Abstract
Objective
Although deficits in cognitive control are thought to contribute to the diverse cognitive and behavioral abnormalities in individuals with schizophrenia, the neural mechanisms underlying these deficits remain unclear. In this event-related functional magnetic resonance imaging (fMRI) study, the authors tested the hypothesis that during cognitive control tasks, impaired activation of the dorsolateral prefrontal cortex in schizophrenia patients is associated with disrupted coordinated activity between this prefrontal region and a distributed brain network that supports cognitive control.
Method
Through the use of an event-related design, 25 patients with first-episode schizophrenia and 24 healthy comparison subjects, matched on demographic characteristics, were assessed while performing a version of the AX continuous performance task. Functional neuroimaging data were analyzed using 1) univariate (region-of-interest blood-oxygen-level-dependent [BOLD] time series and whole brain voxel-wise regression) analysis to confirm the presence of dorsolateral prefrontal cortex dysfunction and 2) multivariate analysis to examine dorsolateral prefrontal cortex functional connectivity. In addition, correlations between dorsolateral prefrontal cortex functional connectivity and the following variables were investigated: clinical symptoms, task performance, and coordinated brain activity associated with cognitive control.
Results
Schizophrenia patients exhibited a specific deficit in cognitive control, with significantly reduced accuracy in the BX condition relative to any other condition. Univariate fMRI revealed dorsolateral prefrontal cortex dysfunction during the high cognitive control condition. Multivariate analysis revealed significant impairment in functional connectivity between the dorsolateral prefrontal cortex and task-relevant brain regions. Significant correlations were also found between dorsolateral prefrontal cortex functional connectivity and cognitive performance, behavioral disorganization, and global functioning.
Conclusions
These findings suggest that there is an association between decreased dorsolateral prefrontal cortex activity and connectivity and a task-related neural network. This deficit in coordinated brain activity may result in the disabling disorganization symptoms related to impaired cognition in individuals with schizophrenia.
Kraepelin (1) described the behavioral disorganization and cognitive deficits in schizophrenia as “an orchestra without a conductor,” which presciently suggests that cognitive control—the coordination of thoughts and actions that facilitates goal-oriented behavior—is one of the key higher-order cognitive processes impaired in individuals with schizophrenia. The findings of basic studies in cognitive neuroscience suggest that the neural correlate of cognitive control is the coordinated activity of task-relevant brain regions, and the dorsolateral prefrontal cortex plays a key role in this process (2–4).
Although dorsolateral prefrontal cortex dysfunction has been one of the most replicated findings in schizophrenia research, particularly during tasks that require cognitive control (5–11), the mechanisms by which impairment of this prefrontal region translates into cognitive control deficits and related behavioral abnormalities in schizophrenia remain poorly understood. The objective of the present study was to examine one potential mechanism by testing the hypothesis that reduced dorsolateral prefrontal cortex activity is associated with altered coordinated activity of task-relevant cortical processing across brain regions in individuals with schizophrenia.
In this functional magnetic resonance imaging (fMRI) study, 25 first-episode schizophrenia patients and 24 healthy comparison subjects were assessed while they completed the AX continuous performance task, which allows the measurement of performance and brain activity associated with specific aspects of cognitive control (6, 10, 12, 13). This paradigm was optimized in order to accommodate an event-related functional connectivity analysis while providing efficient sampling of both A and B cue trials. Under conditions that required greater cognitive control, we expected schizophrenia patients to show reduced dorsolateral prefrontal cortex engagement and reduced correlated activity in the distributed brain network that supports task performance. We investigated the clinical significance of the result by measuring the association of the deficit in top-down modulation with behavioral performance and clinical measures. We predicted that altered top-down modulation would be significantly correlated with impaired behavioral performance and clinical measures of disorganization in schizophrenia patients.
Method
Participants
Twenty five patients with schizophrenia and related disorders (schizophrenia, 23 patients; schizophreniform disorder, 1 patient; schizoaffective disorder, 1 patient) and 24 healthy comparison subjects were recruited from the community. The age range for participants in both study groups was between 14 and 32 years. Demographic and clinical characteristics of the two groups are provided in Table 1. At the time of testing, all subjects in the schizophrenia group were outpatients and within the first year of illness onset. To confirm the diagnosis of schizophrenia in patients and to exclude the presence of a major psychiatric illness in comparison subjects, all subjects were evaluated using the Structured Clinical Interview for DSM-IV-TR. Schizophrenia patients who were <16 years old were assessed using the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version. Master’s- and doctoral-level clinicians conducted the diagnostic evaluations, and diagnoses were confirmed via consensus conference. Illness onset was determined by establishing the time at which DSM-IV criteria A and B for schizophrenia were met. Individuals with an illness onset >12 months from the time of testing were excluded from the study. Symptoms were quantified using the following assessment measures: 24-item Brief Psychiatric Rating Scale (BPRS), Scale for the Assessment of Negative Symptoms (SANS), Scale for the Assessment of Positive Symptoms (SAPS), Strauss Carpenter Outcome Scale, and Global Assessment Scale (GAS). Subscores from the BPRS, SANS, and SAPS were used to derive indexes for the following three major domains of symptoms: reality distortion, disorganization, and negative symptoms (12). Nine schizophrenia patients were medication-naive. The remaining 16 patients were being treated with antipsychotics (risperidone [N=10], aripiprazole [N=4], quetiapine [N=2], olanzapine [N=1]) at the time of testing, with one subject receiving two neuroleptics. Exclusion criteria for comparison subjects were 1) the presence of a lifetime diagnosis of an axis I disorder or 2) a first-degree relative with a psychotic disorder. Exclusion criteria for both study groups were as follows: 1) IQ <70, 2) history of drug or alcohol dependence or abuse within the 3 months before the start of the study or a positive urine drug screen at the start of the study, 3) significant head trauma, or 4) any known contraindication to MRI. Both groups were well-matched, except in years of education (schizophrenia group versus comparison group: 12.4 years [SD=2.7] versus 14.6 years [SD=4.0], p<0.05) and IQ score (schizophrenia group versus comparison group: 98.2 [SD=13.3] versus 112.4 [SD=8.5], p<0.01). After complete description of the study was given, written informed consent was obtained. The study was approved by the University of California Davis Institutional Review Board.
