Abstract
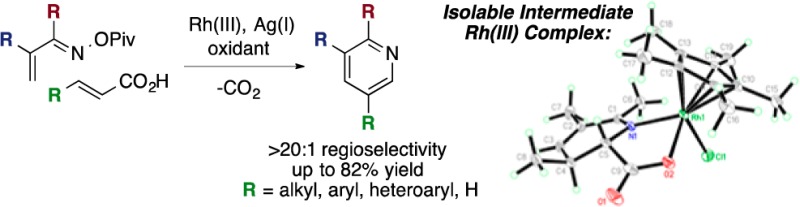
α,β-Unsaturated carboxylic acids undergo Rh(III)-catalyzed decarboxylative coupling with α,β-unsaturated O-pivaloyl oximes to provide substituted pyridines in good yield. The carboxylic acid, which is removed by decarboxylation, serves as a traceless activating group, giving 5-substituted pyridines with very high levels of regioselectivity. Mechanistic studies rule out a picolinic acid intermediate, and an isolable rhodium complex sheds further light on the reaction mechanism.
Substituted pyridines are among the most prevalent scaffolds encountered in medicinal chemistry.1 For this reason, a wealth of research has focused on the construction of these heterocycles.2 Nonetheless, access to desired substitution patterns often remains a challenge with traditional multicomponent approaches to pyridine synthesis.3 Classic condensation protocols rely on aldol and Michael-type steps, and the position and identity of product substituents are dictated by the activating groups required for reactivity.4 Intermolecular [2 + 2 + 2] cycloadditions of nitriles and alkynes often afford regioisomeric mixtures,5 an obstacle that is typically circumvented by tethering strategies.6 Novel approaches to diversely substituted pyridines are highly desirable, and several impressive reports highlight the recent advances in this field.7
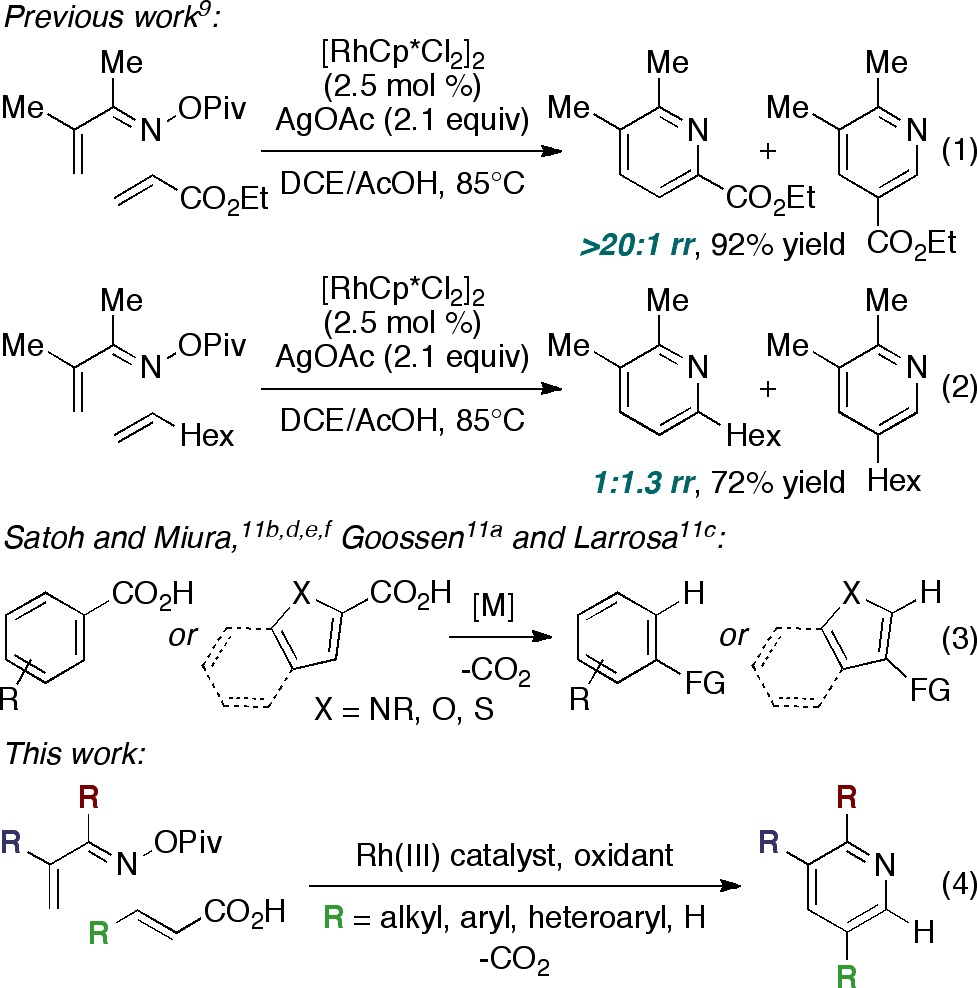
Our efforts in the area of pyridine synthesis have exploited rhodium-catalyzed coupling of α,β-unsaturated oxime esters8 and alkenes to access 6-substituted pyridines.9 During the course of this study, we discovered that selectivity depends crucially on the nature of the alkene substrate. Namely, activated alkenes react with exquisite regioselectivity (eq 1) while unactivated alkenes incorporate to give mixtures of regioisomeric products (eq 2). This limitation prompted us to investigate whether the carboxylic acid moiety of acrylic acid derivatives