Abstract
Ca2+/Calmodulin-dependent protein kinase II (CaMKII) has been shown to play a major role in establishing memories through complex molecular interactions including phosphorylation of multiple synaptic targets. However, it is still controversial whether CaMKII itself serves as a molecular memory because of a lack of direct evidence. Here, we show that a single holoenzyme of CaMKII per se serves as an erasable molecular memory switch. We reconstituted Ca2+/Calmodulin-dependent CaMKII autophosphorylation in the presence of protein phosphatase 1 in vitro, and found that CaMKII phosphorylation shows a switch-like response with history dependence (hysteresis) only in the presence of an N-methyl-D-aspartate receptor-derived peptide. This hysteresis is Ca2+ and protein phosphatase 1 concentration-dependent, indicating that the CaMKII memory switch is not simply caused by an N-methyl-D-aspartate receptor-derived peptide lock of CaMKII in an active conformation. Mutation of a phosphorylation site of the peptide shifted the Ca2+ range of hysteresis. These functions may be crucial for induction and maintenance of long-term synaptic plasticity at hippocampal synapses.
Introduction
In the brain, Ca2+/Calmodulin (Ca2+/CaM)-dependent protein kinase II (CaMKII) has been shown to play a critical role in learning and memory, including synaptic long-term potentiation (LTP) (1–3). CaMKII has been theoretically hypothesized to be a memory switch, which transforms a transient signal into persistent LTP. CaMKII forms a large symmetrical holoenzyme in which 12 kinase domains are tightly packed around a central ring-shaped scaffold (4,5), and the cooperative autophosphorylation at threonine 286 (T286) by neighboring subunits form two stable steady states: a dephosphorylated OFF state and a phosphorylated ON state (6,7). Upon LTP induction, memories are established via a transient Ca2+ signal that switches CaMKII from the dephosphorylated OFF state to the phosphorylated ON state.
To realize such a memory switch at synapses, the interaction between CaMKII and the N-methyl-D-aspartate receptor subunit GluN2B has been shown to be critically important. The interaction between CaMKII and GluN2B is required for persistent activation and translocation of CaMKII (8,9), persistent T286 phosphorylation (10), and LTP maintenance (11,12). Thus, the interaction between CaMKII and GluN2B is a key factor in memory formation. However, the interaction between CaMKII and GluN2B as a memory switch has not been well described.
Using an in vitro reconstitution system (13), we report that CaMKII operates as a memory switch only in the presence of a GluN2B-derived peptide (residues 1289−1310; N2Bs). Hysteresis, which is the dependence of a state on both its current and past environment (14), shows that the Ca2+ range of the memory switch is regulated by phosphorylation of N2Bs at serine 1303 (S1303). Our results show that this CaMKII system can be a minimal component of an erasable memory switch, and no complex molecular interaction with multiple synaptic targets is needed for enabling this function.
Materials and Methods
CaMKII expression and purification
Wild-type (WT) rat alpha-CaMKII cDNA and baculovirus containing alpha-CaMKII cDNA were kindly provided by Dr. Yasunori Hayashi (RIKEN Brain Science Institute) (15) and Haruhiko Bito (The University of Tokyo). The virus containing alpha-CaMKII was added to 175-cm2 monolayer cultures of Sf21 cells for 60–72 h, and the cells were then harvested and stored at −80°C until protein purification (13,16–19).
Alpha-CaMKII was purified essentially as described previously (13,16–19). Briefly, cell pellets were resuspended in lysis buffer (50 mM PIPES at pH 7.0, 1 mM EGTA, 1 mM EDTA, 2.5% Betaine, and 1× complete protease inhibitor cocktail (Roche, Basel, Switzerland)) (16), lysed by brief sonication, and clarified by centrifugation at 100,000 × g, 4°C for 0.5 h. The supernatant was loaded onto a 2 mL phosphocellulose column (P11 cation exchange resin; GE Healthcare, Buckinghamshire, UK) equilibrated with wash buffer (50 mM PIPES at pH 7.0, 200 μM EDTA, 100 mM NaCl, and 1× complete protease inhibitor cocktail) (19). The column was washed in wash buffer, and CaMKII was eluted with wash buffer plus 500 mM NaCl. The eluted fractions were then applied to a Sephacryl S300 Sepharose gel filtration column (GE healthcare) that had been equilibrated in gel filtration buffer (30 mM MOPS at pH 7.2, 200 mM KCl, 10 μM EDTA, and 1 mM 2-mercaptoethanol) (19). The CaMKII-containing fractions were collected and concentrated by a centrifugal filter unit (NMWL: 100 kDa, Amicon Ultra-4; Millipore, Bedford, MA) with gel filtration buffer plus 50% glycerol. 30–50 μM CaMKII (subunit concentration) was subdivided into 1.5 mL tubes, and stored at −80°C until use.