TABLE 1.
Demographic and Clinical Characteristics of Schizophrenia Patients and Healthy Comparison Subjects
| Characteristic | Group
|
|||
|---|---|---|---|---|
| Schizophrenia Patients (N=25) | Healthy Comparison Subjects (N=24) | |||
|
| ||||
| Mean | SD | Mean | SD | |
| Age (years) | 19.6 | 3.80 | 21.6 | 4.24 |
| Parental education (years) | 15.0 | 2.37 | 15.1 | 2.88 |
| Participant education (years) | 12.4 | 2.71 | 14.6 | 4.02 |
| WAIS IQ scorea | 98.2 | 13.93 | 112.4 | 8.46 |
| Symptom severity (scale scores) | ||||
| BPRS | 46.5 | 8.95 | -- | -- |
| SANS | 30.1 | 13.31 | -- | -- |
| SAPS | 30.4 | 16.99 | -- | -- |
| Strauss Carpenter Outcome Scale | 9.9 | 2.84 | -- | -- |
| Global Assessment Scale | 53.7 | 10.15 | -- | -- |
|
| ||||
| N | % | N | % | |
|
| ||||
| Male | 17 | 68 | 13 | 54 |
| Right-handed | 24 | 96 | 23 | 96 |
| Antipsychotic medication | ||||
| Atypical | 16 | 64 | -- | -- |
| Typical | 0 | 0 | -- | -- |
| Unmedicated | 9 | 36 | -- | -- |
| History of substance abuse or dependence | 7 | 28 | 3 | 12.5 |
Significant between-group difference (p≤0.01).
Cognitive Task
Subjects completed the AX continuous performance task, which has been demonstrated to reliably measure specific cognitive control deficits in individuals with schizophrenia (10–12). In this performance task, the cue letter is presented for a 500-msec duration. Following a 3,500-msec delay, the probe letter is shown for a duration of 500 msec. Subjects are required to make a target response (using a right-index-finger button press) to the probe letter X only when it follows the cue letter A. All other stimuli require a nontarget response (using a right-middle-finger button press), including trials in which the letter X is preceded by any letter other than A (collectively referred to as B). Trials with target cue-probe pairings (e.g., AX) occur with high frequency (70%) and set up the tendency for the subject to make a target response to the probe letter X. The condition with the highest cognitive control demand is the BX cue-probe trial, in which subjects must overcome the tendency to make a target response to X.
Neuroimaging
Acquisition
Coplanar T1-weighted structural images were obtained prior to echoplanar imaging scans. Functional scans (T2-weighted echoplanar imaging: TR=2,000 msec, echo time=40 msec, flip angle=90°, field of view=22 cm) were acquired using a 1.5T GE scanner. Twenty-four contiguous 4.0-mm axial slices (with a 3.4-mm2 in-plane resolution from 80 mm above to 16 mm below the anterior commissure-posterior commissure line) were also obtained.
Preprocessing
Preprocessing steps, implemented using Automated Image Registration software (http://bishopw.loni.ucla.edu/AIR), were as follows: 1) removal of linear tendencies and significant outliers in the time series, 2) spatial realignment, 3) normalization to the Montreal Neurological Institute (MNI) template through the use of a nonlinear warping algorithm, and 4) spatial smoothing through the use of a Gaussian 8-mm full-width half-maximum kernel. Two comparison subjects were excluded because of a >4-mm within-run movement. A multivariate analysis of variance (MANOVA) on the linear and angular movement parameters revealed no significant effect by group.
Statistical Analysis
Dorsolateral prefrontal cortex region-of-interest time series
We conducted a region-of-interest analysis with a functionally defined dorsolateral prefrontal cortex region of interest identified in a previous study (14). Trial-averaged blood-oxygen-level-dependent (BOLD) time series were generated for each subject and averaged by group for statistical comparison. For this and all functional neuroimaging analyses, only correct trials were assessed.
