell activating factor in the early post-transplant period compared to MUD recipients.34 B cell activating factor production by monocytes was not evaluated therefore further study is required to determine whether monocytes contribute to elevated B cell numbers or contribute to relapse in CBT patients.
We hypothesize that B cells may attenuate T cell responses in CBT patients. B cell recovery has been correlated with an improvement in cGVHD;35, 36 patients with cGVHD have decreased numbers of B cell precursors even in the absence of steroid treatment.36 The incidence of cGVHD was similar in all HSCT recipients; however, the incidence of severe cGVHD was low in CB recipients. IgMposIgDposCD38posCD5pos B cells are in circulation in both CBT recipients and BMT recipients in the first few months post-transplant37, 38. In BMT patients, CD5pos B cells are thought to represent a transitional B cell population and the percent of CD5pos B cells decreases as the number of mature B cells increases.39 We analyzed CD5pos B cells from three CBT recipients for the expression of CD24 (also known as heat stable antigen), a molecule that is highly expressed by transitional B cells.39 IgMposIgDposCD38posCD5pos B cells in these patients were CD24lo (data not shown) therefore they are unlikely to be transitional B cells. Recently, IgMposIgDposCD1dposCD5pos Breg that produce IL-10 have been described in mice.22, 40 The phenotype of Breg cells in humans has yet to be fully elucidated; however, human B cells capable of suppressing T cell responses have been described.41, 42 CD5pos B cells positive for intracellular IL-10 at 90 and 180 days were present in 2 patients receiving a RIC regimen supporting the possibility that these cells are Breg. However, the IL-10pos B cells were not present at 270 or 360 days in these 2 patients or in patients that received a myeloablative conditioning regimen. From these data, we cannot determine whether the number of IL-10pos B cells declines over time or whether circulating IL-10pos B cells are present only in RIC patients. More patients must be analyzed to distinguish between these possibilities. Future studies characterizing the function of the CD5pos B cell population in CBT patients will determine whether or not this population of B cells is capable of producing cytokines that limit T cell expansion thus reducing the severity of GVHD but increasing susceptibility to infection.
Supplementary Material
Acknowledgements
We thank Shi-kang Zhai for expert technical assistance with PCR and DNA sequencing and Patricia Simms of the flow cytometry core facility for assistance with data acquisition and analysis. We thank Karen Rychlik of the Biostatistics core facility for assistance with statistical analyses. We are indebted to the Division of Hematology/Oncology staff for consenting patients, collecting blood, and organizing data. This work was supported by Illinois Regenerative Medicine Institute (63080019 to PJS), and National Institutes of Health Grants (AG023809 to PTL), (AI068390 to KLK), and (NHLBI F32HL096278 to BCBZ). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Footnotes
Contributions
BCBZ wrote the manuscript, performed the research, and analyzed the data; PTL and KLK designed the research study and critically reviewed the manuscript; S Zhang performed the research and analyzed the data; SZ consented patients and collected data; MP collected and analyzed data; and PJS designed the research study, consented patients, and critically reviewed the manuscript.
Conflict-of-interest: The authors declare no competing financial interests.
