Abstract
Integrins are heterodimeric cell surface adhesion receptors essential for multicellular life. They connect cells to the extracellular environment and transduce chemical and mechanical signals to and from the cell. Intracellular proteins that bind the integrin cytoplasmic tail regulate integrin engagement of extracellular ligands as well as integrin localization and trafficking. Cytoplasmic integrin-binding proteins also function downstream of integrins, mediating links to the cytoskeleton and to signaling cascades that impact cell motility, growth, and survival. Here, we review key integrin-interacting proteins and their roles in regulating integrin activity, localization, and signaling.
Integrins make up a family of transmembrane heterodimeric receptors with crucial roles in a wide variety of cellular functions, including adhesion, migration, mechanotransduction, growth, and survival.1 The integrin family contains 18 α-subunits and eight β-subunits, whose αβ pairings determine ligand specificity and influence intracellular adhesion complex formation and subsequent signaling. Each integrin heterodimer consists of a large multidomain extracellular region that interacts with extracellular matrix (ECM) ligands, two single-pass transmembrane helices (one in each subunit), and short (typically 20–70 amino acids) α- and β-subunit cytoplasmic tails that mediate interactions with intracellular cytoskeletal and signaling proteins. Integrins thus provide a physical connection between the ECM and the actin cytoskeleton and participate in bidirectional signaling across the plasma membrane. Binding of cytoplasmic proteins to integrin cytoplasmic tails can trigger a change in integrin affinity for extracellular ligand (inside-out signaling/activation), and ECM ligand binding can promote downstream intracellular signaling (outside-in signaling). The structural and biochemical basis for binding of integrin to ECM ligands is increasingly well understood,2 as are the conformational rearrangements in the extracellular and transmembrane domains associated with integrin activation.3,4 The tissue-specific distribution of integrin subunits, and of their cytosolic binding partners, allows for fine-tuning of interactomes and consequent signaling. In this review, we discuss the advances in our understanding of how various integrin cytoplasmic interactions contribute to diverse molecular signals and biological functions. We review the rapidly growing list of intracellular proteins that directly interact with integrin cytoplasmic tails1,5 to regulate integrin activation, surface expression and localization, cytoskeletal remodeling, mechanotransduction, and downstream signaling cascades.
Integrin Cytoplasmic Tails
Cytoplasmic tails are essential for integrin function, and their mutation or deletion, especially in the β-subunit, alters integrin affinity for extracellular ligands and perturbs intracellular signaling cascades.6 Nuclear magnetic resonance (NMR) and X-ray crystallographic studies have shown integrin tails to be conformationally flexible; depending on their interaction partner, tails can form α-helices (e.g., in the context of integrin heterodimers7−9) or β-strands (e.g., when in complex with talin or filamin10,11). Integrin tails bind to a functionally diverse set of intracellular proteins to promote downstream signaling,1,12 and at least 40 direct interactors have been reported with many more, potentially indirect, partners recently identified in proteomic screens.13 With only tens of amino acids per tail and substantially more interactors, access of cytoplasmic binding partners to the integrin tail must be finely tuned and carefully regulated.5,13 This seems to occur primarily through competition among proteins for similar binding sites and post-translational modifications of the tail that impact affinity for interactors.14 In addition, hidden or cryptic integrin tail-binding sites in partners can be exposed by mechanical force, proteolysis, or phosphorylation, and the local concentration of binding partners can be regulated by membrane or other subcellular localization signals.5,6,15
Aside from a conserved membrane-proximal GFFKR motif, integrin α-tails have little similarity to one another. In contrast, most integrin β-tails are fairly well conserved and are thus subject to similar modes of regulation (Figure 1). Perhaps this explains why β-tail interactions are generally better understood than α-tail interactions. Most β-tails contain two NPxY motifs, and as discussed below, numerous proteins compete to interact with these sites. As such, the Tyr residues of NPxY motifs are key regulatory sites on the integrin tail. For example, NPxY motif tyrosine phosphorylation by Src family kinases (SFK) may positively or negatively regulate interactions with phosphotyrosine-binding (PTB) domain-containing proteins [e.g., talin and Dok1 (Figure 2, top)].16 Tyrosine phosphorylation of NPxY motifs can also protect integrin tails from calpain cleavage, as has been shown for β3, providing another means of regulating integrin tail availability for cytoplasmic interactions.17 A serine/threonine-rich motif located between the NPxY motifs serves as an additional regulatory point, as phosphorylation of these residues by an unidentified kinase inhibits filamin binding but promotes 14-3-3 binding.18 Integrin tail phosphorylation has been carefully reviewed elsewhere,14 but the continued identification and characterization of regulatory kinases are crucial to a thorough understanding of adhesion dynamics and the role of post-translational modifications in the regulation of the affinity of integrin for the extracellular ligand.
Figure 1.
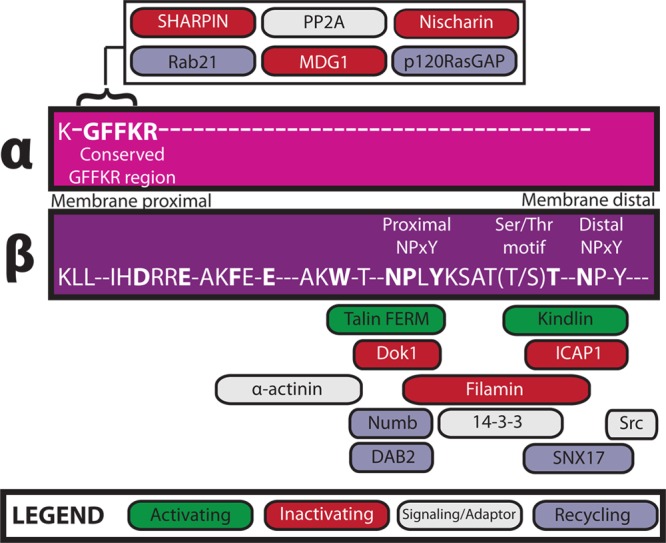
Direct integrin cytoplasmic tail interactors. Interactions between integrin cytoplasmic tails and intracellular proteins regulate integrin activity, surface expression, and downstream signaling. Here we depict known direct integrin interactors, their sites of interaction on α- or β-tails, and their functional role by color, as indicated in the legend. Conserved tail residues are displayed in uppercase letters; highly conserved residues are shown in bold.
Figure 2.
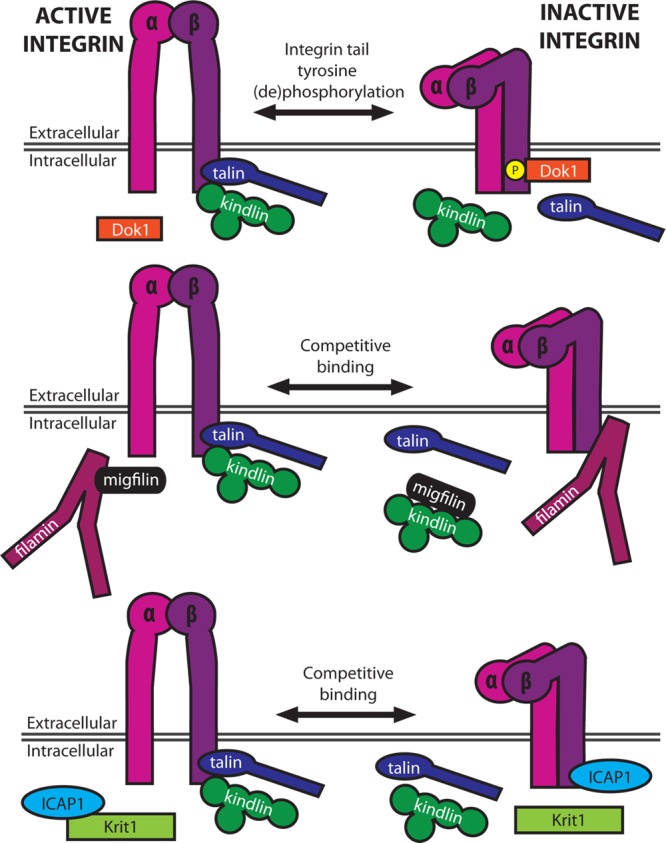
Regulation of integrin activation. Integrin activation may be regulated by post-translational modifications of the tail (e.g., phosphorylation) and through competition between activators and inhibitors for binding the β-tail. Inhibitors of integrin activation may be competed off integrin tails by other intracellular proteins (e.g., migfilin–filamin and Krit1–ICAP1 binding).
Integrin Activators
Activation is the process by which integrins switch from conformations with relatively low affinity for ECM to those with high ligand binding affinity. Tight regulation of integrin activation is essential for a variety of developmental and physiological processes,6 and while integrins can be activated and clustered by ECM itself, here we focus on cytoplasmic interactions that allosterically drive receptor–ligand engagement.