Whole brain analysis
In this exploratory voxel-wise analysis, conducted using Statistical Parametric Mapping-2 (http://www.fil.ion.ucl.ac.uk/spm), we performed a multiple regression, with covariates representing cue and probe events. In the first-level analysis, covariates were convolved with a canonical hemodynamic response function, using the temporal derivative to account for intersubject variability in BOLD signal time to peak (13, 15). In the second-level analysis, we conducted a random-effects comparison of the linear contrast between B and A cue trials between the two study groups. For between-group comparisons, nontask-related regions were excluded by imposing a mask of significant regions of activity from either group. Thresholding of all maps followed the procedure for determining cluster-level significance (corrected for multiple comparisons at p<0.05 family-wise error outlined in Friston et al. [16]).
Dorsolateral prefrontal cortex-correlated brain network activity
We measured functional connectivity of the dorsolateral prefrontal cortex using the beta series correlation method (17). Hemodynamic response function-convolved trial-specific covariates modeled activity during the component stages of each individual trial in order to generate beta series. Voxel-wise bivariate Pearson’s correlations between a “seed” region (i.e., dorsolateral prefrontal cortex) and all other brain regions were computed for a task component and condition of interest (e.g., B cue). Between-group random-effects analysis was then conducted for group-averaged correlations. The same procedure used for determining univariate whole brain analysis was also used to determine statistically significant activity. All correlation analyses were conducted using Statistical Parametric Mapping-2, with Matlab (MathWorks, Natick, Mass.) scripts specifically designed for the present study.
Results
AX Continuous Performance Task In-Scanner Performance
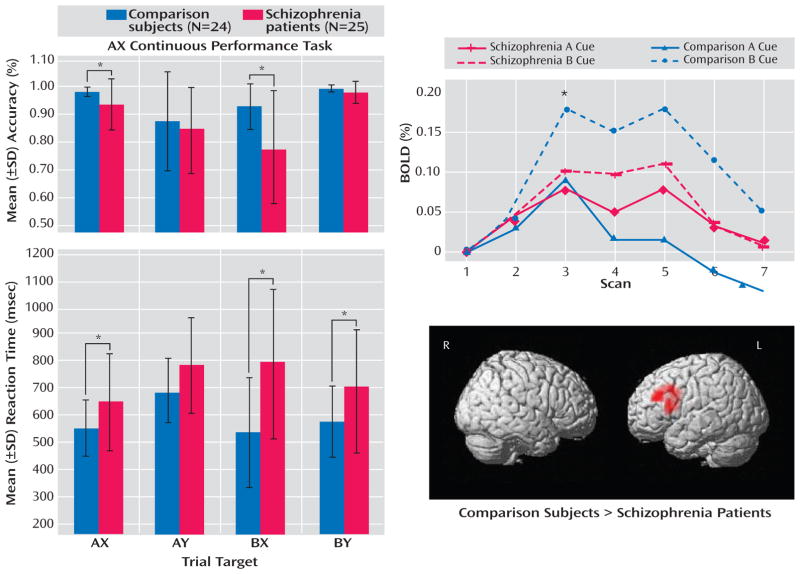
Accuracy results revealed a specific deficit in context processing in schizophrenia patients (Figure 1). Analysis of variance (ANOVA), with between-subject factor by group and within-subject factor by condition (AX, AY, BX, and BY), showed a significant main effect by group (F= 9.240, df=1, 47, p=0.004) (with schizophrenia patients performing worse than comparison subjects) and condition (F=16.982, df=3, 141, p<0.001) and a significant group-by-condition interaction (F=4.033, df=3, 141, p=0.009). Post hoc two-sample t tests demonstrated significantly better cognitive control performance among healthy comparison subjects relative to schizophrenia patients in the BX (t=3.349, df=47, p=0.002) and AX conditions (t=2.295, df= 47, p=0.03) but not in the BY or AY conditions (p>0.15).
FIGURE 1.
AX Continuous Performance Task In-Scanner Behavioral and Univariate fMRI Results in Schizophrenia Patients and Healthy Comparison Subjectsa
aAccuracy and reaction times are displayed for all conditions of the AX continuous performance task in the left column. The overall pattern shows a selective deficit in context processing in schizophrenia patients, with the worst performance occurring in the BX condition. The percent change in the BOLD signal in the left dorsolateral prefrontal cortex in schizophrenia patients and healthy comparison subjects for the B and A cue conditions are shown in the top right. Note that this time series was derived from a functional region of interest identified in healthy subjects in a previous study and not from the dorsolateral prefrontal cortex region identified from the whole brain analysis in the present study. Healthy comparison subjects exhibited the predicted up-regulation of dorsolateral prefrontal cortex activity during the B cue relative to the A cue conditions, while patients did not demonstrate this effect. Results of whole brain, voxel-wise multiple regression analysis are shown in the lower right. These surface-rendered t maps of the contrast B cue condition minus A cue condition, thresholded at the cluster level of p<0.05 (corrected), depict regions showing significantly greater activity in healthy comparison subjects relative to schizophrenia patients.