Protein phosphatase 1 expression and purification
The His-tagged protein phosphatase 1a (PP1a) catalytic subunit cDNA in the vector pDR540 was kindly provided by Dr. Angus Clark Nairn (Yale University) (13,20,21). Escherichia coli BL21 cells harboring the vector were grown at room temperature in 1 L Lennox broth (LB) medium with 50 mg/mL ampicillin and 100 mM CoCl2 until OD600 = 0.4, and the expression of PP1 was induced overnight with 0.5 mM isopropyl β-D-thiogalactoside. The cells were harvested and resuspended in resuspension buffer (10 mM Tris-HCl at pH 8.0, 30 mM imidazole, 10% glycerol, 300 mM NaCl, 1 mM CoCl2, and 1 mM phenylmethylsulfonyl fluoride). The cell suspension was lysed by 30 s sonication, and centrifuged at 30,000 × g, 4°C for 30 min. The supernatant was loaded onto a 2 mL Ni-NTA agarose column (Qiagen, Hilden, Germany) that had been equilibrated in resuspension buffer. The column was washed with wash buffer (30 mM MOPS at pH 7.2, 200 mM KCl, and 10% glycerol), and PP1 was eluted with wash buffer plus 400 mM imidazole. The PP1-containing fractions were pooled, applied to a HiTrap desalting column (GE Healthcare), equilibrated with wash buffer to remove imidazole, and concentrated using a centrifugal filter unit (NMWL: 10 kDa, Amicon Ultra-4; Millipore). Approximately 5 μM PP1 was subdivided into 1.5 mL tubes and stored at −80°C until use. Our PP1 had Km = 10 μM and kcat = 0.043 s−1 for dephosphorylation of CaMKII at T286.
Preparation of CaMKII phosphorylated at T286
After incubation with 19 μM free Ca2+ for 10 min at 4°C, almost all CaMKII showed an upward band shift in Phos-tag Western blotting (22), indicating phosphorylation. The shifted band reacted to the mouse monoclonal antibody 22B1, which specifically recognizes CaMKII phosphorylation at T286. We used this preparation of CaMKII phosphorylated at T286 as a phosphorylated ON state for coming down experiments. We used a twofold concentrated system of Ca2+-EGTA buffer for the preparation of indicated free Ca2+ solutions (see Figs. 1–3; Fig. S1 and Fig. S2) (13) and calculated the free Ca2+ concentrations based on a fluorescence titration with Fluo-4 (the dissociation constant of Ca2+ for EGTA, 110 nM; Invitrogen, Carlsbad, CA).
Figure 1.
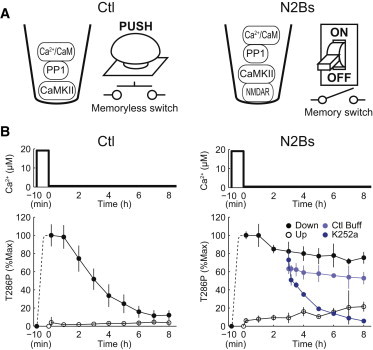
In vitro reconstitution of a memory switch comprising CaMKII and N2Bs. (A) In vitro reconstitution system with a Ctl peptide (GluN2B 1095−1119), where CaMKII functions as a memoryless switch (left), and with N2Bs (GluN2B 1289−1310), where CaMKII functions as a memory switch (right). (B) Ca2+ pulse stimulation (black); 20 μM Ca2+ for 10 min and 0.56 μM (basal Ca2+ concentration) afterward (top). T286 phosphorylation of CaMKII (T286P) with Ctl (bottom, left) or with N2Bs (bottom, right) in response to the pulse stimulation (solid circles) and to the basal Ca2+ concentration (open circles). The CaMKII kinase inhibitor (K252a) (dark blue circles) or control buffer (light blue circles) was added at 3 h. Error bars indicate mean ± SD (n ≥ 3). To see this figure in color, go online.
Figure 2.
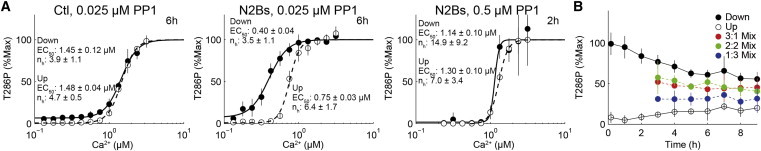
Hysteresis of T286 phosphorylation in the presence of N2Bs. (A) Coming down Ca2+ dose-response curve of T286 phosphorylation (T286P) from a phosphorylated ON state (solid circles) and that of going up from a dephosphorylated OFF state (open circles) in the absence (left) or the presence of N2Bs (middle and right). The concentrations of PP1 were 0.025 μM (left and middle) and 0.5 μM (right), and the incubation time was 6 h. Solid and dashed lines denote the best fits to the Hill equation. The EC50s and Hill coefficients (nh) are indicated with 95% confidence intervals. (B) T286 phosphorylated (black) and dephosphorylated (white) CaMKII at 3 h was mixed with ratios of 3:1 (red), 2:2 (green), or 1:3 (blue). To see this figure in color, go online.
Figure 3.