References
- 1.Hao QL, Shah AJ, Thiemann FT, Smogorzewska EM, Crooks GM. A functional comparison of CD34 + CD38- cells in cord blood and bone marrow. Blood. 1995;86(10):3745–53. [PubMed] [Google Scholar]
- 2.Albano MS, Taylor P, Pass RF, Scaradavou A, Ciubotariu R, Carrier C, et al. Umbilical cord blood transplantation and cytomegalovirus: Posttransplantation infection and donor screening. Blood. 2006;108(13):4275–82. doi: 10.1182/blood-2006-04-020313. [DOI] [PubMed] [Google Scholar]
- 3.Eapen M, Rocha V, Sanz G, Scaradavou A, Zhang MJ, Arcese W, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol. 2010;11(7):653–60. doi: 10.1016/S1470-2045(10)70127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laughlin MJ, Eapen M, Rubinstein P, Wagner JE, Zhang MJ, Champlin RE, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351(22):2265–75. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- 5.Rocha V, Labopin M, Sanz G, Arcese W, Schwerdtfeger R, Bosi A, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004;351(22):2276–85. doi: 10.1056/NEJMoa041469. [DOI] [PubMed] [Google Scholar]
- 6.Almyroudis NG, Fabian J, Hahn T, Segal BH, Wetzler M, McCarthy PL., Jr. Late infectious complications after cord blood stem cell transplantation. Eur J Clin Microbiol Infect Dis. 2009;28(11):1405–8. doi: 10.1007/s10096-009-0789-2. [DOI] [PubMed] [Google Scholar]
- 7.Ooi J, Iseki T, Takahashi S, Tomonari A, Nagayama H, Ishii K, et al. A clinical comparison of unrelated cord blood transplantation and unrelated bone marrow transplantation for adult patients with acute leukaemia in complete remission. Br J Haematol. 2002;118(1):140–3. doi: 10.1046/j.1365-2141.2002.03650.x. [DOI] [PubMed] [Google Scholar]
- 8.Rocha V, Cornish J, Sievers EL, Filipovich A, Locatelli F, Peters C, et al. Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood. 2001;97(10):2962–71. doi: 10.1182/blood.v97.10.2962. [DOI] [PubMed] [Google Scholar]
- 9.Rocha V, Gluckman E. Eurocord, European B, Marrow Transplant G. Clinical use of umbilical cord blood hematopoietic stem cells. Biol Blood Marrow Transplant. 2006;12(1 Suppl 1):34–41. doi: 10.1016/j.bbmt.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Barker JN, Hough RE, van Burik JA, DeFor TE, MacMillan ML, O'Brien MR, et al. Serious infections after unrelated donor transplantation in 136 children: impact of stem cell source. Biol Blood Marrow Transplant. 2005;11(5):362–70. doi: 10.1016/j.bbmt.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Eapen M, Rubinstein P, Zhang MJ, Stevens C, Kurtzberg J, Scaradavou A, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369(9577):1947–54. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 12.Parody R, Martino R, Rovira M, Vazquez L, Vazquez MJ, de la Camara R, et al. Severe infections after unrelated donor allogeneic hematopoietic stem cell transplantation in adults: comparison of cord blood transplantation with peripheral blood and bone marrow transplantation. Biol Blood Marrow Transplant. 2006;12(7):734–48. doi: 10.1016/j.bbmt.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Hentschke P, Omazic B, Mattsson J, Nasman-Bjork I, Lundkvist I, Gigliotti D, et al. T-cell receptor Vbeta repertoire after myeloablative and reduced intensity conditioning allogeneic haematopoietic stem cell transplantation. Scand J Immunol. 2005;61(3):285–94. doi: 10.1111/j.1365-3083.2005.01564.x. [DOI] [PubMed] [Google Scholar]
- 14.Verfuerth S, Peggs K, Vyas P, Barnett L, O'Reilly RJ, Mackinnon S. Longitudinal monitoring of immune reconstitution by CDR3 size spectratyping after T-cell-depleted allogeneic bone marrow transplant and the effect of donor lymphocyte infusions on T-cell repertoire. Blood. 2000;95(12):3990–5. [PubMed] [Google Scholar]
- 15.Wu CJ, Chillemi A, Alyea EP, Orsini E, Neuberg D, Soiffer RJ, et al. Reconstitution of T-cell receptor repertoire diversity following T-cell depleted allogeneic bone marrow transplantation is related to hematopoietic chimerism. Blood. 2000;95(1):352–9. [PubMed] [Google Scholar]