*p<0.05.
fMRI
Dorsolateral prefrontal cortex region-of-interest time series
Healthy comparison subjects exhibited robust enhancement in dorsolateral prefrontal cortex activity during the B cue condition relative to the A cue condition, with maximal difference occurring at scan 3 (the expected peak of cue-related activity). Schizophrenia patients showed minimal enhancement of dorsolateral prefrontal cortex activity across cue types (Figure 1). ANOVA of the BOLD signal at scan 3 revealed a significant main effect of group (F=33.725, df=1, 47, p<0.001) and condition (F=11.014, df=1, 47, p=0.002) and a significant group-by-condition interaction (F=4.685, df=1, 47, p=0.04). Across scans 1 to 3, there was a significant group-by-condition-by-scan interaction (F=3.172, df=2, 94, p=0.05). A two-tailed, paired-sample t test showed that the scan 3 signal was significantly greater during the B cue trial relative to the A cue trial in comparison subjects (t=4.437, df=23, p<0.001) but not in schizophrenia patients (t=0.74, df=24, p=0.47). A two-tailed, two-sample t test of the difference between B and A cue signals during scan 3 revealed a significant difference between groups (t=2.164, df=47, p= 0.04). ANOVA of the dorsolateral prefrontal cortex signal in schizophrenia patients, with regard to medication status, revealed no significant effect of medication on the signal at scan 3 (p=0.26) or scans 1 to 3 (p=0.42).
Whole brain analysis
To confirm the differential involvement of the dorsolateral prefrontal cortex in healthy comparison subjects relative to schizophrenia patients, we conducted a whole brain voxel-wise analysis, an approach complementary to the region-of-interest BOLD time-series analysis (Table 2, Figure 1). In comparison subjects, a diverse network involving the middle frontal gyrus, anterior and posterior cingulate, and precuneus was engaged. In schizophrenia patients, a network limited to subcortical regions—the thalamus and putamen—was activated. Notably, there was an absence of engagement of the middle frontal gyrus in schizophrenia patients. This difference between groups in middle frontal gyrus activity was confirmed by the between-group results. In the B cue minus A cue contrast, only the left middle frontal gyrus and inferior frontal gyrus showed significantly greater activity in healthy comparison subjects relative to schizophrenia patients, while no regions showed greater activity in schizophrenia patients relative to healthy comparison subjects.
TABLE 2.
Univariate Voxel-Wise Whole Brain fMRI Analysis in Schizophrenia Patients (N=25) and Healthy Comparison Subjects (N=24)
| Group and Brain Region | MNI Coordinates (x, y, z) | Analysis
|
|
|---|---|---|---|
| t | z | ||
| Healthy comparison subjects | |||
| Left posterior cingulate | −3, −72, 12 | 5.81 | 4.51 |
| Left middle frontal gyrus | −45, 52, 12 | 5.80 | 4.51 |
| Right middle frontal gyrus | 45, 17, 32 | 5.49 | 4.34 |
| Right precuneus | 21, −72, 48 | 5.39 | 4.29 |
| Anterior cingulate | −3, 34, 40 | 3.89 | 3.38 |
| Schizophrenia patients | |||
| Left thalamus | −7, −7, 4 | 4.44 | 3.76 |
| Right thalamus | 31, −14, 0 | 3.91 | 3.40 |
| Healthy comparison subjects > schizophrenia patientsa | |||
| Left middle frontal gyrus | 48, 31, 28 | 3.89 | 3.61 |
| Left inferior frontal gyrus | 41, 10, 16 | 3.63 | 3.39 |
There were no regions in which greater activity was seen in schizophrenia patients relative to healthy comparison subjects.
Dorsolateral prefrontal cortex-correlated brain network activity
We conducted a dorsolateral prefrontal cortex functional connectivity analysis using the beta series method and predicted that schizophrenia patients would show diminished dorsolateral prefrontal cortex functional connectivity (Table 3, Figure 2). In healthy comparison subjects, the right inferior parietal lobule and left premotor cortex (including the frontal eye fields) were regions showing greater functional connectivity with the dorsolateral prefrontal cortex in the B cue condition relative to the A cue condition. In schizophrenia patients, no regions exhibited enhanced dorsolateral prefrontal cortex functional connectivity. In a direct comparison between the two groups in B cue minus A cue contrast, the left pre-motor cortex and right inferior parietal lobule showed greater dorsolateral prefrontal cortex connectivity in healthy comparison subjects. The identification of the right inferior parietal lobule raises questions as to whether deficits in this region rather than the dorsolateral prefrontal cortex regulate network connectivity differences, given that the connectivity measure cannot provide directionality of influences between these two higher-order brain regions. To address this issue, we conducted a logistic regression analysis in order to predict diagnostic status for dorsolateral prefrontal cortex and right inferior parietal lobule activity. Activity in the dorsolateral prefrontal cortex was a significant negative predictor of schizophrenia (beta=−2.67, p=0.009), with every unit increase in dorsolateral prefrontal cortex activity resulting in a >14:1 decrease in the odds of schizophrenia. Activity in the right inferior parietal lobule was not a significant predictor (beta=0.12, p=0.85).
TABLE 3.