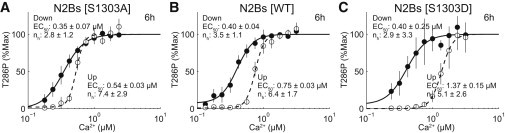
N2Bs phosphorylation state regulates the Ca2+ range of hysteresis. Going up (solid circles) and coming down (open circles) Ca2+ dose-response curves in the presence of the dephosphomimetic mutant peptide (S1303A) (A), the WT peptide (B), or the phosphomimetic peptide (S1303D) (C). Solid and dashed lines denote the best fits to the Hill equation. The EC50s and Hill coefficients (nh) are indicated with 95% confidence intervals.
CaMKII autophosphorylation assay
CaMKII autophosphorylation experiments were performed based on a previous study (13) at 4°C in 20 μL of 2 μM CaM, 0.025 μM PP1, 2 μM CaMKII (subunit concentration), 50 μM N2Bs or Ctl, 5 mM Ca2+-EGTA, 4 mM MgCl2, 2 mM ATP, 200 mM KCl, 30 mM MOPS at pH 7.2, and 0.1% w/v bovine serum albumin (BSA) (standard condition). All reactions in this study were performed under this standard condition, unless specified otherwise. N2Bs and Ctl peptides were derived from residues 1289−1310 (KAQKK NRNKL PRQHS YDTFV DL) and 1095−1119 (SAKSR REFDE IELAY RRRPP RSPDH) of GluN2B, respectively (GL Biochem, Shanghai, China) (8), and CaM was purchased from Wako Pure Chemicals (Osaka, Japan). For coming down experiments from a phosphorylated ON state of CaMKII, 8.4 μL of solution A (2.38× solution of CaM, PP1, CaMKII, N2Bs/Ctl, and BSA in 400 mM KCl and 30 mM MOPS at pH 7.2) was first added to 5 μL of solution B (2× solution of Ca2+-EGTA in 30 mM MOPS at pH 7.2) (Calcium Calibration Buffer Kit, Invitrogen). Then, 1.6 μL of solution C (12.5× solution of MgCl2 and ATP in 200 mM KCl and 30 mM MOPS at pH 7.2) was added to initiate a reaction for preparation of a phosphorylated ON state of CaMKII. The mixture of solutions A, B, and C gave 19 μM free Ca2+ in 15 μL of 2.7 μM CaM, 0.33 μM PP1, 2.7 μM CaMKII, 67 μM N2Bs or Ctl, 3.3 mM Ca2+-EGTA, 5.3 mM MgCl2, 2.7 mM ATP, 267 mM KCl, 30 mM MOPS at pH 7.2, and 0.13% w/v BSA. CaMKII was phosphorylated at T286 for 10 min incubation (22). Then, 5 μL of solution D (2× solution of K+-EGTA and Ca2+-EGTA mixture in 30 mM MOPS at pH 7.2) (Calcium Calibration Buffer Kit, Invitrogen) was added to obtain the final reaction condition with the indicated free Ca2+ concentration. For going up experiments from a dephosphorylated OFF state of CaMKII, the order of addition of solutions C and D was reversed, and solutions A, B, D, and C were sequentially added.
Autophosphorylation reactions were stopped by applying the reaction buffer to Laemmli’s sodium dodecyl sulfate (SDS) sample buffer, and the samples were subjected to SDS polyacrylamide gel electrophoresis, followed by immunoblotting with the mouse monoclonal antibody 22B1. T286 phosphorylation was quantified by the software Phoretix 1D (TotalLab, Newcastle upon Tyne, UK). The Ca2+ dose-responses of CaMKII phosphorylation were fitted by the Hill equation:
where Amax and Amin are the maximum and minimum of T286 phosphorylation, respectively, nh is the Hill coefficient, and EC50 is the half maximal effective concentration.
For the kinase inhibitor (K252a) experiment, 4 μL of 50 μM K252a in 25% v/v DMSO was added to 40 μl of the solution mixture 3 h after initiating the reaction (Fig. 1 B). A control experiment was performed using the same solution without K252a.
Results
Memory switch devices usually have two stable states (ON and OFF) and store information by switching between them. A push button is a typical memoryless switch because it responds just during stimulation (Fig. 1 A, left), whereas a toggle switch is a typical memory switch because it maintains a persistent response even after stimulation (Fig. 1 A, right). To examine whether CaMKII can actually serve as a memory switch, we reconstituted CaMKII phosphorylation in vitro based on an earlier study (13). Purified CaMKII together with CaM, PP1, ATP, and MgCl2 was incubated in a Ca2+-EGTA buffer system at 4°C where free Ca2+ concentration could be controlled (Fig. 1, A and B). We examined CaMKII phosphorylation in the presence of a control peptide (residues 1095−1119 of GluN2B, Ctl) or the GluN2B-derived peptide that has been shown to interact with CaMKII (residues 1289−1310, N2Bs) (8). Consistent with the previous finding (13), in the presence of Ctl, the addition of a Ca2+ pulse transiently increased phosphorylation of CaMKII at T286, which subsequently decreased to a basal level (Fig. 1 B, left black line). By contrast, in the presence of N2Bs, phosphorylation of CaMKII at T286 increased and was persistently retained (Fig. 1 B, right black line). The persistent phosphorylation was blocked by the addition of K252a, a CaMKII inhibitor, indicating that the persistent phosphorylation requires the kinase activity of CaMKII and is not due to irreversibility or slow dephosphorylation. This persistent phosphorylation is consistent with the previous finding that N2Bs locks CaMKII in an active conformation in vitro (8,9). However, unlike the previous finding, the persistent phosphorylation required basal Ca2+ concentration (0.56 μM). If the basal Ca2+ concentration was set below 0.2 μM, the phosphorylation of CaMKII at T286 decreased to a basal level within 6 h even in the presence of N2Bs (Fig. S1 B, T286P at 0.13 μM Ca2+).