Direct binding of the cytoskeletal protein talin to integrin β-tails is now recognized as the key final step in integrin activation19 and has been extensively reviewed elsewhere.15,20,21 The central role of talin in integrin activation is supported by talin knock-down, knock-out, and knock-in phenotypes15 and can be reconstituted in vitro.22 Talin is composed of an N-terminal atypical, four-subdomain (F0–F3) FERM (4.1 band, ezrin, radixin, moesin) domain (the talin head), and a C-terminal rod consisting of 13 helical bundle repeats.15 Briefly, direct binding of the talin head to integrin β-tails disrupts an inhibitory association between the membrane-proximal portions of α- and β-tails, changing the tilt of integrin transmembrane domains and triggering activation.4 Talin binds the membrane-proximal NPxY motif in integrin β1−β3, β5, and β7 tails and probably also in β6 tails.5,23 As β4 and β8 tails lack this motif, the activation of αvβ8 and α6β4 integrins is likely to be talin-independent.
Talin–integrin interactions are regulated in a variety of ways.15 Upstream, GTP-bound Rap1 triggers integrin activation via its effector, Rap1-interacting adaptor molecule (RIAM). Rap1 targets a Rap1–RIAM–talin complex to the plasma membrane to promote talin–integrin binding. Recent reports indicate that while RIAM recruits talin to nascent adhesions, binding of vinculin to talin then displaces RIAM, stabilizing active integrins within focal adhesions (FAs).26,27 Conformational rearrangement of talin provides another means of regulating activation. The integrin-binding site within the talin F3 subdomain is normally masked by an intramolecular interaction with a portion of the C-terminal talin rod.28−30 Phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] binding to the F3 subdomain helps displace the rod, unmasking the integrin-binding site. Additional lipid-binding sites throughout the talin head are important for talin-mediated integrin activation, presumably because they help target talin to the membrane and properly orient it to increase the affinity for the β-integrin cytoplasmic tail.24,25,31 In this way, interactions of talin with PIPs allow for efficient talin-mediated integrin activation. Finally, phosphorylation of the talin FERM domain by cyclin-dependent kinase 5 (Cdk5) protects talin from smurf1-mediated ubiquitinylation and proteosomal degradation, positively contributing to adhesion stability.32
While talin binding is central to integrin activation, other integrin-binding proteins have also been implicated. Chief among these are the members of the recently reviewed kindlin family of proteins.15,21,33−35 Kindlin, like the talin head, is composed of an atypical FERM domain but also includes a nested, lipid-binding pleckstrin homology (PH) domain. Kindlins bind to the membrane-distal NPxY motif and a S/T-containing motif that lies between the two tyrosines on β-integrins (Figure 1). While all three kindlin isoforms bind β1, β2, and β3 integrins, it was recently shown that β6 tails preferentially bind kindlin-1 over kindlin-2.37 This impacts kindlin isoform localization in keratinocytes, but whether it has a consequence on β6 integrin activation remains to be determined.
Overexpression, knock-out, knock-down, and disease mutations clearly establish kindlins as regulators of integrin activation, integrin surface expression levels, and integrin signaling. At least one kindlin isoform (kindlin-1) was identified as a phosphoprotein,38 but the functional relevance of this phosphorylation remains to be determined. While the exact mechanisms of effects of kindlin on activation are unknown, direct binding to the integrin β-tail is required, and scaffolding of additional integrin-associated, kindlin-binding proteins, migfilin and integrin-linked kinase (ILK), may be involved. Kindlin regulates the dynamics of recruitment of migfilin to adhesions,39,40 which may be important as migfilin can enhance talin-mediated activation by competing away filamin, a suppressor of integrin activation (Figure 2, middle).11,41−43 ILK was first identified as an integrin β-tail-binding protein44 and is also thought to regulate β1 and β3 integrin activation,45,46 so it is conceivable that effects of kindlin on integrin activation may at least in part be ILK-mediated.
Although integrin activation is often described as increasing the ligand binding affinity of a single receptor, activated receptors also cluster, resulting in an increased avidity for ECM ligand. Talin binding clearly enhances monomeric affinity,22,47 but overexpression of the talin head also increases the level of clustering of αVβ3 integrins, a process that requires binding of PI(4,5)P2 to talin.48 Most notably, a recent paper reports that kindlin works by clustering talin-activated αIIbβ3 integrins while having no impact on monomeric affinity.47 Consistent with this, kindlin has been implicated in the clustering of hematopoetic integrins.49 While little is known about exactly how integrin microclusters form or are stabilized, kindlins may serve as adaptors that recruit proteins to support clustering. The kindlin-binding protein, ILK, has also been implicated in enhancing clustering and the connection to the cytoskeleton.50
Talin and kindlin are well-established integrin activators, but others have been proposed, including the β-tail-binding proteins, β3-endonexin and cytohesin, and α-tail binders, CIB1 (calcium- and integrin-binding protein 1) and RapL.6,51 However, the true role of these proteins in integrin function remains unclear, as there are little recent data to suggest they are direct activators. Two other adhesion proteins, Zasp (Z-band alternatively spliced PDZ motif-containing protein) and vinculin, have been shown to enhance talin-mediated integrin activation,27,52,53 but it is likely that neither requires direct interactions with integrin cytoplasmic tails.
Suppressors of Integrin Activation
Proper integrin function requires careful temporal and spatial regulation of integrin activation. It is now recognized that, in addition to direct integrin activators, both α- and β-tail-binding proteins can suppress integrin activation.54,55 Thus, integrin regulation occurs through competition between inhibitory and activating proteins for binding to cytoplasmic tails and may be further controlled by phosphorylation of inhibitor- or activator-binding motifs within tails as well as the inhibitors or activators themselves.
Filamin, a large actin-cross-linking protein important for the regulation of cell spreading and migration,56 is among the best characterized integrin inhibitors. It contains an actin-binding domain as well as a series of 24 tandem immunoglobulin (Ig)-like repeats, the last of which allows for homodimerization.11 Filamin binds the first NPxY motif and the subsequent threonine-rich region in the integrin β-tail (Figure 1). This overlaps with the talin- and kindlin-binding sites, thus suppressing integrin activation.11,15,18 As noted above, migfilin can compete with β-tails for filamin, relieving inhibition and enhancing integrin activation (Figure 2, middle).42,43 Kinases also modulate filamin interactions and hence integrin activation, as phosphorylation of β2 integrin tails at T758 inhibits filamin binding but promotes binding of the scaffolding protein 14-3-3, thereby regulating T-cell adhesion.18 A similar phosphoswitch may exist for β1 and β3, given that filamin and 14-3-3 also interact with these integrins.5 Further, molecular dynamics studies suggest that, in the presence of force, Ser2152 phosphorylation of filamin A Ig repeat 20 regulates the exposure of the cryptic integrin-binding site.57
Another talin competitor specifically regulated by phosphorylation of the β-tail is Dok1, a PTB domain-containing scaffolding protein shown to bind β2, β3, β5, and β7 integrin tails.23 SFK-mediated phosphorylation of the talin-binding NPxY motif in β-tails inhibits talin binding and favors Dok1 binding, suppressing activation.16 Surprisingly, despite the apparent importance of tyrosine phosphorylation of NPxY motifs, mice carrying knock-in phospho-blocking β1 integrin Y/F mutations are largely normal58,59 and are still susceptible to Src-mediated and focal adhesion kinase (FAK)-mediated tumorigenesis.60
Most recently, a mechanism that regulates another PTB domain-containing integrin inhibitor, ICAP1 (integrin cytoplasmic domain-associated protein 1), has been elucidated.61 By binding to the β1 tail at the distal NPxY motif, ICAP1 occupies the kindlin-binding site and sterically hinders talin binding.61 Much like the effect of migfilin on filamin-mediated β-integrin suppression, Krit-1 (Krev/Rap1 interaction trapped-1) can compete with β1 for ICAP1 binding, thus increasing the level of integrin activation (Figure 2, bottom).61 Calcium/calmodulin-dependent protein kinase type II (CaMKII), a kinase implicated in regulation of integrin-mediated adhesion, phosphorylates ICAP1 on Thr38, enhancing binding of ICAP1 to β1 tails, reducing the level of activation, and downregulating focal adhesion assembly.62 Other β-tail-binding proteins may also act as integrin inhibitors, but their mechanisms of action require further study. For example, the actin-binding protein α-actinin has been reported to suppress αIIbβ3 activation, presumably through competition with talin, but α-actinin is also reported to enhance the binding of talin to β1, increasing the level of integrin activation.63,64
Finally, in addition to β-tail-binding inhibitors, a growing body of work has explored the roles of α-tail-binding integrin suppressors. These proteins, which include Nischarin, MDGI (mammary-derived growth inhibitor), and SHARPIN (SHANK-associated RH domain-interacting protein), have been thoroughly reviewed elsewhere.54,55 Typically, they bind the conserved GFFKR region of the α-tail, either directly stabilizing the inhibitory α-tail−β-tail association or indirectly preventing binding of talin to the β-tail.
Regulators of Integrin Surface Expression and Localization
In addition to modulating integrin activation, cells regulate adhesion by controlling the availability of integrins at the cell surface and within adhesive structures. Integrin turnover determines the direction and speed of cell migration and, if misregulated, contributes to metastasis and invasion.65 While considerable effort has been devoted to developing therapeutics that inhibit integrin-ligand interactions, there is also recent interest in modulating integrin trafficking.66 Here we will focus on endocytic and recycling proteins that directly interact with integrin cytoplasmic tails to spatially and temporally regulate the delivery of integrin to the plasma membrane and turnover within adhesions.