Multivariate Dorsolateral Prefrontal Cortex Functional Connectivity Analysis in Schizophrenia Patients (N=25) and Healthy Comparison Subjects (N=24)
| Group and Brain Region | MNI Coordinates (x, y, z) | Analysis
|
|
|---|---|---|---|
| t | z | ||
| Healthy comparison subjects | |||
| Right inferior parietal lobule | 43, −44, 45 | 5.97 | 4.59 |
| Left premotor cortex | −16, −6, 57 | 5.84 | 4.53 |
| Healthy comparison subjects > schizophrenia patients | |||
| Right inferior parietal lobule | 43, −44, 45 | 4.30 | 3.93 |
| Left premotor cortex | −19, −6, 65 | 4.17 | 3.83 |
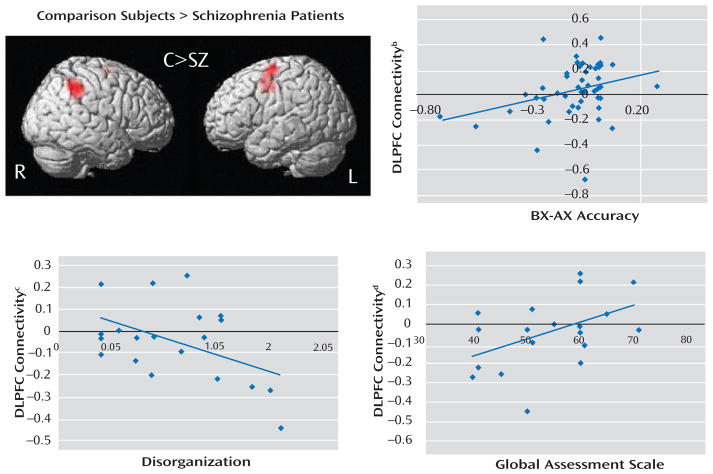
FIGURE 2.
Dorsolateral Prefrontal Cortex Functional Connectivity Analysis in Schizophrenia Patients (N=25) and Healthy Comparison Subjects (N=24)a
a A surface-rendered correlation map showing regions with significantly greater functional connectivity to the dorsolateral prefrontal cortex in healthy comparison subjects relative to schizophrenia patients is illustrated in the top left (p<0.05, corrected). (See Table 3 for regions and Montreal Neurological Institute coordinates.)
b Significant correlation between dorsolateral prefrontal cortex connectivity and BX performance, expressed as the difference between BX and AX accuracy (r=0.31, p=0.03).
c Significant correlation between dorsolateral prefrontal cortex connectivity and disorganization (r=−0.47, p=0.04).
d Significant correlation between dorsolateral prefrontal cortex connectivity and global level of functioning as indexed by the Global Assessment Scale (r=0.47, p=0.04).
To evaluate the possibility that the functional connectivity results were confounded by low univariate activity in the “seed” (i.e., dorsolateral prefrontal cortex) region in schizophrenia patients, we compared trial-to-trial variability of the BOLD signal in the left dorsolateral prefrontal cortex. We found equivalent variance between groups during A and B cues (p=0.75 and p=0.31, respectively). In other words, the lower mean BOLD signal in schizophrenia patients did not constrain the functional connectivity analysis, since patients and healthy comparison subjects exhibited equivalent levels of trial-to-trial variability of the BOLD signal.
Behavioral and clinical correlations with dorsolateral prefrontal cortex functional connectivity measures
To evaluate the relevance of our dorsolateral prefrontal cortex functional connectivity results, we calculated the correlation between dorsolateral prefrontal cortex connectivity and behavior (Figure 2). Dorsolateral prefrontal cortex connectivity showed moderate correlation with performance on the AX continuous performance task across all subjects, and thus connectivity was significantly associated with better performance in the BX condition, with either BX accuracy (r=0.35, p=0.01) or the difference in accuracy between the BX and AX conditions (r= 0.31, p=0.03). There was a significant inverse correlation between disorganization and dorsolateral prefrontal cortex connectivity (r=−0.47, p=0.04). This relationship was specific in that the correlation between dorsolateral prefrontal cortex connectivity and other symptom domains (behavioral poverty and reality distortion) was low and nonsignificant (behavioral poverty: r=0.12, p=0.63; reality distortion: r=−0.29, p=0.21). Finally, dorsolateral prefrontal cortex connectivity was significantly correlated with global level of functioning as assessed by GAS (r=0.47, p= 0.04).
Discussion
In a large sample of subjects with first-episode schizophrenia, we demonstrated a specific deficit in cognitive control, dysfunction of the dorsolateral prefrontal cortex, and significant correlations between impaired dorsolateral prefrontal cortex functional connectivity and behavior and clinical status. Schizophrenia patients exhibited poor performance during the BX condition, a condition that requires a high degree of cognitive control. However, patients did not differ from healthy comparison subjects in the AY and BY trials (12). Schizophrenia patients were unable to up-regulate activity in the dorsolateral prefrontal cortex during B cues, as indicated by the results of the following two separate lines of analyses: BOLD time series derived from an a priori-identified dorsolateral prefrontal cortex region of interest and whole brain voxel-wise regression analysis. Additionally, there was a significant reduction in dorsolateral prefrontal cortex connectivity in the schizophrenia group, which suggests a deficit in dorsolateral prefrontal cortex top-down modulation. Across all subjects, we found significant correlations between the magnitude of dorsolateral prefrontal cortex connectivity and behavioral performance during the AX continuous performance task. In schizophrenia patients, we found significant correlations between connectivity and clinical measures (a positive correlation with GAS scores and an inverse correlation with disorganization). Lower dorsolateral prefrontal cortex connectivity was associated with worse performance, greater disorganization, and lower global functioning. To our knowledge, this is the first study to report an association between dorsolateral prefrontal cortex dysfunction and global measures of impairment.