Hysteresis is an essential property of a memory switch. Therefore, we examined whether reconstituted CaMKII phosphorylation exhibits hysteresis, which can be seen as a distinctive splitting of Ca2+ dose-response curves for coming down from a phosphorylated ON state and for going up from a dephosphorylated OFF state (13,14). We measured the going up Ca2+ dose-response from a dephosphorylated OFF state to a phosphorylated ON state by increasing Ca2+, and the coming down Ca2+ dose-response from a phosphorylated ON state to a dephosphorylated OFF state by decreasing Ca2+ (Fig. 2 A). As previously described (13), the going up and coming down Ca2+ dose-response curves in the presence of Ctl exhibited similar switch-like responses with comparable EC50 values (concentration of Ca2+ that gives 50% of the maximum response) (Fig. 2 A, left; Fig. S1 A). Although both Ca2+ dose-response curves exhibited similar switch-like responses in the presence of N2Bs, the coming down Ca2+ dose-response curve shifted to the left of the going up Ca2+ dose-response curve (Fig. 2 A, center; Fig. S1 B). This result indicates that CaMKII phosphorylation exhibits hysteresis. Six hours after the initiation of a reaction, CaMKII phosphorylation was maintained at a Ca2+ concentration between 0.2 and 0.75 μM in the coming down Ca2+ dose-response curve, which is lower than the EC50 of the going up Ca2+ dose-response curve. Thus, CaMKII phosphorylation can remember the previous Ca2+ concentration (23). In addition to being a Ca2+ concentration-dependent process, hysteresis of CaMKII phosphorylation is also dependent on PP1 concentration. Increasing the concentration of PP1 caused the hysteresis to completely disappear, independent of the presence of N2Bs (Fig. 2 A, right; Fig. S1 C). Thus, hysteresis of CaMKII phosphorylation is a Ca2+- and PP1-dependent reversible process. We saw persistent hysteresis for up to 17 h, although the difference in Ca2+ sensitivities (EC50) gradually decreased (Fig. S1).
CaMKII kinase activity was required for persistent phosphorylation (Fig. 1 B). This suggests that the persistent phosphorylation is due to a regenerative cycle of phosphorylation and dephosphorylation, rather than direct CaMKII activation by N2Bs binding. It has been reported that once CaMKII becomes activated by Ca2+/CaM in the absence of PP1, N2Bs keeps CaMKII persistently activated even after Ca2+ removal by the addition of EGTA (8). This irreversible activation of CaMKII by N2Bs binding is either a Ca2+- or PP1-independent process. Therefore, the hysteresis in this study is likely to be a different process from the previous observation in terms of Ca2+- and PP1-dependent reversibility (8). In addition, we did not observe the PP1 dose-dependent shift of the EC50, unlike the findings of a previous study (13). This PP1 dose-dependency of the EC50 may be due to the effect of imidazole in the PP1 solution, because imidazole has a large ionic strength and chelates Ca2+, which lowers the free Ca2+ concentration. Thus, imidazole may shift the apparent EC50 (13). We removed imidazole with a desalting column and did not see the PP1-dependency of the EC50.
We then analyzed whether persistent phosphorylation occurs in an intermolecular or intramolecular fashion. Phosphorylated ON and dephosphorylated OFF states of CaMKII were mixed in various ratios. The ratio between phosphorylated and nonphosphorylated CaMKII did not change for >6 h after mixing (Fig. 2 B), suggesting that cross-phosphorylation between holoenzymes did not occur and that the persistent phosphorylation occurred only by autophosphorylation within a holoenzyme. This result is consistent with the previous observation that cross-phosphorylation between holoenzymes does not occur ((24), but see (25)).