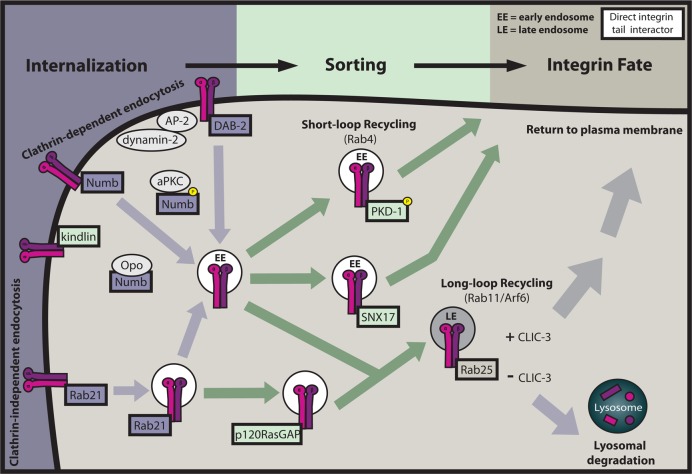
Endocytosis of integrins occurs via both clathrin-dependent and -independent pathways and has been extensively reviewed elsewhere.65−67 Microtubule-mediated focal adhesion (FA) disassembly is a clathrin- and dynamin-dependent process.67 Dynamin deficiency results in increased cell surface integrin levels, enlarged FAs, and impaired endothelial branching morphogenesis with accumulation of integrin at sites of failed angiogenic sprouting.68 Two clathrin adaptors, disabled-2 (DAB2) and Numb, bind NPxY motifs in β3 and β5 tails via their PTB domains.23 DAB2 colocalizes in FAs with clathrin, the primary clathrin adaptor AP-2, and dynamin-2 to facilitate β1 integrin internalization and FA disassembly.69 Numb also localizes at adhesions, and its binding to integrin is regulated by phosphorylation of Numb by atypical protein kinase C.70 More recently, the NPxY motif-containing protein Opo was shown to regulate epithelial morphogenesis by regulating integrin endocytosis through sequestration of Numb or NumbL.71 It has yet to be determined whether other clathrin adaptors that bind NPxY motifs and are important for integrin internalization (e.g., autosomal recessive hypercholesterolemia) also directly bind β-integrin tails.67 Integrins also undergo endocytosis in clathrin-independent ways, and although our understanding of these mechanisms is much more limited, binding of Rab21 to the conserved membrane-proximal portion of integrin α-tails is known to promote clathrin-independent internalization of some β1 heterodimers.72,73
Following internalization, integrins typically face one of two fates: recycling back to the plasma membrane or degradation in lysosomes. Recycling may occur via rapid “short-loop” or slower “long-loop” Rab GTPase-dependent recycling pathways (Figure 3).65,66 αVβ3 integrins recycle though the Rab4 short-loop pathway, in which integrins are returned to the plasma membrane directly from Rab4-enriched early endosomes.74 This pathway is important for directional migration and requires protein kinase D1 (PKD1) binding to the 15 C-terminal amino acids of the β3 tail.75,76 β3–PKD1 association requires PKD1 Ser916 autophosphorylation, which occurs downstream of growth factor stimulation. PKD1 also interacts with β1, making it possible that PKD1 similarly regulates β1 internalization.77
Figure 3.
Integrin interactors involved in sorting and surface expression. Integrin surface expression is carefully regulated by clathrin-dependent and -independent endocytosis, short-loop Rab4-dependent sorting, and long-loop Rab11-dependent sorting. Direct integrin interactors have been implicated as regulators throughout these pathways, as indicated by thick rectangular outlines.
In long-loop recycling, integrins are transported to ADP ribosylation factor 6 (Arf6) and Rab11-enriched vesicles of the perinuclear recycling compartment before returning to the membrane. Rab25, another GTPase that localizes to recycling endosomes as well as late endosomes, directly interacts with β1 integrin and has recently been shown to sort active α5β1 integrins to late endosomes and lysosomes.78,79 In the presence of chloride intracellular channel 3 (CLIC3), these integrins are recycled to the plasma membrane, but in the absence of CLIC3, Rab25 leads to lysosomal degradation of the integrin.79 In addition to mediating β1 integrin endocytosis, Rab21 also controls β1 integrin recycling. This is regulated by competition between Rab21 and p120RasGAP for binding to the membrane-proximal portion of integrin α-tails.80 p120RasGAP normally promotes the recycling of integrin to the plasma membrane, and in its absence, the Rab21-bound integrins are retained in endosomes.
While it is well-established that components of the endocytic machinery are crucial to integrin recycling, integrin activators themselves, such as talin and kindlin, also contribute to this dynamic process. Talin-1 depletion, or mutagenesis of the membrane-proximal NPxY motif in the β1 tail, reduces the level of α5β1 internalization, but not recycling.81 Kindlin-2 depletion, or mutagenesis of the β1 membrane-distal NPxY motif, promotes degradation of α5β1, suggesting a role for kindlin-2 in protecting integrins from degradation.81 Interestingly, it was recently found that sorting nexin 17 (SNX17), a FERM domain-containing protein important for recycling cargo to the plasma membrane, binds the β1 membrane-distal NPxY motif, probably following kindlin dissociation, protecting β1 from lysosomal degradation.82,83 How competition between kindlin and SNX17 for β integrin is regulated has yet to be elucidated.
Cytoskeletal Linkages and Mechanotransduction
Complex cellular processes often require integration of multiple signaling cascades, sensing and transduction of mechanical force, and cytoskeletal reorganization. As well as providing a physical connection to the ECM, integrins transmit chemical and mechanical cues that spatially and temporally regulate cytoskeletal dynamics.84−86 Here we highlight integrin tail-binding proteins that transmit signals from integrins to the cytoskeleton and back again.
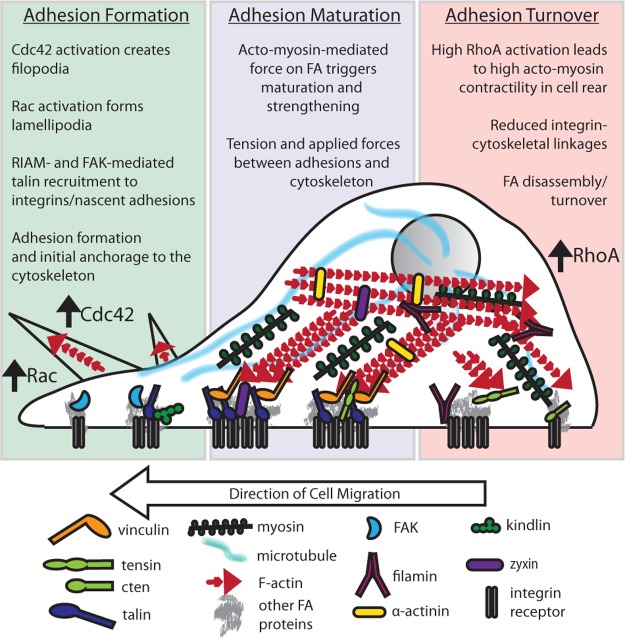
The most direct link between integrins and the cytoskeleton is through direct actin-binding proteins, such as talin, filamin, tensin, and α-actinin (Figures 4 and 5). Talin binds actin via a C-terminal domain87 but can also indirectly associate with actin through vinculin. Forces transmitted to talin result in repeated talin stretching and expose otherwise buried vinculin-binding sites in the talin rod.15,88,89 This is important for force-dependent reinforcement of integrin–cytoskeletal bonds90 and for transduction of mechanical information during force sensing.91
Figure 4.
Integrin–cytoskeletal connections. The dynamic connection between integrins and the cytoskeleton is mediated by a host of adhesion proteins. Here, we show how integrin-binding proteins contribute to the integrin–cytoskeletal connection to orchestrate directed migration.
Figure 5.
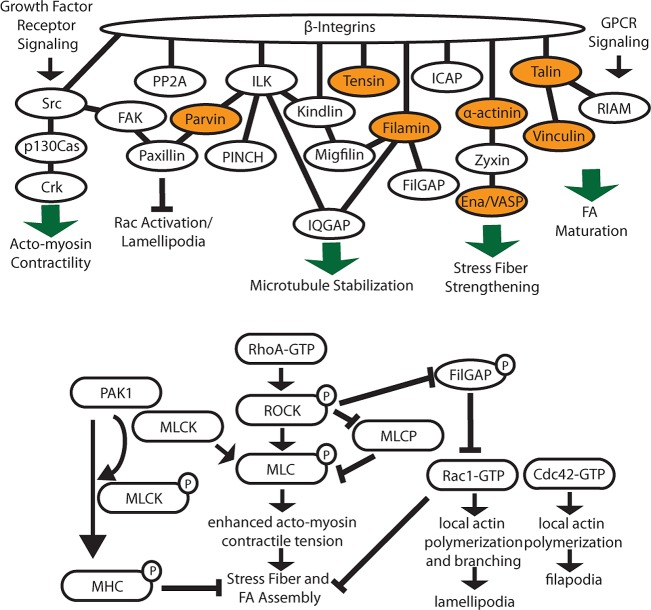
Integrin interactions and downstream signaling. In the top panel, β-integrin interactions are highly interconnected and play important roles when signaling to the cytoskeleton. Orange proteins indicate direct actin binders. In the bottom panel, many integrin-binding proteins impact the spatial and temporal regulation of Rho family GTPases to affect cytoskeletal organization.