The present study was motivated by empirical and theoretical research supporting the central involvement of the dorsolateral prefrontal cortex in cognitive control (2–4). Miller and Cohen proposed a model in which the dorsolateral prefrontal cortex biases activity in the posterior cortex, which is necessary for controlled performance (3). For example, during the delay period of a face working memory task, it is thought that the prefrontal cortex up-regulates the activity of face processing regions in the visual cortex when the neural representation of the to-be-remembered face must be maintained (18, 19). Recent animal and human studies have reported results that are consistent with this finding by demonstrating that lateral prefrontal cortex activity precedes and presumably guides the parietal region during controlled processing (20, 21).
A logical extension of this line of reasoning would be to propose that the magnitude of top-down modulation is a function of control demands. In the AX continuous performance task, we predicted that B cues would engage the dorsolateral prefrontal cortex in healthy comparison subjects to support task-appropriate processing and reduce BX error rates through top-down modulation. We examined this process using the beta correlation method combined with event-related analysis, which allows measurement of functional connectivity during discrete stages of task performance. Enhanced functional connectivity during the B cue condition relative to the A cue condition was observed in healthy comparison subjects, while there was a marked inability to enhance dorsolateral prefrontal cortex connectivity in schizophrenia patients. Previous studies have also shown altered interactions between cortical regions, suggesting the presence of disordered connectivity in schizophrenia (22–25). Accordingly, not only are our results consistent with the hypothesis that connectivity is impaired in individuals with schizophrenia, but they suggest that there is a potential fundamental neural mechanism by which dorsolateral prefrontal cortex dysfunction leads to cognitive impairment (i.e., top-down modulation through impaired connectivity with the dorsolateral prefrontal cortex).
As a measure of covariance, the beta series correlation method cannot determine the directionality of interactions. However, the fact that 1) only the dorsolateral prefrontal cortex (not other elements of the task-related neural network) showed differential activity in the univariate contrast in schizophrenia patients and healthy comparison subjects and 2) logistic regression analysis selectively associated decreased dorsolateral prefrontal cortex activity but not right inferior parietal lobule activity with a schizophrenia diagnosis provides converging support for our model. However, we cannot rule out the possibility that altered function in another element of the distributed network, such as the right inferior parietal lobule, underlies the altered pattern of connectivity. Future studies using tasks that selectively engage prefrontal and parietal cognitive control functions or other methods capable of detecting directionality of effects are needed to address this issue in a more definitive manner.
There may be concerns that our functional connectivity results were confounded by decreased dorsolateral prefrontal cortex activity in schizophrenia patients. However, the most important parameter constraining dorsolateral prefrontal cortex connectivity is not its mean activity but rather the magnitude of trial-to-trial variability. In fMRI, these are largely independent parameters, and lower dorsolateral prefrontal cortex connectivity in patients is not necessarily the result of low univariate activity. This was verified in our data by the demonstration of equivalent dorsolateral prefrontal cortex variance between groups. Other examples of the divergence between the mean univariate and functional connectivity properties of a region are present in the extant literature (17).
In the present study, we included adolescent subjects in order to ensure that we investigated a representative sample of first-episode schizophrenia patients (26). In this study and a previous report (27), we closely matched the study groups on age to ensure that there were no developmental differences, aside from those that may be related to the illness, between the groups. Further, in a reanalysis of our data in the present study, which excluded subjects <16 years old, we found an identical pattern of results. To address the possibility that lower dorsolateral prefrontal cortex activity in schizophrenia patients might have been the result of lower accuracy on the AX continuous performance task among these individuals (and hence a lower number of correct BX trials), we conducted an analysis in which only performance-matched subjects were included. This analysis yielded fMRI results that were identical to those from the full sample.
An examination of the putative functions of cortical regions identified in the dorsolateral prefrontal cortex connectivity analysis revealed that each of these regions may support task-relevant processes during the AX continuous performance task. The right inferior parietal lobule has strong anatomical connections to the dorsolateral prefrontal cortex (28), and recent functional neuroimaging studies in humans suggest that the right inferior parietal lobule supports sustained attention (29, 30). Similarly, the frontal eye field is thought to support visual attention, in addition to its well-recognized role in eye-movement motor control (31, 32). It is possible that functional impairment in one of these regions could lead to disrupted connectivity with the dorsolateral prefrontal cortex.
Limitations to the present study include the inferences that could be made pertaining to the relative importance of brain regions other than the dorsolateral prefrontal cortex (33–35) in the broader pathophysiology of schizophrenia. Although the demonstration of a significant association between dorsolateral prefrontal cortex connectivity and behavioral and clinical impairments (particularly with disorganization symptoms and GAS) suggests broad relevance of dorsolateral prefrontal cortex dysfunction, the determination of the relative importance of the dorsolateral prefrontal cortex compared with other regions involving other cognitive processes needs to be examined in future studies.