could serve as a ‘traceless’ activating group.10 Indeed, this strategy has been utilized by a number of research groups in the context of C–H functionalization (eq 3).11,12 In these examples, carboxylate ligation imparts selectivity to the C–H activation step, and the acid residue is ultimately cleaved via in situ decarboxylation. In a similar manner, we envisioned that the carboxylic acid moiety would direct regioselective alkene incorporation and then be removed by decarboxylation (eq 4).13 This work would complement our previously reported rhodium-catalyzed pyridine synthesis, since 5-substituted pyridines14 could be prepared with high selectivity without the constraint of activating group incorporation in the products.
 |
1 |
We evaluated the feasibility of the proposed approach in the reaction of α,β-unsaturated O-pivaloyl oxime 1a and crotonic acid (2a) (Table 1). An initial screen with catalytic [RhCp*Cl2]2 and AgOAc as an oxidant identified hexafluoroisopropanol (HFIP) as the optimal solvent, furnishing the desired 3aa in 60% yield (entry 1). Importantly, no 6-substituted regioisomer (not shown) was observed. In an effort to avoid superstoichiometric silver reagents, we conducted a screen of other oxidants, which revealed potassium persulfate (K2S2O8) to be modestly effective (entry 2). However, the desired pyridine 3aa was formed in a 1:1 mixture with picolinic acid 4aa, the nondecarboxylated product of the desired coupling. Addition of a catalytic amount of silver p-toluenesulfonate (AgOTs) afforded a 10:1 mixture of products (entry 3), and increasing the amount of AgOTs gave full conversion to 3aa as a single product (entry 4). Changing to [RhCpCF3Cl2]2 (CpCF3 = tetramethyl(trifluoromethyl)cyclopentadienyl) was necessary for increasing reactivity with aryl acrylic acid substrates such as 2k (Table 1, entries 5 and 6, and Supporting Information (SI)), and we thus chose this catalyst for further development.
Table 1. Reaction Optimizationa.