Filamin is another mechanosensitive protein that directly binds integrins and actin.92 Notably, the integrin-binding site in filamin is partially obscured by an adjacent Ig-like repeat domain,93 and force applied to filamin can expose this site and enhance integrin binding.94,95 Strain on the filamin–actin network also releases filaminA-associated Rho GTPase activating protein (FilGAP), a Rac inactivator, from filamin,95 providing another means to mechanically modulate cytoskeletal dynamics (Figure 5).
The direct association of the actin-bundling protein, α-actinin, with integrins provides another link to the cytoskeleton.12 One recent model suggests that α-actinin displaces talin from integrins in nascent β3-rich adhesions, triggering focal adhesion (FA) maturation and leaving α-actinin as the main force transducer between adhesions and the cytoskeleton.63 α-Actinin also interacts with the mechanosensor zyxin, and both α-actinin and zyxin become post-translationally modified and relocalize to stress fibers in response to mechanical stress.96,97 α-Actinin then cooperates with zyxin to recruit Ena/VASP (vasodilator-stimulated phosphoprotein) to strengthen stress fibers (Figure 4).97,98
Finally, the FA protein, tensin, can also potentially directly link integrins to actin.23 Though the level of tensin phosphorylation increases as cells contact ECM or in response to oncogenes,99 the functional consequence of that phosphorylation is not clear. All tensin isoforms bind integrins via a PTB domain. Three of the four tensin isoforms directly bind actin and may act to stabilize stress fibers, anchoring them to β1- and β3-rich FAs.99 The fourth isoform (cten or tensin4) does not bind actin, and in more migratory cell lines, its expression is upregulated in response to growth factor stimulation.100 Concomitantly, tensin3 expression is downregulated; stress fibers disassemble, and there are fewer FAs.99,100 Therefore, one tensin isoform may act to interrupt the transmission of force between FAs and the actin cytoskeleton while others can act to preserve it.
Integrin can also be indirectly linked to actin-binding proteins. One of the best examples of this is vinculin, which is recruited to adhesions via mechanically activated talin (Figure 4).15,88 A recent model based on structure and adhesion dynamics suggests the Rap effector, RIAM, initially activates talin, which then activates integrins in nascent adhesions. There, applied forces inhibit RIAM binding, freeing the vinculin-binding site on talin and triggering FA maturation.26,27 Vinculin itself also transmits force at adhesions; studies have quantified the force applied to vinculin during cell migration.101 This underscores the central role of vinculin in the “molecular clutch” that governs coupling between adhesions and actin filaments and therefore traction force, adhesion strengthening, and cell motility.102,103 Notably, vinculin also binds α-actinin, and molecular dynamics modeling suggests that mechanical force is required to expose the vinculin-binding site on α-actinin.104 Thus, α-actinin may also provide a mechanosensitive route for vinculin recruitment.
ILK provides another potential indirect link to actin that has been extensively reviewed elsewhere.105−107 Yeast two-hybrid data suggest that this pseudokinase directly interacts with integrin β-tails and functions as an adaptor to regulate downstream cytoskeletal dynamics as well as cell polarity and migration. ILK does not directly bind actin, and the cytoskeletal linkage is likely to require its obligate binding partners PINCH and parvin.105 Of these, the calponin homology domain-containing protein, parvin, may directly bind F-actin.105 ILK may also impact microtubule polarity108 by interacting with IQGAP1 to localize it to nascent adhesions, stabilizing microtubule cytoskeletal dynamics.109 ILK may also indirectly impact cytoskeletal dynamics via RhoA signaling and has been implicated in regulating cell contractility.105,106 Indirect integrin–actin links may also pass through kindlin, which binds ILK,15 or via an integrin–kindlin–migfilin–filamin linkage.39,40
In addition to the links to actin discussed above, integrins can impact cytoskeletal dynamics, organization, cell contractility, and force sensing by altering spatiotemporal regulation of Rho family GTPases. Arp2/3 complex-mediated actin nucleation and polymerization take place downstream of GTP-bound Rac or Cdc42, forming lamellipodia or filopodia. GTP-bound RhoA activates Rho kinase (ROCK) and inactivates myosin light chain phosphatase. This promotes phosphorylation of myosin light chain (MLCP), enhancing acto-myosin contractility and FA and stress fiber formation.110 Rho family GTPase signaling is triggered by complex, interconnected pathways beyond the scope of this review.110,111 Here we limit our discussion to direct tail-binding proteins that influence Rho signaling.
As noted above, filamin can inhibit Rac through FilGAP (Figure 5)112 or via a filamin–IQGAP1–RacGAP1 complex.113 Kindlins also regulate actin dynamics and cell spreading,34 presumably though an indirect regulation of Rho,114 Rac,115 and Cdc42.116 How this occurs remains to be shown. Finally, binding of paxillin to α4 integrin tails inhibits the formation of lamellipodia by recruiting ArfGAP to reduce Arf activity and thereby block Rac.117 This is regulated by protein kinase A-mediated α4 tail phosphorylation, which occurs at the leading edge, locally inhibiting paxillin-mediated Rac inactivation and restricting the formation of lamellipodia to the front of migrating cells.118
Integrin Adhesion Signaling
Following adhesion to the ECM, integrins participate in a multitude of signaling cascades mediated by interactions with cytoplasmic proteins.1 Typically termed outside-in signaling, these cascades regulate growth, survival, cytoskeletal dynamics, and motility and are required for normal cellular responses to the local environment. While numerous integrin-mediated signaling pathways have been described, there are still considerable gaps between initial adhesion events and the effects on downstream molecules. Here we limit the discussion to a few adhesion-related signaling molecules proximal to the integrin cytoplasmic tail.
Focal adhesion kinase (FAK) is one of the best-characterized adhesion-related signaling molecules, known to be activated downstream of integrin-mediated adhesion and strongly expressed in numerous cancers.119 FAK is a cytoplasmic tyrosine kinase, and while it has been reported to interact directly with β1 tails,120 it is thought to be recruited via interactions with paxillin or talin.119 However, FAK can be recruited to nascent adhesions by an unknown mechanism and in turn help in the recruitment of talin.121 Integrin engagement triggers FAK autophosphorylation on tyrosine 397, creating a binding site for the SH2 (Src homology 2) domain of Src, allowing for further tyrosine phosphorylation of FAK by Src, and initiation of numerous growth and survival pathways via Rac1, paxillin, and β-Pix, among others.122 In addition to their roles in FAK signaling, SFKs can directly bind integrin cytoplasmic tails, resulting in activation of SFK activity and cell spreading.123 As discussed previously, SFKs also phosphorylate tyrosines on β-integrin tails, thus regulating interactions with cytoplasmic signaling molecules.15
In addition to FAK and Src, a number of other kinases (and phosphatases) are known to interact with integrins, ultimately effecting Rac1- and Cdc42-regulated cytoskeletal remodeling, as well as kinase-regulated growth pathways. β1 integrin has recently been shown to interact directly with Arg kinase, activating p190RhoGAP, which inactivates RhoA GTPase.124 Direct phosphorylation-independent engagement of integrin β-tails by the SH2 domain of Syk and Zap70 kinases leads to kinase activation.125 Members of the p21-activated kinase (PAK) family are also activated downstream of integrin-mediated adhesion, and PAK4, which is activated following αVβ5-mediated adhesion to vitronectin and implicated in regulation of integrin turnover, forms a complex with β5 integrin tails.126 However, a direct in vitro interaction between PAKs and integrins has yet to be established. Phosphatase 2A (PP2A), which dephosphorylates serine/threonine residues, binds to the KVGFFKR sequence of αIIb to block downstream ERK (extracellular signal-regulated kinase) signaling.127 Additional studies will be necessary to fully understand the requisite interactions of integrin with kinases and phosphatases and their ultimate downstream effects.
Integrin signaling often occurs in concert with other receptors, including growth factor receptors, G-protein-coupled receptors (GPCRs), and even other integrins. Integrins and growth factor receptors have been shown to independently and collaboratively activate the same pathways, such as FAK and Src-mediated signaling, though varied evidence exists for whether integrins interact directly with growth factor receptors.128 A growing list of GPCR-related proteins have been reported to interact directly with β-integrin tails, including the cytohesin family of guanine nucleotide exchange factors (GEFs)12 and, more recently, the G-protein Gα13, also implicated in the activation of Src family kinase signaling.129 Signaling downstream of integrins may also be regulated by other integrins, as is evidenced by trans-dominant effects on β1 by β3 cytoplasmic tails.130
Conclusion and Future Perspectives
Though integrin cytoplasmic tails are typically short, they mediate a host of critical interactions. These direct interactions thus regulate cytoskeletal dynamics, response to growth factor signals, transcriptional regulation, and integrin activation, localization, and surface expression. Our understanding of the importance of tail-binding proteins in a variety of signaling pathways is rapidly growing, but there are still many unanswered questions, including the general applicability of findings to specific integrins and cell types. Furthermore, while we know many key players, the structural basis for integrin binding or its functional significance often remains to be determined. Our growing body of knowledge facilitates a more precise examination of how aberrant integrin signaling contributes to disease, pointing the field toward elements of the “integrin cytoplasmic interactome” that might serve as therapeutic targets to modulate integrin activity or signaling. In the future, innovative technologies (like super-resolution imaging or genomic editing in situ) combined with more interdisciplinary approaches are likely to drive adhesion research. While once each aspect of integrin regulation and function was examined in isolation (activation, recycling, localization, signal transduction, etc.), we can now begin synthesizing information from diverse research fields to generate more holistic models of dynamic integrin signaling in cell and tissue homeostasis.