Other possible limitations are the effects of medication on fMRI measures. The available evidence argues against medication effects as a major contributor to our findings. Prior studies have shown that antipsychotic drugs do not suppress the prefrontal cortex BOLD signal during higher-order cognition (27, 36). In the present study, there were no differences between medicated and unmedicated patients in cue-related dorsolateral prefrontal cortex activity. Furthermore, since our patients were very early in the course of illness, it is reasonable to conclude that impaired dorsolateral prefrontal cortex function is fundamental and unrelated to the long-term effects of medication exposure or illness chronicity.
Although the physiological mechanisms underlying impaired dorsolateral prefrontal cortex activity are unknown, the asynchronous activity of neurons as the result of perturbations in the local circuitry is a cogent potential mechanism (37, 38). Simultaneous recording and fMRI studies in primates suggest that synchronous firing of cortical neurons, particularly in the high-frequency range (40–80 Hz or gamma), is highly correlated with BOLD response (39). In a recent EEG study, which parallels the present fMRI study, we reported a decrease in induced gamma-band activity associated with impaired cognitive control performance and disorganization in schizophrenia patients (40). The results of the present study are consistent with the findings of our previous EEG study and with a model of impaired synchronous neuronal firing in the dorsolateral prefrontal cortex, which is associated with disruption in top-down-regulation of neural networks that support cognitive control.
The convergence of behavioral, functional neuroimaging, and clinical findings in the present study supports the dorsolateral prefrontal cortex dysfunction model of cognitive control deficits in schizophrenia. Furthermore, the reported functional connectivity results provide new insights into how dorsolateral prefrontal cortex dysfunction may lead to impaired cognitive control, behavioral disorganization, and functional impairment in individuals with schizophrenia by suggesting a physiological model in which a failure to recruit and maintain a coordinated network may underlie the cognitive impairment and related aspects of the psychopathology of schizophrenia.
Acknowledgments
Supported by NIH grant R01 MH-66629, NIMH KO2 award, and the Burroughs Wellcome Foundation Translational Clinical Scientist Award (Dr. Carter).
Footnotes
Previously presented at the International Congress of Schizophrenia Research Biennial Meeting, Colorado Springs, Colo., 2007.
Dr. Minzenberg is a shareholder with Elan Pharmaceuticals. Dr. Carter has served as a consultant to Pfizer, Hoffman La Roche, and Eli Lilly. Drs. Yoon, Ursu, Wendelken, and Ragland and Mr. Walters report no competing interests.
References
- 1.Kraepelin E. Dementia Praecox and Paraphrenia. New York: Robert D. Krieger Publishing; 1919. [Google Scholar]
- 2.Desimone R. Neural mechanisms for visual memory and their role in attention. Proc Natl Acad Sci U S A. 1996;93:13494–13499. doi: 10.1073/pnas.93.24.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 4.Tomita H, Ohbayashi M, Nakahara K, Hasegawa I, Miyashita Y. Top-down signal from prefrontal cortex in executive control of memory retrieval. Nature. 1999;401:699–703. doi: 10.1038/44372. [DOI] [PubMed] [Google Scholar]
- 5.Berman KF, Zec RF, Weinberger DR. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia, II: role of neuroleptic treatment, attention, and mental effort. Arch Gen Psychiatry. 1986;43:126–135. doi: 10.1001/archpsyc.1986.01800020032005. [DOI] [PubMed] [Google Scholar]
- 6.MacDonald AW, 3rd, Carter CS, Kerns JG, Ursu S, Barch DM, Holmes AJ, Stenger VA, Cohen JD. Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. Am J Psychiatry. 2005;162:475–484. doi: 10.1176/appi.ajp.162.3.475. [DOI] [PubMed] [Google Scholar]
- 7.Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, Goldberg TE, Weinberger DR. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- 8.Cannon TD, Glahn DC, Kim J, Van Erp TG, Karlsgodt K, Cohen MS, Nuechterlein KH, Bava S, Shirinyan D. Dorsolateral prefrontal cortex activity during maintenance and manipulation of information in working memory in patients with schizophrenia. Arch Gen Psychiatry. 2005;62:1071–1080. doi: 10.1001/archpsyc.62.10.1071. [DOI] [PubMed] [Google Scholar]
- 9.Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, Velligan DI. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25:60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A, 3rd, Noll DC, Cohen JD. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry. 2001;58:280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- 11.MacDonald AW, 3rd, Carter CS. Event-related fMRI study of context processing in dorsolateral prefrontal cortex of patients with schizophrenia. J Abnorm Psychol. 2003;112:689–697. doi: 10.1037/0021-843X.112.4.689. [DOI] [PubMed] [Google Scholar]
- 12.Barch DM, Carter CS, MacDonald AW, 3rd, Braver TS, Cohen JD. Context-processing deficits in schizophrenia: diagnostic specificity, 4-week course, and relationships to clinical symptoms. J Abnorm Psychol. 2003;112:132–143. [PubMed] [Google Scholar]