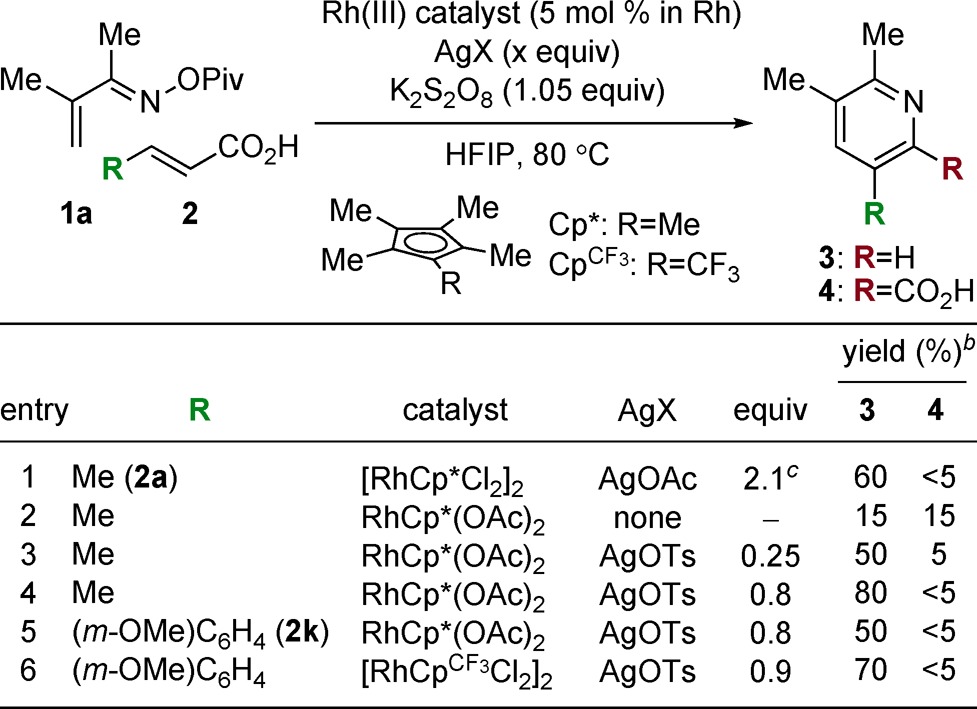
1.2 equiv of 2, 0.3 M.
Determined by 1H NMR.
Without K2S2O8.
We examined the scope of the reaction with these optimized conditions (Chart 1). Oxime esters 1 are easily synthesized from the corresponding enones with hydroxylamine hydrochloride and pivaloyl chloride. Knoevenagel condensation of the appropriate aldehyde and malonic acid conveniently accesses acrylic acid derivatives 2.15 The reaction of various α,β-unsaturated O-pivaloyl oximes (1) and nonenoic acid (2b) affords the 5-substituted pyridines in good yields.16 Both primary and secondary alkyl acrylic acids undergo the desired coupling efficiently; notably, alkyl chloride 2d is tolerated under the Ag(I) conditions. The acrylic acid may also bear aryl or heteroaryl substitution (2h–2m). While the alkene contains two possible activating groups in these cases, 5-substituted products are formed with complete regioselectivity. Finally, acrylic acid (2n) also undergoes decarboxylative coupling to furnish 2,3-disubstituted 3bn in good yield.
Chart 1. Reaction Scopea.
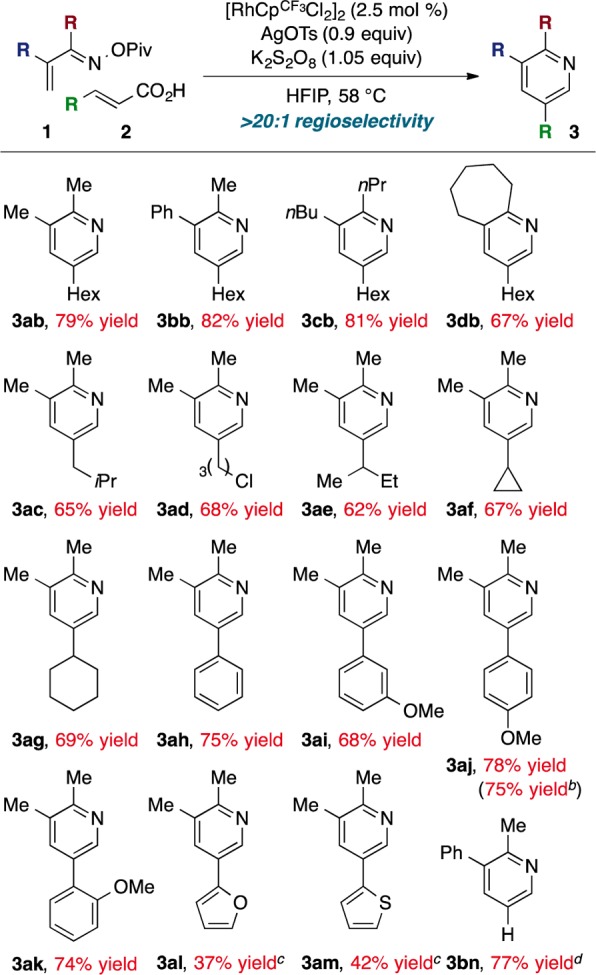
a Conditions: 1.2 equiv of 2, 0.3 M. b 1 mmol scale. c 0.5 equiv of AgOTs. d In TFE at 74 °C.