N2Bs contains the region of GluN2B with serine 1303 (S1303), a major CaMKII phosphorylation site, and S1303 phosphorylation has been reported to interfere with CaMKII binding to GluN2B (26–28). Indeed, the phosphomimetic (S1303D) and dephosphomimetic (S1303A) mutants of N2Bs have been shown to have lower and higher binding affinities to CaMKII, respectively (26–28). We asked whether these N2Bs mutants affected the hysteresis of CaMKII phosphorylation. Compared with WT N2Bs (see Fig. 3 B, dashed line; Fig. S2 B), the going up Ca2+ dose-response curve shifted left in the presence of N2Bs dephosphomimetic (S1303A) (Fig. 3 A, dashed line; Fig. S2 A) and shifted right in the presence of N2Bs phosphomimetic (S1303D) (Fig. 3 C, dashed line; Fig. S2 C). This suggests that dephosphorylated N2Bs is more effective than phosphorylated N2Bs at triggering CaMKII phosphorylation, consistent with the previous observation of lower and higher binding affinities of dephosphomimetic and phosphomimetic N2Bs to CaMKII, respectively (26–28). By contrast, the coming down Ca2+ dose-response curves appeared the same in the presence of any type of N2Bs (Fig. 3, solid lines; Fig. S2), indicating that the mutation of N2Bs only affects the going up process. Of importance, the hysteresis occurred regardless of the amino acid mutation at S1303 (Fig. 3; Fig. S2). As a result, CaMKII hysteresis had the narrower Ca2+ range in the presence of N2Bs dephosphomimetic (S1303A), whereas CaMKII hysteresis had the broader Ca2+ range of hysteresis in the presence of N2Bs phosphomimetic (S1303D). This heterogeneity in the Ca2+ range of hysteresis may contribute to a modifiable Ca2+ threshold for memory induction and persistent memory maintenance such as metaplasticity (29) in concert with other signaling mechanisms (30).
Discussion
Accumulating experimental evidence has clearly shown that the interaction between CaMKII and GluN2B is required in hippocampal LTP. The disruption of the interaction between CaMKII and GluN2B prevents synaptic translocation of CaMKII (9), persistent T286 phosphorylation (10), LTP induction (10,31), LTP maintenance (11,12), and extracellular signal-regulated kinase-dependent synaptic structural plasticity (32). The CaMKII and GluN2B interaction also controls spatial learning in behaving mice (10,33). Together with the function of the postsynaptic density to protect CaMKII from dephosphorylation (34), the CaMKII memory switch can provide a theoretical basis for the maintenance of modifiable and reversible LTP in the hippocampus (11,12). Maintenance of modifiable and reversible LTP cannot be attained by simple intermolecular irreversible binding reactions. Such irreversible binding reactions easily fall in a single stable steady state by noisy molecular interactions, and the coupled molecules do not function as a memory device. By contrast, the CaMKII memory switch accompanies Ca2+ and PP1 concentration-dependent hysteresis (Figs. 2 and 3). With this hysteresis, the dephosphorylated OFF state of CaMKII is robust against temporal noise of basal Ca2+ concentrations, and the dephosphorylated OFF state switches to a phosphorylated ON state only when the Ca2+ signal exceeds a modifiable threshold for LTP. Similarly, the phosphorylated ON state of CaMKII is robust against temporal noise of basal Ca2+ concentrations, and the phosphorylated ON state switches to a dephosphorylated OFF state only when PP1 concentration increases. Such phosphatase-dependent reversal of LTP (depotentiation) has been observed (35,36), and modeled (37).
A fluorescence resonance energy transfer experiment has shown that CaMKII is not persistently but transiently activated in spines by LTP inducing stimulation (38), suggesting that CaMKII does not serve as a memory switch. Similarly, a theoretical study has revealed that a CaMKII memory switch without GluN2B does not operate under physiological conditions (39). Our result indicates that the memory switch of CaMKII phosphorylation can be seen only in the presence of a GluN2B-derived peptide (N2Bs). This result supports the idea that GluN2B-associated CaMKII constitutes only a subpopulation of total spine population in LTP, and the signal of GluN2B-associated CaMKII activation is below the detectable level of multiphoton microscopy (3,10,38). Although this idea can account for the discrepancy among experiments (10,11,38), further studies in actual synapses are necessary to clarify the precise roles of CaMKII and GluN2B in LTP.
Hysteresis in Ca2+ sensitivity eventually disappeared under our experimental conditions (Fig. S1 and Fig. S2). This may raise the possibility that hysteresis is derived from slow convergence to a monostable state, rather than the bistable dynamics in the dynamical systems theory. However, considering that phosphorylation/dephosphorylation is a stochastic event, stochastic phosphorylation/dephosphorylation may account for the disappearance of hysteresis. Miller et al. (40) computationally analyzed the stochastic transition between the ON and OFF states of CaMKII phosphorylation, and they estimated that the ON/OFF state of T286 phosphorylation is stable over a few years. They obtained this long lifetime where the PP1 activity was saturated. In our experimental conditions, however, the PP1 activity was not saturated (2 μM CaMKII where Km = 10 μM of PP1 for dephosphorylation). With unsaturated PP1, the lifetime of the ON/OFF state can be much shorter (40). This short lifetime may explain the gradual decrease of hysteresis (Fig. S1 and Fig. S2).
Hysteresis in Ca2+ sensitivity occurred at Ca2+ concentrations between 0.2 and 0.75 μM, in the presence of 2 μM CaM (Fig. 2 A). This Ca2+ range is above the basal Ca2+ concentration in neurons (0.05−0.1 μM) (39,41), therefore one may think that the CaMKII memory switch does not operate in neurons. However, when CaM concentration was increased to 10 μM, hysteresis appeared between 0.12 and 0.5 μM Ca2+ (data not shown), and the region of hysteresis is expected to cover 0.05−0.1 μM Ca2+ with the 100 μM CaM in neurons (42). Thus, CaMKII can operate as a memory switch under physiological conditions.