- 13.Barch DM, Mathews JR, Buckner RL, Maccotta L, Csernansky JG, Snyder AZ. Hemodynamic responses in visual, motor, and somatosensory cortices in schizophrenia. Neuroimage. 2003;20:1884–1893. doi: 10.1016/s1053-8119(03)00449-x. [DOI] [PubMed] [Google Scholar]
- 14.MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 15.Ford JM, Johnson MB, Whitfield SL, Faustman WO, Mathalon DH. Delayed hemodynamic responses in schizophrenia. Neuroimage. 2005;26:922–931. doi: 10.1016/j.neuroimage.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD. Detecting activations in PET and fMRI: levels of inference and power. Neuroimage. 1996;4(3 pt 1):223–235. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- 17.Rissman J, Gazzaley A, D’Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 18.Gazzaley A, Cooney JW, McEvoy K, Knight RT, D’Esposito M. Top-down enhancement and suppression of the magnitude and speed of neural activity. J Cogn Neurosci. 2005;17:507–517. doi: 10.1162/0898929053279522. [DOI] [PubMed] [Google Scholar]
- 19.Ranganath C, DeGutis J, D’Esposito M. Category-specific modulation of inferior temporal activity during working memory encoding and maintenance. Brain Res Cogn Brain Res. 2004;20:37–45. doi: 10.1016/j.cogbrainres.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 20.Brass M, Ullsperger M, Knoesche TR, von Cramon DY, Phillips NA. Who comes first? The role of the prefrontal and parietal cortex in cognitive control. J Cogn Neurosci. 2005;17:1367–1375. doi: 10.1162/0898929054985400. [DOI] [PubMed] [Google Scholar]
- 21.Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- 22.Fletcher P, McKenna PJ, Friston KJ, Frith CD, Dolan RJ. Abnormal cingulate modulation of fronto-temporal connectivity in schizophrenia. Neuroimage. 1999;9:337–342. doi: 10.1006/nimg.1998.0411. [DOI] [PubMed] [Google Scholar]
- 23.Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, Berman KF. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry. 2005;62:379–386. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- 24.Wolf DH, Gur RC, Valdez JN, Loughead J, Elliott MA, Gur RE, Ragland JD. Alterations of fronto-temporal connectivity during word encoding in schizophrenia. Psychiatry Res. 2007;154:221–232. doi: 10.1016/j.pscychresns.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friston KJ, Frith CD. Schizophrenia: A disconnection syndrome? Clin Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- 26.an der Heiden W, Hafner H. The epidemiology of onset and course of schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2000;250:292–303. doi: 10.1007/s004060070004. [DOI] [PubMed] [Google Scholar]
- 27.Snitz BE, MacDonald A, 3rd, Cohen JD, Cho RY, Becker T, Carter CS. Lateral and medial hypofrontality in first-episode schizophrenia: functional activity in a medication-naive state and effects of short-term atypical antipsychotic treatment. Am J Psychiatry. 2005;162:2322–2329. doi: 10.1176/appi.ajp.162.12.2322. [DOI] [PubMed] [Google Scholar]
- 28.Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey, I: parcellation of areas based on distinctive limbic and sensory corticocortical connections. J Comp Neurol. 1989;287:393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- 29.Husain M, Nachev P. Space and the parietal cortex. Trends Cogn Sci. 2007;11:30–36. doi: 10.1016/j.tics.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Husain M, Rorden C. Non-spatially lateralized mechanisms in hemispatial neglect. Nat Rev Neurosci. 2003;4:26–36. doi: 10.1038/nrn1005. [DOI] [PubMed] [Google Scholar]
- 31.Thompson KG, Biscoe KL, Sato TR. Neuronal basis of covert spatial attention in the frontal eye field. J Neurosci. 2005;25:9479–9487. doi: 10.1523/JNEUROSCI.0741-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wardak C, Ibos G, Duhamel JR, Olivier E. Contribution of the monkey frontal eye field to covert visual attention. J Neurosci. 2006;26:4228–4235. doi: 10.1523/JNEUROSCI.3336-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dierks T, Linden DE, Jandl M, Formisano E, Goebel R, Lanfermann H, Singer W. Activation of Heschl’s gyrus during auditory hallucinations. Neuron. 1999;22:615–621. doi: 10.1016/s0896-6273(00)80715-1. [DOI] [PubMed] [Google Scholar]
- 34.Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, Alpert NM. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci. 1998;1:318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- 35.Onitsuka T, Shenton ME, Salisbury DF, Dickey CC, Kasai K, Toner SK, Frumin M, Kikinis R, Jolesz FA, McCarley RW. Middle and inferior temporal gyrus gray matter volume abnormalities in chronic schizophrenia: an MRI study. Am J Psychiatry. 2004;161:1603–1611. doi: 10.1176/appi.ajp.161.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Honey GD, Bullmore ET, Soni W, Varatheesan M, Williams SC, Sharma T. Differences in frontal cortical activation by a working memory task after substitution of risperidone for typical antipsychotic drugs in patients with schizophrenia. Proc Natl Acad Sci U S A. 1999;96:13432–13437. doi: 10.1073/pnas.96.23.13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 38.Woo TU, Whitehead RE, Melchitzky DS, Lewis DA. A subclass of prefrontal gamma-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proc Natl Acad Sci U S A. 1998;95:5341–5346. doi: 10.1073/pnas.95.9.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 40.Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci U S A. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]