Glossary
Abbreviations
- Arf
ADP ribosylation factor
- Arp2/3
actin-related proteins 2 and 3
- CaMKII
calcium/calmodulin-dependent protein kinase type II
- Cdk5
cyclin-dependent kinase 5
- CH
calponin homology
- CIB1
calcium and integrin-binding protein 1
- DAB2
disabled-2
- ECM
extracellular matrix
- ERK
extracellular signal-regulated kinase
- FA
focal adhesion
- F-actin
filamentous actin
- FAK
focal adhesion kinase
- FERM
4.1 protein, ezrin, radixin, moesin
- FilGAP
filaminA-associated Rho GTPase activating protein
- GAP
GTPase activating protein
- GEF
guanine nucleotide exchange factor
- GPCR
G-protein-coupled receptor
- GTPase
guanosine triphosphate hydrolase
- ICAP
integrin cytoplasmic domain-associated protein 1
- Ig
immunoglobulin G
- ILK
integrin-linked kinase
- IQGAP1
IQ motif containing GTPase activating protein isoform 1
- Krit1
Krev/Rap1 interaction trapped-1
- MDGI
mammary-derived growth inhibitor
- MHC
myosin heavy chain
- MLC
myosin light chain
- MLCK
myosin light chain kinase
- NMR
nuclear magnetic resonance
- PH
pleckstrin homology
- PI(4,5)P2
phosphatidylinositol 4,5-bisphosphate
- PKD1
protein kinase D1
- PP2A
phosphatase 2A
- PTB
phosphotyrosine-binding
- RIAM
Rap1-interacting adaptor molecule
- ROCK
Rho kinase
- SFK
Src family kinase
- SH2
Src homology 2
- SHARPIN
SHANK-associated RH domain-interacting protein
- VASP
vasodilator-stimulated phosphoprotein
- Zasp
Z-band alternatively spliced PDZ motif-containing protein.
Author Contributions
E.M.M. and N.N.B. contributed equally to this work.
This work was funded in whole or in part by National Institutes of Health Grants T32 GM-007223 and RO1 GM-068600, by American Cancer Society Grant RSG-12-053-01, and by National Science Foundation Graduate Research Fellowship DGE-1122492.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Harburger D. S.; Calderwood D. A. (2009) Integrin signalling at a glance. J. Cell Sci. 122, 159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu G.; Wang W.; Luo B. H. (2012) Overview: Structural biology of integrins. Methods Mol. Biol. 757, 81–99. [DOI] [PubMed] [Google Scholar]
- Zhu J.; Zhu J.; Springer T. A. (2013) Complete integrin headpiece opening in eight steps. J. Cell Biol. 201, 1053–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.; Ye F.; Hu X.; Ginsberg M. H. (2012) Talin activates integrins by altering the topology of the β transmembrane domain. J. Cell Biol. 197, 605–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legate K. R.; Fassler R. (2009) Mechanisms that regulate adaptor binding to β-integrin cytoplasmic tails. J. Cell Sci. 122, 187–198. [DOI] [PubMed] [Google Scholar]
- Calderwood D. A. (2004) Integrin activation. J. Cell Sci. 117, 657–666. [DOI] [PubMed] [Google Scholar]
- Vinogradova O.; Velyvis A.; Velyviene A.; Hu B.; Haas T.; Plow E.; Qin J. (2002) A structural mechanism of integrin αIIbβ3 “inside-out” activation as regulated by its cytoplasmic face. Cell 110, 587–597. [DOI] [PubMed] [Google Scholar]
- Bhunia A.; Tang X. Y.; Mohanram H.; Tan S. M.; Bhattacharjya S. (2009) NMR solution conformations and interactions of integrin αLβ2 cytoplasmic tails. J. Biol. Chem. 284, 3873–3884. [DOI] [PubMed] [Google Scholar]
- Metcalf D. G.; Moore D. T.; Wu Y.; Kielec J. M.; Molnar K.; Valentine K. G.; Wand A. J.; Bennett J. S.; DeGrado W. F. (2010) NMR analysis of the αIIbβ3 cytoplasmic interaction suggests a mechanism for integrin regulation. Proc. Natl. Acad. Sci. U.S.A. 107, 22481–22486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alvarez B.; de Pereda J. M.; Calderwood D. A.; Ulmer T. S.; Critchley D.; Campbell I. D.; Ginsberg M. H.; Liddington R. C. (2003) Structural determinants of integrin recognition by talin. Mol. Cell 11, 49–58. [DOI] [PubMed] [Google Scholar]
- Kiema T.; Lad Y.; Jiang P.; Oxley C. L.; Baldassarre M.; Wegener K. L.; Campbell I. D.; Ylanne J.; Calderwood D. A. (2006) The molecular basis of filamin binding to integrins and competition with talin. Mol. Cell 21, 337–347. [DOI] [PubMed] [Google Scholar]
- Liu S.; Calderwood D. A.; Ginsberg M. H. (2000) Integrin cytoplasmic domain-binding proteins. J. Cell Sci. 113(Part 20), 3563–3571. [DOI] [PubMed] [Google Scholar]
- Geiger T.; Zaidel-Bar R. (2012) Opening the floodgates: Proteomics and the integrin adhesome. Curr. Opin. Cell Biol. 24, 562–568. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G.; Fagerholm S. C.; Nurmi S. M.; Chavakis T.; Marchesan S.; Gronholm M. (2009) Regulation of integrin activity and signalling. Biochim. Biophys. Acta 1790, 431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood D. A.; Campbell I. D.; Critchley D. R. (2013) Talins and kindlins: Partners in integrin-mediated adhesion. Nat. Rev. Mol. Cell Biol. 14, 503–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthis N. J.; Haling J. R.; Oxley C. L.; Memo M.; Wegener K. L.; Lim C. J.; Ginsberg M. H.; Campbell I. D. (2009) β integrin tyrosine phosphorylation is a conserved mechanism for regulating talin-induced integrin activation. J. Biol. Chem. 284, 36700–36710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi X.; Flevaris P.; Stojanovic A.; Chishti A.; Phillips D. R.; Lam S. C.; Du X. (2006) Tyrosine phosphorylation of the integrin β3 subunit regulates β3 cleavage by calpain. J. Biol. Chem. 281, 29426–29430. [DOI] [PubMed] [Google Scholar]
- Takala H.; Nurminen E.; Nurmi S. M.; Aatonen M.; Strandin T.; Takatalo M.; Kiema T.; Gahmberg C. G.; Ylanne J.; Fagerholm S. C. (2008) β2 integrin phosphorylation on Thr758 acts as a molecular switch to regulate 14-3-3 and filamin binding. Blood 112, 1853–1862. [DOI] [PubMed] [Google Scholar]
- Tadokoro S.; Shattil S. J.; Eto K.; Tai V.; Liddington R. C.; de Pereda J. M.; Ginsberg M. H.; Calderwood D. A. (2003) Talin binding to integrin β tails: A final common step in integrin activation. Science 302, 103–106. [DOI] [PubMed] [Google Scholar]
- Shattil S. J.; Kim C.; Ginsberg M. H. (2010) The final steps of integrin activation: The end game. Nat. Rev. Mol. Cell Biol. 11, 288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F.; Kim C.; Ginsberg M. H. (2011) Molecular mechanism of inside-out integrin regulation. J. Thromb. Haemostasis 9(Suppl. 1), 20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F.; Hu G.; Taylor D.; Ratnikov B.; Bobkov A. A.; McLean M. A.; Sligar S. G.; Taylor K. A.; Ginsberg M. H. (2010) Recreation of the terminal events in physiological integrin activation. J. Cell Biol. 188, 157–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood D. A.; Fujioka Y.; de Pereda J. M.; Garcia-Alvarez B.; Nakamoto T.; Margolis B.; McGlade C. J.; Liddington R. C.; Ginsberg M. H. (2003) Integrin β cytoplasmic domain interactions with phosphotyrosine-binding domains: A structural prototype for diversity in integrin signaling. Proc. Natl. Acad. Sci. U.S.A. 100, 2272–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goult B. T.; Bouaouina M.; Elliott P. R.; Bate N.; Patel B.; Gingras A. R.; Grossmann J. G.; Roberts G. C.; Calderwood D. A.; Critchley D. R.; Barsukov I. L. (2010) Structure of a double ubiquitin-like domain in the talin head: A role in integrin activation. EMBO J. 29, 1069–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D. T.; Nygren P.; Jo H.; Boesze-Battaglia K.; Bennett J. S.; DeGrado W. F. (2012) Affinity of talin-1 for the β3-integrin cytosolic domain is modulated by its phospholipid bilayer environment. Proc. Natl. Acad. Sci. U.S.A. 109, 793–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goult B. T.; Zacharchenko T.; Bate N.; Tsang R.; Hey F.; Gingras A. R.; Elliott P. R.; Roberts G. C.; Ballestrem C.; Critchley D. R.; Barsukov I. L. (2013) RIAM and vinculin binding to talin are mutually exclusive and regulate adhesion assembly and turnover. J. Biol. Chem. 288, 8238–8249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. S.; Anekal P.; Lim C. J.; Liu C. C.; Ginsberg M. H. (2013) Two modes of integrin activation form a binary molecular switch in adhesion maturation. Mol. Biol. Cell 24, 1354–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goksoy E.; Ma Y. Q.; Wang X.; Kong X.; Perera D.; Plow