The CaMKII memory switch was seen only in the presence of a GluN2B-derived peptide (N2Bs). Although the CaMKII memory switch could be explained by the conventional bistable mechanism in the dynamical systems theory (6,7,40,43), such bistable dynamics do not require GluN2B. Therefore, the CaMKII memory switch may actually be based on other mechanisms. Although the mechanisms are unknown, the slow T286 dephosphorylation caused by GluN2B binding to CaMKII (44) and the decreased dissociation rate of Ca2+/CaM from CaMKII (8) may not be the direct causes of hysteresis. The CaMKII memory switch required not only the presence of a GluN2B-derived peptide (N2Bs), but also >0.2 μM Ca2+ for CaMKII activation (Fig. 1 B, right; Fig. 2 A). If the Ca2+ concentration was below 0.2 μM, the dephosphorylation rates of CaMKII in the presence and absence of N2Bs was similar (Fig. S1). This result suggests that hysteresis requires both N2Bs binding and a Ca2+ concentration >0.2 μM. T286 phosphorylation leads to >1000-fold decrease in the dissociation rate of Ca2+/CaM from CaMKII (45), whereas N2Bs leads to only a 20-fold decrease (8). Despite the much stronger effect of T286 phosphorylation relative to N2Bs, the hysteresis did not appear in the absence of N2Bs, even when T286 phosphorylation occurred (Fig. 2, A and B) (13). This suggests that hysteresis does not occur by simply decreasing the dissociation rate of Ca2+/CaM from CaMKII.
Memory switches using artificial gene regulatory networks (46) and engineered molecules (47) have been proposed; however, few examples of memory switches in natural signaling systems have been clearly described (48,49). To our knowledge, this is the first in vitro reconstitution and description of an erasable memory switch based on enzymatic reactions of a natural signaling system. The CaMKII memory switch occurred within a single holoenzyme, and no cross-phosphorylation between holoenzymes occurred. This indicates that the memory density of CaMKII can be extremely high (i.e., each CaMKII holoenzyme can remember different information in a solution). Thus, the CaMKII memory switch provides a design principle for memory devices that are more energy efficient, high-speed, and high-density memory devices than switches using artificial gene regulatory networks.
Acknowledgments
We thank Y. Hayashi and H. Bito for CaMKII cDNA and baculovirus, and A.C. Nairn for His-tagged PP1 cDNA.
This work was supported by the Next Generation Integrated Living Matter Simulation, a Grant-in-Aid for Young Scientists (B) (#23700371), and the Strategic Research Program for Brain Sciences (SRPBS), by the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT), and by the Creation of Fundamental Technologies for Understanding and Control of Biosystem Dynamics, CREST, from the Japan Science and Technology (JST).
Contributor Information
Hidetoshi Urakubo, Email: urakubo-h@sys.i.kyoto-u.ac.jp.
Shinya Kuroda, Email: skuroda@bi.s.u-tokyo.ac.jp.
Supporting Material
References
- 1.Lisman J., Schulman H., Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat. Rev. Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- 2.Coultrap S.J., Bayer K.U. CaMKII regulation in information processing and storage. Trends Neurosci. 2012;35:607–618. doi: 10.1016/j.tins.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lisman J., Yasuda R., Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat. Rev. Neurosci. 2012;13:169–182. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolodziej S.J., Hudmon A., Stoops J.K. Three-dimensional reconstructions of calcium/calmodulin-dependent (CaM) kinase IIalpha and truncated CaM kinase IIalpha reveal a unique organization for its structural core and functional domains. J. Biol. Chem. 2000;275:14354–14359. doi: 10.1074/jbc.275.19.14354. [DOI] [PubMed] [Google Scholar]
- 5.Chao L.H., Stratton M.M., Kuriyan J. A mechanism for tunable autoinhibition in the structure of a human Ca2+/calmodulin- dependent kinase II holoenzyme. Cell. 2011;146:732–745. doi: 10.1016/j.cell.2011.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhabotinsky A.M. Bistability in the Ca2+/calmodulin-dependent protein kinase-phosphatase system. Biophys. J. 2000;79:2211–2221. doi: 10.1016/S0006-3495(00)76469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okamoto H., Ichikawa K. Switching characteristics of a model for biochemical-reaction networks describing autophosphorylation versus dephosphorylation of Ca2+/calmodulin-dependent protein kinase II. Biol. Cybern. 2000;82:35–47. doi: 10.1007/PL00007960. [DOI] [PubMed] [Google Scholar]
- 8.Bayer K.U., De Koninck P., Schulman H. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature. 2001;411:801–805. doi: 10.1038/35081080. [DOI] [PubMed] [Google Scholar]