Several key experiments contributed to our current understanding of the reaction mechanism. We initially considered a pathway involving decarboxylation of a picolinic acid intermediate 4 (eq 5, Scheme 1a). Ag(I)-catalyzed decarboxylation is a well-known process17 and has been demonstrated with aryl18 and heteroaryl19 carboxylic acids at elevated temperatures. To test this hypothesis, we synthesized possible intermediate 4ca and subjected it to the reaction conditions (eq 6). In the event, we observed no formation of decarboxylated 3ca, ruling out the intermediacy of picolinic acid 4ca.
Scheme 1. Mechanistic Studies.
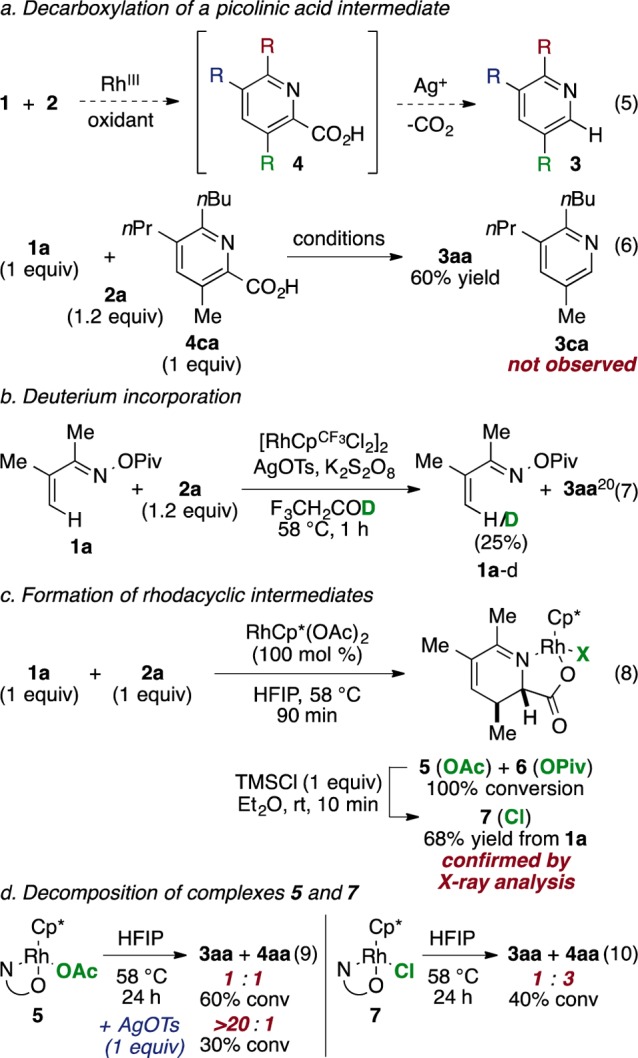
We gained further mechanistic insight from isotope and stoichiometric experiments (Scheme 1b–d). Reaction of 1a and 2a in trifluoroethanol-d1 performed to ∼30% conversion results in partial deuteration at the β-position of the remaining 1a, an observation consistent with a reversible C–H activation step (eq 7). After a mixture of 1a, 2a and a stoichiometric amount of RhCp*(OAc)2 was heated for 90 min, the major products observed are rhodium carboxylate complexes 5 and 6 (eq 8). Importantly, in 5 and 6, C–N bond formation and N–O bond cleavage have taken place but decarboxylation has not yet occurred, clarifying the timing of the decarboxylation step. Treatment of 5 and 6 with TMSCl affords chloride complex 7, an orange solid that is isolated by filtration. The structure given in Scheme 1c was confirmed by single crystal X-ray analysis of 7 (Figure 1). Acetate complex 5 is converted to a 1:1 mixture of 3aa and 4aa upon heating (eq 9), implicating 5 as a common intermediate of both observed products. In agreement with earlier observations, addition of AgOTs to the reaction leads to exclusive formation of 3aa (eq 9). Interestingly, heating chloride complex 7 results in a 1:3 mixture of 3aa and 4aa (eq 10), suggesting that X ligand identity at this stage influences the divergence of reaction pathways to the two products.