E. F.; Qin J. (2008) Structural basis for the autoinhibition of talin in regulating integrin activation. Mol. Cell 31, 124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X.; Yang J.; Hirbawi J.; Ye S.; Perera H. D.; Goksoy E.; Dwivedi P.; Plow E. F.; Zhang R.; Qin J. (2012) A novel membrane-dependent on/off switch mechanism of talin FERM domain at sites of cell adhesion. Cell Res. 22, 1533–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goult B. T.; Bate N.; Anthis N. J.; Wegener K. L.; Gingras A. R.; Patel B.; Barsukov I. L.; Campbell I. D.; Roberts G. C.; Critchley D. R. (2009) The structure of an interdomain complex that regulates talin activity. J. Biol. Chem. 284, 15097–15106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott P. R.; Goult B. T.; Kopp P. M.; Bate N.; Grossmann J. G.; Roberts G. C.; Critchley D. R.; Barsukov I. L. (2010) The structure of the talin head reveals a novel extended conformation of the FERM domain. Structure 18, 1289–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.; Rajfur Z.; Yousefi N.; Chen Z.; Jacobson K.; Ginsberg M. H. (2009) Talin phosphorylation by Cdk5 regulates Smurf1-mediated talin head ubiquitylation and cell migration. Nat. Cell Biol. 11, 624–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahme N. N.; Calderwood D. A. (2012) Cell adhesion: A FERM grasp of the tail sorts out integrins. Curr. Biol. 22, R692–R694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakose E.; Schiller H. B.; Fassler R. (2010) The kindlins at a glance. J. Cell Sci. 123, 2353–2356. [DOI] [PubMed] [Google Scholar]
- Larjava H.; Plow E. F.; Wu C. (2008) Kindlins: Essential regulators of integrin signalling and cell-matrix adhesion. EMBO Rep. 9, 1203–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay A.; Rothschild G.; Kim S.; Calderwood D. A.; Raghavan S. (2012) Functional differences between kindlin-1 and kindlin-2 in keratinocytes. J. Cell Sci. 125, 2172–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz C.; Aumailley M.; Schulte C.; Schlotzer-Schrehardt U.; Bruckner-Tuderman L.; Has C. (2006) Kindlin-1 is a phosphoprotein involved in regulation of polarity, proliferation, and motility of epidermal keratinocytes. J. Biol. Chem. 281, 36082–36090. [DOI] [PubMed] [Google Scholar]
- Tu Y.; Wu S.; Shi X.; Chen K.; Wu C. (2003) Migfilin and Mig-2 link focal adhesions to filamin and the actin cytoskeleton and function in cell shape modulation. Cell 113, 37–47. [DOI] [PubMed] [Google Scholar]
- Brahme N. N.; Harburger D. S.; Kemp O’Brien K.; Stewart R.; Raghavan S.; Parsons M.; Calderwood D. A. (2013) Kindlin binds migfilin tandem LIM domains and regulates migfilin focal adhesion localization and recruitment dynamics. J. Biol. Chem. 288, 35604–35616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M.; Ithychanda S. S.; Qin J.; Plow E. F. (2011) Migfilin and filamin as regulators of integrin activation in endothelial cells and neutrophils. PLoS One 6, e26355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ithychanda S. S.; Das M.; Ma Y. Q.; Ding K.; Wang X.; Gupta S.; Wu C.; Plow E. F.; Qin J. (2009) Migfilin, a molecular switch in regulation of integrin activation. J. Biol. Chem. 284, 4713–4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lad Y.; Jiang P.; Ruskamo S.; Harburger D. S.; Ylanne J.; Campbell I. D.; Calderwood D. A. (2008) Structural basis of the migfilin-filamin interaction and competition with integrin β tails. J. Biol. Chem. 283, 35154–35163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannigan G. E.; Leung-Hagesteijn C.; Fitz-Gibbon L.; Coppolino M. G.; Radeva G.; Filmus J.; Bell J. C.; Dedhar S. (1996) Regulation of cell adhesion and anchorage-dependent growth by a new β1-integrin-linked protein kinase. Nature 379, 91–96. [DOI] [PubMed] [Google Scholar]
- Honda S.; Shirotani-Ikejima H.; Tadokoro S.; Maeda Y.; Kinoshita T.; Tomiyama Y.; Miyata T. (2009) Integrin-linked kinase associated with integrin activation. Blood 113, 5304–5313. [DOI] [PubMed] [Google Scholar]
- Tucker K. L.; Sage T.; Stevens J. M.; Jordan P. A.; Jones S.; Barrett N. E.; St-Arnaud R.; Frampton J.; Dedhar S.; Gibbins J. M. (2008) A dual role for integrin-linked kinase in platelets: Regulating integrin function and α-granule secretion. Blood 112, 4523–4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F.; Petrich B. G.; Anekal P.; Lefort C. T.; Kasirer-Friede A.; Shattil S. J.; Ruppert R.; Moser M.; Fassler R.; Ginsberg M. H. (2013) The mechanism of kindlin-mediated activation of integrin αIIbβ3. Curr. Biol. 23, 2288–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltel F.; Mortier E.; Hytonen V. P.; Jacquier M. C.; Zimmermann P.; Vogel V.; Liu W.; Wehrle-Haller B. (2009) New PI(4,5)P2- and membrane proximal integrin-binding motifs in the talin head control β3-integrin clustering. J. Cell Biol. 187, 715–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigelson S. W.; Grabovsky V.; Manevich-Mendelson E.; Pasvolsky R.; Shulman Z.; Shinder V.; Klein E.; Etzioni A.; Aker M.; Alon R. (2011) Kindlin-3 is required for the stabilization of TCR-stimulated LFA-1:ICAM-1 bonds critical for lymphocyte arrest and spreading on dendritic cells. Blood 117, 7042–7052. [DOI] [PubMed] [Google Scholar]
- Wang H. V.; Chang L. W.; Brixius K.; Wickstrom S. A.; Montanez E.; Thievessen I.; Schwander M.; Muller U.; Bloch W.; Mayer U.; Fassler R. (2008) Integrin-linked kinase stabilizes myotendinous junctions and protects muscle from stress-induced damage. J. Cell Biol. 180, 1037–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri K.; Maeda A.; Shimonaka M.; Kinashi T. (2003) RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nat. Immunol. 4, 741–748. [DOI] [PubMed] [Google Scholar]
- Ohmori T.; Kashiwakura Y.; Ishiwata A.; Madoiwa S.; Mimuro J.; Honda S.; Miyata T.; Sakata Y. (2010) Vinculin activates inside-out signaling of integrin αIIbβ3 in Chinese hamster ovary cells. Biochem. Biophys. Res. Commun. 400, 323–328. [DOI] [PubMed] [Google Scholar]
- Bouaouina M.; Jani K.; Long J. Y.; Czerniecki S.; Morse E. M.; Ellis S. J.; Tanentzapf G.; Schock F.; Calderwood D. A. (2012) Zasp regulates integrin activation. J. Cell Sci. 125, 5647–5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvard D.; Pouwels J.; De Franceschi N.; Ivaska J. (2013) Integrin inactivators: Balancing cellular functions in vitro and in vivo. Nat. Rev. Mol. Cell Biol. 14, 430–442. [DOI] [PubMed] [Google Scholar]
- Pouwels J.; Nevo J.; Pellinen T.; Ylanne J.; Ivaska J. (2012) Negative regulators of integrin activity. J. Cell Sci. 125, 3271–3280. [DOI] [PubMed] [Google Scholar]
- Baldassarre M.; Razinia Z.; Burande C. F.; Lamsoul I.; Lutz P. G.; Calderwood D. A. (2009) Filamins regulate cell spreading and initiation of cell migration. PLoS One 4, e7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. S.; Kolahi K. S.; Mofrad M. R. (2009) Phosphorylation facilitates the integrin binding of filamin under force. Biophys. J. 97, 3095–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.; Zou Z.; Sarratt K. L.; Zhou D.; Zhang M.; Sebzda E.; Hammer D. A.; Kahn M. L. (2006) In vivo β1 integrin function requires phosphorylation-independent regulation by cytoplasmic tyrosines. Genes Dev. 20, 927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuchra A.; Meyer H.; Legate K. R.; Brakebusch C.; Fassler R. (2006) Genetic analysis of β1 integrin “activation motifs” in mice. J. Cell Biol. 174, 889–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves A.; Geiger T.; Zanivan S.; DiGiovanni J.; Mann M.; Fassler R. (2011) β1 integrin cytoplasmic tyrosines promote skin tumorigenesis independent of their phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 108, 15213–15218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.; Draheim K. M.; Zhang R.; Calderwood D. A.; Boggon T. J. (2013) Mechanism for KRIT1 release of ICAP1-mediated suppression of integrin activation. Mol. Cell 49, 719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millon-Fremillon A.; Brunner M.; Abed N.; Collomb E.; Ribba A. S.; Block M. R.; Albiges-Rizo C.; Bouvard D. (2013) Calcium and calmodulin-dependent serine/threonine protein kinase type II (CaMKII)-mediated intramolecular opening of integrin cytoplasmic domain-associated protein-1 (ICAP-1α) negatively regulates β1 integrins. J. Biol. Chem. 288, 20248–20260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca-Cusachs P.; del Rio A.; Puklin-Faucher E.; Gauthier N. C.; Biais N.; Sheetz M. P. (2013) Integrin-dependent force transmission to the extracellular matrix by α-actinin triggers adhesion maturation. Proc. Natl. Acad. Sci. U.S.A. 110, E1361–E1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadokoro S.; Nakazawa T.; Kamae T.; Kiyomizu K.; Kashiwagi H.; Honda S.; Kanakura Y.; Tomiyama Y. (2011) A potential role for α-actinin in inside-out αIIbβ3 signaling. Blood 117, 250–258. [DOI] [PubMed] [Google Scholar]