- 16.Klein AK, Patel DD, Gooding ME, Sempowski GD, Chen BJ, Liu C, et al. T-Cell recovery in adults and children following umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2001;7(8):454–66. doi: 10.1016/s1083-8791(01)80013-6. [DOI] [PubMed] [Google Scholar]
- 17.Talvensaari K, Clave E, Douay C, Rabian C, Garderet L, Busson M, et al. A broad T-cell repertoire diversity and an efficient thymic function indicate a favorable long-term immune reconstitution after cord blood stem cell transplantation. Blood. 2002;99(4):1458–64. doi: 10.1182/blood.v99.4.1458. [DOI] [PubMed] [Google Scholar]
- 18.Hamza NS, Lisgaris M, Yadavalli G, Nadeau L, Fox R, Fu P, et al. Kinetics of myeloid and lymphocyte recovery and infectious complications after unrelated umbilical cord blood versus HLA-matched unrelated donor allogeneic transplantation in adults. Br J Haematol. 2004;124(4):488–98. doi: 10.1046/j.1365-2141.2003.04792.x. [DOI] [PubMed] [Google Scholar]
- 19.Ottinger HD, Beelen DW, Scheulen B, Schaefer UW, Grosse-Wilde H. Improved immune reconstitution after allotransplantation of peripheral blood stem cells instead of bone marrow. Blood. 1996;88(7):2775–9. [PubMed] [Google Scholar]
- 20.Petersen SL, Ryder LP, Bjork P, Madsen HO, Heilmann C, Jacobsen N, et al. A comparison of T-, B- and NK-cell reconstitution following conventional or nonmyeloablative conditioning and transplantation with bone marrow or peripheral blood stem cells from human leucocyte antigen identical sibling donors. Bone Marrow Transplant. 2003;32(1):65–72. doi: 10.1038/sj.bmt.1704084. [DOI] [PubMed] [Google Scholar]
- 21.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28(5):639–50. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Yang M, Sun L, Wang S, Ko KH, Xu H, Zheng BJ, et al. Novel function of B cell- activating factor in the induction of IL-10-producing regulatory B cells. J Immunol. 2010;184(7):3321–5. doi: 10.4049/jimmunol.0902551. [DOI] [PubMed] [Google Scholar]
- 23.Saavedra S, Sanz GF, Jarque I, Moscardo F, Jimenez C, Lorenzo I, et al. Early infections in adult patients undergoing unrelated donor cord blood transplantation. Bone Marrow Transplant. 2002;30(12):937–43. doi: 10.1038/sj.bmt.1703764. [DOI] [PubMed] [Google Scholar]
- 24.Giraud P, Thuret I, Reviron D, Chambost H, Brunet C, Novakovitch G, et al. Immune reconstitution and outcome after unrelated cord blood transplantation: a single paediatric institution experience. Bone Marrow Transplant. 2000;25(1):53–7. doi: 10.1038/sj.bmt.1702089. [DOI] [PubMed] [Google Scholar]
- 25.Komanduri KV, St John LS, de Lima M, McMannis J, Rosinski S, McNiece I, et al. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood. 2007;110(13):4543–51. doi: 10.1182/blood-2007-05-092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gokmen E, Raaphorst FM, Boldt DH, Teale JM. Ig heavy chain third complementarity determining regions (H CDR3s) after stem cell transplantation do not resemble the developing human fetal H CDR3s in size distribution and Ig gene utilization. Blood. 1998;92(8):2802–14. [PubMed] [Google Scholar]
- 27.Suzuki I, Milner EC, Glas AM, Hufnagle WO, Rao SP, Pfister L, et al. Immunoglobulin heavy chain variable region gene usage in bone marrow transplant recipients: lack of somatic mutation indicates a maturational arrest. Blood. 1996;87(5):1873–80. [PubMed] [Google Scholar]
- 28.D'Sa S, Peggs K, Pizzey A, Verfuerth S, Thuraisundaram D, Watts M, et al. T- and B-cell immune reconstitution and clinical outcome in patients with multiple myeloma receiving T-cell-depleted, reduced-intensity allogeneic stem cell transplantation with an alemtuzumab-containing conditioning regimen followed by escalated donor lymphocyte infusions. Br J Haematol. 2003;123(2):309–22. doi: 10.1046/j.1365-2141.2003.04612.x. [DOI] [PubMed] [Google Scholar]