Figure 1.
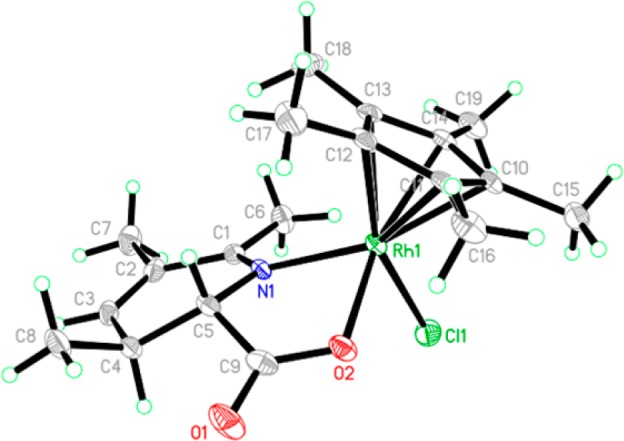
X-ray crystal structure of 7 with thermal ellipsoids drawn at the 50% probability level.
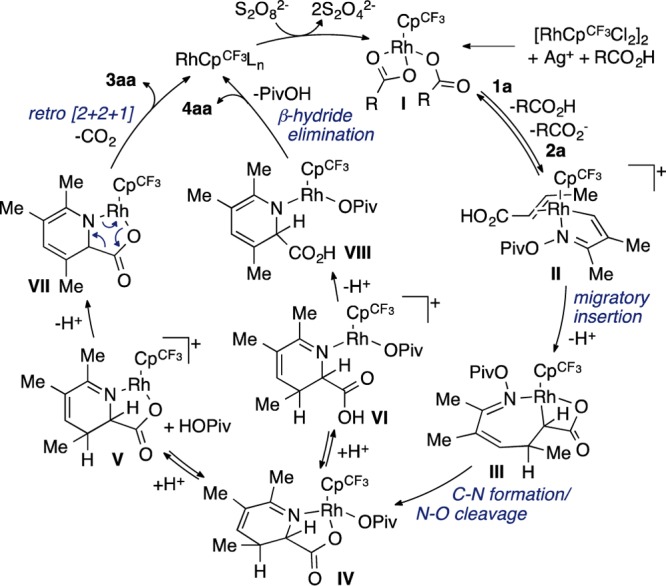
Based on the described mechanistic observations, we propose that pyridine and picolinic acid formation proceeds by the mechanism depicted in Scheme 2. After generation of active catalyst I, reversible C–H activation at the β-position of 1a and ligand exchange provide cationic complex II. Migratory insertion and deprotonation give rhodacycle III. C–N bond formation and N–O bond cleavage afford intermediate IV that is analogous to observable complexes 5 and 6. From IV, proton assisted ionization may occur to liberate either of the two carboxylate ligands, giving cationic complex V or VI.21 Deprotonation of V leads to metallacycle VII, which can undergo a retro [2 + 2 + 1] cycloaddition to extrude CO2 and provide pyridine 3aa and a Rh(I) species.22 Alternatively, deprotonation of VI forms intermediate VIII from which β-hydride elimination gives picolinic acid 4aa. Reductive elimination of the resultant Rh(III) hydride gives a Rh(I) complex that is oxidized to regenerate the active catalyst.
Scheme 2. Proposed Mechanism.
In conclusion, we have developed a rhodium-catalyzed decarboxylative coupling of α,β-unsaturated O-pivaloyl oximes and acrylic acid derivatives. This method takes advantage of a carboxylic acid as a traceless activating group to produce 5-substituted pyridines with complete regioselectivity. Mechanistic studies suggest that decarboxylation does not occur via a picolinic acid intermediate. We identified significant rhodacyclic intermediates that clarify the order of C–N bond formation and decarboxylation.
Acknowledgments
We thank NIGMS (GM80442) for support and Johnson Matthey for a generous loan of rhodium salts.