- 9.Bayer K.U., LeBel E., De Koninck P. Transition from reversible to persistent binding of CaMKII to postsynaptic sites and NR2B. J. Neurosci. 2006;26:1164–1174. doi: 10.1523/JNEUROSCI.3116-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halt A.R., Dallapiazza R.F., Hell J.W. CaMKII binding to GluN2B is critical during memory consolidation. EMBO J. 2012;31:1203–1216. doi: 10.1038/emboj.2011.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanhueza M., Fernandez-Villalobos G., Lisman J. Role of the CaMKII/NMDA receptor complex in the maintenance of synaptic strength. J. Neurosci. 2011;31:9170–9178. doi: 10.1523/JNEUROSCI.1250-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanhueza M., McIntyre C.C., Lisman J.E. Reversal of synaptic memory by Ca2+/calmodulin-dependent protein kinase II inhibitor. J. Neurosci. 2007;27:5190–5199. doi: 10.1523/JNEUROSCI.5049-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradshaw J.M., Kubota Y., Schulman H. An ultrasensitive Ca2+/calmodulin-dependent protein kinase II-protein phosphatase 1 switch facilitates specificity in postsynaptic calcium signaling. Proc. Natl. Acad. Sci. USA. 2003;100:10512–10517. doi: 10.1073/pnas.1932759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrell J.E., Jr. Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr. Opin. Cell Biol. 2002;14:140–148. doi: 10.1016/s0955-0674(02)00314-9. [DOI] [PubMed] [Google Scholar]
- 15.Okamoto K., Narayanan R., Hayashi Y. The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure. Proc. Natl. Acad. Sci. USA. 2007;104:6418–6423. doi: 10.1073/pnas.0701656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brickey D.A., Colbran R.J., Soderling T.R. Expression and characterization of the alpha-subunit of Ca2+/calmodulin-dependent protein kinase II using the baculovirus expression system. Biochem. Biophys. Res. Commun. 1990;173:578–584. doi: 10.1016/s0006-291x(05)80074-9. [DOI] [PubMed] [Google Scholar]
- 17.Putkey J.A., Waxham M.N. A peptide model for calmodulin trapping by calcium/calmodulin-dependent protein kinase II. J. Biol. Chem. 1996;271:29619–29623. doi: 10.1074/jbc.271.47.29619. [DOI] [PubMed] [Google Scholar]
- 18.Singla S.I., Hudmon A., Schulman H. Molecular characterization of calmodulin trapping by calcium/calmodulin-dependent protein kinase II. J. Biol. Chem. 2001;276:29353–29360. doi: 10.1074/jbc.M101744200. [DOI] [PubMed] [Google Scholar]
- 19.Bradshaw J.M., Hudmon A., Schulman H. Chemical quenched flow kinetic studies indicate an intraholoenzyme autophosphorylation mechanism for Ca2+/calmodulin-dependent protein kinase II. J. Biol. Chem. 2002;277:20991–20998. doi: 10.1074/jbc.M202154200. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe T., da Cruz e Silva E.F., Nairn A.C. Preparation and characterization of recombinant protein phosphatase 1. Methods Enzymol. 2003;366:321–338. doi: 10.1016/s0076-6879(03)66024-6. [DOI] [PubMed] [Google Scholar]
- 21.Kelker M.S., Page R., Peti W. Crystal structures of protein phosphatase-1 bound to nodularin-R and tautomycin: a novel scaffold for structure-based drug design of serine/threonine phosphatase inhibitors. J. Mol. Biol. 2009;385:11–21. doi: 10.1016/j.jmb.2008.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinoshita E., Kinoshita-Kikuta E., Koike T. Phosphate-binding tag, a new tool to visualize phosphorylated proteins. Mol. Cell. Proteomics. 2006;5:749–757. doi: 10.1074/mcp.T500024-MCP200. [DOI] [PubMed] [Google Scholar]
- 23.Ferrell J.E., Jr. How regulated protein translocation can produce switch-like responses. Trends Biochem. Sci. 1998;23:461–465. doi: 10.1016/s0968-0004(98)01316-4. [DOI] [PubMed] [Google Scholar]
- 24.Hanson P.I., Meyer T., Schulman H. Dual role of calmodulin in autophosphorylation of multifunctional CaM kinase may underlie decoding of calcium signals. Neuron. 1994;12:943–956. doi: 10.1016/0896-6273(94)90306-9. [DOI] [PubMed] [Google Scholar]
- 25.Rose J., Jin S.X., Craig A.M. Heterosynaptic molecular dynamics: locally induced propagating synaptic accumulation of CaM kinase II. Neuron. 2009;61:351–358. doi: 10.1016/j.neuron.2008.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strack S., McNeill R.B., Colbran R.J. Mechanism and regulation of calcium/calmodulin-dependent protein kinase II targeting to the NR2B subunit of the N-methyl-D-aspartate receptor. J. Biol. Chem. 2000;275:23798–23806. doi: 10.1074/jbc.M001471200. [DOI] [PubMed] [Google Scholar]
- 27.Raveendran R., Devi Suma Priya S., Omkumar R.V. Phosphorylation status of the NR2B subunit of NMDA receptor regulates its interaction with calcium/calmodulin-dependent protein kinase II. J. Neurochem. 2009;110:92–105. doi: 10.1111/j.1471-4159.2009.06108.x. [DOI] [PubMed] [Google Scholar]