- Bridgewater R. E.; Norman J. C.; Caswell P. T. (2012) Integrin trafficking at a glance. J. Cell Sci. 125, 3695–3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera Y.; Nam J. M.; Sabe H. (2013) Intracellular trafficking of integrins in cancer cells. Pharmacol. Ther. 140, 1–9. [DOI] [PubMed] [Google Scholar]
- Ezratty E. J.; Bertaux C.; Marcantonio E. E.; Gundersen G. G. (2009) Clathrin mediates integrin endocytosis for focal adhesion disassembly in migrating cells. J. Cell Biol. 187, 733–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.; Skoura A.; Park E.; Landskroner-Eiger S.; Jozsef L.; Luciano A.; Murata T.; Pasula S.; Dong Y.; Bouaouina M.; Calderwood D. A.; Ferguson S. M.; De Camilli P.; Sessa W. C. (2014) Dynamin-2 regulation of integrin endocytosis, but not VEGF signaling, is critical for developmental angiogenesis. Development in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao W. T.; Kunz J. (2009) Focal adhesion disassembly requires clathrin-dependent endocytosis of integrins. FEBS Lett. 583, 1337–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T.; Kaibuchi K. (2007) Numb controls integrin endocytosis for directional cell migration with aPKC and PAR-3. Dev. Cell 13, 15–28. [DOI] [PubMed] [Google Scholar]
- Bogdanovic O.; Delfino-Machin M.; Nicolas-Perez M.; Gavilan M. P.; Gago-Rodrigues I.; Fernandez-Minan A.; Lillo C.; Rios R. M.; Wittbrodt J.; Martinez-Morales J. R. (2012) Numb/Numbl-Opo antagonism controls retinal epithelium morphogenesis by regulating integrin endocytosis. Dev. Cell 23, 782–795. [DOI] [PubMed] [Google Scholar]
- Pellinen T.; Tuomi S.; Arjonen A.; Wolf M.; Edgren H.; Meyer H.; Grosse R.; Kitzing T.; Rantala J. K.; Kallioniemi O.; Fassler R.; Kallio M.; Ivaska J. (2008) Integrin trafficking regulated by Rab21 is necessary for cytokinesis. Dev. Cell 15, 371–385. [DOI] [PubMed] [Google Scholar]
- Pellinen T.; Arjonen A.; Vuoriluoto K.; Kallio K.; Fransen J. A.; Ivaska J. (2006) Small GTPase Rab21 regulates cell adhesion and controls endosomal traffic of β1-integrins. J. Cell Biol. 173, 767–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M.; Barry S.; Woods A.; van der Sluijs P.; Norman J. (2001) PDGF-regulated rab4-dependent recycling of αvβ3 integrin from early endosomes is necessary for cell adhesion and spreading. Curr. Biol. 11, 1392–1402. [DOI] [PubMed] [Google Scholar]
- Woods A. J.; White D. P.; Caswell P. T.; Norman J. C. (2004) PKD1/PKCmu promotes αvβ3 integrin recycling and delivery to nascent focal adhesions. EMBO J. 23, 2531–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. P.; Caswell P. T.; Norman J. C. (2007) αvβ3 and α5β1 integrin recycling pathways dictate downstream Rho kinase signaling to regulate persistent cell migration. J. Cell Biol. 177, 515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros R. B.; Dickey D. M.; Chung H.; Quale A. C.; Nagarajan L. R.; Billadeau D. D.; Shimizu Y. (2005) Protein kinase D1 and the β1 integrin cytoplasmic domain control β1 integrin function via regulation of Rap1 activation. Immunity 23, 213–226. [DOI] [PubMed] [Google Scholar]
- Caswell P. T.; Spence H. J.; Parsons M.; White D. P.; Clark K.; Cheng K. W.; Mills G. B.; Humphries M. J.; Messent A. J.; Anderson K. I.; McCaffrey M. W.; Ozanne B. W.; Norman J. C. (2007) Rab25 associates with α5β1 integrin to promote invasive migration in 3D microenvironments. Dev. Cell 13, 496–510. [DOI] [PubMed] [Google Scholar]
- Dozynkiewicz M. A.; Jamieson N. B.; Macpherson I.; Grindlay J.; van den Berghe P. V.; von Thun A.; Morton J. P.; Gourley C.; Timpson P.; Nixon C.; McKay C. J.; Carter R.; Strachan D.; Anderson K.; Sansom O. J.; Caswell P. T.; Norman J. C. (2012) Rab25 and CLIC3 collaborate to promote integrin recycling from late endosomes/lysosomes and drive cancer progression. Dev. Cell 22, 131–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai A.; Veltel S.; Pellinen T.; Padzik A.; Coffey E.; Marjomaki V.; Ivaska J. (2011) Competitive binding of Rab21 and p120RasGAP to integrins regulates receptor traffic and migration. J. Cell Biol. 194, 291–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margadant C.; Kreft M.; de Groot D. J.; Norman J. C.; Sonnenberg A. (2012) Distinct roles of talin and kindlin in regulating integrin α5β1 function and trafficking. Curr. Biol. 22, 1554–1563. [DOI] [PubMed] [Google Scholar]
- Steinberg F.; Heesom K. J.; Bass M. D.; Cullen P. J. (2012) SNX17 protects integrins from degradation by sorting between lysosomal and recycling pathways. J. Cell Biol. 197, 219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottcher R. T.; Stremmel C.; Meves A.; Meyer H.; Widmaier M.; Tseng H. Y.; Fassler R. (2012) Sorting nexin 17 prevents lysosomal degradation of β1 integrins by binding to the β1-integrin tail. Nat. Cell Biol. 14, 584–592. [DOI] [PubMed] [Google Scholar]
- Hoffman B. D.; Grashoff C.; Schwartz M. A. (2011) Dynamic molecular processes mediate cellular mechanotransduction. Nature 475, 316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legate K. R.; Wickstrom S. A.; Fassler R. (2009) Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 23, 397–418. [DOI] [PubMed] [Google Scholar]
- Schiller H. B.; Fassler R. (2013) Mechanosensitivity and compositional dynamics of cell-matrix adhesions. EMBO Rep. 14, 509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras A. R.; Bate N.; Goult B. T.; Hazelwood L.; Canestrelli I.; Grossmann J. G.; Liu H.; Putz N. S.; Roberts G. C.; Volkmann N.; Hanein D.; Barsukov I. L.; Critchley D. R. (2008) The structure of the C-terminal actin-binding domain of talin. EMBO J. 27, 458–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio A.; Perez-Jimenez R.; Liu R.; Roca-Cusachs P.; Fernandez J. M.; Sheetz M. P. (2009) Stretching single talin rod molecules activates vinculin binding. Science 323, 638–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margadant F.; Chew L. L.; Hu X.; Yu H.; Bate N.; Zhang X.; Sheetz M. (2011) Mechanotransduction in vivo by repeated talin stretch-relaxation events depends upon vinculin. PLoS Biol. 9, e1001223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannone G.; Jiang G.; Sutton D. H.; Critchley D. R.; Sheetz M. P. (2003) Talin1 is critical for force-dependent reinforcement of initial integrin-cytoskeleton bonds but not tyrosine kinase activation. J. Cell Biol. 163, 409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carisey A.; Tsang R.; Greiner A. M.; Nijenhuis N.; Heath N.; Nazgiewicz A.; Kemkemer R.; Derby B.; Spatz J.; Ballestrem C. (2013) Vinculin regulates the recruitment and release of core focal adhesion proteins in a force-dependent manner. Curr. Biol. 23, 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razinia Z.; Makela T.; Ylanne J.; Calderwood D. A. (2012) Filamins in mechanosensing and signaling. Annu. Rev. Biophys. 41, 227–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lad Y.; Kiema T.; Jiang P.; Pentikainen O. T.; Coles C. H.; Campbell I. D.; Calderwood D. A.; Ylanne J. (2007) Structure of three tandem filamin domains reveals auto-inhibition of ligand binding. EMBO J. 26, 3993–4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognoni L.; Stigler J.; Pelz B.; Ylanne J.; Rief M. (2012) Dynamic force sensing of filamin revealed in single-molecule experiments. Proc. Natl. Acad. Sci. U.S.A. 109, 19679–19684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlicher A. J.; Nakamura F.; Hartwig J. H.; Weitz D. A.; Stossel T. P. (2011) Mechanical strain in actin networks regulates FilGAP and integrin binding to filamin A. Nature 478, 260–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard M.; Zumbrunn J.; Jaquemar D.; Kuhn M.; Walter U.; Trueb B. (1999) An α-actinin binding site of zyxin is essential for subcellular zyxin localization and α-actinin recruitment. J. Biol. Chem. 274, 13410–13418. [DOI] [PubMed] [Google Scholar]