- 29.Omazic B, Hentschke P, Nasman-Bjork I, Mattsson J, Oxelius VA, Ringden O, et al. Reconstitution of the Ig heavy chain CDR3 repertoire after allogeneic haematopoietic stem cell transplantation with myeloablative or reduced-intensity conditioning regimens. Scand J Immunol. 2005;61(1):72–81. doi: 10.1111/j.0300-9475.2005.01528.x. [DOI] [PubMed] [Google Scholar]
- 30.Batten M, Groom J, Cachero TG, Qian F, Schneider P, Tschopp J, et al. BAFF mediates survival of peripheral immature B lymphocytes. J Exp Med. 2000;192(10):1453–66. doi: 10.1084/jem.192.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rolink AG, Tschopp J, Schneider P, Melchers F. BAFF is a survival and maturation factor for mouse B cells. Eur J Immunol. 2002;32(7):2004–10. doi: 10.1002/1521-4141(200207)32:7<2004::AID-IMMU2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 32.Fu L, Lin-Lee YC, Pham LV, Tamayo A, Yoshimura L, Ford RJ. Constitutive NF-kappaB and NFAT activation leads to stimulation of the BLyS survival pathway in aggressive B-cell lymphomas. Blood. 2006;107(11):4540–8. doi: 10.1182/blood-2005-10-4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novak AJ, Bram RJ, Kay NE, Jelinek DF. Aberrant expression of B-lymphocyte stimulator by B chronic lymphocytic leukemia cells: a mechanism for survival. Blood. 2002;100(8):2973–9. doi: 10.1182/blood-2002-02-0558. [DOI] [PubMed] [Google Scholar]
- 34.Jacobson CA, Turki AT, McDonough SM, Stevenson KE, Kim HT, Kao G, et al. Immune reconstitution after double umbilical cord blood stem cell transplantation: comparison with unrelated peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2012;18(4):565–74. doi: 10.1016/j.bbmt.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abrahamsen IW, Somme S, Heldal D, Egeland T, Kvale D, Tjonnfjord GE. Immune reconstitution after allogeneic stem cell transplantation: the impact of stem cell source and graft-versus-host disease. Haematologica. 2005;90(1):86–93. [PubMed] [Google Scholar]
- 36.Sanchez-Garcia J, Serrano J, Gomez P, Martinez F, Martin C, Roman-Gomez J, et al. The impact of acute and chronic graft-versus-host disease on normal and malignant B-lymphoid precursors after allogeneic stem cell transplantation for B- lineage acute lymphoblastic leukemia. Haematologica. 2006;91(3):340–7. [PubMed] [Google Scholar]
- 37.Small TN, Keever CA, Weiner-Fedus S, Heller G, O'Reilly RJ, Flomenberg N. B-cell differetiation following autologous, conventional, or T-cell depleted bone marrow transplantation: a recapitulation of normal B-cell ontogeny. Blood. 1990;76(8):1647–56. [PubMed] [Google Scholar]
- 38.Villasenor-Bustamante S, Alvarado-De La Barrera C, Richaud-Patin Y, Martinez-Ayala H, Llorente L. Possible role of interleukin-10 in autoantibody production and in the fate of human cord blood CD5+ B lymphocytes. Scand J Immunol. 1999;49(6):629–32. doi: 10.1046/j.1365-3083.1999.00545.x. [DOI] [PubMed] [Google Scholar]
- 39.Marie-Cardine A, Divay F, Dutot I, Green A, Perdrix A, Boyer O, et al. Transitional B cells in humans: characterization and insight from B lymphocyte reconstitution after hematopoietic stem cell transplantation. Clin Immunol. 2008;127(1):14–25. doi: 10.1016/j.clim.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 40.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16(2):219–30. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 41.Bouaziz JD, Calbo S, Maho-Vaillant M, Saussine A, Bagot M, Bensussan A, et al. IL-10 produced by activated human B cells regulates CD4(+) T-cell activation in vitro. Eur J Immunol. 2010;40(10):2686–91. doi: 10.1002/eji.201040673. [DOI] [PubMed] [Google Scholar]
- 42.Tretter T, Venigalla RK, Eckstein V, Saffrich R, Sertel S, Ho AD, et al. Induction of CD4+ T-cell anergy and apoptosis by activated human B cells. Blood. 2008;112(12):4555–64. doi: 10.1182/blood-2008-02-140087. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.