- 28.O’Leary H., Liu W.H., Bayer K.U. Nucleotides and phosphorylation bi-directionally modulate Ca2+/calmodulin-dependent protein kinase II (CaMKII) binding to the N-methyl-D-aspartate (NMDA) receptor subunit GluN2B. J. Biol. Chem. 2011;286:31272–31281. doi: 10.1074/jbc.M111.233668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Y.Y., Colino A., Malenka R.C. The influence of prior synaptic activity on the induction of long-term potentiation. Science. 1992;255:730–733. doi: 10.1126/science.1346729. [DOI] [PubMed] [Google Scholar]
- 30.Abraham W.C. Metaplasticity: tuning synapses and networks for plasticity. Nat. Rev. Neurosci. 2008;9:387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- 31.Barria A., Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 32.El Gaamouch F., Buisson A., Nicole O. Interaction between αCaMKII and GluN2B controls ERK-dependent plasticity. J. Neurosci. 2012;32:10767–10779. doi: 10.1523/JNEUROSCI.5622-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou Y., Takahashi E., Silva A.J. Interactions between the NR2B receptor and CaMKII modulate synaptic plasticity and spatial learning. J. Neurosci. 2007;27:13843–13853. doi: 10.1523/JNEUROSCI.4486-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mullasseril P., Dosemeci A., Griffith L.C. A structural mechanism for maintaining the ‘on-state’ of the CaMKII memory switch in the post-synaptic density. J. Neurochem. 2007;103:357–364. doi: 10.1111/j.1471-4159.2007.04744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang C.C., Liang Y.C., Hsu K.S. Characterization of the mechanism underlying the reversal of long term potentiation by low frequency stimulation at hippocampal CA1 synapses. J. Biol. Chem. 2001;276:48108–48117. doi: 10.1074/jbc.M106388200. [DOI] [PubMed] [Google Scholar]
- 36.Kang-Park M.H., Sarda M.A., Wilson W.A. Protein phosphatases mediate depotentiation induced by high-intensity theta-burst stimulation. J. Neurophysiol. 2003;89:684–690. doi: 10.1152/jn.01041.2001. [DOI] [PubMed] [Google Scholar]
- 37.Pi H.J., Lisman J.E. Coupled phosphatase and kinase switches produce the tristability required for long-term potentiation and long-term depression. J. Neurosci. 2008;28:13132–13138. doi: 10.1523/JNEUROSCI.2348-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee S.J., Escobedo-Lozoya Y., Yasuda R. Activation of CaMKII in single dendritic spines during long-term potentiation. Nature. 2009;458:299–304. doi: 10.1038/nature07842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michalski P.J. The delicate bistability of CaMKII. Biophys. J. 2013;105:794–806. doi: 10.1016/j.bpj.2013.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller P., Zhabotinsky A.M., Wang X.J. The stability of a stochastic CaMKII switch: dependence on the number of enzyme molecules and protein turnover. PLoS Biol. 2005;3:e107. doi: 10.1371/journal.pbio.0030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maravall M., Mainen Z.F., Svoboda K. Estimating intracellular calcium concentrations and buffering without wavelength ratioing. Biophys. J. 2000;78:2655–2667. doi: 10.1016/S0006-3495(00)76809-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faas G.C., Raghavachari S., Mody I. Calmodulin as a direct detector of Ca2+ signals. Nat. Neurosci. 2011;14:301–304. doi: 10.1038/nn.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Graupner M., Brunel N. STDP in a bistable synapse model based on CaMKII and associated signaling pathways. PLOS Comput. Biol. 2007;3:e221. doi: 10.1371/journal.pcbi.0030221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheriyan J., Kumar P., Omkumar R.V. Calcium/calmodulin dependent protein kinase II bound to NMDA receptor 2B subunit exhibits increased ATP affinity and attenuated dephosphorylation. PLoS ONE. 2011;6:e16495. doi: 10.1371/journal.pone.0016495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meyer T., Hanson P.I., Schulman H. Calmodulin trapping by calcium-calmodulin-dependent protein kinase. Science. 1992;256:1199–1202. doi: 10.1126/science.256.5060.1199. [DOI] [PubMed] [Google Scholar]
- 46.Purnick P.E., Weiss R. The second wave of synthetic biology: from modules to systems. Nat. Rev. Mol. Cell Biol. 2009;10:410–422. doi: 10.1038/nrm2698. [DOI] [PubMed] [Google Scholar]
- 47.Bashor C.J., Helman N.C., Lim W.A. Using engineered scaffold interactions to reshape MAP kinase pathway signaling dynamics. Science. 2008;319:1539–1543. doi: 10.1126/science.1151153. [DOI] [PubMed] [Google Scholar]
- 48.Xiong W., Ferrell J.E., Jr. A positive-feedback-based bistable ‘memory module’ that governs a cell fate decision. Nature. 2003;426:460–465. doi: 10.1038/nature02089. [DOI] [PubMed] [Google Scholar]
- 49.Ozbudak E.M., Thattai M., Van Oudenaarden A. Multistability in the lactose utilization network of Escherichia coli. Nature. 2004;427:737–740. doi: 10.1038/nature02298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