- Hoffman L. M.; Jensen C. C.; Chaturvedi A.; Yoshigi M.; Beckerle M. C. (2012) Stretch-induced actin remodeling requires targeting of zyxin to stress fibers and recruitment of actin regulators. Mol. Biol. Cell 23, 1846–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshigi M.; Hoffman L. M.; Jensen C. C.; Yost H. J.; Beckerle M. C. (2005) Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J. Cell Biol. 171, 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo S. H. (2004) Tensin. Int. J. Biochem. Cell Biol. 36, 31–34. [DOI] [PubMed] [Google Scholar]
- Mouneimne G.; Brugge J. S. (2007) Tensins: A new switch in cell migration. Dev. Cell 13, 317–319. [DOI] [PubMed] [Google Scholar]
- Grashoff C.; Hoffman B. D.; Brenner M. D.; Zhou R.; Parsons M.; Yang M. T.; McLean M. A.; Sligar S. G.; Chen C. S.; Ha T.; Schwartz M. A. (2010) Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466, 263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K.; Ji L.; Applegate K. T.; Danuser G.; Waterman-Storer C. M. (2007) Differential transmission of actin motion within focal adhesions. Science 315, 111–115. [DOI] [PubMed] [Google Scholar]
- Dumbauld D. W.; Lee T. T.; Singh A.; Scrimgeour J.; Gersbach C. A.; Zamir E. A.; Fu J.; Chen C. S.; Curtis J. E.; Craig S. W.; Garcia A. J. (2013) How vinculin regulates force transmission. Proc. Natl. Acad. Sci. U.S.A. 110, 9788–9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shams H.; Golji J.; Mofrad M. R. (2012) A molecular trajectory of α-actinin activation. Biophys. J. 103, 2050–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghatak S.; Morgner J.; Wickstrom S. A. (2013) ILK: A pseudokinase with a unique function in the integrin-actin linkage. Biochem. Soc. Trans. 41, 995–1001. [DOI] [PubMed] [Google Scholar]
- Qin J.; Wu C. (2012) ILK: A pseudokinase in the center stage of cell-matrix adhesion and signaling. Curr. Opin. Cell Biol. 24, 607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grashoff C.; Aszodi A.; Sakai T.; Hunziker E. B.; Fassler R. (2003) Integrin-linked kinase regulates chondrocyte shape and proliferation. EMBO Rep. 4, 432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar N.; Streuli C. H. (2013) An integrin-ILK-microtubule network orients cell polarity and lumen formation in glandular epithelium. Nat. Cell Biol. 15, 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstrom S. A.; Lange A.; Hess M. W.; Polleux J.; Spatz J. P.; Kruger M.; Pfaller K.; Lambacher A.; Bloch W.; Mann M.; Huber L. A.; Fassler R. (2010) Integrin-linked kinase controls microtubule dynamics required for plasma membrane targeting of caveolae. Dev. Cell 19, 574–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak R. C.; Chang K. H.; Vaitinadin N. S.; Cancelas J. A. (2013) Rho GTPases control specific cytoskeleton-dependent functions of hematopoietic stem cells. Immunol. Rev. 256, 255–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. (2012) Rho family GTPases. Biochem. Soc. Trans. 40, 1378–1382. [DOI] [PubMed] [Google Scholar]
- Nakamura F. (2013) FilGAP and its close relatives: A mediator of Rho-Rac antagonism that regulates cell morphology and migration. Biochem. J. 453, 17–25. [DOI] [PubMed] [Google Scholar]
- Jacquemet G.; Morgan M. R.; Byron A.; Humphries J. D.; Choi C. K.; Chen C. S.; Caswell P. T.; Humphries M. J. (2013) Rac1 is deactivated at integrin activation sites through an IQGAP1-filamin-A-RacGAP1 pathway. J. Cell Sci. 126, 4121–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Has C.; Herz C.; Zimina E.; Qu H. Y.; He Y.; Zhang Z. G.; Wen T. T.; Gache Y.; Aumailley M.; Bruckner-Tuderman L. (2009) Kindlin-1 is required for RhoGTPase-mediated lamellipodia formation in keratinocytes. Am. J. Pathol. 175, 1442–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung G. Y.; Park Y. J.; Han J. S. (2011) Mediation of Rac1 activation by kindlin-2: An essential function in osteoblast adhesion, spreading, and proliferation. J. Cell. Biochem. 112, 2541–2548. [DOI] [PubMed] [Google Scholar]
- Xue Z. H.; Feng C.; Liu W. L.; Tan S. M. (2013) A role of kindlin-3 in integrin αMβ2 outside-in signaling and the Syk-Vav1-Rac1/Cdc42 signaling axis. PLoS One 8, e56911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiya N.; Kiosses W. B.; Han J.; Ginsberg M. H. (2005) An α4 integrin-paxillin-Arf-GAP complex restricts Rac activation to the leading edge of migrating cells. Nat. Cell Biol. 7, 343–352. [DOI] [PubMed] [Google Scholar]
- Goldfinger L. E.; Han J.; Kiosses W. B.; Howe A. K.; Ginsberg M. H. (2003) Spatial restriction of α4 integrin phosphorylation regulates lamellipodial stability and α4β1-dependent cell migration. J. Cell Biol. 162, 731–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S. K.; Schlaepfer D. D. (2006) Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 18, 516–523. [DOI] [PubMed] [Google Scholar]
- Schaller M. D.; Otey C. A.; Hildebrand J. D.; Parsons J. T. (1995) Focal adhesion kinase and paxillin bind to peptides mimicking β integrin cytoplasmic domains. J. Cell Biol. 130, 1181–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson C.; Lim S. T.; Uryu S.; Chen X. L.; Calderwood D. A.; Schlaepfer D. D. (2012) FAK promotes recruitment of talin to nascent adhesions to control cell motility. J. Cell Biol. 196, 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huveneers S.; Danen E. H. (2009) Adhesion signaling: Crosstalk between integrins, Src and Rho. J. Cell Sci. 122, 1059–1069. [DOI] [PubMed] [Google Scholar]
- Arias-Salgado E. G.; Lizano S.; Sarkar S.; Brugge J. S.; Ginsberg M. H.; Shattil S. J. (2003) Src kinase activation by direct interaction with the integrin β cytoplasmic domain. Proc. Natl. Acad. Sci. U.S.A. 100, 13298–13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren M. S.; Bradley W. D.; Gourley S. L.; Lin Y. C.; Simpson M. A.; Reichardt L. F.; Greer C. A.; Taylor J. R.; Koleske A. J. (2012) Integrin β1 signals through Arg to regulate postnatal dendritic arborization, synapse density, and behavior. J. Neurosci. 32, 2824–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodside D. G.; Obergfell A.; Talapatra A.; Calderwood D. A.; Shattil S. J.; Ginsberg M. H. (2002) The N-terminal SH2 domains of Syk and ZAP-70 mediate phosphotyrosine-independent binding to integrin β cytoplasmic domains. J. Biol. Chem. 277, 39401–39408. [DOI] [PubMed] [Google Scholar]
- Li Z.; Lock J. G.; Olofsson H.; Kowalewski J. M.; Teller S.; Liu Y.; Zhang H.; Stromblad S. (2010) Integrin-mediated cell attachment induces a PAK4-dependent feedback loop regulating cell adhesion through modified integrin αvβ5 clustering and turnover. Mol. Biol. Cell 21, 3317–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gushiken F. C.; Patel V.; Liu Y.; Pradhan S.; Bergeron A. L.; Peng Y.; Vijayan K. V. (2008) Protein phosphatase 2A negatively regulates integrin αIIbβ3 signaling. J. Biol. Chem. 283, 12862–12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivaska J.; Heino J. (2011) Cooperation between integrins and growth factor receptors in signaling and endocytosis. Annu. Rev. Cell Dev. Biol. 27, 291–320. [DOI] [PubMed] [Google Scholar]
- Shen B.; Delaney M. K.; Du X. (2012) Inside-out, outside-in, and inside-outside-in: G protein signaling in integrin-mediated cell adhesion, spreading, and retraction. Curr. Opin. Cell Biol. 24, 600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M.; Subbayya Ithychanda S.; Qin J.; Plow E. F. (2014) Mechanisms of talin-dependent integrin signaling and crosstalk. Biochim. Biophys. Acta 1838, 579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]