Supporting Information Available
Experimental procedures, compound characterization, additional experiments, and crystallographic data. This information is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- a Roughley S. D.; Jordan A. M. J. Med. Chem. 2011, 54, 3451. [DOI] [PubMed] [Google Scholar]; b Carey J. S.; Laffan D.; Thomson C.; Williams M. T. Org. Biomol. Chem. 2006, 4, 2337. [DOI] [PubMed] [Google Scholar]
- a Hill M. D. Chem.—Eur. J. 2010, 16, 12052. [DOI] [PubMed] [Google Scholar]; b Henry G. D. Tetrahedron 2004, 60, 6043. [Google Scholar]; c Joule J. A.; Mills K.. Heterocyclic Chemistry, 4th ed.; Blackwell Science: Malden, MA, 2000; pp 63–120. [Google Scholar]
- For reviews of pyridine synthesis by direct functionalization of the heterocycle, see:; a Bull J. A.; Mousseau J. J.; Pelletier G.; Charette A. B. Chem. Rev. 2012, 112, 2642. [DOI] [PubMed] [Google Scholar]; b Nakao Y. Synthesis 2011, 3209. [Google Scholar]; c Schlosser M.; Mongin F. Chem. Soc. Rev. 2007, 36, 1161. [DOI] [PubMed] [Google Scholar]
- a Allais C.; Liéby-Muller F.; Rodriguez J.; Constantieux T. Eur. J. Org. Chem. 2013, 4131. [Google Scholar]; b Allais C.; Liéby-Muller F.; Constantieux T.; Rodriguez J. Adv. Synth. Catal. 2012, 354, 2537. [Google Scholar]; For reviews of pyridine syntheses by condensation methods, see:; c Reference (2c).; d Sausins A.; Duburs G. Heterocycles 1988, 27, 269. [Google Scholar]; e Bagley M. C.; Glover C.; Merritt E. A. Synlett 2007, 2459. [Google Scholar]
- a Knoch F.; Kremer F.; Schmidt U.; Zenneck U.; Le Floch P.; Mathey F. Organometallics 1996, 15, 2713. [Google Scholar]; b Diversi P.; Ermini L.; Ingrosso G.; Lucherini A. J. Organomet. Chem. 1993, 447, 291. [Google Scholar]
- For a review of pyridine syntheses by [2 + 2 + 2] cycloaddition, see:; a Varela J. A.; Saá C. Chem. Rev. 2003, 103, 3787. [DOI] [PubMed] [Google Scholar]; For selected examples, see:; b Wang C.; Li X.; Wu F.; Wan B. Angew. Chem., Int. Ed. 2011, 50, 7162. [DOI] [PubMed] [Google Scholar]; c Chang H.-T.; Jeganmohan M.; Cheng C.-H. Org. Lett. 2007, 9, 505. [DOI] [PubMed] [Google Scholar]; d McCormick M. M.; Duong H. A.; Zuo G.; Louie J. J. Am. Chem. Soc. 2005, 127, 5030. [DOI] [PubMed] [Google Scholar]; For a transition metal-free protocol, see:; e Kral K.; Hapke M. Angew. Chem., Int. Ed. 2011, 50, 2434. [DOI] [PubMed] [Google Scholar]
- For leading examples, see:; a Stark D. G.; Morrill L. C.; Yeh P.-P.; Slawin A. M. Z.; O’Riordan T. J. C.; Smith A. D. Angew. Chem., Int. Ed. 2013, 52, 11642. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lei C.-H.; Wang D.-X.; Zhao L.; Zhu J.; Wang M.-X. J. Am. Chem. Soc. 2013, 135, 4708. [DOI] [PubMed] [Google Scholar]; c Wei Y.; Yoshikai N. J. Am. Chem. Soc. 2013, 135, 3756. [DOI] [PubMed] [Google Scholar]; d Michlik S.; Kempe R. Angew. Chem., Int. Ed. 2013, 52, 6326. [DOI] [PubMed] [Google Scholar]; e Loy N. S. Y.; Singh A.; Xu X.; Park C.-M. Angew. Chem., Int. Ed. 2013, 52, 2212. [DOI] [PubMed] [Google Scholar]; f He Z.; Dobrovolsky D.; Trinchera P.; Yudin A. K. Org. Lett. 2013, 15, 334. [DOI] [PubMed] [Google Scholar]; g Yamamoto S.-I.; Okamoto K.; Murakoso M.; Kuninobu Y.; Takai K. Org. Lett. 2012, 14, 3182. [DOI] [PubMed] [Google Scholar]; h Gati W.; Rammah M. M.; Rammah M. B.; Couty F.; Evano G. J. Am. Chem. Soc. 2012, 134, 9078. [DOI] [PubMed] [Google Scholar]; i Chen M. Z.; Micalizio G. C. J. Am. Chem. Soc. 2012, 134, 1352. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Ohashi M.; Takeda I.; Ikawa M.; Ogoshi S. J. Am. Chem. Soc. 2011, 133, 18018. [DOI] [PubMed] [Google Scholar]; k Wang Y.-F.; Toh K. K.; Ng E. P. J.; Chiba S. J. Am. Chem. Soc. 2011, 133, 6411. [DOI] [PubMed] [Google Scholar]; l Wang Y.-F.; Chiba S. J. Am. Chem. Soc. 2009, 131, 12570. [DOI] [PubMed] [Google Scholar]; m Manning J. R.; Davies H. M. L. J. Am. Chem. Soc. 2008, 130, 8602. [DOI] [PMC free article] [PubMed] [Google Scholar]; n Liu S.; Liebeskind L. S. J. Am. Chem. Soc. 2008, 130, 6918. [DOI] [PMC free article] [PubMed] [Google Scholar]; o Movassaghi M.; Hill M. D.; Ahmad O. K. J. Am. Chem. Soc. 2007, 129, 10096. [DOI] [PubMed] [Google Scholar]; p Movassaghi M.; Hill M. D. J. Am. Chem. Soc. 2006, 128, 4592. [DOI] [PubMed] [Google Scholar]
- α,β-Unsaturated oximes have been coupled to alkynes by Rh(III) catalysis to form polysubstituted pyridines. See:; a Martin R. M.; Bergman R. G.; Ellman J. A. J. Org. Chem. 2012, 77, 2501. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Hyster T. K.; Rovis T. Chem. Commun. 2011, 47, 11846. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Too P. C.; Noji T.; Lim Y. J.; Li X.; Chiba S. Synlett 2011, 2789. [Google Scholar]; d Parthasarathy K.; Cheng C.-H. Synthesis 2009, 1400. [Google Scholar]; e Parthasarathy K.; Jeganmohan M.; Cheng C.-H. Org. Lett. 2008, 10, 325. [DOI] [PubMed] [Google Scholar]; For the analogous reaction of α,β-unsaturated imines, see:; f Colby D. A.; Bergman R. G.; Ellman J. A. J. Am. Chem. Soc. 2008, 130, 3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely J. M.; Rovis T. J. Am. Chem. Soc. 2013, 135, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For a review of the use of removable directing groups, see:Rousseau G.; Breit B. Angew. Chem., Int. Ed. 2011, 50, 2450. [DOI] [PubMed] [Google Scholar]
- a Bhadra S.; Dzik W. I.; Goossen L. J. Angew. Chem., Int. Ed. 2013, 52, 2959. [DOI] [PubMed] [Google Scholar]; b Morimoto K.; Itoh M.; Hirano K.; Satoh T.; Shibata Y.; Tanaka K.; Miura M. Angew. Chem., Int. Ed. 2012, 51, 5359. [DOI] [PubMed] [Google Scholar]; c Cornella J.; Righi M.; Larrosa I. Angew. Chem., Int. Ed. 2011, 50, 9429. [DOI] [PubMed] [Google Scholar]; d Mochida S.; Hirano K.; Satoh T.; Miura M. J. Org. Chem. 2011, 76, 3024. [DOI] [PubMed] [Google Scholar]; e Mochida S.; Hirano K.; Satoh T.; Miura M. Org. Lett. 2010, 12, 5776. [DOI] [PubMed] [Google Scholar]; f Maehara A.; Tsurugi H.; Satoh T.; Miura M. Org. Lett. 2008, 10, 1159. [DOI] [PubMed] [Google Scholar]
- Transition-metal-catalyzed decarboxylative coupling is also an established method for ipso substitution. For selected recent examples, see:; a Haley C. K.; Gilmore C. D.; Stoltz B. M. Tetrahedron 2013, 69, 5732. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Bhadra S.; Dzik W. I.; Goossen L. J. J. Am. Chem. Soc. 2012, 134, 9938. [DOI] [PubMed] [Google Scholar]; c Goossen L. J.; Lange P. P.; Rodríguez N.; Linder C. Chem.—Eur. J. 2010, 16, 3906. [DOI] [PubMed] [Google Scholar]; d Goossen L. J.; Rodríguez N.; Linder C. J. Am. Chem. Soc. 2008, 130, 15248. [DOI] [PubMed] [Google Scholar]; e Goossen L. J.; Rodríguez N.; Melzer B.; Linder C.; Deng G.; Levy L. M. J. Am. Chem. Soc. 2007, 129, 4824. [DOI] [PubMed] [Google Scholar]; f Becht J.-M.; Catala C.; Le Drian C.; Wagner A. Org. Lett. 2007, 9, 1781. [DOI] [PubMed] [Google Scholar]; g Forgione P.; Brochu M.-C.; St-Onge M.; Thesen K. H.; Bailey M. D.; Bilodeau F. J. Am. Chem. Soc. 2006, 128, 11350. [DOI] [PubMed] [Google Scholar]; h Goossen L. J.; Deng G.; Levy L. M. Science 2006, 313, 662. [DOI] [PubMed] [Google Scholar]; For a review of decarboxylative coupling, see:; i Rodríguez N.; Goossen L. J. Chem. Soc. Rev. 2011, 40, 5030. [DOI] [PubMed] [Google Scholar]
- a Yang Y.; Yao J.; Zhang Y. Org. Lett. 2013, 15, 3206. [DOI] [PubMed] [Google Scholar]; b Yang Y.; Xie C.; Xie Y.; Zhang Y. Org. Lett. 2012, 14, 957. [DOI] [PubMed] [Google Scholar]; c Šmejkal T.; Breit B. Angew. Chem., Int. Ed. 2008, 47, 3946. [DOI] [PubMed] [Google Scholar]
- For recent approaches to 5-substituted pyridines by derivatization of a pyridine core, see:; a Ye M.; Gao G.-L.; Edmunds A. J. F.; Worthington P. A.; Morris J. A.; Yu J.-Q. J. Am. Chem. Soc. 2011, 133, 19090. [DOI] [PubMed] [Google Scholar]; b Ye M.; Gao G.-L.; Yu J.-Q. J. Am. Chem. Soc. 2011, 133, 6964. [DOI] [PubMed] [Google Scholar]; c Li B.-J.; Shi Z.-J. Chem. Sci. 2011, 2, 488. [Google Scholar]
- a Reference (13c).; b Szymanski W.; Wu B.; Weiner B.; de Wildeman S.; Feringa B. L.; Janssen D. B. J. Org. Chem. 2009, 74, 9152. [DOI] [PubMed] [Google Scholar]
- 1-Cyclohexene-1-carboxaldehyde O-pivaloyl oxime eliminates PivOH under the reaction conditions, giving the corresponding nitrile (50% yield). The reaction of 1-penten-3-one O-pivaloyl oxime results in low conversion to the desired product (10% yield).
- For selected examples, see:; a Liu X.; Wang Z.; Cheng X.; Li C. J. Am. Chem. Soc. 2012, 134, 14330. [DOI] [PubMed] [Google Scholar]; b Citterio A.; Minisci F.; Franchi F. J. Org. Chem. 1980, 45, 4752. [Google Scholar]; c Anderson J. M.; Kochi J. K. J. Am. Chem. Soc. 1970, 92, 1651. [Google Scholar]
- a Goossen L. J.; Linder C.; Rodríguez N.; Lange P. P.; Fromm A. Chem. Commun. 2009, 7173. [DOI] [PubMed] [Google Scholar]; b Cornella J.; Sanchez C.; Banawa D.; Larrosa I. Chem. Commun. 2009, 7176. [DOI] [PubMed] [Google Scholar]; c References (11a)–11e.
- a Lu P.; Sanchez C.; Cornella J.; Larrosa I. Org. Lett. 2009, 11, 5710. [DOI] [PubMed] [Google Scholar]; b References 11d,11f.
- Partial deuteration of the 2-Me and 6-H of 3aa is also observed; see SI.
- The exact role played by Ag+ in increasing selectivity for 3 is not fully understood at this time.
- A stepwise process of CO2 extrusion and reductive elimination is another possibility.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


